Abstract
The use of graphene quantum dots as biomedical device and drug delivery system has been increasing. This nanoplatform of pure carbon has showed unique properties and showed to be safe for human use. The imatinib is a molecule designed to specifically inhibit the tyrosine kinase, used for leukemia treatment. In this study, we successfully decorated the graphene quantum dots (GQDs@imatinb) by a carbodiimide crosslinking reaction. The GQDs@imatinb were characterized by FTIR and AFM. The nanoparticles’ in vitro behaviors were evaluated by cellular trafficking (internalization) assay and cell viability and apoptosis assays in various cancer cell lines, including suspension (leukemia) cells and adherent cancer cells. The results showed that the incorporation of the imatinib on the surface of the graphene quantum dots did not change the nanoparticles’ morphology and properties. The GQDs@imatinb could be efficiently internalized and kill cancer cells via the induction of apoptosis. The data indicated that the prepared GQDs@imatinb might be a great drug nano-platform for cancer, particularly leukemia treatments.
Keywords: Drug delivery, Graphene quantum dot, Biomedical device, Smart material, Nanoparticle
1. Introduction
Carbon-based nanomaterials are nanomaterials with a high possibility of structures in diverse geometries and chemical bonds (sp1, sp2, sp3), which significantly changes their properties. The carbon-based nanomaterials include: fullerenes (0D), carbon nanotubes (1D) and more recently (<10 –y) graphene (2D) [1,2]. The latter consists of a monolayer of sp2 carbon atoms arranged in a hexagonal crystal lattice. Graphene has unique properties related to its dimensionality, such as electronic, optical, mechanical and thermal. The surface of pure graphene usually interacts with other molecules via physical adsorption (π-π interactions). To allow the graphene surface to be more reactive, defects or surface functional groups are generally introduced, like carboxyl, carbonyl and amino groups can adjust the surface properties and electronic properties of graphene. The electrical conductivity (up to 2104 S/cm) can be 100 times higher than silicon [3–8].
A more recent variation of the carbon-based nanomaterials is the graphene quantum dots (GQDs). Among all the carbon-based family the GQDs are the most prominent in the use for biomedical applications, especially due the distinctive and tunable photoluminescence properties, physicochemical properties, high photostability, good biocompatibility, and small size. In general, GQDs are composed of a few layers of graphene fragments, with an absorption in the UV region (230–300 nm), which may change according to the size, since the quantum confinement effect and density of sp2 sites are size-dependent [9–11]. Finally, GQDs presents low toxicity for cells and in vivo, corroborating their use for biomedical application [12–14].
Leukemia is defined as an aberrant hyper-proliferation of immature blood cells that do not form solid tumor masses (i.e., liquid cancer). Usually, leukemia could be either of the myeloid or lymphoid lineages, and is classified as acute or chronic in nature. Leukemia is the most common type of cancer in children and adolescents, accounting for between 25% and 35% of all pediatric neoplasms. Leukemias arise in cells of the hematopoietic system. They originate from immature cells of the bone marrow. In the early stage of lymphoblast development, for example, a rapid onset disease called acute lymphoid leukemia results. When it originates in cells related to the development of granulocytic and monocytes cells, they are called acute myeloid leukemia. In most countries, acute lymphoid leukemia is more common among children under 5 years of age, its frequency decreases with increasing age and accommodates more boys than girls. For acute myeloid leukemia, these rates are five times lower than all leukemia’s combined. Is important to notice that chronic leukemias tend to have more mature cells and are rare in pediatric patients, while the acute ones are typically less mature and commonly occur in patients of all ages and are potentially rapidly fatal if not readily treated [15]. Also, some studies have shown that GQDs are capable to interact with a great variety of molecules, drugs, DNA and radioactive materials, with a wide range of application including: i) bioimaging and biosensing, ii) drug delivery, and ii) photothermal and photodynamic therapy [12,13]. Finally, the GQDs presents low toxicity for cells and in vivo, corroborating their use for biomedical application [14–16].
In this study we have developed, characterized and tested in vitro the potential use of graphene quantum dots decorated with imatinib as a nanodrug for leukemia therapy.
2. Materials and methods
The both synthesis and characterization of graphene are comprehensively described in Menezes et al. [18]. Briefly, electrochemically green route was used to produce the graphene. Initially, graphite rod and a platinum, respectively anode and cathode were used in a buffer solution of citric acid: sodium citrate. The decomposition was performed using a constant current of 190 mA for 24 h (ICEL PS-1500). After electrolysis the dispersion was filtered and dried at 60 °C. Then, 50 mL of pure ethanol was added and the upper phase (purified graphene) was collected and dried again at 60 °C. All the characterization methods included atomic force microscopy (AFM) (Bruker, Santa Barbara) (scan resolution of 256 × 256 lines and a frequency of 0.5 Hz), UV–Visible (Cary 60 UV–Vis, Agilent) (emission at wavelength of 395 nm), fluorescence spectrophotometer (Ocean Optics HR2000), powder X-ray diffraction (PXRD) (Bruker AXS D8) using a CuKα radiation source and Raman analysis were performed by Horiba-Jobin-Yvon triple spectrometer (model T64000) [18].
2.1. Graphene quantum dots decoration process with imatinib
Imatinib decorated graphene quantum dots (GQDs@imatinib) was obtained via a carbodiimide functionalization reaction. For the reaction, GQDs (Graphene Quantum Dot) (13,5 mg) previously produced and described [16] was dispersed in 10 mL of water by ultrasonication (5 min, 155w of potency, 50/60 Hz of frequency) at room temperature. A solution by 12,5 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Oakwood chemical) and 38,2 mg of N-hidroxisuccimida (NHS) (Alfa Aesar) was prepared in 2,5 ml of distilled water and then added of 13 mg of GQDs dispersion. Subsequently, the mixture was stirred for 4 h at room temperature. Then, 12,9 mg of imatinib (Sigma-Aldrich) was diluted in 1 ml of distilled water and add to the mixture and stirred for 18 h. The resulted material was centrifuged (13 300 rpm, 5 min) and washed three times with distilled water to remove unbound reagents. The quality of the reaction has been confirmed by Fourier-transformed infrared spectroscopy (FTIR) (IR-tracer-100 Simadzu).
2.2. FTIR spectroscopy analysis
Infrared absorption spectra were captured using the method FTIR with UATR accessory (universal sampling arm accessory with pressure) were reached at room temperature on a spectrophotometer with transformed by Fourier from PerkinElmer (model: Spectrum 400, Serial No: 82 287) in the region between 4000 and 400 cm-1. The spectral resolution was 2 cm-1 and 32 accumulations.
2.3. Atomic force microscopy
Samples of Imatinib decorated graphene quantum dots (GQDs@imatinib) were solubilized in distilled water and 10 μm was dropped in fresh cleaved mica and rest until complete drying. The AFM measurements of graphene QDs film was performed using a Multimode 8 microscope (Bruker, Santa Barbara, CA) in QNM (Quantitative Nanomechanics) mode using probes model Scanasyst Air, with nominal spring constant of 0.4 N/m and nominal tip radio of 2 nm approximately. The particle and section analysis was performed with NanoScope Analysis 1.50 software and the images resolution was 256 samples per lines.
2.4. Cell viability assay
To study the anticancer activity of imatinib, GQD, and GQDs@imatinib, the human myeloma cells (RPMI 8226) and ovarian cancer cells (NCI-ADR/RES) were grown at 3 × 103 cells/well in 96-well plates. The imatinib, GQD, or GQDs@imatinib was incubated with the cells at various concentrations (0.1–1000 μg/mL) for 72 h. The cytotoxicity was evaluated by the CellTiter-Blue® Cell Viability Assay [17]. Briefly, 10 μL of the reagent was diluted with 90 μL of the complete growth medium per well and incubated with the cells at 37 °C for 2 h. Then, the fluorescence intensity was determined at λex = 560 nm and λem = 590 nm on a microplate reader. The four-parameter dose-response curves were made and the corresponding half maximal inhibitory concentrations (IC50) were calculated by GraphPad Prism software v8.3 (GraphPad Software).
