Abstract
Climate change has been linked to poor childhood growth and development through maternal stress, nutritional insults related to lean harvests, and exposure to infectious diseases. Vulnerable populations are often most susceptible to these stressors. This study tested whether susceptibility to linear growth faltering is higher among Peruvian children from indigenous, rural, low-education, and low-income households. High-resolution weather and household survey data from Demographic and Health Survey 1996–2012 were used to explore height-for-age z-scores (HAZ) at each year of life from 0 to 5. Rural, indigenous children at age 0–1 experience a HAZ reduction of 0.35 units associated with prenatal excess rainfall which is also observed at age 4–5. Urban, non-indigenous children at age 4–5 experience a HAZ increase of 0.07 units associated with postnatal excess rainfall, but this advantage is not seen among rural, indigenous children. These findings highlight the need to consider developmental stage and social predictors as key components in public health interventions targeting increased climate change resilience.
Keywords: Climate change, Stunting, Thousand days, DHS, Social determinants of health
Introduction
Climate change is expected to increase global temperatures, cause unpredictable precipitation, and increase the frequency of weather anomalies (Collins et al., 2013). The health implications of climate change are wide ranging. Heat waves have been linked to impaired cognitive function and increased mortality (Madrigano et al., 2013; Cedeño Laurent et al., 2018). Unpredictable precipitation has been linked to increases in vector-borne diseases, communicable diseases, impaired crop yields from flooding, and widespread famine from lost agricultural productivity during droughts (Rose et al., 2001; Hales et al., 2002; Kovats et al., 2003; del Ninno & Lundberg, 2005). Both the frequency and severity of temperature and rainfall anomalies are expected to increase (Meehl & Tebaldi, 2004; Milly et al., 2002).
Though these impacts are experienced globally, some populations are more vulnerable than others. Key examples include the elderly who experience higher influenza-related mortality during winter, urban residents who are comparatively more vulnerable to heat stress than rural residents, low-income families in housing with poor weather cladding, and children whose growth and development depend on adequate nutrition and disease-free environments (Keatinge et al., 2000; Smoyer et al., 2000; Reichert et al., 2004; Phalkey et al., 2015). By 2050, climate change is predicted to increase global rates of stunting by one million children in Latin America and the Caribbean alone, underscoring the importance of studies examining determinants of childhood growth (Nelson et al., 2009; Phalkey et al., 2015).
Linear growth is a valuable marker for childhood health and is associated with 53% of infectious-disease-related deaths in lower- and middle-income countries (LMICs) (Schaible & Kaufmann, 2007). Children who are stunted experience delayed developmental milestones, late school enrollment, impaired cognitive ability, decreased fine motor skills, and have a mortality risk two to four times higher than children who are not stunted (Black et al., 2008; Caulfield et al., 2004; Woldehanna, 2010). The long-term effects of childhood stunting are far-reaching, and childhood linear growth by age three strongly predicts adult health (Christian et al., 2013; Prentice et al., 2013; Prendergast & Humphrey, 2014). The largest contributor to stunting is calorie deficiency, due to both food insecurity (issues related to food availability, accessibility, and affordability) and chronic diarrheal disease (FAO, 2010). Weather variability and extreme weather events directly affect crop yields and the spread of infectious diseases and are considered important contributors to childhood stunting (Porter & Semenov, 2005; Semenza & Menne, 2009).
Developmental stage is an important moderator of the climate-stunting relationship. The first 1000 days of growth, the period beginning at conception and ending at the start of the third postnatal year, is the most critical and active period for neurological development (Cusick & Georgieff, 2016). However, environmental stressors experienced during pregnancy operate via different mechanisms compared with stressors experienced postnatally. During pregnancy, poor maternal health can compromise critical brain development with implications for medical and psychosocial health (Schwarzenberg & Georgieff, 2018). High temperature and low precipitation during pregnancy have been linked to low birthweight and preterm birth, which are both associated with stunting and other poor health outcomes (Davenport et al., 2017; Molina & Saldarriaga, 2017). Extreme heat exposure during pregnancy can also damage the placenta leading to increased risk of preterm births, stillbirths, and placental abruptions (He et al., 2018).
Postnatal environmental stressors imposed by climate change occur at a time when children are particularly sensitive to nutrition and disease (Lloyd et al., 2011). Children experience higher climate exposure per unit body weight, have limited physiological adaptive capacity, depend heavily on caregivers, and are highly vulnerable in the early, rapid development phase (Sheffield & Landrigan, 2011). Several mechanisms linking climate and stunting have been proposed. Linear growth has been linked to agricultural productivity using the intensity of green vegetation as a proxy for climate-dependent crop yields (Shively et al., 2015). Similarly, the global rise in atmospheric carbon dioxide is linked to reduced soil concentrations of zinc, iron, and key proteins which exacerbate stunting related to micronutrient deficiencies (Myers et al., 2014). Other mechanisms implicate the role of foregone workplace productivity and loss of parental livelihoods following natural disasters and the timing of women’s pregnancy given the impact of climate change on agricultural and domestic labor responsibilities (Akresh et al., 2011; Rodriguez-Llanes et al., 2011; Davenport et al., 2017; MacVicar et al., 2017; Randell & Gray, 2019).
Social predictors such as socioeconomic and demographic characteristics also moderate the climate-stunting relationship. At the household level, the number of total residents and number of children < 5 years affect the risk for stunting under environmental duress partly due to resource allocation constraints (Huss-Ashmore & Curry, 1994; Brentlinger et al., 1999). Familial livelihoods can also be protective against stunting associated with climate exposure (Grace et al., 2012; Jankowska et al., 2012). Urbanicity, water source as a socioeconomic indicator, household demographics, and maternal education are other household factors that can modify the relationship between climate exposures and stunting (Stewart et al., 1990; Panter-Brick, 1997; Brentlinger et al., 1999; Kaufmann, 2008; Woldehanna, 2010; Chotard et al., 2011; Grace et al., 2012; Jankowska et al., 2012). However, the number of studies that have directly tested these moderating effects remains small.
This paper explores the climate-growth relationship in Peru from 1996 to 2012, a period characterized by rapid reductions in child stunting as well as the acceleration of climate change (Mejía Acosta & Haddad, 2014; Huicho et al., 2017). We ask if past weather anomalies undermined these gains, and thereby provide insight into the human costs of ongoing changes to the climate. Despite these gains, Peru experiences one of the highest rates of stunting in Latin America, affecting roughly one in four children (Larrea & Freire, 2002; Marini & Rokx, 2016; Wagstaff & Watanabe, 2000). Rates of stunting are higher among children in households with low socioeconomic status, with less educated mothers, in rural communities, and those who are indigenous (Johnson & Brown, 2014; Shin, 2007; Urke et al., 2013). Vulnerable children are disproportionately located in the Andean highlands and Amazon lowlands as compared with the coastal lowlands, but all three regions are expected to be vulnerable to climate change (Perry et al., 2017; Leal Filho & Freitas, 2018; Veettil & Kamp, 2019). Rural children and children in the Andean and Amazonian regions experience higher rates of stunting than those in urban areas or in coastal regions (Chaparro & Estrada, 2012; Larrea & Freire, 2002; Pomeroy et al., 2014; Urke et al., 2013). Indigenous communities, especially those located in the highlands and Amazon, have a stunting rate double those of non-indigenous households (Larrea & Freire, 2002). Some attribute this difference between lowland and highland stunting to differences in environmental stress exposure (Pomeroy et al., 2014). They characterize the highlands as a “multi-stress environment” given the colder temperatures, higher aridity, lower oxygen availability, poorer diets, higher levels of physical duress, and limited access to health care and education (Pomeroy et al., 2014).
Other studies explain this regional difference in terms of socioeconomic status. One study in Peru showed a clear relationship between rates of poverty and stunting among children under five (Chaparro & Estrada, 2012; Larrea & Freire, 2002). The highest prevalence of stunting was found in the highland department of Huancavelica (54.6%), the area with highest rates of poverty in the country. In contrast, the central and southern coastal areas have both poverty rates and stunting rates lower than 15%. However, others have also found that some of the greatest reductions in stunting prevalence in the last decade have been in the same predominantly rural Amazonian and Andean departments with high baseline poverty rates, including Cusco, Amazonas, Puno, Huanuco, and Ancash, highlighting the importance of geographic variability (Huicho et al., 2017).
