Abstract
Deep-sea hydrothermal vent habitats are small, rare and support unique species through chemosynthesis. As this vulnerable ecosystem is increasingly threatened by human activities, management approaches should address biodiversity conservation. Diversity distribution data provide a useful basis for management approaches as patterns of β-diversity (the change in diversity from site to site) can guide conservation decisions. Our question is whether such patterns are similar enough across vent systems to support a conservation strategy that can be deployed regardless of location. We compile macrofaunal species occurrence data for vent systems in three geological settings in the North Pacific: volcanic arc, back-arc and mid-ocean ridge. Recent discoveries in the Mariana region provide the opportunity to characterize diversity at many vent sites. We examine the extent to which diversity distribution patterns differ among the systems by comparing pairwise β-diversity, nestedness and their additive components. A null model approach that tests whether species compositions of each site pair are more or less similar than random provides insight into community assembly processes. We resolve several taxonomic uncertainties and find that the Mariana arc and back-arc share only 8% of species despite their proximity. Species overlap, species replacement and richness differences create different diversity distributions within the three vent systems; the arc system exhibits much greater β-diversity than both the back-arc and mid-ocean ridge systems which, instead, show greater nestedness. The influence of nestedness on β-diversity also increased from the arc to back-arc to ridge. Community assembly processes appear more deterministic in the arc and ridge systems while back-arc site pairs deviate little from the null expectation. These analyses reflect the need for a variety of management strategies that consider the character of diversity distribution to protect hydrothermal vents, especially in the context of mining hydrothermal deposits.
Introduction
Conservation of biodiversity underlies policies for sustainable approaches to live with and use natural systems [1]. Basic information on species arrangement over the landscape is fundamental to detecting change and predicting responses to threats [2]. Asaad et al. [3] describe eight relevant ecological criteria requiring species occurrence data, including endemicity, geographic range and species richness. Local species occurrences can differ among sites, both in richness (alpha(α)-diversity) and composition. This variability is captured as beta(β)-diversity, the facet of regional diversity encompassing differences among local assemblages [4]. Socolar et al. [5] and Carlos-Júnior et al. [6] outline use of β-diversity in conservation, including management planning, such as choosing which sites to protect.
As many ecological phenomena can shape β-diversity, patterns can reveal key processes. By separating β-diversity into its components, ecologists can both describe diversity patterns and test hypotheses regarding mechanisms that shape them [7–9]. The specific formulation to identify components is debated (e.g., [10–12]), but we find the recent SET framework [13] derives a clear scenario. This framework introduces the concept of pairwise pattern components (PPCs) that isolate the community response pattern between every pair of sites examined for species presence/absence. It also incorporates the role of nestedness in the concepts of ‘intersection of nestedness and β-diversity’ and ‘the relative complement of nestedness in β-diversity’. Partitioning β-diversity into its components helps to describe the regional pattern and to support hypotheses around the mechanisms that shape diversity distributions [7]. A conservation approach can target the underlying mechanism if one component dominates a region. For example, where nestedness is low, selection pressures have likely driven species substitution (e.g., [9]), and conservation may need to target several representative sites rather than one larger area. Combined with the null model approach of Raup and Crick [14], these tools provide insight into the processes shaping differences between sites [15].
The remoteness of the deep ocean no longer buffers human impacts. Less than 10% of the deep ocean is classified as “wilderness”, with only 0.5% in Marine Protected Areas [16]. As climate change, plastic pollution and ocean dumping impacts increase, exploitation for food, natural products and mineral resources is expanding in the deep sea [17–19]. So far, hydrothermal vents remain relatively untouched, as reflected in the similar contributions of common and rare species to functional diversity, in contrast to disrupted terrestrial systems [20]. However, the seafloor massive sulphides formed at vents has attracted mining interests despite the minimal projected economic returns [21]. Beyond national jurisdictions, the International Seabed Authority has awarded seven exploration contracts for massive sulphides to date (www.isa.org.jm/exploration-contracts/polymetallic-sulphides). Immediate development of management plans should include, inter alia, designating conservation areas (e.g., [22]) for which knowledge of species diversity and distributions is a critical component. As most vent studies focus on single sites, regional β-diversity is poorly understood, although a few applications have revealed local [23, 24] to broader patterns [25].
The reduced compounds dissolved in vent water sustain microbial chemosynthesis and associated lush animal communities; most animals at hot vents are known nowhere else [26]. While vents occur on spreading centres and subsea volcanoes in every ocean, and appear to be abundant [27], the habitat extent is highly constrained to fluid outlets; consequently, the global vent ecosystem is very small [28]. Vents are insular habitats distributed along geologic structures where inter-site distances can exceed 100s of km. Site stability varies from frequent volcanic disruption [29] to millennia-long fluid delivery [30]. Nonetheless, vent habitats tend to be characterized as unstable, disturbed, and short-lived with inhabitants adapted to such conditions [31–33], where a single management strategy may seem appropriate. Despite common basic characteristics, similar ecosystem types vary among regions in their β-diversity patterns [34, 35], thus conservation approaches for vent habitats may need to target differing underlying causes.
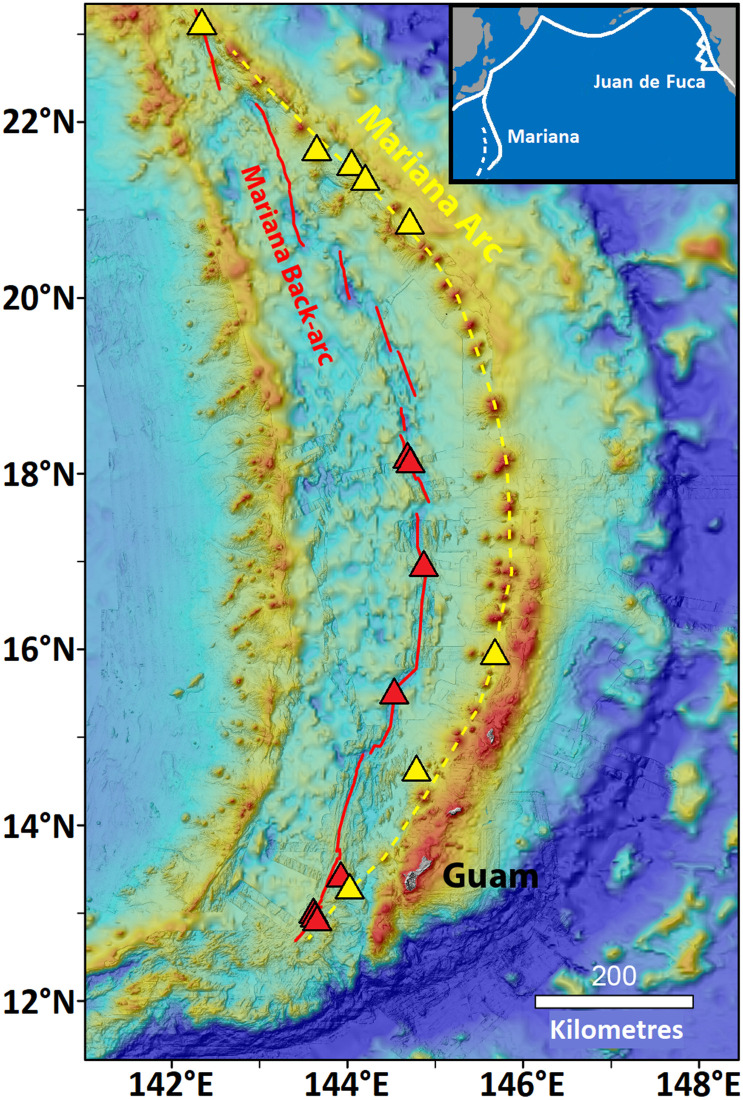
Vent species are distributed among biogeographic regions following vicariant patterns related to tectonic history [26, 36]. The region lying westward of the Mariana Trench includes the Mariana back-arc spreading centre and the Mariana volcanic arc (Fig 1). The 1500 km long Mariana arc has a northward extension as the Izu-Bonin arc. Many of the submarine volcanoes are hydrothermally active with fluids enriched in CO2 and SO2 [37–39] and notable variability in both fluid and faunal characteristics among sites [40]. Faunal diversity along the arc is incompletely documented [41]. The 1300 km long Mariana back-arc is located in the Mariana Trough where the spreading axis is highly segmented; magmatic influence from the adjacent arc increases from central to southern segments [42] where hydrothermal fluids reflect input from magmatic volatiles [43]. Kojima and Watanabe [44] review faunal samples from 1987 to 2010 at six sites on these segments and describe a distinct back-arc fauna with low similarity to the adjacent Mariana-Izu-Bonin arc system. Many faunal records from the Mariana back-arc are not resolved to species level, and there is low confidence in some names assigned from decades past. Some Mariana species names appear in lists from other biogeographic regions suggesting broad distributions, but closer study may detect greater regionalism (e.g., [45]).
Fig 1. Map of the Mariana region and its known hydrothermal vent sites.
The Mariana tectonic region includes back-arc and volcanic arc vent systems. The red and yellow lines trace the back-arc and arc respectively, and coloured triangles indicate locations of hydrothermal vent sites in this study. From north to south, the red triangles represent Alice Springs/Illium, Burke (overlapping), Hafa Adai, Perseverance, Forecast, then Snail, Archaean and Urashima/Pika (overlapping). From north to south, the yellow triangles represent Nikko, Kasuga-2, NW Eifuku, Daikoku, Chamorro, E Diamante, NW Rota and Seamount X. The last site lies only 20 km from Forecast. Inset shows locations of the Mariana and Juan de Fuca vent systems in the North Pacific. Map produced by WW Chadwick using data from GEBCO (British Oceanographic Data Centre) and from NOAA surveys.
Since 2004, the Vents Program and Ocean Exploration Program of the National Oceanic and Atmospheric Administration (NOAA) have supported research cruises in the region. One outcome of the discoveries was the declaration, in 2009, of the Mariana Trench Marine National Monument (MTMNM) that includes the Volcanic Unit encompassing the known vent sites. Two recent missions investigated hydrothermalism in the southern Mariana back-arc. The first located water column signatures from 19 possible seafloor vent sites [46]. The densest cluster occurs in the south where distance to the arc falls and spreading rate increases to 56 mm yr-1. Work with a remotely operated vehicle (ROV) the following year confirmed two new vent sites in the south-central axis plus a newly eruptive site where venting was short-lived [47].
Our study documents diversity at the new back-arc sites and compiles a regional species check-list for all known sites in the Mariana region. We examine the hypothesis that there is an along-strike faunal shift within the Mariana back-arc using measures of α- and β-diversity and explore some potential drivers. Lastly, we investigate the relative partitions of β-diversity in three hydrothermal systems of similar extent but differing geological settings: back-arc, volcanic arc and mid-ocean ridge. This last region lies in the Northeast Pacific: the Juan de Fuca-Explorer Ridge complex. We examine the extent to which these hydrothermal systems show similar patterns given that vents host relatively low diversity communities with a similar basis in chemosynthesis. We assess the extent to which such analyses can inform conservation management of this unusual, but vulnerable, ecosystem.
Methods
Collection in the Mariana Trench Marine National Monument was conducted with on board oversight by the National Oceanic and Atmospheric Administration of the USA. Collection on the Juan de Fuca Ridge was conducted under permit #16-OPAC-00002EHV.
Study locations
A vent ‘system’ is an array of vent sites on a distinct geologic structure, such as a spreading centre or volcanic arc. A ‘site’ refers to a broad area of venting influenced by an underlying geological process. A site may have more than one vent ‘field’, each of which appears to have a discrete heat source. Vents form around discrete fluid outlets on the seafloor. In our study, fields less than 3 km apart were combined into one site.
