Abstract
Objective
To compare the perioperative parameters between single- and triple-port video-assisted thoracoscopic surgery (VATS) lobectomy in the treatment of lung cancer.
Methods
The Pubmed, Embase, Cochrane library, and the Web of Science databases were electronically searched from inception to September 2019 for all relevant studies. Study quality was evaluated using the Jadad scale or the Newcastle-Ottawa scale. The results were pooled using the generic inverse-variance method and expressed as mean differences or risk ratios, with 95% confidence intervals.
Results
Three randomized controlled trials (RCTs) and ten cohort studies with 2,278 subjects were included in the meta-analysis. Whether based on RCTs or cohort studies, the pooled results showed no significant difference in the operation time, chest tube duration, intraoperative blood loss, postoperative hospital stays, lymph node dissection number, postoperative drainage volume, and postoperative complications between single- and triple-port VATS lobectomy (P > 0.05). Single-port VATS could relieve postoperative pain better than triple-port VATS, especially in the first day and fifth day (P < 0.05). No evidence of significant publication bias was found (P > 0.05).
Conclusion
Single-port VATS lobectomy can yield similar perioperative results to those of triple-port VATS lobectomy and is more effective in relieving postoperative pain.
Keywords: VATS, single-port, triple-port, lung cancer, meta-analysis
1. Introduction
Lung cancer is the most fatal cancer worldwide, which is characterized by uncontrolled cell growth in the lung tissues. It is estimated that 2.09 million new cases of lung cancer occurred globally in 2018, ranking first among all cancer types [1]. The etiology of lung cancer is not yet clear, and the myriad risk factors for lung cancer most commonly include lifestyle, environmental, and occupational exposures [2]. Although the survival rates for all cancers have improved in recent years, lung cancer survival remains at a relatively low level in China. In 2012–2015, the lung cancer survival rate in men and women were 16.8 and 25.1%, respectively [3]. Lung cancer poses a significant public health burden.
Lung cancer is broadly classified into two types, which grow and spread differently: small cell lung carcinoma and non-small cell lung carcinoma (NSCLC). Treatment options for lung cancer include surgery, radiation therapy, chemotherapy, and targeted therapy [4]. Although long-term survival remains poor for patients with metastasis, complete surgical resection is potentially curative for patients with early-stage lung cancer [5]. Video-assisted thoracoscopic surgery (VATS) is a type of thoracic surgery performed using a small video camera that is introduced into the patient’s chest via small incisions and has become a major surgical method in chest surgery [6]. Early in 2007, the American College of Chest Physicians’ evidence-based clinical practice guidelines for the treatment of stage I and II NSCLC consider VATS lobectomy to be an acceptable alternative to open thoracotomy [7]. Formerly, there was much debate about the feasibility of the technique in cancer surgery and proper lymph node handling [8]. Now, it is generally accepted that the outcome of a VATS procedure is at least not inferior to a resection via a traditional thoracotomy in the treatment of lung cancer [8].
VATS incision has many options, and the most frequently used option is one observation hole and 2–3 operation holes [9]. With the development of laparoscopic instrument technology, VATS is gradually reduced from multiple incisions to double incision, namely single utility port thoracoscopic surgery [10]. Early in 2011, Gonzalez-Rivas et al. reported their experience of single-port VATS lobectomy, the first worldwide-published study on major lung resection [11]. Single-port VATS has been developed in recent years, featured by minimal invasion and operation difficulty [12]. Both randomized controlled trial (RCT) and cohort study have reported that single-port VATS lobectomy showed similar results as triple-port VATS in safety and efficacy [13,14], indicating that single-port VATS lobectomy is a feasible and safe option for lung cancer patients. To the best of our knowledge, no systematic review has evaluated the curative effect between single-port and triple-port VATS for the surgical resection of lung cancer based on different study design. In this regard, the present systematic review aimed to compare the perioperative parameters between single- and triple-port VATS lobectomy for lung cancer treatment, so as to provide recommendation statement outlining VATS implication in lung cancer treatment.
2. Materials and methods
2.1. Literature search
This systematic review and meta-analysis followed the PRISMA statement and guidelines [15]. The literature research was performed using Pubmed, Embase, Cochrane library, and the Web of Science before September 2019. The search terms combined the following items: (“lung cancer” OR “lung carcinoma” OR “pulmonary cancer” OR “pulmonary carcinoma” OR “lung neoplasm” OR “pulmonary neoplasm”) AND (“thoracoscopic” OR “thoracoscopy” OR “thoracoscope”) AND (“Lobectomy” OR “pneumonectomy” OR “lung resection”). The search strategy applied a combination of title and abstract and used the Mesh Term (Table S1).
Reviewers were divided into two groups that worked in parallel. The reviewers independently screened each record by title, keywords, and abstract against the eligibility criteria. Full texts were referred to when information in the records was inadequate for determination. Any disagreement between the two groups of reviewers was resolved by an additional reviewer. Hand searching was performed by reviewing the references of included studies.
2.2. Selection criteria
The eligible studies included in this systematic review and meta-analysis met the following inclusion criteria: (1) patients with lung cancer; (2) different treatment groups adopted single-port or triple-port VATS lobectomy; (3) RCTs or cohort studies (retrospective and prospective); (4) the perioperative parameters were measured; (5) the language was restricted to English or Chinese.
The studies were excluded if: (1) case reports, conference abstracts, editorials, expert opinions, protocol, and commentaries; (2) lung cancer patients with other tumors; (3) studies with duplicate data reported in multiple studies by the same research group.
2.3. Data extraction
An extraction form was designed to extract data, including general information, methodological quality, clinical characteristics, and data of treatment outcomes. The data extraction procedure was also implemented independently by the two parallel groups of reviewers. Any disagreement was resolved by an additional reviewer.
2.4. Quality assessment
The quality of RCTs was evaluated using the Jadad scale, which was presented as a total scale of 1–5 based on assessment of randomization method, blinding, and descriptions of withdrawals and dropouts [16]. The quality of cohort studies was evaluated using the Newcastle-Ottawa scale (NOS) with the following domains: selection, comparability, and ascertainment of exposure/outcome [17].
