Abstract
Cysteinyl leukotriene (cysLT) overproduction and eosinophil activation are hallmarks of aspirin-exacerbated respiratory disease (AERD). However, pathogenic mechanisms of AERD remain to be clarified. Here, we aimed to find the significance of transforming growth factor beta 1 (TGF-β1) in association with cysteinyl leukotriene E4 (LTE4) production, leading to eosinophil degranulation. To evaluate levels of serum TGF-β1, first cohort enrolled AERD (n = 336), ATA (n = 442) patients and healthy control subjects (HCs, n = 253). In addition, second cohort recruited AERD (n = 34) and ATA (n = 25) patients to investigate a relation between levels of serum TGF-β1 and urinary LTE4. The function of TGF-β1 in LTE4 production was further demonstrated by ex vivo (human peripheral eosinophils) or in vivo (BALB/c mice) experiment. As a result, the levels of serum TGF-β1 were significantly higher in AERD patients than in ATA patients or HCs (P = .001; respectively). Moreover, levels of serum TGF-β1 and urinary LTE4 had a positive correlation (r = 0.273, P = .037). In the presence of TGF-β1, leukotriene C4 synthase (LTC4S) expression was enhanced in peripheral eosinophils to produce LTE4, which sequentially induced eosinophil degranulation via the p38 pathway. When mice were treated with TGF-β1, significantly induced eosinophilia with increased LTE4 production in the lung tissues were noted. These findings suggest that higher levels of TGF-β1 in AERD patients may contribute to LTE4 production via enhancing LTC4S expression which induces eosinophil degranulation, accelerating airway inflammation.
Introduction
Aspirin-exacerbated respiratory disease (AERD) typically presents moderate-to-severe phenotypes of asthma, chronic rhinosinusitis (CRS) and/or nasal polyps with persistent eosinophilia in the upper and lower airway mucosa. In addition, cysteinyl leukotriene (cysLT) overproduction is a hallmark of AERD, and the increased level of cysLTs derived from mast cells and eosinophils is a characteristic feature of AERD, in which leukotriene C4 synthase (LTC4S) is a key enzyme for converting arachidonic acid to cysLTs [1]. To date, type 1 cysteinyl leukotriene receptor (cysLT1R) antagonists and 5-lipoxygenase inhibitors have been used in the management of AERD [2]; however, there are unmet needs for its pathogenic mechanisms and therapeutic targets.
Eosinophilia in peripheral blood and upper/lower airway mucosa are commonly found in AERD patients [3]. Although both mast cells and eosinophils are critical for inducing airway inflammation in the pathogenesis of AERD, emerging evidence supports an important role of eosinophils in its pathogenesis [4,5]. AERD patients have shown that significantly elevated levels of eosinophil-derived granule proteins, such as eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN), compared to aspirin-tolerant asthma (ATA) patients [6,7]. The direct effect of aspirin on eosinophil activation on releasing granule proteins has previously been demonstrated [8]. In addition, some studies have suggested that granule proteins play a crucial role in enhancing Th2 immune response among allergic diseases [9,10].
Transforming growth factor beta 1 (TGF-β1) has also been suggested to contribute to immune responses and structural changes in the lungs of asthmatic patients [11]. Moreover, this mediator is strongly expressed in nasal mucosa in response to inflammation, but not in normal nasal mucosa [12]. So far, the role of TGF-β1 in airway remodeling has mostly been highlighted; however, a few studies have shown that TGF-β1 down-regulates cyclooxygenase (COX)-2 in airway epithelial cells and then reduces prostaglandin E2 production [13]. Furthermore, enhanced LTC4S expression in fibroblasts and monocytes in the presence of TGF-β1 has been revealed [14,15], suggesting that TGF-β1 may contribute to cysLT production in AERD patients. Considering that persistent eosinophilic inflammation is a key feature of AERD and that studies evaluating the function of TGF-β1 in eosinophilic airway inflammation are still lacking, the present study focuses on the effect of TGF-β1 on eosinophil activation in AERD pathogenesis.
We hypothesized that TGF-β1 plays an important role in the severity of eosinophilic airway inflammation in AERD patients. This study compared the levels of serum TGF-β1 between AERD and ATA patients, investigated the association between TGF-β1 and LTE4 in the clinical cohort, and evaluated the effect of TGF-β1 on LTE4 production ex vivo or in vivo.
Materials and methods
Ethics
Two cohorts of adult asthmatic patients were assessed in this study approved by the Institutional Review Board of Ajou University Hospital (AJIRB-GEN-SMP-13-108; AJIRB-BMR-SUR-15-498). All patients provided written informed consent to participate in this study by signing the consent form.
Patient cohorts, clinical parameters and serum cytokine levels
The clinical significance of TGF-β1 level in AERD was evaluated in which AERD (n = 336), ATA (n = 442) patients and healthy control subjects (HCs; n = 253) were recruited. Among AERD and ATA patients enrolled in the first cohort, we enrolled AERD (n = 34) and ATA (n = 25) patients who had wanted to participate voluntarily in the second cohort study to investigate the role of TGF-β1 in association with LTE4 metabolite levels/eosinophil activation markers. AERD were diagnosed according to clinical features previously described [16]. The diagnosis of AERD was based on a positive response to lysine-aspirin bronchoprovocation test (L-ASA BPT). The presence of CRS and nasal polyps was confirmed using paranasal sinus X-rays, CT scans and/or rhinoscopy as well as clinical symptoms. The degree of airway obstruction was evaluated using spirometry. The degree of airway hyperresponsiveness was examined by methacholine bronchial challenge test. Atopy status was defined as previously described [17]. The levels of serum IgE were quantified using UniCAP® system (ThermoFisher Scientific, Waltham, MA, USA). The levels of serum TGF-β1 from every study subject were measured using ELISA (R&D systems, Minneapolis, MN, USA). Sputum collection and neutrophils/eosinophils counting were performed as previously described [18], which were always the same between the first and second cohorts. To determine TGF-β1-low/high groups, the cutoff value (48.1 ng/mL) was set at mean plus 2 standard deviations of the test values. In the second cohort, the urine and serum of each patient were simultaneously collected in the morning time during the enrollment period. The urinary LTE4 metabolite levels were measured using ultra-high-performance liquid chromatography system as previously described [19]. In addition, the levels of serum EDN were measured using the ELISA kit (SKIMS-BIO, Seoul, Korea).