Mariana back-arc spreading centre
In 2016, the Schmidt Ocean Institute Research Vessel Falkor mapped and sampled four vent sites from 15.5°N to 18.2°N with the Remotely Operated Vehicle (ROV) SuBastian: two newly discovered sites, Perseverance and Hafa Adai (Fig 1), and two previously visited sites, Alice Springs/Illium and Burke [48]. We sampled three additional sites further south with ROV Jason-2 (Forecast in 2006, Snail and Urashima/Pika in 2014); other species reports from these sites, plus Archaean, derive from Kojima and Watanabe [44]. Table 1 presents site characteristics.
Table 1. Environmental variables for the Mariana back-arc vent sites.
| Alice Springs/Illium | Burke | Hafa Adai | Perseverance | Forecast | Snail | Archaean | Urashima/Pika | |
|---|---|---|---|---|---|---|---|---|
| Coordinates | 18°12.71’N 144°42.45’E | 18°10.95’N 144°43.19’E | 16°57.68’N 144°52.15’E | 15°28.80’N 144°30.46’E | 13°24’N 143°55’E | 12°57.20’N 143°37.20’E | 12°56’N 143°38’E | 12°55.10’N 143°38.90’E |
| Depth (m) | 3597 | 3630 | 3279 | 3910 | 1470 | 2850 | 2990 | 2956 |
| Distance from arc (km) | 109 | 108 | 101 | 97 | 23 | 11 | 8 | 6 |
| Distance to next site south (km) | 3.5 | 136.7 | 169.2 | 239.9 | 59.1 | 2.7 | 2.3 | NA |
| Max temperature (°C) | 165 (287a) | 50 | 345 | 264 | 136 (210b) | 214 (248b) | (345b) | 196 (330b) |
| Est venting area (m 2 ) | 2270 | 2625 | 5165 | 510 | 2510c | 1183c | 1840c | 1995c |
Vent sites of the Mariana back-arc spreading centre and their respective geographic coordinates. The environmental variables included were those suspected to drive the diversity distribution patterns in this vent system; therefore, these variables were analyzed with the associated α- and β-diversity data of each site.
The highest temperature measured during the “Ring of Fire” cruises is shown with past highest temperatures in brackets.
a Maximum temperature measurement from [49].
b Maximum temperature measurements from [43].
c Estimated venting area using JAMSTEC imagery.
Mariana volcanic arc
Biological sampling at eight seamounts with vents was uneven as the six expeditions (2004–2014) differed in objectives. Descriptions of all sites are available [37, 40, 43, 50–52], including in cruise reports (www.pmel.noaa.gov). Vent sites (Fig 1) are located on seamount summits, some of which are recently volcanically active (NW Rota, Daikoku). A key feature of the arc vents is variability in venting characteristics in which values for pH, CO2, SO2, H2S and S0 differed markedly among sites [39, 52].
Juan de Fuca ridge
We use data from seven sites along the Juan de Fuca and Explorer Ridges in the Northeast Pacific for comparison (Fig 1 inset). This mid-ocean ridge system has several large vent sites including a central active volcano (Axial Seamount), extensive fields of black smokers (Endeavour) and a sedimented massive sulphide deposit (Middle Valley). Data derive from many studies (e.g. [25, 53–56]) in addition to work at individual vents and in systematic descriptions. Vent assemblages of the southern and northern sites (South Cleft and Explorer) are less well assessed. While Explorer Ridge is separated from the Juan de Fuca by a 150 km long transform fault, we treat these seven sites as a single system.
Data collection
In general, methods in all locations and years were similar. Pilots of the ROVs ROPOS, Jason-2 or SuBastion executed the collections as guided by scientists. Imagery acquisition used digital still cameras and video cameras of increasing quality over the years to the current high definition systems. Due to rough terrain on different habitat types, a quantitative approach was not possible. Manipulator arms collected animals and substratum either directly or with scoops to deposit them in closable, sealed boxes. A suction sampler pulled mobile or small animals into swivelling sealed jars. Sampling was more consistent among sites at the JdF and, largely, at the Mariana back-arc as most diversity was associated with one foundation species. However, for the Mariana arc sites, the high variability in sample retrieval methods did not support sample standardization for richness estimates. At some sites, collection was not possible at all major vents. Thus, diversity is very likely higher than represented, especially for Nikko and Daikoku. Nonetheless, as similar collection approaches were used, an effort to establish overall patterns in diversity distributions is warranted.
Samples were stored in 75–80% ethanol or 7% buffered formalin on board ship. On shore, a 1 mm sieve separated macrofauna from meiofauna for complete sorting of all samples. Identifications followed published descriptions and consultations with systematics experts, some of whom used molecular approaches. We sent some specimens from the Mariana arc and back-arc to BOLD, the Centre for Biodiversity Genomics (boldsystems.org), to compare COI barcodes with previous work.
Dive imagery was reviewed for the Mariana back-arc, including that available from the JAMSTEC-EDI system (www.godac.jamstec.go.jp/jedi/e/) for missions conducted with Japanese vehicles. Larger species and several smaller species in high definition are distinctive and added to the records. We include taxa from Kojima and Watanabe [44] who summarize all reports to that time. Of the 47 macrofauna listed in their study, 18 have species identities. We cannot match the remainder to our species list as different authors may have made conflicting designations, especially those listed as con forma (cf.) or “sp.”. Thus, we use a conservative list in which species are identified consistently along the back-arc for our analyses. For the Mariana arc, our data are supplemented for the northern seamounts from Watanabe et al. [41].
Data analysis
Diversity
Following Whittaker [4], α- and γ-diversity represent “local” and “regional” species richness, respectively, where the number of species at a site determines the α-diversity value, and γ-diversity is the number of species present at all sites in the system. We also make a larger ‘region’ by combining all Mariana data in a separate analysis. We used the Chao1 estimator [57] in PAST 2.17 [58] to estimate an upper species number for the four Mariana back-arc vent sites sampled in 2016. Only macrofaunal vent-associated species are used.
As data are restricted to site-by-species presence matrices, we use the Jaccard family of indices [59, 60]. Using the POD and SET frameworks [12, 13, 61], we identify the diversity patterns in each vent system by quantifying the pairwise pattern components (PPCs) using the ‘beta.div.comp’ function in the ‘adespatial’ R package [62, 63]. The PPCs are represented as species overlap (OJ), richness difference (DJ) and replacement (RJ), which can occur singly or in combination [13]. As they correspond with the SDR-simplex indices developed by Podani and Schmera [61], we generated simplex plots for each vent system using the ‘TernaryPlot’ function in the ‘Ternary’ R package [64]. We calculate βJ-diversity (βJ = 1 –OJ = RJ + DJ) and nestedness (NJ = 1 –RJ = OJ + DJ). Dendrograms for each vent system, generated using the ‘average’ method and ‘hclust’ function in the ‘vegan’ R package [63, 65], illustrate relative similarities of vent sites. We calculated the LCBD (Local Contribution to Beta Diversity) using the ‘beta.div’ function in the ‘adespatial’ R package [62, 63, 66] to determine which sites contribute most to the overall βJ-diversity of each system.
Following the SET framework, we calculate the intersection (IJ) of nestedness and β-diversity and the relative complement (RCJ) of nestedness in β-diversity. Given that DJ is used to calculate both βJ and NJ, the relativized richness difference is the intersection of nestedness and β-diversity (DJ = IJ), but only when OJ > 0; otherwise, IJ = 0 and RCJ = 1 [13]. The βratio (IJ / βJ) for each pair of sites is an indication of which additive component plays the dominant role in shaping β-diversity [67].
The pairwise Raup-Crick dissimilarity index (βRC) is a measure of the probability that an observed βJ-diversity value would occur by chance using a null model that controls for richness differences [15, 68]. βRC values falling beyond the 95% confidence intervals (CIs) exhibit significant deviation from the null expectation and may implicate deterministic mechanisms in community assembly [15]. We calculated βRC values using the ‘raupcrick’ function in the ‘vegan’ R package [63] and converted output values to a scale of negative one to positive one [15]. To illustrate the similarities of vent sites relative to the expectation of random assembly (βRC), we generated non-metric multi-dimensional scaling (nMDS) plots generated using the ‘metaMDS’ function in the ‘vegan’ R package [63, 65].
Between-system comparisons
Since the Mariana volcanic arc and back-arc share some species, we combine data to calculate PPC and βRC values and thereby illustrate dissimilarity patterns over the entire Mariana region. We also compared the pairwise βJ-diversity, its additive components, the pairwise nestedness and βRC-diversity values among the three vent systems [69], while acknowledging inherent limitations [70]. The method allows application of significance tests through permutation ANOVA and permutation t-tests using the ‘perm.oneway.anova’ function in the ‘rcompanion’ R package [71] and the ‘perm.t.test’ function in the ‘RVAideMemoire’ R package [72], respectively; the probability threshold for all significance tests was set at 0.05.
Environmental analyses
We examined environmental variables that may affect diversity on the Mariana back-arc (Table 1); similar consistent data were not available for the volcanic arc or Juan de Fuca systems. Depth is a well-known diversity driver [73]. Distance from the volcanic arc affects magmatic activity and topography [42, 74], therefore influencing hydrothermalism and local currents for dispersing larvae. Distance between sites affects connectivity, while the maximum fluid temperature (measured at any visit) is an indication of relative hydrothermal vigour at the site. Habitat area, another diversity predictor [75], was estimated using maps in cruise reports and, for Forecast, in Fujikura et al. [76]. By integrating video footage and dive track maps, we used ImageJ to create polygons over areas with hydrothermal indicators such as venting fluids, bacterial mats, and vent animals.
We calculated Kendall rank correlation coefficients using the ‘cor.test’ function in R [63] to assess associations between α-diversity and each environmental variable over the sites. For the significant explanatory variables (two total), simple regressions and generalized linear models, generated in R using the ‘lm’ function and the ‘glm’ function of the ‘mgcv’ R package [77], determined if the significant correlations were linear. We used dbMEM analysis using the ‘adespatial’ R package [66] to compare the β-diversity values to the environmental variables. As β-diversity values correspond with pairs of sites, relevant environmental variables were pairwise comparisons (differences in depth, arc distance, temperature and area; distance between sites).
Results
Mariana back-arc
A brief description and images of the back-arc habitats appears in S1 Text in S1 File. The four sampled sites returned 28 macrofaunal species (Table 2). The hairy snail, Alviniconcha hessleri, acts as a foundation species expanding surface area for other species, especially crabs (Austinograea williamsi) and shrimp (Rimicaris vandoverae). Sampling the snails resulted in the recovery of 16 macrofaunal and four meiofaunal species. Overall, among the 2,038 specimens collected, over 47% were individuals of four species: A. hessleri, Neoverruca brachylepadoformis, Lepetodrilus aff. schrolli MT and R. vandoverae. Meiofauna were rare: of the 181 meiofaunal specimens, 71% (128/181) were nematodes, and nearly all the remainder were copepods (mostly dirivultids and harpacticoids).