2.5. Statistical analysis
Weighted mean differences (WMDs) in continuous variables and risk ratios (RRs) in dichotomous variables, with 95% confidence intervals (CIs) and P values, were calculated to assess effects of single- and triple-port VATS lobectomy for lung cancer. The inverse-variance method in continuous variables and Mantel-Haenszel method in dichotomous variables were used to combine data and generate the overall effect estimate. Heterogeneity was assessed by Q test and I 2 statistic, and P value of <0.10 or an I 2 value of >50% indicated substantial heterogeneity, thus determining the use of a fixed-effects model or random-effects model. Sensitivity analysis was performed based on cohort studies with propensity-matched analysis or not. Subgroup analysis was performed according to: (1) location (China, Korea), (2) sample size (≥200, <200), and (3) quality score (≥8, < 8). Meta-regression analysis was used to explore the potential source of heterogeneity. If more than 10 trials were included in the meta-analysis, publication bias was assessed using funnel plots and Egger’s test. Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for data syntheses. STATA version 15.1 (College Station, TX, USA) was used to analyze meta-regression analysis and publication bias.
3. Results
3.1. Characteristics of studies
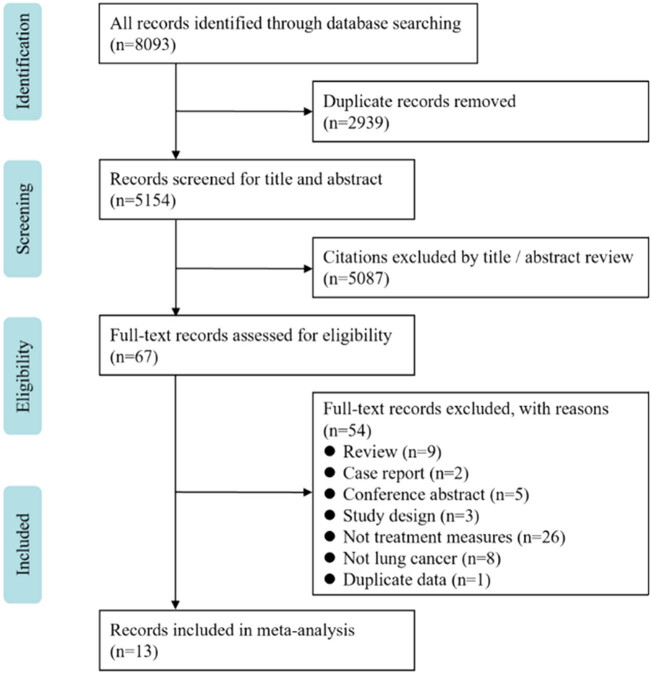
As illustrated in Figure 1, our initial search identified 5,154 potentially references from four databases, from which 5,084 were excluded after screening the titles and abstracts. Ultimately, 13 studies were eligible for inclusion [13,14,18,19,20,21,22,23,24,25,26,27,28]. This selection consisted of 3 articles designed as RCTs [13,25,27] and 10 articles designed as cohort studies [14,18,19,20,21,22,23,24,26,28] (with 2 propensity-matched cohort studies [18,23]), with approximately 2,278 participants. Among the included studies, seven published in Chinese [19,21,22,24,25,26,27] and six in English [13,14,18,20,23,28]. Eleven studies were conducted in China [13,14,19,21,22,23,24,25,26,27,28], whereas others were performed in Korea [18,20]. There were four studies with sample sizes greater than 200 [19,20,21,26]. The characteristics of the articles are listed in Table 1.
Figure 1.
PRISMA flow chart for literature search.
Table 1.
General information of all the included studies
| Author, year | Type of study | Location | Study period | Groups | No. of cases | Age (years)a | Genderb | Tumor locationc | Pathological typesd | TNM stagee | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al. 2018 | RCT | China | 2012/01–2016/01 | Single-port | 31 | 58.8 ± 13.7 | 25/6 | 11/20 | 8/21/2 | 13/14/4 | 4* |
| [25] | Triple-port | 31 | 56.3 ± 11.9 | 22/9 | 12/17 | 8/21/2 | 14/12/5 | ||||
| Shi et al. 2018 | RCT | China | 2017/02–2017/10 | Single-port | 80 | 61.77 ± 10.07 | 34/46 | 28/52 | 8/65/7 | 62/14/4 | 4* |
| [27] | Triple-port | 96 | 60.04 ± 9.15 | 51/45 | 39/57 | 10/81/5 | 77/9/10 | ||||
| Ye et al. 2019 | RCT | China | 2015/07–2017/01 | Single-port | 74 | 62.67 ± 9.16 | 43/31 | 24/50 | 10/59/5 | 59/15/0 | 5* |
| [13] | Triple-port | 82 | 61.83 ± 8.26 | 59/23 | 29/53 | 14/61/7 | 64/18/0 | ||||
| Han et al. 2017 | Cohort study | Korea | 2006/01–2015/06 | Single-port | 203 | 62.9(33–84) | 132/71 | 79/124 | 46/113/8 | 120/33/14 | 8** |
| [20] | Triple-port | 168 | 64.1(40–86) | 105/63 | 73/95 | 49/86/19 | 89/33/23 | ||||
