Abstract
Background and Aims
Secondary metabolites are integral to multiple key plant processes (growth regulation, pollinator attraction and interactions with conspecifics, competitors and symbionts) yet their role in plant adaptation remains an underexplored area of research. Carnivorous plants use secondary metabolites to acquire nutrients from prey, but the extent of the role of secondary metabolites in plant carnivory is not known. We aimed to determine the extent of the role of secondary metabolites in facilitating carnivory of the Cape sundew, Drosera capensis.
Methods
We conducted metabolomic analysis of 72 plants in a time-series experiment before and after simulated prey capture. We used ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and the retention time index to identify compounds in the leaf trap tissue that changed up to 72 h following simulated prey capture. We identified associated metabolic pathways, and cross-compared these compounds with metabolites previously known to be involved in carnivorous plants across taxa.
Key Results
For the first time in a carnivorous plant, we have profiled the whole-leaf metabolome response to prey capture. Reliance on secondary plant metabolites was higher than previously thought – 2383 out of 3257 compounds in fed leaves had statistically significant concentration changes in comparison with unfed controls. Of these, ~34 compounds are also associated with carnivory in other species; 11 are unique to Nepenthales. At least 20 compounds had 10-fold changes in concentration, 12 of which had 30-fold changes and are typically associated with defence or attraction in non-carnivorous plants.
Conclusions
Secondary plant metabolites are utilized in plant carnivory to an extent greater than previously thought – we found a whole-metabolome response to prey capture. Plant carnivory, at the metabolic level, likely evolved from at least two distinct functions: attraction and defence. Findings of this study support the hypothesis that secondary metabolites play an important role in plant diversification and adaptation to new environments.
Keywords: Drosera capensis, secondary plant metabolites, carnivorous plant, plant–insect interactions, metabolomics
INTRODUCTION
Secondary plant metabolites are integral for plant fitness and are a key aspect of plant evolutionary adaptation (Duplais et al., 2020). They are involved in a wide range of key plant processes such as growth regulation (Erb and Kliebenstein, 2020), pollinator attraction (Hartmann, 2007; Kessler and Baldwin, 2007), facilitating interactions between conspecifics (Catola et al., 2018), competitors and symbionts (van Dam and Bouwmeester, 2016), and as defence against abiotic and biotic stress (Agrawal, 2011). Their importance is clearly defined for only a narrow selection of these processes, predominantly pollination, stress and defence (Kessler and Baldwin, 2007; Ramakrishna and Ravishankar, 2011; Jander, 2014); the extent of their function outside of these processes is, however, not well understood. Plant carnivory is an example of a life-history strategy that relies on secondary metabolites (Hatcher et al., 2020), and for which convergent evolution of secondary metabolites appears to be likely. The extent to which carnivorous plants rely on secondary metabolites and the diversity of compounds used to facilitate carnivory remain an important area to be determined.
Carnivorous plants are a diverse, polyphyletic group of flowering plants adapted, in general, to nutrient-poor environments (Darwin, 1875; Albert et al., 1992; Ellison and Gotelli, 2001). Plant carnivory involves adaptation to facilitate the key processes of prey attraction, capture, digestion and assimilation (Ellison and Adamec, 2018). The morphological and physiological basis for these adaptations is well known (Gibson and Waller, 2009; Król et al., 2012; Gaume et al., 2016), with some clear examples of convergent and divergent evolution (e.g. pitcher plants, Thorogood et al., 2017). There is clear evidence that secondary metabolites play a key role in some of these processes, such as olfactory cues, prey capture signalling, trap movement and defence of plant tissue from decaying prey (see review by Hatcher et al., 2020). An understanding of biochemical profiles of the process of plant carnivory is, however, currently not determined. Most studies focusing on the role of secondary metabolites in plant carnivory are targeted analyses of known compounds. Jasmonates, for example, are typically involved in stress response but in Drosera spp. they instigate leaf bending within an hour following prey capture (Krausko et al., 2017). We lack, however, a more focused understanding of the biochemical responses to prey capture of a carnivorous plant. Unsupervised metabolomic methods provide an effective strategy for determining the extent of involvement of secondary metabolites in carnivorous plants. Understanding the scale of the role of metabolites in a plant’s carnivory provides insight into the importance of secondary plant metabolites for diversification into new environments and the capacity for these compounds to act as conduits for evolutionary adaptation in areas outside of pollination, stress and defence.
To establish the potential extent of the biochemical role of secondary plant metabolites in carnivory, we measured the metabolic profile of the carnivorous herb Cape sundew, Drosera capensis, before and after the addition of rehydrated Drosophila melanogaster powder (‘insect substrate’ herein) to the traps. Up- or downregulation of compounds following insect substrate addition indicates the involvement of that compound in carnivory (as per Kreuzwieser et al., 2014). We predicted that there is a whole-leaf metabolic response to substrate addition incorporating multiple secondary metabolites to facilitate carnivory. Specifically, we hypothesize that (1) compounds involved in leaf bending, e.g. jasmonates, will increase in concentration within 45 min and the increase will be sustained for the remainder of the experiment; (2) compounds involved in attraction will decrease in concentration following nutrient addition as the benefit of these compounds is reduced; and (3) there will be a latency of certain compounds before upregulation, these compounds being suggested to be compounds involved in the digestion or assimilation of nutrients from prey.
MATERIALS AND METHODS
Plant rearing and preparation
Drosera capensis L. plants were purchased from an established nursery. Prior to this, seeds were grown in an open polytunnel on a standard medium of peat:sand:perlite (6:1:1 respectively) in individual pots. Plants were able to catch available prey during growth to maturity. On 1 July, 4 weeks prior to the experiment, 500 mature individuals were translocated to a greenhouse at Loughborough University. Exposure to prey and prey items on leaves were restricted as much as possible; the few prey that were captured were removed during daily inspections. The greenhouse was maintained at ambient temperature, with supplementary heating to prevent the temperature dropping below 18 °C, additional fluorescent lighting on a light:dark cycle of 12:12 h was used and plants were watered regularly with de-ionized water when required. To prevent allocation to carnivory being confounded by reproductive investment, flower stems were removed when visible during this time up to the final week before the experiment, when removal of stems may have mitigated a stress response. Plants were selected at random from the 500 individuals in the greenhouse for use in this experiment.
Experimental design
The metabolic response of D. capensis to prey capture was determined by adding insect substrate to one leaf-trap of an individual plant; this leaf was subsequently harvested for analysis. Leaf response to prey is known to initiate within the first 6 h after prey capture but can take place over multiple days or weeks to completion (Adamec, 2002; Nakamura et al., 2013; Krausko et al., 2017). To capture these short- and longer-term plant responses, we measured changes across a time-series of harvests from insect substrate addition. Plants were harvested at 0.75, 1.5, 3, 6, 24, 48 and 72 h after insect substrate addition. Circadian variability in the metabolome of plants is common (Kim et al., 2017). We therefore staggered insect substrate addition throughout the day (prey additions from 0515 to 1250 h) to keep harvest times in the shortest possible time window. Plant harvests were therefore all carried out between 1030 and 1330 h. Each time interval had six replicates. These replicates were spread evenly throughout the harvest period and at least one unfed control was harvested within 10 min of a fed plant being harvested to account for natural circadian changes and comparison of the metabolite profile with that of unfed plants (total number of controls = 32). There was a total of 74 plant samples. Due to the amount of work involved, the experiment was carried out in two overlapping sections. The first started on day 1 (2 August) and finished on day 4, and the second started on day 2 and finished on day 5 (6 August).
Prey addition
To simulate an accurate and standardized response of D. capensis to prey capture, fruit fly powder (Drosophila melanogaster) was used as the prey addition (fed) treatment using the protocol outlined by Gao et al. (2015). Fruit flies were reared on a standard medium, freeze-dried and ground into a homogenized powder using a ball mill. Insect substrate addition consisted of 25 mg of fruit fly powder in 100 µL of de-ionized water spread evenly and carefully across the trap using a pipette, intentionally stimulating the leaf-trap tentacles mechanically.
