Abstract
Vitamin D (VitD) deficiency has been shown to be a risk factor for a plethora of disorders. We have shown that dogs with clinical leishmaniasis presented lower VitD serum levels than non-infected dogs, and even lower than those with asymptomatic infection. However, if VitD deficiency is a risk factor to develop clinical leishmaniasis remains to be answered. It is also unknown if VitD participates in Leishmania control. First, we retrospectively analysed VitD concentration in serum samples from 36 healthy dogs collected in different periods of the year concluding that there isn’t a seasonal variation of this vitamin in dogs. We also included 9 dogs with clinical leishmaniasis and 10 non-infected healthy dogs, in which we measured VitD levels at the beginning of the study, when all dogs were negative for serology and qPCR, and 1 year later. Whereas non-infected dogs showed no change in VitD levels along the study, those developing clinical leishmaniasis showed a significant VitD reduction at the end of the study (35%). When we compared VitD concentration between the two groups at the beginning of the study, no differences were detected (43.6 (38–59) ng/mL, P = 0.962). Furthermore, an in vitro model using a canine macrophage cell line proved that adding active VitD leads to a significant reduction in L. infantum load (31.4%). Analyzing expression of genes related to VitD pathway on primary canine monocytes, we showed that CBD103 expression was significantly enhanced after 1,25(OH)2D addition. Our results show that VitD concentration is neither seasonal nor a risk factor for developing canine leishmaniasis, but it diminishes with the onset of clinical disease suggesting a role in parasitic control. Our in vitro results corroborate this hypothesis and point out that VitD regulates infection through CBD103 expression. These results open the possibility for studies testing VitD as an adjuvant in leishmaniasis therapy.
Author summary
Vitamin D (VitD), the precursor of the powerful steroid hormone calcitriol, has been widely known to regulate the calcium and phosphate homeostasis. Several studies have shown that VitD plays also an important role on innate immunity. The mechanisms by which VitD modulates the immune function have been studied within different contexts involving multiple pathogens, but not Leishmania sp. It is known that VitD strengthens the innate immune system by inducing the expression of anti-microbial peptides in Mycobacterium tuberculosis-infected human macrophages. Antimicrobial peptides act on the bacterial wall, increasing the formation of reactive oxygen species, modulating cytokine expression, and inducing autophagy. Immune system plays a key role in leishmaniasis disease control, thus VitD could have a relevant contribution in leishmaniasis. In a previous study, we shown that clinical leishmaniasis is associated with VitD deficiency. This research aims to determine whether vitamin D is seasonal and a risk factor for developing canine leishmaniasis and, also, to study the possible VitD anti-parasitic effect and the expression of genes related to VitD pathway. The results show that VitD in canine leishmaniasis is neither seasonal nor a risk factor for developing clinical disease. However, its role in reducing parasite load suggests that VitD could be an adjuvant in leishmaniasis therapy.
Introduction
Leishmaniases are a group of neglected vector-borne diseases caused by obligate intracellular protozoan parasites of the genus Leishmania (Trypanosomatida: Trypanosomatidae). The disease is considered endemic in tropical and subtropical regions of the Palearctic and Neotropic ecozones, and in ecoregions around the Mediterranean Basin. Human visceral leishmaniasis (VL) can be fatal if left untreated, resulting in 26 000–65 000 deaths per year (World Health Organization, 2019). Canids are the main reservoir and hosts of L. infantum, the causative agent of zoonotic VL in the Mediterranean Basin [1]. Only in western Mediterranean countries, there is an estimate of 2.5 million infected dogs [2]. Most Leishmania-infected humans and dogs show an asymptomatic infection, with only 5–20% of the cases developing the patent disease over a variable period of time [3,4]. The mechanisms that regulate the final outcome of the infection remain unknown, although it appears that asymptomatic infections are associated with a strong specific cell-mediated immunity and a Th1-proinflamatory immune response [5]. Some studies show that Th17 cells act synergistically with the Th1 population to control L. infantum growth by modulating some key regulatory cytokines [6]. However, exacerbated Th1 and Th17 may be responsible for excessive inflammation and pathology [7,8]. Susceptible individuals develop progressive disease with increasing parasite burden, concomitant to high antibody levels and a progressive Th2-deactivating immune response in the presence of a strong inflammatory reaction [9,10]. Innate immune system plays an important role by promoting the appropriate adaptive cellular immune response against the Leishmania infection [11]. Macrophages are central players in innate immune response and the main host cell for Leishmania spp.
Several studies have shown the important role that vitamin D (VitD) plays on innate immunity [12–14]. In humans, active VitD can be obtained from a small number of foods, from dietary supplements or photochemically through conversion of provitamin D by UVB rays exposure [15], resulting in a poorer VitD status in non-summer seasons [16]. Previous studies suggest that there would be no seasonal variations in vitamin D levels in dogs due to inadequate cutaneous synthesis [17], but this should be confirmed. The effects of VitD are mediated by its binding to the vitamin D receptor (VDR) which is expressed in several antigen-presenting cells and acts as a transcription factor specifically modulating the expression of many genes [12,18]. In human macrophages, toll-like receptors (TLR) activation by Mycobacterium tuberculosis antigens increases the expression of VDR and 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) genes, resulting in an over-expression of target genes, including antimicrobial peptides (APM) such as cathelicidins and β-defensins genes [19]. These peptides carry out several anti-microbial functions by increasing the formation of reactive oxygen species, modulating cytokine expression and inducing autophagy [20,21]. In the last decade, other transcriptional regulators that participate in the secondary gene regulatory response to VitD have been also described [22]. Also, it is well established that VitD plays a role modulating adaptive immune response against infection by reducing the production of pro-inflammatory Th1 and Th17 derived cytokines [23,24], IgG secretion and maintaining B-cell homeostasis [25].
