Abstract
Mycobacterium ulcerans is the causative agent of Buruli ulcer, a rare but chronic debilitating skin and soft tissue disease found predominantly in West Africa and Southeast Australia. While a moderate body of research has examined the distribution of M. ulcerans, the specific route(s) of transmission of this bacterium remain unknown, hindering control efforts. M. ulcerans is considered an environmental pathogen given it is associated with lentic ecosystems and human-to-human spread is negligible. However, the pathogen is also carried by various mammals and invertebrates, which may serve as key reservoirs and mechanical vectors, respectively. Here, we examine and review recent evidence from these endemic regions on potential transmission pathways, noting differences in findings between Africa and Australia, and summarising the risk and protective factors associated with Buruli ulcer transmission. We also discuss evidence suggesting that environmental disturbance and human population changes precede outbreaks. We note five key research priorities, including adoption of One Health frameworks, to resolve transmission pathways and inform control strategies to reduce the spread of Buruli ulcer.
Author summary
Buruli ulcer is a debilitating skin and soft tissue disease characterised by large ulcerative wounds that are treated with antibiotics or with adjunctive surgery for advanced cases. Found predominantly in West Africa and Southeast Australia, the causative agent is the environmental bacterial pathogen Mycobacterium ulcerans. Lack of understanding of transmission pathways, combined with the absence of a vaccine, has hindered efforts to control the spread of M. ulcerans. Here, in order to identify probable transmission pathways and inform future studies, we review literature linking M. ulcerans to environmental reservoirs, mammalian hosts, and potential invertebrate vectors. We also summarise factors and behaviours that reduce the risk of developing Buruli ulcer, to inform effective prevention strategies and further shed light on transmission pathways.
Introduction
Buruli ulcer is a rare but devastating bacterial skin and soft tissue infection caused by Mycobacterium ulcerans. It usually presents as cutaneous lesions that necrotise and progress to painless ulcers. Despite being treatable with antibiotics, 31% of cases exhibit severe lesions that can be disabling and stigmatising [1]. Reported in at least 33 countries, the pathogen has been most reported and intensively studied in West Africa and Southeast Australia [2,3]. Strategies for effective control of the disease depend on a clear understanding of the transmission pathways of M. ulcerans. Yet, despite a body of research spanning more than 80 years examining Buruli ulcer risk factors and prevention methods, the mode(s) of transmission remain unresolved [4,5]. Investigation into M. ulcerans transmission by tracing clusters and cases back to the source of infection is further complicated by the relative rarity of Buruli ulcer (approximately 3,000 to 4,000 reported global cases annually [1]; less than 2% of annual leprosy case numbers [6]) and a 5-month incubation period [7]. The absence of an effective vaccine against M. ulcerans further heightens the importance of disease control and prevention in the management of Buruli ulcer worldwide.
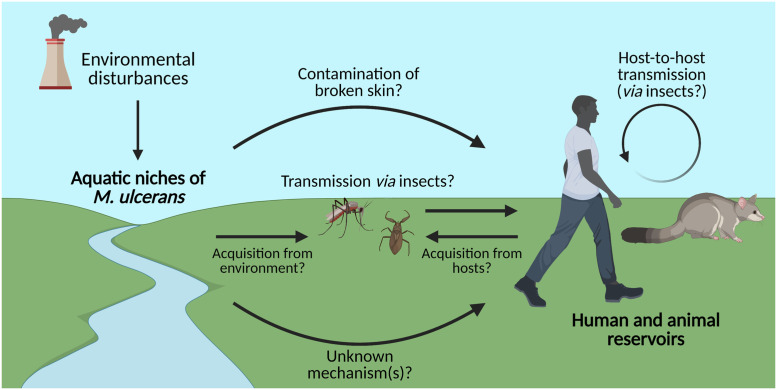
Several possible modes of transmission have been proposed (Fig 1), with evidence for these scenarios varying by region, as summarised in Table 1. Generally, M. ulcerans is considered an environmental pathogen associated with freshwater ecosystems. However, it remains unclear whether the pathogen can be directly transmitted from the environment to humans, for example, from contaminated water, sediments, vegetation, or aerosols, to broken skin [4,5]. Increasing evidence suggests that certain mammals can serve as reservoirs for the pathogen (which we define as hosts or environments in which M. ulcerans lives and reproduces), while aquatic insects and mosquitoes may serve as carriers and potentially mechanical vectors [8,9]. In this review, we summarise the current evidence surrounding the involvement of known reservoirs, the likelihood of different transmission pathways based on experimental studies and observed risk factors, and how environmental disturbance may be connected to Buruli ulcer outbreaks. Furthermore, we propose directions for future research targeting the resolution of M. ulcerans transmission to enable clear recommendations for efficient disease control in vulnerable areas.
Fig 1. Proposed mechanisms of transmission of Mycobacterium ulcerans, the causative agent of Buruli ulcer.
The mode(s) of transmission of Buruli ulcer are still uncertain and are likely to differ between West Africa and Southeast Australia. However, hypotheses such as M. ulcerans contamination via aquatic niches [10], host-to-host transmission between humans and animal reservoirs (including possums) [8], and transmission via insect mechanical vectors (including mosquitoes) [9] have been proposed. These hypotheses are explored in this review. Created with BioRender.com.
Table 1. Summary of current evidence regarding proposed routes of transmission of Mycobacterium ulcerans in West Africa and Southeast Australia.
| Route of transmission | West Africa | Southeast Australia |
|---|---|---|
| Contamination of broken skin | Unknown. Inoculation of M. ulcerans on to abraded skin does not cause infection in guinea pigs [11]. However, proper hygiene and wound care are associated with reduced odds of developing infection in Africa [12]. | |
| Breaking of contaminated skin | Possible. Needle puncture was sufficient to allow M. ulcerans to enter skin and cause infection in mice [9]. Infection has been reported following breaking of skin via human bite [13,14]. | |
| Via aquatic environments | Likely. Environmental M. ulcerans DNA identical to genetic profiles of local human Buruli ulcer cases [15]. Transmission mechanism unknown. | Possible. Buruli ulcer case numbers increase in rainy seasons [16]. Transmission mechanism unknown. |
| Human to human | Unlikely. Analysis of familial clusters suggests that infections were due to genetic predispositions, rather than human-to-human transmission [17]. | Unlikely. Analysis of familial clusters found that family members were infected with different strains of M. ulcerans, suggesting that human-to-human transmission had not occurred [18]. |
| Vertebrate to human | Unlikely. M. ulcerans has not been recovered from domestic animal samples [19]. M. ulcerans reported in wild grasscutters [20,21]. | Unknown. Possums are hosts of M. ulcerans strains also isolated from human patients [8], part of same transmission chain. |
| Aquatic insect vectors | Possible. M. ulcerans DNA has been recovered from African insects in endemic areas [22]. Insects have been shown to carry M. ulcerans and transmit to mice [23]. | Unknown. Studies have not been conducted. |
| Mosquito vectors | Unlikely. M. ulcerans has not been recovered from mosquito populations [24], and bacilli are not maintained through mosquito life cycle [25]. | Likely. Very strong geographical correlation between M. ulcerans detection in mosquito populations and human Buruli ulcer cases [26]. Mosquito bites can facilitate M. ulcerans infection in mice [9]. |
Inferences from phylogenomic studies
Examining the evolution and spread of M. ulcerans across different regions can help to elucidate the transmission and movement of this pathogen between environments and reservoirs. Comparative genomic studies indicate that M. ulcerans likely evolved from an ancestor of Mycobacterium marinum [27,28]. M. ulcerans and M. marinum are both bacteria associated with aquatic environments that cause opportunistic animal and human infections [10,29]. Yet, while their core genomes share 97% nucleotide identity [28], the M. ulcerans genome contains various signatures of a recent evolutionary bottleneck, characterised by an increased proportion of pseudogenes and substantial chromosomal deletions [30]. Through deletions and mutations, M. ulcerans has lost various genes required for survival in diverse aquatic habitats, for example, those associated with anaerobic energy conservation and light-dependent pigment biosynthesis [31]. This suggests that M. ulcerans has become a more specialist bacterium that primarily or solely grows in host-associated niches [30,32]. Importantly, M. ulcerans has also acquired the virulence plasmid pMUM001 that confers the ability to synthesise the cytotoxic and immunosuppressive toxin mycolactone and thus cause more serious ulcerative disease than M. marinum [28].
Further bacterial evolution and dissemination events gave rise to the M. ulcerans lineage primarily responsible for causing Buruli ulcer today [28,33]. Genomic analysis of West African strains of M. ulcerans suggests that the bacterium was introduced into Africa at least twice, first circa 68 BC and second during the 1800s [34]. Both introductions were highly geographically localised, but there was significant proliferation and expansion concurrent with the human colonisation of these regions [34]. Through phylogenetic, spatial, and temporal analyses, it has been shown that M. ulcerans migrated through Victoria, Australia, following an increase in human population size [35]. M. ulcerans and Buruli ulcer disease appear to have been introduced into east Australia in the early 1800s, before spreading westwards in the 1980s towards more populous cities, such as Melbourne, Victoria [35]. Similar analyses have shown that cases of Buruli ulcer rose in regions of central Africa following European colonisation [36]. In West Africa, the different genetic lineages of M. ulcerans are generally localised to specific regions and areas, which supports the possibility of an aquatic reservoir restricting bacterial movement between these regions [34,37–39]. Yet, at smaller scales, different genotypes of M. ulcerans in Africa have been found in areas dominated by another genotype [40], demonstrating the ability of the pathogen to mobilise more locally, potentially via a vector. The emergence patterns in both continents suggests that M. ulcerans may be able to mobilise and spread along gradients of human population and activity, though increased detection may also be a consequence of increased disease presentation in people.
