Abstract
Simple Summary
Abiotic and biotic stresses are a major challenge for agricultural production. To deal with stressed conditions, many techniques, including the use of nanoparticles (NPs), could be considered to mitigate the adversities mediated by these stresses. The application of silicon (Si) and Si-NPs has emerged as a common agronomic technique as it is regarded as a sustainable option. Because of their innumerable benefits, the usage of Si and Si-NPs has attracted a great deal of interest. As a result, their application has been found to minimize the detrimental effects of various stressors by modifying morpho-physiological indices in plants and rhizospheric microbiome characteristics.
Abstract
Silicon (Si) is considered a non-essential element similar to cadmium, arsenic, lead, etc., for plants, yet Si is beneficial to plant growth, so it is also referred to as a quasi-essential element (similar to aluminum, cobalt, sodium and selenium). An element is considered quasi-essential if it is not required by plants but its absence results in significant negative consequences or anomalies in plant growth, reproduction and development. Si is reported to reduce the negative impacts of different stresses in plants. The significant accumulation of Si on the plant tissue surface is primarily responsible for these positive influences in plants, such as increasing antioxidant activity while reducing soil pollutant absorption. Because of these advantageous properties, the application of Si-based nanoparticles (Si-NPs) in agricultural and food production has received a great deal of interest. Furthermore, conventional Si fertilizers are reported to have low bioavailability; therefore, the development and implementation of nano-Si fertilizers with high bioavailability could be crucial for viable agricultural production. Thus, in this context, the objectives of this review are to summarize the effects of both Si and Si-NPs on soil microbes, soil properties, plant growth and various plant pathogens and diseases. Si-NPs and Si are reported to change the microbial colonies and biomass, could influence rhizospheric microbes and biomass content and are able to improve soil fertility.
Keywords: silicon, Si-NPs, rhizosphere, microbes, soil properties, abiotic stressors
1. Introduction
Silicon (Si) is not regarded as a necessary element for plants; however, some recent studies reported this element to be beneficial for plant growth. Si is one of the most abundant elements in the Earth’s crust and around 70% of soil mass is made up of Si [1,2,3]. Exposure to Si imparts uncountable beneficial effects on various plants, especially in gramineous and cyperaceous plants [4,5]. In addition, it could alleviate the detrimental consequences of biotic and abiotic stresses that directly or indirectly increase the plants’ resistance to external adversities. For example, it promotes the elongation of roots and alleviates salt stress by reducing NaCl accumulation [2,6]. Si plays a crucial role in several physiological and metabolic processes in plants [7]. For example, in a study, positive effects (enhanced seed germination and chlorophyll content) of Si-based nanoparticles (Si-NPs) on Zea mays were observed [8]. Exogenous treatment with Si-NPs reduced salt stress in Glycine max by increasing the antioxidant activities, K+ concentration and non-enzymatic components, and decreasing lipid peroxidation, reactive oxygen species (ROS) generation and Na+ intracellular concentration [9].
Recently, Si-NPs have been documented as a novel Si source that can be used to enhance plant resistance under unfavorable environmental conditions. However, the shape, size and other characteristics of Si-NPs are reported to impact directly or indirectly the responses of plants to Si-NP application [10]. Regarding the efficacy of Si-NPs, it is observed that the soil-applied were more effective than foliar-applied Si-NPs [11]. Si-NP treatment improved the growth and oil content in Cymbopogon citratus [12]. It enhanced the growth of Avena sativa and led to lignification in plant tissues [13]. It was reported that a nano-silica fertilizer improved the leaf area index, net assimilation rate, relative growth rate and yield of G. max [14]. Seed priming and seed soaking of Helianthus annuus in Si-NPs improved seedlings’ shoot and root length, biomass and vigor index [15].
Seed germination and seedling growth of Agropyron elongatum have been found to be improved by silicon dioxide nanoparticles (SiO2-NPs) [16]. Nano-SiO2-based fertilizers are determined to be beneficial for crops as they minimize fertilizer loss such as nitrogen and phosphorus by controlled release [17]. The application of SiO2-NPs could improve the photosynthetic pigments and increase the photosynthetic rate [2,3,18]. It also improved seed germination in Solanum lycopersicum; the net photosynthetic rate, photochemical efficiency, photosystem II (PSII) activity, electron transport rate, carbonic anhydrase activity, photochemical quenching, stomatal conductance and transpiration rate in Indocalamus barbatus and Cucurbita pepo [19,20]; and it also increased the growth, chlorophyll and carotenoid contents of Solanum tuberosum tubers [21]. Plants absorb Si in the form of mono-silicic acid and it accumulates in different tissues [18,22], and its deposition may occur in the leaf, stem and vascular tissues [1,23] and cuticles of plants [24]. In plants, there are Si transporters in the cell membrane (low silicon; Lsi1, Lsi2) and these transporters function as influx and efflux transporters [1,25,26].
After a review of the literature, some authors observed that Si is beneficial in controlling a variety of plant diseases by triggering the host defense system [27,28]. The effects of nano-silica (i.e., synthesized using O. sativa husk) and conventional Si on the bacterial population and seed germination of Z. mays, as well as the soil properties, have been evaluated [29]. Soil treated with sodium silicate hindered the colonization of plant-growth-promoting rhizobacteria, whereas the application of nano-silica enhanced the bacterial population. As a result, Si could boost plant resistance to bacteria, fungus, nematodes and viruses [30]. Si is reported to modulate the signaling systems that normalize the expression of defense genes related to proteins, the structural modification of cell walls, antimicrobial compound synthesis, hypersensitivity responses and hormone synthesis [31]. It was found that Si stimulated resistance in Solanum lycopersicum against Ralstonia solanacearum by upregulating defense gene expression [32]. The application of Si changed 26 proteins in S. lycopersicum inoculated with R. solanacearum, and it also changed the protein level in the host plants [33]. It was also noticed that the application of Si reduced the infection of Magnaporthe oryzae in O. sativa [34]. The application of SiO2-NPs was observed in the management of a Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani-mediated disease complex in Beta vulgaris L.; thus, its potential to reduce disease severity has been revealed [30]. Therefore, these research outcomes have shown that Si could reduce pathogen invasion in plants [35].
Therefore, this review article aims to assess the impacts of Si and Si-NPs on soil microbes, soil properties and their effect on plant growth and diseases.
2. Source of Si and Si-NPs and Their Uptake
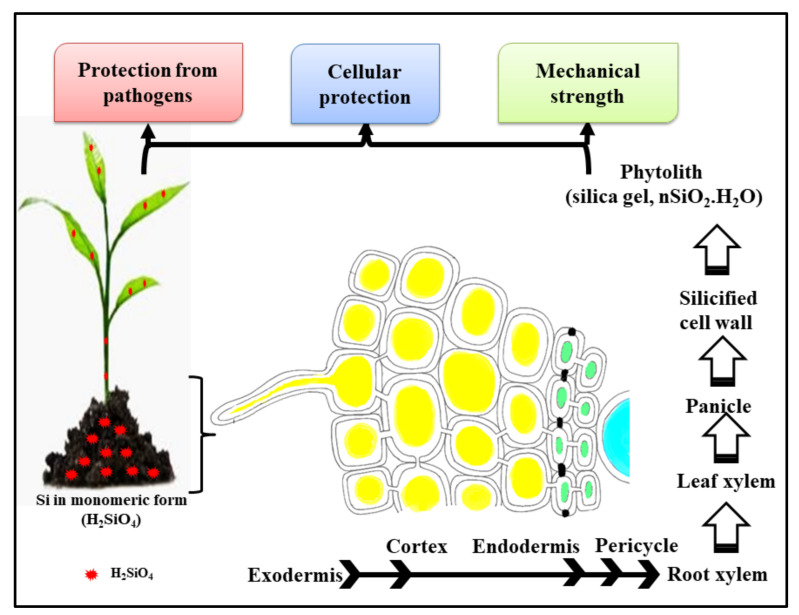
Si is an element with a Van der Waals radius of 210 pm that exists as different forms in the environment, while Si nanoparticles are synthesized particles that are smaller than 100 nm with special properties. The unique characteristics of Si nanoparticles have made them effective reagents in agricultural applications. Unlike bulk silicon, a very dull material, ultrasmall silicon nanoparticles are extremely efficient at ameliorating soil properties [36]. Si could be found in the soil solution in a variety of forms, including monomeric (H4SiO4; monosilicic acid), oligomeric and polysilicic acid. The monomeric form is readily bioavailable for plants [25]. Plants are recorded to accumulate Si up to a significant amount and earlier studies revealed that there are three different modes of Si uptake, namely active, passive and rejective [37]. In the active mode, plants absorb Si at a faster rate than water, resulting in a lower Si concentration in the uptake solution; yet, in passive mode, Si absorption by plants is similar to water uptake. Thus, in passive uptake, there are no discernible changes in the concentration of Si in the uptake solution. Plants that uptake using a rejective mode prefer to exclude Si, as seen by the increasing concentration of Si in the uptake solution [38]. However, there is still great room for further investigation to depict the mechanisms involved in the different uptake modes of Si.
In the roots, after the uptake of Si-NPs, their transport to other aerial parts is reported by three routes viz., cell wall pores, the apoplastic pathway and the symplastic pathway (Figure 1). The Si-NPs are recorded to travel either intracellularly or extracellularly before they enter the xylem, according to the existing literature [39]. It was noted that the critical value of soil-available Si content for O. sativa is 300 mg SiO2 kg−1.
Figure 1.
Diagrammatic presentation of silicon transport in plants.
Based on Si uptake accumulation, plants are categorized as high, intermediate and low Si accumulators. It is assumed that in high Si accumulators, the amount of H4SiO4 taken up by active mechanisms is greater than concentrations taken up by mass flow due to the high density of Si transporters in roots and shoots expediting H4SiO4 movement through root cell membranes (Figure 1) [37,40]. The transport of Si is a multi-step process and, from roots to shoots, silicic acid crosses the plasma membrane at biological pH. The first Si transporter (Lsi1) was discovered in O. sativa and belongs to the Nod26-like major intrinsic protein subfamily [41]. According to the findings of previous studies, the site of Si uptake is in the mature regions of the roots rather than the root tips due to the higher expression of Lsi1 genes than the apical region. Further, the expression of Lsi1 in rice at various growth stages was found to be transiently increased around the heading stage. It has also been shown that the maximal amount of Si was taken up during the reproductive stage from panicle initiation to heading in O. sativa [42].