Some observational, preclinical, and clinical studies have shown that low levels of VitD increase the risk of developing multiple diseases in humans (cancer, diabetes, autoimmune diseases and infectious diseases such as toxoplasmosis, AIDS, influenza or malaria) [26–32]. However, few studies have investigated VitD and leishmaniasis, with contradictory results depending on the animal model and Leishmania species [33–36]. We previously showed, for the first time, that dogs with clinical leishmaniasis and also those with asymptomatic infection presented lower VitD serum concentration than non-infected dogs. Vitamin D concentration were strongly negatively correlated with clinical severity, parasite load, and anti-Leishmania IgG levels. However, no association was found between VitD and T-cell response. Therefore, we reported that progression of clinical CanL was strongly associated with VitD deficiency in dogs [37].
The aims of the current study were i) to evaluate if VitD concentration in dogs shows a seasonal variation as it is the case for humans; ii) to investigate whether low VitD concentration is a risk factor for developing canine leishmaniasis (CanL); and iii) to determine if VitD has an anti-parasitic effect in Leishmania-infected macrophages and analyze RNA expression of components of VitD pathway.
Methods
Ethics statement
The research protocol was submitted to the Ethics Committee on Animal Experimentation (CEEA) of Universitat de Barcelona which, in compliance with national (Royal Decree 1201/2005) and European Union regulations (European Directive 86/609/CE) for projects using animals for research purpose, considered that an ethical approval was not required for this study. The project was also submitted to and approved by ISGlobal Internal Scientific Committee (ISC). All dog owners were informed about the research protocol and signed an informed consent allowing for sample and data collection.
Dog population included in seasonality study
In order to know if there is a seasonal variation in vitamin D levels in dogs, we included serum samples from thirty-six dogs from different breeds and ages living in Spain, which remained clinically healthy and Leishmania-free thorough one year (2016–2017). Serum samples from these dogs obtained in three timing points (February 2016, May/June 2016 and January 2017) were analysed. All dogs were fed commercial dry diet during the entire studied period. No changes in diet were made between timing points.
Dog population included in longitudinal study
To study the relationship between VitD levels and evolution of L. infantum infection we included retrospective serum samples from 19 dogs belonging to a longitudinal study of CanL living in a highly endemic area (Spain). Nine of these dogs became Leishmania infected, while 10 remained healthy and Leishmania-free along the study. This studied population included 60% males and 40% females from different breeds, and ages ranging from 1 to 11 years (4 ± 3.3 years). Samples at Spring times T1 and T2 were analysed.
Clinicopathological characterization
Clinical signs, clinical chemistry, and haematological values were scored using a clinicopathological score (CPS) as previously described [38]. Briefly, clinical signs compatible with leishmaniasis such as cutaneous lesions, ocular signs, and epistaxis were scored 0–3. Both clinical chemistry and haematological results scored 1 point for each abnormal value. These scores were added to obtain an overall clinicopathological score for each dog.
Samples
Serum was obtained after centrifugation of peripheral blood at 1600 g for 10 min and stored at -20°C until serology and biochemical analyses were performed. Popliteal lymph node samples collected by fine needle aspiration in sterile 0.9% NaCl solution were frozen at -20°C until DNA extraction. Peripheral Blood Mononuclear Cells (PBMCs) were isolated from blood in heparin tubes and preserved in liquid nitrogen until processing.
Crude total L. infantum antigen (CTLA)-based ELISA
B cell function was analysed through anti-Leishmania antibody concentration using an ELISA technique, as previously described with minor modifications [39]. Briefly, microtiter plates were coated with 2 μg of CTLA per well and sequentially incubated with sera and protein A conjugated to horseradish peroxidase (Pierce). Working dilutions were 1/400 and 1/10 000 for sera and protein A-HRP, respectively. Absorbance values were read at 450 nm in an automatic microELISA reader (Spark 10M, Tecan). Results were expressed in ELISA units (EU), referred to a known positive serum used as a calibrator and arbitrarily set to 1 EU. Cut-off value (mean + 3 SD) for 76 dogs from a non-endemic area was 0.200 OD.
Real-Time PCR amplification of Leishmania DNA
Parasite load was determined by qPCR in lymph nodes. DNA was extracted using the High Pure PCR Template Preparation Kit (Roche). L. infantum DNA was specifically detected and quantified with a TaqMan qPCR Assay (Applied Biosystems) following a previously reported protocol [40] with some modifications. The qPCR assay was designed to target conserved DNA regions of the kinetoplast from L. infantum genome. Primer sequences were LEISH-1 5′-AAC TTT TCT GGT CCT CCG GGT AG-3′, LEISH-2 5′-ACC CCC AGT TTC CCG CC-3′, and the TaqMan-MGB probe FAM-5′-AAA AAT GGG TGC AGA AAT-3′- MGB. The thermal cycling profile was 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 s, and 60°C for 1 min. Analyses were performed in a StepOnePlus Real Time PCR System device (Applied Biosystems). Each sample plus a negative control was analysed in triplicate. Parasite quantification was performed by comparison with a standard curve generated with L. infantum DNA extracted from 1 × 107 parasites by using serial dilutions from 103 to 10−3 parasites. This technique was sensitive enough to detect 0.001 parasites per reaction with a dynamic range of 107. The median slope of three different standard curves was −3.44, and the qPCR efficiency was 98%. Quantification was linear between 103 and 10−2 parasites per reaction tube (correlation = 0.99).