Plant harvests
At each harvest, leaf and root tissue was cut from the plant and washed immediately in de-ionized water to remove prey residue and soil. The leaf was then flash-frozen in liquid nitrogen (−196 °C). The time from cutting to freezing was always <30 s. Tissue samples were then weighed before being placed in a Precellys homogenizer tube (Bertin Technologies, Sain Quentin en Yvelines, France) in a −80 °C freezer until analysis.
Sample preparation, UHPLC-MS analysis and processing for metabolite profile
For a more in-depth description of these methods, please see Supplementary Data Methods Detail and data available at Hatcher et al. (2021). Monophasic extractions used acetonitrile:methanol:water 2:2:1 as the final solvent. Equal volumes of the supernatant were stored at −80 °C. LC–MS samples were reconstituted in 50 µL of 20 % aqueous methanol each, and 10 μL each was pooled into one quality control (QC) sample per sample type, giving 16 QC samples in total.
The samples were run in controlled randomized order, with QC samples equidistant between them. They were analysed by ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS). Quality control sample 02 contains data-dependent MS/MS data and is used with the retention time index to annotate metabolites. Data were collected in positive ion and profile mode, m/z 100–1000, at 70 k resolution (see Supplementary Data Fig. S1 for total ion chromatogram for a blank and a QC run).
Data processing and QC methods followed guidelines outlined by Di Guida et al. (2016) and Kirwan et al. (2014) using NBAF-B in-house scripts in MatLab (v8.1; The MathWorks, Natick, MA, USA), the SIMStitch pipeline. Briefly, an R (3.2.0)-based XCMS/CAMERA script was used for alignment and resulted in an intensity matrix in a csv file (9309 features). Following replicate and blank filtering, samples were filtered with a 2-ppm mass error and a 75 % filter. Data were normalized using probabilistic quotient normalization (PQN) and missing values were filled using the k-nearest neighbours (KNN) algorithm (k = 5). This matrix was used for univariate statistics including fold changes. Generalized logarithm (g-log) transformation was applied to all samples. This matrix was used for multivariate statistics.
Statistical analysis and identification of metabolites
We initially used principal component analysis (PCA) to assess the overall metabolic differences among the control and time-after-feeding treatments in an unbiased manner using the ‘prcomp’ function in R (R Core Team, 2016). Following this, there was obvious separation of the treatments into three groups: controls, ≤6 h and ≥24 h. Further analyses were, therefore, conducted for these three pooled groups. PCA of the samples highlighted a clear single anomalous control sample, which we excluded from all analyses.
We subsequently performed three separate supervised multivariate analyses using orthogonal partial least-squares discriminant analysis (OPLS-DA) using Simca-P+ (v14, Umetrics Umeå, Sweden) to find the direction of maximum covariance: between controls and both treatments (≤6 h or ≥24 h); among grouped treatments (≤6 h and ≥24 h); and between all three groups of treatments. OPLS-DA separates the difference between groups of observations by rotating PCA components such that maximum separation among groups is obtained and identifies variables that are most contributing to this separation. Simca-P+ validates the OPLS-DA model by removing one-seventh of the data and producing a model for the six-sevenths of remaining data. The new data are then predicted using the new model, continuing in this process until all the data have been predicted. We evaluated the contribution of each metabolite to the separation of treatments using S-plots and derived metabolites of biological relevance indicated from these plots. S-plots were analysed for metabolites that had the greatest selectivity and sensitivity in discriminating between the data, presented as points at the upper right and lower left corners of the plots (Supplementary Data Figs S2–S9).
We used univariate statistical analyses to identify changes in individual mass-spectral signals between unfed (controls) and grouped fed treatments (≤6 h’ or ≥24 h). A series of filters (ANOVA, t-tests and fold changes) were applied to the data. Metabolites that passed all three filters (i.e. were statistically significant in ANOVA, t-test and with a fold change greater than ±2) are presented and considered in more detail.
Firstly, we used analysis of variance (ANOVA) to determine if any compounds across all fed treatments changed significantly from the unfed controls. A Benjamini–Hochberg correction was applied to limit the false discovery rate (FDR) to 5 %. These identified compounds were compared with previously identified metabolites in other studies highlighted in Hatcher et al. (2020). Following this, we used multiple Welch’s t-tests to determine pairwise differences between controls and the ≤6 h or ≥24 h treatment group. We used Benjamini–Hochberg correction to control the FDR of 5 % to correct for multiple hypothesis testing of all treatment peak intensities compared with control to identify any changes in compound intensities across all treatments due to the large number of tests. These were then combined with log-fold changes to identify the significant and intense signal changes between control samples and the ≤6 h or ≥24 h treatments presented as volcano plots. Conservative thresholds of ±2 for the log2 fold change and 2 for the −log FDR-adjusted P value (P < 0.01) were used to highlight those features that showed the largest differences (Grace and Hudson, 2016). Of the significant compounds (high statistical significance and fold change), the 100 most significant compounds are presented in a heat map to illustrate plant metabolome response.
Metabolites that are highlighted by OPLS-DA as well as univariate analyses as statistically significant (as defined above) were cross-examined (Supplementary Data Table S1) and presented in box plots to inspect individual metabolite patterns.
Annotation summary and pathway analysis
Annotation of metabolites were assigned from tandem MS (MS/MS) and metabolite databases. We used the in-house MIPack software to match 419 signals of the total of 3257 signals to the BioCyc/Arabidopsis thaliana database (5 ppm error), and 1290 signals up to m/z 600 to the KEGG database with 2 ppm error and including molecular formula search (as per Weber and Viant, 2010). Choline was annotated manually.
Compounds annotated and found to be biologically important from the analyses were inserted into the MetaboAnalyst pathway generator. MetaboAnalyst was implemented using the PrimeFaces library (v6.1) based on the JavaServer Faces Technology. The communication between Java and R is established through TCP/IP using the Rserve program (Li et al., 2018). The pathway library selected was ‘Arabidopsis thaliana’. The over-representation analysis method was ‘hypergeometric test’. The node importance measure for topological analysis was ‘relative betweenness centrality’.
Metabolite comparisons with other carnivorous plants
We cross-checked the compounds with a library of metabolites that have previously been associated with a role in carnivory (Hatcher et al., 2020). Exact compounds or isomers were identified and their regulation before and after prey capture and the statistical significance of these changes in the experiment were produced in a table and compared with previously annotated compounds. Metabolites derived from carnivorous plants purely for pharmaceutical use are not discussed in this paper as their derivation is stimulated under conditions not analogous to the plant’s ecology and therefore do not assist in the identification of compounds likely to function in plant carnivory.
RESULTS
In response to simulated prey capture, D. capensis upregulated and downregulated a large number of secondary plant metabolites over the course of 72 h. Of the 3257 analytical features present, statistically significant (ANOVA, adjusted P < 0.05) changes in intensity were found in 2383 peaks in at least one time point compared with controls (Supplementary Data Table S2). Among the 2383 compounds where change was detected, some clear patterns were identified. Generally, compounds that were upregulated increased in concentration within 0.75 h following substrate addition. After this point, in general, concentrations stopped increasing and the elevated concentration was maintained for the duration of the experiment. In some cases, metabolites rapidly increased at 0.75 h, then slowly increased for the duration of the experiment; other metabolites had a more consistent rate of increase over time which lasted the duration of the experiment. For a small proportion of compounds, increases in concentration were very large; >20 compounds had 10-fold increases and were statistically significant compared with controls, and 12 of these had >30-fold increases compared with controls. Decreases in concentration of downregulated compounds were mostly small in the first 6 h, followed by much larger decreases in concentration for the remaining 66 h. In very few cases, there were compounds that decreased in concentration initially, and then had an overall increase in concentration by the end of the experiment.