Methods for Mycobacterium ulcerans surveillance
Resolving the transmission pathways of M. ulcerans depends on being able to detect and isolate the pathogen from diverse sample types. Most surveillance studies of M. ulcerans rely on quantitative PCR (qPCR)-based detection of specific markers. For clinical diagnosis, TaqMan qPCR assays targeting the multicopy IS2404 insertion sequence (with 213 copies in the M. ulcerans genome [30,41]) remain the gold standard for detecting Buruli ulcer due to their high specificity, high sensitivity, and rapid turnaround time [42,43]. For environmental surveys, a multiplex qPCR assay is typically used to simultaneously detect IS2404, IS2606 (another multicopy insertion sequence), and ketoreductase-B (KR-B) DNA (required for mycolactone biosynthesis). Using these markers, M. ulcerans DNA has been reliably detected by PCR or qPCR across diverse waters, soils, invertebrates, vertebrates, and plant-associated samples [8,42,44–47]. However, as previously noted [5], the information gained from such detection is limited. Most importantly, it is difficult to discern whether the small quantities of DNA often detected are relevant for resolving transmission cycles. Through such methods alone, it is also impossible to determine whether the pathogen is viable in different samples (in contrast to cultivation) or comes from the same lineage as infected patients (in contrast to genomics and amplicon sequencing).
At present, the challenges associated with cultivating M. ulcerans mean that we lack a strong understanding of its viability and population structure in environmental and animal samples. The pathogen typically takes 2 to 3 months to grow on an agar plate and will be rapidly outgrown by bacterial and fungal contaminants if decontamination steps aren’t taken [48]. Moreover, its abundance is often too low in environmental or animal samples to be reliably cultivated [49]. As a result, there are few examples of M. ulcerans being isolated in axenic cultures from aquatic sources [49,50]. However, there may be potential to improve cultivation methods to better select and differentiate M. ulcerans. For example, a recent study combined a selective medium (Middlebrook 7H10 agar with chlorhexidine decontamination) and a differential readout (F420-based autofluorescence) to cultivate M. ulcerans from the faeces of wild grasscutters (greater cane rats; Thryonomys swinderianus) [20]. There is also the potential to leverage recent advances in culture-independent sequencing, including metagenomic mapping and assembly or tiled amplicon sequencing, to better resolve population structure and potential transmission dynamics of M. ulcerans between human, animal, and environmental sources.
Humans as reservoirs of Mycobacterium ulcerans
The most frequently reported host of M. ulcerans is humans, although direct human-to-human transmission has not been demonstrated or documented and is considered an unlikely route in endemic regions of both Africa and Australia. Although one case of Buruli ulcer was reported following a human bite in Benin, it is thought that the pathogen entered the wound from an environmental rather than oral source [13,14]. In the absence of confirmed transmission and experimental studies, familial and genetic analyses of infected individuals and causative strains of M. ulcerans can provide insight into the possibility of transmission between humans. A case–control study conducted in Benin found that individuals were five times more likely to develop Buruli ulcer if a family member had previously contracted the disease; however, this was attributed to genetic predispositions to infection rather than familial transmission, though cases were not genotypically matched to controls [17]. Several alleles and genetic polymorphisms have been predicted to confer an increased susceptibility to developing symptomatic Buruli ulcer, which may contribute to the emergence of familial clusters of this disease [51,52]. A more recent study conducted in Victoria, Australia examined familial clusters of Buruli ulcer infections and found that M. ulcerans isolates differed in genotype between family members, resolving that transmission between family members had not occurred [53]. This study also found that family members were diagnosed at similar times (shorter than the median incubation time of approximately 4.5 months), suggesting they acquired infection from a common environmental source within weeks of each other [53]. The absence of evidence for human-to-human transmission suggests that M. ulcerans is not transmitted between humans, implicating environmental and other potential routes of transmission.
Environmental reservoirs of Mycobacterium ulcerans
To understand the enigma of Buruli ulcer transmission, consideration of the environmental reservoirs in which M. ulcerans is found is critical, as these may overlap with human activity and present opportunities for infection. M. ulcerans is considered an environmental pathogen, given evidence from epidemiological studies from both Southeast Australia and West Africa that Buruli ulcer primarily occurs in areas with swamps and slow-flowing water, and the lack of evidence of human-to-human spread [10,15,17,53–55]. Indeed, fine spatial modelling suggests that proximity to waterbodies and related factors were the strongest predictors of Buruli ulcer occurrence and M. ulcerans suitability [56,57]. Likewise, from a social perspective, residents in the Ivory Coast (a Buruli ulcer–endemic region of Africa) also associate unclean water with acquiring Buruli ulcer [58].
Such inferences are supported to some extent by molecular evidence on the distribution of M. ulcerans in aquatic habitats. Multiple studies have detected M. ulcerans DNA in aquatic ecosystems, such as stagnant water sources, in West Africa and French Guiana [59–61]. For example, Bratschi and colleagues tested samples from shallow water holes in Cameroon over a period of more than 2 years and found that M. ulcerans DNA was consistently present over the entire duration of the study [10]. An important feature of this study was that the sample collection sites were water sources used by villagers for bathing and washing, supporting the hypothesis of environmental transmission through aquatic reservoirs. Environmental detection of M. ulcerans DNA in West Africa is positively correlated with the incidence of Buruli ulcer in an area, supporting the possibility of an environmental transmission route [62]. M. ulcerans is also detected more frequently during wet (rainy) seasons, in endemic regions of both Africa and Australia, with Buruli ulcer case numbers increasing during these periods [16,54,63,64]. The increased environmental load of M. ulcerans reported during these rainy seasons likely contributes to the observed increase in case numbers [63]. This is reminiscent of the transmission of leptospirosis, which increases after flooding or heavy rain [65]. Other than water bodies, M. ulcerans is known to persist for extended periods in various other environments [66,67]. M. ulcerans DNA has additionally been detected in West Africa in diverse aquatic plant species [15,46,68,69] and animals, including snails, fish, and amphibians [47,70,71], which may contribute to hosting and maintaining M. ulcerans in an aquatic environment. M. ulcerans DNA has also been detected in amoebae, though at a low prevalence based on PCR screening only [72,73].
An important caveat is that most environmental surveys solely rely on qPCR-based detection of M. ulcerans DNA. As noted above, such approaches do not provide information on whether the pathogen is viable, transmissible, and shares genetic history with human isolates in samples it is detected from. Some studies have partially genotyped samples (e.g., through variable number of tandem repeats (VNTR) profiling) but have not precisely resolved phylogenetic relationships between M. ulcerans in human and environmental sources [55,74,75]. Also in counterpoint, a study in Ghana by Pileggi and colleagues found a negative association between the wetness index of an area and the odds of M. ulcerans DNA presence, seemingly contradicting the accepted paradigm of M. ulcerans as an aquatic pathogen [76]. However, aquatic sites were only sampled over a single day and selected sites were restricted to large bodies of water, excluding many smaller niches. It also remains unclear whether M. ulcerans can be directly transmitted from water sources independently of a vector. Studies in guinea pigs found that bacteria inoculated onto abraded skin did not lead to infection [11]. Rather, disease in guinea pigs was only observed after subcutaneous bacterial inoculation, a finding reinforced from infection studies in mice that showed infection was only established after needle puncture (or mosquito blood feeding) [9]. It is possible that infections may be acquired directly from the environment via deeper cuts and more exposed wounds, similar to leptospirosis where existing lacerations increase the risk of infection from a contaminated environment [65], though experimental studies demonstrating this in Buruli ulcer are lacking.
Aquatic insects in Mycobacterium ulcerans transmission
Given M. ulcerans is linked with lentic systems and has signatures of a host-adapted lifestyle, it has been proposed that water bugs and other aquatic insects serve as vectors. Several studies have focused on elucidating the role of aquatic insects in the transmission of M. ulcerans in West Africa, though corresponding research efforts have not been conducted in Australia. The association of M. ulcerans DNA with aquatic insects has been confirmed in endemic regions of Africa, particularly Benin [22]. In addition, a recent model of aquatic insect distribution in West Africa predicted that insects likely to be involved in the transmission of Buruli ulcer were those which had adapted to environments of two highly affected regions, Ghana and Cameroon [77]. Also consistently, aquatic insects are one of the main perceived causes of Buruli ulcer by residents in the Ivory Coast [58], and receiving insect bites near a river has recently been identified as a risk factor for developing Buruli ulcer in Togo [78].
A 2014 case report describes a 6-year-old girl in Benin who presented with ulcers and recalled being bitten by an aquatic insect of the Belostomatidae (giant water bug) family at the site of ulceration [79]. This occurred near an aquatic environment, so it is not clear whether the source of infection was the insect vector or if the bite simply facilitated entry from the environment. No other direct evidence of insect transmission to a human has been reported to date. Experimentally, African aquatic insects of the Belostomatidae family (Appasus spp., Diplonychus spp.) have been shown to carry M. ulcerans and transmit bacteria to their larvae [80]. A major study isolated an M. ulcerans strain from water striders (Gerridae family, Gerris spp.); this strain shared genotypic and phenotypic features to human isolates and caused severe infection in a mouse footpad model [49]. Notably, this is the only report of successful axenic cultivation of M. ulcerans from an environmental sample worldwide. Naucoridae (creeping water bugs) can also carry M. ulcerans and, in an experimental model, transmit the bacteria to mice by biting, further supporting the possibility of environmental transmission via insects [23,81].
Despite the above evidence, aquatic insects have been described as “unlikely vectors” for M. ulcerans on several grounds [82]. A field study in Ghana collected over 22,000 aquatic insects and found no difference in numbers of insect communities positive for M. ulcerans between endemic and nonendemic sites (less than 2% positive across the study), which questions the relationship of these insects with carriage and transmission of Buruli ulcer in West Africa [82]. There is also conflicting evidence whether the abundance of these insects correlates with Buruli ulcer incidence [82–84]. Moreover, Belostomatidae and Naucoridae rarely bite people and their trophic ecology is atypical of disease vectors [5,82]. Mathematical modelling has also shown that spatial and temporal variations of Buruli ulcer incidence are better explained by environmental rather than water bug transmission [85]. A systematic review further critiques the evidence for the role of aquatic insects and proposes establishing more rigorous criteria for vector incrimination [5].