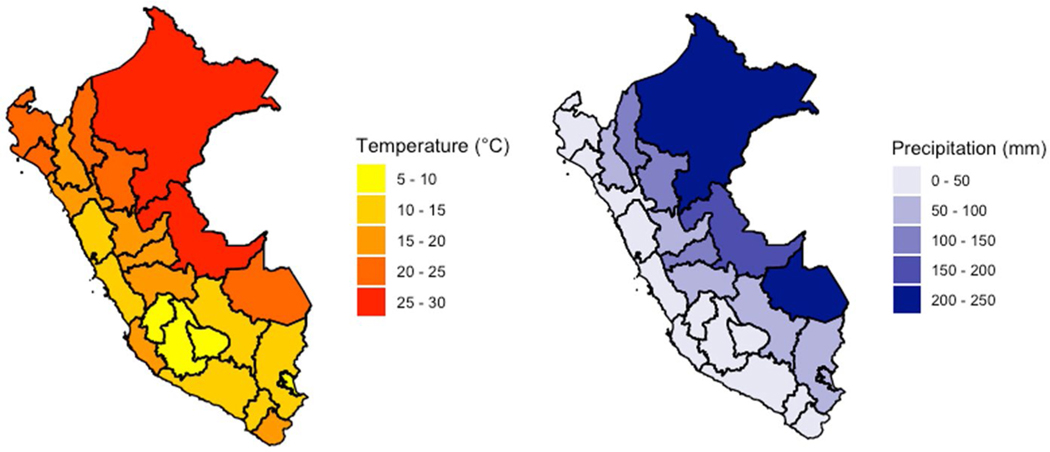
Climate vulnerability is a concern throughout Peru. Over seventy percent of the global tropical glacier supply is found in Peru, and its low-lying coastal zones are prone to flooding during seasonal melts (USAID, 2017; Leal Filho & Freitas, 2018; Veettil & Kamp, 2019). Rising temperatures have accelerated Peru’s glacial retreat, resulting in a 40% loss of glacial density since 1970, flooding during the rainy season, and less water for irrigation in the dry season (USAID, 2017). Additionally, 80% of Peruvian farmers, many of whom live in rural, indigenous communities, practice subsistence farming using rainfed agriculture (USAID, 2017). Unpredictable changes in streamflow can also lead to decreased yearly supplies of drinking water in these higher-altitude regions (Bradley et al., 2006; Perry et al., 2017). Peru’s vulnerability to natural disasters such as flooding, droughts, and landslides is additionally compounded by the effects of the El Niño Southern Oscillation (Ministerio del Ambiente, 2016). In the next 50–100 years, Peru is expected to witness an average increase of 2–4 °C and 50 cm in sea level, further amplifying the frequency and severity of these climate-related events (USAID, 2017). Historical monthly averages for precipitation and temperature are shown in Fig. 2.
Fig. 2.
Monthly average for precipitation and temperature for all departments in Peru over the study period (1981–2012)
This investigation of the effect of climate anomalies on childhood linear growth in Peru from 1996 to 2012 makes three key contributions. First, this paper assesses the climate-growth relationship across biologically meaningful periods of childhood development. While controlling for time- and place-dependent contributors to growth faltering (e.g., national economic growth and a 30-year climate average), these models test specifically for vulnerability during the in utero prenatal period and across each subsequent year of life. Second, this paper tests whether the climate-growth relationship is influenced by social modifiers such as education, indigeneity, and urban/rural designation. In addition to the biologically meaningful cutpoints of childhood developmental stage, this approach is inclusive of socially meaningful class memberships relevant to Peruvian children. Lastly, this paper explores the impact of rainfall anomalies in a region of high precipitation variability and over a long period of time. Shifts in rainfall exhibit greater spatial and temporal variation compared with those in temperature, and the impacts of rainfall variations often have significant impact on rain-fed agricultural regions (Lu et al., 2014; Issahaku et al., 2016; Omiat & Shively, 2020). Understanding the relationship between weather anomalies and growth faltering is important to understand how future climate change will impact stunting rates (Aragón et al., 2018).
Methods
Data
Demographic and anthropometric data are extracted from six rounds of the Demographic and Health Survey (DHS): 1996, 2000, 2004–2008, 2009, 2011, and 2012. The Peruvian survey has been conducted by the Instituto Nacional de Estadística e Informática and uses a three-stage, community-based cluster sampling design to select women and households for interviews from each of the 25 Peruvian departments (regions). Callao, a small administrative unit near Lima, was only incorporated in 2002 and is merged into the adjacent Lima department in this study. The department level was chosen as the unit of analysis for this study because it is the smallest scale at which household locations can be identified across these survey rounds. Although within-country DHS often conducts recurrent rounds, DHS does not purposively sample the same households and is not longitudinal. The analytic sample of children ages 0–60 months was restricted to rounds in which anthropometry was collected (1996–2012) and observations with non-missing data for interview month and year, urbanicity, department, maternal age and education, household density, wealth quintile, child sex, age, height, and weight, and indigeneity (n = 69,850). The sample is described in Table 1.
Table 1.
Sample-weighted summary statistics for climate exposures, growth outcome, and sociodemographic covariates. Table shows variable mean, standard deviation, and definition
| Variable | Units | Mean | SD | Definition |
|---|---|---|---|---|
| Rain prenatal | z-score | –0.079 | 0.95 | Rain anomaly during prenatal period |
| Temp prenatal | z-score | 0.01 | 0.94 | Temp anomaly during prenatal period |
| Rain 0–1 for 1–2 | z-score | 0.085 | 0.96 | Rain anomaly during ages 0–1 for ages 1–2 |
| Temp 0–1 for 1–2 | z-score | –0.225 | 0.74 | Temperature anomaly during ages 0–1 for ages 1–2 |
| Rain 0–2 for 2–3 | z-score | 0.124 | 0.84 | Rain anomaly during ages 0–2 for age 2–3 |
| Temp 0–2 for 2–3 | z-score | 0.023 | 0.71 | Temperature anomaly during ages 0–2 for ages 2–3 |
| Rain 0–3 for 3–4 | z-score | 0.112 | 0.83 | Rain anomaly during ages 0–3 for ages 3–4 |
| Temp 0–3 for 3–4 | z-score | 0.225 | 0.85 | Temperature anomaly during ages 0–3 for ages 3—4 |
| Rain 0–4 for 4–5 | z-score | –0.024 | 0.82 | Rain anomaly during ages 0–4 for ages 4–5 |
| Temp 0–4 for 4–5 | z-score | 0.127 | 0.74 | Temperature anomaly during ages 0–4 for ages 4–5 |
| HAZ | z-score | –0.928 | 1.29 | Height-for-age z-score |
| Child age | Years | 2.47 | 1.45 | Child’s reported age |
| Female | 0/1 | 0.495 | 0.50 | Child’s reported sex |
| Indigenous | 0/1 | 0.128 | 0.33 | Household indigeneity status by language |
| Rural | 0/1 | 0.388 | 0.49 | Urban or rural designation |
| Maternal age | Years | 29.50 | 6.96 | Maternal age on survey date |
| Maternal education | ||||
| No education | 0/1 | 0.055 | 0.23 | No formal education reported (mother) |
| Primary education | 0/1 | 0.346 | 0.48 | Some or completed primary education (mother) |
| Secondary education | 0/1 | 0.408 | 0.49 | Some or completed secondary school (mother) |
| Higher education | 0/1 | 0.191 | 0.39 | Completed higher than secondary school (mother) |
| Household size | # of indiv | 5.89 | 2.34 | Number of household members |
| Wealth quintile | ||||
| 1 | 0/1 | 0.242 | 0.43 | First (lowest) wealth quintile based on household income and assets |
| 2 | 0/1 | 0.238 | 0.43 | Second wealth quintile |
| 3 | 0/1 | 0.217 | 0.41 | Third wealth quintile |
| 4 | 0/1 | 0.172 | 0.38 | Fourth wealth quintile |
| 5 | 0/1 | 0.129 | 0.34 | Fifth (highest) wealth quintile |
| Time (linear) | Months | 24,072 | 72.0 | Continuous time trend linked to interview month and year (12*year+month) |
Data for monthly precipitation and temperature were extracted from the Climatic Research Unit-time series (CRU-TS) database for the years 1981 onwards. The CRU-TS dataset provides monthly, high-frequency information on temperature and rainfall on a 0.5 × 0.5 degree grid at approximately 50-km resolution. Because not all Peru DHS rounds have published household GPS locations, the department-level is the smallest spatial unit at which DHS households can be located across all rounds. Departments are equivalent to large counties in the US context and are large relative to the CRU pixel size; thus, the departments represent the primary constraint on spatial resolution in this context. CRU-TS uses spatial and temporal interpolation of weather station data to account for missing data and poor station coverage (Harris et al., 2020). These data were extracted as a spatial mean for each department in each month and attached to each person-month of life for the sample children living in that department. These values were then transformed to standardized climate anomalies as described below. This measure of within-area variation in atmospheric conditions represents a frequently used proxy for climate change but is not itself a direct measure of climate change (Cooper et al., 2019).