2.5. Apoptosis analysis
The cell apoptosis was analyzed by the Annexin V-FITC and propidium iodide (PI) double staining [18]. Briefly, the RPMI 8226 cells (6 × 103 cells/well) were incubated with imatinib, GQD, or GQDs@imatinib at 100 μg/mL in the complete growth medium for 48 h. The treated cells were collected by centrifugation and resuspended in 100 μL of the binding buffer. The cells were stained with 10 μL annexin V-FITC and 10 μL PI for 15 min at room temperature and analyzed by the flow cytometry.
2.6. Trafficking assay
MDA-MB-231 (ATCC® HTB-26™) were seeded at 2500 cells/cm2 and reaching 60% confluence, exposed to 20 μg/ml graphene quantum dots for, alternatively, 30 min or 2 h. Following, cells were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2), post-fixed in 1% osmium tetroxide (OsO4) and 0.8% potassium ferrocyanide in 0.1 M cacodylate buffer during 40 min, dehydrated in a series of acetone gradation (70–90-100%), and embedded in epoxy resin. Ultrathin sections (60–70-nm thick) were collected, stained with uranyl acetate and lead citrate. At the end, the samples were analyzed using a Tecnai Spirit TEM (FEI Co. The Netherlands).
3. Results
3.1. Graphene quantum dots decoration process with imatinib
The synthesis of graphene quantum dots@imatinib (GQDs@imatinib) was performed by GQDs functionalization with EDC (ethyl-3-(3-dimethylaminopropyl)carbodiimide) and NHS (N-hydroxysuccinimide) to activate carboxyl groups of GQDs (Fig. 1). FTIR spectroscopy analysis GQDs (Fig. 2) shows adsorption bands at 3480–3520 cm-1, 1762 cm-1 and 1041 cm-1, corresponding to OH, C=O and >O, respectively. The chemical changes of graphene quantum dots@imatinib (GQDs@imatinib) were analyzed comparing the FITR spectra of the decorated GQDs with the FITR spectra of the pure GQDs.
Fig. 1.
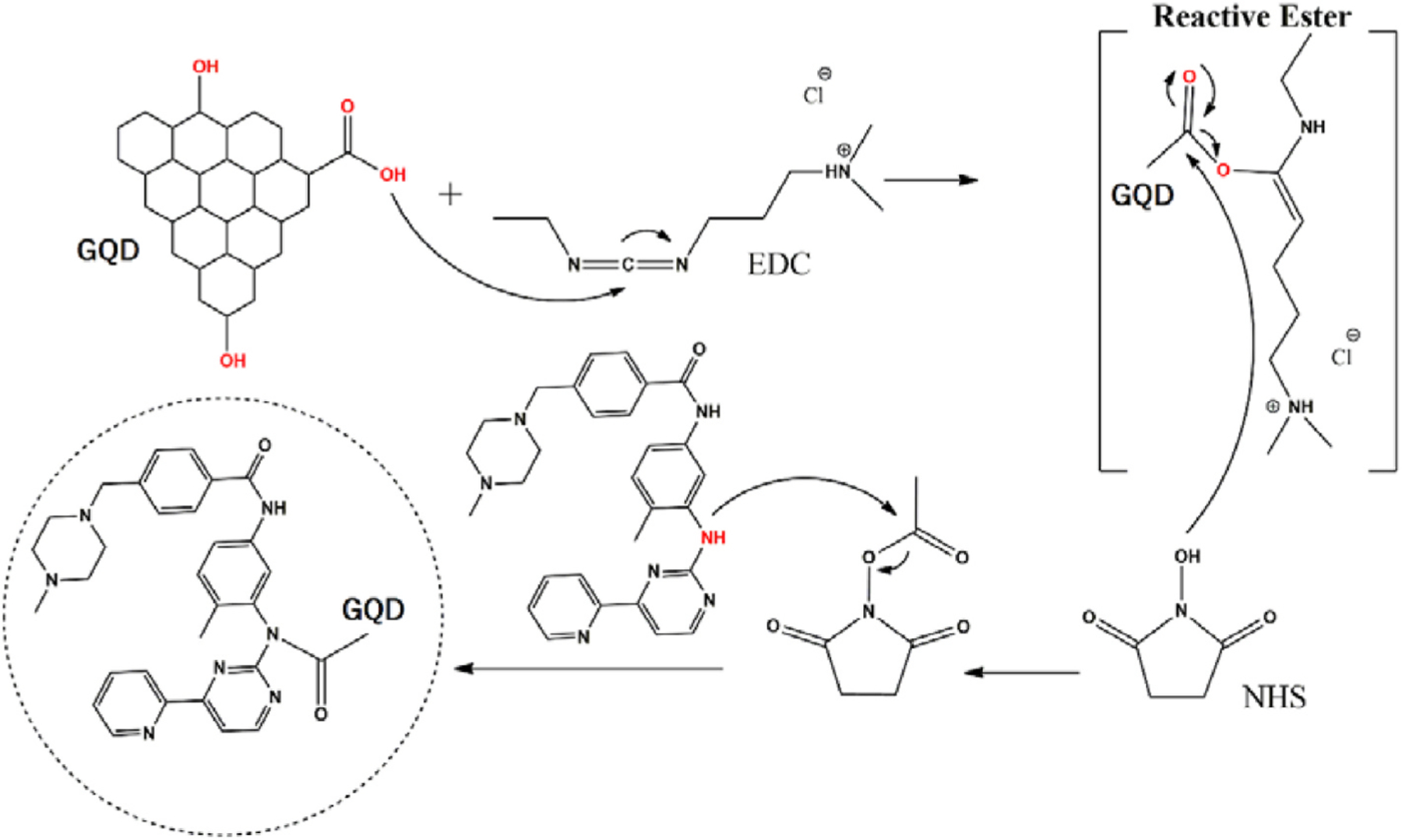
Functionalization using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and 38,2 mg N-hidroxisuccimida (NHS) and decoration with imatinib.
Fig. 2.