Mosquitoes in Mycobacterium ulcerans transmission
Molecular studies have implicated mosquitoes as potential mechanical vectors for M. ulcerans in Australia, where M. ulcerans DNA has been recovered from mosquito populations in endemic areas [26,86]. In particular, Lavender and colleagues collected data simultaneously with human Buruli ulcer case data in Victoria and found a very strong positive dose–response relationship between M. ulcerans DNA detection in mosquitoes and Buruli ulcer cases in each town (r = 0.99, p < 0.001) [26]. Specifically, mosquitoes were more likely positive for M. ulcerans DNA in Point Lonsdale, the town with the greatest number of cases in the study. A case–control study in Southeast Australia found that a history of mosquito bites was associated with an increased risk of Buruli ulcer, while use of insect repellents and long-sleeved clothing was associated with a reduced risk of infection [87]. A role for mosquitoes in the spread of Buruli ulcer is supported further by the fact that Buruli ulcer lesions develop preferentially on extremities of limbs, which are often exposed and susceptible to mosquito bites [88]. It has also been found in Victoria, Australia that the majority of Buruli ulcer infections occur in the warmer months, when the abundance of mosquitoes is highest and individuals are more likely to wear clothing that leaves skin and limbs exposed to mosquitoes and other biting insects [88]. Contrastingly, an environmental survey of mosquitoes in Queensland, Australia found that while mosquitoes were positive for genetic markers of the pathogen, genetic analysis revealed divergence between human pathogenic strains and those isolated from mosquitoes [89]. Further genetic comparisons of clinical and mosquito-associated strains of M. ulcerans may clarify the role mosquitoes play in transmission in various endemic regions of Australia.
A recent study by Wallace and colleagues demonstrated that Australian mosquito bites (Aedes notoscriptus) facilitate M. ulcerans infection in a mouse tail model [9]. Mouse tails were coated with M. ulcerans and subjected to mosquito biting for 20 minutes. This was sufficient to cause infection, demonstrating that mosquitoes can facilitate the entry of M. ulcerans through the skin. However, it remains unclear whether mosquitoes can cause infection in natural settings or in humans, whether by providing an entry route for the bacterium when it is already present on the skin, or by acting as a mechanical vector by providing both the entry route and the bacterium. This study also found that M. ulcerans has a low infectious dose, which is a commonly observed feature of vector-borne diseases [9]. Other experimental studies suggest that mycolactone may serve as a potential attractant for mosquitoes, thereby facilitating M. ulcerans transmission [90,91]. Ultimately, further experimental and observational studies are needed to establish vector competence.
In contrast, the evidence for mosquito transmission is lacking in West Africa. Environmental surveillance studies have failed to detect both M. ulcerans bacteria and DNA in mosquito populations of endemic areas of Africa, suggesting that mosquitoes do not play a major role in the carriage or transmission of M. ulcerans as they appear to in some areas of Australia [22,24]. Additionally, experimental studies have shown that while African mosquito larvae (Anopheles gambiae) can ingest and host M. ulcerans during larval developmental stages, bacilli are absent by the adult stage where they would be transmitted to offspring, so biological transmission via mosquitoes appears to be unlikely in West Africa [24,25]. Due to the long incubation time of Buruli ulcer, it is difficult to trace an infection back to a brief event such as a mosquito bite. More rigorous surveillance of endemic regions and mosquito activity in West Africa is needed before any conclusions can be made. Currently, the contrasting evidence between Southeast Australian and West African studies suggests that the mode(s) of transmission of Buruli ulcer may be different, with mosquitoes playing a more significant role in Australia. This could be a consequence of different bacterial strains, mosquito species/populations, environmental factors, and/or social behaviours between the two continents. Direct comparisons of Australian and African mosquito species or M. ulcerans strains may further resolve this difference.
Mammalian reservoirs of Mycobacterium ulcerans
In addition to humans, several other mammals have also been shown to become infected with M. ulcerans [92]. Overall, evidence for animals as hosts of M. ulcerans is stronger for Australian animals than for African animals, which may reflect geographical differences in M. ulcerans lineages and/or routes of transmission. In Point Lonsdale, a Buruli ulcer–endemic area of Southeast Australia, Fyfe and colleagues found that over one-fifth of Australian native possums (Pseudocheirus peregrinus and Trichosurus vulpecula) were positive for M. ulcerans DNA and shed bacterial DNA in their faecal pellets, and several showed lesions characteristic of symptomatic Buruli ulcer [8]. This high prevalence, especially in comparison to observations in other wildlife, points to possums as important reservoirs of M. ulcerans [92,93]. An isolate was also obtained from a ringtail possum, and subsequent whole genome sequencing revealed that the isolate was virtually identical to human clinical isolates of the same endemic region (differing by only two SNPs), indicating that possums and humans were part of the same transmission chain [8].
Case reports of Buruli ulcer have also been described in a number of other animals in endemic regions of Australia, including koalas [94,95], horses [96], dogs [97], a cat [98], and alpacas [99], where swabs from lesions or ulcers were positive for M. ulcerans by PCR. These findings highlight the importance of M. ulcerans as both a human and an animal pathogen, though provide only incomplete evidence for infection in each of these species. Recently in Australia, bandicoots have been investigated as hosts of M. ulcerans, though studies conducted to date have only identified a total of four faecal samples containing M. ulcerans DNA [89,100]. One bandicoot has been reported presenting with lesions characteristic of Buruli ulcer, though swabs of these lesions were all negative for M. ulcerans [100], and local veterinarians did not report any Buruli ulcer cases in bandicoots. Furthermore, molecular detection in faecal samples may be indicative of exposure to M. ulcerans rather than infection.
In contrast, there does not appear to be an equivalent vertebrate host in endemic regions of Africa. Grasscutters have recently been proposed as potential hosts of M. ulcerans in endemic regions of Africa. In the Ivory Coast, almost 20% faecal samples and 80% spleens surveyed were positive for M. ulcerans DNA, though it is likely that prevalence was overestimated as the criteria for a positive sample in this study were less rigorous than many other studies (one of the IS2404, IS2606, and KR-B M. ulcerans genetic markers positive by PCR analysis, rather than all three simultaneously) [21]. An M. ulcerans isolate has also been cultivated from grasscutter stool, though was not genotypically or phenotypically characterised [20]. Thus, it remains uncertain what role these animals play in pathogen ecology. In contrast to possums, Buruli lesions have not been observed in grasscutters. In most cases, rodents likely ingest M. ulcerans while consuming aquatic plants without developing systemic infections [101,102]. There is minimal evidence that other animals in West Africa may be hosts of M. ulcerans. One case study in Benin reported a goat and a dog each presenting with lesions positive for M. ulcerans DNA, though this is the only report of infection in such animals in Africa [103]. Tobias and colleagues conducted a large-scale study of domestic animal faecal samples in endemic villages in Ghana and did not recover a single positive sample, indicating that it is unlikely that domestic animals play a role in harbouring M. ulcerans in West Africa [19]. Likewise, a survey of 565 small mammals trapped in Benin did not identify M. ulcerans [104].
Environmental disturbance precedes Buruli ulcer outbreaks
Several studies have reported that Buruli ulcer often follows environmental disturbance. Deforestation and a lack of forested land cover have been associated with M. ulcerans emergence in several regions of West Africa [76,105–107]. Specifically in Ghana, Buruli ulcer incidence is positively associated with urbanisation and mining [105,106]. Similar trends have been observed in the Ivory Coast, where areas in the vicinity of man-made dams and cultivated crop fields were found to be high-risk areas for contracting Buruli ulcer [108,109]. In addition, the emergence of M. ulcerans has recently been reported in Cameroon as a result of the damming of the Mapé river [110]. Fewer studies on environmental factors facilitating M. ulcerans emergence have been conducted in Australia. However, a large, localised outbreak in Phillip Island was strongly linked to the construction of a golf course and wetland [111,112]. Based on PCR data, both the golf course irrigation system and wetland were highly contaminated with M. ulcerans [45,113], with a strong correlation observed between inferred pathogen numbers and reported Buruli ulcer cases observed both spatially and temporally. It has been proposed that M. ulcerans was transmitted to humans from either the irrigation system through aerosolised droplets or from the wetland via an insect vector [5,45].
These various disturbances to the environment may contribute to the emergence of M. ulcerans in several ways. As discussed earlier, given M. ulcerans is primarily found in and adapted to aquatic niches, human activities may create appropriate aquatic environments where M. ulcerans and potentially their vectors can thrive [16]. Additionally, both increased interactions of humans with aquatic environments and increased proximity of settlements to waterways create opportunities for infections to occur. A study by Morris and colleagues found that human-driven land use changes and deforestation had ecologically significant impacts on the abundance of potential animal hosts of M. ulcerans within aquatic ecosystems in French Guiana, subsequently increasing the bacterial load within an ecosystem and potentially facilitating transmission to humans via these hosts [114]. Specifically, these environmental disturbances impacted freshwater food webs, causing complex downstream effects in trophic ecology that together favour the emergence and transmission of M. ulcerans. Overall, human interventions generally diminish local biodiversity and often compromise animal health, creating more favourable conditions for M. ulcerans to cause infection and potentially vectors to thrive [115,116]. Given the rarity and uncertainty of Buruli ulcer data, environmental and landscape factors such as those described above could be used to predict M. ulcerans presence, emergence, and potential high-risk areas for Buruli ulcer, which may assist in preventing environmental transmission to humans [105]. Previous reviews provide additional perspectives on the effects of environmental disturbance and potentially global change in M. ulcerans ecology [5,117].