Stimulation of peripheral eosinophils from asthmatic patients
Peripheral eosinophils were isolated from asthmatic patients as previously described [20]. To stimulate eosinophils, the cells (1×106) were seeded on a 24-well plate and maintained in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 2% fetal bovine serum (FBS; ThermoFisher Scientific). Then, the cells were treated with human recombinant 10 ng/mL IL-5 (Sigma-Aldrich) and 5 ng/mL TGF-β1 (R&D systems). To investigate the effect of cysLTs on eosinophil degranulation, the cells were treated with LTE4 (Cayman Chemical, Ann Arbor, MI, USA) for 4 hours in the presence of 10 ng/mL IL-5 (Sigma-Aldrich). The function of montelukast (Sigma-Aldrich; 0.1 and 1 μM) against LTE4 was also investigated. To confirm eosinophil degranulation, eosinophils were seeded on Poly L-lysine-coated slides (Polysciences, Warrington, PA, USA). Then the cells were incubated overnight with anti-eosinophil peroxidase antibody (Cell Signaling, Minneapolis, MN, USA), followed by Alexa fluor 488 donkey anti-rabbit (ThermoFisher Scientific) for 1 hour. 4’,6-diamidino-2-phenylindole (Sigma-Aldrich), and was observed using a Zeiss LSM710 confocal microscope (Carl Zeiss AG, Oberkochen, Germany).
Interactions between eosinophils and airway epithelial cells
A549 cells (American Type Culture Collection, Manassas, VA, USA) were used to investigate the role of airway epithelial cells in eosinophilic airway inflammation. The cells (5×105) were seeded on a 24-well plate in RPMI with 10% FBS. Then RPMI with 2% was used when A549 cells were treated with peripheral eosinophils (1×106) from asthmatic patients for 24 hours. In addition, A549 cells were treated with EDN (Athens Research & Technology, Athens, GA, USA; 1, 10 and 100 ng/mL) for 24 hours to demonstrate the effect of granule proteins on airway epithelial cell stimulation. To collect supernatant, culture medium was centrifuged at 12,000 rpm for 20 min at 4°C.
Polymerase chain reaction
Total RNA was isolated from human peripheral eosinophils using TRIzol® (ThermoFisher Scientific), according to the manufacturer’s instructions. Then, 1 μg of total RNA was synthesized to the single-stranded cDNA using primers (LTC4S, Forward: 5’-AGGTGGGCTGGTTCCTATCTA-3’ and Reverse: 5′-CCCATGGCTATCCTACCATTT-3′; GAPDH, Forward: 5’-GCAAAGTCAAGGCTGAGAAC-3’ and Reverse: 5’-ATGGTGGTGAAGACGCCAGT-3’). The PCR products were separated by electrophoresis using a 1% ethidium bromide-stained agarose gel and visualized by ultraviolet transillumination.
Western blot analysis
To separate proteins (total protein concentration of cell lysate; 50 μg), 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis was used. Then the gels were transferred to PVDF membrane (BIO-RAD, Hercules, CA, USA). The antibodies used were as follows: TGF-β1 receptor (TGFR1; Abcam, Cambridge, United Kingdom; 1:1,000; 45 kDa), TGF-β2 receptor (TGFR2; Abcam; 1:1,000; 75 kDa), LTC4S (Sigma-Aldrich; 1:500; 40 kDa), p38 (Cell Signaling Technology; 1:1000; 38 kDa), phospho-p38 (Cell Signaling Technology; 1: 500; 38 kDa), and actin (Santa Cruz, Dallas, TX, USA; 1:1,000; 42 kDa).
In vivo mouse model
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Ajou University (IACUC-2017-0067). Female 6-week-old BALB/c wild-type mice (Jackson Laboratory, Bar Harbor, ME, USA) were maintained under specific pathogen-free conditions. To demonstrate the effect of TGF-β1 on LTE4 production, mice (n = 6 mice per group) were intranasally injected with 0.1 μg of mouse recombinant TGF-β1 (R&D systems) for 5 days. Eosinophil numbers in bronchoalveolar lavage fluid (BALF) were determined by Diff-quick staining (Dade Behring, Dudingen, Switzerland). Moreover, LTE4 (MyBioSource, San Diego, CA, USA) and EDN (LifeSpan BioSciences, Seatle, WA, USA) in BALF were measured using ELISA kits.
Statistical analysis
All statistical analyses were performed using IBM SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). P values < .05 was considered statistically significant. GraphPad Prism 8.0 software (GraphPad Inc., San Diego, CA, USA) was used to create graphs.