Measures and methods
Height-for-age z-scores (HAZ) were calculated using the zanthro package in Stata 13.1 and are based on World Health Organization (WHO) standards for childhood growth (Vidmar et al., 2010). Biologically implausible HAZ of ± 5 were excluded (n = 232). HAZ was chosen as the primary outcome instead of weight-for-height-z-scores (WHZ) for two reasons. First, while WHZ is a marker of acute starvation, HAZ is a marker of long-term nutritional deprivation and thus matches the long-term postnatal weather exposures used in this study. Second, Peru’s historically high ratio of stunting (low HAZ) to wasting (low WHZ) implicates long-term nutritional deprivation versus short-term acute malnutrition as a key health challenge. Respondents were classified as indigenous based on the language(s) spoken in the household as previously documented (Mensch et al., 1996; Terborgh et al., 1995; Valdivia, 2004).
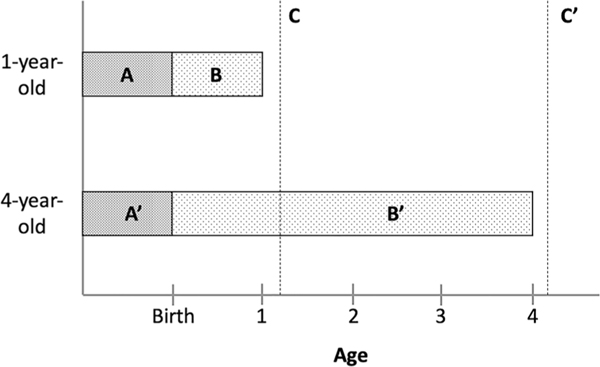
To account for extreme variability in climate conditions across Peru as well as for differing vulnerabilities over the life course, monthly climate values were transformed into child-specific standardized climate anomalies that capture the relative deviation from historical conditions. Relative to raw climate values, standardized climate anomalies have multiple advantages for analyses of climatic impacts on health: they are locally meaningful deviations from familiar conditions, they can be interpreted as exogenous shocks, and they are stronger predictors of social outcomes (Gray & Wise, 2016; Nordkvelle et al., 2017). Specifically, we first calculate 9-month running mean temperature and precipitation values in the department-month dataset and then standardize these values against all other 9-month periods in that dataset. We then attach these standardized values to the prenatal period of each child based on month and place of birth. We repeat this procedure for periods of 12, 24, and 36 and 48 months and attach these values respectively to the first year of life beginning in the month of birth for children ages 12–23 months, the first through second year of life for children ages 24–35 months, the first through third of life for children ages 36–47 months, and finally the first through fourth year of life for children ages 48–59 months. Figure 1 shows a breakdown of climate anomalies for two example children at age one and four, respectively. Each regression model below includes a prenatal measure and the age-appropriate postnatal measure, which can be interpreted as the extent to which these life periods for that child differed from the historical climate of that district. For example, a rainfall exposure of + 2 for a child’s first 2 years of life corresponds to precipitation levels two standard deviations higher than the department-specific average over the study period.
Fig. 1.
Climate anomaly exposures for two example children ages 1 and 4 years. Period A and A′ represent prenatal climate anomalies, while B and B′ represent postnatal climate anomalies for 1- and 4-year-olds, respectively. C and C′ represent the point at which height measurements were taken on corresponding DHS survey dates
All models include department, urban/rural designation, maternal age and education, household size and wealth, child age, sex, and indigeneity as controls. DHS wealth indices are based on a range of selected household assets such as electronic devices and kitchen appliances (DHS Program). With the exception of Peru’s DHS rounds 2000 and 2004–2008 which are referenced to one another, these are round-specific measures. Wealth quintiles capturing categorical (versus continuous) wealth distributions are used in this manuscript to facilitate pooled comparisons. A linear time trend based on survey date and fixed effects at the department level are also included. The inclusion of the time trend and department fixed effects accounts for all national-scale, linear time trends as well as all time-stable characteristics of departments, respectively, that might confound the effects of climate. As robustness checks for the linear time trend, we tested childhood date of birth as reference dates and specifying a quadratic linear time trend. Yearly and monthly fixed effects were not included because these effects would absorb all year-to-year, national-level climate variation as well as all seasonality in prenatal climate exposures, both important sources of climate variation that are relevant to our research questions. All analyses incorporate the DHS sampling weights and are corrected for clustering at the department level. Fit statistics for linear models were compared with non-linear quadratic models to identify the best exposure-outcome relationship. Linear models were retained based on BIC and AIC model fit statistics (values shown in Supplementary Table 1). The analysis is stratified by child age (0–1, 1–2, 2–3, 3–4 and 4–5) to account for differing vulnerabilities across the life course and the potential for catch-up growth. Two-way interactions with climate were also tested for education, wealth, indigeneity, and urban/rural. Finally, one three-way interaction between climate, indigeneity, and urban/rural was also tested.
Results
Results from models testing the association between weather exposures and HAZ throughout the prenatal period and first 5 years of life are shown in Table 2. Prenatal rain and temperature z-scores correspond to climate exposures experienced in the 9 months preceding birth for all children from DHS 1996–2012. Postnatal rain and temperature z-scores are cumulative measures of climate exposure spanning the postpartum period up until the survey date for all kids from DHS 1996–2012. At age 0–1, higher prenatal temperature is weakly associated (p < 0.10) with reduced HAZ (β = − 0.066, CI (− 0.14, 0.009)). Beyond age 1–2, prenatal temperature is not associated with linear growth. Contrary to prenatal temperature, the prenatal rain effect size grows between ages 0–1 and 4–5. At age 1–2, higher prenatal rainfall is significantly associated (p < 0.01) with increased HAZ (β = 0.038, CI (0.12, 0.63)), but by ages 4–5, higher prenatal rainfall is significantly associated (p < 0.05) with reduced HAZ (β = − 0.033, CI (− 0.062, − 0.004)). At age 2–3, higher postnatal rainfall is significantly associated (p < 0.05) with increased HAZ (β = 0.052, CI (0.005, 0.10)). Postnatal temperature is not associated with HAZ from birth through ages 3–4. By age 4–5, higher postnatal rainfall (β = 0.05, CI (0.006, 0.094)) and temperature (β = 0.069, CI (0.015, 0.122)) are significantly associated (p < 0.05) with increased HAZ. As model covariates, being indigenous, living in rural areas, and having lower wealth quintiles and maternal education were consistently and significantly associated with reduced HAZ. Disaggregated postnatal yearly weather exposures for each year of life are included in Supplementary Table 2. These results are robust to models excluding children of mothers who have moved within the past five years, as shown in Supplementary Table 3.
Table 2.