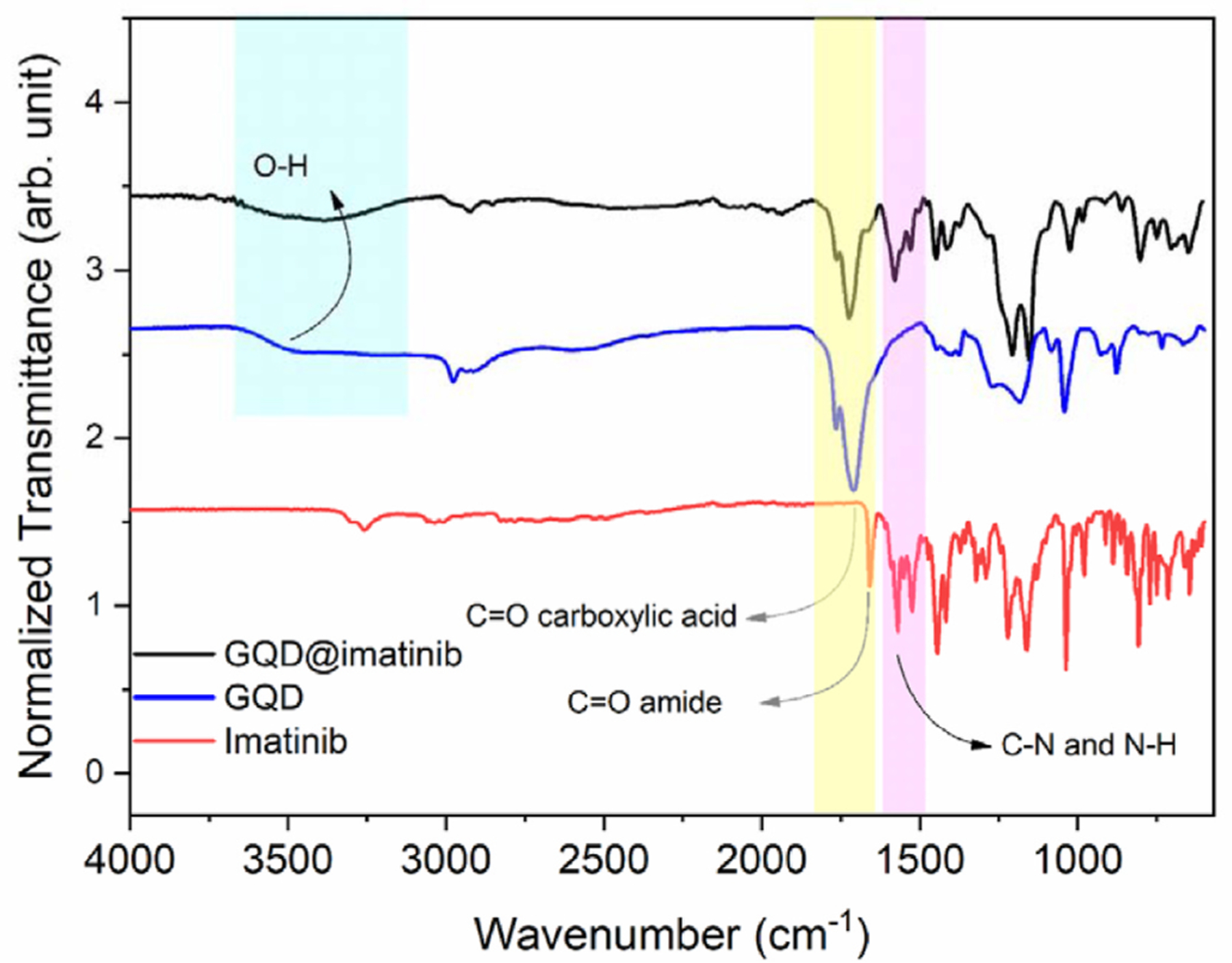
Fourier-transform infrared spectroscopy analysis comparing the pure GQDs, the GQDs decorated with imatinib and the pure imatinib.
The data showed the formation of a new band at 1664 cm-1 in the GQDs@imatinib spectra, corresponding to amide vibration, which suggest the covalent ligation formed between amine from imatinib group with the active carboxyl groups from GQDs. Also there are peaks at 1530 cm-1 e 1576 cm-1 which corresponds to C-N and N-H vibration.
3.2. Atomic force microscopy
The atomic force microscopy (Fig. 3) showed quantum dot property of the GQDs and corroborated the topographical 2D. The QNM analysis with energy dispersion evidenced the complexation of imatinib with the graphene quantum dot corroborating the FTIR analysis.
Fig. 3.
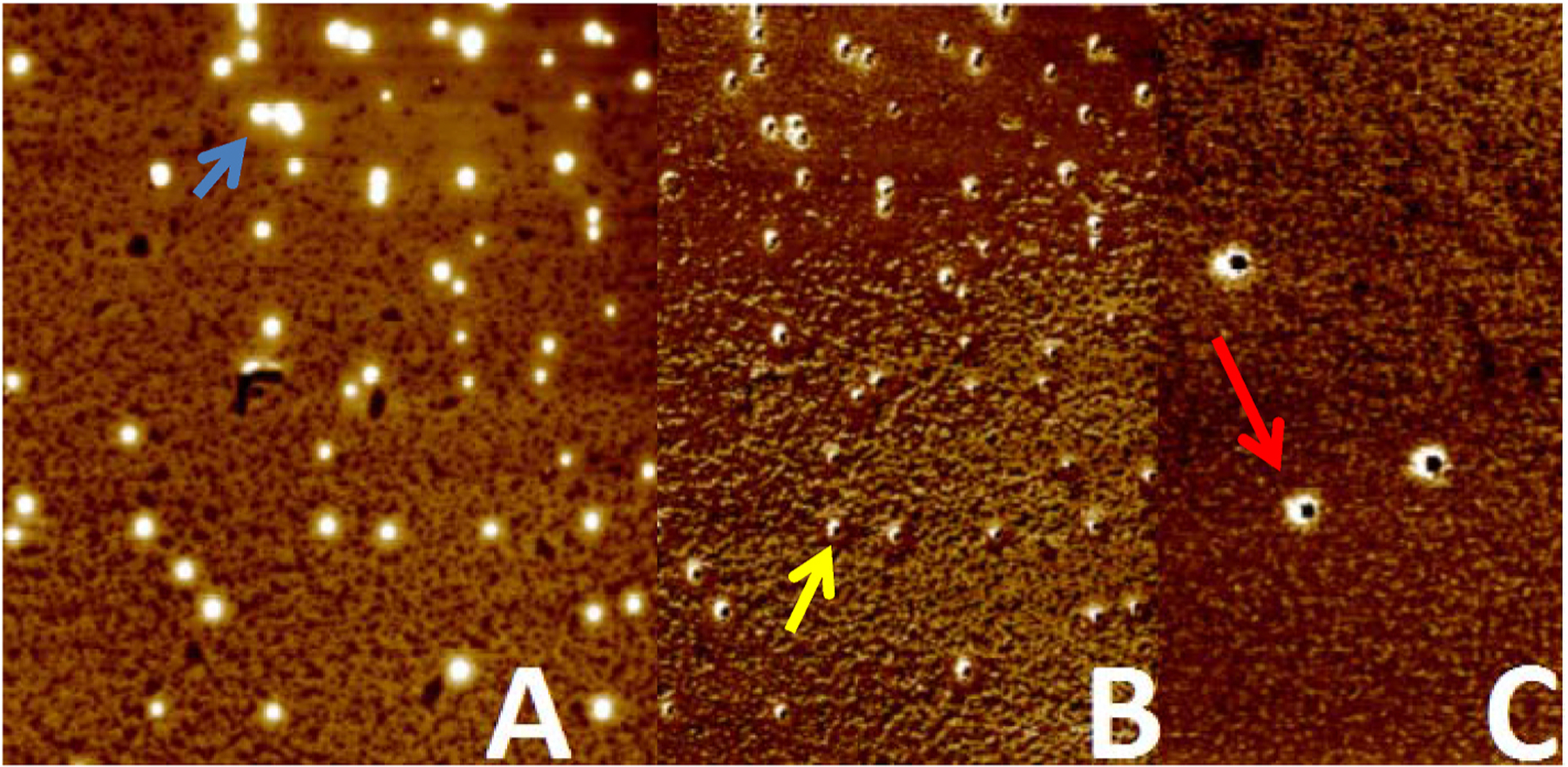
A: Overview analysis. B: Topographical analysis and C: QNM mode with energy dispersion analysis. The blue arrow shows the quantum property. The yellow arrow shows the height of the graphene quantum dot and the red arrow shows the energy dispersion regarded to the imatinib complexation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Cellular trafficking assay
In order to establish the permeation of the GQDs@imatinib into cells and predicts it behavior we performed cellular trafficking assay. Nonetheless, is important to fully understand if GQDs@imatinib is capable to enter cells cytoplasm and block effectively the Bcr-Abl tyrosine kinase, since imatinib inhibits Bcr–Abl kinase, mainly presented in cytoplasm, causing apoptosis [19–21].
First, was evaluated the behavior of GQDs@imatinib into the cells medium. In this direction, 1 mg/ml of GQDs@imatinib was resuspended in culture medium Dulbecco’s Modified Eagle’s medium (DMEM) high glucose supplemented with 10% serum fetal bovine. The influence on the particle was a partial agglomeration with size ranging from 40 to 200 nm (Fig. 4).
Fig. 4.