Strategies for controlling Buruli ulcer
Given our understanding of the mode(s) of transmission of Buruli ulcer remains low, control of this disease is difficult as specific transmission pathways cannot be interrupted. Rather, evidence taken directly from prevention studies can inform effective strategies for disease control and further elucidate transmission pathways. Several prevention case–control studies conducted in Buruli ulcer–endemic regions of Africa have explored factors that reduce the risk of contracting Buruli ulcer (Table 2), providing insight into potential transmission routes. Contact with water sources in various settings is consistently associated with increased odds of infection, while reducing contact with these environments is protective [118–121]. This is especially important in agricultural settings where individuals may be working in these areas for long periods of time. The use of adequate waterproof protective equipment and sleeved clothing reduces these risk factors and, therefore, the likelihood of developing an infection. A similar approach is recommended to reduce the risk of leptospirosis infection, which is also associated with flooding, wet environments, and existing wounds as potential entry points for infection [65,122]. There is also the potential to develop physical interventions to reduce contact with environmental water sources. For example, a recent study showed a strong negative correlation between the introduction of new wells and the incidence of Buruli ulcer in Benin [123]; an associated case–control study showed that regular use of water from the wells (for washing, bathing, drinking, or cooking) was a protective factor against infection [123].
Table 2. Summary of activities and behaviours that influence odds of developing Buruli ulcer infection.
Data were taken from case–control studies conducted in regions of Africa endemic for Buruli ulcer. OR refers to the odds of developing Buruli ulcer in cases compared to noninfected controls for “risk factors” and the odds of not developing Buruli ulcer in noninfected individuals compared to infected cases for “protective factors”.
| Factor | OR | 95% Confidence Interval | Reference |
|---|---|---|---|
| Risk factor | |||
| Agricultural contact with surface water | 6.3 | 1.8–21.9 | N’Krumah et al. [118] |
| Recreational contact with surface water | 5.7 | 1.6–20 | Pouillot et al. [119] |
| Washing/bathing in surface water | 7.5 | 2.0–27.8 | N’Krumah et al. [118] |
| 6.9 | 1.4–34.7 | Landier et al. [12] | |
| Absence of protective clothing during agricultural activities | 18.5 | 5.2–66.7 | N’Krumah et al. [118] |
| 15 | 4.2–58 | Pouillot et al. [119] | |
| Receiving insect bites near a river | 7.8 | 1.5–41.2 | Maman et al. [78] |
| Report scratching wounds after insect bites | 2.7 | 1.4–5.4 | Landier et al. [12] |
| Treating wounds with adhesive bandages | 6.4 | 2.2–19 | Pouillot et al. [119] |
| Protective factor | |||
| Infrequent contact with flowing water | 2.1* | 1.4–3.3 | Nackers et al. [120] |
| Washing, bathing, drinking, or cooking with well water | 10* | 2.3–25 | Degnonvi et al. [123] |
| Footwear use | 6.7* | 3.4–13 | Tomczyk et al. [124] |
| Regular use of bed nets | 2.6 | 1.2–6.0 | Pouillot et al. [119] |
| 2.5* | 1.1–5 | Landier et al. [12] | |
| Regularly washing clothing | 5.1 | 1.5–17 | Pouillot et al. [119] |
| Using rubbing alcohol on wounds | 2.2 | 1.0–4.6 | Pouillot et al. [119] |
| Use of soap and good general hygiene | 10* | 3.3–33 | Landier et al. [12] |
| 2.4* | 1.5–4.0 | Nackers et al. [120] | |
| 4.0* | 2.7–5.9 | Nackers et al. [120] | |
| Secondary education or above | 3.6 | 1.3–9.8 | Pouillot et al. [119] |
| Good knowledge of risks that may result in Buruli ulcer | 3.3* | 1.3–10 | N’Krumah et al. [118] |
| Good knowledge of causes of Buruli ulcer | 10* | 3.3–50 | N’Krumah et al. [118] |
*Original data reported as odds of developing Buruli ulcer in cases compared to noninfected controls, converted to odds of not developing Buruli ulcer in noninfected individuals compared to cases.
OR, odds ratio.
In addition, efforts should be dedicated to ensuring hygiene is maintained in areas of West Africa where Buruli ulcer is endemic. As M. ulcerans can cause infection via exposed wounds, adequate and proper wound care should be maintained to prevent infection [12,119,120]. Additionally, the regular use of bed nets and insect repellents has been associated with a lower incidence of Buruli ulcer, which may be a consequence of deterring contact with potential insect vectors [12,119]. These observations support a possible role of insects and mosquitoes in the mechanical transmission of M. ulcerans, where insect bites puncturing the skin may facilitate the colonisation and entry of the pathogen [9]. Future studies may investigate other biting insects to explore this hypothesis further and uncover any other potential vectors.
An important observation from several studies in West African countries is that education, both general and Buruli ulcer specific, is associated with a lower incidence of Buruli ulcer [118,119]. Improving access to education and educational facilities in endemic regions may be a significant and effective intervention in reducing case numbers [125,126]. More specifically, targeted educational programmes about Buruli ulcer and the causes and risks should be implemented to increase awareness of this disease and improve daily decision-making to help individuals protect themselves from contracting Buruli ulcer. This could influence whether individuals spend time in water, the protective measures they employ in these environments, and their hygiene practices to minimise the risk of environmental transmission. Early identification of lesions and disease diagnosis is an important strategy in ensuring treatment is prompt in preventing severe disease progression. Various community service and surveillance programmes have already been established throughout West Africa, which have seen increased clinical presentations of cases in early stages where treatment is more effective [127–131]. Additionally, programmes such as these could be extended in promoting community education of the various risk factors associated with Buruli ulcer to prevent infection and reduce the burden of infection in these communities.
Corresponding prevention case–control studies have not yet been conducted in Southeast Australia. However, given M. ulcerans is found in aquatic sites in endemic regions of Australia and evidence of vector transmission via mosquitoes, certain preventive measures identified by West African studies would likely be effective in preventing infection in Southeast Australia [16,26]. Specifically, reducing contact with water sources, maintaining adequate wound care, and the use of sleeved clothing and insect repellents may be effective strategies. However, evidence of mosquito vectors and mammalian reservoirs such as possums is stronger in Southeast Australia than in West Africa [8,19]. Thus, different control measures that interrupt specific transmission routes may be required in Southeast Australia. This may include epidemiological tracking of M. ulcerans among important animal reservoirs and reducing human contact with these species. Targeted mosquito control trials are planned to determine whether mosquito reductions are associated with reduced Buruli ulcer incidence in the Mornington and Bellarine peninsulas [132].
Future directions
Research to decipher the modes of transmission of M. ulcerans is expansive and ongoing, but, ultimately, the precise transmission pathways to humans remain unclear. Aquatic environments play a key role in sustaining M. ulcerans and appear to be a major route of infection, posing challenges in rural and agricultural communities in West Africa. Vertebrates and insects can carry M. ulcerans bacilli, but whether this is a risk factor for human infection has not been adequately demonstrated. Further studies elucidating the roles of animal hosts and potential insect vectors in this environmental transmission pathway would provide valuable evidence into the more specific routes of transmission of M. ulcerans and may identify further environmental risks to humans. As there is strong evidence in Australia for mosquitoes as vectors of M. ulcerans, future research should explore this relationship further to establish vector competence and resolve the mechanism and nature of mosquito carriage of M. ulcerans, including the mode of bacterial persistence within mosquitoes [5]. Studies in West Africa are also needed to investigate transmission via mosquitoes further and compare this with findings in Australia. We suggest five key research priority areas (Box 1) that require attention to advance understanding of Buruli ulcer transmission and suggest that future studies on pathogen transmission and prevention should operate under a One Health framework. This reflects that Buruli ulcer is evidently a disease of environmental origin in both humans and mammals, and environmental disturbance is linked to outbreaks. With a vaccine not presently available, efforts to prevent infection in vulnerable communities should also be a priority, including improving access to adequate sanitation and education on the risks and causes of Buruli ulcer.
Box 1. Buruli ulcer transmission: Current research priorities
Adoption of One Health frameworks
M. ulcerans can reside in humans, animals, the environment, and other sources. However, the transmission and control of Buruli ulcer is rarely investigated through One Health frameworks. Unified approaches that concurrently monitor M. ulcerans in several sources are needed to guide environmental survey designs and studies inferring transmission using bacterial phylogenomics. Integrating prevalence and genomic information from multiple reservoirs, rather than focusing on only one, will aid in understanding transmission between them. Moreover, with evidence that Buruli ulcer incidence is linked with environmental disturbances, it is critical to assess how changes in environmental and animal health impact Buruli ulcer transmission. A better integrated understanding of disease transmission would inform policies and interventions to reduce Buruli ulcer spread.
Establishing vector competence
There is good evidence for an association between mosquitoes, M. ulcerans, and human Buruli ulcer cases in areas of Southeast Australia. Initial experimental studies on vector competence of local mosquitoes have been performed, but further observational and laboratory studies are required to define the precise roles that mosquitoes play as vectors in the transmission of M. ulcerans to humans. These studies should be combined with additional surveillance of mosquitoes and native possum populations to monitor these associations and perhaps build predictive mathematical models that can guide targeted mosquito control trials. In African countries, despite several large-scale insect surveys, there is minimal evidence for mosquitoes as a vector for M. ulcerans, but new vector competence studies are required to better understand the roles other invertebrates (e.g., water bugs) might play in pathogen transmission on that continent.
Resolving transmission chains within and between animal reservoirs
Australian native possums are reservoirs of M. ulcerans in Australia. However, the links between chains of transmission are not well established. It is not clear if M. ulcerans is transmitted from possums to other hosts or vectors, or how possums become infected. Bacterial comparative genomics and phylogeographic studies may help elucidate how M. ulcerans is spread between hosts, identifying intervention points for disease control.