Height-for-age z-score (HAZ) and climate exposure across periods of childhood. Table shows beta coefficients when HAZ is regressed on climate anomalies and sociodemographic covariates. Joint tests of significance for climate are listed below
| Variable | HAZ (B) | ||||
|---|---|---|---|---|---|
|
| |||||
| Age 0–1 | Age 1–2 | Age 2–3 | Age 3–4 | Age 4–5 | |
| Prenatal rain | 0.016 | 0.038** | 0.026 | − 0.001 | − 0.033* |
| Prenatal temp | − 0.066 + | − 0.006 | − 0.001 | 0.014 | 0.023 |
| Postnatal rain | – | 0.008 | 0.052* | 0.049 | 0.05* |
| Postnatal temp | – | 0.012 | − 0.013 | 0 | 0.069* |
| Child age | − 0.039*** | − 0.025*** | − 0.012** | 0.012** | − 0.009** |
| Female | 0.155*** | 0.129** | 0.068*** | 0.090*** | 0.068*** |
| Indigenous | − 0.222* | − 0.205** | − 0.205* | − 0.205* | − 0.161* |
| Rural | − 0.003 | − 0.079 | − 0.083 | − 0.127* | − 0.114* |
| Maternal age | − 0.001 | 0.002 | 0.008** | 0.005** | 0.006** |
| Maternal education | |||||
| None | − 0.13 + | − 0.210** | − 0.272* | − 0.131 + | − 0.187** |
| Primary | |||||
| Secondary | 0.16** | 0.221*** | 0.270*** | 0.308*** | 0.259*** |
| Higher | 0.272*** | 0.430*** | 0.432*** | 0.481*** | 0.459*** |
| Household size | − 0.024*** | − 0.035*** | − 0.051*** | − 0.041*** | − 0.046*** |
| Wealth quintile | |||||
| 1 | − 0.341*** | − 0.333*** | − 0.460*** | − 0.444*** | − 0.414*** |
| 2 | − 0.142*** | − 0.184*** | − 0.255*** | − 0.199*** | − 0.227*** |
| 3 | - | - | - | - | - |
| 4 | − 0.051 + | 0.169** | 0.257*** | 0.323*** | 0.248*** |
| 5 | 0.099* | 0.337*** | 0.409** | 0.547*** | 0.537*** |
| Time | − 0.002*** | 0.00 | 0.002*** | 0.002*** | 0.002*** |
| n | 15,452 | 13,569 | 13,484 | 13,501 | 13,844 |
| Joint test of climate | 1.73 | 5.02** | 3.09* | 1.71 | 5.44** |
Models also controlled for Census-based regions (departments)
p < 0.10
p < 0.05
p < 0.01
p < 0.001,—reference
Results from interactions with weather and indigeneity, urban/rural, wealth, education, and indigeneity with urban/rural are presented in Table 3. Results are shown for age 0–1 and age 4–5 only. Intervening ages are included in Supplementary Table 4. At age 0–1, the association with prenatal temperature and reduced HAZ is concentrated among children who are more socioeconomically advantaged. These include children who are non-indigenous (β = − 0.08, CI (− 0.15, − 0.015)), who live in urban areas (β = − 0.11, CI (− 0.17, − 0.06)), are wealthier (β for quintile 3 = − 0.17, CI (− 0.26, − 0.08)), and have higher maternal education (β for higher education = − 0.085, CI (− 0.17, − 0.0001)). At age 0–1, three-way interactions between rainfall and temperature, indigeneity, and urban/rural designation reveal that for non-indigenous urban-dwelling children, higher prenatal temperature is significantly associated (p < 0.01) with reduced HAZ (β = − 0.11, CI (− 0.17, − 0.04)). For indigenous urban-dwelling children, higher prenatal rainfall is associated with increased HAZ (β = 0.35, CI (0.02, 0.69)) but for indigenous rural-dwelling children, higher prenatal rainfall is associated with reduced HAZ (β = − 0.35, CI (− 0.67, − 0.03)).
Table 3.
Height-for-age z-score (HAZ) outcome interactions between climate and sociodemographic variables. Table shows beta coefficients with significance of interactions along with chi squared joint test of interactions below each section
| Variable interactions | Age 0–1 | Age 4–5 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Prenatal | Prenatal | Postnatal | ||||
|
|
|
|
||||
| Rain | Temp | Rain | Temp | Rain | Temp | |
| Indigenous | ||||||
| No | 0.013 | − 0.083* | − 0.029 + | 0.021 | 0.056* | 0.079** |
| Yes | 0.037 | 0.052 | − 0.063 | 0.051 | 0.022 | 0.032 |
| Joint test of interactions | 3.33 + | 4.94** | ||||
| Urban/Rural | ||||||
| Urban | 0.024 | − 0.111** | − 0.017 | 0.021 | 0.069** | 0.072* |
| Rural | 0.009 | 0.006 | − 0.056* | 0.024 | 0.014 | 0.060 + |
| Joint test of interactions | 9.18** | 2.89* | ||||
| Wealth | ||||||
| Quintile 1 | 0.002 | − 0.022 | − 0.023 | 0.051 + | 0.020 | 0.109** |
| Quintile 2 | 0.044 + | − 0.009 | − 0.019 | − 0.010 | 0.006 | 0.002 |
| Quintile 3 | − 0.016 | − 0.166** | − 0.036 | 0.032 | 0.091** | 0.037 |
| Quintile 4 | 0.005 | − 0.088 | − 0.011 | − 0.034 | 0.055* | 0.060 + |
| Quintile 5 | 0.073 + | − 0.061 | − 0.078** | 0.069** | 0.166*** | 0.036 |
| Joint test of interactions | 6.93*** | 13.57*** | ||||
| Education | ||||||
| None | − 0.020 | 0.043 | 0.023 | − 0.049 | 0.014 | 0.059 |
| Primary | 0.010 | − 0.060 | − 0.021 | 0.026 | − 0.014 | 0.046 |
| Secondary | 0.019 | − 0.073* | − 0.058** | 0.043 + | 0.100*** | 0.072* |
| Higher | 0.030 | − 0.085* | − 0.028 | 0.002 | 0.070 + | 0.094* |
| Joint test of interactions | 0.79 | 6.12*** | ||||
| Indigenous * Urban/Rural | ||||||
| Non-indigenous urban | 0.019 | − 0.11** | − 0.019 | 0.019 | 0.069** | 0.075* |
| Non-indigenous rural | − 0.013 | 0.074* | − 0.029 | 0.004 | − 0.040 | 0.007 |
| Indigenous urban | 0.35* | − 0.265 | 0.074 | 0.152* | − 0.137 | − 0.079 |
| Indigenous rural | − 0.35* | 0.393 | − 0.105 + | − 0.139 | 0.086 | 0.027 |
| Joint test of interactions | 4.12** | 6.81*** | ||||
p < 0.10
p < 0.05
p < 0.01
p < 0.001
By age 4–5, higher prenatal rainfall is weakly associated (p < 0.10) with reduced HAZ for non-indigenous children (β = − 0.03, CI (− 0.06, 0.001)). Higher prenatal rainfall is also associated with reduced HAZ for children in rural areas (β = −0.06, CI: (−0.10, −0.01)), in wealthier households (β for quintile 5 = − 0.08, CI (− 0.15, − 0.01)), and with higher maternal education (β for secondary education = − 0.06, CI (− 0.09, − 0.02)). By age 4–5, higher prenatal temperature is significantly associated with increased HAZ for children in higher-income households (β quintile 5 = 0.07, CI (0.023, 0.12) and indigenous urban-dwelling children (β = 0.15, CI (0.014, 0.29)) and weakly associated with increased HAZ (p < 0.10) for kids with higher maternal education (β secondary education = 0.04, CI: (− 0.002, 0.09)). Higher cumulative postnatal rainfall by age 4–5 is significantly associated with increased HAZ among children considered socioeconomically advantaged. Children who are non-indigenous (β = 0.06, CI (0.012, 0.10)), live in urban areas (β = 0.07, CI (0.019, 0.12)), wealthier (β wealth quintile 5 = 0.17, CI: (0.086, 0.26)), and have higher maternal education (β higher education = 0.07, CI (− 0.003, 0.14)) all experience increased linear growth concurrent with higher postnatal rainfall. Higher cumulative postnatal temperature is associated with increased HAZ among non-indigenous children (β = 0.08, CI (0.03, 0.13)), urban children (β = 0.07, CI (0.012, 0.13)), and children in low-income households (β wealth quintile 1 = 0.11, CI (0.05, 0.17)). In three-way interactions with climate, indigeneity, and urban/rural, only non-indigenous urban-dwelling children have increased HAZ with elevated postnatal rainfall (β = 0.07, CI (0.018, 0.12)) and with elevated postnatal temperature (β = 0.08, CI (0.012, 0.14)).
Discussion
This research reveals strong associations between weather anomalies and childhood growth in Peru when stratifying by childhood developmental stage and with strong effect measure modification by indigeneity and urbanicity. Most previous studies find a significant association between stunting and climate. This relationship is dependent on the severity and timing of shocks as well as local geography and child and household characteristics (Skoufias & Vinha, 2012; Johnson & Brown, 2014; Phalkey et al., 2015; Tiwari et al., 2017). Consistent with most of this study’s findings, others have found that temperature anomalies increase the likelihood of stunting while additional precipitation can be beneficial for children’s health (Woldehanna, 2010; Phalkey et al., 2015). However, as also shown in this study, increased rainfall has also been found to increase growth faltering among certain subpopulations (Skoufias & Vinha, 2012). For example, children in India born during monsoon months are more likely to be stunted, highlighting the importance of magnitude and timing of rainfall (Lokshin & Radyakin, 2012). In Latin America, lower temperatures have also been linked to impaired linear growth at the same rates as exposure to heat waves (Skoufias & Vinha, 2012; Andalón et al., 2016).