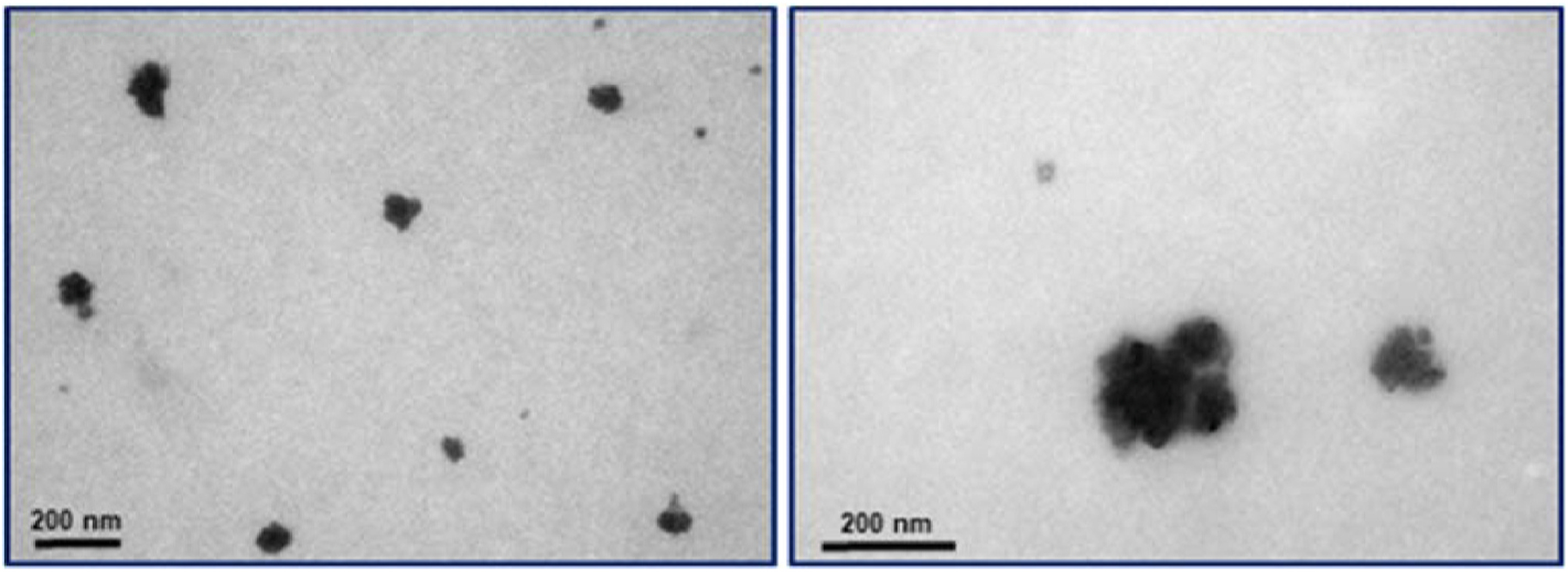
Physicochemical characterization pure graphene quantum dots in medium culture. Representative STEM images of graphene quantum dots agglomerates.
In order to verify cellular internalization of GQDs@imatinib MDA-MB-231 (ATCC® HTB-26™), mammary cell line derived from metastatic site were exposed to 20 μg/ml GQDs@imatinib during 1 and 24 h (Fig. 5). Recently, a study performed by Chevalier et al. [22] correlated the presence of ABL tyrosine kinase in MBA-MB-231 cells, which influenced our choice for this cell line to perform this assay.
Fig. 5.
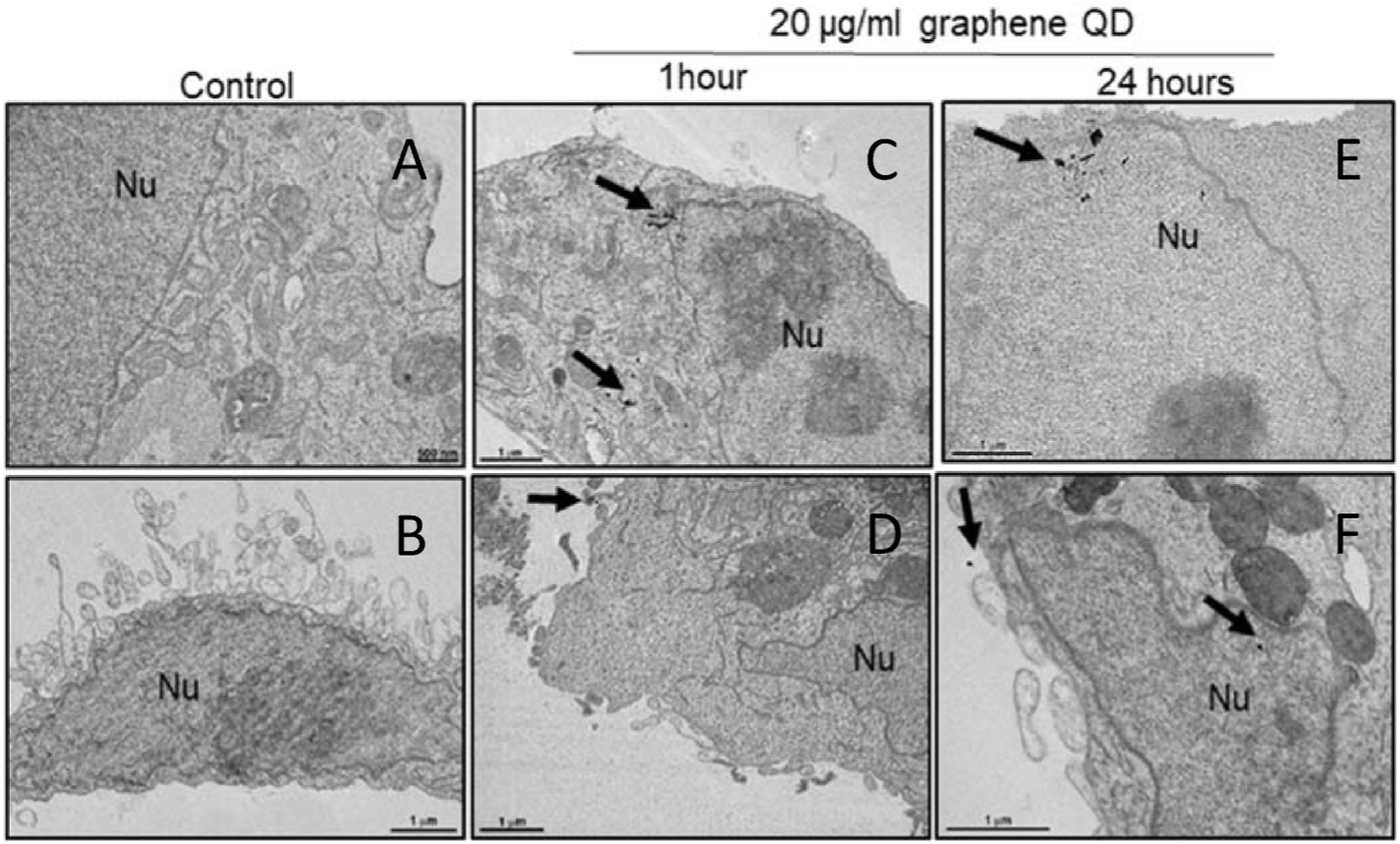
Electron micrographs of MDA-MB-231 (ATCC® HTB-26™): (A and B) untreated cells (C and D) 1 h exposed (E and F) 24 h exposed to graphene quantum dots. Note graphene quantum dots presence (black arrow) in cytoplasm after 1 h’s exposition and nuclear translocation after 24 h exposition. Nu- Nucleus.
3.4. Cell viability assay
The cytotoxicity of imatinib, GQD, and GQDs@imatinib was evaluated by the CellTiter-Blue® Cell Viability Assay in both adherent cells (NCI-ADR/RES) and suspension cells (RPMI 8226) (Fig. 6). The imatinib showed the highest cytotoxicity among the treatments in both cell lines. The GQDs@imatinib showed a slightly decreased cytotoxicity, which was probably due to the delayed drug release/dissociation. In contrast, the GQDs were very safe, indicating its safety as a delivery nanocarrier. The calculated IC50s showed the same results (Table 1).