Determination of Vitamin D levels in serum samples
The prehormone 25-hydroxyvitamin D (25(OH)D) is the most reliable estimate of overall VitD status because this is a stable circulating metabolite of VitD, and its concentration is nearly 1000-fold higher than the biologically active form 1,25-dihydroxyvitamin D (1,25(OH)2D). Thus, 25(OH)D levels were assessed in serum samples using a competitive direct enzyme-linked immuno-sorbent assay (IDS 25-Hydroxy Vitamin D Direct EIA kit, Immunodiagnostic Systems Ltd.) according to the manufacturer’s instructions and employing an automatic micro-ELISA reader (Spark 10M, Tecan). The concentration of 25(OH)D in each sample was calculated using a four-parameter logistic curve fit (Prism 5, GraphPad Software), and results were expressed in ng/mL units.
In vitro evaluation of Vitamin D effect on L. infantum parasite killing
L. infantum promastigotes of the MCAN/ES/92/BCN-83/MON-1 strain were cultured at 26°C in R15 medium [RPMI 1640 medium (Gibco) supplemented with 15% heat-inactivated fetal bovine serum (FBS) (Gibco), 2% HEPES 1M (Gibco) and 1% 10000 U/mL penicillin with 10 000 μg/mL streptomycin (Gibco)]. Weekly passages were performed. Metacyclic promastigotes for in vitro infections were obtained from a 6-days-old stationary culture. DH82 dog macrophages kindly provided by Dr. Javier Moreno (Instituto de Salud Carlos III, Spain). Cells were cultured in R-10 media [RPMI 1640 medium (Gibco) supplemented with 10% FBS (Gibco) and 1% Penicillin/Streptomycin (Gibco)] and kept in a humid atmosphere at 37°C and in 5% CO2. The day of the experiment, cells were cultured in a 24-well plate (250 000 cells per well) and left to adhere for 2h. Different concentrations of 1,25-dihydroxyvitamin D (CAS N 250-963-8, Sigma-Aldrich) were added to DH82 cells in triplicate (0.01 μM, 0.1 μM and 1 μM) and plates were incubated for 24 h. After 24 hours, pre-treated DH82 cells were infected with metacyclic promastigotes at a parasite:cell ratio of 5:1, incubated for 24 h and then, washed with 1× PBS to discard non-internalized promastigotes. Cells were treated with trypsin-EDTA 0.05% (Gibco) and plated on microscopic slides by cytocentrifugation (Thermo Scientific Shandon Cytospin 4). Preparations were fixed with methanol and stained with Giemsa 10%. The number of infected macrophages and of intracellular parasites were recorded by direct microscopic count of 200 cells per sample. Values of infected macrophages and parasite burden were expressed as absolute number per 100 macrophages.
Monocytes isolation
Monocytes were isolated from 2 groups of dogs: a) Nine dogs from the field study, 4 Leishmania-infected dogs and 5 non-infected dogs at T2; b) Six healthy dogs whose buffy coats were obtained from the animal blood bank of Spain (Banco de Sangre Animal SL). Buffy coats were obtained by centrifugation (Megafuge 40R, Thermo Scientific) of 450 ml whole blood bags at slow speeds (4000 rpm, 17 min) at 22° C, after which the buffy coat was transferred into an attached satellite bag. First, PBMCs were isolated using Ficoll density gradient method. Samples were diluted (1:3) with 1x PBS, gently layered over 15 mL of Ficoll Paque Plus solution (GE Healthcare) and centrifuged at 400 g for 30 min. The buffy coat cells collected at the interface were washed with 1× PBS and treated with 4 mL of Ammonium-Chloride-Potassium Lysing Buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate and 0.1 mM EDTA) and washed again with 1× PBS. Cells were resuspended in R-10 medium. The differential counting was determined by haematological analyser (XN-1500, Sysmex Europe GmbH). PBMCs from dogs of the field study were frozen in liquid nitrogen until use. PBMCs were cultivated in 24-well plates (2.5 × 106 cells/well) and incubated for 18 h at 37°C in humidified incubator (5% CO2) for adherence. Later, cells were washed to obtain (a) only monocytes and RNA was directly extracted or (b) monocytes were treated with 1,25(OH)2D at a concentration of 0.1 μM of Lipopolysaccharides from Escherichia coli O111:B4 (LPS) (EC N 297-473-0, Sigma-Aldrich) at 0.1 μg/mL as positive control for 24 h. Plates were centrifuged at 400 g for 10 min, washed once with 1x PBS and monocytes were collected with 1 mL TRI Reagent (Ambion) and stored at -80°C until RNA extraction.
Gene expression analysis of Vitamin D pathway
RNA was extracted from monocytes using the RiboPure RNA Purification Kit (Ambion) following manufacturer’s instructions and measured with a NanoDrop-2000 Spectrophotometer (Isogen Life Science B.V). Retro-transcription was carried out by using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) following a thermal profile of 25°C for 10 min, 37°C for 120 min, 95°C for 5 min. Levels of CAMP, CBD103, CYP24A1, CYP27B1, NOS2 and VDR expression were determined in addition to RPL18 as a housekeeping gene. To ensure amplification of cDNA sequences derived from retro-transcription of mRNA of interest, primers were designed including exon boundary (Table 1). Amplification of each sample was carried out in triplicate by using SYBR Select Master Mix reagents (Applied Biosystems) with the aid of Applied Biosystems StepOnePlus PCR instrument and StepOnePlus Software v2.3 (Applied Biosystems). The thermal cycling profile was 10 min at 50°C, followed by 40 cycles of 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. Melting curves assessed the specificity of our amplification products. Cytokine mRNA expression levels were calculated by relative quantification using the 2−ΔΔCT method [41].