Developmental stage is a key determinant of the climate-HAZ relationship. During ages 0–1, each additional standard deviation in prenatal temperature anomalies was associated with a 6.6% HAZ reduction. This finding was most prominent among non-indigenous, urban, and wealthier children with higher maternal educations. One potential reason that these comparatively more privileged children experienced growth faltering from increased prenatal temperature is the urban heat island effect. Urban heat islands are defined by increased sensible heat stemming from reduced heat flux as vegetation and evaporating soils are replaced by building materials with low albedo (Imhoff et al., 2010). Given the density of wealthier, more educated, and non-indigenous populations in urban centers in Peru, a given temperature increase may be compounded in urban centers, increasing heat stress for mothers and infants. A second potential explanation might be the conferred resilience of adaptive strategies to climate change exhibited by rural, low-income communities. For example, in anticipation of a climate shock, farmers in the Andes can diversify their crops and activities to manage risk by ensuring more predictable income streams throughout the year (Valdivia et al., 2010). Fava beans and barley, for example, are preferentially rotated on croplands in order to increase food security and the resilience of local farming systems (Capparelli et al., 2005; Perez et al., 2010). After a climate shock, these communities cope by liquidating assets, temporary migration, pausing children’s schooling, relying on remittances, and building relationships with neighboring communities to barter labor, land, food, and animals (Valdivia et al., 2010). Peruvian highland farmers cope with cold weather anomalies by controlling soil moisture through irrigation, terracing, landscaping with trees, and selective use of frost and drought-resistant seeds (Perez et al., 2010). These adaptive strategies may protect rural, indigenous children from in utero temperature anomalies.
However, by age 4–5 prenatal temperature anomalies are not associated with HAZ. Life course theory, which posits that organisms allocate energy at various life stages to maximize fitness and reproductive health, suggests that one reason for this might be the energetic tradeoffs between linear growth and immune/cognitive development (Urlacher et al., 2018). That is, the deleterious effects of increasing temperature anomalies may have shifted to cognitive and immune function as the body prioritizes somatic growth during early life. In fact, by age 4–5, prenatal temperature is mildly beneficial for linear growth across a range of social predictors (lowest economic quintile, highest economic quintile, higher maternal education, and indigenous urban children). These findings may be driven by children who experience prenatal climate stressors and live through age 4–5, conferring increased resilience. Children who did not survive to age 4–5 are not represented in this sample and may otherwise have attenuated this positive relationship with HAZ.
Increased prenatal rainfall is associated with reduced HAZ at age 0–1 and age 4–5. Prenatal rainfall anomalies are associated with 35% HAZ reduction among indigenous, rural children in the first year of life, representing the single largest climate-growth association observed in this study. However, indigenous urban children experienced a 35% HAZ increase over the same period, suggesting a protective effect against rainfall excess. By age 4–5, most children experience reduced HAZ with increasing prenatal rainfall with indigenous, rural children experiencing the greatest effect size; a 10.5% HAZ reduction per z-score increase in prenatal rainfall. Unlike the effect of prenatal rainfall anomalies at age 0–1, no protective effect for urban-dwelling indigenous children is observed by age 4–5. One potential mechanism may be the sustained effects of pathogenic exposures related to poor water and sanitation experienced both in utero that can contribute to complications during pregnancy and childbirth (Benova et al., 2014). During excess rainfall, standing floodwater, untreated water in cisterns, and poor water infrastructure can contribute to unsafe drinking and sanitation, leading to diarrheal illness and poor maternal health (Prüss-Üstün et al., 2008). Urban children, irrespective of indigeneity, do not share this HAZ reduction from excess rainfall, potentially because of better water infrastructure and healthcare access.
By age 4–5, postnatal rainfall anomalies are associated with increased HAZ exclusively among privileged groups. Urban, non-indigenous, wealthier children with higher maternal education all experience increases in linear growth with higher postnatal rainfall. Children in the highest economic quintile experience the largest effect; a 16.6% HAZ increase with each z-score increase in postnatal rainfall. One potential explanation may include the combined buffering against negative effects and benefits gained from excess rain not shared by low-income, rural, indigenous children with lower maternal education. Buffering against negative effects include the improved shelter, cisterns, plumbing, water treatment, and healthcare infrastructure seen in urban settings. Benefits from consistent, reliable rains include increased agricultural yield leading to increased economic output and sustained lower food prices. While urban residents might experience both of these, rural residents may experience one or none. For example, rural, low-income farming communities might share in the experience of a windfall from higher agricultural output but not protection against excess rain from adequate infrastructure. Lastly, by age 4–5, postnatal temperature is associated with increased HAZ among non-indigenous, urban-dwelling children. One factor driving this finding might be that warming weather is beneficial among children living in colder, urban regions where warming temperatures may lead to more fruitful crop yields and less exposure to adverse low temperatures.
This study has several limitations. Firstly, DHS data publish dichotomous urban/rural designations that some have noted may not capture the essence of urbanicity (Dorélien et al., 2013). This imposes a limitation in comparisons across urban and rural households, especially given that distinctions between the two have evolved over time. However, defining custom urban/rural indicators as useful comparison measures is not feasible for three reasons. First, the creation of study-specific urban/rural designation does not easily facilitate comparison with the existing literature in Peru (let alone outside of Peru). Second, there is no standardized method for the selection of annually time varying spatial data use to measure urbanicity over time. Thirdly, this is made impossible by the lack of GPS data for some DHS rounds.
Additionally, the ideal methodological approach when using spatiotemporally aggregated household data is to measure weather exposures at the level of the DHS cluster. In this study, we measure weather exposures at the department-level for several reasons. Firstly, only three DHS rounds (2000, 2004–2008, and 2009) offer household GPS locations. Locating households in departments precludes the need to sacrifice temporal resolution for spatial resolution. Secondly, because geocoded household locations are not longitudinal across Peru’s DHS rounds, clusters are not static, and a fixed-effects analysis would still require a prescribed administrative unit such as Peru’s departments. Lastly, a primary benefit of increased spatial resolution is to avoid excessive smoothing of weather exposures. However, standardized weather exposures such as rainfall and temperature anomalies are uncorrelated on average with raw weather values and vary relatively little within departments at a single point in time. For these reasons, households were grouped at the department level in this analysis. We highlight the need for future research utilizing a larger dataset in which GPS locations can be compared with administrative units for the full sample.
Given the nature of DHS survey data, the comparisons of developmental stages across time are not longitudinal comparisons. Rather they are comparisons of how climate impacts linear growth at various ages across time. This means that cohort-specific cumulative effects of climate cannot be examined. While traditional longitudinal analyses account for time-varying social and economic trends that shape birth cohorts and their developmental outcomes, the developmental stage comparisons in this study do not. A linear time trend and department fixed effects were included in this analysis to account for these regional and time-dependent trends.
Another data limitation is the exclusion of children without mothers in the household in the 1996 and 2000 surveys. DHS collected child height data as part of the mother survey and so maternal orphans and children not living in the same household as their mothers were omitted from the survey. This exclusion of young children without mothers at home and the patterning of the characteristics shared by these children may have dampened the observed effects in some sociodemographic groups. In all subsequent DHS rounds, the Peruvian survey transitioned to a household-based approach that includes children without mothers living in the same home. Next, the DHS uses primary language spoken at home as a proxy for indigeneity. Though this proxy is commonly used to assess indigenous status, there is potential for underestimation of the indigenous population (e.g., Spanish identified as the primary language due to cultural assimilation) (Mensch et al., 1996; Terborgh et al., 1995; Valdivia, 2004). However, this undercounting would attenuate the significance of this study’s findings. Given the significant findings across indigenous groups in this study, this suggests an even stronger effect size by indigeneity.
A key strength of this study is the use of developmentally specific climate exposures measured across 16 years of DHS surveys. Of similar studies included in a recent systematic review, most measured the seasonality, level, and variability in rainfall; some defined extreme events (droughts and floods); and few defined changes in ambient temperature (Phalkey et al., 2015). For example, some studies considered the timing and level of rainfall as indicators of variability with many focusing specifically on rainfall anomalies during the growing season (Shively, 2017; Tiwari et al., 2017; Bakhtsiyarava et al., 2018). The choice of climate variable is driven by data availability as well as what is appropriate according to local geography (Molina & Saldarriaga, 2017; Tiwari et al., 2017). Given Peru’s topographic diversity and the importance of both rainfall and temperature exposures for stunting outcomes, this study utilizes rainfall and temperature data to specify continuous measures of climate anomalies over 30 years, providing a more comprehensive view of climatic changes. The findings presenting in this paper that rainfall and temperature impact HAZ differently highlight the need for continued use of highly detailed, time-varying climate data in future studies.