Table 2. Macrofauna collected from the four northern-most vent sites in the Mariana back-arc.
| Class | Group | Family | Species | Notes |
|---|---|---|---|---|
| Anthozoa | Actinaria | Kadosactinidae |
Marianactis bythios Fautin & Hessler 1989 |
|
| Anthozoa | Zoantharia | Epizoanthidae | Epizoanthus aff. sp. nov.a | 1st coll. |
| Aplacophora | Solenogastres | Simrothiellidae | Helicoradomenia sp. nov. | [78] |
| Bivalvia | Mytilida | Mytilidae |
Bathymodiolus septemdierumb Hashimoto & Okutani 1994 |
[79] |
| Gastropoda | Abyssochrysoidea | Provannidae |
Alviniconcha hessleri Okutani & Ohta 1988 |
COI |
| Gastropoda | Abyssochrysoidea | Provannidae |
Provanna nassariaeformis Okutani 1990 |
|
| Gastropoda | Abyssochrysoidea | Provannidae |
Desbruyeresia marianaensis (Okutani 1990) |
COI |
| Gastropoda | Abyssochrysoidea | Provannidae |
Desbruyeresia chamorrensis Chen, Ogura & Okutani 2016 |
COI |
| Gastropoda | Lepetellida | Lepetodrilidae | Lepetodrilus aff. schrolli MTb | [80] COI |
| Gastropoda | Lepetellida | Lepetodrilidae |
Pseudorimula marianae McLean 1989 |
COI |
| Gastropoda | Lottoidea | Pectinodontidae | Bathyacmaea sp.b | COI |
| Gastropoda | Cycloneritida | Phenacolepadidae | Shinkailepas sp. nov.a | |
| Gastropoda | Neomphaloidea | Neomphalidae |
Symmetromphalus regularis McLean 1990 |
|
| Gastropoda | Neogastropoda | Raphitomidae |
Phymorhynchus wareniab Sysoev & Kantor 1995 |
|
| Hexanauplia | Cirripedia | Neoverrucidae |
Neoverruca brachylepadoformis Newman 1989 |
COI |
| Hexanauplia | Cirripedia | Eolepadidae |
Vulcanolepas verenaea Watanabe, Chen & Chan 2021 |
1st coll. |
| Malacostraca | Decapoda | Alvinocarididae |
Rimicaris vandoverae (Martin & Hessler 1990) |
COI |
| Malacostraca | Decapoda | Alvinocarididae |
Rimicaris cf. variabilis (Komai & Tsuchida 2015) |
COI |
| Malacostraca | Decapoda | Alvinocarididae |
Rimicaris falkorae Komai & Giguère 2019 |
COI |
| Malacostraca | Decapoda | Bythograeidae |
Austinograea williamsi Hessler & Martin 1989 |
COI |
| Pycnogonida | Pantapoda | Ammotheidae |
Sericosura cochleifovea Child 1989 |
|
| Polychaeta | Errantia | Polynoidae |
Levensteiniella raisae Pettibone 1989 |
|
| Polychaeta | Errantia | Polynoidae |
Lepidonotopodium minutum Pettibone 1989 |
|
| Polychaeta | Errantia | Polynoidae |
Branchinotogluma marianus (Pettibone 1989) |
|
| Polychaeta | Errantia | Hesionidae |
Sirsoe hessleri (Blake 1991) |
|
| Polychaeta | Sedentaria | Spionidae | Laonice sp. nov. | |
| Polychaeta | Sedentaria | Alvinellidae |
Paralvinella hessleri Desbruyères & Laubier 1989 |
|
| Polychaeta | Sedentaria | Ampharetidae | Amphisamytha sp. nov.ab |
Abbreviations: 1st coll., first time collected but seen (undescribed) by Hessler and Lonsdale [81]; COI, cytochrome c oxidase subunit I barcode sequence available.
a A gene sequenced by collaborators.
b Identity update from prior report(s).
Our work at the four northern sites resolved several macrofaunal species identities to the regional list. Table 2 notes changes in species names from taxa previously reported in this region including two undescribed species, a Lepetodrilus limpet and an Amphisamytha polychaete, originally identified with names from other vent provinces [82]. Komai and Giguère [83] distinguish two additional shrimp species from the R. vandoverae originally noted. A spionid polychaete and mite are new reports, and one galatheid did not match described species. Noting four additional species from prior reports, we find 32 species in the four northern sites; individual-based rarefaction (not shown) on the macrofauna indicates a range for estimated species between 32 and 40 for these sites. Given that the two new sites contained no species that were not also present in Burke or Alice Springs/Illium, the current α-diversity numbers may not increase much.
Table 3 presents diversity numbers for all eight sites (complete list in S4 Table in S1 File) averaging ~20 species per site with Alice Springs/Illium having the greatest number. Six species occurred at all sites, while eight (21%) were found at only one site each. γ-diversity of the system is 39 species.
Table 3. Species richness measures for the three study vent systems.
| Mariana Back-Arc (γ = 39) | Mariana Volcanic Arc (γ = 45) | Juan de Fuca Ridge (γ = 71) | ||||||
| 599 km | 1107 km | 565 km | ||||||
| Site | Abbr | α | Site | Abbr | α | Site | Abbr | α |
| Alice/Illiuma | AI | 29 | Nikkoa | Nk | 12 | Explorera | Ex | 29 |
| Burkea | Bk | 23 | Kasuga-2 | K2 | 12 | Middle Valleya | MV | 46 |
| Hafa Adaib | HA | 25 | NW Eifukua | NWE | 21 | Endeavoura | En | 45 |
| Perseveranceb | Pv | 13 | Daikokua | Dk | 14 | CoAxial | CA | 27 |
| Forecasta | Fc | 20 | Chamorro | Ch | 5 | Axiala | Ax | 40 |
| Snaila | Sn | 21 | East Diamantea | ED | 16 | North Clefta | NC | 28 |
| Archaeana | Ar | 13 | NW Rota | NWR | 9 | South Cleft | SC | 16 |
| Urashima/Pikaa | UP | 15 | Seamount Xa | SX | 14 | |||
| spp/site (sd) | 19.9 (5.8) | 12.9 (4.7) | 35.0 (11.0) | |||||
| γ / | 1.96 | 3.49 | 2.15 | |||||
α-diversity for sites on each hydrothermal system. The second line is the along-structure distance between the farthest sites.
Abbreviations: Abbr, abbreviations for vent site names; γ, gamma diversity; α, alpha diversity; γ/, “true beta-diversity” sensu Whittaker [4].
a The minimum combination of sites that hold all species known in the respective hydrothermal system.
b Newly assessed vent sites.
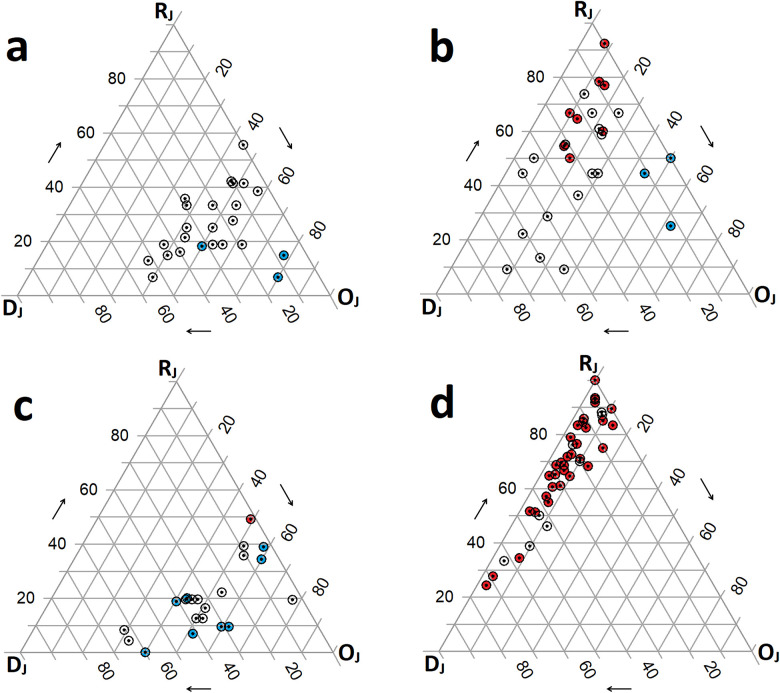
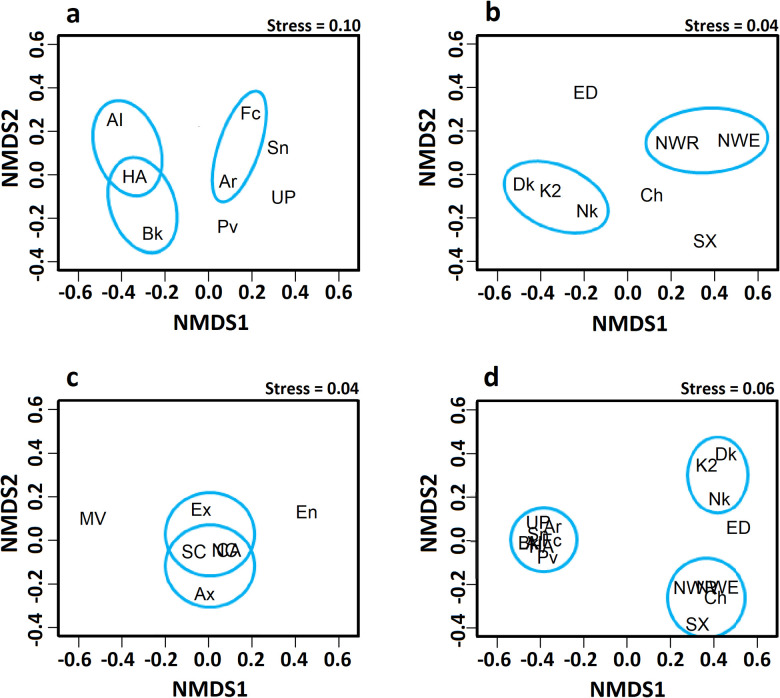
The three northern sites (Alice Springs/Illium, Burke, Hafa Adai) form the tightest cluster (Fig 2). Comparisons between sites on the Mariana back-arc show a ‘complex’ pattern (sensu Schmera et al. [13]), in which all three PPCs are present in the site-by-species matrix, though not in all site pairs. The overlap PPC (OJ) contributes most to the pattern with pairwise site values clustered in the lower-right corner of the simplex plot (Fig 3a). Pairwise βJ values range from 0.2 to 0.65 and indicate that, on average, these sites share ~49% of their species (Table 4). Perseverance is the only site with a significant local contribution to β-diversity (LCBD; p < 0.05). Pairwise NJ values are generally higher than the βJ-diversity, ranging from 0.44 to 0.93. The average βratio for these sites is 0.49, indicating that the two components of βJ-diversity (IJ and RCJ) have nearly equal relative contributions overall. The average βRC value is -0.33, and only ~11% (3/28) of these values fall beyond the 95% CIs (Table 4, S11 Table in S1 File). Hence, most paired sites are no more similar or different from each other than expected by random chance. Significant similarities are Hafa Adai with both Alice Springs/Illium and Burke, and Archaean with Forecast (Fig 4a).
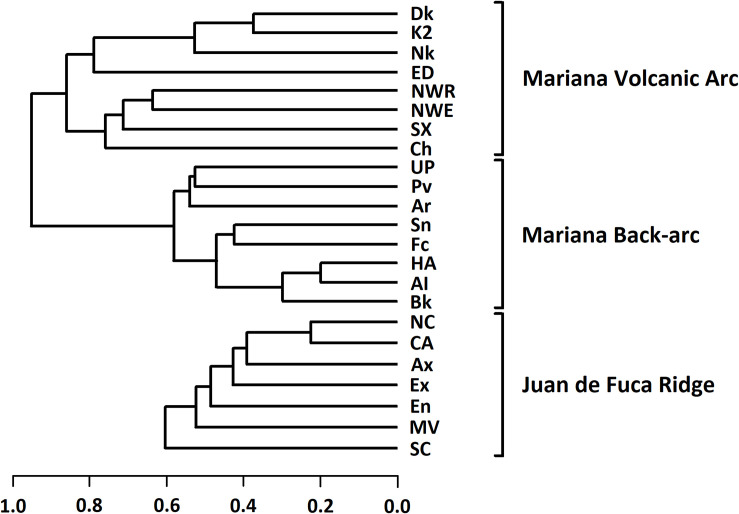
Fig 2. Dendrograms of hydrothermal vent macrofauna assemblages in the Mariana region and Juan de Fuca ridge.