Fig. 6.
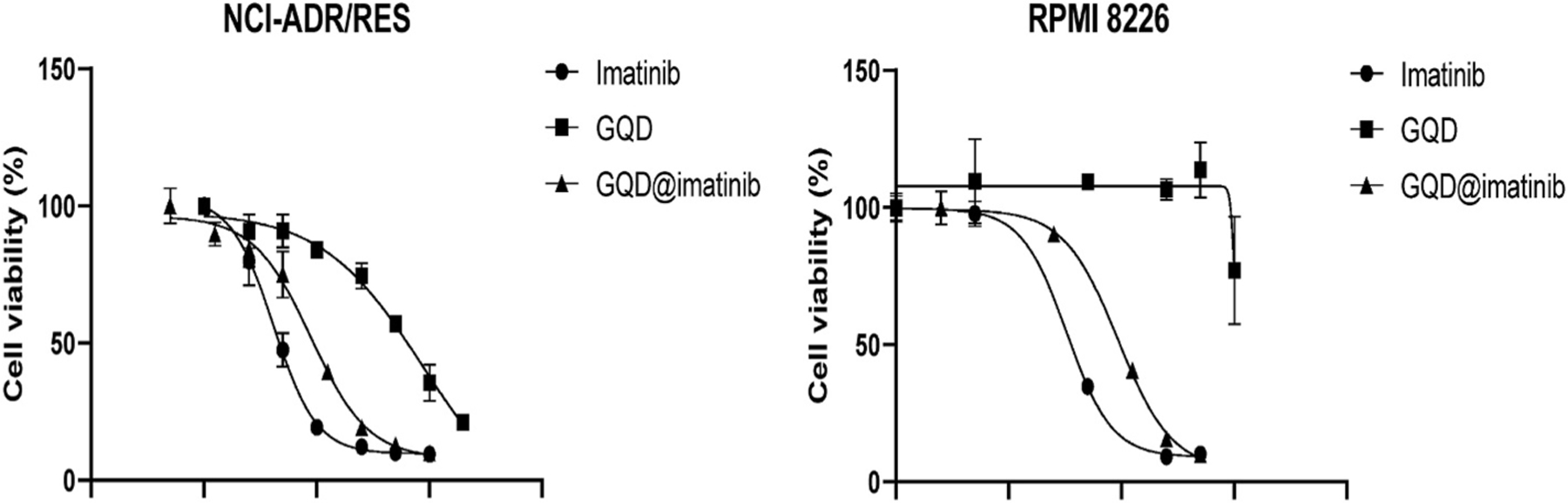
The cell viability assay after 72 h treatments in the NCI-ADR/RES (A) and RPMI 8226 cells (B).
Table 1.
The IC50s of the treatments (μg/mL).
| Cell lines | Imatinib | GQD | GQDs@imatiniba |
|---|---|---|---|
| RPMI 8226 | 32.61 | 991.1 | 93.59 |
| NCI-ADR/RES | 41.35 | 849.2 | 88.93 |
Equivalent imatinib dose.
3.5. Apoptosis analysis
The apoptotic and dead cells were stained by the annexin V-FITC and PI and analyzed by flow cytometry. In the RPMI 8226 cells, at 48 h, the number of the apoptotic cells (annexin V positive) upon imatinib and GQDs@Imatinib treatments significantly increased compared to those of the untreated cells (Fig. 7A). The number of the late apoptotic and dead cells (PI positive) upon the imatinib treatment significantly increased compared to the untreated cells, while the GQDs@imatinib only caused the mild cell death (Fig. 7B), in consistent with the cytotoxicity data. In contrast, the GQDs didn’t cause significant cell apoptosis/death.
Fig. 7.
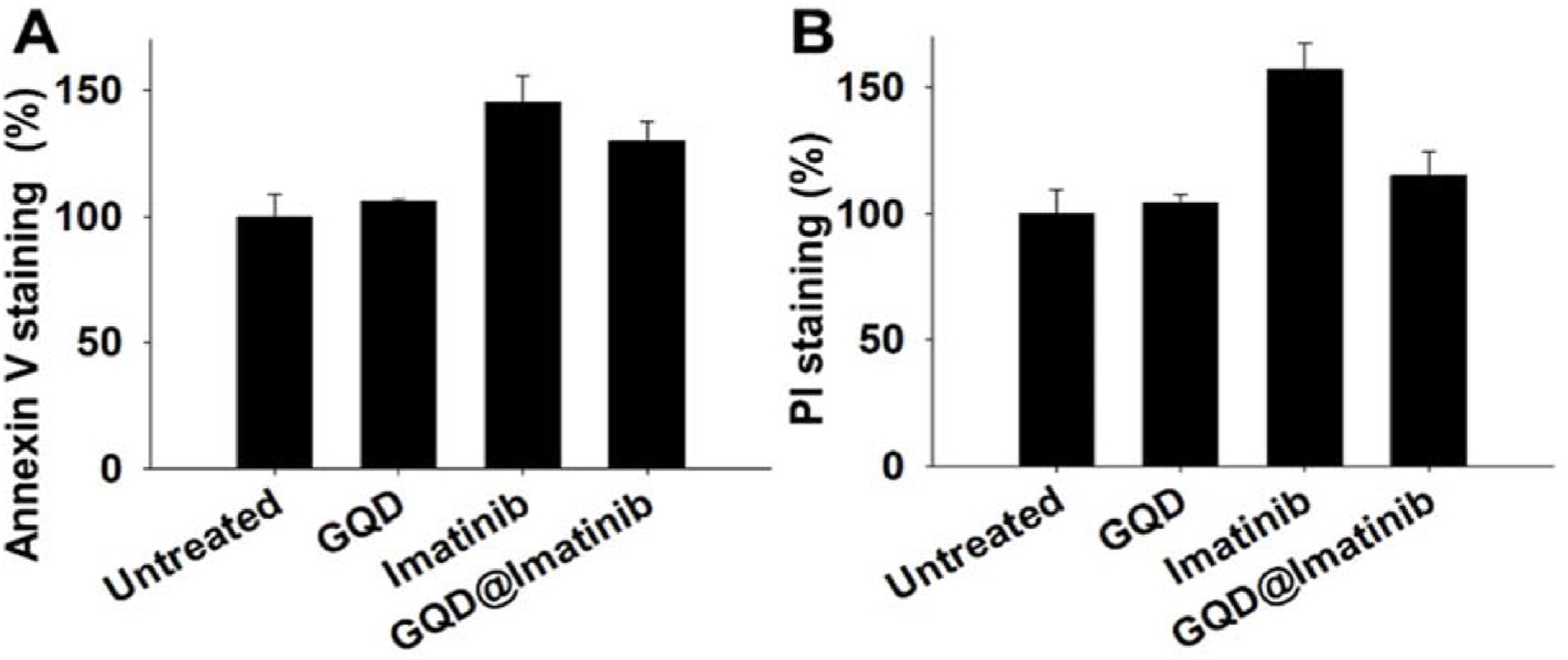
The apoptosis and death of the RPMI 8226 cells analyzed by the annexin V (A) and PI (B) double staining.
4. Discussion
According to Dreyer et al. [23] graphene and the reduced form (graphene oxide) can easily perform reduction reactions due the disrupted sp2 bonding networks. Aside this, graphene shows reactive oxygen: carboxylic acids (in the edges) and epoxy and hydroxyl groups (in the basal planes) [24,25] (Fig. 8). The presence of the carboxylic acids and hydroxyl groups allows the carbodiimide crosslink reaction using EDC (ethyl-3-(3-dimethylaminopropyl)carbodiimide) and NHS (N-hydroxysuccinimide).
Fig. 8.