Another strength of this study is its consideration of both prenatal and postnatal developmental periods. While earlier studies often measure only one, the approach outlined here uses biologically meaningful cutpoints to examine climate impacts across childhood developmental stages. The magnitude and significance of the findings presented are age-dependent, underscoring the importance of stratifying by developmental stage in future studies. Finally, this study recognizes the importance of social modifiers in climate-health relationships. Not all climate exposures are borne equally across a population, and some groups may be better resourced to adapt to these changes. This treatment of social predictors as potential modifiers and not only as controlled covariates strengthens the relevance of these findings in Peru.
Conclusion
This paper shows that the association between temperature and rainfall anomalies and linear growth among Peruvian children is dependent on childhood developmental stage, geography, and sociodemographic traits. Rural, indigenous children experience the greatest deleterious climate effects across all age groups from 1996 to 2012. Prenatal rainfall anomalies are strongly associated with reduced linear growth in the first year of life for rural, indigenous children. This is the only group for whom this finding is observed through the first 5 years of life. While postnatal rainfall anomalies are strongly associated with increased linear growth, social interaction models revealed that this applies only to non-indigenous, urban, wealthy children with higher maternal education. These findings suggest that indigenous, rural children have experienced most of the negative health consequences and none of the benefits of rainfall anomalies. Climate change is expected to increase the range of weather anomalies globally. For Peru, with its topographic and climatic diversity, the long-term health consequences of climate change should be considered in future public health policymaking. Lastly, given the vulnerability of lower-resourced populations to climate anomalies, these public health policies may strengthen their effectiveness by targeting rural, indigenous communities.
Supplementary Material
Acknowledgments
Funding This research is funded by the Carolina Population Center for training support (T32 HD007168) and general support (P2C HD050924).
Footnotes
Supplementary information The online version contains supplementary material available at https://doi.org/10.1007/s11111-021-00376-8.
Declarations
Conflict of interest The authors declare that they have no conflict of interest.
References
- Akresh R, Verwimp P, & Bundervoet T (2011) Civil War, Crop Failure, and Child Stunting in Rwanda. EconomicDevelopment and Cultural Change, 59(4):777–810. [Google Scholar]
- Andalón M, Azevedo JP,Rodríguez-Castelán C, Sanfelice V, & Valderrama-González D. (2016) Weather Shocks and Health at Birth in Colombia. World Development,82(November 2012): 69–82. [Google Scholar]
- Aragón FM, Oteiza F, & Rud JP (2018) Climate change and agriculture: farmer adaptation to extreme heat IFS Working Paper W18/06 Climate Change and Agriculture: Farmer Adaptation to Extreme Heat *. [Google Scholar]
- Bakhtsiyarava M, Grace K, & Nawrotzki RJ (2018) Climate, birth weight, and agricultural livelihoods in Kenya and Mali. American Journal of Public Health, 108(S2): S144–S150. available at 10.2105/AJPH.2017.304128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benova L, Cumming O, & Campbell OMR (2014), Systematic review and meta-analysis: Association between water and sanitation environment and maternal mortality. Tropical Medicine & International Health, 19(4): 368–387. available at 10.1111/tmi.12275 [18 November 2018]. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, & Rivera J. (2008) Maternal and child undernutrition: Global and regional exposures and health consequences. The Lancet, 371(9608): 243–260. available at https://pubmed.ncbi.nlm.nih.gov/18207566/ [2 July 2020]. [DOI] [PubMed] [Google Scholar]
- Bradley RS, Vuille M, Diaz HF, & Vergara W. (2006) Threats to water supplies in the tropical Andes., 312(June): 1755–1757. [DOI] [PubMed] [Google Scholar]
- Brentlinger PE, Hernan MA, Hernandez-Diaz S, Azaroff LS, & McCall M. (1999) Childhood malnutrition and postwar reconstruction in Rural El Salvador. A Community-Based Survey. Journal of the American Medical Association, 281(2): 184–190. available at https://pubmed.ncbi.nlm.nih.gov/9917125/ [2 July 2020]. [DOI] [PubMed] [Google Scholar]
- Capparelli A, Lema V, Giovannetti M, & Raffino R. (2005) The introduction of old world crops (wheat, barley and peach) in Andean Argentina during the 16th century a.d.: Archaeobotanical and Ethnohistorical Evidence. Vegetation History and Archaeobotany, 14(4): 472–484. [Google Scholar]
- Caulfield LE, de Onis M,Blössner M, & Black RE (2004) Undernutrition as an UnderlyingCause of Child Deaths Associated with Diarrhea, Pneumonia, Malaria, andMeasles. The American journal of clinical nutrition, 80(1): 193–198. [DOI] [PubMed] [Google Scholar]
- Cedeño Laurent JG, Williams A, Oulhote Y, Zanobetti A, Allen JG, & Spengler JD (2018) Reduced cognitive function during a heat wave among residents of non-air-conditioned buildings: An observational study of young adults in the summer of 2016 (Patz JA, Ed.). PLOS Medicine, 15(7): e1002605. available at 10.1371/journal.pmed.1002605 [12 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro MP, & Estrada L. (2012) Mapping the nutrition transition in Peru: Evidence for decentralized nutrition policies. Revista Panamericana de Salud Pública, 32(3): 241–244. available at http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1020-49892012000900010&lng=en&nrm=iso&tlng=en [DOI] [PubMed] [Google Scholar]
- Chotard S, Mason JB, Oliphant NP, Mebrahtu S. & Hailey P. (2011) Fluctuations in wasting in vulnerable child populations in the Greater Horn of Africa. Food and Nutrition Bulletin, 32(3 SUPPL.). available at https://pubmed.ncbi.nlm.nih.gov/21049843/ [2 July 2020]. [DOI] [PubMed] [Google Scholar]
- Christian P, Lee SE, Angel MD, Adair LS, Arifeen SE, Ashorn P, Barros FC,Fall CHD, Fawzi WW, Hao W, Hu G, Humphrey JH,Huybregts L, Joglekar CV, Kariuki SK, Kolsteren P,Krishnaveni GV, Liu E, Martorell R, Osrin D,Persson LA, Ramakrishnan U, Richter L, Roberfroid D,Sania A, Kuile FOT, Tielsch J, Victora CG, Yajnik CS, Yan H, Zeng L, & Black RE (2013), Risk ofChildhood Undernutrition Related to Small-for-Gestational Age and Preterm Birthin Low- and Middle-Income Countries. International Journal of Epidemiology,42(5): 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne JL, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner MF, Allen MR, Andrews T, Beyerle U, Bitz CM, Bony S, & Booth BBB (2013) Long-Term Climate Change: Projections, Commitments and Irreversibility, 1029–1136, in: Stocker Thomas F., Qin Dahe, Plattner Gian-Kasper, Tignor Melinda M.B., Allen Simon K., Boschung Judith, Alexander, Xia Yu, Bex Vincent, Midgley Pauline M. Stocker Thomas F., Qin Dahe, Plattner Gian-Kasper, Tignor Melinda M.B., Allen Simon K., Judit PMM (Ed.), Climate Change 2013—The physical science basis. New York NY USA: Cambridge University Press. available at https://research.monash.edu/en/publications/long-term-climate-change-projections-commitments-and-irreversibil [7 July 2020]. [Google Scholar]
- Cooper MW, Brown ME, Hochrainer-Stigler S, Pflug G, McCallum I, Fritz S, Silva J, & Zvoleff A. (2019) Mapping the effects of drought on child stunting. Proceedings of the National Academy of Sciences of the United States of America, 116(35): 17219–17224. available at http://www.ncbi.nlm.nih.gov/pubmed/31405971 [13 September 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick SE, & Georgieff MK(2016) The Role of Nutrition in Brain Development: The Golden Opportunity ofthe “First 1000 Days”. Journal of Pediatrics, 175: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport F, Grace K, Funk C, & Shukla S. (2017) Child Health Outcomes in Sub-Saharan Africa: AComparison of Changes in Climate and Socio-Economic Factors. GlobalEnvironmental Change, 46(August): 72–87. [Google Scholar]
- DHS Program Wealth-Index-Construction. (2020) Available at https://dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm [16 November 2020].
- Dorélien A, Balk D, & Todd M. (2013) What Is Urban? Comparing a Satellite View with the Demographic and Health Surveys. Population and Development Review, 39(3): 413–439. [Google Scholar]
- FAO. (2010) Household food security and community nutrition. available at http://www.fao.org/ag/agn/nutrition/household_en.stm [2 July 2020].