Dendrograms illustrating the relative dissimilarity (β-diversity using the Jaccard Index) of vent sites in each of the three vent systems investigated in this study. Site name abbreviations match those in Table 3. The Mariana systems are illustrated by a single dendrogram because they share six species. No species are shared across the North Pacific.
Fig 3. Simplex plots of hydrothermal vent macrofauna assemblages in the Mariana region and Juan de Fuca ridge.
Simplex plots sensu Podani & Schmera [61] to illustrate the relative importance of additive pairwise pattern components (PPCs) sensu Schmera et al. [13] in diversity distributions. PPCs include species overlap (OJ), relativized species replacement (RJ) and relativized species richness difference (DJ). Values are calculated for every site pair within each vent system. Red points indicate site pairs within which differences are significant, as calculated by the Raup-Crick Index. Blue points indicate pairs with significant similarity, while clear points represent pairs with no significant deviance from the expectation of random chance. Arrows show direction in which to read each axis. a) Mariana back-arc ridge system; b) Mariana volcanic arc system; c) Juan de Fuca/Explorer mid-ocean ridge system; d) Mariana region with back-arc and volcanic arc combined, illustrating between-system values only. Here, 16 between-system pairs that share no species lie at the top apex. Thus, the intersection of nestedness and β-diversity (IJ) and the relative complement of nestedness in β-diversity (RCJ) are used for calculation as OJ = 0 [13].
Table 4. Species diversity measures for the three study vent systems.
| Measures | Mariana back-arc | Mariana volcanic arc | Juan de Fuca ridge | Mariana Region (arc and back-arc) |
|---|---|---|---|---|
| βJ | 0.51 (0.53) | 0.78 (0.82)** | 0.50 (0.49) | 0.81 (0.90) |
| NJ | 0.74 (0.77) | 0.49 (0.48)** | 0.80 (0.81) | 0.45 (0.44) |
| IJ | 0.26 (0.27) | 0.27 (0.23) | 0.30 (0.33) | 0.22 (0.19) |
| RCJ | 0.26 (0.23) | 0.51 (0.52)** | 0.20 (0.19) | 0.58 (0.61) |
| βratio | 0.49 (0.54) | 0.35 (0.3) | 0.57 (0.66) | 0.30 (0.27) |
| βRC | -0.33 (-0.34) | 0.24 (0.65)** | -0.56 (-0.88) | 0.23 (0.86) |
| % significant βRC values | 10.7 | 46.4 | 47.6 | 75 |
Abbreviations.
βJ, pairwise Jaccard dissimilarity which is the inverse of the overlap (OJ) PPC (βJ = 1 –OJ).
NJ, relativized nestedness sensu Schmera et al. [13].
IJ, intersection of nestedness and β-diversity, which equals richness difference (DJ) PPC when species overlap is > 0.
RCJ, relative complement of nestedness in β-diversity (IJ + RCJ = βJ); it is identical to the replacement (RJ) PPC when species overlap is > 0.
βratio, the proportional contribution of IJ in βJ sensu Dobrovolski et al. [67].
βRC, Raup-Crick index; significance is 95% CI on 9,999 permutations.
**Significant differences of βJ, NJ, DJ/IJ, RJ/RCJ, and βRC values among the three systems; significance p < 0.05.
Fig 4. nMDS plots illustrating the βRC-diversity values of hydrothermal vent assemblages in the Mariana back-arc, volcanic arc and Juan de Fuca ridge.
Non-metric multidimensional scaling (nMDS) plots illustrating the dissimilarity of vent sites in each system relative to the null expectation of random assembly (βRC-diversity). Site name abbreviations match those in Table 3. Blue ellipses represent the sites that are significantly more similar to each other (βRC-diversity). (a) Mariana back-arc ridge system; (b) Mariana volcanic arc system; (c) Juan de Fuca/Explorer mid-ocean ridge system; (d) Mariana region including the back-arc and volcanic arc.
Mariana volcanic arc
We identified 45 species, less than half of which have definitive species-level assignment (S5 Table in S1 File). For another eight, experts indicate ‘new species’ status, while the remainder require further assessment. Some better sampled sites returned fewer species than those with less sampling (e.g. 27 samples on NW Rota returned only nine species, while East Diamante had 16 species in nine samples). The expansive vent fields of Nikko and Daikoku need more investigation. The average number of species per site was 12.6 (Table 3). Only one species appears at all sites: the crab Gandalfus yunohana, while 47% (21/45) of species are known from only one site. Six species occur on both arc and back-arc: Bathymodiolus septemdierum (mussel), L. aff. schrolli MT (limpet), B. marianus, Levensteiniella raisae (both scaleworms), D. marianaensis and P. cf. nassariaeformis (both snails). While it is likely that further sampling will augment diversity and alter the specific results here, we highlight the overall pattern that is revealed in comparison to the back-arc that was sampled in a similar fashion dictated by the field conditions.
Dissimilarity among sites in the arc is generally greater than in the back-arc; the two systems form two high level clusters (Fig 2). The volcanic arc sites also present a ‘complex’ pattern; however, the replacement PPC (RJ) contributes most to this pattern. Pairwise values on the simplex plot (Fig 3b) are less clustered than the back-arc (Fig 3a) and are more concentrated near the RJ apex. βJ values range from 0.38 to 0.94 and indicate that sites, on average, share 22% of species. LCBD is not significant for any sites. NJ values are notably lower than the βJ-diversity, ranging from 0.08 to 0.91. Nestedness contributes less to β-diversity than its relative complement (Table 4), although it strongly influences some β-diversity values given that βratio values range from 0 to 0.89. Unlike the back-arc, the average βRC value is positive (Table 4), and about 46% (13/28) of pairwise values fall beyond the 95% CIs. While three sites (Daikoku, Kasuga-2 and Nikko) and a pair (NW Eifuku and NW Rota2) are significantly more similar to each other (Fig 4b), most significant βRC values are positive (S12 Table in S1 File).
Juan de Fuca (JdF) ridge
This mid-ocean ridge system in the northeast Pacific is more speciose with 72 macrofaunal species identified–nearly all to species-level (S6 Table in S1 File). Average species per site is 30.4. The only sedimented site, Middle Valley, is one of the poorest sampled, yet returned the greatest species number (Table 3). At least half the species along the ridge are associated with the foundation species Ridgeia piscesae (tubeworm). Eleven species occurred at all seven sites, while 39% (28/71) are currently known only at one site, usually Middle Valley or Endeavour.
The JdF shows a cascade pattern of clusters with overall dissimilarity similar to the Mariana back-arc (Fig 2). Again, a ‘complex’ pattern is present, and, like the Mariana back-arc, OJ is the dominant PPC. Pairwise values also cluster near the OJ apex of the simplex plot (Fig 3c). βJ values range from 0.23 to 0.71 with ~50% of species shared on average (Table 4). LCBD is significant for South Cleft and Middle Valley sites (p < 0.001 and p < 0.05 respectively). NJ values generally exceed the β-diversity, ranging from 0.51 to 1 (perfect nestedness). As the average βratio is 0.57, nestedness contributes to the overall β-diversity more than its relative complement (Table 4). However, βratio values nearly span the full scale between 0.03 and 1. The average βRC is -0.56, and ~48% (10/21) of the pairwise values fall beyond the 95% CIs, most of which are negative, although Endeavour and Middle Valley are significantly different (Fig 4c; S13 Table in S1 File).
Between-system comparisons
βJ values differ among the three vent systems (F = 48.3; p = 0.001). Both the back-arc and JdF systems have significantly lower βJ values than the Mariana arc (both p < 0.001) (Table 4), although there is little difference between the Mariana back-arc and JdF Ridge (p = 0.98). The RCJ values of the arc are also significantly higher than those of the back-arc and JdF (both p < 0.001). However, IJ value differences are not significant among the three vent systems (F = 0.4; p = 0.68). Therefore, species overlap and replacement distinguish the volcanic arc from the two spreading ridges. Consistent with these results, βRC values also differ among the three systems (F = 10.8; p = 0.001) with the arc having significantly higher βRC values than the back-arc and JdF systems (p = 0.002 and p < 0.001, respectively), indicating that sites within the arc are more compositionally dissimilar relative to the random expectation compared to those of the other two systems. Although the proportion of significant βRC values was notably higher in JdF than the back-arc, average values did not differ (p = 0.13) (Table 4).
In the combined Mariana region (volcanic arc and back-arc systems), RJ is the dominant influence on the ‘complex’, between-system pattern, while OJ had the smallest influence (Fig 3d). Between-system species replacement is high because only six of the total 75 species in the Mariana region are shared across the two systems. Overall, βJ-diversity values between arc and back-arc sites range from 0.85 to 1. Compared to the lower among-system βJ values within these systems (Table 4), the average between-system βJ value is 0.95. Hafa Adai and NW Eifuku share the greatest proportion of species (15%) among between-system pairs, but 16 such pairs share no species. Overall, six sites have significant LCBDs, all of which are arc-hosted (Nikko, Daikoku, Chamorro, East Diamante, NW Rota and Seamount X; p < 0.05), thus reflecting the significantly greater dissimilarity among arc sites (Table 4).
Both the average between-system NJ and βratio values are low (Table 4), consistent with our observation of apparent species substitutions between the two systems. For example, bythograeid crabs occupy every Mariana site, but the species differs between systems. Similarly, several other related taxon pairs occupy similar niches on the arc and back-arc. The pairwise, between-system NJ (0.03 to 0.76) and βratio (0 to 0.75) values indicate that nestedness influences β-diversity in some between-system pairs more than the relative complement. However, large richness differences contribute most to the high between-system nestedness values.
In the combined species pool of the Mariana region, 75% (90/120) of the βRC values are significant (S14 Table in S1 File). In this context, within-system βRC values for the back-arc all exceed the negative CI, and 21% (6/28) of the within-system βRC values for the volcanic arc are also more similar than random (Fig 4d) with one arc pair significantly different. In contrast, 86% (55/64) of the between-system βRC values exceed the positive CI, emphasizing the differences between the arc and back-arc.
Environmental drivers
For the Mariana back-arc, the non-linear correlation between habitat area and α-diversity lies on the threshold of significance (Kendall tau, α = 0.05, p = 0.05). No other environmental variables correlate with α-diversity. The dbMEM analyses also found no significant correlation between the abiotic variables and the β-diversity values.
Discussion
We examined vent assemblages in three geotectonic settings: back-arc, volcanic arc and mid-ocean ridge. Differing diversity characteristics emerge among the systems, especially in the β-diversity partitions. Whittaker’s diversity index is similar between the back-arc and mid-ocean ridge systems, but much higher for the volcanic arc. In each, the relative contribution of each pairwise pattern component differs such that the βratio decreases from JdF to Mariana back-arc to volcanic arc reflecting the increasing role of the replacement PPC while richness difference plays a larger role in the JdF system. A study of the invertebrates of Finland streams in eight regions [84] resembles ours in both assemblage type and outcomes, but we find a greater range in mean overlap and replacement PPCs in only three equivalent ‘regions’.