Results
Higher levels of serum TGF-β1 in AERD patients
Demographic data from the study subjects of the first cohort are described in Table 1. The presence of nasal polyps and decrease in FEV1 (%) after lysine-aspirin bronchoprovocation test were significantly higher in AERD patients than in ATA patients (P = .001 and P = .001, respectively). In addition, lower baseline FEV1 (%) and PC20 methacholine values were noted in the AERD patients compared to the ATA patients (P = .026 and P = .001, respectively), whereas total IgE, total eosinophil count and sputum eosinophil/neutrophils (%) were not significantly different between the 2 groups. However, the levels of serum TGF-β1 were significantly higher in the AERD patients than in the ATA patients (P = .001). When asthmatic patients were divided into the TGF-β1-low and -high subgroups (the cutoff value, 48.1 ng/mL), the TGF-β1-high subgroup showed lower baseline FEV1 (%) than the TGF-β1-low subgroup within the AERD group (P = .034), while no differences were found within the ATA group (Table 2). In this study, we enrolled the second cohort to verify the reproducibility of clinical data. As in the result of the first cohort, the levels of serum TGF-β1 were also significantly higher in the AERD group than in the ATA group (P = .026; Table 3). In addition, the levels of urinary LTE4 were significantly higher in the AERD group than those in the ATA group (P = .001; Table 3). These findings indicate that higher levels of TGF-β1 may have an important role in AERD pathogenesis.
Table 1. Demographic data from the study subjects enrolled in the first cohort of adult asthmatic patients.
| Variables | AERD (n = 336) | ATA (n = 442) | HCs (n = 253) | P value | ||
|---|---|---|---|---|---|---|
| AERD vs.ATA | AERD vs. HCs | ATA vs. HCs | ||||
| Age (y) | 42.2 ± 13.9/336 | 44.7 ± 14.4/442 | 31.6 ± 10.6/253 | .018 | .001 | .001 |
| Female sex (%) | 64.9/336 | 61.9/442 | 54.7/253 | .385 | .012 | .065 |
| Atopy (%) | 52.1/330 | 48.8/412 | 28.0/200 | .367 | .001 | .001 |
| Nasal polyp (%) | 42.1/271 | 18.2/148 | NA | .001 | NA | NA |
| Severe asthma (%) | 22.5/329 | 16.8/440 | NA | .048 | NA | NA |
| Baseline FEV1 (%) | 85.3 ± 20.2/316 | 88.8 ± 19.6/335 | NA | .026 | NA | NA |
| Fall of FEV1 (%) | 16.1 ± 5.7/189 | 6.5 ± 3.8/185 | NA | .001 | NA | NA |
| PC20 (mg/mL) | 3.2 ± 4.6/239 | 5.3 ± 5.7/246 | NA | .001 | NA | NA |
| Total IgE (kU/L) | 358.4 ± 550.1/328 | 365.9 ± 590.0/410 | 74.0 ± 111.6/66 | .857 | .001 | .001 |
| TEC (/μL) | 413.9 ± 412.2/310 | 384.0 ± 368.1/344 | NA | .327 | NA | NA |
| Sputum Eos (%) | 23.9 ± 35.2/217 | 21.6 ± 32.5/216 | NA | .470 | NA | NA |
| Sputum Neu (%) | 57.5 ± 34.6/166 | 59.6 ± 33.2/192 | NA | .561 | NA | NA |
| TGF-β1 (ng/mL) | 33.1 ± 14.2/191 | 28.4 ± 15.7/304 | 22.5 ± 11.3/175 | .001 | .001 | .021 |
P values were obtained by Pearson’s Chi-square test for categorical variables (sex, atopy, nasal polyp, severe asthma, baseline FEV1, Fall of FEV1, sputum Eos and Neu) and Student’s t test for continuous variables (age, PC20, total IgE, TEC, TGF-β1).
AERD, aspirin-exacerbated respiratory disease; ATA, aspirin-tolerant asthma; HCs, healthy control subjects; FEV1, forced expiratory volume in 1 s; PC20, the provocative concentration of methacholine required to cause a 20% fall in FEV1; Fall of FEV1; decrease in FEV1 after the inhalation of lysin aspirin; IgE, immunoglobulin E; TEC, total eosinophil count; Eos, eosinophils; Neu, neutrophils; NA, not available.
Table 2. Characteristics of asthmatic patients with high (≥48.1 ng/mL) and low TGF-β1 (<48.1 ng/mL) levels in the first cohort.
| Variables | AERD | P value | ATA | P value | ||
|---|---|---|---|---|---|---|
| High (n = 23) | Low (n = 168) | High (n = 27) | Low (n = 277) | |||
| Age (y) | 40.0 ± 13.8/23 | 43.9 ± 13.2/168 | .192 | 41.5 ± 11.9/27 | 45.8 ± 14.7/277 | .149 |
| Female sex (%) | 56.5/23 | 70.8/168 | .164 | 59.3/27 | 60.3/277 | .917 |
| Atopy (%) | 43.5/23 | 46.7/165 | .774 | 54.2/24 | 50.0/256 | .696 |
| Nasal polyps (%) | 57.1/21 | 47.5/118 | .413 | 42.9/7 | 14.0/57 | .056 |
| Severe asthma (%) | 30.4/23 | 29.3/167 | .914 | 25.9/27 | 19.3/274 | .414 |
| Baseline FEV1 (%) | 75.4 ± 19.2/21 | 85.5 ± 20.2/153 | .034 | 82.5 ± 23.6/16 | 89.2 ± 20.0/207 | .207 |
| PC20 (mg/mL) | 2.1 ± 2.7/16 | 3.5 ± 4.8/115 | .278 | 7.2 ± 7.0/23 | 6.2 ± 6.2/255 | .577 |
| Total IgE (kU/L) | 303.5 ± 285.5/23 | 363.4 ± 619.2/164 | .649 | 553.8 ± 910.6/22 | 371.7 ± 599.0/255 | .193 |
| TEC (/μL) | 352.3 ± 282.4/23 | 425.9 ± 410.2/150 | .408 | 496.7 ± 370.4/13 | 368.1 ± 352.5/210 | .204 |
| Sputum Eos (%) | 26.6 ± 39.5/19 | 21.1 ± 34.1/122 | .520 | 24.5 ± 30.6/10 | 19.0 ± 32.1/141 | .602 |
| Sputum Neu (%) | 58.0 ± 39.2/13 | 54.4 ± 34.2/95 | .727 | 62.3 ± 29.8/9 | 62.7 ± 33.8/118 | .973 |
P values were obtained by Pearson’s Chi-square test for categorical variables and Student’s t test for continuous variables.