Culturing M. ulcerans from environmental samples
Detection of M. ulcerans in the environment is challenging, with qPCR-based assays providing limited information and isolation in pure culture achieved in only one published study. It is critical to develop approaches to determine the viability (e.g., improved cultivation methods) and population structure (e.g., culture-independent genomics) of M. ulcerans in the environment. These will be important steps in standardising and accelerating Buruli ulcer research and will aid in studies aimed at resolving pathogen transmission between humans, other animals, and the environment.
Comparing different modes of transmission between continents
Current evidence suggests different routes of transmission between West Africa and Southeast Australia, though studies have not directly compared transmission between these two continents. Comparative research employing standardised and consistent protocols for vector competence, comparative genomics, and direct detection of M. ulcerans in complex environmental samples are needed to confirm whether transmission routes vary within and between countries and why this is so, guiding best practice for M. ulcerans prevention in all endemic locations.
Key Learning Points
M. ulcerans is an environmental pathogen found predominantly in West Africa and Southeast Australia. Contamination of broken skin in these environments seems to be a major risk factor for developing Buruli ulcer.
In Southeast Australia, mammals such as native possums are symptomatic hosts of Mycobacterium ulcerans, and genotyping confirms that they are part of the same transmission network as humans. Evidence for mammalian hosts in West Africa is weaker.
In Southeast Australia, mosquitoes likely mediate transmission of M. ulcerans. There is no evidence for mosquitoes spreading Buruli ulcer in African countries, although other biting aquatic insects may play a role.
In African countries, outbreaks of Buruli ulcer are sometimes preceded by human disturbances to the environment, creating favourable environments for M. ulcerans.
Top Five Papers
Fyfe JAM, Lavender CJ, Handasyde KA, Legione AR, O’Brien CR, Stinear TP, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010;4(8).
Lavender CJ, Fyfe JAM, Azuolas J, Brown K, Evans RN, Ray LR, et al. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in Southeastern Australia. PLoS Negl Trop Dis. 2011;5(9).
Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden B, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11(4):e0005553.
Maman I, Tchacondo T, Kere AB, Beissner M, Badziklou K, Tedihou E, et al. Molecular detection of Mycobacterium ulcerans in the environment and its relationship with Buruli ulcer occurrence in Zio and Yoto districts of maritime region in Togo. PLoS Negl Trop Dis. 2018;12(5).
Degnonvi H, Fleuret S, Coudereau C, Gnimavo R, Giffon S, Yeramian E, et al. Effect of well drilling on Buruli ulcer incidence in Benin: a case-control, quantitative survey. Lancet Planet Health. 2019;3(8):e349-e56.
Funding Statement
C.G. received funding for this work through an EL2 Fellowship (grant number APP1178715) from the National Health and Medical Research Council (https://www.nhmrc.gov.au/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Omansen TF, Erbowor-Becksen A, Yotsu R, van der Werf TS, Tiendrebeogo A, Grout L, et al. Global epidemiology of Buruli ulcer, 2010–2017, and analysis of 2014 WHO programmatic targets. Emerg Infect Dis. 2019;25(12):2183–90. doi: 10.3201/eid2512.190427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson H, Deribe K, Tabah EN, Peters A, Maman I, Frimpong M, et al. Mapping the global distribution of Buruli ulcer: a systematic review with evidence consensus. Lancet Glob Health. 2019;7(7):e912–e22. doi: 10.1016/S2214-109X(19)30171-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Röltgen K, Pluschke G. Epidemiology and disease burden of Buruli ulcer: a review. Res Rep Trop Med. 2015;6:59–73. [Google Scholar]
- 4.Zingue D, Bouam A, Tian RBD, Drancourt M. Buruli ulcer, a prototype for ecosystem-related infection, caused by Mycobacterium ulcerans. Clin Microbiol Rev. 2018;31(1). doi: 10.1128/CMR.00045-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merritt RW, Walker ED, Small PL, Wallace JR, Johnson PD, Benbow ME, et al. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis. 2010;4(12):e911. doi: 10.1371/journal.pntd.0000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation. Global leprosy (Hansen disease) update, 2019: time to step-up prevention initiatives. World Health Organisation; 2020 Sep 4. Contract No.: 36.
- 7.Trubiano JA, Lavender CJ, Fyfe JAM, Bittmann S, Johnson PDR. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS Negl Trop Dis. 2013;7(10):e2463–e. doi: 10.1371/journal.pntd.0002463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fyfe JAM, Lavender CJ, Handasyde KA, Legione AR, O’Brien CR, Stinear TP, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden B, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11(4):e0005553. doi: 10.1371/journal.pntd.0005553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bratschi MW, Ruf MT, Andreoli A, Minyem JC, Kerber S, Wantong FG, et al. Mycobacterium ulcerans persistence at a village water source of Buruli ulcer patients. PLoS Negl Trop Dis. 2014;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson HR, Mosi L, Donnell R, Aqqad M, Merritt RW, Small PLC. Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: implications for transmission. PLoS Negl Trop Dis. 2014;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landier J, Boisier P, Piam F, Noumen-Djeunga B, Simé J, Wantong FG, et al. Adequate wound care and use of bed nets as protective factors against Buruli ulcer: Results from a case control study in Cameroon. PLoS Negl Trop Dis. 2011;5(11). doi: 10.1371/journal.pntd.0001392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debacker M, Zinsou C, Aguiar J, Meyers W, Portaels F. Mycobacterium ulcerans disease (Buruli ulcer) following human bite. Lancet. 2002;360(9348):1830. doi: 10.1016/S0140-6736(02)11771-5 [DOI] [PubMed] [Google Scholar]
- 14.Debacker M, Zinsou C, Aguiar J, Meyers WM, Portaels F. First case of Mycobacterium ulcerans disease (Buruli ulcer) following a human bite. Clin Infect Dis. 2003;36(5):e67–e8. doi: 10.1086/367660 [DOI] [PubMed] [Google Scholar]
- 15.Maman I, Tchacondo T, Kere AB, Beissner M, Badziklou K, Tedihou E, et al. Molecular detection of Mycobacterium ulcerans in the environment and its relationship with Buruli ulcer occurrence in Zio and Yoto districts of maritime region in Togo. PLoS Negl Trop Dis. 2018;12(5). doi: 10.1371/journal.pntd.0006455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Ravensway J, Benbow ME, Tsonis AA, Pierce SJ, Campbell LP, Fyfe JA, et al. Climate and landscape factors associated with Buruli ulcer incidence in Victoria, Australia. PLoS ONE. 2012;7(12):e51074. doi: 10.1371/journal.pone.0051074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sopoh GE, Barogui YT, Johnson RC, Dossou AD, Makoutodé M, Anagonou SY, et al. Family relationship, water contact and occurrence of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2010;4(7). doi: 10.1371/journal.pntd.0000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien DP. Unlocking of the secrets of Mycobacterium ulcerans disease transmission. Lancet Planet Health. 2017;1(2):e52–e3. doi: 10.1016/S2542-5196(17)30026-8 [DOI] [PubMed] [Google Scholar]
- 19.Tobias NJ, Ammisah NA, Ahortor EK, Wallace JR, Ablordey A, Stinear TP. Snapshot fecal survey of domestic animals in rural Ghana for Mycobacterium ulcerans. PeerJ. 2016;2016(6). doi: 10.7717/peerj.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zingue D, Panda A, Drancourt M. A protocol for culturing environmental strains of the Buruli ulcer agent, Mycobacterium ulcerans. Sci Rep. 2018;8(1):6778. doi: 10.1038/s41598-018-25278-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammoudi N, Dizoe AS, Regoui S, Davoust B, Drancourt M, Bouam A. Disseminated Mycobacterium ulcerans infection in wild grasscutters (Thryonomys swinderianus), Côte d’Ivoire. Am J Trop Med Hyg. 2019;101(3):491–3. doi: 10.4269/ajtmh.19-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zogo B, Djenontin A, Carolan K, Babonneau J, Guegan JF, Eyangoh S, et al. A field study in Benin to investigate the role of mosquitoes and other flying insects in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2015;9(7). doi: 10.1371/journal.pntd.0003941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsollier L, Robert R, Aubry J, Saint André J-P, Kouakou H, Legras P, et al. Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol. 2002;68(9):4623. doi: 10.1128/AEM.68.9.4623-4628.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djouaka R, Zeukeng F, Daiga Bigoga J, N’Golo Coulibaly D, Tchigossou G, Akoton R, et al. Evidences of the low implication of mosquitoes in the transmission of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Can J Infect Dis Med Microbiol. 2017:2017. doi: 10.1155/2017/1324310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace JR, Gordon MC, Hartsell L, Mosi L, Benbow ME, Merritt RW, et al. Interaction of Mycobacterium ulcerans with mosquito species: implications for transmission and trophic relationships. Appl Environ Microbiol. 2010;76(18):6215–22. doi: 10.1128/AEM.00340-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavender CJ, Fyfe JAM, Azuolas J, Brown K, Evans RN, Ray LR, et al. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in Southeastern Australia. PLoS Negl Trop Dis. 2011;5(9). doi: 10.1371/journal.pntd.0001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yip MJ, Porter JL, Fyfe JA, Lavender CJ, Portaels F, Rhodes M, et al. Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J Bacteriol. 2007;189(5):2021–9. doi: 10.1128/JB.01442-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doig KD, Holt KE, Fyfe JA, Lavender CJ, Eddyani M, Portaels F, et al. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genomics. 2012;13:258. doi: 10.1186/1471-2164-13-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurokawa S, Kabayama J, Hwang SD, Nho SW, Hikima J, Jung TS, et al. Comparative genome analysis of fish and human isolates of Mycobacterium marinum. Mar Biotechnol (N Y). 2013;15(5):596–605. doi: 10.1007/s10126-013-9511-6 [DOI] [PubMed] [Google Scholar]