| Hao et al. 2017 | Cohort study | China | 2015/03–2015/12 | Single-port | 208 | 59.2 ± 5.3 | 110/98 | 78/130 | 80/118/10 | 154/24/31 | 7** |
| [21] | Triple-port | 103 | 59.7 ± 5.1 | 61/42 | 41/62 | 42/56./5 | 67/16/20 | ||||
| Li et al. 2013 | Cohort study | China | 2011/02–2013/01 | Single-port | 87 | 63.86 ± 12.10 | 65/22 | 34/53 | 39/48/0 | 31/38/18 | 7** |
| [24] | Triple-port | 75 | 66.20 ± 8.72 | 52/23 | 31/44 | 42/33/0 | 30/29/16 | ||||
| Mu et al. 2015 | Cohort study | China | 2014/11–2015/05 | Single-port | 47 | 56.67 ± 11.62 | 25/22 | 18/29 | 2/45/0 | 27/9/2 | 9** |
| [23] | Triple-port | 47 | 60.77 ± 11.04 | 14/33 | 20/30 | 5/42/0 | 26/5/1 | ||||
| Rao et al. 2019 | Cohort study | China | 2017/08–2018/03 | Single-port | 153 | 56.1 ± 8.5 | 67/86 | — | 54/87/12 | — | 7** |
| [26] | Triple-port | 102 | 54.4 ± 7.4 | 43/59 | — | 41/56/5 | — | ||||
| Song et al. 2017 | Cohort study | Korea | 2011/12–2016/08 | Single-port | 26 | 64.8 ± 9.7 | 15/11 | 8/18 | 8/17/1 | 17/9/0 | 9** |
| [18] | Triple-port | 26 | 65.0 ± 9.4 | 15/11 | 13/13 | 7/19/0 | 20/5/1 | ||||
| Wang et al. 2017 | Cohort study | China | 2015/01–2015/12 | Single-port | 73 | 57.12 ± 6.43 | 31/42 | — | 16/34/23 | 27/21/3 | 9** |
| [14] | Triple-port | 98 | 61.32 ± 7.54 | 53/45 | — | 13/52/33 | 34/30/7 | ||||
| Wang 2018 | Cohort study | China | 2016/01–2017/08 | Single-port | 153 | 61.52 ± 9.70 | 82/71 | 57/96 | 26/115/12 | 115/14/24 | 7** |
| [19] | Triple-port | 113 | 62.27 ± 10.08 | 69/44 | 47/66 | 25/81/7 | 72/10/31 | ||||
| Xu et al. 2018 | Cohort study | China | 2017/09–2017/11 | Single-port | 60 | 61.4 ± 10.8 | 33/27 | 27/33 | 10/47/3 | 35/14/11 | 6** |
| [22] | Triple-port | 60 | 63.5 ± 9.6 | 31/29 | 27/33 | 8/48/4 | 34/16/10 | ||||
| Zhu et al. 2015 | Cohort study | China | 2014/08–2014/10 | Single-port | 33 | 62(25–79) | 11/22 | 14/19 | 5/26/2 | 23/8/2 | 8** |
| [28] | Triple-port | 49 | 59(31–81) | 19/30 | 20/29 | 11/35/3 | 34/11/4 |
Abbreviation: RCT, randomized controlled trial; TNM, tumor node metastasis.
aValues are presented as mean ± standard deviations or median (interquartile range); bMale/Female; cRight/Left; dSquamous carcinoma/Adenocarcinoma/Others; estage I/stage II/stage III.
*Assessed using the Jadad scale; **Assessed using NOS.
3.2. Quality of studies
Based on the Jadad scale, one study had a total score of 5 [13] and two studies had a total score of 4 (Table 1) [25,27]. The scores of the NOS quality assessment in cohort studies ranged from 6 to 9, four of them had scores greater than 8 [14,18,20,23,28]. All scores are listed in Table 1. Overall, most of the studies demonstrated a good or moderate methodology.
3.3. Perioperative parameters
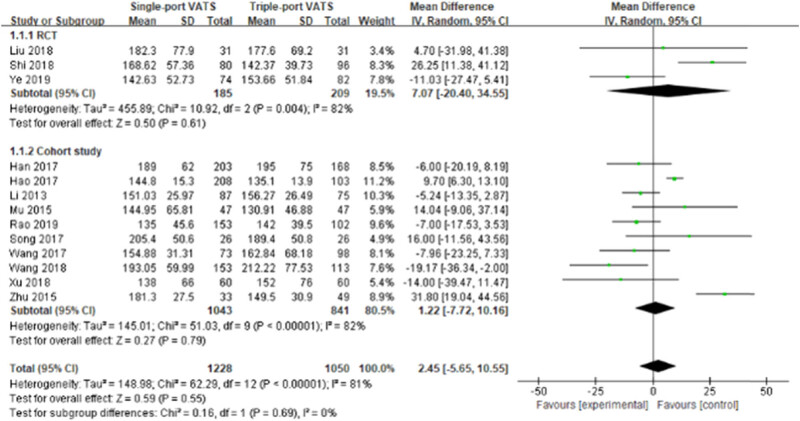
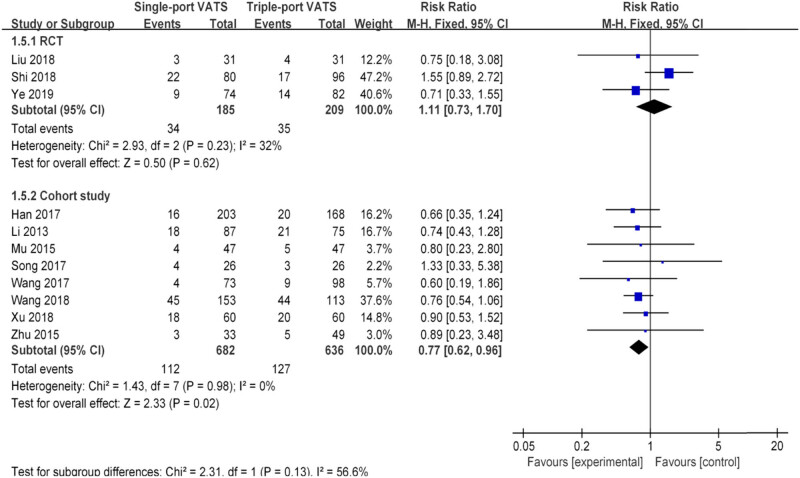
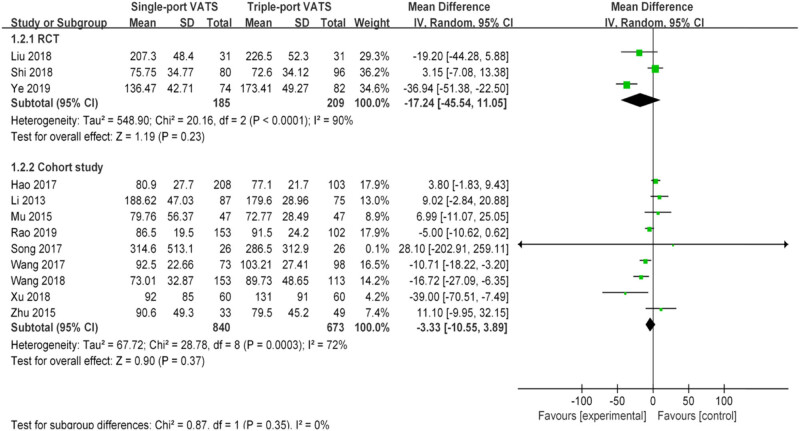
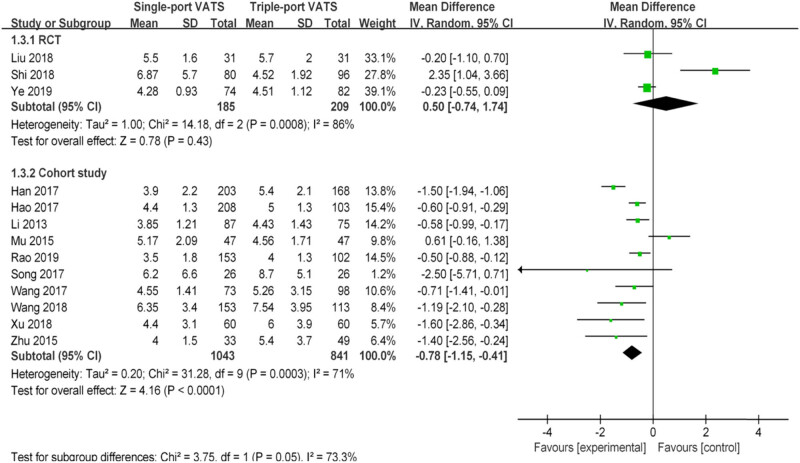
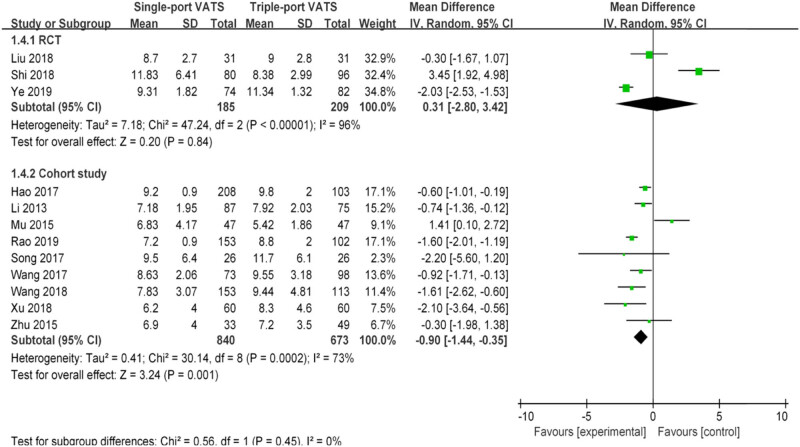
Nearly 20 perioperative parameters were reported in thirteen studies, of which the most frequently reported outcomes were operation time, chest tube duration, intraoperative blood loss, postoperative hospital stays, lymph node dissection number, postoperative pain score, postoperative drainage volume, postoperative complications, pulmonary leakage, pulmonary infection, atelectasis, arrhythmia, chylothorax, etc. Whether based on RCTs or cohort studies, the pooled results indicated that there was no significant difference in multiple perioperative parameters between single- and the triple-VATS