As Lsi1 are responsible for the influx of silicic acid from external media to cells, similarly, for the efflux of Si, there are Lsi2 transporters. The mechanism of Si transport mediated by Lsi2 is an energy-dependent active process, i.e., led by the proton gradient. Lsi2 is expressed in the roots, similarly to Lsi1 [43]. Thus, the expression of Lsi1 and Lsi2 is reported to be regulated in a similar manner. Both Lsi1 and Lsi2 are localized at the exodermis and the endodermis cells of the roots. The main difference is in their localization; Lsi1 is localized on the distal side, while Lsi2 is localized on the proximal side of the exodermis and the endodermis cells [44]. Si influx has also been identified in other plants, e.g., Z. mays (ZmLsi1) and Hordeum vulgare (HvLsi1), whilst very few efflux transporters in other plants have been identified so far [45].
In the soil solution, certain dissolved silicon acid forms organic and inorganic compound complexes. Silcretes are a form of derived soil that contains a significant amount of Si. In petrocalcic horizons, the Si amount is much smaller than in silcrete (8%), while it is significantly lower in minerals of certain heavily weathered oxisols such as bauxites and ferricretes [46]. Most of the soils are rich in Si; however, some soils are poor, especially the plant-available type of Si [47]. The oxisols and ultisols are heavily weathered, leached, acidic and also display poor base saturation [48]. Meanwhile, the histosols possess a great deal of organic matter and very low mineral content [49]. Furthermore, soils with a high proportion of quartz sand and those that have been subjected to long-term crop productivity have low plant-available Si [46,50].
Among these, silica is listed as one of the crystalline types of Si in the solid phase fraction [51]. The primary and secondary crystalline silicates, which are abundant in mineral soils formed from rocks and sediments, were previously the only crystalline types [52]. Quartz and disordered silica make up the majority of silica products. The Si fractions in the solid phase also include amorphous, poorly crystalline and microcrystalline shapes [52,53]. The liquid and adsorbed phases of Si are identical, with the exception that the liquid phase components are dissolved in the soil solution, while the adsorbed phase components are retained on soil particles as well as on Fe and Al oxides/hydroxides.
Si content and its abundance in soils are closely dependent on processes of soil formation and consequently on the soil type. Except for organic soils (histosols), most mineral soils are made up of sands (mostly SiO2), different forms of primary crystalline (e.g., olivine, augite, hornblende, quartz, feldspars-orthoclase, plagioclase, albite, and mica) and secondary minerals of silicate including clay minerals such as illite, vermiculite, montmorillonite, chlorite and kaolinite. These silicate compounds are, in most cases, not very soluble and are biogeochemically inert. In soil, polymerized silicic acid is only partly water-soluble, while H4SiO4 is the water-soluble type of Si. Water-soluble Si can be adsorbed onto the surfaces of inorganic, organic and organic–inorganic complexes in soil [25,47,52,54].
3. Impacts of Si-NPs on Soil Properties
Natural nano-sized materials found in soil include Si, Al, K, Na, Ca, Fe, Ba, Sr, Rb, as well as silicates, carbonates, sulphates, oxides, hydroxides and phosphates [55]. The soil properties viz., soil texture, pH, soil salinity (EC), soil organic matter, cation exchange capacity (CEC), etc., control the fate and behavior of any element in the soil rhizosphere [56]. Moreover, the rhizosphere itself can also regulate the movement of the elements. Thus, cultivated plants also might impact the uptake of the element in the soil, even when the elements are in nano-form (Table 1).
Table 1.
The impacts of soil properties on cultivated plants and their links to applied nano-silica.
| Details of Si-NPs | Soil Properties | Main Effect | Reference | ||||
|---|---|---|---|---|---|---|---|
| Nano-Si Dose and Type of Preparation | Size (nm) | Texture | pH | SOM (g kg−1) |
EC (dS m−1) |
||
| Chemical nano-SiO2 (1.5 mM mg Si L−1) | 20–35 | Clay loam | 7.84 | 10.3 | 1.03 | Improves growth and oil yield of coriander under drought stress | [62] |
| Chemical nano-SiO2 fertilizer (2%) | 80–90 | Silty clay | 7.99 | 9.2 | 1.1 | Records the best yield of wheat under water deficit | [63] |
| Chemical nano-SiO2 (60 mg L−1) |
20 | Conditioned soil (data not available) | Mitigates stress in rice by enhancing antioxidant system | [64] | |||
| Chemical chitosan-Si-nano-fertilizer (from 0.01 to 0.16%, w/v) | 100 | Clay | 8.20 | 13.78 | 0.56 | Enhances maize growth and yield by inducing antioxidant defense system | [65] |
| Chemical thiol functionalized nano-SiO2 (4%) | 20 | Silt loam | 7.93 | 13.78 | NA | Remediates polluted soil from heavy metals and improves growth of lettuce | [66] |
| Biological Si-NPs (2.5 and 5.0 mmol L−1) | 38.78 | Loamy | 7.30 | 6.88 | 7.81 | Promotes common bean under saline and polluted soil with Pb, Ni and Cd | [67] |
| Chemical SiO2-NPs (0.75, 1.5 and 2.25 mM) | 10–20 | Sandy loam | 7.10 | NA | 1.20 | Mitigates Cd stress by improving antioxidants and growth of summer savory | [68] |
| Chemical Si-NPs (100–200 mg kg−1) |
8.3 | Clay loam | 6.60 | 7.0 | 0.70 | The 200 mg kg−1 nano-Si + PGPB recorded highest level of Si-soluble and exchangeable fractions under water deficit stress | [69] |
| Chemical SiO2-NPs (150–2000 mg kg−1) | 10 | Commercial soil | 7.35 | NA | NA | Treatment of 500 mg kg−1 lowered the content of As and Cd to 70 and 50% under the water regimes in rice shoots | [70] |
| Chemical SiO2-NPs (at 2 mM) | 30 | Clay loam | 7.40 | 2.80 | 1.7 | Improves maize yield under applied nano-Si in combined with Zn nutrient | [71] |
| Surface-modified nano-silica (3.0%) | 18.0 | NA | 7.61 | 16.9 | NA | Immobilizes bioavailable As, Pb, Cd, by 85, 97.1, 80.1%, res. in polluted soil | [72] |
| Chemical nano-Si complex with glycine, glutamine, histidine | 10–40 | Silty loam | 7.02 | NA | 0.62 | Nano-Si enhances growth of feverfew under drought stress at foliar 1.5 or 3 mM | [73] |
| Chemical Si-NPs (at 1 and 2 mM) | 20 | Loamy | 8.08 | 8.0 | 1.11 | Improves antioxidants to protect sugar beet plants from water deficiency stress | [74] |
| Chemical mercapto-functionalized nano-silica (0.2 to 0.1%) | 20–30 | NA | 8.12 | 19.6 | NA | Increases wheat grain yield by 33.5% and soil dehydrogenase by 43.4% under Cd stress | [75] |
| Chemical nano-SiO2 (500 mg kg−1) | NA | Sandy loam | 7.67 | 2.54 | NA | Enhances remediation by Erigeron annuus L. grown in polluted soil by PAH (150 mg kg−1) | [76] |
Abbreviations: PGPB: plant-growth-promoting bacteria (Pseudomonas19 sp.); NA: not available; PAH: phenanthrene.
Three main types of Si in the solid state in the soils are amorphous (i.e., poorly crystalline), microcrystalline and crystalline. The crystalline types of Si are primarily used as silicates and silica materials (primary and secondary), and they account for the majority of Si in the solid phase. The primary mineral-bearing silicates inherent in soils are contained in sand and silt particles, while the secondary silicates are found in clay particles formed by pedogenic processes involving phyllosilicates and Al-Fe oxides/hydroxides [52]. The Si exists as poorly crystalline and microcrystalline types, such as short-range ordered silicates, chalcedony and secondary quartz [52].
Amorphous forms are biogenic and lithogenic and are available at quantities of up to 30 mg g−1 total soil. The biogenic types, which consist of plant residues and the remains of microorganisms, are called biogenic opals. These biogenic opals are formed when the soluble Si in the soil is supersaturated [57]. Plants accumulate Si as phytoliths in their leaves, culms and stems, while microorganisms contribute as microbial and protozoic Si [52,58]. The solubility of various Si types in the solid stage significantly affects the concentration of their soil solution. The solubility of minerals containing silica depends on the density and range of the silica tetrahedrals [57,59]. Further, amorphous silica is anticipated to contribute to higher solubility than quartz. However, quartz is extremely stable and thermodynamically resistant to weathering; its solubility ranges from 0.10 to 0.25 mM Si [57]. Thus, if the quartz is abundant in residual compounds, then its contribution to Si in soil solution is negligible [60].
The solubility of both amorphous and crystalline silica is documented to be nearly constant at around pH 2.0 and 8.5. However, their solubility quickly increases at pH 9.0 as the concentration of H4SiO4 decreases in the soil solution due to the dissociation of H4SiO4 into H3SiO4− and H+ at pH 9.0 [61]. This allows the crystalline and amorphous silica to dissolve in order to replenish or buffer the decreased concentration of H4SiO4 in the soil solution [47]. The plant-available forms of Si present in soil range from 10 to 100 mg kg−1. In soil, less than 20 mg kg−1 Si is considered a Si deficit and the amendment of Si is recommended [54].
In addition, Si-NPs can inhibit the leaching and movement of heavy metals in soil. For instance, it was confirmed that the application of Si-NPs subsequently improves the stable concentrations of Cu, Zn and Ti oxides [77]. To provide detailed insights, in Table 1, we summarize the research outcomes of recent studies that demonstrate the influences of soil properties in cultivated plants and their association with the application of Si-NPs.