- Grace K, Davenport F, Funk C, & Lerner AM (2012) Child Malnutrition and Climate in Sub-SaharanAfrica: An Analysis of Recent Trends in Kenya. Applied Geography,35(1–2): 405–413. [Google Scholar]
- Gray C, & Wise E. (2016) Country-Specific Effects of Climate Variability on Human Migration. ClimaticChange, 135(3–4): 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales S, De Wet N, Maindonald J, & Woodward A. (2002) Potential Effect of Population and Climate Changes on Global Distribution of Dengue Fever: An Empirical Model. Lancet,360(9336): 830–834. [DOI] [PubMed] [Google Scholar]
- Harris I, Osborn TJ, Jones P, & Lister D. (2020) Version 4 of the CRU TS Monthly High-Resolution GriddedMultivariate Climate Dataset. Scientific Data, 7(1): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Kosatsky T, Smargiassi A,Bilodeau-Bertrand M, & Auger N. (2018) Heat and Pregnancy-Related Emergencies: Risk of Placental Abruption during Hot Weather. EnvironmentInternational, 111: 295–300. [DOI] [PubMed] [Google Scholar]
- Huicho L, Huayanay-Espinoza CA, Herrera-Perez E, Segura ER, Niño de Guzman J, Rivera-Ch M, & Barros AJD (2017) Factors behind the success story of under-five stunting in Peru: A district ecological multilevel analysis. BMC Pediatrics, 17(1): 1–9. available at 10.1186/s12887-017-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss-Ashmore R, & Curry JJ (1994) Diet, nutrition, and agricultural development in Swaziland. 3. Household economics and demography. Ecology of Food and Nutrition, 33(1–2): 107–121. available at 10.1080/03670244.1994.9991419 [2 July 2020]. [DOI] [Google Scholar]
- Imhoff ML, Zhang P, Wolfe RE & Bounoua L. (2010) Remote Sensing of the Urban Heat Island Effectacross Biomes in the Continental USA. Remote Sensing of Environment,114(3): 504–513. [Google Scholar]
- Issahaku AR, Campion BB, & Edziyie R. (2016) Rainfall and temperature changes and variability in the Upper East Region of Ghana. Earth and Space Science, 3(8): 284–294. available at 10.1002/2016EA000161 [26 March 2020]. [DOI] [Google Scholar]
- Jankowska MM, Lopez-Carr D, Funk C, Husak GJ, & Chafe ZA (2012) Climate Change and HumanHealth: Spatial Modeling of Water Availability, Malnutrition, and Livelihoodsin Mali, Africa. Applied Geography, 33(1): 4–15. [Google Scholar]
- Johnson K, & Brown ME (2014) Environmental risk factors and child nutritional status and survival in a context of climate variability and change. Applied Geography, 54: 209–221. available at 10.1016/j.apgeog.2014.08.007 [DOI] [Google Scholar]
- Kaufmann S. (2008), The nutrition situation in Northern Laos—Determinants of malnutrition and changes after four years of intensive interventions. PhD Thesis ((Justus Liebig University Giessen, Giessen, Germany: ). [Google Scholar]
- Keatinge WR, Donaldson GC, Bucher K,Jendritzky G, Cordioli E, Martinelli M, Katsouyanni K, Kunst AE,McDonald C, Näyhä S, & Vuori I. (2000), Winter Mortality in Relation toClimate. International journal of circumpolar health, 59(3–4): 154–159. [PubMed] [Google Scholar]
- Kovats RS, Bouma MJ, Hajat S, Worrall E, & Haines A. (2003) El Niño and Health. Lancet,362(9394): 1481–1489. [DOI] [PubMed] [Google Scholar]
- Larrea C, & Freire W. (2002) Social inequality and child malnutrition in four Andean countries. Revista Panamericana de Salud Pública, 11(5–6): 356–364. available at http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1020-49892002000500010&lng=en&nrm=iso&tlng=en [DOI] [PubMed] [Google Scholar]
- Leal Filho W, & Freitas LE (2018) Climate change Adaptation in Latin America : Managing vulnerability, fostering resilience. [Google Scholar]
- Lloyd SJ, Sari Kovats R, & Chalabi Z. (2011) Climate Change, Crop Yields, and Undernutrition: Development of aModel to Quantify the Impact of Climate Scenarios on Child Undernutrition. EnvironmentalHealth Perspectives, 119(12): 1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokshin M, & Radyakin S. (2012) Month of birth and children’s health in India Author ( s ): Michael Lokshin and Sergiy Radyakin Published by : University of Wisconsin Press Stable URL : http://www.Jstor.Com/Stable/23214385 Month of Birth and Children ‘ s Health in India. , 47(1): 174–203. [Google Scholar]
- Lu H, Jing W, Zhao J, Liu X, & Huang Z. (2014) Characteristics of the temporal variation in temperature and precipitation in China’s Lower Yellow River Region. Advances in Meteorology, 2014. [Google Scholar]
- MacVicar S, Berrang-Ford L, Harper S, Huang Y, Namanya Bambaiha D, & Yang S. (2017) Whether weather matters: Evidence of association between in utero meteorological exposures and foetal growth among indigenous and non-indigenous mothers in rural Uganda (Rosenfeld CS, Ed.). PLOS One, 12(6): e0179010. available at 10.1371/journal.pone.0179010 [2 July 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J, Mittleman MA, Baccarelli A, Goldberg R, Melly S, von Klot S, & Schwartz J. (2013) Temperature, myocardial infarction, and mortality. Epidemiology, 24(3): 439–446. available at http://journals.lww.com/00001648-201305000-00017 [12 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A, & Rokx C. (2016) Standing tall: Peru’s success in overcoming its stunting crisis. Public Dissclosure. [Google Scholar]
- Meehl GA, & Tebaldi C. (2004) More Intense, More Frequent, and Longer Lasting Heat Waves in the 21st Century.Science, 305(5686): 994–997. [DOI] [PubMed] [Google Scholar]
- Mejía Acosta A, & Haddad L. (2014), The Politics of Success in the Fight against Malnutrition in Peru. FoodPolicy, 44: 26–35. [Google Scholar]
- Mensch B, Arends-Kuenning M, & Jain A. (1996) The impact of the quality of family planning services on contraceptive use in Peru. [PubMed] [Google Scholar]
- Milly PCD, Wetherald RT, Dunne KA, & Delworth TL (2002), Increasing Risk of Great Floods in a ChangingClimate. Nature, 415(6871): 514–517. [DOI] [PubMed] [Google Scholar]
- del Ambiente Ministerio (2016), El Perú y El Cambio Climático: Tercera Comunicación Nacional Del Perú a La Convención Marco de Las Naciones Unidas Sobre El Cambio Climático. Convención Marco de las Naciones Unidas sobre Cambio Climático: 662. available at http://www.minam.gob.pe/wp-content/uploads/2016/05/Tercera-Comunicación.pdf [Google Scholar]
- Molina O, & Saldarriaga V. (2017) The perils of climate change: In utero exposure to temperature variability and birth outcomes in the Andean Region. Economics and Human Biology. [DOI] [PubMed] [Google Scholar]
- Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey ADB, Bloom AJ, Carlisle E,Dietterich LH, Fitzgerald G, Hasegawa T, Holbrook NM, Nelson RL, Ottman MJ, Raboy V, Sakai H, Sartor KA, Schwartz J, Seneweera S, Tausz M, & Usui Y. (2014) Increasing CO2 Threatens Human Nutrition. Nature,510(7503):139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GC, Rosegrant MW, Koo J, Robertson RD, Sulser T, Zhu T, Ringler C, Msangi S, Palazzo A, Batka M, Magalhaes M, Valmonte-Santos R, Ewing M, Lee D, Nelson G, Rosegrant M, Koo J, Robertson R, Sulser T, Zhu T, Ringler C, Msangi S, Palazzo A, Batka M, Magalhaes M, Valmonte-Santos R, Ewing M, & Lee D. (2009) Climate change: Impact on agriculture and costs of adaptation. International Food Policy Research Institute (IFPRI). available at https://econpapers.repec.org/RePEc:fpr:fprepo:21 [2 July 2020]. [Google Scholar]
- del Ninno C, & Lundberg M. (2005) Treading Water. The Long-Term Impact of the 1998 Flood on Nutrition inBangladesh. Economics and Human Biology, 3(1): 67–96. [DOI] [PubMed] [Google Scholar]
- Nordkvelle J, Rustad SA, & Salmivalli M. (2017) Identifying the Effect of Climate Variability onCommunal Conflict through Randomization. Climatic Change, 141(4):627–639. [Google Scholar]
- Omiat G, & Shively G. (2020) Rainfall and Child Weight in Uganda. Economics and Human Biology, 38:100877. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C. (1997) Seasonal growth patterns in Rural Nepali children. Annals of Human Biology, 24(1): 1–18. available at https://pubmed.ncbi.nlm.nih.gov/9022902/ [2 July 2020]. [DOI] [PubMed] [Google Scholar]