Table 1. Sequences of primers used for gene expression determinations.
| Gene | Primer sequence | ||
| CAMP | F | 5’-AGGACACGGGCTACTTTGAC-3’ | |
| R | 5’-TTTCGCCAATCTTCTGCCCC-3’ | ||
| CBD103 | F | 5’-GCCGCTGCTTACTTGTACCT-3’ | |
| R | 5’-CCTCATGACCAACAGGCTTC-3’ | ||
| CYP24A1 | F | 5’-ACTCCTTCGGAAGAATGCGG-3’ | |
| R | 5’-CGACCGGGGTTACCATCATC-3’ | ||
| CYP27B1 | F | 5’-GGCACACCTGACCTACTTCC-3’ | |
| R | 5’-AGAGCGTGTTGGATACCGTG-3’ | ||
| NOS2 | F | 5’-CACAGGATGACCCCAAGTGTC-3’ | |
| R | 5’-CAGCTGGCTTGATTGTGGATTC-3’ | ||
| VDR | F | 5’-TATCACCAAGGACAACCGCC-3’ | |
| R | 5’-CAGGATCATCTCCCGCTTCC -3’ | ||
| RPL18 | F | 5’-GTCGACATCCGCCACAACAA-3’ | |
| R | 5’-AGGTAGAGTTGGTTCGTCTGG-3’ | ||
Initials: F and R mean forward primer and reverse primer, respectively.
Data analysis
In the unadjusted analysis, the comparisons between different groups were performed using unpaired t-test and comparisons between same groups but different times using paired t-test. For analysis in which distribution does not conform to parametric criterion we used Wilcoxon signed-rank test to compare related samples and Mann-Whitney U test for independent samples. One-way ANOVA was used for multiple comparisons in dose-response experiments. All statistical tests were performed using GraphPad Prism 9.0 software. A P-value ≤ 0.05 was considered significant.
Results
Vitamin D seasonality in healthy dogs
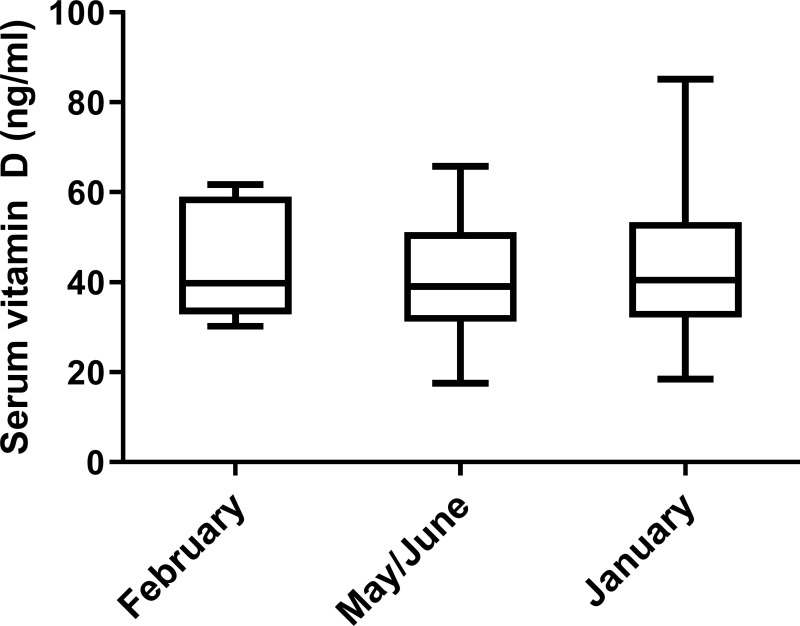
The mean 25(OH)D concentration of healthy dogs in the three points evaluated were 44.6 ± 12.5 ng/mL, 41.28 ± 12.4 ng/mL and 43.15 ± 14.8 ng/mL for February, May/June and January, respectively. No statistically significant variation was observed in any of the times studied (Wilcoxon matched-pairs signed rank test) (Fig 1).
Fig 1. Serum Vitamin D levels of 36 healthy dogs living in Spain at three different time points.
Vitamin D status in dogs was assessed according to the serum levels of 25-hydroxyvitamin D estimated with an ELISA test.
Vitamin D concentration as a risk factor for CanL
We selected 9 Leishmania-infected dogs presenting clinicopathological symptoms compatible with this disease (median CPS = 4) and which tested positive for qPCR analysis and/or CTLA-serology at T2 (“Infected” group) and 10 dogs which consistently tested negative in both tests (“Non-Infected” group). The baseline characteristics of both groups at the starting and end points are shown in Table 2.
Table 2. Characteristics of the dog population used in the current study at the two points studied.