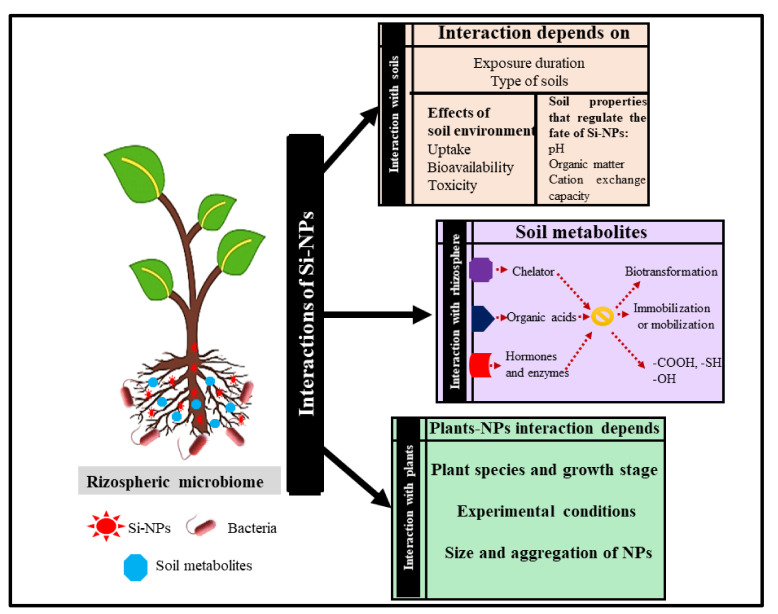
4. Effects of Si-NPs on the Rhizospheric Microbiome
Soil is a reservoir of water and nutrients for plants and is, therefore, indispensable for the plants’ normal functional processes. The surrounding area of the roots of plants is known as the rhizosphere. In the rhizosphere complex, biotic and abiotic relations exist. Thus, the rhizospheric microbiome comprises many microbial organisms, including archaea, viruses, fungus, bacteria as well as eukaryotic microorganisms, which are directly linked to the plant roots in a compact region of soil. It is also documented that approximately up to 1011 microbial cells g−1 of the root are present in this rhizospheric unit, accounting for more than 30,000 prokaryotes [78]. Moreover, the rhizospheric component of the soil is characterized as a zone that is influenced by exudates and roots’ secretions, which are vital for plant growth and health along with the microbial community [79]. The release of a variety of soil metabolites viz., organic acids, inorganic acids, siderophores, sugars, vitamins, amino acids, purines, nucleosides, polysaccharide mucilage, etc., is reported by different researchers. Thus, this subset of soil microbial diversity is reported to be sensitive to numerous chemical substances, including NP application and other physicochemical changes in the rhizosphere that subsequently favor the selective enrichment of certain microbial communities over others [80].
The microflora present in this region tends to be pathogenic as well as beneficial [81]. The beneficial rhizospheric microorganisms play a pivotal role in the immobilization/cycling of nutrients along with the detoxification or degradation of pollutants, which results in improved soil health (Figure 2).
Figure 2.
Schematic representation of interactions of Si-NPs with rhizospheric microbiome.
The rhizosphere contains advantageous microorganisms such as phosphate-solubilizing bacteria (PSB) and nitrogen-fixing microbes [82]. These microbes are plant growth promoters and thus can exert modulatory impacts on the biological and chemical properties of soils. Silicate-solubilizing bacteria (SSB) are also present in soil and they could convert insoluble silicates into soluble Si and alleviate Si content in the soil. Thus, the rhizosphere plays a vital role in the maintenance of soil properties and plant health [83]. In this context, treatment with Si-NPs and conventional Si is found to enhance the microbial biomass in the soils and the availability of Si to plants. Further, Si-NPs play an important role in influencing microbial biota and soil nutrient content; hence, their application is recorded to promote the growth of crops [84]. In this study, the PSB population (3.8 × 104 CFU g−1) was observed to increase after Si-NP treatment, whilst there was no impact on the population of SSB. Likewise, in a study, it was reported that the application of Si-NPs had a significant impact on soil nutrient content and microbial biota and thus improved Z. mays growth [11].
Nitrogen-fixing bacteria have a high population among the Si amendment soil. The foliar application of SiO2-NPs increased the bacterial communities of Paenibacillus and Rhodobacteraceae [85], and also improved Chaetomium fungal genera in the rhizosphere. Moreover, in this study, the comparative profusion of the genus Paenibacillus in the phylum Firmicutes was approximately 16% higher in the soil with NPs than in the control. The genus of Paenibacillus includes plant growth-promoting bacteria, which encourage plant growth via different mechanisms, such as nutrient solubility, biological nitrogen-fixing, induction of systemic resistance and plant growth regulators and organic acid production [86]. These microbes are vital for the nitrogen and carbon cycles. Si- and SiO2-NP uptake and their impact on soil microbial colonies require a thorough and deep investigation. Thus, treatment with Si and Si-NPs can be beneficial due to their direct or indirect influences on the economic productivity of plants.
Soil metabolites are important intermediates in many soils’ productivity and fertility processes. Thus, shifts in the root exudate will affect the plants’ health and growth levels or composition by attenuating soil fertility. In this context, soil metabolomics provides a potential method for soil characterization and the evaluation of the soil microbial community’s metabolic status, as shown in a high-performance study on small molecular organic compounds [87]. In another study, it was indicated that the rhizosphere metabolite profile was significantly influenced following the foliar exposure of SiO2-NPs [85]. The considerable increase in the relative profusion of numerous metabolites, including sugar and sugar alcohol, fatty acids and small organic acids, confirmed the influences of NPs on carbon and nitrogen pools in the rhizosphere. Others noted the impact of some NPs such as Si-NPs and their results indicated that oversaturation of these NPs reduced dehydrogenase and urease activity as well as bacterial and archaeal amoA gene abundance in soil [81]; it was confirmed that a mixture of Cu, Ag and Si decreased C and N biomass and changed the microbial community structure in soil [88].
5. Effects of Si-NPs on Plant Growth and Development
Nanotechnology is reported as a cutting-edge technique that has been proven to be more efficient for phytoremediation along with its application in stress mitigation [2,89]. Si-NPs may improve crop yield by influencing nutrient availability in rhizospheric soil and absorption by the plants. Si-NPs improve the nutrient bioavailability in plants, thus acting as a primary reason for the increased plant growth following NP application [6,90]. Due to the importance of Si in plants, similar to other essential macronutrients, as an agricultural nutrient, scientists have focused on applying Si-NPs in the soil in order to improve plant growth. Moreover, Si deficiency has been linked to nutritional imbalances, resulting in poor growth [59].
In a recent study, it was found that Si-NP priming of different seeds viz., T. aestivum, Pisum sativum and Brassica improved the parameters of seed germination and seedling growth [91]. Several reports have revealed that Si-NPs act as a fertilizer [3,20,92]. In addition, treatment with Si-NPs reduced the negative effects of salt stress on vegetative growth and soil relative water content, resulting in a considerable improvement in plant height, fresh and dry weights, total yield and seed quality. The various indices related to Z. mays plants’ physiology and anatomy were considerably altered after exposure to Si-NPs at 20–40 nm with a high surface area.
The impact of Si-NPs on plants is affected by various factors, i.e., the size, shape, application phase and biomechanical and physical properties [10]. According to some reports, Si-NPs can communicate directly with plants, altering their morphological behavior and physiological activity in different ways [20]. Si-NPs, on the other hand, have been shown in numerous experiments to be harmful to plants [54,93]. Therefore, some important studies involving plants and Si-NPs are shown in Table 2 to provide a better understanding.
Table 2.
Role of silicon nanoparticles in plant growth and development.
| Structure of Si-NPs |
Crop | Concentration | Adaptive Mechanism | Reference |
|---|---|---|---|---|
| SiO2 (chemical) |
Saccharum officinarum L. | 300 ppm | Improves leaf photosynthetic responses, chlorophyll fluorescence yield, photosynthetic pigments and photosynthetic apparatus (PS II) during chilling stress | [94] |
| SiO2 (chemical) |
Glycine max L. | 100–2000 ppm | Si-NPs increase plant performance and reduce the uptake of Hg in epidermis and pericycle of roots and stems. They enhance photosynthetic content and antioxidant enzyme activities in soybean during exposure to mercury (Hg) | [95] |
| SiO2 (chemical) |
Hordeum vulgare L. | 125–250 ppm | Improves plant development, green pigments, photosynthetic activities, plant osmolyte and metabolite profiles, cellular damage and membrane stability indicators, and antioxidant enzymes are all affected. | [96] |
| Si-NPs (chemical) |
Oryza sativa L. | 1 mM | Enhances gene expression and transportation of cadmium to vacuoles. | [97] |
| SiO2 (commercial) |
Trigonella foenumL. | 0–2.5 mM | Increases nanoparticle translocation, accumulation, Si uptake, cell wall lignification and the formation of stress-related enzymes during metal toxicity (cadmium) | [98] |
| Si-NPs (chemical) |
Triticum aestivumL. | 10 µM | Mitigates negative effects of UV radiation on plants | [99] |
| Mesoporous Si-NPs (chemical) |
Triticum spp. L., Lupinus polyphyllusL. | 200–2000 ppm | Nanoparticles upregulate leaf gas exchange responses and growth development performance of plants | [100] |
| SiO2 (commercial) |
Pisum sativum L. | 10 µM | Protects plant seedlings and increases enzymatic activities | [101] |
Si-NPs can also act as a strengthening substance that is responsible for improving disease resistance by preventing fungal, bacterial and nematode infections. Si-NPs can also reduce the transpiration rate of the plant, rendering it more resistant to limited water supply (drought), high temperature and humidity [18,46,93,102]. Except for a few scientific papers indicating that Si-NPs have a negative impact on plant performance, most of the studies found Si-NPs to be beneficial or ineffectual for plants by either promoting plant growth or having no impact [7,11,102].
Plants produce naturally mineralized NPs for proper growth and development when subjected to different stresses [93]. The use of Si-NPs ensures better plant performance and yield during unfavorable environmental conditions. The high surface-to-volume ratio of Si-NPs is reported to increase their reactivity and biochemical activities, which is responsible for their favorable effects on plants [89,103].
6. Role of Si and Si-NPs in Abiotic Stress Tolerance in Plants
Drought, heat, salinity, heavy metals and salt contamination of soil are all critical environmental stressors that severely affect the productivity and quality of agricultural species around the world. Plants’ physiology, morphology and biochemistry are altered by abiotic stresses, resulting in reduced growth and economic output [18,93,104]. Thus, Si-NPs have been reported to serve indispensable roles by mitigating different abiotic stress-induced consequences [4,70,105,106]. For example, metal toxicity can be minimized and plant growth can be improved by the amendment of Si-rich materials in soils [18,93]. Si-NPs can significantly decrease the heavy metal content in plants. In a study, it was reported that the application of Si-NPs diminished the content of Pb in the different tissues of Brassica chinensis L. as compared to the control [85]. These results suggest that the exogenous application of Si-NPs can minimize heavy metal uptake in plants [97,107].
Cadmium is one of the most hazardous toxic metals on the planet [108]. It inhibits plant growth, photosynthesis and yield [2,109]. Si-NP treatment of Cd-stressed plants increased the plant growth and biomass [110]. Moreover, it was concluded that Si-NP treatment of the soil could promote plant growth indicators and photosynthesis while lowering Cd levels in plants’ tissues, particularly in grains that are or are not experiencing drought stress [30]. Moreover, Si-NPs lessened oxidative stress, as evidenced by decreased H2O2 generation, electrolyte leakage and malondialdehyde levels, as well as increasing superoxide dismutase and peroxidase activity. Further, Si treatment protects cell membranes from injury [111]. The application of Si-NPs reduced reactive oxygen species (ROS) levels and boosted antioxidative defense components in Cd-stressed plants [68].