- Perez C, Nicklin C, Dangles O, Vanek S, Sherwood S, Halloy S, Garrett K, & Forbes G. (2010) Climate change in the High Andes: Implications and adaptation strategies for small-scale farmers. The International Journal of Environmental, Cultural, Economic and Social Sustainability, 6(5): 71–88. available at http://www.ijs.cgpublisher.com/product/pub.41/prod.727 [Google Scholar]
- Perry LB, Seimon A, Andrade-Flores MF, Endries JL, Yuter SE, Velarde F, Arias S, Bonshoms M, Burton EJ, Winkelmann IR, Cooper CM, Mamani G, Rado M, Montoya N, & Quispe N. (2017) Characteristics of precipitating storms in glacierized tropical Andean Cordilleras of Peru and Bolivia. Annals of the American Association of Geographers, 107(2): 309–322. available at 10.1080/24694452.2016.1260439 [29 April 2019]. [DOI] [Google Scholar]
- Phalkey RK, Aranda-Jan C, Marx S, Höfle B, & Sauerborn R. (2015) Systematic review of current efforts to quantify the impacts of climate change on undernutrition. Proceedings of the National Academy of Sciences, 112(33): E4522–E4529. available at 10.1073/pnas.1409769112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy E, Stock JT, Stanojevic S,Miranda JJ, Cole TJ, & Wells JCK (2014), Stunting,Adiposity, and the Individual-Level ‘Dual Burden’ among Urban Lowland and RuralHighland Peruvian Children. American Journal of Human Biology, 26(4):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JR, & Semenov MA (2005) Crop Responses to Climatic Variation. Philosophical Transactions of theRoyal Society B: Biological Sciences, 360(1463): 2021–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast Andrew J., & Humphrey JH (2014) The stunting syndrome in developing countries. Paediatrics and International Child Health, 34(4): 250–265. available at 10.1179/2046905514Y.0000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Ward K, Goldberg G,Jarjou L, Moore S, Fulford A, & Prentice A. (2013) Critical Windows for Nutritional Interventions against Stunting. AmericanJournal of Clinical Nutrition, 98(3): 854–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss-Üstün A, Bos R, Gore F, & Bartram J. (2008) Safer water, better health: costs, benefits and sustainability of interventions to protect and promote health. World Health Organization. available at http://apps.who.int/iris/bitstream/handle/10665/43840/9789241596435_eng.pdf;jsessionid=8B46DF2A4D2B44908ECDADDF05A4F834?sequence=1 [24 October 2018]. [Google Scholar]
- Randell H, & Gray C. (2019) Climate change and educational attainment in the global tropics. Proceedings of the National Academy of Sciences of the United States of America, 116(18): 8840–8845. available at https://www.pnas.org/content/116/18/8840 [2 July 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, & Miller MA (2004) Influenza and the Winter Increase in Mortality in the United States, 1959–1999.American Journal of Epidemiology, 160(5): 492–502. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Llanes JM, Ranjan-Dash S, Degomme O, Mukhopadhyay A, & Guha-Sapir D. (2011) Child Malnutrition and Recurrent Flooding in Rural Eastern India: ACommunity-Based Survey. BMJ Open, 1(2): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JB, Epstein PR, Lipp EK, Sherman BH, Bernard SM, & Patz JA (2001) Climate variability and change in the United States: Potential impacts on water- and foodborne diseases caused by microbiologic agents. Environmental Health Perspectives, 109(suppl 2): 211–221. available at 10.1289/ehp.01109s2211 [12 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, & Kaufmann SHE (2007) Malnutrition and infection: Complex mechanisms and global impacts. PLoS Medicine, 4(5): e115. available at 10.1371/journal.pmed.0040115 [2 July 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberg SJ, & Georgieff MK (2018) Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics, 141(2). [DOI] [PubMed] [Google Scholar]
- Semenza JC, & Menne B. (2009) Climate Change and Infectious Diseases in Europe. The Lancet InfectiousDiseases, 9(6): 365–375. [DOI] [PubMed] [Google Scholar]
- Sheffield PE, & Landrigan PJ(2011) Global Climate Change and Children’s Health: Threats and Strategies forPrevention. Environmental Health Perspectives, 119(3): 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. (2007), Child health in Peru: Importance of regional variation and community effects on children’s height and weight. Journal of Health and Social Behavior, 48(4): 418–433. available at https://pubmed.ncbi.nlm.nih.gov/18198688/ [2 July 2020]. [DOI] [PubMed] [Google Scholar]
- Shively GE (2017) Infrastructure mitigates the sensitivity of child growth to local agriculture and rainfall in Nepal and Uganda. Proceedings of the National Academy of Sciences, 114(5): 903–908. available at 10.1073/pnas.1524482114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively G, Sununtnasuk C, & Brown M. (2015) Environmental variability and child growth in Nepal. Health and Place. [DOI] [PubMed] [Google Scholar]
- Skoufias E, & Vinha K. (2012) Climate Variability and Child Height in Rural Mexico. Economics and HumanBiology, 10(1): 54–73. [DOI] [PubMed] [Google Scholar]
- Smoyer KE, Rainham DGC, & Hewko JN (2000) Heat-Stress-Related Mortality in Five Cities inSouthern Ontario: 1980–1996. International Journal of Biometeorology,44(4): 190–197. [DOI] [PubMed] [Google Scholar]
- Stewart MK, Fauveau V, Chakraborty J, Briend A, Yunus MD, & Sarder AM (1990) Post-Flood Nutritional Anthropometry of Children in Matlab, Bangladesh. Ecology of Food and Nutrition, 24(2): 121–131. [Google Scholar]
- Terborgh A, Rosen JE, Santiso G, Roberto T, Willy BJT, & Bull SE (1995) Family planning among indigenous populations in Latin America. International Family Planning Perspectives, 21(4): 143–149. available at https://www.jstor.org/stable/2133321 [30 March 2020]. [Google Scholar]
- Tiwari S, Jacoby HG, & Skoufias E. (2017) Monsoon Babies: Rainfall Shocks and Child Nutrition in Nepal. EconomicDevelopment and Cultural Change, 65(2): 167–188. [Google Scholar]
- Urke HB, Mittelmark MB, & Valdivia M. (2013) Trends in Stunting and Overweight in Peruvian Pre-Schoolers from 1991 to 2011: Findings from the Demographic and Health Surveys. Public Health Nutrition, 17(11): 2407–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlacher SS, Ellison PT,Sugiyama LS, Pontzer H, Eick G, Liebert MA,Cepon-Robins TJ, Gildner TE, & Josh Snodgrass J. (2018) Tradeoffs between Immune Function and Childhood Growth among AmazonianForager-Horticulturalists. Proceedings of the National Academy of Sciencesof the United States of America, 115(17): E3914–E3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USAID (2017), Climate change risk in Peru: Country risk profile, (February): 5. available at https https://www.climatelinks.org/sites/default/files/asset/document/2017_ClimateChangeRiskProfile_Peru.pdf
- Valdivia C, Seth A, Gilles JL,García M, Jiménez E, Cusicanqui J, Navia F, & Yucra E. (2010) Adapting to Climate Change in Andean Ecosystems: Landscapes,Capitals, and Perceptions Shaping Rural Livelihood Strategies and LinkingKnowledge Systems. Annals of the Association of American Geographers,100(4): 818–834. [Google Scholar]
- Valdivia M(2004), Poverty, health infrastructure and the nutrition of Peruvian children. Economics and Human Biology, 2(3 SPEC. ISS.): 489–510. [DOI] [PubMed] [Google Scholar]
- Veettil BK, & Kamp U. (2019) Global Disappearance of Tropical Mountain Glaciers: Observations,Causes, and Challenges. Geosciences (Switzerland), 9(5): 196. [Google Scholar]
- Vidmar S, Cole T, & Pan H. (2010) Standardizing anthropometric measures in children and adolescents with functions for Egen: update. Stata Journal, 13(2): 366–378. available at http://ideas.repec.org/a/tsj/stataj/v7y2007i4p465-506.html [Google Scholar]
- Wagstaff A, & Watanabe N. (2000) Socioeconomic inequalities in child malnutrition in the developing world. Program: 53. [Google Scholar]
- Woldehanna T. (2010) Do pre-natal and post-natal economic shocks have a long-lasting effect on the height of 5-year-old children? Evidence from 20 sentinel sites of rural and urban Ethiopia. Young Lives. available at https://ora.ox.ac.uk/objects/uuid:2044e932-9375-4c78-abb6-04befff9ac6f [2 July 2020]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.