| PARAMETERS | GROUPS OF ANIMALS | Ref. RANGE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Infected T1 | Infected T1 | Non-Infected T2 | Infected T2 | ||||||
| Median [IQR] | (+) | Median [IQR] | (+) | Median [IQR] | (+) | Median [IQR] | (+) | ||
| CPS | 0.0 [0.00–0.00] | 0% | 0.0 [0.00–0.00] | 0% | 0.0 [0.00–0.00] | 0% | 6 [4.00–9.00] | 100.0% | ≥ 4 |
| Anti-Leishmania Antibodies (EU) | 4.8 [3.55–6.56] | 0% | 7.8 [7.04–8.41] | 0% | 5.2 [4.68–5.56] | 0% | 58.9 [34.91–124.40] | 77.8% | ≥ 20 |
| Parasite Load in LN (pp/mL) | 0.0 [0.00–0.00] | 0% | 0.0 [0.00–0.00] | 0% | 0.0 [0.00–0.00] | 0% | 1195.1 [4.5–5862.5] | 75.0% | ≥ 1 |
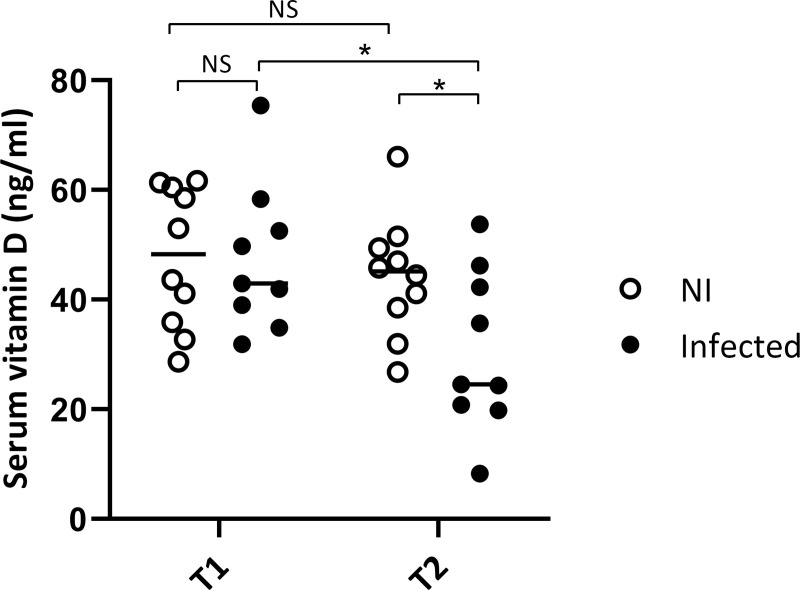
No statistically significant differences in VitD levels were observed between groups at the beginning of study (T1) (unpaired t-test; P = 0.9619) (Fig 2). Likewise, there were no statistically significant differences between initial and final 25(OH)D levels in the healthy group (paired t-test; P = 0.1828). Conversely, infected dogs showed statistically significant lower concentration of 25(OH)D in serum at the end of the study (T2) than at the beginning (T1) (paired t-test; P = 0.0396). At the end of the study, healthy animals showed higher 25(OH)D levels in serum than infected dogs (unpaired t-test; P = 0.0032). Therefore, sick animals show a greater reduction in VitD (-35.37%) than healthy ones (-7.18%) after a year. The median [interquartile range] levels of 25(OH)D in Non-Infected dogs at the beginning and at the end of the study were 48.3 [37.19–60.00] and 45.2 [39.22–48.80], respectively, and in Infected dogs were 42.9 [39.03–52.57] and 24.5 [20.86–42.27] ng/ml, respectively.
Fig 2. Serum vitamin D concentration in a dog population living in Spain.
Vitamin D status in dogs was assessed according to the serum levels of 25-hydroxyvitamin D estimated with an ELISA test. Comparison between Non-Infected dogs (NI) and Infected dogs during the longitudinal study (Infected) at the inclusion point (T1) and at the end of the study (T2) (* P < 0.05).
In vitro effect of vitamin D in L. infantum parasite killing
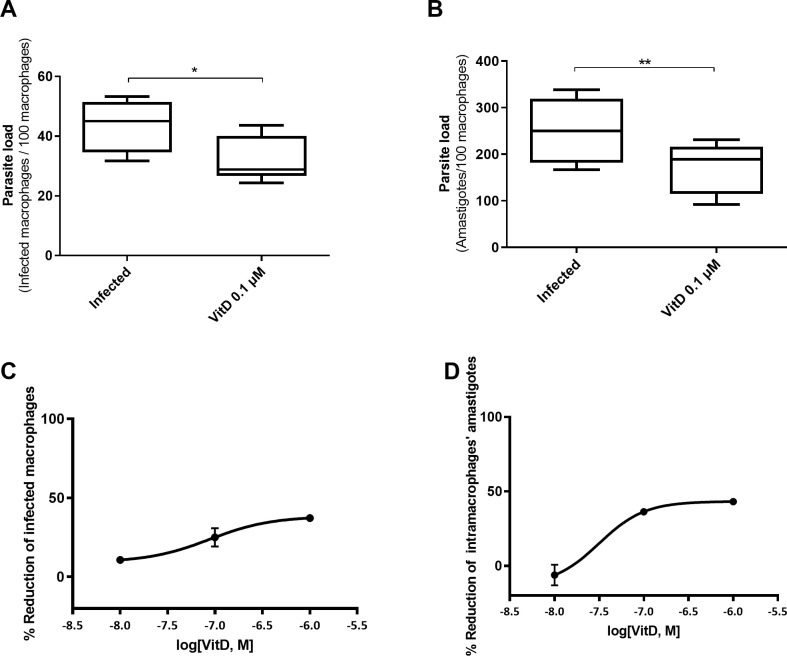
In an in vitro model using a canine macrophage cell line (DH82) infected by L. infantum we found that addition of active VitD lead to a significant reduction in parasite load (Fig 3). Pretreatment of canine macrophages cell line with 1,25(OH)2D at a dose of 0.1 μM, achieved a reduction of 26.5% in the number of infected macrophages (Wilcoxon matched pairs signed rank test; P = 0.0156) and 31.4% in the number of amastigotes per 100 macrophages (Wilcoxon matched pairs signed rank test; P = 0.0078) (Fig 3A and 3B). These reductions follow a dose-response effect (ANOVA test; P = 0.0285 and P = 0.0107, for infected macrophages and number of amastigotes reduction, respectively) (Fig 3C and 3D).
Fig 3. Vitamin D effect on L. infantum parasite load in macrophages.