- 30.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007;17(2):192–200. doi: 10.1101/gr.5942807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Röltgen K, Pluschke G. Mycobacterium ulcerans disease (Buruli ulcer): potential reservoirs and vectors. Curr Clin Microbiol Rep. 2015;2(1):35–43. [Google Scholar]
- 32.Demangel C, Stinear TP, Cole ST. Buruli ulcer: reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat Rev Microbiol. 2009;7(1):50–60. doi: 10.1038/nrmicro2077 [DOI] [PubMed] [Google Scholar]
- 33.Röltgen K, Stinear TP, Pluschke G. The genome, evolution and diversity of Mycobacterium ulcerans. Infect Genet Evol. 2012;12(3):522–9. doi: 10.1016/j.meegid.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 34.Vandelannoote K, Meehan CJ, Eddyani M, Affolabi D, Phanzu DM, Eyangoh S, et al. Multiple introductions and recent spread of the emerging human pathogen Mycobacterium ulcerans across Africa. Genome Biol Evol. 2017;9(3):414–26. doi: 10.1093/gbe/evx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buultjens AH, Vandelannoote K, Meehan CJ, Eddyani M, de Jong BC, Fyfe JAM, et al. Comparative genomics shows that Mycobacterium ulcerans migration and expansion preceded the rise of Buruli ulcer in southeastern Australia. Appl Environ Microbiol. 2018;84(8). doi: 10.1128/AEM.02612-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandelannoote K, Phanzu DM, Kibadi K, Eddyani M, Meehan CJ, Jordaens K, et al. Mycobacterium ulcerans population genomics to inform on the spread of Buruli ulcer across central Africa. mSphere. 2019;4(1). doi: 10.1128/mSphere.00472-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandelannoote K, Jordaens K, Bomans P, Leirs H, Durnez L, Affolabi D, et al. Insertion sequence element single nucleotide polymorphism typing provides insights into the population structure and evolution of Mycobacterium ulcerans across Africa. Appl Environ Microbiol. 2014;80(3):1197–209. doi: 10.1128/AEM.02774-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coudereau C, Besnard A, Robbe-Saule M, Bris C, Kempf M, Johnson RC, et al. Stable and local reservoirs of Mycobacterium ulcerans inferred from the nonrandom distribution of bacterial genotypes, Benin. Emerg Infect Dis. 2020;26(3):491–503. doi: 10.3201/eid2603.190573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ablordey AS, Vandelannoote K, Frimpong IA, Ahortor EK, Amissah NA, Eddyani M, et al. Whole genome comparisons suggest random distribution of Mycobacterium ulcerans genotypes in a Buruli ulcer endemic region of Ghana. PLoS Negl Trop Dis. 2015;9(3):e0003681. doi: 10.1371/journal.pntd.0003681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolz M, Bratschi MW, Kerber S, Minyem JC, Um Boock A, Vogel M, et al. Locally confined clonal complexes of Mycobacterium ulcerans in two Buruli ulcer endemic regions of Cameroon. PLoS Negl Trop Dis. 2015;9(6):e0003802. doi: 10.1371/journal.pntd.0003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stinear T, Ross BC, Davies JK, Marino L, Robins-Browne RM, Oppedisano F, et al. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol. 1999;37(4):1018–23. doi: 10.1128/JCM.37.4.1018-1023.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fyfe JAM, Lavender CJ, Johnson PDR, Globan M, Sievers A, Azuolas J, et al. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol. 2007;73(15):4733. doi: 10.1128/AEM.02971-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips R, Horsfield C, Kuijper S, Lartey A, Tetteh I, Etuaful S, et al. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an assay using punch biopsy specimens for diagnosis of Buruli ulcer. J Clin Microbiol. 2005;43(8):3650. doi: 10.1128/JCM.43.8.3650-3656.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson PDR, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;13(11):1653–60. doi: 10.3201/eid1311.061369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stinear T, Davies JK, Jenkin GA, Hayman JA, Oppedisano F, Johnson PDR. Identification of Mycobacterium ulcerans in the environment from regions in southeast Australia in which it is endemic with sequence capture-PCR. Appl Environ Microbiol. 2000;66(8):3206. doi: 10.1128/AEM.66.8.3206-3213.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsollier L, Stinear T, Aubry J, Saint André JP, Robert R, Legras P, et al. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol. 2004;70(2):1097–103. doi: 10.1128/AEM.70.2.1097-1103.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eddyani M, Ofori-Adjei D, Teugels G, De Weirdt D, Boakye D, Meyers WM, et al. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): An environmental study. Appl Environ Microbiol. 2004;70(9):5679–81. doi: 10.1128/AEM.70.9.5679-5681.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bratschi MW, Bolz M, Grize L, Kerber S, Minyem JC, Um Boock A, et al. Primary cultivation: factors affecting contamination and Mycobacterium ulcerans growth after long turnover time of clinical specimens. BMC Infect Dis. 2014;14(1):636. doi: 10.1186/s12879-014-0636-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portaels F, Meyers WM, Ablordey A, Castro AG, Chemlal K, de Rijk P, et al. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl Trop Dis. 2008;2(3):e178–e. doi: 10.1371/journal.pntd.0000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aboagye SY, Danso E, Ampah KA, Nakobu Z, Asare P, Otchere ID, et al. Isolation of nontuberculous mycobacteria from the environment of Ghanian communities where Buruli ulcer is endemic. Appl Environ Microbiol. 2016;82(14):4320–9. doi: 10.1128/AEM.01002-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capela C, Dossou AD, Silva-Gomes R, Sopoh GE, Makoutode M, Menino JF, et al. Genetic variation in autophagy-related genes influences the risk and phenotype of Buruli ulcer. PLoS Negl Trop Dis. 2016;10(4):e0004671. doi: 10.1371/journal.pntd.0004671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stienstra Y, van der Werf TS, Oosterom E, Nolte IM, van der Graaf WT, Etuaful S, et al. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun. 2006;7(3):185–9. doi: 10.1038/sj.gene.6364281 [DOI] [PubMed] [Google Scholar]
- 53.O’Brien DP, Wynne JW, Buultjens AH, Michalski WP, Stinear TP, Friedman ND, et al. Exposure risk for infection and lack of human-to-human transmission of Mycobacterium ulcerans disease, Australia. Emerg Infect Dis. 2017;23(5):837–40. doi: 10.3201/eid2305.160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garchitorena A, Guégan JF, Léger L, Eyangoh S, Marsollier L, Roche B. Mycobacterium ulcerans dynamics in aquatic ecosystems are driven by a complex interplay of abiotic and biotic factors. eLife. 2015;4:e07616. doi: 10.7554/eLife.07616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dassi C, Mosi L, Narh CA, Quaye C, Konan DO, Djaman JA, et al. Distribution and risk of mycolactone-producing mycobacteria transmission within Buruli ulcer endemic communities in Côte d’Ivoire. Trop Med Infect Dis. 2017;2(1). doi: 10.3390/tropicalmed2010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simpson H, Tabah EN, Phillips RO, Frimpong M, Maman I, Ampadu E, et al. Mapping suitability for Buruli ulcer at fine spatial scales across Africa: a modelling study. PLoS Negl Trop Dis. 2021;15(3):e0009157. doi: 10.1371/journal.pntd.0009157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jagadesh S, Combe M, Couppié P, Le Turnier P, Epelboin L, Nacher M, et al. Emerging human infectious diseases of aquatic origin: a comparative biogeographic approach using Bayesian spatial modelling. Int J Health Geogr. 2019;18(1):23. doi: 10.1186/s12942-019-0188-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konan DO, Mosi L, Fokou G, Dassi C, Narh CA, Quaye C, et al. Buruli ulcer in southern Côte D’ivoire: dynamic schemes of perception and interpretation of modes of transmission. J Biosoc Sci. 2019;51(4):520–33. doi: 10.1017/S0021932018000317 [DOI] [PubMed] [Google Scholar]
- 59.Garchitorena A, Roche B, Kamgang R, Ossomba J, Babonneau J, Landier J, et al. Mycobacterium ulcerans ecological dynamics and its association with freshwater ecosystems and aquatic communities: results from a 12-month environmental survey in Cameroon. PLoS Negl Trop Dis 2014;8(5):e2879. doi: 10.1371/journal.pntd.0002879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris A, Gozlan R, Marion E, Marsollier L, Andreou D, Sanhueza D, et al. First detection of Mycobacterium ulcerans DNA in environmental samples from South America. PLoS Negl Trop Dis. 2014;8(1):e2660. doi: 10.1371/journal.pntd.0002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandelannoote K, Durnez L, Amissah D, Gryseels S, Dodoo A, Yeboah S, et al. Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of Mycobacterium ulcerans in the environment. FEMS Microbiol Lett. 2010;304(2):191–4. doi: 10.1111/j.1574-6968.2010.01902.x [DOI] [PubMed] [Google Scholar]