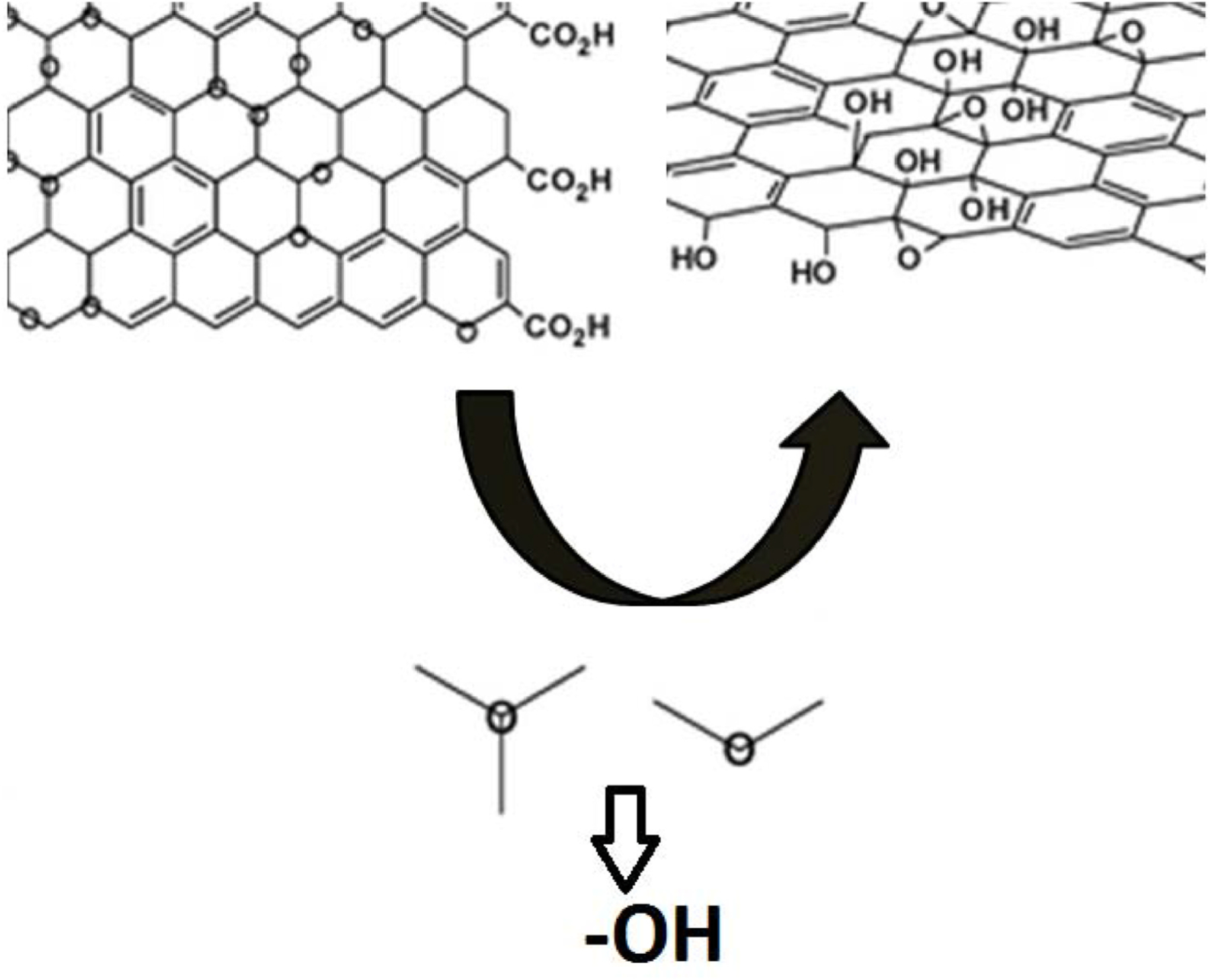
Schematic representation of the presence of carboxylic acid (edge) and hydroxyl groups in graphene adapted from Ref. [26].
The activation of acid group by the 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and subsequent addition of esters (nucleophilic specie) by the use of NHS (N-hydroxysuccinimide) is a common methodology to covalent functionalization, and the final product may be used as a drug delivery device [27–29].
The EDC carbodiimide/NHS crosslink reaction used in this study efficiently produced graphene quantum dots functionalized with imatinib. This reaction active the carboxyl groups from GQDs assisting the formation of amide bond, which increase the applicability for insertion of proteins, peptides and chemotherapeutics that are rich substrates with a great variety of functional groups in their chemical structure [30–32]. The FITR analysis compared pure GQDs, GQDs decorated with imatinib and the pure imatinib. The results showed peaks that evidenced the presence of groups OH and C=O and a new band at 1664 cm-1 corresponding to amide bond formed in the conjugated GQDs@imatinib.
The atomic force microscopy (AFM) analysis, especially the QNM analysis corroborated the functionalization of the GQDs with imatinib. Also showed that functionalization of GQDs with imatinib did not interfere in the size of the GQDs. The AFM analysis showed a GQD with a size range from 162 to 287 nm with a height of 5 nm, practically the same result described by Menezes et al. [33].
The trafficking assay showed cells internalization of GQDs@imatinib nanoparticles with morphology compatible with graphene quantum dots. These particles appeared after 1 h in cell cytoplasm and after 24 h images shows nuclear translocation (Fig. 5). The presence of GQDs@imatinib nanoparticles into the nucleus is an advantage. According to Huang et al. [34], Bcr-Abl has three nuclear localization signals and one nuclear export signal. Nonetheless, although Bcr-Abl is mainly located in the cytoplasm, the c-Abl shuttles between the nucleus and cytoplasm [35–37]. Thus the presence of GQDs@imatinib, a potent inhibitor of Bcr-Abl and c-ABL in the nucleus may predicts a stronger effect of the nanosystem when compared to the solely effect of pure imatinib.
The cell viability data and apoptosis analysis showed that: i) the graphene quantum dot (GQD) alone is harmless for the cells tested and ii) the GQDs@imatinib was less efficient than the pure imatinib. The first finding is quite desirable and demonstrated that any effect caused by the GQDs@imatinib is due to the presence of the imatinib. Also corroborated the low cytotoxic of the pure GQDs [38]. Regarding the second result, the lower cytotoxic effect of the conjugated imatinib (GQDs@imatinib) observed comparing with the free imatinib can be explained by the delayed release/dissociation of imatinib from the GQDs. According to Liu et al. [39], the similar result was observed using paclitaxel (PTX) nanoconjugate (PEG2000-peptide-PTX), in which the drug release process (cleavage) delayed the PTX’s action. As explained above the functionalization of the GQDs with imatinib occurred by a covalent-bond crosslinking reaction. The formed amide bond may be relatively stable and requires a longer contact time for the cleavage. However, controversially with the lower efficacy when compared with the free imatinib, the GQDs@imatinib was able to produce significant cancer cell-killing effects, supporting the use of this platform as a drug delivery system. Here, we speculate that in an in vivo biological environment (human or animals) the GQDs@imatinib’s anticancer effects would be more significant, considering the myriads of endogenous factors as the EPR effects and tumor microenvironment, such as pH variation, temperature variation and enzymes [40,41]. Our findings are corroborated by Jian et al. [42], which have used metal-organic framework composite associated with graphene quantum dots for leukemia treatment with positive results. Also, the studies performed by Russier et al. [43] demonstrated that few-layer graphene (FLG) dispersions are capable to specifically kill myelomonocytic leukemia. Thus, the use of GQDs@imatinib may represent a safety and new approach for leukemia treatment.
5. Conclusion
The study showed that is possible to functionalize the graphene quantum dot with a small molecule as imatinib using a rapid carbodiimide crosslinking reaction. Also the data showed that conjugation of the imatinib with the GQDs did not change the size as the shape. The trafficking assay corroborates the internalization of the GQDs@imatinib. The cytotoxicity as the apoptosis assay showed that GQDs@imatinib are capable to produce a remarkable cancer cell proliferation.