Number of infected macrophages per 100 macrophages (A) and amount of intracellular amastigotes per 100 macrophages (B) counted in Giemsa stained-preparations from DH82 macrophages infected with L. infantum at ratio 5:1 and pre-treated or not (Control) with 1,25(OH)2D at 0.1 μM 24 h before infection (* P < 0.05, ** P < 0.01). Dose response curves showing inhibitory rates of L. infantum-infected macrophages (C) and intracellular amastigote growth (D) after 24h of treatment with 1,25(OH)2D based on the values for the untreated controls.
VitD pathway in primary canine monocytes
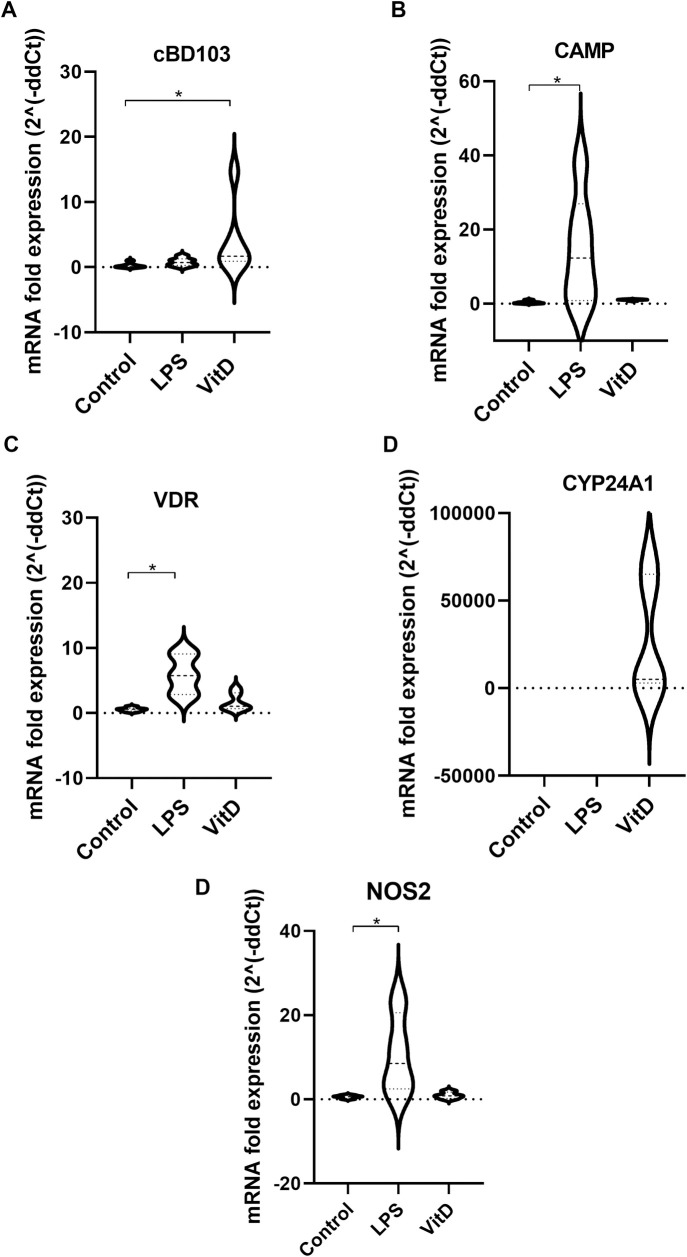
In a model using monocytes obtained from buffy coat of healthy blood donor dogs from an animal blood bank we found that 24 h treatment with 1,25(OH)2D lead to a statistically significant increase of the AMP β-defensin CBD103 gene compared to the basal expression of untreated monocytes (Wilcoxon signed rank test; P = 0.0313). No differences were detected in cathelicidin AMP CAMP gene expression (Wilcoxon signed rank test; P = 0.0625) neither to the VDR, CYP24A1 and NOS2 gene expression (Wilcoxon signed rank test; P = 0.0938 and P = 0.0625, respectively) (Fig 4). Addition of LPS at a concentration of 0.1 μg/mL greatly increased CAMP, VDR and NOS2 expression (Wilcoxon signed rank test; P = 0.0313, both), but not that of CBD103 and CYP24A1 (Wilcoxon signed rank test; P = 0.0938 and P = 0.8125, respectively).
Fig 4.
Fold expression of CBD103 (A), CAMP (B), VDR (C), CYP24A1 (D) and NOS2 (E) genes. mRNA fold-increase from VitD treated samples was calculated with reference to its negative control (cells of same extraction but untreated). mRNA expression from 0 condition was normalized from a control with a lowest value (* P < 0.05).
Gene expression of monocytes from retrospective field longitudinal study
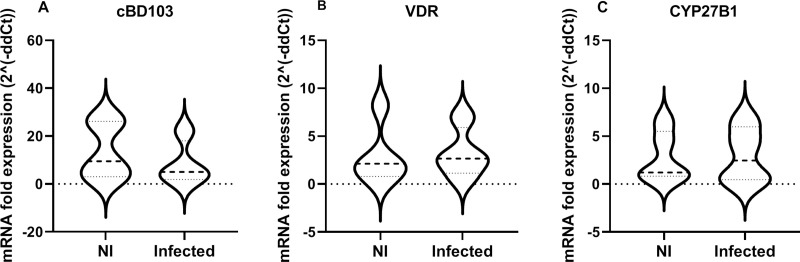
We have shown that CBD103 expression increases with the presence of VitD, so that it could play a key role in the antiparasitic activity derived from the action of VitD. For this reason, we analysed mRNA expression of CAMP, CBD103, CYP24A1, CYP27B1 and VDR in monocytes from 9 dogs included in the retrospective longitudinal study from which we were able to collect PBMC samples (4 dogs for Infected group and 5 for the Non-Infected group). We found no statistically significant differences in any of the genes studied. However, results suggest that at the endpoint healthy animals have higher expression of β-defensin CBD103, while animals suffering leishmaniasis have higher expression of CYP27B1. In the case of VDR there are no indications of a differential trend between groups (Fig 5). CAMP expression levels were very close to the quantification limit, and reliable results could not be obtained. CYP24A1 was undetectable.