- 62.Williamson HR, Benbow ME, Campbell LP, Johnson CR, Sopoh G, Barogui Y, et al. Detection of Mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2012;6(1):e1506. doi: 10.1371/journal.pntd.0001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aboagye SY, Ampah KA, Ross A, Asare P, Otchere ID, Fyfe J, et al. Seasonal pattern of Mycobacterium ulcerans, the causative agent of Buruli ulcer, in the environment in Ghana. Microb Ecol. 2017;74(2):350–61. doi: 10.1007/s00248-017-0946-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yerramilli A, Tay EL, Stewardson AJ, Fyfe J, O’Brien DP, Johnson PDR. The association of rainfall and Buruli ulcer in southeastern Australia. PLoS Negl Trop Dis. 2018;12(9):e0006757. doi: 10.1371/journal.pntd.0006757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naing C, Reid SA, Aye SN, Htet NH, Ambu S. Risk factors for human leptospirosis following flooding: A meta-analysis of observational studies. PLoS ONE. 2019;14(5):e0217643–e. doi: 10.1371/journal.pone.0217643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian RD, Lepidi H, Nappez C, Drancourt M. Experimental survival of Mycobacterium ulcerans in watery soil, a potential source of Buruli ulcer. Am J Trop Med Hyg. 2016;94(1):89–92. doi: 10.4269/ajtmh.15-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris A, Guégan JF, Benbow ME, Williamson H, Small PL, Quaye C, et al. Functional diversity as a new framework for understanding the ecology of an emerging generalist pathogen. Ecohealth. 2016;13(3):570–81. doi: 10.1007/s10393-016-1140-x [DOI] [PubMed] [Google Scholar]
- 68.Tano MB, Dassi C, Mosi L, Koussémon M, Bonfoh B. Molecular characterization of mycolactone producing mycobacteria from aquatic environments in Buruli ulcer non-endemic areas in Côte d’Ivoire. Int J Environ Res Public Health. 2017;14(2). doi: 10.3390/ijerph14020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McIntosh M, Williamson H, Benbow ME, Kimbirauskas R, Quaye C, Boakye D, et al. Associations between Mycobacterium ulcerans and aquatic plant communities of West Africa: implications for Buruli ulcer disease. Ecohealth. 2014;11(2):184–96. doi: 10.1007/s10393-013-0898-3 [DOI] [PubMed] [Google Scholar]
- 70.Marsollier L, Sévérin T, Aubry J, Merritt RW, André JPS, Legras P, et al. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl Environ Microbiol. 2004;70(10):6296–8. doi: 10.1128/AEM.70.10.6296-6298.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drancourt M, Jarlier V, Raoult D. The environmental pathogen Mycobacterium ulcerans grows in amphibian cells at low temperatures. Appl Environ Microbiol. 2002;68(12):6403–4. doi: 10.1128/AEM.68.12.6403-6404.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gryseels S, Amissah D, Durnez L, Vandelannoote K, Leirs H, de Jonckheere J, et al. Amoebae as potential environmental hosts for Mycobacterium ulcerans and other mycobacteria, but doubtful actors in Buruli Ulcer epidemiology. PLoS Negl Trop Dis. 2012;6(8). doi: 10.1371/journal.pntd.0001764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amissah NA, Gryseels S, Tobias NJ, Ravadgar B, Suzuki M, Vandelannoote K, et al. Investigating the role of free-living amoebae as a reservoir for Mycobacterium ulcerans. PLoS Negl Trop Dis. 2014;8(9). doi: 10.1371/journal.pntd.0003148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeukeng F, Ablordey A, Kakou-Ngazoa SE, Ghogomu SM, N’Golo Coulibaly D, Nsoga MTN, et al. Community-based geographical distribution of Mycobacterium ulcerans VNTR-genotypes from the environment and humans in the Nyong valley, Cameroon. Trop Med Health. 2021;49(1):41. doi: 10.1186/s41182-021-00330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williamson HR, Benbow ME, Nguyen KD, Beachboard DC, Kimbirauskas RK, McIntosh MD, et al. Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl Trop Dis. 2008;2(3):e205. doi: 10.1371/journal.pntd.0000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pileggi SM, Jordan H, Clennon JA, Whitney E, Benbow ME, Merritt R, et al. Landscape and environmental influences on Mycobacterium ulcerans distribution among aquatic sites in Ghana. PLoS ONE. 2017;12(4):e0176375. doi: 10.1371/journal.pone.0176375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cano J, Rodríguez A, Simpson H, Tabah EN, Gómez JF, Pullan RL. Modelling the spatial distribution of aquatic insects (Order Hemiptera) potentially involved in the transmission of Mycobacterium ulcerans in Africa. Parasit Vectors. 2018;11(1). doi: 10.1186/s13071-018-3066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maman I, Tchacondo T, Kere AB, Piten E, Beissner M, Kobara Y, et al. Risk factors for Mycobacterium ulcerans infection (Buruli Ulcer) in Togo ─ a case-control study in Zio and Yoto districts of the maritime region. BMC Infect Dis. 2018;18(1):48. doi: 10.1186/s12879-018-2958-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marion E, Chauty A, Yeramian E, Babonneau J, Kempf M, Marsollier L. A case of guilt by association: water bug bite incriminated in M. ulcerans infection. Int J Mycobacteriol. 2014;3(2):158–61. doi: 10.1016/j.ijmyco.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 80.Mosi L, Williamson H, Wallace JR, Merritt RW, Small PLC. Persistent association of Mycobacterium ulcerans with West African predaceous insects of the family belostomatidae. Appl Environ Microbiol. 2008;74(22):7036–42. doi: 10.1128/AEM.01234-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ebong SM, García-Peña GE, Pluot-Sigwalt D, Marsollier L, Le Gall P, Eyangoh S, et al. Ecology and feeding habits drive infection of water bugs with Mycobacterium ulcerans. Ecohealth. 2017;14(2):329–41. doi: 10.1007/s10393-017-1228-y [DOI] [PubMed] [Google Scholar]
- 82.Benbow ME, Williamson H, Kimbirauskas R, McIntosh MD, Kolar R, Quaye C, et al. Aquatic invertebrates as unlikely vectors of Buruli ulcer disease. Emerg Infect Dis. 2008;14(8):1247–54. doi: 10.3201/eid1408.071503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carolan K, Ebong SMA, Garchitorena A, Landier J, Sanhueza D, Texier G, et al. Ecological niche modelling of Hemipteran insects in Cameroon; the paradox of a vector-borne transmission for Mycobacterium ulcerans, the causative agent of Buruli ulcer. Int J Health Geogr. 2014;13:44. doi: 10.1186/1476-072X-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benbow EM, Kimbirauskas R, McIntosh MD, Williamson H, Quaye C, Boakye D, et al. Aquatic macroinvertebrate assemblages of Ghana, West Africa: understanding the ecology of a neglected tropical disease. Ecohealth. 2014;11(2):168–83. doi: 10.1007/s10393-013-0886-7 [DOI] [PubMed] [Google Scholar]
- 85.Garchitorena A, Ngonghala CN, Texier G, Landier J, Eyangoh S, Bonds MH, et al. Environmental transmission of Mycobacterium ulcerans drives dynamics of Buruli ulcer in endemic regions of Cameroon. Sci Rep. 2015;5:18055. doi: 10.1038/srep18055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A, McBride WJH, Govan B, Pearson M, Ritchie SA. A survey on Mycobacterium ulcerans in mosquitoes and march flies captured from endemic areas of northern Queensland, Australia. PLoS Negl Trop Dis. 2019;13(2). doi: 10.1371/journal.pntd.0006745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quek TYJ, Athan E, Henry MJ, Pasco JA, Redden-Hoare J, Hughes A, et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis. 2007;13(11):1661–6. doi: 10.3201/eid1311.061206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yerramilli A, Tay EL, Stewardson AJ, Kelley PG, Bishop E, Jenkin GA, et al. The location of Australian Buruli ulcer lesions—implications for unravelling disease transmission. PLoS Negl Trop Dis. 2017;11(8). doi: 10.1371/journal.pntd.0005800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Röltgen K, Pluschke G, Johnson PDR, Fyfe J. Mycobacterium ulcerans DNA in bandicoot excreta in Buruli ulcer–endemic area, Northern Queensland Australia. Emerg Infect Dis. 2017;23(12):2042–5. doi: 10.3201/eid2312.170780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanders ML, Jordan HR, Serewis-Pond C, Zheng L, Benbow ME, Small PL, et al. Mycobacterium ulcerans toxin, mycolactone may enhance host-seeking and oviposition behaviour by Aedes aegypti (L.) (Diptera: Culicidae). Environ Microbiol. 2017;19(5):1750–60. doi: 10.1111/1462-2920.13629 [DOI] [PubMed] [Google Scholar]
- 91.Mashlawi AM, Jordan HR, Crippen LT, Tomberlin JK. Impact of mycolactone produced by Mycobacterium ulcerans on life-history traits of Aedes aegypti (L.) and resulting habitat selection for oviposition. Trop Biomed. 2020;37(4):973–85. doi: 10.47665/tb.37.4.973 [DOI] [PubMed] [Google Scholar]
- 92.Singh A, McBride WJH, Govan B, Pearson M. Potential animal reservoir of Mycobacterium ulcerans: a systematic review. Trop Med Infect Dis. 2018;3(2). doi: 10.3390/tropicalmed3020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Brien CR, Handasyde KA, Hibble J, Lavender CJ, Legione AR, McCowan C, et al. Clinical, microbiological and pathological findings of Mycobacterium ulcerans infection in three Australian possum species. PLoS Negl Trop Dis. 2014;8(1):e2666. doi: 10.1371/journal.pntd.0002666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McOrist S, Jerrett IV, Anderson M, Hayman J. Cutaneous and respiratory tract infection with Mycobacterium ulcerans in two koalas (Phascolarctos cinereus). J Wildl Dis. 1985;21(2):171–3. doi: 10.7589/0090-3558-21.2.171 [DOI] [PubMed] [Google Scholar]
- 95.Mitchell PJ, McOrist S, Bilney R. Epidemiology of Mycobacterium ulcerans infection in koalas (Phascolarctos cinereus) on Raymond Island, southeastern Australia. J Wildl Dis. 1987;23(3):386–90. doi: 10.7589/0090-3558-23.3.386 [DOI] [PubMed] [Google Scholar]