lobectomy, except the other three outcomes (chest tube duration, postoperative hospital stays, and postoperative complications, Table 2, Figures 2–6). Based on the cohort studies and compared to the triple-VATS lobectomy, the single-VATS lobectomy showed shorter chest tube duration (days), shorter postoperative hospital stays (days), and lower risk of postoperative complications (WMD = −0.78, 95% CI: −1.15, −0.41, P < 0.01; WMD = −0.90, 95% CI: −1.44, −0.35, P < 0.01; RR = 0.77, 95% CI: 0.62, 0.96, P = 0.02, respectively; Table 2). However, combined results from RCTs showed that no significant difference in those outcomes was found between single- and the triple-VATS lobectomy (P > 0.05, Table 2). Results from combined RCTs and one cohort study showed that single-port VATS was better at reducing postoperative pain scores than triple-port VATS, especially in the first day and third day (first day for RCTs: WMD = −0.99, 95% CI: −1.25, −0.74, P < 0.01; first day for cohort: WMD = −0.28, 95% CI: −0.51, −0.05, P = 0.02; third day for RCTs: WMD = −0.90, 95% CI: −1.52, −0.28, P < 0.01, respectively; Table 2).
Table 2.
Summary of perioperative outcomes between single- and the triple-VATS lobectomy based on different study designs
| Perioperative parameters | RCTs | Cohort studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Participants | MD (95% CI) | P | I2 (%) | Studies | Participants | MD (95% CI) | P | I2 (%) | |
| Operation time (min) | 3 | 394 | 7.07 (−20.40, 34.55) | 0.61 | 82 | 10 | 1,884 | 1.22 (−7.72, 10.16) | 0.79 | 82 |
| Intraoperative blood loss (mL) | 3 | 394 | −17.24 (−45.54, 11.05) | 0.23 | 90 | 9 | 1,513 | −3.33 (−10.55, 3.89) | 0.37 | 72 |
| Chest tube duration (days) | 3 | 394 | 0.50 (−0.74, 1.74) | 0.43 | 86 | 10 | 1,884 | −0.78 (−1.15, −0.41) | <0.01 | 71 |
| 2* | 146 | −0.54 (−3.48, 2.40) | 0.72 | 71 | ||||||
| Postoperative hospital stays (days) | 3 | 394 | 0.31 (−2.80, 3.42) | 0.84 | 96 | 9 | 1,513 | −0.90 (−1.44, −0.35) | <0.01 | 73 |
| 2* | 146 | −0.04 (−3.51, 3.43) | 0.98 | 74 | ||||||
| Lymph node dissection number | 2 | 332 | −0.32 (−1.15, 0.50) | 0.44 | 24 | 10 | 1,884 | 0.10 (−0.10, 0.29) | 0.32 | 13 |
| Postoperative drainage volume (mL) | 1 | 156 | −11.33 (−24.50, 1.84) | 0.09 | — | 2 | 428 | −147.21 (−339.25, 44.83) | 0.13 | 78 |
| Postoperative pain score (first day) | 3 | 394 | −0.99 (−1.25, −0.74) | <0.01 | 0 | 1 | 266 | −0.28 (−0.51, −0.05) | 0.02 | — |
| Postoperative pain score (third day) | 3 | 394 | −0.90 (−1.52, −0.28) | <0.01 | 84 | — | — | — | — | — |
| Studies | Participants | RR (95% CI) | P | I2 (%) | Studies | Participants | RR (95% CI) | P | I 2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Postoperative complications | 3 | 394 | 1.11 (0.73, 1.70) | 0.62 | 32 | 8 | 1,318 | 0.77 (0.62, 0.96) | 0.02 | 0 |
| 1* | 94 | 0.80 (0.23, 2.80) | 0.73 | — | ||||||
| Pulmonary leakage | 2 | 332 | 1.17 (0.24, 5.70) | 0.85 | 0 | 9 | 1,513 | 0.82 (0.55, 1.21) | 0.31 | 0 |
| Pulmonary infection | 1 | 156 | 0.22 (0.01, 4.54) | 0.33 | — | 8 | 1,351 | 0.76 (0.48, 1.19) | 0.23 | 0 |
| Atelectasis | 2 | 332 | 0.77 (0.22, 2.67) | 0.68 | 0 | 6 | 1,208 | 0.63 (0.37, 1.09) | 0.10 | 0 |
| Arrhythmia | 2 | 332 | 0.76 (0.32, 1.79) | 0.53 | 0 | 6 | 1,112 | 0.91 (0.57, 1.44) | 0.68 | 0 |
| Chylothorax | — | — | — | — | — | 4 | 914 | 0.75 (0.21, 2.72) | 0.66 | 0 |
Abbreviation: RCTs, randomized controlled trials; MD, mean difference; CI: confidence interval; RR, risk ratio.