Table 3. Demographic data of the study subjects enrolled in the second cohort.
| Variables | AERD (n = 34) | ATA (n = 25) | P value |
|---|---|---|---|
| Age (y) | 44.5 ± 10.3/34 | 49.2 ± 19.1/25 | .266 |
| Female sex (%) | 70.6/34 | 76.0/25 | .770 |
| Atopy (%) | 32.4/34 | 40.0/25 | .544 |
| Nasal polyp (%) | 64.0/25 | 18.2/11 | .014 |
| Severe asthma (%) | 52.9/34 | 32.0/25 | .109 |
| Baseline FEV1 (%) | 86.6 ± 20.3/30 | 94.5 ± 15.3/15 | .195 |
| Fall of FEV1 (%) | 17.8 ± 4.5/21 | 5.4 ± 2.1/9 | .001 |
| PC20 (mg/mL) | 3.2 ± 4.3/25 | 4.8 ± 5.6/16 | .308 |
| Total IgE (kU/L) | 232.7 ± 242.9/32 | 280.2 ± 312.8/20 | .542 |
| TEC (/μL) | 493.3 ± 292.9/30 | 428.9 ± 280.5/16 | .475 |
| Sputum Eos (%) | 30.8 ± 41.6/25 | 20.8 ± 32.3/15 | .429 |
| Sputum Neu (%) | 36.6 ± 37.0/19 | 59.8 ± 34.0/14 | .411 |
| TGF-β1 (ng/mL) | 36.9 ± 15.2/34 | 27.7 ± 15.3/25 | .026 |
| LTE4 (ng/mL creatinine) | 0.4 ± 0.3/34 | 0.1 ± 0.2/25 | .001 |
P values were obtained by Pearson’s Chi-square test for categorical variables and Student’s t test for continuous variables.
Function of TGF-β1 in LTC4S expression and LTE4 production
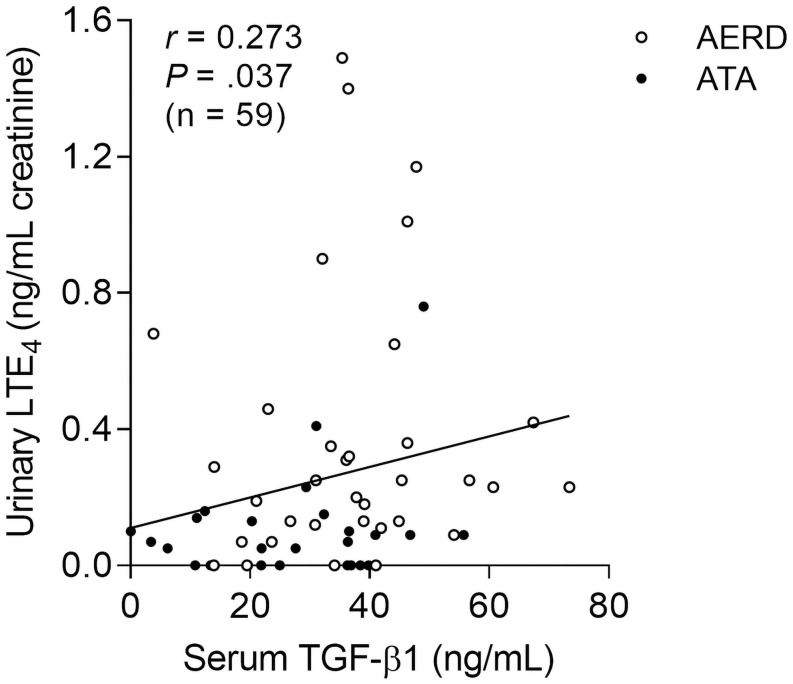
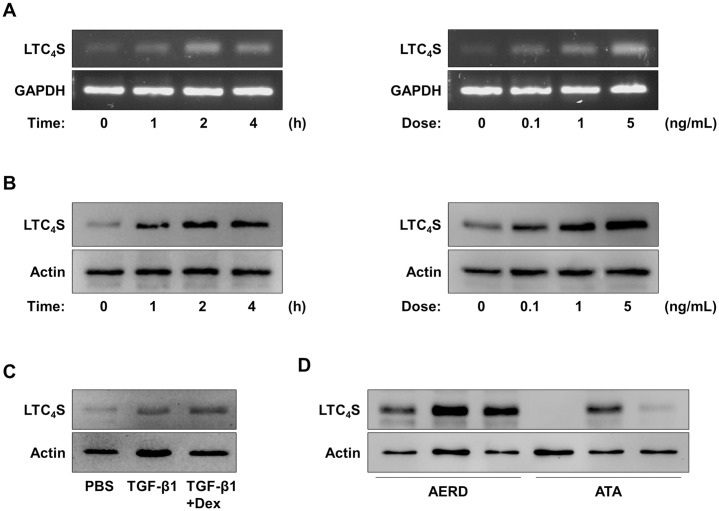
The levels of serum TGF-β1 and urinary LTE4 showed a significantly positive correlation (r = 0.273, P = .037; Fig 1). When peripheral eosinophils from asthmatic patients were treated with TGF-β1, expression of LTC4S in the cells was markedly upregulated (Fig 2A). In addition, levels of LTC4S in the cells were enhanced by TGF-β1 treatement (Fig 2B), but dexamethasone did not effectively reduce levels of LTC4S (Fig 2C). In the presence of TGF-β1, high levels of LTC4S with increased in peripheral eosinophils from the AERD patients was noted compared to those of the ATA patients (Fig 2D). Moreover, the TGF-β1 receptor (TGFR1) within eosinophils was highly expressed in peripheral eosinophils from the AERD patients than those from the ATA patients, while TGFR2 was not (S1 Fig). These results imply that eosinophils from AERD patients may be more sensitive to TGF-β1 in association with LTE4 production because of highly expressed TGFR1 on the surface of eosinophils.