The foliar application of Si-NPs significantly reduced the accumulation of Pb in the leaves of O. sativa [110]. Treatment with Si-NPs in soil was also used as a unique strategy to alleviate Al phytotoxicity in acidic soils, and a thorough view of the cellular and biochemical mechanisms behind this mitigation process was provided. Arsenic is a metalloid that is toxic to plants and adversely affects plant growth [112,113]. Si-NP treatment reduced As stress-mediated vulnerabilities in O. sativa [114]. Si-NPs were used to prevent damage and restore the photosynthetic mechanism. Si-NPs also enhanced the activities of antioxidant enzymes to counter ROS generation to reestablish cellular homeostasis [112].
Here, we consider the consequences of chromium stress-induced responses in plants. Generally, Cr is reported to accumulate in plants, causing changes in photosynthetic activity, nutrient uptake and plant development [115]. Si-NPs are reported to improve P. sativum seedlings’ growth under Cr stress. It was found that the application of Si-NPs to Cr-stressed plants ameliorated Cr-induced phytotoxicity symptoms, i.e., pigment content, chlorophyll fluorescence, proteins level and nutrient status, resulting in improved growth [112]. The reduction in Cr accumulation in plant organs is followed by an improvement in physiological indices. The ability of Si to upregulate the expression of osNAC proteins, which are responsible for the upregulation of genes involved in stress tolerance, proline synthesis, soluble sugar biosynthesis and redox homeostasis, could be a reflection of Si-NPs’ increased stress tolerance. The significant roles of Si-NPs in mitigating metal toxicity in maize (Zea mays L.) plants were recorded, which resulted in enhanced photosynthesis responses, reduced oxidative stress, i.e., ROS, H2O2 and malondialdehyde (MDA) content, and maintained antioxidative defense mechanisms. The application of Si-NPs decreased MDA content during metal toxicity and positively improved cell wall breadth in the epidermis of roots, while also downregulating metal ion absorption and the accumulation rate [99]. Si-NPs are easily absorbed by plants as compared to inorganic Si and protect against excess metal ion toxicity in crop plants [116].
Si-NPs significantly boost the germination and vigor index in Cucurbita plants during saline stress conditions [20]. Si is reported to balance the homeostasis of ions and mitigate abiotic stresses. Si-NPs provided a favorable environment for seed germination under salinity stress [19]. The enhancement of growth indices might reflect the photosynthetic functions of Si-NPs, which is needed for photosynthetic leaf gas exchange and the assimilation of nitrate [104,117,118]. However, Si-NPs have been implicated in the synthesis of proteins and amino acids and in nutrient uptake; the strength and rigidity of plants are improved through Si-NP deposition in different plant organs [43]. Si-NPs increased the morphological and photosynthetic traits of plants via enhancing organic compound production, i.e., proteins, pigments and phenols, relative to bulk particles [4,12]. Under unfavorable environmental conditions, plants synthesize compatible solutes, i.e., glycine betaine and proline, to maintain the osmotic potential within plant cells. The level of proline is enhanced with Si-NPs. Proline is a universal osmoprotectant, acts as an antioxidative and energy source [119] and regulates the expression of genes, leading to osmotic adjustment [19,120].
Si-NPs have a potential role in S. lycopersicum germination (%), time, index, vigor index, fresh and air-dried biomass of plants [20]. Under saline conditions, the amendment of nutrient media with Si-NPs enhanced the seed germination and seedling early growth of Lens culinaris [15]. Si-NPs decreased the effects of saline toxicity in Ocimum basilicum and enhanced the fresh and dry mass of plant organs, leaf chlorophyll index and proline level [46]. In another study, Si-NPs were used to enhance the photosynthetic capacity and mitigate the seed germination and plant growth inhibition caused by salinity in S. lycopersicum plants [121]. Salt-stressed genes, i.e., AREB, TAS14, NCED3 and CRK1, were found to increase their expression in S. lycopersicum subjected to saline conditions with the application of Si-NPs, while RBOH1, APX2, MAPK2, ERF5, MAPK3 and DDF2 genes were noted to be downregulated [121]. Si triggered modifications in plant cell metabolism, a decline in heavy metal uptake by roots and the exudation of specific chemicals such as organic acids and phenols [122,123].
In addition, plants can use Si-NPs as a carrier of important macronutrients (N, P and K) and as a slow-release Si supplement to help them cope with salt stress [124]. Thus, in this context, these materials can be considered growth-promoting agents. Si supplementation is considered to inhibit salinity in plants, so Si-NPs have been used to improve salinity tolerance in plants [124]. Moreover, for regulated release, NPK fertilizers and Si-NPs should be carried inside the core of controlled-release fertilizers, which can be coated with chitosan as the first semipermeable coating and sodium alginate and kaolin as the outermost superabsorbent coating [125]. These artificial beds could slowly disperse nutrients, allowing plants to hold enormous amounts of water, manage salinity and thrive in drought circumstances [124].
7. Role of Si and Si-NPs in Plant Biotic Stress Management
The application of Si decreases biotic stress severity in many plants. Si has the potential to help plants to avoid pathogen penetration by forming physical barriers and suppressing pathogen colonization by boosting systemic acquired resistance. Moreover, Si protects plants by strengthening the host plant’s cuticle and cell walls, as well as producing silicate papillae, which impede the spread of pathogenic structures. Si accumulation in the cell wall of the host plant to form a double layer of silica makes the penetration of pathogens difficult [126]. Si accumulation can lead to the deposition of a double layer beneath the cuticle, preventing pathogen penetration and lowering disease incidence.
Si may play an active prophylactic role in plants. Phenolic-like compounds were found at a high level in Si-treated plants upon infection with pathogens [127]. Si binds with pectins, polyphenols and hemicellulose in cell walls and improves the mechanical strength of the plant cell wall [112,128]. Si provides rigidity to the cell wall. Si treatment in rice induced resistance against pathogens and reduced blast disease [126]. In T. aestivum leaves, Si application inhibited the hyphae penetration of Pyricularia oryzae, while, in the absence of Si, fungal hyphae penetrated successfully [105]. Similarly, Si treatment in O. sativa reduced the infection and leaf lesions caused by Rhizoctonia solani and Pyricularia grisea [129,130]. Moreover, P. oryzae penetration in O. sativa tissues decreased after Si treatment and it was suggested that the presence of the Si layer helped in blocking or delaying pathogen penetration [131]. Si reduced sheath blight disease of O. sativa in treated plants [129]. After treatment with Si, the number of Podosphaera fuliginea colonies was reduced by 43–94% in Cucumis sativus [132]. Si treatment reduced the blast lesion length in O. sativa by 40–80% [130].
Si-NPs exhibit potent antibacterial properties against a variety of plant diseases and are thought to help in the regulation of soil N levels [133]. Si-NPs could hamper the growth of pathogenic fungus (Fusarium oxysporum f. sp. niveum) [134] and may reduce the growth of plant parasitic nematodes (Meloidogyne incognita), bacteria (Pectobacterium betavasculorum) and fungus (Rhizoctonia solani) [30]. In a study, Si-NP treatment improved the growth of P. sativum, but the highest growth was recorded when SiO2-NPs and N-fixing bacteria (Rhizobium leguminosarum) were applied together [135]. SiO2-NP treatment reduced the bacterial blight disease complex of P. sativum caused by a plant parasitic nematode (Meloidogyne incognita) and bacterium (Pseudomonas syringae pv. pisi). Detailed studies of the effects of Si and Si-NPs on plant biotic stress management, especially diseases, are listed in Table 3.
Table 3.
Effects of Si and Si-NPs on plant biotic stress management.
| Nanoparticle Type | Pathogen | Concentration | Effect | Reference |
|---|---|---|---|---|
| Si-NPs | Fusarium oxysporum f. sp. niveum | 100 mg L−1 | Enhances biomass and fruit yield in comparison to untreated plants | [134] |
| SiO2-NPs | Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani | 100, 200 mg L−1 | Si-NPs were most effective against test pathogens | [30] |
| SiO2-NPs | Xanthomonas campestris pv. carotae, Pectobacterium carotovorum and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci | 100 mg L−1 | Inhibits the growth of all tested pathogens | [23] |
| SiO2-Ag composites | Xanthomonas oryzae >pv. oryzae | 50, 100 and 200 μg mL−1 | Displays antibacterial activity against the tested pathogen | [136] |
| Si | Puccinia melanocephala | 400, 1200 mg L−1 | Reduces disease in sugarcane and induces resistance | [137] |
| Si-NPs | Fusarium oxysporum and Aspergillus niger | 5, 10, 15 kg ha−1 | Reduces the growth of pathogens | [138] |
| Si | Hemileia vastatrix | 0.24 and 0.30 mg kg−1 | Inhibits infection of fungus Hemileia vastatrix and urediniospore germination | [28] |
| Si | Colletotrichum sublineolum | 2 mmol L−1 | Reduces growth by around 20%, acervuli found smaller in size | [139] |
| Si | Podosphaera pannosa | 1 mg mL−1 | Reduces disease severity by 46% and induces phenolic acid formation | [123] |
| Si | Fusarium sulphureum | 100 and 200 mM | Decreases pathogen growth and reduces disease | [140] |
| SiO2 | Sclerosporagraminicola | 5, 10, 15 mM | Inhibits the growth of the fungal pathogen | [141] |
8. Molecular Mechanism of Si and Si-NP Uptake and Their Applications in Agriculture
In plants, variations in the accumulation of Si occur. In the bryophytes and pteridophytes, high Si accumulation occurs as compared to the angiosperms. In angiosperm families, the genera of Poaceae and Cyperaceae have been reported to accumulate high amounts of silicon, whilst the Urticaceae, Commelinaceae and Cucurbitaceae families have intermediate silicon accumulation [142,143]. Rice belongs to the Poaceae family and it can accumulate around 10% Si. Hodson et al. [143] performed a meta-analysis that recorded the following order of Si accumulation in various plant groups (from high concentrations to low): liverworts > horsetails > clubmosses > mosses > angiosperms > gymnosperms > ferns. Silicon uptake in plants is attributed to specific transporters.