*: Only included cohort studies with propensity-matched analysis.
Figure 2.
Forest plots of operation time for single- and triple-VATS lobectomy based on different research designs. For each study, the estimated OR (shown as square) and its 95% CI (shown as horizontal line) were plotted. The pooled OR and 95% CI were plotted as black diamond.
Figure 6.
Forest plots of postoperative complications for single- and triple-VATS. For each study, the estimated OR (shown as square) and its 95% CI (shown as horizontal line) were plotted. The pooled OR and 95% CI were plotted as black diamond.
Figure 3.
Forest plots of intraoperative blood loss for single- and triple-VATS lobectomy. For each study, the estimated OR (shown as square) and its 95% CI (shown as horizontal line) were plotted. The pooled OR and 95% CI were plotted as black diamond.
Figure 4.
Forest plots of chest tube duration for single- and triple-VATS lobectomy. For each study, the estimated OR (shown as square) and its 95% CI (shown as horizontal line) were plotted. The pooled OR and 95% CI were plotted as black diamond.
Figure 5.
Forest plots of postoperative hospital stays for single- and triple-VATS lobectomy. For each study, the estimated OR (shown as square) and its 95% CI (shown as horizontal line) were plotted. The pooled OR and 95% CI were plotted as black diamond.
3.4. Sensitivity analysis
Sensitivity analysis was performed on three outcomes with statistical differences between RCTs and cohort studies, namely, chest tube duration, postoperative hospital stays, and postoperative complications. When only propensity-matched cohort studies were included, the combined results showed no difference between the single- and triple-VATS lobectomy groups (chest tube duration: WMD = −0.54, 95% CI: −3.48, 2.40, P = 0.72; postoperative hospital stays: WMD = −0.04, 95% CI: −3.51, 3.43, P = 0.98; postoperative complications: RR = 0.80, 95% CI: 0.23, 2.80, P = 0.73; Table 2). The results were consistent with those of RCTs. These results showed that the pooled estimates were statistically significant in the sensitivity analysis.
3.5. Subgroup and meta-regression analysis
Subgroup and meta-regression analysis were performed to explore the potential source of heterogeneity in cohort studies where more than 10 studies were included. The results of subgroup analysis were summarized in Table 3. Further results from the univariate meta-regression analysis based on three grouping factors (location, sample size, and quality score) showed that there was no significant correlation between any of the covariates and the three perioperative outcomes (P > 0.05, Table 3).
Table 3.
Subgroup and meta-regression analysis in cohort studies included
| Perioperative parameters | Variables | Classification | Subgroup analysis | Meta-regression | |||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Participants | MD (95% CI) | P | I2 (%) | t | P | |||
| Operation time (min) | Location | China | 8 | 1,461 | 1.01 (−9.15, 11.16) | 0.85 | 85 | −0.13 | 0.902 |
| Korea | 2 | 423 | −1.39 (−14.01, 11.23) | 0.83 | 48 | ||||
| Sample size | ≥200 | 4 | 1,203 | −4.23 (−17.78, 9.33) | 0.54 | 86 | −1.00 | 0.345 | |
| <200 | 6 | 681 | 5.80 (−10.09, 21.70) | 0.47 | 83 | ||||
| Quality score | ≥8 | 5 | 770 | 9.22 (−8.76, 27.20) | 0.31 | 82 | 1.50 | 0.172 | |
| <8 | 5 | 1,114 | −5.09 (−16.67, 6.49) | 0.39 | 86 | ||||
| Chest tube duration (days) | Location | China | 8 | 1,461 | −0.61 (−0.93, −0.30) | <0.01 | 55 | 1.90 | 0.093 |
| Korea | 2 | 423 | −1.52 (−1.95, −1.08) | <0.01 | 0 | ||||
| Sample size | ≥200 | 4 | 1,203 | −0.90 (−1.39, −0.41) | <0.01 | 79 | −0.53 | 0.612 | |
| <200 | 6 | 681 | −0.69 (−1.33, −0.06) | 0.03 | 67 | ||||
| Quality score | ≥8 | 5 | 770 | −0.86 (−1.80, 0.08) | 0.07 | 83 | −0.08 | 0.941 | |
| <8 | 5 | 1,114 | −0.62 (−0.82, −0.42) | <0.01 | 6 | ||||
| Lymph node dissection number | Location | China | 8 | 1,461 | 0.10 (−0.10, 0.29) | 0.33 | 32 | 0.01 | 0.993 |
| Korea | 2 | 423 | 0.06 (−1.81, 1.92) | 0.95 | 0 | ||||
| Sample size | ≥200 | 4 | 1,203 | −0.00 (−0.22, 0.21) | 0.99 | 38 | −1.54 | 0.162 | |
| <200 | 6 | 681 | 0.45 (0.04, 0.87) | 0.03 | 0 | ||||
| Quality score | ≥8 | 5 | 770 | 0.45 (−0.49, 1.39) | 0.35 | 0 | 0.66 | 0.527 | |
| <8 | 5 | 1,114 | 0.02 (−0.37, 0.42) | 0.91 | 50 | ||||
Abbreviation: MD, mean difference; CI: confidence interval.