Multivariate analyses
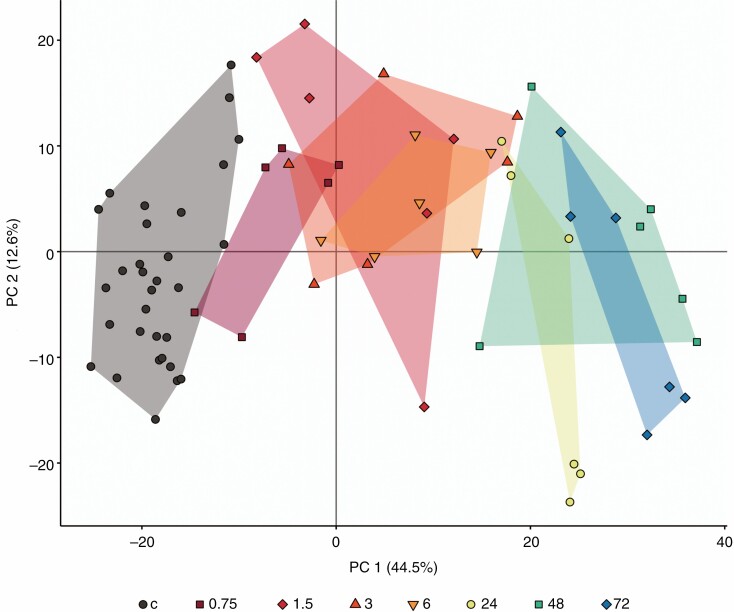
The PCA of the metabolome of plant samples showed a clear separation between treatment time points along PC1, increasing in time from left to right. PCA axes 1 and 2 explained 57·1 % (44·4 and 12·7 %, respectively) of the variance. Control groups were clustered at the left side, shorter times (0, 75, 1.5, 3, 6 h) clustered in the middle of the ordination, and longer treatment times (24, 48, 72 h) clustered at the right side of the ordination (Fig. 1). Further analysis, therefore, focused on compounds within these three clustered time range groups.
Fig. 1.
Ordination of the first two principle components from a PCA of metabolite profiles of D. capensis following insect substrate addition, with unfed controls. Treatments indicated are hours after substrate addition (c, unfed controls). Presented are the PC1 and PC2 scores for each plant, with convex hull colour for each treatment group. Different symbols represent each treatment time point. The presented PCA is without inclusion of the anomalous result (one control sample).
S-plots of metabolites between controls and ≤6 h highlighted 28 upregulated and 8 downregulated compounds. S-plots of metabolites between controls and ≥24 h highlighted ten upregulated and four downregulated compounds. OPLS-DA comparing ≤6 h with ≥24 h highlighted 13 upregulated and three downregulated compounds in ≥24 h compared with ≤6 h . A final OPLS-DA comparing all three groups (unfed, ≤6 h after feeding and ≥24 h after feeding) highlighted four compounds that were highly associated specifically with the ≥24 h after feeding group (Supplementary Data Figs S2–S9; annotated metabolites from these analyses are compiled in Supplementary Data Table S1).
Compounds with high fold changes and importance (volcano plot)
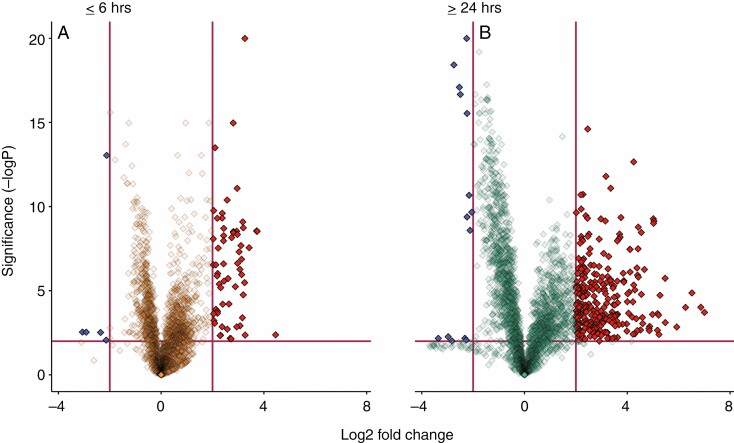
In the ≤6 h group compared with unfed controls, 62 compounds were upregulated and 5 downregulated, with a statistically significant fold change >4. In the ≥24 h group compared with unfed controls, 287 compounds were upregulated and 14 were downregulated, with a statistically significant fold change >4. All of the 62 upregulated compounds in the ≤6 h group were also identified among the 287 upregulated compounds in the ≥24 h group. None of the six downregulated compounds in the <6 h’ group was present in the statistically significant downregulated compounds in the ≥24 h group (Fig. 2 and Supplementary Data Table S1).
Fig. 2.
Number and proportion of discriminatory features in treatment groups compared with the control group. Shown here are log fold changes (x-axis), in comparison with unfed control plants, and the y-axis displays the negative log of the P-value from a two-sample t-test. Data points that are far from the origin (near the top or far left and right) are considered important variables with potentially high biological relevance. Blue and red features (downregulated and upregulated respectively) are those chosen based on the presented criteria of thresholds of 2 and −2 for the log2 fold change and 2 for the −log FDR-adjusted P-value (P < 0.01, indicated with red margins) to highlight those features that showed the largest differences (Grace and Hudson, 2016).
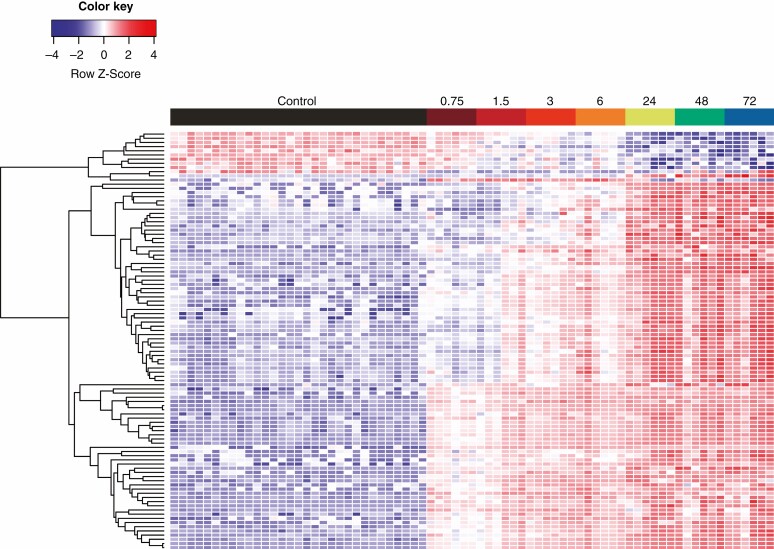
Compounds identified from fold change and significance (fold change >4 and α < 0.01 from adjusted Welch’s t-test) were investigated further to illustrate the general metabolic change in D. capensis following prey capture. A heat map with a compound dendrogram shows a clear treatment grouping of controls, followed by the treatments up to 6 h, and then the treatment times >24 h (Fig. 3). There are four clear separations of metabolite patterns. There are compounds that are downregulated 24 h after prey capture, compounds that have a gradual change in concentration over the course of the experiment, and some compounds that appear to have a high increase in concentration initially, followed by a steady increase in concentration for the remainder of the experiment. The results of the volcano plot also confirmed the significance of compounds highlighted in OPLS-DA, a sample of which are included in Fig. 4A–I, M–P.
Fig. 3.
Metabolic patterns over time after prey substrate addition. Presented are the 100 most important features, based on statistical significance and fold change. Colour and colour intensity represent the Z score for the change in peak intensity from the mean of the compound (one compound per row). Each column represents a single plant; these are grouped according to treatment and harvest time point (randomly assigned within these). Each row represents a single metabolite grouped as a dendrogram by similarity in change of intensity.
Pathway analysis of biologically important metabolites
The compounds involved in carnivory are derived from multiple metabolic pathways (Table 1). Four pathways were found to be significantly involved in the D. capensis response to prey addition: flavone and flavonol biosynthesis, phenylalanine metabolism, isoquinoline alkaloid biosynthesis and flavonoid biosynthesis. Sixteen other metabolic pathways were associated with compounds found to significantly change in response to simulated prey capture in this experiment, but these pathways were not statistically significant. They are included here for future work.
Table 1.