Fig 1. Association between levels of serum TGF-β1 and urinary LTE4 in the study subjects.
Data are represented as Spearman correlation coefficient r (P value).
Fig 2. Effect of TGF-β1 on LTC4S expression in human peripheral eosinophils.
Effect of TGF-β1 on (A) LTC4S expression and (B) LTC4S levels in peripheral eosinophils in a time- or dose-dependent manner (samples from 3 asthmatic patients were pooled). (C) Function of dexamethasone against TGF-β1 treatment (samples from 3 asthmatic patients were pooled). (D) Comparison of LTC4S levels between ARED and ATA patients (n = 3 asthmatic patients per group).
Induction of eosinophil degranulation by LTE4 treatment
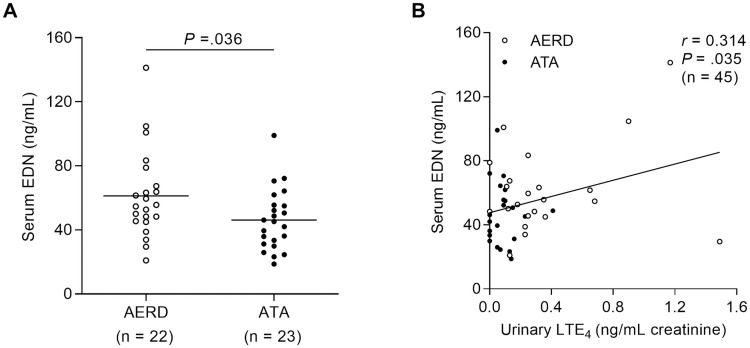
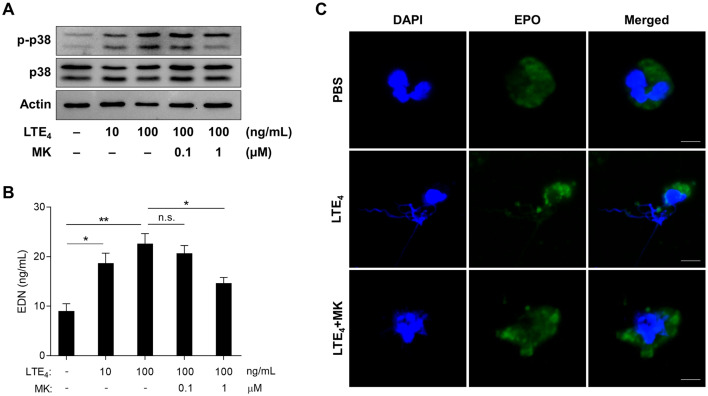
As TGF-β1 markedly enhanced LTE4 production by eosinophils, sequential effects of LTE4 on peripheral eosinophils were further investigated. In this study, we found significantly elevated levels of serum EDN in the AERD group compared to the ATA group (P = .036; Fig 3A). In addition, the levels of urinary LTE4 were positively correlated with serum EDN (r = 0.314, P = .035; Fig 3B). When peripheral eosinophils from asthmatic patients were treated with LTE4, phosphorylation of p38 was significantly elevated in the cells; however, montelukast (cysLT receptor 1 antagonist) could inhibit phosphorylation of signaling molecules (Fig 4A). In addition, LTE4 enhanced levels of EDN released from the eosinophils (Fig 4B). The eosinophils observed using confocal microscopy also showed granule proteins released by LTE4 stimulation (Fig 4C), indicating that LTE4 is important for inducing eosinophil degranulation through the p38 pathway.
Fig 3. Relation between levels of urinary LTE4 and serum EDN.
(A) Levels of serum EDN in the study subjects. Data are presented as mean. P values were obtained by Student’s t test. (B) A correlation between the levels of serum EDN and urinary LTE4. Data are represented as Spearman correlation coefficient r (P value).
Fig 4. Function of LTE4 in eosinophil degranulation.
(A) Phosphorylation of p38 in peripheral eosinophils (samples from 3 asthmatic patients were pooled). (B) Levels of EDN released from the cells. Data are presented as mean ± SD, n = 5. *P < .05 and **P < .01 were obtained by the Mann-Whitney test. n.s., not significant. (C) Images of eosinophils observed using confocal microscopy. Scale bar, 5 μm. DAPI, 4′,6-diamidino-2-phenylindole (blue); EPO, eosinophil peroxidase (green); MK, montelukast.
Effect of granule proteins in airway epithelial cells
When airway epithelial cells (A549 cells) were co-cultured with peripheral blood eosinophils with/without LTE4, significantly elevated levels of TGF-β1 in culture supernatant were noted; however, the effect of eosinophils in airway epithelial cells was partially attenuated by montelukast treatment (S2 Fig). In particular, EDN could enhance TGF-β1 production from airway epithelial cells. Although dexamethasone tends to inhibit the effect of granule proteins in airway epithelial cells, it could not fully attenuate TGF-β1 production from the cells (S3 Fig), suggesting limited action of corticosteroids against eosinophil granule proteins.