3.6. Publication bias analysis
Publication bias was examined only for three outcomes, namely, operation time, chest tube duration, and lymph node dissection number in cohort studies. The distribution of dots on the funnel plot was not significantly asymmetric (data not shown) and the results of Egger test were not significant (P = 0.287, P = 0.483, and P = 0.772 in operation time, chest tube duration, and lymph node dissection number, respectively), indicating the absence of publication bias in the present meta-analysis.
4. Discussion
This systematic review compared the perioperative parameters between single- and triple-port thoracoscopic lobectomy in lung cancer treatment. The combined results including 3 RCTs and 10 cohort studies showed that there was no statistical difference in the perioperative parameters of the two surgical methods, except postoperative pain score. Our results are consistent with the major findings of previous original studies with limited sample size [13,14,18,19,20,21,22,23,24,25,26,27,28]. Single-port VATS lobectomy is only performed in a limited number of hospitals in several countries due to its technical difficulty [18]. Our meta-analysis suggests that there was no obvious difference between single-port and triple-port thoracoscopic lobectomy, which laid the foundation for further promotion of single-port VATS lobectomy in the world.
Compared to conventional surgery, triple-port VATS thoracoscopic surgery has many advantages, such as less intraoperative blood lost, less pain, shorter duration of hospitalization, more rapid postoperative recovery [29], and long-term survival similar to that of conventional open surgery [30]. It is now generally accepted that the outcome of a VATS procedure is at least not inferior to a resection via a traditional thoracotomy [8]. Single-port VATS is a less invasive approach that allows major thoracic operations to be performed through a single small incision of about 4 cm, which can further reduce wounds and achieve the same effect as triple-port VATS thoracoscopic surgery [14]. Although our present study showed that there was no difference in postoperative indicators, combined results from RCTs and one cohort study both indicated that single-port VATS could relieve postoperative pain better than triple-port VATS, especially in the first day and fifth day. This may be one of the advantages of single-port VATS. Furthermore, the included RCTs also showed that single-port VATS could reduce trauma during surgery, reduce stress response, facilitate the recovery of postoperative quality of life, shorten the incision length, and improve scar appearance [13,27]. All these indicate that single-port VATS lobectomy is a feasible and safe option for lung cancer patients and should be popularized with its merits of minimal invasiveness.
The present meta-analysis included both RCTs and cohort studies with inconsistent results in chest tube duration, postoperative hospital stays, and postoperative complications. Although the results of well-designed observational studies (such as cohort study) do not systematically overestimate the magnitude of the effects of treatment as compared with those in RCTs, confounding is still a typical hazard of observational clinical research [31,32]. Propensity score methodology is a common approach to control confounding in nonexperimental studies of treatment effects via matching, stratification, regression adjustment, or any combination of these strategies [33]. When only propensity-matched cohort studies were included, there was no difference between the combined results from the RCTs and those from the cohort studies, further indicating that no difference was found in perioperative parameters between single-port and triple-port thoracoscopic lobectomy for lung cancer treatment. Nevertheless, when cohort studies are conducted in the future, various potential confounding biases should be controlled as much as possible.
There were several limitations of this systematic review and meta-analysis, as follows: (1) This study only analyzed perioperative parameters and failed to analyze long-term efficacy parameters. Even if we did not use the outcomes as a search term, few literatures with long-term outcome were found in literature screening. Future studies should clarify these questions when including single-VATS as a standard in thoracic procedures [20]. (2) Although the necessary data were not available, subgroup and meta-regression analyses were performed in three factors to explore the source of heterogeneity in cohort studies. We did not find any valuable heterogeneity factors. More extensive exploration may be needed, especially the differences in lung cancer subtypes. (3) Although extensive search strategies were used in present study, only Korean and Chinese studies were included at last. On the one hand, it may be related to the incomplete search of the database; on the other hand, it may be related to the epidemiologic distribution of lung cancer. Because China is one of the countries with a high incidence of lung cancer [3], there are a large number of cases for clinical study. We did not search the Chinese database because the quality of articles in Chinese journals has aroused concern in Chinese society [34]. Moreover, the methodological quality of those articles is often poor. There is also a selective publication bias in favor of positive results [35,36].
This systematic review and meta-analysis also showed some significant advantages. Most importantly, compared with published systematic review and meta-analysis, literature retrieval in this study was more comprehensive, and both RCTs and cohort studies were included with larger number of literatures. Therefore, the evidence was more reliable and scientific. Second, more adequate outcome indicators were analyzed in this meta-analysis. Thus, the stability of our research results was demonstrated. Finally, this systematic review and meta-analysis were performed following the PRISMA statement strictly, and the content was comprehensive.
In conclusion, the present systematic review and meta-analysis review comprehensively compare the perioperative parameters between single-port and triple-port thoracoscopic lobectomy for lung cancer treatment. Based on the combined results, single-port VATS lobectomy is a feasible option for lobectomy in lung cancer and may yield similar perioperative results to those of triple-port VATS lobectomy, and the former is more effective in relieving postoperative pain.
Footnotes
Funding information: This study was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (grant no 2017D01C388).
Author contributions: Y.G. and D.L.: designed the study; Y.G., A.A., D.H., and A.R.: collected and assembled the data; G.Y., A.A., and D.L.: analyzed and interpreted the data; Y.G. and D.L.: wrote the manuscript.
Conflict of interest: The authors declare no conflicts of interest.
Data availability statement: All data and models used during the study are provided in the article [and in supplementary information files].