Si is present in the soil and its uptake and transport in plants depend on the chemical composition and plant roots. Si is taken up by plant roots as monosilicic acid and transported to the shoots. Deposition of Si mostly occurs in leaf epidermal cells, in the outer epidermal cells of inflorescence bracts and in the root endodermis. Due to the transpiration, Si concentrates and polymerizes into colloidal silica gel (SiO2·nH2O). Uptake of Si in rice plants is faster, indicating the presence of Si transporters across the cell membrane [142]. To understand the molecular mechanism of Si uptake and transport in rice, a mutagenic approach has been used and it was found that the transporter gene Lsi1 plays an important role in Si transport. Shortly after the discovery of Lsi1, a second Si transporter gene, Lsi2, was discovered. Lsi2 has 9–12 transmembrane domains and it is an anion transporter coupled with a proton antiport. The discovery of these transporters has explained the molecular mechanism of Si uptake and transport in rice plants [1,2,142]. In Z. mays plants, Si uptake and deposition were mediated by ZmLsi1 and ZmLsi6 genes. ZmLsi1 is found to play a key role in the uptake of Si by roots. ZmLsi6 is located in the parenchyma cells of Z. mays leaves and it is responsible for Si unloading in xylem. Homologs of Lsi2 and HvLsi2 genes have also been reported in H. vulgare plants [144].
Si and Si-NPs have promising applications in agriculture. Si-NPs have a beneficial impact in the agriculture sector and they may improve yields, leading to increased productivity [145]. Moreover, additional applications of Si-NPs include pesticides, fertilizers and herbicides. Si-NPs could be used to develop NPs. Several studies have shown that nano-silica increases the efficiency and durability of pesticides. Si-NPs could be used for the target-specific delivery of fertilizers and herbicides. Mesoporous silica NPs with a pore size of 2–10 nm served as an efficient delivery vector for boron, urea and nitrogenous fertilizers. Li et al. [146] observed that Si-NPs increased the photostability and sustained the release of an avermectin pesticide.
9. Conclusions and Future Perspectives
The benefits of Si to a variety of crops have long been known, implying the importance of Si fertilization as a long-term option for viable agriculture. Agricultural lands with rigorous cultivation systems, particularly those that are naturally low in soluble Si soils, can be modified with Si-rich materials to ensure productivity. Recently, the use of Si in soil fertilization has emerged as a common agronomic practice in several parts of the world. Despite significant advances in Si research and soil science in the development and standardization of multiple processes for the extraction and quantification of various soil Si fractions, their use in soil fertility and nutrient management is limited. The soil interpretation test could be used to assess whether Si-based fertilization is necessary or not, but it does not provide the exact Si concentration required to increase Si availability to the optimal limit, nor does it indicate whether the affected plant will respond to Si fertilization. Thus, in the near future, soil science-based Si research studies are expected to make substantial progress in current soil Si knowledge and provide crop-producing fertilization recommendations.
Acknowledgments
The research was financially supported by the Ministry of Science and Higher Education of the Russian Federation (no. 0852-2020-0029).
Author Contributions
Conceptualization, V.D.R., T.M., M.F., A.K. and M.K.; methodology, V.D.R. and A.K.; software, V.D.R.; validation, V.D.R. and T.M.; formal analysis, V.D.R., T.M., M.F., A.K. M.K., S.M., S.S., H.E.-R., K.K.V. and A.S.; investigation, V.D.R., T.M., M.F., A.K. and M.K.; resources, V.D.R., T.M. and S.M.; data curation, V.D.R., M.F., A.K., M.K., R.C., R.K.S. and H.S.J.; writing—original draft preparation, V.D.R., T.M., M.F., A.K., M.K., H.E.-R., A.K. and K.K.V.; writing—review and editing, V.D.R., T.M., M.F., A.K., M.K., A.K., S.M., S.S. and E.D.v.H.; visualization, V.D.R. and T.M.; supervision, V.D.R. and T.M.; project administration, V.D.R. and T.M.; funding acquisition, V.D.R. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
Not Applicable. This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ma J.F., Yamaji N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant. Sci. 2006;11:392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui H., Ahmed K.B.M., Sami F., Hayat S. Silicon nanoparticles and plants: Current knowledge and future perspectives. In: Hayat S., Pichtel J., Faizan M., Fariduddin Q., editors. Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development. Springer International Publishing; Cham, Switzerland: 2020. pp. 129–142. [Google Scholar]
- 3.Verma K.K., Song X.-P., Li D.-M., Singh M., Rajput V.D., Malviya M.K., Minkina T., Singh R.K., Singh P., Li Y.-R. Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change. Plants. 2020;9:1055. doi: 10.3390/plants9091055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma K.K., Song X.-P., Verma C.L., Chen Z.-L., Rajput V.D., Wu K.-C., Liao F., Chen G.-L., Li Y.-R. Functional Relationship Between Photosynthetic Leaf Gas Exchange in Response to Silicon Application and Water Stress Mitigation in Sugarcane. Biol. Res. 2021;54:15. doi: 10.1186/s40659-021-00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang Y., Wong J.W., Wei L. Silicon-mediated Enhancement of Cadmium Tolerance in Maize (Zea mays L.) Grown in Cadmium Contaminated Soil. Chemosphere. 2005;58:475–483. doi: 10.1016/j.chemosphere.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Liu P., Yin L., Wang S., Zhang M., Deng X., Zhang S., Tanaka K. Enhanced Root Hydraulic Conductance by Aquaporin Regulation Accounts for Silicon Alleviated Salt-Induced Osmotic Stress in Sorghum Bicolor L. Environ. Exp. Bot. 2015;111:42–51. doi: 10.1016/j.envexpbot.2014.10.006. [DOI] [Google Scholar]
- 7.Bao-Shan L., Shao-Qi D., Chun-Hui L., Li-Jun F., Shu-Chun Q., Min Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004;15:138–140. doi: 10.1007/BF02856749. [DOI] [Google Scholar]
- 8.Yuvakkumar R., Elango V., Rajendran V., Kannan N.S., Prabu P. Influence of Nanosilica Powder on the Growth of Maize Crop (Zea Mays L.) Int. J. Green Nanotechnol. 2011;3:180–190. doi: 10.1080/19430892.2011.628581. [DOI] [Google Scholar]
- 9.Farhangi-Abriz S., Torabian S. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma. 2018;255:953–962. doi: 10.1007/s00709-017-1202-0. [DOI] [PubMed] [Google Scholar]
- 10.Rastogi A., Zivcak M., Sytar O., Kalaji H.M., He X., Mbarki S., Brestic M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017;5 doi: 10.3389/fchem.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suriyaprabha R., Karunakaran G., Yuvakkumar R., Rajendran V., Kannan N. Foliar Application of Silica Nanoparticles on the Phytochemical Responses of Maize (Zea mays L.) and Its Toxicological Behavior. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2014;44:1128–1131. doi: 10.1080/15533174.2013.799197. [DOI] [Google Scholar]
- 12.Mukarram M., Khan M.M.A., Corpas F.J. Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J. Hazard. Mater. 2021;412:125254. doi: 10.1016/j.jhazmat.2021.125254. [DOI] [PubMed] [Google Scholar]
- 13.Asgari F., Majd A., Jonoubi P., Najafi F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.) Plant Physiol. Biochem. 2018;127:152–160. doi: 10.1016/j.plaphy.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Suciaty T., Purnomo D., Sakya A.T., Supriyadi The effect of nano-silica fertilizer concentration and rice hull ash doses on soybean (Glycine max (L.) Merrill) growth and yield. IOP Conf. Ser. Earth Environ. Sci. 2018;129:012009. doi: 10.1088/1755-1315/129/1/012009. [DOI] [Google Scholar]
- 15.Janmohammadi M., Sabaghnia N. Effect of Pre-Sowing Seed Treatments with Silicon Nanoparticles on Germinability of Sunflower (Helianthus Annuus) Botanica. 2015;21:13–21. doi: 10.1515/botlit-2015-0002. [DOI] [Google Scholar]
- 16.Azimi R., Borzelabad M.J., Feizi H., Azimi A. Interaction of SiO Nanoparticles with Seed Prechilling on Germination and Early Seedling Growth of Tall Wheatgrass (Agropyron Elongatum L.) Pol. J. Chem. Technol. 2014;16:25–29. doi: 10.2478/pjct-2014-0045. [DOI] [Google Scholar]
- 17.Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 18.Verma K.K., Song X.-P., Lin B., Guo D.-J., Singh M., Rajput V.D., Singh R.K., Singh P., Sharma A., Malviya M.K., et al. Silicon Induced Drought Tolerance in Crop Plants: Physiological Adaptation Strategies. Silicon. 2021 doi: 10.1007/s12633-021-01071-x. [DOI] [Google Scholar]
- 19.Siddiqui M.H., Al-Whaibi M.H., Faisal M., Al Sahli A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014;33:2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui M.H., Al-Whaibi M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.) Saudi J. Biol. Sci. 2014;21:13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mushinskiy A.A., Aminova E.V., Korotkova A.M. Evaluation of tolerance of tubers Solanum tuberosum to silica nanoparticles. Environ. Sci. Pollut. Res. Int. 2018;25:34559–34569. doi: 10.1007/s11356-018-3268-4. [DOI] [PubMed] [Google Scholar]
- 22.Sahebi M., Hanafi M.M., Siti Nor Akmar A., Rafii M.Y., Azizi P., Tengoua F.F., Nurul Mayzaitul Azwa J., Shabanimofrad M. Importance of silicon and mechanisms of biosilica formation in plants. Biomed. Res. Int. 2015;2015:396010. doi: 10.1155/2015/396010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui Z.A., Hashmi A., Khan M.R., Parveen A. Management of bacteria Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci with silicon dioxide nanoparticles on carrot. Int. J. Veg. Sci. 2020;26:547–557. doi: 10.1080/19315260.2019.1675843. [DOI] [Google Scholar]