Despite their proximity, Mariana volcanic arc and back-arc differ in both overall species composition (as noted by Kojima and Watanabe [44]) and spatial arrangement of diversity. βJ-diversity (dissimilarity) is much higher on the arc compared to greater nestedness on the back-arc. Combining to a single Mariana region emphasizes the strong replacement component across systems. Phylogenetically related species occupy similar hydrothermal niches in the arc and back-arc; however, their origins likely reflect differing vicariant histories. The bythograeid crabs in arc and back-arc both have closer relatives in the south-west Pacific vent settings [85], and arc and back-arc Alviniconcha snails show markedly different phylogenetic divergence times [86]. While bathymetric differences between arc and back-arc sites may discourage faunal crossover, we note that A. hessleri larvae have been detected at 500 m depth [87]. It is likely that many vent species rise to the surface to feed, thus facilitating wider dispersion [88]. However, while surface dispersal occurs in the limpet Shinkailepas myojinensis on the Izu-Bonin arc [89] (contiguous with the Mariana arc), this species is replaced by others of the genus in each of the Mariana arc and back-arc sites. Thus, larval dispersability may not assure connectivity. Furthermore, Seamount X (arc) and Forecast (back-arc) are separated by only 20 km distance and less than 200 m depth, yet they share only 3 of the 33 species that we record at these sites. A study of ε-proteobacteria also noted the marked difference of microbial composition in Forecast fluids from those in arc fluids [52].
The differing interactions with underlying heat sources of the two systems affects the fundamental character of venting fluids [90], including compounds (e.g. CO2, CH4, H2S) that control toxicity and microbial productivity. The Mariana back-arc is characterized by extensive deposits of mineralized sulphides compared to few deposits on the arc; instead many sites are paved with elemental sulphur [51]. The differing substrata reflect excess sulphur (SO2 and SO4-2) in the arc volcanoes compared to the back-arc [39, 49, 51]. Abundant CO2 also reduces relative pH in arc fluids [91] compared to the back-arc. Detailed study that includes fluid constituents may point to factors that affect habitat suitability for colonizing fauna.
Among volcanic arc sites, the replacement PPC is also strong: over 46% (13/28) of site pair comparisons were significantly different from random (βRC). βRC-diversity values suggest strong deterministic processes in this system. The species replacement has no distinct pattern along the arc where similar variability is reflected in three microbial studies at many of the same sites: microbial mats [92], fluid bacteria [52] and fluid protists [93]. Several factors may affect these site to site differences. First, in a study of the northern Mariana and Izu-Bonin arcs, Watanabe et al. [41] find that water depth may explain the similarities among the three northern Mariana arc sites (<600 m), whereas nearby NW Eifuku fauna is distinctly different (1600 m). However, we note this latter site is most similar to shallow NW Rota (550 m) at the southern end of the arc, suggesting that depth may not be the single factor. Second, reduced connectivity may contribute to observed diversity patterns as currents flow across the arc, not along the structure [94], thus impeding larval exchange. Metaxas [95] finds larval behaviours that favour local retention on two arc volcanoes. Thirdly, in general, arc volcanoes can vary markedly as venting fluids have different rock and magma influences [90]. The three northern sites with similar faunae all have sulphur-rich fluids [51], whereas NW Eifuku and NW Rota emit CO2 dominated fluids [37].
Mariana back-arc βJ-diversity is more similar to JdF Ridge, northeast Pacific, in that nestedness (overlap and richness difference PPCs together) is strong. On both systems, connectivity may be enhanced by the topographic structure of an axial valley that directs currents along-strike [96]. Some widespread species show little genetic structure among populations such as R. piscesae on JdF Ridge [97]. Nestedness on the back-arc may result from past exchanges among sites where extensive extinct chimneys once supported vigorous hydrothermal emissions. The waning sites, Perseverance and Urashima/Pika, have low α-diversity values, yet they show no significant (dis)similarity with other sites. Receding hydrothermal flow could generate a random sampling effect in these sites, similar to the effects of a bleaching event on coral species where α-diversity declined, but βRC was unchanged [15]. As few back-arc site pairs were significant (βRC), stochastic processes dominate in community assembly. This back-arc is a young system (~3 MA) [98], which may influence gamma diversity compared to the 28 MA-old JdF [99] with greater species accumulation.
Nestedness in JdF reflects a ‘core species set’ stretching across the system. The high level of significant similarity of pairs among five sites suggests deterministic processes influencing community assembly. Three sites have experienced eruptions at least once in the past 30 years where disturbance-adapted species colonize rapidly [53, 100]. Eruptive disturbance could impose an ecological filter, reducing α-diversity as described by Chase et al. [15]. In contrast, Middle Valley and Endeavour host large sulphide deposits where venting has persisted over thousands of years [30]. However, the sediments of Middle Valley and bare rock of Endeavour support significantly different assemblages from each other, again implicating the role of substratum in diversity patterns. The Endeavour site has maintained activity with no known species loss over 35 years of observation. Stability and habitat complexity likely contribute to diversity accumulation: here, α-diversity is highest, and some eruption site species are replaced. Long-term stability is also a feature of vent sites in other locations [101]. Vent systems display a range of disturbance-stability reflecting the underlying dynamics of the hydrothermal heat source [32, 102], and our three systems show varying degrees of disturbance from eruptive activity. We visited Alice Springs, Mariana back-arc, 30 years after discovery [81], to find it virtually unchanged, but three arc sites have experienced eruptions since 2006 (e.g. NW Rota multiple times [29]).
The role of foundation species could affect differences in average α-diversity among systems. In the back-arc, the hairy snail Alviniconcha hessleri forms low mounds providing additional surface area for associated species while foundation species are absent from most arc sites. In contrast, the siboglinid tubeworm (Ridgeia piscesae) on JdF creates complex bush-like structures at every site that greatly expand the surface area accessing vent fluids and support a complex association of microbes and fauna [56]. For the Mariana back-arc, we found no environmental factors to explain differences in α-diversity and also no correlation between β-diversity and site distances, thus no support for the hypothesis of an along-strike faunal shift. Overall, the differences we observe in patterns of diversity distribution suggest that community assembly processes are not simple at hydrothermal vent ecosystems.
Our results are relevant to nations considering protection or mining in their waters and to the International Seabed Authority, the agency that will enable exploitation in seven (at present) high seas contracts. β-diversity analyses can help address criteria that identify significant areas for protection. Interactions among habitat suitability, geographic location and dispersal in the vent ecosystem influence the maintenance of metacommunities [103] in which species distribution reflects past or current connectivity. β-diversity patterns identify site linkages that can be tested with both genetic and network models to examine models of resilience to mining intrusions. For example, Suzuki et al. [104] use a dispersal model to predict very short recovery times for the Marianas, but it assumed incorrectly that any given species occurs at all sites on the volcanic arc and back-arc combined. Such models need grounding in distribution data to identify key nodes to maintain metacommunities. Dunn et al. [22] present a well-reasoned approach to placing broad conservation areas along the Mid-Atlantic Ridge where three ISA contracts exist. Citing the lack of available information on diversity distribution, they develop a framework based in habitat indicators and biodiversity drivers to address CBD criteria. A β-diversity analysis of the vent system here could test which siting scenarios would best support target criteria. Compiling data from expeditions of several nations and contractors remains a key requirement. Bonifácio et al. [105] demonstrate the role of such approaches, reporting high species replacement across mining contract areas in an abyssal manganese nodule province. Incorporating functional β-diversity into conservation planning would be highly effective [106, 107], but decisions on mining these deep-sea areas may not wait for acquisition of more than compositional data.
Most sites we examined are under some degree of protection (Mariana Trench Marine National Monument, Endeavour Hot Vents Marine Protected Area). Canada proposes to expand the Endeavour Hot Vents MPA to include Middle Valley and Explorer vent sites [108]. These three sites hold 89% of the known diversity in the region. While the remaining sites fall in international waters, protection of the three northern sites ensures habitat “safety” at large, relatively stable sites. As small geographic range is a superior predictor of extinction risk [109], conservation managers should consider that 75% of species occurring in our study area are endemic to the spreading ridges of the northeast Pacific. The results of our study provide strong support for the extended MPA and underscore the recommendation that siting MPAs consider β-diversity patterns [110]. The US Marine National Monument does not include the newly discovered sites on the Mariana back-arc. Here, Hafa Adai hosts the most extensive venting known in this system with a high faunal diversity. While Perseverance appears relatively depauperate, it is the only site with a significant local contribution to β-diversity due to a unique combination of species. We recommend inclusion of these sites in the Monument. Our study emphasizes just how limited distributions of these uniquely adapted species are; at least 60% of the Mariana back-arc species are known only from the very small habitat areas that we measured to a total of only about.02 km2.
The outcomes of β-diversity analyses provide decision support tools to achieve conservation objectives including species richness, site representativity, site replication and presence of species with restricted ranges [3]. Pairwise measures, such as PPCs, provide greater insight into diversity patterns than overall dissimilarity measures [111] by examining β-diversity partitions. The extent to which species among sites are similar, are replaced, or differ in species number, influences conservation choices. For example, the nested structure of birds on Amazonian cangas pointed to larger sites as targets for conservation with secondary sites to meet breeding needs [112]. We find that, on JdF Ridge, higher nestedness among sites suggests protection can focus on the more species-rich sites. The ratio of richness difference to replacement can identify the dominant PPC components in a system. With βratio < 0.5, a system such as the Mariana arc may require relatively more sites under protection to capture regional diversity [5]. Si et al. [9] recommend similar multi-site protection for lizard and bird communities on islands due to dominance of the replacement component. We supplemented the SET analysis with the Raup-Crick index (βRC) to identify pairs of sites that are less, or more, similar than random while controlling for richness difference. This index is often used to assess temporal changes in ecosystems under disturbance (e.g. [113]), thus may be applicable for long-term assessments of the impacts of seabed mining on diversity patterns. As βRC also can determine whether community assembly over an ecosystem is deterministic, a search for possible drivers can begin. Knowledge of those drivers may help in deciding placement of conservation areas (such as vent sites that are sources for larvae for other sites [114]). Analyses that can document environmental drivers of observed patterns may simplify decision-making when inventories of species are not available. A predictive model, such as that developed for β-diversity in tree species [115], can identify environmental surrogates for conservation targets as the network of habitat sites expands. Another measure derived from β-diversity data is calculation of the “minimum set” of sites that include all species [116]. Our study finds 75% (6/8) of Mariana back-arc, 63% (5/8) of Mariana arc, and 71% (5/7) of JdF sites; these proportions are notably higher than a mean of 41% for 97 habitat island datasets [117]. Such values may be an intrinsic feature of hydrothermal vent systems which requires consideration in setting environmental objectives. Assessment of LCBD (local contribution to β-diversity) analyses provides more perspective to understand the differential roles of sites [66] and can complement minimum set analyses; they can also be partitioned to determine site contributions to both replacement and richness difference [12]. Hill et al. [118] demonstrate that replacement is dominant in diversity of urban ponds while identifying which ponds are most significant for conservation at the landscape scale. Overall, β-diversity approaches provide considerable information to reach a variety of conservation objectives in area selection, especially for networks of protected areas. Once established, β-diversity analysis can support monitoring approaches such as protected area effectiveness assessments (e.g. [119]) or to establish baselines for long-term trends within the designated area [120].