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancer in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed]; Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancer in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- [2].Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1):8. [DOI] [PMC free article] [PubMed]; Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1):8. doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. [DOI] [PMC free article] [PubMed]; Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. doi: 10.1111/1759-7714.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856(2):189–210. [DOI] [PMC free article] [PubMed]; Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856(2):189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu CC, Wang BY, Shih CS, Pennarun N, Lim LC, Gao SY, et al. Comparison of survival between lung cancer patients receiving single or multiple-incision thoracoscopic surgery. J Thorac Dis. 2018;10(2):930–40. [DOI] [PMC free article] [PubMed]; Liu CC, Wang BY, Shih CS, Pennarun N, Lim LC, Gao SY. et al. Comparison of survival between lung cancer patients receiving single or multiple-incision thoracoscopic surgery. J Thorac Dis. 2018;10(2):930–40. doi: 10.21037/jtd.2018.01.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ng CS, Wan S, Lee TW, Wan IYP, Arifi AA, Yim APC. Video-assisted thoracic surgery in spontaneous pneumothorax. Can Respir J. 2002;9(2):122–7. [DOI] [PubMed]; Ng CS, Wan S, Lee TW, Wan IYP, Arifi AA, Yim APC. Video-assisted thoracic surgery in spontaneous pneumothorax. Can Respir J. 2002;9(2):122–7. doi: 10.1155/2002/672953. [DOI] [PubMed] [Google Scholar]
- [7].Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. American college of chest physicians. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edn). Chest. 2007;132(3 suppl):234s–42s. [DOI] [PubMed]; Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. American college of chest physicians. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edn) Chest. 2007;132(3 suppl):234s–42s. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- [8].Dziedzic D, Orlowski T. The role of VATS in lung cancer surgery: current status and prospects for development. Minim Invasive Surg. 2015;2015:938430. [DOI] [PMC free article] [PubMed]; Dziedzic D, Orlowski T. The role of VATS in lung cancer surgery: current status and prospects for development. Minim Invasive Surg. 2015;2015:938430. doi: 10.1155/2015/938430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang X, Wang L, Zhang H, Li K, Gong X. Feasibility and application of single-hole video-assisted thoracoscope in pulmonary peripheral tumors. Oncol Lett. 2016;12(6):4957–60. [DOI] [PMC free article] [PubMed]; Wang X, Wang L, Zhang H, Li K, Gong X. Feasibility and application of single-hole video-assisted thoracoscope in pulmonary peripheral tumors. Oncol Lett. 2016;12(6):4957–60. doi: 10.3892/ol.2016.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ng CS, Gonzalez-Rivas D, D’Amico TA, Rocco G. Uniportal VATS-a new era in lung cancer surgery. J Thorac Dis. 2015;7(8):1489–91. [DOI] [PMC free article] [PubMed]; Ng CS, Gonzalez-Rivas D, D’Amico TA, Rocco G. Uniportal VATS-a new era in lung cancer surgery. J Thorac Dis. 2015;7(8):1489–91. doi: 10.3978/j.issn.2072-1439.2015.08.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gonzalez-Rivas D, de la Torre M, Fernandez R, Mosquera VX. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg. 2011;13(5):539–41. [DOI] [PubMed]; Gonzalez-Rivas D, de la Torre M, Fernandez R, Mosquera VX. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg. 2011;13(5):539–41. doi: 10.1510/icvts.2011.274746. [DOI] [PubMed] [Google Scholar]
- [12].Lin F, Zhang C, Zhang Q, Cheng K, Zhao Y. Uniportal video-assisted thoracoscopic lobectomy: an alternative surgical method for pulmonary carcinoma. Pak J Med Sci. 2016;32(5):1283–5. [DOI] [PMC free article] [PubMed]; Lin F, Zhang C, Zhang Q, Cheng K, Zhao Y. Uniportal video-assisted thoracoscopic lobectomy: an alternative surgical method for pulmonary carcinoma. Pak J Med Sci. 2016;32(5):1283–5. doi: 10.12669/pjms.325.10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ye Z, Zhang B, Chen Y, Lin J. Comparison of single utility port video-assisted thoracoscopic surgery (VATS) and three-port VATS for non-small cell lung cancer. Oncol Lett. 2019;18(2):1311–7. [DOI] [PMC free article] [PubMed]; Ye Z, Zhang B, Chen Y, Lin J. Comparison of single utility port video-assisted thoracoscopic surgery (VATS) and three-port VATS for non-small cell lung cancer. Oncol Lett. 2019;18(2):1311–7. doi: 10.3892/ol.2019.10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. 2017;17(1):75. [DOI] [PMC free article] [PubMed]; Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. 2017;17(1):75. doi: 10.1186/s12885-017-3069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [PMC free article] [PubMed]; Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. PRISMA Group . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed]; Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed]; Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- [18].Song KS, Park CK, Kim JB. Efficacy of single-port video-assisted thoracoscopic surgery lobectomy compared with triple-port VATS by propensity score matching. Korean J Thorac Cardiovasc Surg. 2017;50(5):339–45. [DOI] [PMC free article] [PubMed]; Song KS, Park CK, Kim JB. Efficacy of single-port video-assisted thoracoscopic surgery lobectomy compared with triple-port VATS by propensity score matching. Korean J Thorac Cardiovasc Surg. 2017;50(5):339–45. doi: 10.5090/kjtcs.2017.50.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang G, Xiong R, Wu H, Xu G, Li C, Sun X, et al. Short-term outcome of uniportal and three portal video-assisted thoracic surgery for patients with non-small cell lung cancer. Chin J Lung Cancer. 2018;21:896–901 [Chinese]. [DOI] [PMC free article] [PubMed]; Wang G, Xiong R, Wu H, Xu G, Li C, Sun X. et al. Short-term outcome of uniportal and three portal video-assisted thoracic surgery for patients with non-small cell lung cancer. Chin J Lung Cancer. 2018;21:896–901. doi: 10.3779/j.issn.1009-3419.2018.12.03. [Chinese]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han KN, Kim HK, Choi YH. Midterm outcomes of single port thoracoscopic surgery for major pulmonary resection. PLoS One. 2017;12(11):e0186857. [DOI] [PMC free article] [PubMed]; Han KN, Kim HK, Choi YH. Midterm outcomes of single port thoracoscopic surgery for major pulmonary resection. PLoS One. 2017;12(11):e0186857. doi: 10.1371/journal.pone.0186857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hao Z, Cai Y, Fu S, Zhang N, Fu X. Modular dissection of mediastinal lymphadenectomy in uniportal video-assisted thoracoscopic surgery for radical resection of lung cancer. Chin J Clin Thorac Cardiovasc Surg. 2017;24(07):527–32.; Hao Z, Cai Y, Fu S, Zhang N, Fu X. Modular dissection of mediastinal lymphadenectomy in uniportal video-assisted thoracoscopic surgery for radical resection of lung cancer. Chin J Clin Thorac Cardiovasc Surg. 2017;24(07):527–32. [Google Scholar]