The results obtained in this study corroborated the potential use of graphene quantum dots decorated with imatinib as a nanosystem for leukemia treatment. The use of a pure-carbon nanosystem, easily decorated, with the ability to penetrates the cancer cell as efficiently kills the target cell is innovative approach in the blood cancer treatment.
Acknowledgement
The authors would like to kindly thank CAPES PROEX-PPGB-UERJ (23038.002824/2018–20) and CNPq Bolsa Produtividade (301069/2018–2) to Dr. Ralph Santos-Oliveira. Also, The work was partially supported by the National Cancer Institute of the National Institutes of Health (R15CA213103) to Dr. Lin Zhu.
Footnotes
Declaration of competing interest
The authors state that do not have any conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jddst.2020.102117.
References
- [1].Raslan Ahmed, Del Burgo Laura Saenz, Ciriza Jesús, Pedraz Jose Luis, Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine, Int. J. Pharm (Mar. 2020) 119226–119240, 10.1016/j.ijpharm.2020.119226. Elsevier BV [DOI] [PubMed] [Google Scholar]
- [2].Xie Zhongjian, Duo Yanhong, Lin Zhitao, Fan Taojian, Xing Chenyang, Yu Li, Wang Renheng, Qiu Meng, Zhang Yupeng, Zhao Yonghua, The rise of 2D photothermal materials beyond graphene for clean water production, Adv. Sci 7 (5) (27 jan. 2020) 1902236–1902250, 10.1002/advs.201902236. Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tade Rahul S., Nangare Sopan N., Patil Ashwini, Pandey Abhijeet, Deshmukh Prashant K., Dilip Ramsing Patil Tanisha Agrawal, Mutalik Srinivas, Patil AM, Mahesh P. More, Recent advancement in bio-precursor derived graphene quantum dots: synthesis, characterization and toxicological perspective, Nanotechnology 10 (4) (16 mar. 2020) 1–70, 10.1088/1361-6528/ab803e. IOP Publishing. [DOI] [PubMed] [Google Scholar]
- [4].Sekiya Ryo, Haino Takeharu, Chemically functionalized two-dimensional carbon materials, Chem. Asian J (4 mar. 2020) 327–345, 10.1002/asia.202000196. Wiley. [DOI] [PubMed] [Google Scholar]
- [5].Yang Gao, Li Lihua, Bun Lee Wing, Man Cheung Ng, Structure of graphene and its disorders: a review, Sci. Technol. Adv. Mater 19 (1) (29 ago. 2018) 613–648, 10.1080/14686996.2018.1494493. Informa UK Limited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Allen Matthew J., Tung Vincent C., Kaner Richard B., Honeycomb carbon: a review of graphene, Chem. Rev 110 (1) (13 jan. 2010) 132–145, 10.1021/cr900070d. American Chemical Society (ACS). [DOI] [PubMed] [Google Scholar]
- [7].Tang Qing, Zhou Zhen, Chen Zhongfang, Graphene-related nanomaterials: tuning properties by functionalization, Nanoscale 5 (11) (2013) 4541–4561, 10.1039/c3nr33218g. Royal Society of Chemistry (RSC). [DOI] [PubMed] [Google Scholar]
- [8].Zhao Guoke, Li Xinming, Huang Meirong, Zhen Zhen, Zhong Yujia, Chen Qiao, Zhao Xuanliang, He Yijia, Hu Ruirui, Yang Tingting, The physics and chemistry of graphene-on-surfaces, Chem. Soc. Rev 46 (15) (2017) 4417–4449, 10.1039/c7cs00256d. Royal Society of Chemistry (RSC). [DOI] [PubMed] [Google Scholar]
- [9].Banszerus Luca, Samuel Möller Eike Icking, Watanabe Kenji, Taniguchi Takashi, Volk Christian, Stampfer Christoph, Single-electron double quantum dots in bilayer graphene, Nano Lett 20 (3) (21 feb. 2020) 2005–2011, 10.1021/acs.nanolett.9b05295. American Chemical Society (ACS). [DOI] [PubMed] [Google Scholar]
- [10].Sun Chang Q., Size and confinement effect on nanostructures, ChemInform 37 (21) (23 maio 2006) 327–345, 10.1002/chin.200621219. Wiley. [DOI] [Google Scholar]
- [11].Huang Zhongkai, Qu Jinfeng, Peng Xiangyang, Liu Wenliang, Zhang Kaiwang, Wei Xiaolin, Zhong Jianxin. Quantum confinement in graphene quantum dots, Phys. Status Solidi Rapid Res. Lett 8 (5) (31 mar. 2014) 436–440, 10.1002/pssr.201409064. Wiley. [DOI] [Google Scholar]
- [12].Chung Seokhwan, Revia Richard A., Zhang Migin, Graphene quantum dots and their applications in bioimaging, biosensing, and therapy, Adv. Mater (2019 Dec 12), e1904362, 10.1002/adma.201904362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daniela Iannazzo, Ida Ziccarelli, Alessandro Pistone.Graphene quantum dots: multifunctional nanoplatforms for anticancer therapy. J. Mater. Chem. B 5(32): 6471–6489. [DOI] [PubMed] [Google Scholar]
- [14].Tade Rahul S., Nangare Sopan N., Patil Ashwini, Pandey Abhijeet, Deshmukh Prashant K., Patil Dilip Ramsing, Agrawal Tanisha, Mutalik Srinivas, Patil AM, Mahesh P. More, Recent advancement in bio-precursor derived graphene quantum dots: synthesis, characterization and toxicological perspective, Nanotechnology 5 (8) (16 mar. 2020) 357–378, 10.1088/1361-6528/ab803e. IOP Publishing. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Hao, Ba Sai, Yang Zhaoqi, Wang Tianxiang, Jasmine Yiqin Lee Tianhu Li, Shao Fangwei. Graphene quantum dot-based nanocomposites for diagnosing cancer biomarker APE1 in living cells, ACS Appl. Mater. Interfaces 5 (10) (10 mar. 2020) 127–145, 10.1021/acsami.9b21385. American Chemical Society (ACS). [DOI] [PubMed] [Google Scholar]
- [16].Liu Jing, Li Chengnan, Brans Toon, Harizaj Aranit, Van De Steene Shana, De Beer Thomas, Stefaan De Smedt, Szunerits Sabine, Boukherroub Rabah, Xiong Ranhua, Surface functionalization with polyethylene glycol and polyethyleneimine improves the performance of graphene-based materials for safe and efficient intracellular delivery by laser-induced photoporation, Int. J. Mol. Sci 21 (4) (24 fev. 2020) 1540–1565, 10.3390/ijms21041540. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Juliusson Gunnar, Hough Rachael, in: Leukemia.Progress in Tumor Research, S. Karger AG, 2016, pp. 87–100, 10.1159/000447076. [DOI] [PubMed] [Google Scholar]
- [18].De Menezes Frederico Duarte, Dos Reis Sara Rhaissa Rezende, Pinto Suyene Rocha, Portilho Filipe Leal, Francisco Do Vale Chaves E. Mello, Helal-Neto Edward, Da Silva De Barros Aline Oliveira, Rebêlo Alencar Luciana Magalhães, De Menezes Alan Silva, dos Santos Clenilton Costa, Graphene quantum dots unraveling: green synthesis, characterization, radiolabeling with 99mTc, in vivo behavior and mutagenicity, Mater. Sci. Eng. C 102 (Set. 2019) 405–414, 10.1016/j.msec.2019.04.058. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- [19].Dai Zhi, Yao Qing, Zhu Lin, MMP2-Sensitive PEG–lipid copolymers: a new type of tumor-targeted P-glycoprotein inhibitor, ACS Appl. Mater. Interfaces 8 (20) (12 maio 2016) 12661–12673, 10.1021/acsami.6b03064. American Chemical Society (ACS). [DOI] [PubMed] [Google Scholar]