- 24.Gattullo C.E., Allegretta I., Medici L., Fijan R., Pii Y., Cesco S., Mimmo T., Terzano R. Silicon dynamics in the rhizosphere: Connections with iron mobilization. J. Plant Nutr. Soil Sci. 2016;179:409–417. doi: 10.1002/jpln.201500535. [DOI] [Google Scholar]
- 25.Frew A., Weston L.A., Reynolds O.L., Gurr G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018;121:1265–1273. doi: 10.1093/aob/mcy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J.F., Yamaji N., Mitani N., Xu X.Y., Su Y.H., McGrath S.P., Zhao F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye M., Song Y., Long J., Wang R., Baerson S.R., Pan Z., Zhu-Salzman K., Xie J., Cai K., Luo S., et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA. 2013;110:E3631–E3639. doi: 10.1073/pnas.1305848110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carré-Missio V., Rodrigues F.A., Schurt D.A., Resende R.S., Souza N.F.A., Rezende D.C., Moreira W.R., Zambolim L. Effect of foliar-applied potassium silicate on coffee leaf infection by Hemileia vastatrix. Ann. Appl. Biol. 2014;164:396–403. doi: 10.1111/aab.12109. [DOI] [Google Scholar]
- 29.Karunakaran G., Suriyaprabha R., Manivasakan P., Yuvakkumar R., Rajendran V., Prabu P., Kannan N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013;7:70–77. doi: 10.1049/iet-nbt.2012.0048. [DOI] [PubMed] [Google Scholar]
- 30.Khan M.R., Siddiqui Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.) Sci. Hortic. 2020;265:109211. doi: 10.1016/j.scienta.2020.109211. [DOI] [Google Scholar]
- 31.Wang M., Gao L., Dong S., Sun Y., Shen Q., Guo S. Role of Silicon on Plant–Pathogen Interactions. Front. Plant. Sci. 2017;8 doi: 10.3389/fpls.2017.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghareeb H., Bozsó Z., Ott P.G., Repenning C., Stahl F., Wydra K. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011;75:83–89. doi: 10.1016/j.pmpp.2010.11.004. [DOI] [Google Scholar]
- 33.Chen Y., Liu M., Wang L., Lin W., Fan X., Cai K. Proteomic characterization of silicon-mediated resistance against Ralstonia solanacearum in tomato. Plant Soil. 2015;387:425–440. doi: 10.1007/s11104-014-2293-4. [DOI] [Google Scholar]
- 34.Brunings A.M., Datnoff L.E., Ma J.F., Mitani N., Nagamura Y., Rathinasabapathi B., Kirst M. Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann. Appl. Biol. 2009;155:161–170. doi: 10.1111/j.1744-7348.2009.00347.x. [DOI] [Google Scholar]
- 35.Chain F., Côté-Beaulieu C., Belzile F., Menzies J.G., Bélanger R.R. A Comprehensive Transcriptomic Analysis of the Effect of Silicon on Wheat Plants Under Control and Pathogen Stress Conditions. Mol. Plant Microbe Interact. 2009;22:1323–1330. doi: 10.1094/MPMI-22-11-1323. [DOI] [PubMed] [Google Scholar]
- 36.Nayfeh M.H., Mitas L. Chapter one—Silicon nanoparticles: New Photonic and electronic material at the transition between solid and molecule. In: Kumar V., editor. Nanosilicon. Elsevier; Amsterdam, The Netherlands: 2008. pp. 1–78. [Google Scholar]
- 37.Kaur H., Greger M. A Review on Si Uptake and Transport System. Plants. 2019;8:81. doi: 10.3390/plants8040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Ramady H., Alshaal T., Elhawat N., El-Nahrawy E., Omara A.E.-D., El-Nahrawy S., Elsakhawy T., Ghazi A., Abdalla N., Fári M. Biological aspects of selenium and silicon nanoparticles in the terrestrial environments. In: Ansari A.A., Gill S.S., Gill R., Lanza G., Newman L., editors. Phytoremediation: Management of Environmental Contaminants. Volume 6. Springer International Publishing; Cham, Switzerland: 2018. pp. 235–264. [Google Scholar]
- 39.Rajput V.D., Minkina T.M., Behal A., Sushkova S.N., Mandzhieva S., Singh R., Gorovtsov A., Tsitsuashvili V.S., Purvis W.O., Ghazaryan K.A., et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018;9:76–84. doi: 10.1016/j.enmm.2017.12.006. [DOI] [Google Scholar]
- 40.Ma J., Miyake Y., Takahashi E. Chapter 2 Silicon as a beneficial element for crop plants. Stud. Plant Sci. 2001;8:17–39. [Google Scholar]
- 41.Jadhao K.R., Bansal A., Rout G.R. Silicon amendment induces synergistic plant defense mechanism against pink stem borer (Sesamia inferens Walker.) in finger millet (Eleusine coracana Gaertn.) Sci. Rep. 2020;10:4229. doi: 10.1038/s41598-020-61182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma J.F., Yamaji N., Tamai K., Mitani N. Genotypic Difference in Silicon Uptake and Expression of Silicon Transporter Genes in Rice. Plant. Physiol. 2007;145:919–924. doi: 10.1104/pp.107.107599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma J.F., Yamaji N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. CMLS. 2008;65:3049–3057. doi: 10.1007/s00018-008-7580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaji N., Sakurai G., Mitani-Ueno N., Ma J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA. 2015;112:11401–11406. doi: 10.1073/pnas.1508987112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitani N., Yamaji N., Ma J.F. Identification of maize silicon influx transporters. Plant. Cell Physiol. 2009;50:5–12. doi: 10.1093/pcp/pcn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rastogi A., Tripathi D.K., Yadav S., Chauhan D.K., Živčák M., Ghorbanpour M., El-Sheery N.I., Brestic M. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019;9:90. doi: 10.1007/s13205-019-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coskun D., Deshmukh R., Sonah H., Menzies J.G., Reynolds O., Ma J.F., Kronzucker H.J., Bélanger R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019;221:67–85. doi: 10.1111/nph.15343. [DOI] [PubMed] [Google Scholar]
- 48.Foy C.D. Soil chemical factors limiting plant root growth. In: Hatfield J.L., Stewart B.A., editors. Limitations to Plant Root Growth. Springer; New York, NY, USA: 1992. pp. 97–149. [Google Scholar]
- 49.Snyder G.H., Jones D.B., Gascho G.J. Silicon Fertilization of Rice on Everglades Histosols. Soil Sci. Soc. Am. J. 1986;50:1259–1263. doi: 10.2136/sssaj1986.03615995005000050035x. [DOI] [Google Scholar]
- 50.Datnoff L.E., Deren C.W., Snyder G.H. Silicon fertilization for disease management of rice in Florida. Crop. Prot. 1997;16:525–531. doi: 10.1016/S0261-2194(97)00033-1. [DOI] [Google Scholar]
- 51.Sauer D., Burghardt W. The occurrence and distribution of various forms of silica and zeolites in soils developed from wastes of iron production. CATENA. 2006;65:247–257. doi: 10.1016/j.catena.2005.11.017. [DOI] [Google Scholar]
- 52.Farooq M.A., Dietz K.-J. Silicon as Versatile Player in Plant and Human Biology: Overlooked and Poorly Understood. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savvas D., Ntatsi G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015;196:66–81. doi: 10.1016/j.scienta.2015.09.010. [DOI] [Google Scholar]
- 54.Zargar S.M., Mahajan R., Bhat J.A., Nazir M., Deshmukh R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech. 2019;9:73. doi: 10.1007/s13205-019-1613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buzea C., Pacheco I. Nanomaterials and their classification. In: Shukla A.K., editor. EMR/ESR/EPR Spectroscopy for Characterization of Nanomaterials. Springer India; New Delhi, India: 2017. pp. 3–45. [Google Scholar]
- 56.Jilling A., Keiluweit M., Contosta A.R., Frey S., Schimel J., Schnecker J., Smith R.G., Tiemann L., Grandy A.S. Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry. 2018;139:103–122. doi: 10.1007/s10533-018-0459-5. [DOI] [Google Scholar]
- 57.Richard Drees L., Wilding L.P., Smeck N.E., Senkayi A.L. Minerals in Soil Environments. Soil Science Society of America, Inc.; Madison, WI, USA: 1989. Silica in soils: Quartz and disordered silica polymorphs; pp. 913–974. [Google Scholar]
- 58.Sommer M., Kaczorek D., Kuzyakov Y., Breuer J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006;169:310–329. doi: 10.1002/jpln.200521981. [DOI] [Google Scholar]
- 59.Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- 60.Fraysse F., Pokrovsky O.S., Schott J., Meunier J.-D. Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim. Cosmochim. Acta. 2006;70:1939–1951. doi: 10.1016/j.gca.2005.12.025. [DOI] [Google Scholar]
- 61.Tubaña B.S., Heckman J.R. Silicon in soils and plants. In: Rodrigues F.A., Datnoff L.E., editors. Silicon and Plant Diseases. Springer International Publishing; Cham, Switzerland: 2015. pp. 7–51. [Google Scholar]
- 62.Afshari M., Pazoki A., Sadeghipour O. Foliar-applied Silicon and its Nanoparticles Stimulate Physio-chemical Changes to Improve Growth, Yield and Active Constituents of Coriander (Coriandrum Sativum L.) Essential Oil Under Different Irrigation Regimes. Silicon. 2021 doi: 10.1007/s12633-021-01101-8. [DOI] [Google Scholar]
- 63.Ahmadian K., Jalilian J., Pirzad A. Nano-fertilizers improved drought tolerance in wheat under deficit irrigation. Agric. Water Manag. 2021;244:106544. doi: 10.1016/j.agwat.2020.106544. [DOI] [Google Scholar]
- 64.Banerjee A., Singh A., Sudarshan M., Roychoudhury A. Silicon nanoparticle-pulsing mitigates fluoride stress in rice by fine-tuning the ionomic and metabolomic balance and refining agronomic traits. Chemosphere. 2021;262:127826. doi: 10.1016/j.chemosphere.2020.127826. [DOI] [PubMed] [Google Scholar]
- 65.Kumaraswamy R.V., Saharan V., Kumari S., Chandra Choudhary R., Pal A., Sharma S.S., Rakshit S., Raliya R., Biswas P. Chitosan-silicon nanofertilizer to enhance plant growth and yield in maize (Zea mays L.) Plant Physiol. Biochem. 2021;159:53–66. doi: 10.1016/j.plaphy.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 66.Lian M., Wang L., Feng Q., Niu L., Zhao Z., Wang P., Song C., Li X., Zhang Z. Thiol-functionalized nano-silica for in-situ remediation of Pb, Cd, Cu contaminated soils and improving soil environment. Environ. Pollut. Barking Essex 1987. 2021;280:116879. doi: 10.1016/j.envpol.2021.116879. [DOI] [PubMed] [Google Scholar]