- [22].Xu GW, Xiong R, Wu HR, Li CW, Xu SB, Xie MR. A prospective comparative study examing the impact of uniportal and three portal video-assisted thoracic surgery on short-term quality of life in lung cancer. Chin J Surg. 2018;56:452–7 [Chinese]. [DOI] [PubMed]; Xu GW, Xiong R, Wu HR, Li CW, Xu SB, Xie MR. A prospective comparative study examing the impact of uniportal and three portal video-assisted thoracic surgery on short-term quality of life in lung cancer. Chin J Surg. 2018;56:452–7. doi: 10.3760/cma.j.issn.0529-5815.2018.06.013. [Chinese]. [DOI] [PubMed] [Google Scholar]
- [23].Mu JW, Gao GS, Xue Q, Zhao J, Li N, Yang K, et al. A matched comparison study of uniportal versus triportal thoracoscopic lobectomy and sublobectomy for early-stage nonsmall cell lung cancer. Chin Med J. 2015;128(20):2731–5. [DOI] [PMC free article] [PubMed]; Mu JW, Gao GS, Xue Q, Zhao J, Li N, Yang K. et al. A matched comparison study of uniportal versus triportal thoracoscopic lobectomy and sublobectomy for early-stage nonsmall cell lung cancer. Chin Med J. 2015;128(20):2731–5. doi: 10.4103/0366-6999.167298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li C, Ma H, He J, Ni B, Xu C, Zhao J. Clinical analysis of thoracoscopic lobectomy in the treatment of peripheral lung cancer with single utility port. Chin J Lung Cancer. 2013;16:487–91 [Chinese]. [DOI] [PMC free article] [PubMed]; Li C, Ma H, He J, Ni B, Xu C, Zhao J. Clinical analysis of thoracoscopic lobectomy in the treatment of peripheral lung cancer with single utility port. Chin J Lung Cancer. 2013;16:487–91. doi: 10.3779/j.issn.1009-3419.2013.09.09. [Chinese]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu Y, Song X, Zhang W. Comparison between single-port and three-port video-assisted thoracoscopic lobectomy in the treatment of lung cancer. Chin J Minim Invas Surg. 2018;18(03):205–8.; Liu Y, Song X, Zhang W. Comparison between single-port and three-port video-assisted thoracoscopic lobectomy in the treatment of lung cancer. Chin J Minim Invas Surg. 2018;18(03):205–8. [Google Scholar]
- [26].Rao S, Huang Y, Ye L, Ruan W, Chen Y, Yang J. Wide exposure in uniportal video-assisted thoracoscopic surgery for radical resection of lung cancer. Chin J Clin Thorac Cardiovasc Surg. 2019;26(04):374–8.; Rao S, Huang Y, Ye L, Ruan W, Chen Y, Yang J. Wide exposure in uniportal video-assisted thoracoscopic surgery for radical resection of lung cancer. Chin J Clin Thorac Cardiovasc Surg. 2019;26(04):374–8. [Google Scholar]
- [27].Shi D, Xu R, Han Y, Shi W. Comparison of efficacy using single-port and three-port thoracoscopic lobectomy in patients with peripheral lung cancer. J China Med Univ. 2018;47(07):609–11, 616.; Shi D, Xu R, Han Y, Shi W. Comparison of efficacy using single-port and three-port thoracoscopic lobectomy in patients with peripheral lung cancer. J China Med Univ. 2018;47(07):609–11, 616. [Google Scholar]
- [28].Zhu Y, Liang M, Wu M, Zheng J, Zheng W, Guo Z, et al. Preliminary results of single-port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med. 2015;3(7):92. [DOI] [PMC free article] [PubMed]; Zhu Y, Liang M, Wu M, Zheng J, Zheng W, Guo Z. et al. Preliminary results of single-port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med. 2015;3(7):92. doi: 10.3978/j.issn.2305-5839.2015.03.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol. 2013;39(9):957–63. [DOI] [PubMed]; Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol. 2013;39(9):957–63. doi: 10.1016/j.ejso.2013.06.016. [DOI] [PubMed] [Google Scholar]
- [30].Yamamoto K, Ohsumi A, Kojima F, Imanishi N, Matsuoka K, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89(2):353–9. [DOI] [PubMed]; Yamamoto K, Ohsumi A, Kojima F, Imanishi N, Matsuoka K. et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89(2):353–9. doi: 10.1016/j.athoracsur.2009.10.034. [DOI] [PubMed] [Google Scholar]
- [31].Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–92. [DOI] [PMC free article] [PubMed]; Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–92. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meuli L, Dick F. Understanding confounding in observational studies. Eur J Vasc Endovasc Surg. 2018;55(5):737. [DOI] [PubMed]; Meuli L, Dick F. Understanding confounding in observational studies. Eur J Vasc Endovasc Surg. 2018;55(5):737. doi: 10.1016/j.ejvs.2018.02.028. [DOI] [PubMed] [Google Scholar]
- [33].Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed]; Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fung ICH. Chinese journals: a guide for epidemiologists. Emerg Themes Epidemiol. 2008;5:20. [DOI] [PMC free article] [PubMed]; Fung ICH. Chinese journals: a guide for epidemiologists. Emerg Themes Epidemiol. 2008;5:20. doi: 10.1186/1742-7622-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tang JL, Hackshaw A, Lao LX, Liu BY, Chung VCH. Improving Res efficacy, effectiveness, harms traditional Chin Med. 2014;2014:657679. [DOI] [PMC free article] [PubMed]; Tang JL, Hackshaw A, Lao LX, Liu BY, Chung VCH. Improving Res efficacy, effectiveness, harms traditional Chin Med. 2014;2014:657679. doi: 10.1155/2014/657679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen Y, Wang C, Shang H, Yang K, Norris SL. Clinical practive guidelines in China. BMJ. 2018;360:j5158. [DOI] [PMC free article] [PubMed]; Chen Y, Wang C, Shang H, Yang K, Norris SL. Clinical practive guidelines in China. BMJ. 2018;360:j5158. doi: 10.1136/bmj.j5158. [DOI] [PMC free article] [PubMed] [Google Scholar]