- [20].Liu Yin, Dai Zhi, Wang Jiao, Tu Ying, Zhu Lin, Folate-targeted pH-sensitive bortezomib conjugates for cancer treatment, Chem. Commun 55 (29) (2019) 4254–4257, 10.1039/c9cc01344j. Royal Society of Chemistry (RSC). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Joensuu Heikki, Dimitrijevic Sasa, Tyrosine kinase inhibitor imatinib (STIS71) as an anticancer agent for solid tumours, Ann. Med 33 (7) (January. 2001) 451–455, 10.3109/07853890109002093. Informa UK Limited. [DOI] [PubMed] [Google Scholar]
- [22].Manley PW, Cowan-Jacob SW, Buchdunger E, Fabbro D, Fendrich G, Furet P, Zimmermann J, Imatinib: a selective tyrosine kinase inhibitor European Journal of Cancer 10.1016/s0959-8049(02)80599-8, 2002, 38, S19–S27. [DOI] [PubMed] [Google Scholar]
- [23].Brama Marina, Basciani Sabrina, Cherubini Sara, Mariani Stefania, Migliaccio Silvia, Arizzi Mario, Rosano Giuseppe, Spera Giovanni, Gnessi Lucio, Osteoblast-conditioned medium promotes proliferation and sensitizes breast cancer cells to imatinib treatment, Endocr. Relat. Canc 14 (1) (Mar. 2007) 61–72, 10.1677/erc.1.01307. Bioscientifica. [DOI] [PubMed] [Google Scholar]
- [24].Chevalier C, Cannet A, Descamps S, Sirvent A, Simon V, Roche S, et al. , ABL tyrosine kinase inhibition variable effects on the invasive properties of different triple negative breast cancer cell lines, PloS One 10 (3) (2015), e0118854, 10.1371/journal.pone.0118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dreyer Daniel R., Park Sungjin, Bielawski Christopher W., Ruoff Rodney S., The chemistry of graphene oxide, Chem. Soc. Rev 39 (1) (2010) 228–240, 10.1039/b917103g. Royal Society of Chemistry (RSC). [DOI] [PubMed] [Google Scholar]
- [26].Lerf Anton, He Heyong, Forster Michael, Klinowski Jacek, Structure of graphite oxide revisitedǁ, J. Phys. Chem. B 102 (23) (Jun. 1998) 4477–4482, 10.1021/jp9731821. American Chemical Society (ACS). [DOI] [Google Scholar]
- [27].Chua Chun Kiang, Pumera Martin, The reduction of graphene oxide with hydrazine: elucidating its reductive capability based on a reaction-model approach, Chem. Commun 52 (1) (2016) 72–75, 10.1039/c5cc08170j. Royal Society of Chemistry (RSC). [DOI] [PubMed] [Google Scholar]
- [28].Dreyer Daniel R., Park Sungjin, Christopher W. Bielawski, Rodney S. Ruoff, The chemistry of graphene oxide, Chem. Soc. Rev 39 (1) (2010) 228–240, 10.1039/b917103g. Royal Society of Chemistry (RSC). [DOI] [PubMed] [Google Scholar]
- [29].Liu Zhuang, Robinson Joshua T., Sun Xiaoming, Hongjie Dai, PEGylated nanographene oxide for delivery of water-insoluble cancer drugs, J. Am. Chem. Soc 130 (33) (2008) 10876–10877, 10.1021/ja803688x. American Chemical Society (ACS). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ionińă Mariana, Vlăsceanu Mihail George, Watzlawek Aiza Andreea, Voicu Stefan Ioan, Burns Jorge S., Horia Iovu, Graphene and functionalized graphene: extraordinary prospects for nanobiocomposite materials, Compos. B Eng 121 (Jul. 2017) 34–57, 10.1016/j.compositesb.2017.03.031. Elsevier BV. [DOI] [Google Scholar]
- [31].Liu Zhuang, Robinson Joshua T., Sun Xiaoming, Hongjie Dai, PEGylated nanographene oxide for delivery of water-insoluble cancer drugs, J. Am. Chem. Soc 130 (33) (2008) 10876–10877, 10.1021/ja803688x. American Chemical Society (ACS). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo Jiubiao, Chan Edward WC, Chen Sheng, Zhenling Zeng, Development of a novel quantum dots and graphene oxide based FRET assay for rapid detection of invA gene of Salmonella, Front. Microbiol 8 (10) (17 jan. 2017) 327–350, 10.3389/fmicb.2017.00008. Frontiers Media SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tian Bowen, Kou Yanxia, Jiang Xiangmei, Lu Jiajia, Xue Yuanyuan, Wang Meijuan, Tan Liang, Ultrasensitive determination of mercury ions using a glassy carbon electrode modified with nanocomposites consisting of conductive polymer and amino-functionalized graphene quantum dots, Microchimica Acta 187 (4) (9 mar. 2020) 357–388, 10.1007/s00604-020-4191-1. Springer Science and Business Media LLC. [DOI] [PubMed] [Google Scholar]
- [34].Slekiene Nora, Snitka Valentinas, Impact of graphene oxide functionalized with doxorubicin on viability of mouse hepatoma MH-22A cells, Toxicol. Vitro (Mar. 2020) 104821–104842, 10.1016/j.tiv.2020.104821. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- [35].Huang Zheng-Lan, Gao Miao, Li Qian-Yin, Tao Kun, Xiao Qing, Cao Wei-Xi, Feng Wen-Li, Induction of apoptosis by directing oncogenic Bcr-Abl into the nucleus, Oncotarget 4 (12) (10 out. 2013) 328–346, 10.18632/oncotarget.1339. Impact Journals, LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dierov Jamil, Dierova Raia, Carroll Martin, BCR/ABL translocates to the nucleus and disrupts an ATR-dependent intra-S phase checkpoint, Canc. Cell 5 (3) (Mar. 2004) 275–285, 10.1016/s1535-6108(04)00056-x. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- [37].Huang Zheng-Lan, Gao Miao, Li Qian-Yin, Tao Kun, Xiao Qing, Cao Wei-Xi, Feng Wen-li, Induction of apoptosis by directing oncogenic Bcr-Abl into the nucleus, Oncotarget 4 (12) (10 out. 2013) 322–342, 10.18632/oncotarget.1339. Impact Journals, LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hantschel Oliver, Wiesner Silke, Güttler Thomas, Mackereth Cameron D., Remsing Rix Lily L., Mikes Zsuzsanna, Dehne Jana, Görlich Dirk, Sattler Michael, Giulio Superti-Furga, Structural basis for the cytoskeletal association of bcr-abl/c-abl, Mol. Cell 19 (4) (2005) 461–473, 10.1016/j.molcel.2005.06.030. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- [39].Arvidsson Rickard, Boholm Max, Johansson Mikael, de Montoya Monica Lindh, “Just carbon”: ideas about graphene risks by graphene researchers and innovation advisors, Nanoethics 12 (3) (22 out. 2018) 199–210, 10.1007/s11569-018-0324-y. Springer Science and Business Media LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhu L, Wang T, Perche F, Taigind A, Torchilin VP, Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety, Proc. Natl. Acad. Sci. Unit. States Am 110 (42) (23 set. 2013) 17047–17052, 10.1073/pnas.1304987110. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nunzio Di Mattia, Valli Veronica, Tomás-Cobos Lidia, Tomas-Chisbert Teresa, Murgui-Bosch Lucía, Danesi Francesca, Bordoni Alessandra, Is cytotoxicity a determinant of the different in vitro and in vivo effects of bioactives? BMC Compl. Alternative Med 17 (1) (7 set. 2017) 327–351, 10.1186/s12906-017-1962-2. Springer Science and Business Media LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jia Qiaojuan, et al. , A γ-cyclodextrin-based metal–organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy, Nanoscale 11 (2019) 20956–20967. [DOI] [PubMed] [Google Scholar]
- [43].Russier Julie, et al. , Few-layer graphene kills selectively tumor cells from myelomonocytic leukemia patients, Angew. Chem., Int. Ed. Engl 56 (11) (2017 Mar 6) 3014–3019. [DOI] [PubMed] [Google Scholar]