- 67.El-Saadony M.T., Desoky E.-S.M., Saad A.M., Eid R.S.M., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Farajzadeh Memari-Tabrizi E., Yousefpour-Dokhanieh A., Babashpour-Asl M. Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.) Plant Physiol. Biochem. 2021;165:71–79. doi: 10.1016/j.plaphy.2021.04.040. [DOI] [PubMed] [Google Scholar]
- 69.Valizadeh-rad K., Motesharezadeh B., Alikhani H.A., Jalali M. Direct and Residual Effects of Water Deficit Stress, Different Sources of Silicon and Plant-Growth Promoting Bacteria on Silicon Fractions in the Soil. Silicon. 2021 doi: 10.1007/s12633-021-01120-5. [DOI] [Google Scholar]
- 70.Wang X., Jiang J., Dou F., Sun W., Ma X. Simultaneous mitigation of arsenic and cadmium accumulation in rice (Oryza sativa L.) seedlings by silicon oxide nanoparticles under different water management schemes. Paddy Water Environ. 2021 doi: 10.1007/s10333-021-00855-6. [DOI] [Google Scholar]
- 71.Asadpour S., Madani H., Mohammadi G.N., Heravan I.M., Abad H.H.S. Improving Maize Yield with Advancing Planting Time and Nano-Silicon Foliar Spray Alone or Combined with Zinc. Silicon. 2020 doi: 10.1007/s12633-020-00815-5. [DOI] [Google Scholar]
- 72.Cao P., Qiu K., Zou X., Lian M., Liu P., Niu L., Yu L., Li X., Zhang Z. Mercapto propyltrimethoxysilane- and ferrous sulfate-modified nano-silica for immobilization of lead and cadmium as well as arsenic in heavy metal-contaminated soil. Environ. Pollut. Barking Essex 1987. 2020;266:115152. doi: 10.1016/j.envpol.2020.115152. [DOI] [PubMed] [Google Scholar]
- 73.Esmaili S., Tavallali V., Amiri B. Nano-Silicon Complexes Enhance Growth, Yield, Water Relations and Mineral Composition in Tanacetum parthenium under Water Deficit Stress. Silicon. 2020 doi: 10.1007/s12633-020-00605-z. [DOI] [Google Scholar]
- 74.Namjoyan S. Nano-silicon protects sugar beet plants against water deficit stress by improving the antioxidant systems and compatible solutes. Acta Physiol. Plant. 2020;42:157. doi: 10.1007/s11738-020-03137-6. [DOI] [Google Scholar]
- 75.Wang Y., Liu Y., Zhan W., Zheng K., Lian M., Zhang C., Ruan X., Li T. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 2020;197:110600. doi: 10.1016/j.ecoenv.2020.110600. [DOI] [PubMed] [Google Scholar]
- 76.Zuo R., Liu H., Xi Y., Gu Y., Ren D., Yuan X., Huang Y. Nano-SiO2 combined with a surfactant enhanced phenanthrene phytoremediation by Erigeron annuus (L.) Pers. Environ. Sci. Pollut. Res. Int. 2020;27:20538–20544. doi: 10.1007/s11356-020-08552-3. [DOI] [PubMed] [Google Scholar]
- 77.Oliveira E.M.D., Garcia-Rojas E.E., Valadão I.C.R.P., Araújo A.D.S.F., Castro J.A.D. Effects of the silica nanoparticles (NPSiO2) on the stabilization and transport of hazardous nanoparticle suspensions into landfill soil columns. REM Int. Eng. J. 2017;70:317–323. doi: 10.1590/0370-44672015700204. [DOI] [Google Scholar]
- 78.Berendsen R.L., Pieterse C.M.J., Bakker P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Santos L.F., Olivares F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021;26:100198. doi: 10.1016/j.cpb.2021.100198. [DOI] [Google Scholar]
- 80.Brolsma K.M., Vonk J.A., Mommer L., Van Ruijven J., Hoffland E., De Goede R.G.M. Microbial catabolic diversity in and beyond the rhizosphere of plant species and plant genotypes. Pedobiologia. 2017;61:43–49. doi: 10.1016/j.pedobi.2017.01.006. [DOI] [Google Scholar]
- 81.McGee C.F., Storey S., Clipson N., Doyle E. Soil microbial community responses to contamination with silver, aluminium oxide and silicon dioxide nanoparticles. Ecotoxicology. 2017;26:449–458. doi: 10.1007/s10646-017-1776-5. [DOI] [PubMed] [Google Scholar]
- 82.Kour D., Kaur T., Devi R., Yadav A., Singh M., Joshi D., Singh J., Suyal D.C., Kumar A., Rajput V.D., et al. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: Present status and future challenges. Environ. Sci. Pollut. Res. 2021;28:24917–24939. doi: 10.1007/s11356-021-13252-7. [DOI] [PubMed] [Google Scholar]
- 83.Barea J.M., Pozo M.J., Azcón R., Azcón-Aguilar C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005;56:1761–1778. doi: 10.1093/jxb/eri197. [DOI] [PubMed] [Google Scholar]
- 84.Theng B.K.G., Yuan G. Nanoparticles in the Soil Environment. Elements. 2008;4:395–399. doi: 10.2113/gselements.4.6.395. [DOI] [Google Scholar]
- 85.Tian L., Shen J., Sun G., Wang B., Ji R., Zhao L. Foliar Application of SiO2 Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2020;54:13137–13146. doi: 10.1021/acs.est.0c03767. [DOI] [PubMed] [Google Scholar]
- 86.Liu X., Li Q., Li Y., Guan G., Chen S. Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ. 2019;7:e7445. doi: 10.7717/peerj.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Withers E., Hill P.W., Chadwick D.R., Jones D.L. Use of untargeted metabolomics for assessing soil quality and microbial function. Soil Biol. Biochem. 2020;143:107758. doi: 10.1016/j.soilbio.2020.107758. [DOI] [Google Scholar]
- 88.Kumar N., Shah V., Walker V.K. Influence of a nanoparticle mixture on an arctic soil community. Environ. Toxicol. Chem. 2012;31:131–135. doi: 10.1002/etc.721. [DOI] [PubMed] [Google Scholar]
- 89.Khan M., Khan A.U., Hasan M.A., Yadav K.K., Pinto M.M.C., Malik N., Yadav V.K., Khan A.H., Islam S., Sharma G.K. Agro-Nanotechnology as an Emerging Field: A Novel Sustainable Approach for Improving Plant Growth by Reducing Biotic Stress. Appl. Sci. 2021;11 doi: 10.3390/app11052282. [DOI] [Google Scholar]
- 90.Liu R., Lal R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015;514:131–139. doi: 10.1016/j.scitotenv.2015.01.104. [DOI] [PubMed] [Google Scholar]
- 91.Chourasiya V., Nehra A., Shukla P., Singh K., Singh P. Impact of Mesoporous Nano-Silica (SiO2) on Seed Germination and Seedling Growth of Wheat, Pea and Mustard Seed. J. Nanosci. Nanotechnol. 2021 doi: 10.1166/jnn.2021.19013. [DOI] [PubMed] [Google Scholar]
- 92.Verma K.K., Song X.-P., Zeng Y., Guo D.-J., Singh M., Rajput V.D., Malviya M.K., Wei K.-J., Sharma A., Li D.-P., et al. Foliar application of silicon boosts growth, photosynthetic leaf gas exchange, antioxidative response and resistance to limited water irrigation in sugarcane (Saccharum officinarum L.) Plant Physiol. Biochem. 2021;166:582–592. doi: 10.1016/j.plaphy.2021.06.032. [DOI] [PubMed] [Google Scholar]
- 93.Rajput V.D., Minkina T., Kumari A., Harish, Singh V.K., Verma K.K., Mandzhieva S., Sushkova S., Srivastava S., Keswani C. Coping with the Challenges of Abiotic Stress in Plants: New Dimensions in the Field Application of Nanoparticles. Plants. 2021;10 doi: 10.3390/plants10061221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elsheery N.I., Sunoj V.S.J., Wen Y., Zhu J.J., Muralidharan G., Cao K.F. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol. Biochem. 2020;149:50–60. doi: 10.1016/j.plaphy.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 95.Li Y., Zhu N., Liang X., Bai X., Zheng L., Zhao J., Li Y.-F., Zhang Z., Gao Y. Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg(ii) in soybean (Glycine max) Environ. Sci. Nano. 2020;7:1807–1817. doi: 10.1039/D0EN00091D. [DOI] [Google Scholar]
- 96.Ghorbanpour M., Mohammadi H., Kariman K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano. 2020;7:443–461. doi: 10.1039/C9EN00973F. [DOI] [Google Scholar]
- 97.Cui J., Liu T., Li F., Yi J., Liu C., Yu H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. Barking Essex 1987. 2017;228:363–369. doi: 10.1016/j.envpol.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 98.Nazaralian S., Majd A., Irian S., Najafi F., Ghahremaninejad F., Landberg T., Greger M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017;115:25–33. doi: 10.1016/j.plaphy.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Tripathi D.K., Singh S., Singh V.P., Prasad S.M., Dubey N.K., Chauhan D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017;110:70–81. doi: 10.1016/j.plaphy.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 100.Sun D., Hussain H.I., Yi Z., Rookes J.E., Kong L., Cahill D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere. 2016;152:81–91. doi: 10.1016/j.chemosphere.2016.02.096. [DOI] [PubMed] [Google Scholar]
- 101.Tripathi D.K., Singh V.P., Prasad S.M., Chauhan D.K., Dubey N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015;96:189–198. doi: 10.1016/j.plaphy.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 102.Adisa I.O., Pullagurala V.L.R., Peralta-Videa J.R., Dimkpa C.O., Elmer W.H., Gardea-Torresdey J.L., White J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano. 2019;6:2002–2030. doi: 10.1039/C9EN00265K. [DOI] [Google Scholar]
- 103.Adrees M., Ali S., Rizwan M., Zia-Ur-Rehman M., Ibrahim M., Abbas F., Farid M., Qayyum M.F., Irshad M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015;119:186–197. doi: 10.1016/j.ecoenv.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Rajput V.D., Harish, Singh R.K., Verma K.K., Sharma L., Quiroz-Figueroa F.R., Meena M., Gour V.S., Minkina T., Sushkova S., et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology. 2021;10:267. doi: 10.3390/biology10040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Sousa A., Saleh A.M., Habeeb T.H., Hassan Y.M., Zrieq R., Wadaan M.A.M., Hozzein W.N., Selim S., Matos M., AbdElgawad H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019;693:133636. doi: 10.1016/j.scitotenv.2019.133636. [DOI] [PubMed] [Google Scholar]
- 106.Gu H.H., Qiu H., Tian T., Zhan S.S., Deng T.H., Chaney R.L., Wang S.Z., Tang Y.T., Morel J.L., Qiu R.L. Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere. 2011;83:1234–1240. doi: 10.1016/j.chemosphere.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 107.Cui J., Li Y., Jin Q., Li F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano. 2020;7:162–171. doi: 10.1039/C9EN01035A. [DOI] [Google Scholar]