We examined only three vent systems, yet they show differing patterns. Unlike a similar study of three forests in which replacement dominates β-diversity [121], we find PPC contributions differ. Thus, vent systems cannot be treated as similar across regions nor can the same management approaches be applied. The differences in diversity distribution patterns suggest that community assembly processes vary at vent ecosystems. Further work can test the role of diversity drivers such as habitat size, habitat complexity, and stability. The best conservation approach for this special and rare ecosystem is to adopt the Vulnerable Marine Ecosystem designation from FAO [122] and place all active vents under protection from anthropogenic impacts, including mining for seafloor deposits. Regional β-diversity analyses for seamounts, another island-like ecosystem, would also support development of conservation plans in the face of fishing and planned mining pressures [123]. As pressures of human activities grow in the deep ocean, compilation and analysis of diversity data are critical to guide management decisions.
Supporting information
a) Low-lying habitat type with weak fluid delivery through cracks in the basalt at Alice Springs. Zonation patterns from higher to lower fluid exposure: Alviniconcha hessleri snails, Neoverruca brachylepadoformis barnacles and Marianactis bythios anemones in peripheral area. Image about 3 m across at bottom. b) A close up of the Sequoia chimney at Hafa Adai, illustrates vigorous fluid delivery as a black smoker. Bacterial mats on the left are grazed by alvinocaridid shrimp, hairy snails cluster in centre while limpets are abundant on the right. Image about 1.5 m across.
(TIF)
(DOCX)
Acknowledgments
Data in this study were collected over the course of 25 years involving the crews of many ships and operators of three submersible systems. Of particular note, is the long term collaboration with colleagues in the NOAA Vents and EOI Programs, especially Robert Embley, William Chadwick and Dave Butterfield. For lab and analysis assistance, we thank Jonathan Boschen-Rose, Nick Brown, John Nelson, Mallory Van Wyngaarden, and Jackson Chu.
Data Availability
Data sources for species occurrences in each vent system in this study are in Supporting Information Tables S2–S4. Pairwise pattern component data for Figure 3 are in Tables S5.1–S5.4. β-diversity data for Figures 2 & 4 are in Tables S6.1–S6.4. COI sequences of Mariana back-arc specimens were deposited at NCBI (https://www.ncbi.nlm.nih.gov/), with the accession numbers of MW807757-MW807758 for Alvinocaris marimonte, MW497300-MW497305 for Austinograea williamsi, MW497306 and MW497307 for Bathyacmaea sp., MW497308 for Desbruyeresia cf. chamorrensis, MW497309-MW497311 for Desbruyeresia marianaensis, MW807760-MW807762 for Laeviphitus cf. japonicus, MW497312-MW497314 & MW807763-MW807765 for Lepetodrilus aff. schrolli MT, MW497315-MW497329 for Neoverruca brachylepadoformis, MW497330-MW497332 for Pseudorimula marianae, MW497333 and MW497334 for Rimicaris falkorae, MW497335-MW497414 for Rimicaris vandoverae, MW497415 for Shinkailepas sp., MW497416 for Symmetromphalus regularis and MZ509425-MZ509427 & MZ509431-MZ509434 for Symphurus thermophiles.
Funding Statement
This study was enabled by support from the Schmidt Ocean Institute to execute the back-arc expeditions FK151121 & FK161129 (schmidtocean.org). Funding for biological sampling was provided by National Geographic Society grant #9953-16 to VT (www.nationalgeographic.org/). Funding for research effort was provided by the Canadian Natural Sciences and Engineering Research Council grant #RG3805 to VT (https://www.nserc-crsng.gc.ca/index_eng.asp. VT acknowledges salary support from the Canada Research Chairs Foundation (www.chairs-chaires.gc.ca/home-accueil-eng.aspx). Funding for molecular sequencing was provided by the University of Victoria Oceans of Biodiversity program, which is part of the University of Guelph’s Food From Thought program, funded by the Canada First Research Excellence Fund (https://www.cfref-apogee.gc.ca). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.CBD. Strategic plan for biodiversity 2011–2020 and the Aichi targets. Report of the Tenth Meeting of the Conference of the Parties to the Convention on Biological Diversity. Montreal, Canada.: Secretariat of the Convention on Biological Diversity,; 2010.
- 2.Hortal J, Bello Fd, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol Syst. 2015;46(1):523–49. [Google Scholar]
- 3.Asaad I, Lundquist CJ, Erdmann MV, Costello MJ. Ecological criteria to identify areas for biodiversity conservation. Biol Conserv. 2017;213:309–16. [Google Scholar]
- 4.Whittaker RH. Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monogr. 1960;30(3):279–338. [Google Scholar]
- 5.Socolar JB, Gilroy JJ, Kunin WE, Edwards DP. How should beta-diversity inform biodiversity conservation? Trends Ecol Evol. 2016;31(1):67–80. doi: 10.1016/j.tree.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Carlos-Júnior LA, Spencer M, Neves DM, Moulton TP, Pires DdO, e Castro CB, et al. Rarity and beta diversity assessment as tools for guiding conservation strategies in marine tropical subtidal communities. Divers Distrib. 2019;25(5):743–57. [Google Scholar]
- 7.Soininen J, Heino J, Wang J. A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob Ecol Biogeogr. 2018;27(1):96–109. [Google Scholar]
- 8.Medeiros CR, Hepp LU, Patrício J, Molozzi J. Tropical estuarine macrobenthic communities are sructured by turnover rather than nestedness. PLoS One. 2016;11(9):e0161082. doi: 10.1371/journal.pone.0161082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Si X, Baselga A, Ding P. Revealing beta-diversity patterns of breeding bird and lizard communities on inundated land-bridge islands by separating the turnover and nestedness components. PLoS One. 2015;10(5):e0127692. doi: 10.1371/journal.pone.0127692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray KA, Baselga A. Reply to Chen and Schmera: Partitioning beta diversity into replacement and nestedness-resultant components is not controversial. Proc Natl Acad Sci U S A. 2015;112(52):E7162–E. doi: 10.1073/pnas.1522279113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podani J, Schmera D. Once again on the components of pairwise beta diversity. Ecol Inform. 2016;32:63–8. [Google Scholar]
- 12.Legendre P. Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr. 2014;23(11):1324–34. [Google Scholar]
- 13.Schmera D, Podani J, Legendre P. What do beta diversity components reveal from presence-absence community data? Let us connect every indicator to an indicandum! Ecol Indic. 2020;117:106540. [Google Scholar]
- 14.Raup DM, Crick RE. Measurement of faunal similarity in paleontology. J Paleontol. 1979:1213–27. [Google Scholar]
- 15.Chase JM, Kraft NJ, Smith KG, Vellend M, Inouye BD. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere. 2011;2(2):1–11. [Google Scholar]
- 16.Jones KR, Klein CJ, Halpern BS, Venter O, Grantham H, Kuempel CD, et al. The location and protection status of Earth’s diminishing marine wilderness. Curr Biol. 2018;28(15):2506–12.e3. doi: 10.1016/j.cub.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Llodra E, Tyler PA, Baker MC, Bergstad OA, Clark MR, Escobar E, et al. Man and the last great wilderness: human impact on the deep sea. PLoS One. 2011;6. doi: 10.1371/journal.pone.0022588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweetman AK, Thurber AR, Smith CR, Levin LA, Mora C, Wei C-L, et al. Major impacts of climate change on deep-sea benthic ecosystems. Elementa (Wash D C). 2017;5:Art. No. 4. [Google Scholar]
- 19.Levin LA, Wei C-L, Dunn DC, Amon DJ, Ashford OS, Cheung WWL, et al. Climate change considerations are fundamental to management of deep-sea resource extraction. Glob Change Biol. 2020;0(0). doi: 10.1111/gcb.15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman ASA, Tunnicliffe V, Bates AE. Both rare and common species make unique contributions to functional diversity in an ecosystem unaffected by human activities. Divers Distrib. 2018;24(5):568–78. [Google Scholar]
- 21.Petersen S, Krätschell A, Augustin N, Jamieson J, Hein JR, Hannington MD. News from the seabed–Geological characteristics and resource potential of deep-sea mineral resources. Mar Policy. 2016;70:175–87. [Google Scholar]
- 22.Dunn DC, Van Dover CL, Etter RJ, Smith CR, Levin LA, Morato T, et al. A strategy for the conservation of biodiversity on mid-ocean ridges from deep-sea mining. Sci Adv. 2018;4(7). doi: 10.1126/sciadv.aar4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen A, Podowski EL, Becker EL, Shearer EA, Gartman A, Yücel M, et al. Community succession in hydrothermal vent habitats of the Eastern Lau Spreading Center and Valu Fa Ridge, Tonga. Limnol Oceanogr. 2014;59(5):1510–28. [Google Scholar]
- 24.Alfaro-Lucas JM, Pradillon F, Zeppilli D, Michel L, Martinez-Arbizu P, Tanaka H, et al. High environmental stress and productivity increase functional diversity along a deep-sea hydrothermal vent gradient. Ecology. 2020:e03144. doi: 10.1002/ecy.3144 [DOI] [PubMed] [Google Scholar]
- 25.Tsurumi M, Tunnicliffe V. Tubeworm-associated communities at hydrothermal vents on the Juan de Fuca Ridge, northeast Pacific. Deep Sea Res 1 Oceanogr Res Pap. 2003;50(5):611–29. [Google Scholar]
- 26.Tunnicliffe V, McArthur AG, McHugh D. A biogeographical perspective of the deep-sea hydrothermal vent fauna. In: Blaxter J.H.S. S AJ, Tyler PA, editors. Adv Mar Biol. Volume 34: Academic Press; 1998. p. 353–442. [Google Scholar]
- 27.Baker ET, Resing JA, Haymon RM, Tunnicliffe V, Lavelle JW, Martinez F, et al. How many vent fields? New estimates of vent field populations on ocean ridges from precise mapping of hydrothermal discharge locations. Earth Planet Sci Lett. 2016;449:186–96. [Google Scholar]
- 28.Van Dover CL, Arnaud-Haond S, Gianni M, Helmreich S, Huber JA, Jaeckel AL, et al. Scientific rationale and international obligations for protection of active hydrothermal vent ecosystems from deep-sea mining. Mar Policy. 2018;90:20–8. [Google Scholar]
- 29.Schnur SR, Chadwick WW Jr, Embley RW, Ferrini VL, de Ronde CE, Cashman KV, et al. A decade of volcanic construction and destruction at the summit of NW Rota-1 seamount: 2004–2014. J Geophys Res Solid Earth. 2017;122(3):1558–84. [Google Scholar]
