Abstract
This article comments on:
Christopher R. Hatcher, Ulf Sommer, Liam M. Heaney and Jonathan Millett, Metabolomic analysis reveals reliance on secondary plant metabolites to facilitate carnivory in the Cape sundew, Drosera capensis, Annals of Botany Volume 128, Issue 3, 26 August 2021, Pages 301–314, https://doi.org/10.1093/aob/mcab065
Keywords: Carnivorous plants, specialized metabolites, sundew, Drosera, Nepenthes
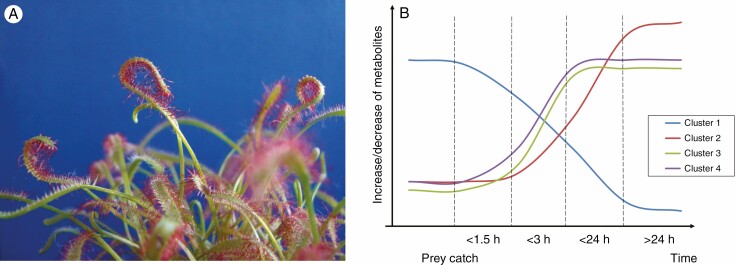
Plants are a rich source of low molecular weight secondary or specialized metabolites with a huge range of bioactivities. This is also the case for carnivorous plants, which typically grow in nutrient-poor environments and capture and digest prey, mainly insects, for additional nutrients such as nitrogen, phosphate and minerals. The role of many of these secondary metabolites in plant carnivory is still not known, but Hatcher et al., 2020 have proposed that they play a key role in the carnivorous process. By focusing on a sundew species, Drosera capensis (Fig. 1A), Hatcher et al. (2021) return to this question in the current issue of Annals of Botany.
Fig 1.
(A) Drosera capensis L. (Cape sundew). The curled flypaper leaves are sticky and catch mainly flying insect prey. Upon capture, the leaves start to bend to enclose and digest the prey. (B) A simplified model for the kinetics of four different metabolite clusters changing in their concentrations upon prey capture found in D. capensis leaves. One cluster is decreasing in metabolite concentrations; three clusters increase but at different speeds. All clusters respond within the first 3 h, reach a certain plateau and keep stable for days, indicating that the digestive and assimilation processes are ongoing.
Carnivorous plants are a polyphyletic group of plants and have fascinated scientists from the times of Charles Darwin, who provided the first reliable evidence for plant insectivory. Due to the development of large-scale ‘-omics’ techniques, our knowledge and understanding of the molecular mechanisms and evolution of the ‘plant carnivorous syndrome’ have significantly improved over the last 20 years (Ellison and Adamec, 2018; Hedrich and Fukushima, 2021). Aggregation of these data is beginning to permit an integrated approach to the identification of particular genes, enzymes and specialized metabolites in the biology of carnivorous plants.
For a long time, and in contrast to primary compounds that are directly involved in plant growth and development, specialized low molecular weight compounds have been viewed as unimportant for plant viability, as indicated by the pejorative adjective ‘secondary’. An appreciation in recent years that these metabolites are also critical for the survival of most plants – in particular in the interactions of plants with the (a)biotic environment – has altered this view. Recently, it also became evident that the current function-based classification of the various metabolites is limited, as many specialized metabolites show multifunctionality and, for example, play roles in primary metabolism and regulation of cellular processes (Erb and Kliebenstein, 2020).
Hatcher et al. (2020) have already hypothesized, based on a comprehensive literature search, that specialized metabolites have an important role in plant carnivory. Within carnivorous plants of the orders Nepenthales (e.g. the genera Dionaea, Drosera and Nepenthes) and Ericales (e.g. Sarracenia), they identified about 170 key metabolites having similar functions in various phases of the carnivorous process, from prey attraction via capture and digestion to nutrient assimilation. Strikingly, 26 compounds were found to play similar roles across independently evolved taxa, which suggested convergent evolution. Although the precise biological role of many of these carnivory-related specialized metabolites remains elusive, and functions and underlying molecular mechanisms need to be confirmed, the data point to the presence of a plant carnivory-metabolome. Some of these metabolites have been co-opted from other processes, such as pollinator attraction or defence, supporting the multifunctionality of certain compounds (Erb and Kliebenstein, 2020). In the present study, Hatcher et al. (2021) used a UHPLC-MS/MS (ultra-high performance liquid chromatography–tandem mass spectrometry) technique in a non-targeted metabolome analysis of insect-fed vs. non-fed D. capensis flypaper-trap leaves, taking samples at eight different time points from 0 to 72 h. The analysis revealed the presence of >3250 features, about 2380 of which were found to be regulated upon prey capture; of which 34 compounds might belong to the plant carnivory-metabolome. For the first time, a time-dependent analysis reveals the dynamics of metabolite regulation, showing four main clustering groups of metabolites with different kinetics in up- (cluster 2–4) and downregulated (cluster1) configurations (Fig. 1B). All clusters respond rapidly within the first 3 h, although with different intensities and some delays (see, for example, cluster 4), reaching a certain plateau and keeping stable for days. This indicates that the digestive and assimilation processes continue, and further suggests that they play distinct roles in different phases of the carnivory process. Importantly, this work also clearly demonstrates the need for identification of all these compounds, which remains a significant challenge.