Results of metabolic pathway analysis of D. capensis after prey substrate addition. Presented are the number of metabolites in certain metabolic pathways. Statistically significant pathways are in bold type; non-significant pathways are included for reference in future studies. Match status is number of metabolites in this experiment/number of compounds involved in a pathway. Holm P is used to control multiple testing. Impact is a combination of centrality and pathway enrichment results calculated as the sum of the importance of each metabolite divided by the sum of all metabolites in each pathway. Statistically significant pathways are indicated in bold.
| Pathway | Match status | P value | −log(P) | Holm P | FDR | Impact |
|---|---|---|---|---|---|---|
| Flavone and flavonol biosynthesis | 4/9 | 0.0005 | 7·7043 | 0·039226 | 0·039226 | 0·8 |
| Phenylalanine metabolism | 3/8 | 0·0046 | 5·3911 | 0·39189 | 0·19822 | 0·16667 |
| Isoquinoline alkaloid biosynthesis | 2/6 | 0·0284 | 3·5625 | 1 | 0·82268 | 0·5 |
| Flavonoid biosynthesis | 5/43 | 0·0456 | 3·0868 | 1 | 0·99287 | 0·00566 |
| Purine metabolism | 5/61 | 0·1497 | 1·8992 | 1 | 1 | 0·04869 |
| Tyrosine metabolism | 2/18 | 0·2031 | 1·5942 | 1 | 1 | 0·45455 |
| Glucosinolate biosynthesis | 4/54 | 0·2407 | 1·4244 | 1 | 1 | 0·00952 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 2/21 | 0·2558 | 1·3635 | 1 | 1 | 0 |
| Indole alkaloid biosynthesis | 1/7 | 0·2845 | 1·2571 | 1 | 1 | 0 |
| Valine, leucine and isoleucine biosynthesis | 2/26 | 0·3438 | 1·0677 | 1 | 1 | 0·01865 |
| Aminoacyl-tRNA biosynthesis | 4/67 | 0·3809 | 0·96519 | 1 | 1 | 0 |
| Nicotinate and nicotinamide metabolism | 1/12 | 0·4373 | 0·82719 | 1 | 1 | 0 |
| Valine, leucine and isoleucine degradation | 2/34 | 0·4768 | 0·74074 | 1 | 1 | 0 |
| Zeatin biosynthesis | 1/19 | 0·5987 | 0·51308 | 1 | 1 | 0 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 1/23 | 0·6694 | 0·40135 | 1 | 1 | 0 |
| α-Linolenic acid metabolism | 1/23 | 0·6694 | 0·40135 | 1 | 1 | 0 |
| Diterpenoid biosynthesis | 1/26 | 0·7143 | 0·33646 | 1 | 1 | 0·01368 |
| Tryptophan metabolism | 1/27 | 0·7279 | 0·31762 | 1 | 1 | 0·17059 |
| Porphyrin and chlorophyll metabolism | 1/29 | 0·7532 | 0·28347 | 1 | 1 | 0·00806 |
| Glycine, serine and threonine metabolism | 1/30 | 0·7649 | 0·26797 | 1 | 1 | 0 |
Convergence of compounds for plant carnivory
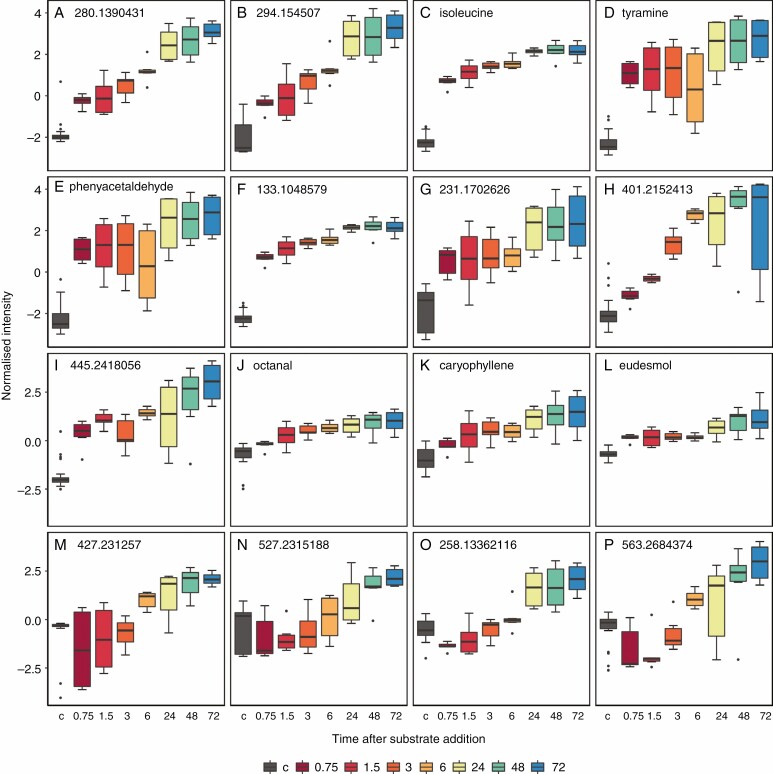
Thirty-four secondary plant metabolites already known to function in plant carnivory were also present in D. capensis in this experiment from putative annotation. Of these, only nonanal (pelargonaldehyde) did not have significant fold changes in concentration after prey substrate addition when compared with the results of the multiple ANOVA. From the volcano plot highlighting high statistical significance and fold changes, five compounds were identified that are also important in carnivory for other carnivorous plants (Fig. 4: C, E, J, K and L). Of the 34 compounds, 21 compounds have been found in D. capensis that are also produced and linked to carnivory in plants that do not share a common carnivorous ancestor (light grey shading in Table 2). Eleven compounds have been identified for the first time in Nepenthales (a carnivorous clade of five genera with multiple trap types) that are also present in an unrelated carnivorous lineage (dark grey shadingrows in Table 2).
Fig. 4.
Normalized intensities of filtered metabolites. (A–I) Compounds highlighted as biologically important by all analyses (volcano plot group ≤6 h and ≥24 h and from OPLS-DA of fed groups compared with unfed controls). These compounds identified by OPLS-DA are therefore confirmed by FDR-adjusted t-tests likely to be of high biological importance. (C, E, J–L) Compounds previously known to be involved in carnivory and confirmed to be significant by volcano plot in this experiment. (M–P) Compounds identified by three-way OPLS-DA as highly associated with a separation of ≥24 h from unfed controls and plants ≤6 h after feeding. Black (labelled c) (box 1) represents unfed controls (n = 32); dark red, red, dark orange and light orange (boxes 2–5) are treatments ≤6 h ; lime green, green and blue (boxes 6–8) are ≥24 h (n = 6 per fed treatment harvest time). Note that the x-axis is not scaled and therefore distances are not proportional between treatment boxes. Box plots present the median, interquartile range, whiskers are values up to 1.5 times the interquartile range, and values exceeding this are outliers. If a metabolite cannot be annotated, its peak is ascribed.
Table 2.