Fig 5. Expression of vitamin D pathway genes.
Fold expression of CBD103 (A) CYP27B1 (B) and VDR (C) genes in Non-Infected (NI) and Infected (Infected) groups at the end of the study (T2).
Discussion
Our previous study showed that dogs with clinical leishmaniasis presented lower VitD serum concentration than non-infected dogs, and even lower than those with asymptomatic infection [37]. However, it was not possible to prove whether the low VitD levels found in dogs with CanL were the consequence or the cause of this parasitic disease. Although VitD levels have been determined in other canine infectious diseases such as spirocercosis [42], no longitudinal studies have been performed. Based on the literature search, this is the first longitudinal study describing VitD concentration in a canine population living in a highly endemic area of leishmaniasis.
In humans, it has been widely described that VitD status is seasonal due to photochemical activation of VitD in skin by UVB rays [15,43], but it remains unclear if the same is true in dogs because of differences in skin hair. Our results show that VitD concentration in dogs does not follow a seasonal pattern, with similar concentration in winter and spring. These results are consistent with the few previous studies that investigated VitD synthesis in dogs. Whatley and Sher (1961) reported low presence of VitD precursors in the skin of healthy dogs [44] and How et al. (1994) detected a low UV-mediated conversion rate of the precursor 7-dihydrocholesterol to VitD in dogs compared to rats [16]. However, only one longitudinal study has been performed, enrolling huskies from polar latitudes and showing an inverse relationship between UVB radiation and VitD status [45]. This result accounted for the VitD rich diet received by these dogs during the winter. These studies pointed out the importance of VitD supplementation in dogs, but they did not rule out some effect of UVB light on this specie. Our results confirm that VitD status in dogs is not influenced by the number of hours of exposure to sunlight and would not matter the season of the year when assessing VitD levels in clinical practice.
In the present retrospective longitudinal study, we showed that non-infected dogs did not present significant changes in VitD concentration between the beginning and the end of the study one year later, whereas those developing clinical leishmaniasis have a significant VitD decrease at the end of the study (35% reduction). When VitD levels at starting points were compared, no differences between groups were detected. Therefore, VitD concentration could not be used as a prognostic marker of clinical leishmaniasis. However, as VitD concentration decreased with the onset of clinical symptoms, we suggest that VitD concentration could be useful as clinical marker for the evolution of this disease. Low VitD concentration in sick dogs could be a consequence of VitD exhaustion due to the inflammatory process, following a similar pattern as the previously described for vitamin A during chickenpox infection [46]. Decreased VitD with the onset of clinical disease would be consistent with studies that have suggested that low VitD concentration is a marker of ill health [47]. However, we cannot rule out that VitD decrease in Leishmania-infected dogs were related to a poor nutritional status, although, only 2 dogs presented a loss of body weight at T2.
One limitation of the present study could be the low number of dogs included in the analysis, but we have to keep in mind that CanL is a disease with a long latent period, and that only 2.5% of dogs is diagnosed for this disease within 12 months in Spain [48]. We are planning future studies including a larger number of animals.
We investigated if VitD plays a role in the control of Leishmania load inside macrophages. Our in vitro model using a canine macrophage cell line showed that this hormone has a parasite killing activity, since addition of active VitD at 0.1 μM led to a significant reduction in L. infantum parasite load at 24 h post-infection. This is in line with the inhibitory effect of 1,25(OH)2D described for Toxoplasma gondii and Mycobacterium tuberculosis intracellular growth [18,29] and it suggests that VitD could have a protective in vivo effect against Leishmania, as it is the case for Trypanosoma cruzi infection [49]. The present study and our previous results [37] pointed out that L. infantum induced VitD deficiency in dogs and at the same time, this deficiency favored parasite dissemination. Other studies investigating the relationship between VitD and response to Leishmania infection yielded discrepant results. Ramos-Martinez et al. (2013) reported a significant reduction in the lesion size in L. mexicana-infected mice treated with 1,25(OH)2D [35], while other studies suggest that VitD deficiency increases resistance to L. major and L. amazonensis [50–52]. However, these results focused on the cutaneous form and/or in a mouse model, which is predisposed to Th1 immune response. A study with VL patients has shown that people suffering from this disease presented significant lower 1,25(OH)D3 serum concentration than healthy people, in agreement with our results [33].