- 96.van Zyl A, Daniel J, Wayne J, McCowan C, Malik R, Jelfs P, et al. Mycobacterium ulcerans infections in two horses in south-eastern Australia. Aust Vet J. 2010;88(3):101–6. doi: 10.1111/j.1751-0813.2009.00544.x [DOI] [PubMed] [Google Scholar]
- 97.O’Brien CR, McMillan E, Harris O, O’Brien DP, Lavender CJ, Globan M, et al. Localised Mycobacterium ulcerans infection in four dogs. Aust Vet J. 2011;89(12):506–10. doi: 10.1111/j.1751-0813.2011.00850.x [DOI] [PubMed] [Google Scholar]
- 98.Elsner L, Wayne J, O’Brien CR, McCowan C, Malik R, Hayman JA, et al. Localised Mycobacterium ulcerans infection in a cat in Australia. J Feline Med Surg. 2008;10(4):407–12. doi: 10.1016/j.jfms.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Brien C, Kuseff G, McMillan E, McCowan C, Lavender C, Globan M, et al. Mycobacterium ulcerans infection in two alpacas. Aust Vet J. 2013;91(7):296–300. doi: 10.1111/avj.12071 [DOI] [PubMed] [Google Scholar]
- 100.Singh A, McBride JHW, Govan B, Pearson M. Survey of local fauna from endemic areas of Northern Queensland, Australia for the presence of Mycobacterium ulcerans. Int J Mycobacteriol. 2019;8(1):48–52. doi: 10.4103/ijmy.ijmy_168_18 [DOI] [PubMed] [Google Scholar]
- 101.Hammoudi N, Dizoe S, Saad J, Ehouman E, Davoust B, Drancourt M, et al. Tracing Mycobacterium ulcerans along an alimentary chain in Côte d’Ivoire: a one health perspective. PLoS Negl Trop Dis. 2020;14(5):e0008228. doi: 10.1371/journal.pntd.0008228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hammoudi N, Fellag M, Militello M, Bouam A, Drancourt M. Translocating Mycobacterium ulcerans: an experimental model. PLoS ONE. 2020;15(12):e0230544. doi: 10.1371/journal.pone.0230544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Djouaka R, Zeukeng F, Bigoga JD, Kakou-Ngazoa SE, Akoton R, Tchigossou G, et al. Domestic animals infected with Mycobacterium ulcerans—Implications for transmission to humans. PLoS Negl Trop Dis. 2018;12(7). doi: 10.1371/journal.pntd.0006572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Durnez L, Suykerbuyk P, Nicolas V, Barrière P, Verheyen E, Johnson CR, et al. Terrestrial small mammals as reservoirs of Mycobacterium ulcerans in Benin. Appl Environ Microbiol. 2010;76(13):4574–7. doi: 10.1128/AEM.00199-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu J, Smithwick EA. Landscape fragmentation as a risk factor for Buruli ulcer disease in Ghana. Am J Trop Med Hyg. 2016;95(1):63–9. doi: 10.4269/ajtmh.15-0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu J, Tschakert P, Klutse E, Ferring D, Ricciardi V, Hausermann H, et al. Buruli ulcer disease and its association with land cover in southwestern Ghana. PLoS Negl Trop Dis. 2015;9(6):e0003840. doi: 10.1371/journal.pntd.0003840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Landier J, Gaudart J, Carolan K, Lo Seen D, Guégan JF, Eyangoh S, et al. Spatio-temporal patterns and landscape-associated risk of Buruli ulcer in Akonolinga, Cameroon. PLoS Negl Trop Dis. 2014;8(9):e3123. doi: 10.1371/journal.pntd.0003123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brou T, Broutin H, Elguero E, Asse H, Guegan JF. Landscape diversity related to Buruli ulcer disease in Côte d’Ivoire. PLoS Negl Trop Dis. 2008;2(7):e271. doi: 10.1371/journal.pntd.0000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.N’Krumah RTAS, Koné B, Cissé G, Tanner M, Utzinger J, Pluschke G, et al. Characteristics and epidemiological profile of Buruli ulcer in the district of Tiassalé, south Côte d’Ivoire. Acta Trop. 2017;175:138–44. doi: 10.1016/j.actatropica.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 110.Vandelannoote K, Pluschke G, Bolz M, Bratschi MW, Kerber S, Stinear TP, et al. Introduction of Mycobacterium ulcerans disease in the Bankim Health District of Cameroon follows damming of the Mapé River. PLoS Negl Trop Dis. 2020;14(9):e0008501. doi: 10.1371/journal.pntd.0008501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veitch MG, Johnson PD, Flood PE, Leslie DE, Street AC, Hayman JA. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol Infect. 1997;119(3):313–8. doi: 10.1017/s0950268897008273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson PDR, Veitch MGK, Leslie DE, Flood PE, Hayman JA. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust. 1996;164(2):76–8. doi: 10.5694/j.1326-5377.1996.tb101352.x [DOI] [PubMed] [Google Scholar]
- 113.Ross BC, Johnson PD, Oppedisano F, Marino L, Sievers A, Stinear T, et al. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol. 1997;63(10):4135–8. doi: 10.1128/aem.63.10.4135-4138.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morris AL, Guégan JF, Andreou D, Marsollier L, Carolan K, Le Croller M, et al. Deforestation-driven food-web collapse linked to emerging tropical infectious disease, Mycobacterium ulcerans. Sci Adv. 2016;2(12):e1600387. doi: 10.1126/sciadv.1600387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–52. doi: 10.1038/nature09575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roche B, Rohani P, Dobson AP, Guégan JF. The impact of community organization on vector-borne pathogens. Am Nat. 2013;181(1):1–11. doi: 10.1086/668591 [DOI] [PubMed] [Google Scholar]
- 117.Combe M, Velvin CJ, Morris A, Garchitorena A, Carolan K, Sanhueza D, et al. Global and local environmental changes as drivers of Buruli ulcer emergence. Emerg Microbes Infect. 2017;6(4):e21. doi: 10.1038/emi.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.N’Krumah RTAS, Kone B, Tiembre I, Cisse G, Pluschke G, Tanner M, et al. Socio-environmental factors associated with the risk of contracting Buruli ulcer in Tiassale, south Cote d’Ivoire: a case-control study. PLoS Negl Trop Dis. 2016;10(1):e0004327. doi: 10.1371/journal.pntd.0004327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pouillot R, Matias G, Wondje CM, Portaels F, Valin N, Ngos F, et al. Risk factors for Buruli ulcer: a case control study in Cameroon. PLoS Negl Trop Dis. 2007;1(3):e101. doi: 10.1371/journal.pntd.0000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nackers F, Johnson RC, Glynn JR, Zinsou C, Tonglet R, Portaels F. Environmental and health-related risk factors for Mycobacterium ulcerans disease (Buruli ulcer) in Benin. Am J Trop Med Hyg. 2007;77(5):834–6. [PubMed] [Google Scholar]
- 121.Aboagye SY, Asare P, Otchere ID, Koka E, Mensah GE, Yirenya-Tawiah D, et al. Environmental and behavioral drivers of Buruli ulcer disease in selected communities along the Densu river basin of Ghana: a case-control study. Am J Trop Med Hyg. 2017;96(5):1076–83. doi: 10.4269/ajtmh.16-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chadsuthi S, Chalvet-Monfray K, Wiratsudakul A, Modchang C. The effects of flooding and weather conditions on leptospirosis transmission in Thailand. Sci Rep. 2021;11(1):1486. doi: 10.1038/s41598-020-79546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Degnonvi H, Fleuret S, Coudereau C, Gnimavo R, Giffon S, Yeramian E, et al. Effect of well drilling on Buruli ulcer incidence in Benin: a case-control, quantitative survey. Lancet Planet Health. 2019;3(8):e349–e56. doi: 10.1016/S2542-5196(19)30110-X [DOI] [PubMed] [Google Scholar]
- 124.Tomczyk S, Deribe K, Brooker SJ, Clark H, Rafique K, Knopp S, et al. Association between footwear use and neglected tropical diseases: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(11):e3285. doi: 10.1371/journal.pntd.0003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nwafor CC, Meka A, Chukwu JN, Ekeke N, Alphonsus C, Mbah O, et al. Assessment of community knowledge, attitude, and stigma of Buruli ulcer disease in Southern Nigeria. Afr Health Sci. 2019;19(2):2100–11. doi: 10.4314/ahs.v19i2.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahorlu CSK, Okyere D, Ampadu E. Implementing active community-based surveillance-response system for Buruli ulcer early case detection and management in Ghana. PLoS Negl Trop Dis. 2018;12(9):e0006776. doi: 10.1371/journal.pntd.0006776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Awah PK, Boock AU, Mou F, Koin JT, Anye EM, Noumen D, et al. Developing a Buruli ulcer community of practice in Bankim, Cameroon: A model for Buruli ulcer outreach in Africa. PLoS Negl Trop Dis. 2018;12(3):e0006238. doi: 10.1371/journal.pntd.0006238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tabah EN, Nsagha DS, Bissek A-CZ-K, Njamnshi AK, Bratschi MW, Pluschke G, et al. Buruli Ulcer in Cameroon: the development and impact of the national control programme. PLoS Negl Trop Dis. 2016;10(1):e0004224. doi: 10.1371/journal.pntd.0004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abass KM, van der Werf TS, Phillips RO, Sarfo FS, Abotsi J, Mireku SO, et al. Buruli ulcer control in a highly endemic district in Ghana: role of community-based surveillance volunteers. Am J Trop Med Hyg. 2015;92(1):115–7. doi: 10.4269/ajtmh.14-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barogui YT, Sopoh GE, Johnson RC, de Zeeuw J, Dossou AD, Houezo JG, et al. Contribution of the community health volunteers in the control of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2014;8(10):e3200. doi: 10.1371/journal.pntd.0003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vouking MZ, Takougang I, Mbam LM, Mbuagbaw L, Tadenfok CN, Tamo CV. The contribution of community health workers to the control of Buruli ulcer in the Ngoantet area, Cameroon. Pan Afr Med J. 2013;16:63. doi: 10.11604/pamj.2013.16.63.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Victoria State Government. Beating Buruli in Victoria: Information about the mosquito control study. Department of Health and Human Services; 2021. Available from: https://www2.health.vic.gov.au/public-health/infectious-diseases/beating-buruli.