Secondary metabolites annotated in this experiment that have been found to have a role in carnivory in a previous study, highlighting occurrence of this compound in other carnivorous plants and whether this experiment shows agreement with previously proposed function. Light-grey bars are metabolites newly found present for Drosera. Dark-grey bars are newly found in Nepenthales. White bars are compounds found previously for D. capensis. Ticks indicate that findings of this experiment are congruent with the previously proposed function. Crosses do not agree with previously proposed function and brackets indicate partial agreement or disagreement and these compounds require further investigation. ns, no significant fold change across any treatment; arrows indicate concentration increase or decrease by the end of the experiment. No. lineages, number of lineages in which the metabolite has been found to have a carnivorous function. Compounds reported from Hotti et al. (2017) and Kreuzwieser et al. (2014) have 70% confidence minimum. The proposed function of all metabolites are either known (i.e. directly tested) or highly likely (but not directly or individually tested). Note that some compound isomers are identified, but in some studies this detail is not included. Volatile organic compound (VOC) is ascribed in Proposed function column if detail on the compound’s scent is not available
| Proposed role | Metabolite | Proposed function | Support for suggested function in this study | Plant genus/ species previously studied | Family | Order | Source | No. lineages | Regulation after prey capture in this study |
|---|---|---|---|---|---|---|---|---|---|
| Attraction | -Eudesmol | Floral or fruit scent | ✗ | Sarracenia spp. | Sarraceniaceae | Ericales | Hotti et al., 2017 | 2 | ↑ |
| Attraction | 2-Phenylethanol (phenylethyl alcohol) | Scent (honey, spice, rose, lilac). Attracts a wide range of taxa (downregulated after prey capture in Venus flytrap) | ✗ | Dionaea muscipula, Nepenthes rafflesiana, Sarracenia flava, S. leucophylla, S. minor | Droseraceae, Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010; Kreuzwieser et al., 2014 | 2 | ↑ |
| Attraction | 2-Phenylethyl acetate (phenethyl acetate) | Scent (fruity, sweet) | (✗) | Nepenthes rafflesiana, Sarracenia flava, S. leucophylla | Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010 | 2 | ↑ |
| Attraction | 5-Hydroxy-methylfurfural | Scent (unknown specific attraction) | ✓ | Sarracenia flava, S. leucophylla, S. minor | Sarraceniaceae | Ericales | Jürgens et al., 2009 | 2 | ↓ |
| Attraction | 6-Methyl-5-hepten-2-one | Scent (unknown specific attraction | (✗) | Dionaea muscipula, Drosera binata, Nepenthes rafflesiana, Sarracenia flava, S. leucophylla, Sarracenia minor, S. purpurea | Droseraceae, Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010; Kreuzwieser et al., 2014 | 2 | ↑ |
| Capture, retention and digestion | Abscisic acid | Trap closure sensitivity in Venus flytrap | ✓ | Dionaea muscipula | Droseraceae | Nepenthales (Caryophyllales) | Escalante-Pérez et al., 2011 | 1 | ↑ |
| Attraction or capture | Apigenin (and derivatives acacetin and 7,4′-dimethyl ether) | Not tested (present in sticky resin) | (✓) | Roridula gorgonias | Roridulaceae | Ericales | Wollenweber, 2007 | 2 | ↓ |
| Attraction | Benzaldehyde | Scent (almond, burnt sugar) attracts Lepidoptera, Pieridae). Emitted from Dionaea muscipula trap but not individually confirmed to attract prey | ✗ | Dionaea muscipula, Nepenthes rafflesiana, Sarracenia flava, S. leucophylla | Droseraceae, Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010; Kreuzwieser et al., 2014 | 2 | ↑ |
| Attraction | Benzyl acetate | Scent (floral; attracts Lepidoptera, Noctuidae) | ✗ | Nepenthes rafflesiana, Sarracenia flava, S. leucophylla | Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010 | 2 | ↑ |
| Attraction | Benzyl alcohol | Sweet, flower scent (emitted from Venus flytrap trap but not individually confirmed to attract prey) | (✗) | Dionaea muscipula, Nepenthes rafflesiana, Sarracenia flava, S. leucophylla | Droseraceae, Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010; Kreuzwieser et al., 2014; Hotti et al., 2017 | 2 | ↑ |
| Attraction | Caryophyllene oxide | Floral or fruit scent (downregulated after prey capture in Venus flytrap) | ✗ | Dionaea muscipula, Nepenthes rafflesiana | Droseraceae, Nepenthaceae | Nepenthales (Caryophyllales) | Di Giusto et al., 2010; Kreuzwieser et al., 2014 | 1 | ↑ |
| Capture | Coniine | Insect-paralysing agent | ✓ | Darlingtonia californica, Heliamphora spp., Sarracenia spp. | Sarraceniaceae | Ericales | Hotti et al., 2017 | 2 | ↑ |
| Attraction | Decanal | Floral or fruit scent (emitted from Venus flytrap trap but not individually confirmed to attract prey) | ✗ | Dionaea muscipula, Nepenthes rafflesiana | Droseraceae, Nepenthaceae | Nepenthales (Caryophyllales) | Di Giusto et al., 2010; Kreuzwieser et al., 2014 | 1 | ↑ |
| Digestion | Gallic acid | Anti-fungal | ✓ | Dionaea muscipula, Drosera capensis, Nepenthes anamensis | Droseraceae, Nepenthaceae | Nepenthales (Caryophyllales) | Kováčik et al., 2012a, b | 1 | ↓ |
| Capture/digestion/retention | Jasmonates (jasmonic acid, 12-oxo-phytodienoic acidic and isoleucine conjugate of jasmonic acid) | Trap seal and secretion of enzymes in Dionaea muscipula. Leaf bending in Drosera spp. after prey capture. Digestive enzyme secretion in Drosera spp., Dionaea muscipula and Nepenthes alata | ✓ | Dionaea muscipula, Drosera capensis, Nepenthes alata | Droseraceae, Nepenthaceae | Nepenthales (Caryophyllales) | Escalante-Pérez et al., 2011; Nakamura et al., 2013; Libiaková et al., 2014; Mithöfer et al., 2014; Yilamujiang et al., 2016; Krausko et al., 2017; Pavlovič et al., 2017; Pavlovič and Mithöfer 2019 | 1 | ↑ |
| Attraction | Isomenthol | VOC (emitted from trap but not individually confirmed to attract prey) | ✗ | Dionaea muscipula | Droseraceae | Nepenthales (Caryophyllales) | Kreuzwieser et al., 2014 | 1 | ↑ |
| Digestion | Isorhamnetin | Not specifically tested | ✗ | Drosera adelae, D. aliciae, D. capensis, D. cuneifolia, D. ramentacea | Droseraceae | Nepenthales (Caryophyllales) | Marczak et al., 2005 | 1 | ↓ |
| Attraction or capture | Kaempferol | Not tested (present in sticky resin) | (✗) | Roridula dentata | Roridulaceae | Ericales | Wollenweber, 2007 | 2 | ↓ |
| Attraction | Linalool | Floral, lavender scent. Attracts Lepidoptera, Noctuidae, Hymenoptera | (✗) | Nepenthes rafflesiana, Sarracenia minor | Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010 | 2 | ↑ |
| Attraction or capture | Luteolin (and derivatives velutin and apometzgerin) | Not tested (present in sticky resin) | (✗) | Roridula gorgonias | Roridulaceae | Ericales | Wollenweber, 2007 | 2 | ↓ |
| Attraction | Methyl salicylate | Scent (peppermint, attracts Lepidoptera, Noctuidae, usually acts as a stress response) | ✓ | Dionaea muscipula, Nepenthes rafflesiana, Sarracenia flava, S. leucophylla | Droseraceae, Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Jürgens et al., 2009; Di Giusto et al., 2010 | 2 | ↓ |
| Digestion | Myricetin | Antibacterial | (✗) | Dionaea muscipula, Drosera adelae, D. aliciae, D. capensis, D. cuneifolia, D. ramentacea | Droseraceae | Nepenthales (Caryophyllales) | Marczak et al., 2005; Krolicka et al., 2008 | 1 | ↓ |
| Attraction | Nonanal | Floral scent | ns | Dionaea muscipula, Nepenthes rafflesiana, Sarracenia spp. | Droseraceae, Nepenthaceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Di Giusto et al., 2010; Kreuzwieser et al., 2014; Hotti et al., 2017 | 2 | ns |
| Attraction | Octanal | VOC (emitted from trap but not individually confirmed to attract prey) | (✗) | Dionaea muscipula | Droseraceae | Nepenthales (Caryophyllales) | Kreuzwieser et al., 2014 | 1 | ↑ |
| Attraction | p-Cymene | Floral or fruit scent (downregulated after prey capture in Venus flytrap) | ✗ | Dionaea muscipula, Sarracenia spp. | Droseraceae, Sarraceniaceae | Nepenthales (Caryophyllales), Ericales | Kreuzwieser et al., 2014; Hotti et al., 2017 | 2 | ↑ |
| Attraction | Phenylacetaldehyde | Scent | ✗ | Heliamphora spp. | Sarraceniaceae | Ericales | Jaffé et al., 1995 | 2 | ↑ |
| Capture and/or digestion | Plumbagin (or isomer 2-methyljuglone) | Toxic or anaesthetic to prey in Nepenthes khasiana. Antibacterial, insecticide | ✓ | Aldrovanda vesiculosa, Dionaea muscipula, Drosera adelae, D. aliciae, D. auriculata, D. binata, D. capensis, D. cistiflora, D. dichotomata, D. indica, D. longifolia, D. lunata, D. ramentacea, D. whitakeri, Drosophyllum lusitanicum, Nepenthes gracilis, N. khasiana, N rafflesiana, Triphyophyllum peltatum | Droseraceae, Drosophyllaceae, Nepenthaceae, Dioncophyllaceae | Nepenthales (Caryophyllales) | Zenk et al., 1969; Cannon et al., 1980; Culham and Gornall, 1994; Bringmann et al., 2000; Rischer et al., 2002; Aung et al., 2002; Marczak et al., 2005; Gonçalves et al., 2008; Krolicka et al., 2008; Raj et al., 2011; Buch et al., 2013 | 1 | ↓ |