Enhanced LTE4 and EDN production by TGF-β1 treatment in vivo
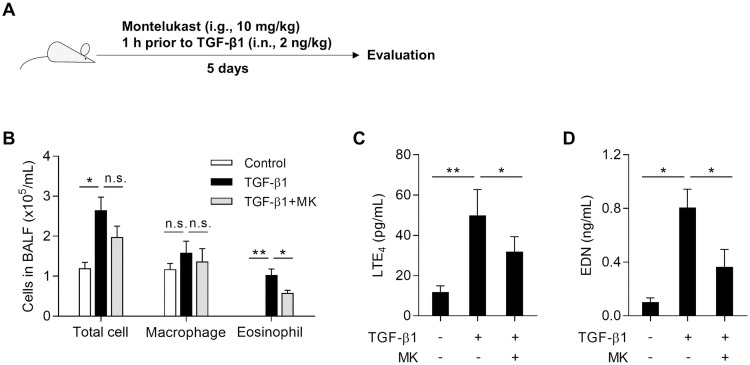
To demonstrate the effect of TGF-β1 on cysLT production, mice were intranasally injected with TGF-β1 with or without montelukast for every 5 days (Fig 5A). As a result, the total cell and eosinophil but not macrophage number in BALF was markedly elevated in mice treated with TGF-β1. Although montelukast did not fully reduce total cell count, the number of eosinophils was significantly decreased (Fig 5B). In addition, production of LTE4 and EDN was markedly elevated when mice were treated with TGF-β1, but montelukast could attenuated these mediators (Fig 5C and 5D). These findings show that TGF-β1 may contribute to eosinophilic airway inflammation through induction of LTE4 production.
Fig 5. Roles of TGF-β1 in the lipoxygenase pathway to produce LTE4 in vivo.
(A) Experimental schedule. (B) Differential cell count. Levels of (C) LTE4 and (D) EDN in bronchoalveolar lavage fluid. Data are presented as mean ± SD, n = 6. *P < .05 and **P < .01 were obtained by the Mann-Whitney test. n.s., not significant. i.n., intranasal injection; i.g., intragastric administration; MK, montelukast.
Discussion
This is the first study to demonstrate the pathophysiological function of TGF-β1 in AERD in the 2 clinical cohorts of adult asthmatic patients. It was found that the levels of serum TGF-β1 were higher in the AERD patients than in the ATA patients. AERD patients with higher TGF-β1 levels had lower FEV1 (%) and PC20 methacholine values, suggesting that TGF-β1 may be involved in the lung. In addition, ex vivo and in vivo studies confirmed an association between TGF-β1 and cysLT overproduction in AERD pathogenesis. Furthermore, increased LTE4 could induce eosinophil degranulation, which further stimulates airway epithelial cells to produce TGF-β1, resulting in the formation of the vicious circle. These provide a new insight into AERD pathogenesis via the TGF-β1-LTE4-eosinophil axis.
In the present study, significantly elevated levels of serum TGF-β1 were noted in the AERD patients compared to the ATA patients having a positive correlation with the levels of urinary LTE4. Previously, TGF-β1 polymorphisms have been suggested as a risk factor for AERD development, and TGF-β1 was associated with the prevalence of CRS in AERD patients, but not in ATA patients [21]. Nevertheless, the function of TGF-β1 in the pathogenesis of AERD has not been fully understood. Therefore, we aimed to find the functional effect of TGF-β1 in eosinophils, especially LTE4 production and eosinophil activation. Although the role of TGF-β1 in the arachidonic acid pathway was not well studied, a previous study revealed that TGF-β1 contributed to changes in LTC4S expression and cysLT1R in astrocytes [22]. Our ex vivo study demonstrated significantly enhanced LTC4S expression and LTE4 production in response to TGF-β1 in peripheral eosinophils from asthmatic patients. These suggest that TGF-β1 may be an essential factor for LTE4 production.
Here, we found highly expressed TGFR1, but not TGFR2 in peripheral eosinophils from AERD patients than from ATA patients. Previously, TGF-β1 has been shown to enhance the expression of TGFR1, and activation of Smad and MAPK/ERK in fibroblasts [23]. These findings are one of the plausible mechanisms explaining how eosinophils could produce more LTE4 in association with an increased level of TGF-β1 in AERD patients. In addition, previous studies have shown that AERD patients present moderate to severe phenotypes with lower levels of FEV1 (%) and PC20 methacholine compared to ATA patients [24,25]. Here, we also showed that AERD patients with higher levels of serum TGF-β1 with lower levels of FEV1 (%), suggesting that TGF-β1 may contribute to presenting more severe phenotypes with lung dysfunction. As conventional anti-inflammatory medications have limited effects in the TGF-β1-mediated inflammatory pathway [2], a new therapeutic strategy to suppress the pathway noted in the present study is required in long-term management of AERD.
Overproduction of cysLTs is the key finding in the pathogenesis and progression of AERD pathogenesis. In particular, LTE4 is involved in persistent eosinophilia via enhancement of eosinophil recruitment to the airway mucosa and bronchoconstriction [26]. In this study, LTE4 could stimulate eosinophils to secrete EDN through p38 phosphorylation, similar to eotaxin (a potent stimulator of eosinophil chemotaxis) which binds to a CC chemokine receptor and induces eosinophil degranulation through activation of the ERK–p38 pathway [27]. However, LTE4 certainly activates eosinophils via cysLT1R rather than other receptors as montelukast reduced levels of granule proteins released from eosinophils. A previous paper has also been shown that LTE4 is able to release granule proteins through binding to cysLT1R (major) and other receptors (minor) [28]. These implicate that increased levels of LTE4 may be responsible for enhancing eosinophil degranulation as well as eosinophil activation/recruitment, exacerbating type 2 airway inflammation in AERD patients where leukotriene receptor antagonists have partially suppressive effects.