- 30.Jamieson JW, Clague DA, Hannington MD. Hydrothermal sulfide accumulation along the Endeavour Segment, Juan de Fuca Ridge. Earth Planet Sci Lett. 2014;395(0):136–48. [Google Scholar]
- 31.Van Dover CL. Impacts of anthropogenic disturbances at deep-sea hydrothermal vent ecosystems: A review. Mar Environ Res. 2014;102:59–72. doi: 10.1016/j.marenvres.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 32.Juniper SK, Tunnicliffe V. Crustal accretion and the hot vent ecosystem. Philos Trans R Soc Lond A. 1997;355(1723):459–74. [Google Scholar]
- 33.Teixeira S, Cambon-Bonavita MA, Serrão EA, Desbruyeres D, Arnaud-Haond S. Recent population expansion and connectivity in the hydrothermal shrimp Rimicaris exoculata along the Mid-Atlantic Ridge. J Biogeogr. 2011;38(3):564–74. [Google Scholar]
- 34.McKnight MW, White PS, McDonald RI, Lamoreux JF, Sechrest W, Ridgely RS, et al. Putting beta-diversity on the map: broad-scale congruence and coincidence in the extremes. PLoS Biol. 2007;5(10):e272. doi: 10.1371/journal.pbio.0050272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian H, Ricklefs RE, White PS. Beta diversity of angiosperms in temperate floras of eastern Asia and eastern North America. Ecol Lett. 2005;8(1):15–22. [Google Scholar]
- 36.Moalic Y, Desbruyères D, Duarte CM, Rozenfeld AF, Bachraty C, Arnaud-Haond S. Biogeography revisited with network theory: retracing the history of hydrothermal vent communities. Syst Biol. 2012;61(1):127–37. doi: 10.1093/sysbio/syr088 [DOI] [PubMed] [Google Scholar]
- 37.Baker ET, Embley RW, Walker SL, Resing JA, Lupton JE, Nakamura K-i, et al. Hydrothermal activity and volcano distribution along the Mariana arc. J Geophys Res Solid Earth. 2008;113:B8. [Google Scholar]
- 38.Resing JA, Baker ET, Lupton JE, Walker SL, Butterfield DA, Massoth GJ, et al. Chemistry of hydrothermal plumes above submarine volcanoes of the Mariana Arc. Geochem Geophys Geosyst. 2009;10(2). [Google Scholar]
- 39.Butterfield DA, Nakamura K-i, Takano B, Lilley MD, Lupton JE, Resing JA, et al. High SO2 flux, sulfur accumulation, and gas fractionation at an erupting submarine volcano. Geology. 2011;39(9):803–6. [Google Scholar]
- 40.Embley RW, Baker ET, Butterfield DA, Chadwick WW Jr, Lupton JE, Resing JA, et al. Exploring the submarine ring of fire: Mariana Arc—Western Pacific. Oceanography. 2007;20:68–79. [Google Scholar]
- 41.Watanabe HK, Shigeno S, Fujikura K, Matsui T, Kato S, Yamamoto H. Faunal composition of deep-sea hydrothermal vent fields on the Izu–Bonin–Mariana Arc, northwestern Pacific. Deep Sea Res 1 Oceanogr Res Pap. 2019;149:103050. [Google Scholar]
- 42.Anderson MO, Chadwick WW Jr, Hannington MD, Merle SG, Resing JA, Baker ET, et al. Geological interpretation of volcanism and segmentation of the Mariana back-arc spreading center between 12.7° N and 18.3° N. Geochem Geophys Geosyst. 2017;18(6):2240–74. [Google Scholar]
- 43.Toki T, Ishibashi J-i, Noguchi T, Tawata M, Tsunogai U, Yamanaka T, et al. Chemical and isotopic compositions of hydrothermal fluids at Snail, Archaean, Pika, and Urashima Sites in the southern Mariana Trough. In: Ishibashi J-i, Okino K, Sunamura M, editors. Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept. Tokyo: Springer Japan; 2015. p. 587–602. [Google Scholar]
- 44.Kojima S, Watanabe H. Vent Fauna in the Mariana Trough. In: Ishibashi J-i, Okino K, Sunamura M, editors. Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept. Tokyo: Springer Japan; 2015. p. 313–23. [Google Scholar]
- 45.Johnson SB, Warén A, Tunnicliffe V, Dover CV, Wheat CG, Schultz TF, et al. Molecular taxonomy and naming of five cryptic species of Alviniconcha snails (Gastropoda: Abyssochrysoidea) from hydrothermal vents. Syst Biodivers. 2014;13(3):278–95. [Google Scholar]
- 46.Baker ET, Walker SL, Resing JA, Chadwick WW, Merle SG, Anderson MO, et al. The efect of arc proximity on hydrothermal activity along spreading centers: new evidence from the Mariana Back Arc (12.7°N–18.3°N). Geochem Geophys Geosyst. 2017;18(11):4211–28. [Google Scholar]
- 47.Chadwick WW, Merle SG, Baker ET, Walker SL, Resing JA, Butterfield DA, et al. A recent volcanic eruption discovered on the central Mariana back-arc spreading center. Front Earth Sci. 2018;6:172. [Google Scholar]
- 48.Hessler RR, Lonsdale P, Hawkins J. Patterns on the ocean floor. New Sci. 1988;1605:47–8. [Google Scholar]
- 49.Ishibashi J-i, Tsunogai U, Toki T, Ebina N, Gamo T, Sano Y, et al. Chemical composition of hydrothermal fluids in the central and southern Mariana Trough backarc basin. Deep Sea Res 2 Top Stud Oceanogr. 2015;121:126–36. [Google Scholar]
- 50.Embley RW, Chadwick WW, Baker ET, Butterfield DA, Resing JA, de Ronde CEJ, et al. Long-term eruptive activity at a submarine arc volcano. Nature. 2006;441(7092):494–7. doi: 10.1038/nature04762 [DOI] [PubMed] [Google Scholar]
- 51.de Ronde C, Chadwick W, Ditchburn R, Embley R, Tunnicliffe V, Baker E, et al. Submarine sulfur lakes: degassing of intraoceanic arc volcanoes. In: D Rouwet RT, Vandelbroulemuck J and Christenson B, editor. Volcanic Lakes. Berlin: Springer; 2015. p. 261–88. [Google Scholar]
- 52.Huber JA, Cantin HV, Huse SM, Mark Welch DB, Sogin ML, Butterfield DA. Isolated communities of Epsilonproteobacteria in hydrothermal vent fluids of the Mariana Arc seamounts. FEMS Microbiol Ecol. 2010;73(3):538–49. doi: 10.1111/j.1574-6941.2010.00910.x [DOI] [PubMed] [Google Scholar]
- 53.Marcus J, Tunnicliffe V, Butterfield DA. Post-eruption succession of macrofaunal communities at diffuse flow hydrothermal vents on Axial Volcano, Juan de Fuca Ridge, Northeast Pacific. Deep Sea Res 2 Top Stud Oceanogr. 2009;56(19–20):1586–98. [Google Scholar]
- 54.Juniper SK, Tunnicliffe V, Southward EC. Hydrothermal vents in turbidite sediments on a Northeast Pacific spreading centre: organisms and substratum at an ocean drilling site. Can J Zool. 1992;70(9):1792–809. [Google Scholar]
- 55.Tunnicliffe V, Botros M, De Burgh ME, Dinet A, Johnson HP, Juniper SK, et al. Hydrothermal vents of Explorer Rdge, northeast Pacific. Deep Sea Res A. 1986;33(3):401–12. [Google Scholar]
- 56.Tunnicliffe V, Cordes EE. The tubeworm forests of hydrothermal vents and cold seeps. In: Rossi S, Bramanti L, editors. Perspectives on the Marine Animal Forests of the World: Springer; 2020. p. in press. [Google Scholar]
- 57.Colwell RK, Chao A, Gotelli NJ, Lin S-Y, Mao CX, Chazdon RL, et al. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol. 2012;5(1):3–21. [Google Scholar]
- 58.Hammer Ø, Harper DA, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4(1):9. [Google Scholar]
- 59.Jaccard P. The distribution of the flora in the alpine zone. New Phytol. 1912;11(2):37–50. [Google Scholar]
- 60.Baselga A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob Ecol Biogeogr. 2012;21(12):1223–32. [Google Scholar]
- 61.Podani J, Schmera D. A new conceptual and methodological framework for exploring and explaining pattern in presence–absence data. Oikos. 2011;120(11):1625–38. [Google Scholar]
- 62.Dray S, Blanchet G, Borcard D, Guenard G, Jombart T, Larocque G, et al. Package ‘adespatial’. R package version. 2018:3–8. [Google Scholar]
- 63.R Core Team. A language and environment for statistical computing 2020 [https://www.R-project.org/.
- 64.Smith M. Ternary: an R package for creating ternary plots. Zenodo French Conseil Européen pour la Recherche Nucléaire (CERN), Geneva, Switzerland doi. 2017;10.
- 65.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara R, et al. Package ‘vegan’. Community ecology package, version. 2013;2(9):1–295. [Google Scholar]
- 66.Legendre P, De Cáceres M. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol Lett. 2013;16(8):951–63. doi: 10.1111/ele.12141 [DOI] [PubMed] [Google Scholar]
- 67.Dobrovolski R, Melo AS, Cassemiro FA, Diniz-Filho JAF. Climatic history and dispersal ability explain the relative importance of turnover and nestedness components of beta diversity. Glob Ecol Biogeogr. 2012;21(2):191–7. [Google Scholar]
- 68.Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, et al. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett. 2011;14(1):19–28. doi: 10.1111/j.1461-0248.2010.01552.x [DOI] [PubMed] [Google Scholar]
- 69.Schmera D, Podani J. Through the jungle of methods quantifying multiple-site resemblance. Ecol Inform. 2018;44:1–6. [Google Scholar]
- 70.Baselga A. Multiple site dissimilarity quantifies compositional heterogeneity among several sites, while average pairwise dissimilarity may be misleading. Ecography. 2013;36(2):124–8. [Google Scholar]
- 71.Mangiafico S, Mangiafico MS. Package ‘rcompanion’. Cran Repos. 2017:1–71.
- 72.Hervé M, Hervé MM. Package ‘RVAideMemoire’. See https://CRANR-projectorg/package=RVAideMemoire. 2020.
- 73.Levin LA, Etter RJ, Rex MA, Gooday AJ, Smith CR, Pineda J, et al. Environmental influences on regional deep-sea species diversity. Annu Rev Ecol Syst. 2001;32:51–93. [Google Scholar]
- 74.Stern RJ, Tamura Y, Masuda H, Fryer P, Martinez F, Ishizuka O, et al. How the Mariana Volcanic Arc ends in the south. Isl Arc. 2013;22(1):133–48. [Google Scholar]
- 75.Rosenzweig ML. Species diversity in space and time. Cambridge: Cambridge University Press; 1995. pp.i-xxi, 1–436 p. [Google Scholar]
- 76.Fujikura K, Yamazaki T, Hasegawa K, Tsunoga iU, Stein R, Ueno H, et al. Biology and earth scientific investigation by the submersible “Shinkai 2000” system of deep-sea hydrothermalism and lithosphore in the Mariana Back-Arc Basin. JAMSTEC J Deep Sea Res. 1997;13:1–20. [Google Scholar]
- 77.Wood S. Mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation and GAMMs by REML/PQL. R package version. 2018:1.8–23.
- 78.Scheltema AH. Biogeography, diversity, and evolution through vicariance of the hydrothermal vent aplacophoran genus Helicoradomenia (Aplacophora, Mollusca). J Shellfish Res. 2008;27(1):91–6. [Google Scholar]
- 79.Breusing C, Johnson SB, Tunnicliffe V, Vrijenhoek RC. Population structure and connectivity in Indo-Pacific deep-sea mussels of the Bathymodiolus septemdierum complex. Conserv Genet. 2015:1–16. [Google Scholar]
- 80.Johnson SB, Warén A, Vrijenhoek RC. DNA barcoding of Lepetodrilus limpets reveals cryptic species. J Shellfish Res. 2008;27(1):43–51. [Google Scholar]
- 81.Hessler RR, Lonsdale PF. Biogeography of Mariana Trough hydrothermal vent communities. Deep Sea Res A. 1991;38:185–99. [Google Scholar]
- 82.Giguère TN. Characterization of hydrothermal vent faunal assemblages in the Mariana Back-arc Spreading Centre [M.Sc. Thesis]. Victoria, BC, Canada: University of Victoria; 2020.