| Attraction or capture | Quercetin | Not tested (present in sticky resin) | (✓) | Roridula dentata | Roridulaceae | Ericales | Wollenweber, 2007 | 2 | ↓ |
| Attraction | Trans-jasmone | Floral or fruit scent | (✗) | Sarracenia spp. | Sarraceniaceae | Ericales | Hotti et al., 2017 | 2 | ↑ |
| Attraction or capture | Tricetin (derivative corymbosin) | Not tested (present in sticky resin) | (✓) | Roridula gorgonias | Roridulaceae | Ericales | Wollenweber, 2007 | 2 | ↓ |
| Attraction | Xylene (o-, m-, p-) | Found on the spoon of the pitcher, assumed to be for attraction | ✗ | Heliamphora heterodoxa, H. tatei | Sarraceniaceae | Ericales | Jaffé et al., 1995 | 2 | ↑ |
DISCUSSION
We have profiled, for the first time, the whole-leaf metabolome response to the addition of animal substrate to a carnivorous plant trap. Though some metabolites have been previously shown to be involved in plant carnivory (Hatcher et al., 2020), we show that there is a substantial metabolic response, which involves a large proportion of known biochemical systems as well as at least 164 unidentified secondary metabolites. The largest response (fold changes and number of metabolites) was of compounds previously associated with defence processes, and compounds that are associated with the attraction of pollinators in other plant systems. This finding is important because it indicates that carnivory, at the metabolic level, is evolved from at least two distinct plant functions: defence and attraction of organisms. Many of the secondary metabolites that demonstrated a statistically significant response had fold increases >30 times the concentration in unfed plants. This demonstrates that there is a clear and substantial metabolic response to prey capture.
Secondary plant metabolites are hypothesized to play an important role in plant diversification and evolution into novel environments (Theis and Lerdau, 2003; Wink, 2003; Lewinsohn and Gijzen, 2009). If secondary metabolites are a conduit for plant evolutionary adaptation, co-occurring metabolites are expected to be present among unrelated taxa that are evolved to occupy similar habitats or possess similar syndromes (Fang et al., 2018). The extent to which convergent evolution of secondary metabolites is true for carnivorous plants is unclear (Ellison and Gotelli, 2009). We identified putatively ~34 compounds present in D. capensis that are hypothesized to be involved in carnivory in other carnivorous plants. Eleven of these compounds are new to the Nepenthales lineage but have been previously identified in other carnivorous lineages (Table 2). Two-thirds of these 34 compounds are found across independent lineages of carnivory. It is likely that the same compounds are used to some extent for the purpose of acquiring nutrients from prey, even in different carnivorous plant lineages i.e. phenylacetaldehyde, nonanal and quercetin. These shared metabolic traits, and the clear potential for them to play a role in carnivory, provide some support to the hypothesis that secondary plant metabolites are important for evolutionary adaptation to specific environments (Theis and Lerdau, 2003; Wink, 2003). This remains speculative until confirmation with robust tests of convergent metabolic evolution for plant carnivory. Some shared metabolites perform other functions and are shared with non-carnivorous plants. For example, compounds found only to increase following prey digestion and assimilation may be a consequence of increased nutrient status, rather than holding a role in the carnivorous habit specifically. Additionally, the compounds we identify in this study are annotated from metabolite databases, and therefore require specific targeted analysis to comprehensively confirm their presence, concentration and function.
Drosera capensis has co-opted or exapted the use of existing secondary plant metabolites known to function in non-carnivorous plant processes for responding to prey capture (Nakamura et al., 2013; Pavlovič and Saganová, 2015). In the present study, some of these compounds increased to considerably higher concentrations and/or were sustained for much longer in response to prey capture than is typical of the known response in non-carnivorous plant functions. Defence-related jasmonates such as isoleucine increased by 3000 % within 24 h of simulated prey capture and were sustained above this level for the full 72-h experiment. Wounding has been shown to instigate a transient, comparatively low change in concentration of jasmonates, and the response of this compound to herbivory peaks similarly to the response to prey capture after 1.5 h but returns to the original concentration by 3 h post-herbivory (Mithöfer et al., 2014). Furthermore, we measured 36-fold increases in the floral scent and flavour phenylacetaldehyde in response to prey capture. Metabolites can have multiple functions within the plant based on biological thresholds. Here, D. capensis appears to have metabolic thresholds for this compound at much higher levels in response to prey capture than has been demonstrated in other plants for different functions. For example, in tomato (Solanum lycopersicum), overexpression of genes controlling phenylacetaldehyde increased concentration by up to ten times (Schwab et al., 2008). It is therefore clear that in D. capensis prey capture instigates a highly complex biochemical response utilizing many compounds across multiple metabolic pathways, differentiating from non-carnivorous functions for the same compounds through either larger fold changes or by sustaining high levels for longer.
The increased concentration of metabolites following prey capture is possibly a result of a compound’s function in carnivory, but alternatively may be the result of increased nutrient status following prey digestion. Nepenthes insignis directly incorporated C2 units, from a solution of sodium acetate and alanine added to the prey-capturing pitchers, for plumbagin synthesis (Rischer et al., 2002). Such metabolic responses have also been identified in non-carnivorous plants, for example Plumbago indica (Jaisi and Panichayupakaranant, 2017). That some metabolites, in this study, had an initial statistically significant response within hours suggests a metabolic response to prey capture rather than because of altered nutrient status. Additionally, some metabolites showed a dynamic response in concentration to prey capture i.e. an initial decrease, followed by a gradual increase in concentration over time (Fig. 4P). These results suggest that many metabolites identified here are a response to prey capture. We cannot, however, rule out the possibility that some metabolite concentration changes may simply be a result of a change in plant nutrient status following prey digestion and assimilation. Thus, further investigation into the function of specific metabolites identified in this study is necessary.
The function of compounds that decrease in concentration may have multiple biological explanations and should be interpreted with caution. Many metabolites in plants are stored as an inactive compound until some form of stimulus instigates the activation of these compounds at specific times, often through glycosylation (Gachon et al., 2005). Important compounds can, as a result, rapidly become active rather than the plant having to synthesize the whole compound on demand. The presentation of these processes would be of one metabolite increasing in concentration whilst another decreases. While it remains reasonable to state that compounds up- and downregulated following prey capture are likely to be involved in some aspect of carnivory, their specific function, particularly for fold decreases, cannot be explicitly determined. It can be stated, however, that due to the high number of compounds involved that change in intensity following prey capture (~2383 features), there is a reliance on secondary plant metabolites to facilitate plant carnivory.
Our results provide a clear focus for future studies, in particular the role of volatile organic compounds (VOCs). Kreuzwieser et al. (2014) hypothesize that VOCs are downregulated following prey capture and speculate that this is because if the compound functions as an attractant to prey it is not necessary to produce it following prey capture. Reduced synthesis of attractants following prey capture is a logical response because a decrease in the production of these compounds minimizes the net cost of production, which may reduce the cost of carnivory (Ellison and Gotelli, 2009; Grace and Hudson, 2016). Evidence from our study is, however, equivocal to Kreuzwieser et al. (2014), as only a subset of identified VOCs decreases following insect substrate addition (e.g. isorhamnetin, tricetin, gallic acid and kaempferol; Table 2). These differences may be explained by sampling approaches; Kreuzwieser et al. (2014) measured volatiles emitted from the leaf, as opposed to, in our study, directly measuring the leaf. Further work is necessary to determine the role of some VOCs in carnivory and may require GC-MS to identify highly volatile VOCs.