Increased eosinophil number and activation markers in blood, sputum and tissues are common characteristics of bronchial asthma. The eosinophils communicate with several cell types involved in the pathogenesis of asthma; however, eosinophil-epithelial cell interactions have been extensively highlighted to play an important role in the processes of chronic airway inflammation as airway epithelial cells are a regulator of both innate and adaptive immune responses to host defence [29,30]. Following stimulation by multiple factors, airway epithelial cells produce large quantities of cytokines, chemokines and growth factors, such as TGF-β1, enhancing type 2 immune response [31,32]. Although the mechanism of TGF-β1 production from airway epithelial cells has not been fully elucidated, eosinophil granule proteins, such as ECP and EDN, are a possible factor contributing to the stimulation of the airway [33–35]. The function of EDN in enhancing airway remodeling in patients with eosinophilic CRS has been shown [36]. Furthermore, a recent study in adult asthmatic cohorts demonstrated that higher serum EDN was associated with severe asthma with asthma exacerbation [37]. In addition, a novel effect of EDN in airway epithelial cells on releasing TGF-β1 was noted in our in vitro study, where steroid may have a limited action. Taken together, these findings provide a possible mechanism of how activated eosinophils to induce TGF-β1 production contributing to chronic progressive type 2 airway inflammation in AERD pathogenesis.
This study has some limitations. First, the effect of TGF-β1 in multiple cells, such as mast cells, neutrophils or platelets, has not been determined. Secondly, further clinical trials in AERD patients according to the results of serum TGF-β1 levels and eosinophil activation status are needed to validate our findings.
In conclusion, TGF-β1 has a novel function contributing to cysLT overproduction through induction of LTC4S expression in eosinophils of AERD patients. Moreover, increased LTE4 induces eosinophil degranulation via the p38 pathway which further stimulates airway epithelial cells, suggesting that TGF-β1 plays a key role in enhancing eosinophilic airway inflammation, leading to poor clinical outcomes of AERD patients.
Supporting information
(n = 3 asthmatic patients per group) TGFR1, TGF-β1 receptor; TGFR2, TGF-β2 receptor.
(PDF)
Levels of TGF-β1 released from A549 cells when co-cultured with peripheral eosinophils with/without LTE4 or montelukast (MK). The data are presented as means ± SD, n = 5. *P < .05 and **P < .01 were obtained by the Mann-Whitney test. n.s., not significant.
(PDF)
The data are presented as means ± SD, n = 5. *P < .05 was obtained by the Mann-Whitney test. n.s., not significant. EDN, eosinophil-derived neurotoxin.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This study was supported by a grant from the Korean Health Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (HI16C0992), and a grant from Dong-A ST Co., Republic of Korea (AJIRB-MED-KSP-18-395). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cahill KN, Boyce JA. Aspirin-exacerbated respiratory disease: Mediators and mechanisms of a clinical disease. J Allergy Clin Immunol. 2017;139(3):764–6. doi: 10.1016/j.jaci.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 2.Buchheit KM, Laidlaw TM. Update on the Management of Aspirin-Exacerbated Respiratory Disease. Allergy Asthma Immunol Res. 2016;8(4):298–304. doi: 10.4168/aair.2016.8.4.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okano M, Kariya S, Ohta N, Imoto Y, Fujieda S, Nishizaki K. Association and management of eosinophilic inflammation in upper and lower airways. Allergol Int. 2015;64(2):131–8. doi: 10.1016/j.alit.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Feng X, Ramsden MK, Negri J, Baker MG, Payne SC, Borish L, et al. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;138(4):1089–97 e3. doi: 10.1016/j.jaci.2016.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi Y, Sim S, Park HS. Distinct functions of eosinophils in severe asthma with type 2 phenotype: clinical implications. Korean J Intern Med. 2020;35(4):823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015;192(6):682–94. doi: 10.1164/rccm.201412-2278OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin SW, Park JS, Park CS. Elevation of Eosinophil-Derived Neurotoxin in Plasma of the Subjects with Aspirin-Exacerbated Respiratory Disease: A Possible Peripheral Blood Protein Biomarker. PLoS One. 2013;8(6):e66644. doi: 10.1371/journal.pone.0066644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol. 2014;193(1):41–7. doi: 10.4049/jimmunol.1301753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102(9):3396–403. doi: 10.1182/blood-2003-01-0151 [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205(1):79–90. doi: 10.1084/jem.20062027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor beta and severe asthma: a perfect storm. Respir Med. 2014;108(10):1409–23. doi: 10.1016/j.rmed.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 12.Elovic A, Wong DT, Weller PF, Matossian K, Galli SJ. Expression of transforming growth factors-alpha and beta 1 messenger RNA and product by eosinophils in nasal polyps. J Allergy Clin Immunol. 1994;93(5):864–9. doi: 10.1016/0091-6749(94)90379-4 [DOI] [PubMed] [Google Scholar]