Although this study is a new and important contribution, the approach did not discriminate between metabolomes of distinct cell types or tissues. Hence, a great amount of information on the spatial function of the specialized metabolites is unavailable. This latter point was addressed recently in another study on carnivorous Nepenthes × ventrata plants. Dávila-Lara et al. (2020) investigated the metabolome of fed- and non-fed pitfall-trap and leaf tissues. They found about 2000 different features, as well as a tissue-specific and higher chemical diversity in leaves compared with pitchers. Upon feeding, only 73 polar pitcher tissue compounds differed significantly. Both studies (Dávila-Lara et al., 2020; Hatcher et al., 2021) complement each other and contribute to the bigger picture. However, we are far from understanding the role of specialized metabolites in plant carnivory, as the plants, trapping types and experimental approaches in these two studies were different. Nevertheless, we can conclude with some confidence that specialized metabolite production upon prey capture is tightly regulated both spatially and temporally.
For more in-depth insights, we need to apply metabolome analyses to other carnivorous plants such as Dionaea muscipula (Venus flytrap), Utricularia spp. (bladderworts) or another pitcher plant, Cephalotus follicularis (Albany pitcher plant), for all of which a sequenced genome is available. As more species are added to the picture, our understanding of the role of specialized metabolites in plant carnivory will increase. Recent studies on other Nepenthes and pitcher plant species, although unrelated to carnivory, will definitely provide important insights (Hotti et al., 2017; Wong et al., 2020; Rosli et al., 2021). It is important that future studies should focus on a clear, indisputable identification of the compounds of interest, based on chemical analysis and reference compound spectra, particularly for metabolites that might belong to the postulated plant carnivory-metabolome. For the final elucidation of the roles of specialized metabolites, a combination of chemical identification, enzymology, genetics and physiology is necessary. This, however, is still complicated by redundancy in both genes and metabolic pathways, protein multifunctionality and the challenge of reliable and simple identification of small molecules. Technically, such analyses are also hampered by poor genome information and the lack of simple genetic manipulation methods for almost all carnivorous plant species, together with the limited availability of metabolite databases providing necessary reference mass spectra. Hopefully these limitations will be addressed by the increasing number of groups that have begun developing transformation techniques for a range of carnivorous plants, and further advances in analytical technologies and computational metabolomics. Overall, specialized metabolites in carnivorous plants represent promising model compounds to explore as drivers of evolution. Both the generation of novel compounds and the co-option of existing metabolites may represent important strategies in responding to environmental challenges.
LITERATURE CITED
- Dávila-Lara A, Rodríguez-López CE, O’Connor SE, Mithöfer A. 2020. Metabolomics analysis reveals tissue-specific metabolite compositions in leaf blade and traps of carnivorous Nepenthes plants. International Journal of Molecular Sciences 21: 4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Adamec L. 2018. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press. [Google Scholar]
- Erb M, Kliebenstein DJ. 2020. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiology 184: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher CR, Ryves DB, Millett J. 2020. The function of secondary metabolites in plant carnivory. Annals of Botany 125: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher CR, Sommer U, Heaney L, Millet J. 2021. Metabolomic analysis reveals reliance on secondary plant metabolites to facilitate carnivory in the Cape sundew, Drosera capensis. Annals of Botany 128: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Fukushima K. 2021. On the origin of carnivory: molecular physiology and evolution of plants on an animal diet. Annual Review of Plant Biology 72: 133–153. [DOI] [PubMed] [Google Scholar]
- Hotti H, Gopalacharyulu P, Seppänen-Laakso T, Rischer H. 2017. Metabolite profiling of the carnivorous pitcher plants Darlingtonia and Sarracenia. PLoS One 12: e0171078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosli MAF, Mediani A, Azizan KA, Baharum SN, Goh HH. 2021. UPLC-TOF-MS/MS-based metabolomics analysis reveals species-specific metabolite compositions in pitchers of nepenthes ampullaria, nepenthes rafflesiana, and their hybrid nepenthes × hookeriana. Frontiers in Plant Science 12: 655004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Ling YS, Wee JLS, Mujahid A, Müller M. 2020. A comparative UHPLC-Q/TOF-MS-based eco-metabolomics approach reveals temperature adaptation of four Nepenthes species. Scientific Reports 10: 21861. [DOI] [PMC free article] [PubMed] [Google Scholar]