The majority (142 of ~170) of secondary plant metabolites involved in carnivory are classified as VOCs and are assumed to be attractants for prey (see review by Hatcher et al., 2020). The assumption that VOCs attract prey may be misleading. Rather than a description of biological relevance, the classification of VOCs is a chemical grouping and these compounds usually have very low volatility under ambient conditions (under European classification VOCs have boiling points <250 °C; US EPA, 2019). In the present experiment, 24 of the 34 compounds also found in other carnivorous plants have previously been suggested to function in attraction (Table 2) (Jürgens et al., 2009; Kreuzwieser et al., 2014; Hotti et al., 2017). If compounds are involved in attraction, it is expected that these compounds will decrease in concentration after prey capture, or at the very least not change in concentration, as found in a study on prey-induced changes to phenolic metabolites in D. capensis (Kováčik et al., 2012b). Of these 24 compounds, however, 18 increase in concentration after prey capture, in some cases by >30 times the concentration in unfed plants (Table 2). This is contradictory to expected responses if the compound is solely involved in the attraction of prey. Such findings may be in contrast to the findings of Kováčik et al. (2012b) due to differences in prey addition method (powdered flies or whole ants) and temporal sampling. These differences may mean certain transient changes in metabolites are not recorded or responses instigated in this experiment do not occur with different prey addition techniques. Involvement of these 24 compounds in carnivory is not disputed here. We propose, however, that these compounds provide an alternative function, at least for D. capensis but probably also for other carnivorous plants, that aids in capture, retention, digestion or assimilation. Volatile organic compounds require further investigation to establish their biological function, rather than relying solely on chemical classification (Table 2).
We conclude that secondary plant metabolites have a substantial role in the multifaceted plant adaptation of carnivory in D. capensis. Response to prey capture involves a whole-metabolome response. Not only does it appear that there is a strong biochemical basis in response to prey capture, but there also appears to be a large diversity of compounds – larger than previously considered – that are important for carnivory in plants. Secondary metabolites may be much more important for plant carnivory than previously thought. In addition, we provide evidence that there is convergence in the secondary metabolites involved in carnivory across independently evolved lineages of carnivorous plant. This work supports the hypothesis of Wagner (2017) that secondary metabolites may therefore be important drivers of plant evolution and diversification into new environments. Future work should focus on characterisation of the metabolomic response across species of carnivorous plant, and identification of biologically relevant metabolites and their functions.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Methods Detail: extractions, UHPLC-MS analysis and data processing. Figure S1: total ion current (TIC) chromatogram for a blank and a QC run. Figures S2–S9: OPLS-DA S-plot and score plots. Table S1: cross-comparison of univariate and OPLS-DA analysis. Table S2: FDR-adjusted ANOVA of fed plants compared with unfed controls.
ACKNOWLEDGEMENTS
We thank South West Carnivorous Plant Nursery for controlled growing of Drosera capensis, Richard Harland for technical support and Dr Amit Chandra for providing liquid nitrogen, and reviewers for their constructive comments.
FUNDING
This work was supported by a Loughborough University PhD studentship to C.R.H. The processing and LC–MS analyses of metabolomic samples were conducted at the Natural Environment Research Council (NERC) Biomolecular Analysis Facility at the University of Birmingham (NBAF-B) (grant number NBAF976).
LITERATURE CITED
- Adamec L. 2002. Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist 155: 89–100. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. 2011. Current trends in the evolutionary ecology of plant defence. Functional Ecology 25: 420–432. [Google Scholar]
- Albert VA, Williams S, Chase M. 1992. Carnivorous plants: phylogeny and structural evolution. Science 257: 1491–1495. [DOI] [PubMed] [Google Scholar]
- Aung H, Chia L, Goh N, et al. 2002. Phenolic constituents from the leaves of the carnivorous plant Nepenthes gracilis. Fitoterapia 73: 445–447. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Rischer H, Wohlfarth M, Schlauer J, Assi LA. 2000. Droserone from cell cultures of Triphyophyllum peltatum (Dioncophyllaceae) and its biosynthetic origin. Phytochemistry 53: 339–343. [DOI] [PubMed] [Google Scholar]
- Buch F, Rott M, Rottloff S, et al. 2013. Secreted pitfall-trap fluid of carnivorous Nepenthes plants is unsuitable for microbial growth. Annals of Botany 111: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J, Lojanapiwatna V, Raston C, Sinchai W, White A. 1980. The quinones of Nepenthes rafflesiana. The crystal structure of 2,5-dihydroxy-3,8-dimethoxy-7-methylnaphtho-1,4-quinone (nepenthone-e) and a synthesis of 2,5-dihydroxy-3-methoxy-7-methylnaphtho-1,4-quinone (nepenthone-c). Australian Journal of Chemistry 33: 1073. [Google Scholar]
- Catola S, Centritto M, Cascone P, et al. 2018. Effects of single or combined water deficit and aphid attack on tomato volatile organic compound (VOC) emission and plant-plant communication. Environmental and Experimental Botany 153: 54–62. [Google Scholar]
- Culham A, Gornall RJ. 1994. The taxonomic significance of naphthoquinones in the Droseraceae. Biochemical Systematics and Ecology 22: 507–515. [Google Scholar]
- van Dam NM, Bouwmeester HJ. 2016. Metabolomics in the rhizosphere: tapping into belowground chemical communication. Trends in Plant Science 21: 256–265. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1875. Insectivorous plants. London: John Murray. [Google Scholar]
- Di Giusto B, Bessière J-M, Guéroult M, et al. 2010. Flower-scent mimicry masks a deadly trap in the carnivorous plant Nepenthes rafflesiana. Journal of Ecology 98: 845–856. [Google Scholar]
- Duplais C, Papon N, Courdavault V. 2020. Tracking the origin and evolution of plant metabolites. Trends in Plant Science 25: 1182–1184. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Adamec L. 2018. Part III: physiology, form and function. In: Carnivorous plants: physiology, ecology, and evolution. Oxford, England: Oxford University Press, 155–281. [Google Scholar]
- Ellison AM, Gotelli NJ. 2001. Evolutionary ecology of carnivorous plants. Trends in Ecology & Evolution 16: 623–629. [Google Scholar]
- Ellison AM, Gotelli NJ. 2009. Energetics and the evolution of carnivorous plants – Darwin’s ‘most wonderful plants in the world’. Journal of Experimental Botany 60: 19–42. [DOI] [PubMed] [Google Scholar]
- Erb M, Kliebenstein DJ. 2020. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiology 184: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Pérez M, Krol E, Stange A, et al. 2011. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proceedings of the National Academy of Sciences of the USA 108: 15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Fernie AR, Luo J. 2018. Exploring the diversity of plant metabolism. Trends in Plant Science 24: 83–98. [DOI] [PubMed] [Google Scholar]
- Gachon CMMM, Langlois-Meurinne M, Saindrenan P. 2005. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends in Plant Science 10: 542–549. [DOI] [PubMed] [Google Scholar]
- Gao P, Loeffler TS, Honsel A, et al. 2015. Integration of trap- and root-derived nitrogen nutrition of carnivorous Dionaea muscipula. New Phytologist 205: 1320–1329. [DOI] [PubMed] [Google Scholar]
- Gaume L, Bazile V, Huguin M, Bonhomme V. 2016. Different pitcher shapes and trapping syndromes explain resource partitioning in Nepenthes species. Ecology and Evolution 6: 1378–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TC, Waller DM. 2009. Evolving Darwin’s ‘most wonderful’ plant: ecological steps to a snap-trap. New Phytologist 183: 575–587. [DOI] [PubMed] [Google Scholar]