- 108.Shoeva O.Y., Khlestkina E.K. Anthocyanins Participate in the Protection of Wheat Seedlings against Cadmium Stress. Cereal Res. Commun. 2018;46:242–252. doi: 10.1556/0806.45.2017.070. [DOI] [Google Scholar]
- 109.Hayat S., Hasan S.A., Hayat Q., Ahmad A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma. 2010;239:3–14. doi: 10.1007/s00709-009-0075-2. [DOI] [PubMed] [Google Scholar]
- 110.Hussain B., Lin Q., Hamid Y., Sanaullah M., Di L., Hashmi M., Khan M.B., He Z., Yang X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.) Sci. Total Environ. 2020;712:136497. doi: 10.1016/j.scitotenv.2020.136497. [DOI] [PubMed] [Google Scholar]
- 111.Merwad A.-R.M.A., Desoky E.-S.M., Rady M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018;228:132–144. doi: 10.1016/j.scienta.2017.10.008. [DOI] [Google Scholar]
- 112.Tripathi D.K., Rai P., Guerriero G., Sharma S., Corpas F.J., Singh V.P. Silicon induces adventitious root formation in rice under arsenate stress with involvement of nitric oxide and indole-3-acetic acid. J. Exp. Bot. 2021;72:4457–4471. doi: 10.1093/jxb/eraa488. [DOI] [PubMed] [Google Scholar]
- 113.Burachevskaya M., Minkina T., Fedorenko A., Fedorenko G., Chernikova N., Rajput V.D., Mandzhieva S., Bauer T. Accumulation, translocation, and toxicity of arsenic in barley grown in contaminated soil. Plant Soil. 2021 doi: 10.1007/s11104-021-05067-9. [DOI] [Google Scholar]
- 114.Liu C., Wei L., Zhang S., Xu X., Li F. Effects of nanoscale silica sol foliar application on arsenic uptake, distribution and oxidative damage defense in rice (Oryza sativa L.) under arsenic stress. RSC Adv. 2014;4:57227–57234. doi: 10.1039/C4RA08496A. [DOI] [Google Scholar]
- 115.Sharma A., Kapoor D., Wang J., Shahzad B., Kumar V., Bali A.S., Jasrotia S., Zheng B., Yuan H., Daoliang Y. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants. 2020;9:100. doi: 10.3390/plants9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suriyaprabha R., Karunakaran G., Yuvakkumar R., Prabu P., Rajendran V., Kannan N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012;14:1294. doi: 10.1007/s11051-012-1294-6. [DOI] [Google Scholar]
- 117.Verma K.K., Song X.-P., Tian D.-D., Singh M., Verma C.L., Rajput V.D., Singh R.K., Sharma A., Singh P., Malviya M.K., et al. Investigation of Defensive Role of Silicon during Drought Stress Induced by Irrigation Capacity in Sugarcane: Physiological and Biochemical Characteristics. ACS Omega. 2021 doi: 10.1021/acsomega.1c02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singhal R.K., Jatav H.S., Aftab T., Pandey S., Mishra U.N., Chauhan J., Chand S., Indu, Saha D., Dadarwal B.K., et al. Roles of Nitric Oxide in Conferring Multiple Abiotic Stress Tolerance in Plants and Crosstalk with Other Plant Growth Regulators. J. Plant. Growth Regul. 2021 doi: 10.1007/s00344-021-10446-8. [DOI] [Google Scholar]
- 119.Matysik J., Alia N., Bhalu B., Mohanty P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002;82:525–532. [Google Scholar]
- 120.Iyer S., Caplan A. Products of Proline Catabolism Can Induce Osmotically Regulated Genes in Rice1. Plant Physiol. 1998;116:203–211. doi: 10.1104/pp.116.1.203. [DOI] [Google Scholar]
- 121.Almutairi Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (’Solanum lycopersicum’ L.) seedlings under salt stress. South. Cross J. 2016;9:106–114. [Google Scholar]
- 122.Wang Y., Stass A., Horst W.J. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol. 2004;136:3762–3770. doi: 10.1104/pp.104.045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shetty R., Fretté X., Jensen B., Shetty N.P., Jensen J.D., Jørgensen H.J.L., Newman M.-A., Christensen L.P. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 2011;157:2194–2205. doi: 10.1104/pp.111.185215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mushtaq A., Jamil N., Rizwan S., Mandokhel F., Riaz M., Hornyak G.L., Najam Malghani M., Naeem Shahwani M. Engineered Silica Nanoparticles and silica nanoparticles containing Controlled Release Fertilizer for drought and saline areas. IOP Conf. Ser. Mater. Sci. Eng. 2018;414:012029. doi: 10.1088/1757-899X/414/1/012029. [DOI] [Google Scholar]
- 125.Faizan M., Rajput V.D., Al-Khuraif A.A., Arshad M., Minkina T., Sushkova S., Yu F. Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum. Biology. 2021;10:666. doi: 10.3390/biology10070666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S.G., Kim K.W., Park E.W., Choi D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathology. 2002;92:1095–1103. doi: 10.1094/PHYTO.2002.92.10.1095. [DOI] [PubMed] [Google Scholar]
- 127.Takó M., Kerekes E.B., Zambrano C., Kotogán A., Papp T., Krisch J., Vágvölgyi C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants. 2020;9:165. doi: 10.3390/antiox9020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guerriero G., Hausman J.-F., Legay S. Silicon and the Plant Extracellular Matrix. Front. Plant Sci. 2016;7:463. doi: 10.3389/fpls.2016.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodrigues F.A., Benhamou N., Datnoff L.E., Jones J.B., Bélanger R.R. Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology. 2003;93:535–546. doi: 10.1094/PHYTO.2003.93.5.535. [DOI] [PubMed] [Google Scholar]
- 130.Seebold K.W., Datnoff L.E., Correa-Victoria F.J., Kucharek T.A., Snyder G.H. Effects of Silicon and Fungicides on the Control of Leaf and Neck Blast in Upland Rice. Plant Dis. 2004;88:253–258. doi: 10.1094/PDIS.2004.88.3.253. [DOI] [PubMed] [Google Scholar]
- 131.Hayasaka T., Fujii H., Ishiguro K. The Role of Silicon in Preventing Appressorial Penetration by the Rice Blast Fungus. Phytopathology. 2008;98:1038–1044. doi: 10.1094/PHYTO-98-9-1038. [DOI] [PubMed] [Google Scholar]
- 132.Menzies J.G., Ehret D.L., Glass A.D.M., Samuels A.L. The influence of silicon on cytological interactions between Sphaerotheca fuliginea and Cucumis sativus. Physiol. Mol. Plant Pathol. 1991;39:403–414. doi: 10.1016/0885-5765(91)90007-5. [DOI] [Google Scholar]
- 133.Ponmurugan P., Manjukarunambika K., Elango V., Gnanamangai B.M. Antifungal activity of biosynthesised copper nanoparticles evaluated against red root-rot disease in tea plants. J. Exp. Nanosci. 2016;11:1019–1031. doi: 10.1080/17458080.2016.1184766. [DOI] [Google Scholar]
- 134.Kang H., Elmer W., Shen Y., Zuverza-Mena N., Ma C., Botella P., White J.C., Haynes C.L. Silica Nanoparticle Dissolution Rate Controls the Suppression of Fusarium Wilt of Watermelon (Citrullus lanatus) Environ. Sci. Technol. 2021 doi: 10.1021/acs.est.0c07126. [DOI] [PubMed] [Google Scholar]
- 135.Kashyap D., Siddiqui Z.A. Effect of silicon dioxide nanoparticles and Rhizobium leguminosarum alone and in combination on the growth and bacterial blight disease complex of pea caused by Meloidogyne incognita and Pseudomonas syringae pv. pisi. Arch. Phytopathol. Plant Prot. 2020:1–17. doi: 10.1080/03235408.2020.1843306. [DOI] [Google Scholar]
- 136.Cui J., Liang Y., Yang D., Liu Y. Facile fabrication of rice husk based silicon dioxide nanospheres loaded with silver nanoparticles as a rice antibacterial agent. Sci. Rep. 2016;6:21423. doi: 10.1038/srep21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramouthar P.V., Caldwell P.M., McFarlane S.A. Effect of silicon on the severity of brown rust of sugarcane in South Africa. Eur. J. Plant Pathol. 2016;145:53–60. doi: 10.1007/s10658-015-0812-7. [DOI] [Google Scholar]
- 138.Suriyaprabha R., Karunakaran G., Kavitha K., Yuvakkumar R., Rajendran V., Kannan N. Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotechnol. 2014;8:133–137. doi: 10.1049/iet-nbt.2013.0004. [DOI] [PubMed] [Google Scholar]
- 139.Resende R.S., Rodrigues F.A., Gomes R.J., Nascimento K.J.T. Microscopic and biochemical aspects of sorghum resistance to anthracnose mediated by silicon. Ann. Appl. Biol. 2013;163:114–123. doi: 10.1111/aab.12040. [DOI] [Google Scholar]
- 140.Li Y.C., Bi Y., Ge Y.H., Sun X.J., Wang Y. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. J. Food Sci. 2009;74:M213–M218. doi: 10.1111/j.1750-3841.2009.01154.x. [DOI] [PubMed] [Google Scholar]
- 141.Deepak S., Manjunath G., Manjula S., Niranjan-Raj S., Geetha N.P., Shetty H.S. Involvement of silicon in pearl millet resistance to downy mildew disease and its interplay with cell wall proline/hydroxyproline-rich glycoproteins. Australas. Plant Pathol. 2008;37:498–504. doi: 10.1071/AP08047. [DOI] [Google Scholar]
- 142.Mandlik R., Thakral V., Raturi G., Shinde S., Nikolić M., Tripathi D.K., Sonah H., Deshmukh R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020;71:6703–6718. doi: 10.1093/jxb/eraa301. [DOI] [PubMed] [Google Scholar]
- 143.Hodson M.J., White P.J., Mead A., Broadley M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005;96:1027–1046. doi: 10.1093/aob/mci255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bokor B., Bokorová S., Ondoš S., Švubová R., Lukačová Z., Hýblová M., Szemes T., Lux A. Ionome and expression level of Si transporter genes (Lsi1, Lsi2, and Lsi6) affected by Zn and Si interaction in maize. Environ. Sci. Pollut. Res. Int. 2015;22:6800–6811. doi: 10.1007/s11356-014-3876-6. [DOI] [PubMed] [Google Scholar]
- 145.Parisi C., Vigani M., Rodríguez-Cerezo E. Agricultural Nanotechnologies: What are the current possibilities? Nano Today. 2015;10:124–127. doi: 10.1016/j.nantod.2014.09.009. [DOI] [Google Scholar]
- 146.Li Z.Z., Chen J.F., Liu F., Liu A.Q., Wang Q., Sun H.Y., Wen L.X. Study of UV-shielding properties of novel porous hollow silica nanoparticle carriers for avermectin. Pest. Manag. Sci. 2007;63:241–246. doi: 10.1002/ps.1301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.