- 13.Takai E, Tsukimoto M, Kojima S. TGF-beta1 downregulates COX-2 expression leading to decrease of PGE2 production in human lung cancer A549 cells, which is involved in fibrotic response to TGF-beta1. PLoS One. 2013;8(10):e76346. doi: 10.1371/journal.pone.0076346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James AJ, Penrose JF, Cazaly AM, Holgate ST, Sampson AP. Human bronchial fibroblasts express the 5-lipoxygenase pathway. Respir Res. 2006;7:102. doi: 10.1186/1465-9921-7-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddick CA, Serio KJ, Hodulik CR, Ring WL, Regan MS, Bigby TD. TGF-beta increases leukotriene C4 synthase expression in the monocyte-like cell line, THP-1. J Immunol. 1999;162(2):1101–7. [PubMed] [Google Scholar]
- 16.Choi Y, Lee DH, Lee JH, Shin YS, Kim SH, Park HS. Immunomodulatory function of surfactant protein D in eosinophilic asthma. Allergy. 2019;74(1):192–5. doi: 10.1111/all.13588 [DOI] [PubMed] [Google Scholar]
- 17.Choi Y, Lee DH, Trinh HKT, Ban GY, Park HK, Shin YS, et al. Surfactant protein D alleviates eosinophil-mediated airway inflammation and remodeling in patients with aspirin-exacerbated respiratory disease. Allergy. 2019;74(1):78–88. doi: 10.1111/all.13458 [DOI] [PubMed] [Google Scholar]
- 18.Nahm DH, Park HS. Analysis of induced sputum for studying allergen-specific IgE antibodies in airway secretion from asthmatic patients. Clin Exp Allergy. 1998;28(6):686–93. doi: 10.1046/j.1365-2222.1998.00291.x [DOI] [PubMed] [Google Scholar]
- 19.Ban GY, Cho K, Kim SH, Yoon MK, Kim JH, Lee HY, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2017;47(1):37–47. doi: 10.1111/cea.12797 [DOI] [PubMed] [Google Scholar]
- 20.Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 2018;50(8):104. doi: 10.1038/s12276-018-0136-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Park HS, Holloway JW, Shin HD, Park CS. Association between a TGFbeta1 promoter polymorphism and rhinosinusitis in aspirin-intolerant asthmatic patients. Respir Med. 2007;101(3):490–5. doi: 10.1016/j.rmed.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 22.Huang XQ, Zhang XY, Wang XR, Yu SY, Fang SH, Lu YB, et al. Transforming growth factor beta1-induced astrocyte migration is mediated in part by activating 5-lipoxygenase and cysteinyl leukotriene receptor 1. J Neuroinflammation. 2012;9:145. doi: 10.1186/1742-2094-9-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CG, Herzog EL, Ahangari F, Zhou Y, Gulati M, Lee CM, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-beta1 signaling. J Immunol. 2012;189(5):2635–44. doi: 10.4049/jimmunol.1201115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L, et al. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116(5):970–5. doi: 10.1016/j.jaci.2005.08.035 [DOI] [PubMed] [Google Scholar]
- 25.Chang HS, Park JS, Jang AS, Park SW, Uh ST, Kim YH, et al. Diagnostic value of clinical parameters in the prediction of aspirin-exacerbated respiratory disease in asthma. Allergy Asthma Immunol Res. 2011;3(4):256–64. doi: 10.4168/aair.2011.3.4.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laitinen A, Lindqvist A, Halme M, Altraja A, Laitinen LA. Leukotriene E(4)-induced persistent eosinophilia and airway obstruction are reversed by zafirlukast in patients with asthma. J Allergy Clin Immunol. 2005;115(2):259–65. doi: 10.1016/j.jaci.2004.10.021 [DOI] [PubMed] [Google Scholar]
- 27.Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95(6):1911–7. [PubMed] [Google Scholar]
- 28.Baptista-dos-Reis R, Muniz VS, Neves JS. Multifaceted roles of cysteinyl leukotrienes in eliciting eosinophil granule protein secretion. Biomed Res Int. 2015;2015:848762. doi: 10.1155/2015/848762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sexton DW, Walsh GM. Eosinophil-epithelial cell interactions: an important facet of asthmatic inflammation. Clin Exp Allergy. 2002;32(6):811–3. doi: 10.1046/j.1365-2745.2002.01428.x [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. 2008;102(7):949–55. doi: 10.1016/j.rmed.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 31.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19(6):711–20. doi: 10.1016/j.coi.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denney L, Byrne AJ, Shea TJ, Buckley JS, Pease JE, Herledan GM, et al. Pulmonary Epithelial Cell-Derived Cytokine TGF-beta1 Is a Critical Cofactor for Enhanced Innate Lymphoid Cell Function. Immunity. 2015;43(5):945–58. doi: 10.1016/j.immuni.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289(25):17406–15. doi: 10.1074/jbc.R113.546218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177(7):4861–9. doi: 10.4049/jimmunol.177.7.4861 [DOI] [PubMed] [Google Scholar]
- 35.Choi Y, Kim YM, Lee HR, Mun J, Sim S, Lee DH, et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy. 2020;75(1):95–103. doi: 10.1111/all.13997 [DOI] [PubMed] [Google Scholar]
- 36.Tsuda T, Maeda Y, Nishide M, Koyama S, Hayama Y, Nojima S, et al. Eosinophil-derived neurotoxin enhances airway remodeling in eosinophilic chronic rhinosinusitis and correlates with disease severity. Int Immunol. 2019;31(1):33–40. doi: 10.1093/intimm/dxy061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Lee JH, Yang EM, Kwon E, Jung CG, Kim SC, et al. Serum Levels of Eosinophil-Derived Neurotoxin: A Biomarker for Asthma Severity in Adult Asthmatics. Allergy Asthma Immunol Res. 2019;11(3):394–405. doi: 10.4168/aair.2019.11.3.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(n = 3 asthmatic patients per group) TGFR1, TGF-β1 receptor; TGFR2, TGF-β2 receptor.
(PDF)
Levels of TGF-β1 released from A549 cells when co-cultured with peripheral eosinophils with/without LTE4 or montelukast (MK). The data are presented as means ± SD, n = 5. *P < .05 and **P < .01 were obtained by the Mann-Whitney test. n.s., not significant.
(PDF)
The data are presented as means ± SD, n = 5. *P < .05 was obtained by the Mann-Whitney test. n.s., not significant. EDN, eosinophil-derived neurotoxin.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.