- 83.Komai T, Giguère T. A new species of alvinocaridid shrimp Rimicaris Williams and Rona, 1986 (Decapoda: Caridea) from hydrothermal vents on the Mariana Back Arc Spreading Center, northwestern Pacific. J Crustac Biol. 2019;39:640–50. [Google Scholar]
- 84.Podani J, Ódor P, Fattorini S, Strona G, Heino J, Schmera D. Exploring multiple presence-absence data structures in ecology. Ecol Modell. 2018;383:41–51. [Google Scholar]
- 85.Mateos M, Hurtado LA, Santamaria CA, Leignel V, Guinot D. Molecular systematics of the deep-sea hydrothermal vent endemic brachyuran Family Bythograeidae: A comparison of three Bayesian species tree methods. PLoS One. 2012;7(3):e32066. doi: 10.1371/journal.pone.0032066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breusing C, Johnson SB, Tunnicliffe V, Clague DA, Vrijenhoek RC, Beinart RA. Allopatric and sympatric drivers of speciation in Alviniconcha hydrothermal vent snails. Mol Biol Evol. 2020. doi: 10.1093/molbev/msaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sommer SA, Van Woudenberg L, Lenz PH, Cepeda G, Goetze E. Vertical gradients in species richness and community composition across the twilight zone in the North Pacific Subtropical Gyre. Mol Ecol. 2017;26(21):6136–56. doi: 10.1111/mec.14286 [DOI] [PubMed] [Google Scholar]
- 88.Gary SF, Fox AD, Biastoch A, Roberts JM, Cunningham SA. Larval behaviour, dispersal and population connectivity in the deep sea. Sci Rep. 2020;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yahagi T, Kayama Watanabe H, Kojima S, Kano Y. Do larvae from deep-sea hydrothermal vents disperse in surface waters? Ecology. 2017;98(6):1524–34. doi: 10.1002/ecy.1800 [DOI] [PubMed] [Google Scholar]
- 90.de Ronde CE, Stucker VK. Seafloor hydrothermal venting at volcanic arcs and backarcs. The Encyclopedia of Volcanoes: Elsevier; 2015. p. 823–49. [Google Scholar]
- 91.Lupton J, Lilley M, Butterfield D, Evans L, Embley R, Massoth G, et al. Venting of a separate CO2-rich gas phase from submarine arc volcanoes: Examples from the Mariana and Tonga-Kermadec arcs. J Geophys Res Solid Earth. 2008;113(B8). [Google Scholar]
- 92.Davis RE, Moyer CL. Extreme spatial and temporal variability of hydrothermal microbial mat communities along the Mariana Island Arc and southern Mariana back-arc system. J Geophys Res Solid Earth. 2008;113(B8). [Google Scholar]
- 93.Murdock SA, Juniper SK. Hydrothermal vent protistan distribution along the Mariana arc suggests vent endemics may be rare and novel. Environ Microbiol. 2019;21(10):3796–815. doi: 10.1111/1462-2920.14729 [DOI] [PubMed] [Google Scholar]
- 94.Kendall M, Poti M. Potential larval sources, destinations, and self-seeding in the Mariana Archipelago documented using ocean drifters. J Oceanogr. 2014;70(6):549–57. [Google Scholar]
- 95.Metaxas A. Spatial and temporal patterns in larval supply at hydrothermal vents in the northeast Pacific Ocean. Limnol Oceanogr. 2004;49(6):1949–56. [Google Scholar]
- 96.Thomson RE, Mihaly SF, Rabinovich AB, McDuff RE, Veirs SR, Stahr FR. Constrained circulation at Endeavour ridge facilitates colonization by vent larvae. Nature. 2003;424:545–9. doi: 10.1038/nature01824 [DOI] [PubMed] [Google Scholar]
- 97.Young CR. Directional dispersal between mid-ocean ridges: deep-ocean circulation and gene flow in Ridgeia piscesae. Mol Ecol. 2008;17:1718–31. doi: 10.1111/j.1365-294X.2008.03609.x [DOI] [PubMed] [Google Scholar]
- 98.Stern RJ, Fouch MJ, Klemperer SL. An overview of the Izu-Bonin-Mariana subduction factory. Geophysical Monographs. 2003;138:175–222. [Google Scholar]
- 99.Mammerickx J, Klitgord KD. Northern East Pacific Rise: evolution from 25 m.y. B.P. to the present. J Geophys Res Solid Earth. 1982;87: 6751–9. [Google Scholar]
- 100.Tunnicliffe V, Embley RW, Holden JF, Butterfield DA, Massoth GJ, Juniper SK. Biological colonization of new hydrothermal vents following an eruption on Juan de Fuca Ridge. Deep Sea Res 1 Oceanogr Res Pap. 1997;44(9–10):1627–44. [Google Scholar]
- 101.Du Preez C, Fisher CR. Long-term stability of back-arc basin hydrothermal vents. Front Mar Sci. 2018;5:54. [Google Scholar]
- 102.Jamieson JW, Gartman A. Defining active, inactive, and extinct seafloor massive sulfide deposits. Mar Policy. 2020;117:103926. [Google Scholar]
- 103.Mullineaux LS, Metaxas A, Beaulieu SE, Bright M, Gollner S, Grupe BM, et al. Exploring the ecology of deep-sea hydrothermal vents in a metacommunity framework. Front Mar Sci. 2018;5(49). [Google Scholar]
- 104.Suzuki K, Yoshida K, Watanabe H, Yamamoto H. Mapping the resilience of chemosynthetic communities in hydrothermal vent fields. Sci Rep. 2018;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonifácio P, Martínez Arbizu P, Menot L. Alpha and beta diversity patterns of polychaete assemblages across the nodule province of the eastern Clarion-Clipperton Fracture Zone (equatorial Pacific). Biogeosciences. 2020;17(4):865–86. [Google Scholar]
- 106.Bevilacqua S, Terlizzi A. Nestedness and turnover unveil inverse spatial patterns of compositional and functional β-diversity at varying depth in marine benthos. Divers Distrib. 2020;26(6):743–57. [Google Scholar]
- 107.Loiseau N, Legras G, Kulbicki M, Mérigot B, Harmelin-Vivien M, Mazouni N, et al. Multi-component β-diversity approach reveals conservation dilemma between species and functions of coral reef fishes. J Biogeogr. 2017;44(3):537–47. [Google Scholar]
- 108.DFO. Biophysical and Ecological Overview of the Offshore Pacific Area of Interest (AOI). DFO Can. Sci. Advis. Sec. Sci. Resp. In: Canada FaO, editor. 2019.
- 109.Harris G, Pimm SL. Range size and extinction risk in forest birds. Conserv Biol. 2008;22(1):163–71. doi: 10.1111/j.1523-1739.2007.00798.x [DOI] [PubMed] [Google Scholar]
- 110.Kenchington E, McLean S, Rice JC. Considerations for Identification of Effective Area-based Conservation Measures. DFO Can. Sci. Advis. Sec. Res. Doc. 2016. p. 53 [Google Scholar]
- 111.Bush A, Harwood T, Hoskins AJ, Mokany K, Ferrier S. Current uses of beta-diversity in biodiversity conservation: A response to. Trends Ecol Evol. 2016;31:337. doi: 10.1016/j.tree.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 112.Borges SH, Santos MP, Soares L, Silva A. Avian communities in the amazonian cangas vegetation: Biogeographic affinities, components of Beta-Diversity and conservation. An Acad Bras Cienc. 2017;89:2167–80. doi: 10.1590/0001-3765201720160048 [DOI] [PubMed] [Google Scholar]
- 113.Tatsumi S, Strengbom J, Čugunovs M, Kouki J. Partitioning the colonization and extinction components of beta diversity across disturbance gradients. Ecology. 2020;101(12):e03183. doi: 10.1002/ecy.3183 [DOI] [PubMed] [Google Scholar]
- 114.Boschen RE, Collins PC, Tunnicliffe V, Carlsson J, Gardner JP, Lowe J, et al. A primer for use of genetic tools in selecting and testing the suitability of set-aside sites protected from deep-sea seafloor massive sulfide mining activities. Ocean Coast Manag. 2016;122:37–48. [Google Scholar]
- 115.McKenzie PF, Iacona GD, Larson ER, Armsworth PR. Partitioning tree diversity patterns to prioritize conservation investments. Environ Conserv. 2021;48(2):75–83. [Google Scholar]
- 116.Watson JE, Grantham HS, Wilson KA, Possingham HP. Systematic conservation planning: past, present and future. Conservation Biogeography. 2011;1:136–60. [Google Scholar]
- 117.Matthews TJ, Cottee-Jones HEW, Whittaker RJ. Quantifying and interpreting nestedness in habitat islands: a synthetic analysis of multiple datasets. Divers Distrib. 2015;21(4):392–404. [Google Scholar]
- 118.Hill MJ, White JC, Biggs J, Briers RA, Gledhill D, Ledger ME, et al. Local contributions to beta diversity in urban pond networks: Implications for biodiversity conservation and management. Divers Distrib. 2021;27(5):887–900. [Google Scholar]
- 119.Beauvais M-P, Pellerin S, Lavoie C. Beta diversity declines while native plant species richness triples over 35 years in a suburban protected area. Biol Conserv. 2016;195:73–81. [Google Scholar]
- 120.Lacharité M, Brown CJ. Utilizing benthic habitat maps to inform biodiversity monitoring in marine protected areas. Aquat Conserv. 2019;29(6):938–51. [Google Scholar]
- 121.Bergamin R, Bastazini V, Vélez-Martin E, Debastiani V, Zanini K, Loyola R, et al. Linking beta diversity patterns to protected areas: lessons from the Brazilian Atlantic Rainforest. Biodivers Conserv. 2017;26(7):1557–68. [Google Scholar]
- 122.FAO. International guidelines for the management of deep-sea fisheries in the high seas. Rome, Italy2009.
- 123.Watling L, Auster PJ. Seamounts on the high seas should be managed as vulnerable marine ecosystems. Front Mar Sci. 2017;4:14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) Low-lying habitat type with weak fluid delivery through cracks in the basalt at Alice Springs. Zonation patterns from higher to lower fluid exposure: Alviniconcha hessleri snails, Neoverruca brachylepadoformis barnacles and Marianactis bythios anemones in peripheral area. Image about 3 m across at bottom. b) A close up of the Sequoia chimney at Hafa Adai, illustrates vigorous fluid delivery as a black smoker. Bacterial mats on the left are grazed by alvinocaridid shrimp, hairy snails cluster in centre while limpets are abundant on the right. Image about 1.5 m across.
(TIF)
(DOCX)
Data Availability Statement
Data sources for species occurrences in each vent system in this study are in Supporting Information Tables S2–S4. Pairwise pattern component data for Figure 3 are in Tables S5.1–S5.4. β-diversity data for Figures 2 & 4 are in Tables S6.1–S6.4. COI sequences of Mariana back-arc specimens were deposited at NCBI (https://www.ncbi.nlm.nih.gov/), with the accession numbers of MW807757-MW807758 for Alvinocaris marimonte, MW497300-MW497305 for Austinograea williamsi, MW497306 and MW497307 for Bathyacmaea sp., MW497308 for Desbruyeresia cf. chamorrensis, MW497309-MW497311 for Desbruyeresia marianaensis, MW807760-MW807762 for Laeviphitus cf. japonicus, MW497312-MW497314 & MW807763-MW807765 for Lepetodrilus aff. schrolli MT, MW497315-MW497329 for Neoverruca brachylepadoformis, MW497330-MW497332 for Pseudorimula marianae, MW497333 and MW497334 for Rimicaris falkorae, MW497335-MW497414 for Rimicaris vandoverae, MW497415 for Shinkailepas sp., MW497416 for Symmetromphalus regularis and MZ509425-MZ509427 & MZ509431-MZ509434 for Symphurus thermophiles.