- Gonçalves S, Gonçalves MA, Ameixa O, Nogueira JMF, Romano A. 2008. Insecticidal activity of leaf extracts from Drosophyllum lusitanicum against Liriomyza trifolii (Burgess) (Diptera: Agromyzidae). Journal of Horticultural Science and Biotechnology 83: 653–657. [Google Scholar]
- Grace SC, Hudson DA. 2016. Processing and visualization of metabolomics data using R. In: JK Prasain, ed. Metabolomics – fundamentals and applications. London, England: IntechOpen. doi: 10.5772/65405 [DOI] [Google Scholar]
- Di Guida R, Engel J, Allwood JW, et al. 2016. Non-targeted UHPLC-MS metabolomic data processing methods: a comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabolomics 12: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T. 2007. From waste products to ecochemicals: fifty years research of plant secondary metabolism. Phytochemistry 68: 2831–2846. [DOI] [PubMed] [Google Scholar]
- Hatcher CR, Ryves DB, Millett J. 2020. The function of secondary metabolites in plant carnivory. Annals of Botany 125: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher CR, Sommer U, Heaney LM, Millett J. 2021. Data for: Metabolomic analysis reveals reliance on secondary plant metabolites to facilitate carnivory in the Cape sundew, Drosera capensis. Loughborough, England: Loughborough University dataset. 10.17028/rd.lboro.14188703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotti H, Gopalacharyulu P, Seppänen-Laakso T, Rischer H. 2017. Metabolite profiling of the carnivorous pitcher plants Darlingtonia and Sarracenia. PLoS ONE 12: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé K, Blum MS, Fales HM, Mason RT, Cabrera A. 1995. On insect attractants from pitcher plants of the genus Heliamphora (Sarraceniaceae). Journal of Chemical Ecology 21: 379–384. [DOI] [PubMed] [Google Scholar]
- Jaisi A, Panichayupakaranant P. 2017. Enhanced plumbagin production in Plumbago indica root cultures by ʟ-alanine feeding and in situ adsorption. Plant Cell, Tissue and Organ Culture 129: 53–60. [Google Scholar]
- Jander G. 2014. Revisiting plant-herbivore co-evolution in the molecular biology era. In: Voelckel C, Jander G, eds. Annual plant reviews: insect-plant interactions. Chichester: John Wiley, 361–384. [Google Scholar]
- Jürgens A, El-Sayed AM, Suckling DM. 2009. Do carnivorous plants use volatiles for attracting prey insects? Functional Ecology 23: 875–887. [Google Scholar]
- Kessler D, Baldwin IT. 2007. Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant Journal 49: 840–854. [DOI] [PubMed] [Google Scholar]
- Kim JA, Kim HS, Choi SH, Jang JY, Jeong MJ, Lee SI. 2017. The importance of the circadian clock in regulating plant metabolism. International Journal of Molecular Sciences. MDPI AG. 18: 2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan JA, Weber RJM, Broadhurst DI, Viant MR. 2014. Direct infusion mass spectrometry metabolomics dataset: a benchmark for data processing and quality control. Scientific Data 1: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováčik J, Klejdus B, Repčáková K. 2012a. Phenolic metabolites in carnivorous plants: inter-specific comparison and physiological studies. Plant Physiology and Biochemistry 52: 21–27. [DOI] [PubMed] [Google Scholar]
- Kováčik J, Klejdus B, Štork F, Hedbavny J. 2012b. Prey-induced changes in the accumulation of amino acids and phenolic metabolites in the leaves of Drosera capensis L. Amino Acids 42: 1277–1285. [DOI] [PubMed] [Google Scholar]
- Krausko M, Perutka Z, Šebela M, et al. 2017. The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis. New Phytologist 213: 1818–1835. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Scheerer U, Kruse J, et al. 2014. The Venus flytrap attracts insects by the release of volatile organic compounds. Journal of Experimental Botany 65: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Król EE, Płachno BJ, Adamec L, et al. 2012. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Annals of Botany 109: 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolicka A, Szpitter A, Gilgenast E, Romanik G, Kaminski M, Lojkowska E. 2008. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzyme and Microbial Technology 42: 216–221. [Google Scholar]
- Lewinsohn E, Gijzen M. 2009. Phytochemical diversity: the sounds of silent metabolism. Plant Science 176: 161–169. [Google Scholar]
- Li C, Soufan O, Chong J, et al. 2018. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research 46: W486–W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libiaková M, Floková K, Novák O, Slováková L, Pavlovič A. 2014. Abundance of cysteine endopeptidase dionain in digestive fluid of Venus flytrap (Dionaea muscipula Ellis) is regulated by different stimuli from prey through jasmonates. PLoS ONE 9: e104424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczak L, Stobiecki M, Łojkowska E. 2005. Secondary metabolites in in vitro cultured plants of the genus Drosera. Phytochemical Analysis 16: 143–149. [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Reichelt M, Nakamura Y. 2014. Wound and insect-induced jasmonate accumulation in carnivorous Drosera capensis : two sides of the same coin. Plant Biology 16: 982–987. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Reichelt M, Mayer VE, Mithöfer A. 2013. Jasmonates trigger prey-induced formation of ‘outer stomach’ in carnivorous sundew plants. Proceedings of the Royal Society B. Biological Sciences 280: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Mithöfer A. 2019. Jasmonate signalling in carnivorous plants: copycat of plant defence mechanisms. Journal of Experimental Botany 70: 3379–3389. [DOI] [PubMed] [Google Scholar]
- Pavlovič A, Saganová M. 2015. A novel insight into the cost-benefit model for the evolution of botanical carnivory. Annals of Botany 115: 1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Jakšová J, Novák O. 2017. Triggering a false alarm: wounding mimics prey capture in the carnivorous Venus flytrap (Dionaea muscipula). New Phytologist 216: 927–938. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2016. R: a language and environment for statistical computing. [Google Scholar]
- Raj G, Kurup R, Hussain AA, Baby S. 2011. Distribution of naphthoquinones, plumbagin, droserone, and 5-O-methyl droserone in chitin-induced and uninduced Nepenthes khasiana: molecular events in prey capture. Journal of Experimental Botany 62: 5429–5436. [DOI] [PubMed] [Google Scholar]
- Ramakrishna A, Ravishankar GA. 2011. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signaling & Behavior 6: 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rischer H, Hamm A, Bringmann G. 2002. Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry 59: 603–609. [DOI] [PubMed] [Google Scholar]
- Schwab W, Davidovich-Rikanati R, Lewinsohn E. 2008. Biosynthesis of plant-derived flavor compounds. Plant Journal 54: 712–732. [DOI] [PubMed] [Google Scholar]
- Theis N, Lerdau M. 2003. The evolution of function in plant secondary metabolites. International Journal of Plant Sciences 164: S93–S102. [Google Scholar]
- Thorogood CJ, Bauer U, Hiscock SJ. 2017. Convergent and divergent evolution in carnivorous pitcher plant traps. New Phytologist 217: 1035–1041. [DOI] [PubMed] [Google Scholar]
- US EPA . 2019. Technical overview of volatile organic compounds. https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds. (4 March 2019, date last accessed). [Google Scholar]
- Wagner A. 2017. The White-Knight hypothesis, or does the environment limit innovations? Trends in Ecology & Evolution 32: 131–140. [DOI] [PubMed] [Google Scholar]
- Weber RJM, Viant MR. 2010. MI-pack: increased confidence of metabolite identification in mass spectra by integrating accurate masses and metabolic pathways. Chemometrics and Intelligent Laboratory Systems 104: 75–82. [Google Scholar]
- Wink M. 2003. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64: 3–19. [DOI] [PubMed] [Google Scholar]
- Wollenweber E. 2007. Flavonoids occurring in the sticky resin on Roridula dentata and Roridula gorgonias (Roridulaceae). Carnivorous Plant Newsletter 36: 77–80. [Google Scholar]
- Yilamujiang A, Reichelt M, Mithöfer A. 2016. Slow food: insect prey and chitin induce phytohormone accumulation and gene expression in carnivorous Nepenthes plants. Annals of Botany 118: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk MH, Fürbringer M, Steglich W. 1969. Occurrence and distribution of 7-methyljuglone and plumbagin in the Droseraceae. Phytochemistry 8: 2199–2200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.