Although the molecules and signals involved in VitD effects against Leishmania infection have not yet been investigated, the mechanism of VitD action against tuberculosis infection is well-known. Following TLR-2 activation of human macrophages by M. tuberculosis antigens, expression of CYP27B1 and VDR increases [19]. This ends up in increased expression of AMPs, mainly cathelicidin [12]. AMPs are important innate immunity mediators against microbial pathogens. They act through direct interaction with and disruption of microbial membranes, and indirectly through modulation of host cell migration and activation [53]. There are evidences that mammalian AMP cathelicidin influences control of cutaneous Leishmania infection; a Leishmania study using a CAMP knock-out mouse model showed that the presence of high inflammatory response in infected animals was CAMP-dependent [54]. In our retrospective longitudinal study, we did not find statistically significant differences in CAMP expression between non-infected and Leishmania-infected groups at the end of the study. We also investigated CAMP expression in primary canine monocytes as an ex vivo model. These experiments also showed no-differences in CAMP expression after addition of 1,25(OH)2D, even though CAMP expression was increase after LPS stimulation. Although in human macrophages 1,25(OH)2D increases the expression of CAMP directly via VitD response elements in the CAMP gene promoter [33,55], the pathway of action of VitD could be different depending on the animal species. In cattle, CAMP was not affected by addition of 1,25(OH)2D [56], but it modulates the immune response by increasing NO production in peripheral blood mononuclear cells [57]. On the contrary, addition of 1,25(OH)2D did not increase the expression of CAMP neither NOS2 in our ex vivo model. For this reason, we investigated other candidates that could explain the parasite killing effect of VitD in Leishmania-infected canine macrophages.
The other group of AMPs that can fend off bacterial and viral infections are β-defensins [58,59]. Antimicrobial response of VitD in human macrophages is also mediated by β-defensins through human TLRs [14,60]. In dogs, canine β-defensin 103 (CBD103) has been found in the epidermis of healthy dogs and its expression was altered in atopic animals [61]. In our study, CBD103 expression was significantly enhanced after 1,25(OH)2D addition on primary canine monocytes from blood donors. In addition, we detected that healthy dogs had slightly higher expression of CBD103 than those suffering from the disease at the end of the study, although this difference was not statistically significant. In agreement with our results, other studies have determined the important role that β-defensins play in host defense against Leishmania protozoa [62,63]. In dogs, some SNP’s in CBD103 gene have been associated with Leishmania infection, suggesting that it could be a marker of susceptibility [62]. The expression of β-defensins in Leishmania-infected human macrophage cell line THP-1 was induced by the cytokine IL-32γ. The inhibition of IL-32 lead to an increase of Leishmania infection index in THP-1 cells whereas its overexpression induced parasite control by AMPs [63]. The detection of IL-32 in canine macrophages would be very useful to determine if β-defensin expression in dogs is also modulated by this cytokine. Functional studies investigating the direct effect of β-defensin on Leishmania growth could confirm this molecule as responsible of the observed antiparasitic activity of VitD in canine monocytes.
LPS stimulation did not induce β-defensin expression in canine monocytes. CBD103 has antimicrobial activity against the respiratory pathogen Bordetella bronchiseptica, but tracheal epithelial cells stimulated with LPS did not increase β -defensins production [64]. Similarly, LPS stimulation was not enough to induce β-defensin expression in cattle monocytes [65]. These authors showed that VitD was the major driver of the β-defensin response of bovine monocytes. These results suggest that VitD pathway in canine macrophages may not be activated via TLR-4, but TLR-2/1 as in humans [66].
After addition of VitD, VDR expression remains unchanged in canine macrophages. Treatment of bovine monocytes with the protein translation inhibitor cycloheximide blocked upregulation of β-defensins in response to 1,25(OH)2D [65]. This suggests that although β-defensins are targets of 1,25(OH)2D in cattle, they are not direct targets of the VDR. Nurminen et al. (2015) identified multiple transcriptional regulators that are direct targets of VitD in the human THP-1 monocyte cell line. They demonstrated that BCL6 mediated the induction of several of the secondary response genes, and concluded that most of the physiological response of human monocytes to 1,25(OH)2D was a secondary response [22]. The same could occur in canine monocytes where we found a significant increase in CBD103 after adding active VitD but not in VDR.
In summary, we have shown that VitD in dogs was not seasonal and was not lower a-priori in dogs that will develop the disease. We have described for the first time the parasite killing activity of VitD addition in Leishmania-infected canine monocytes. Our results suggest that this relevant effect could be due to the induction of expression of genes implicated in host defence, such as the AMP β-defensin 103. A future goal derived from this study would be to investigate if VitD or calcitriol supplementation during clinical disease may mitigate the symptoms and progression of this parasitic infection in dogs. Leishmaniases are of great concern because they deeply intertwine pathogenic protozoa, insect vectors, dogs, humans and environment. The "One Health, One World" approach—the interconnection between human medicine, veterinary medicine, environmental science and wildlife conservation—is particularly suited for the control of this kind of diseases. The demonstration that vitamin D can be useful as a clinical marker, and that it decreases parasite load and may have a protective effect in vivo in dogs suggests that it may play a significant role in this holistic approach.
Acknowledgments
We thank all the dogs included in the study and their owners.
Crude total L. infantum antigen was provided by Dra. Riera and L. infantum BCN strain was provided by the UB Trypanosomatid Cryobank (Dra. Gállego) (Universitat de Barcelona, Spain).
Data Availability
All relevant data are within the manuscript.
Funding Statement
RV. received funding from H2020 Marie Sklodowska-Curie Actions grant 642609. RV and MG were funded by Agència de Gestió d’Ajuts Universitaris i de Recerca grant AGAUR 2017 SGR 924 and Red de Investigación Cooperativa en Enfermedades Tropicales grant RD12/0018/0010. RV and MG. also received funding from Ministerio de Ciencia, Innovación y Universidades, grant CEX2018-000806-S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gállego M. Emerging parasitic zoonoses: Leishmaniosis. OIE Rev Sci Tech. 2004;23(2):661–76. [PubMed] [Google Scholar]
- 2.Moreno J, Alvar J. Canine leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002;18(9):399–405. doi: 10.1016/s1471-4922(02)02347-4 [DOI] [PubMed] [Google Scholar]
- 3.Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, et al. New Perspectives on a Subclinical Form of Visceral Leishmaniasis. J Infect Dis. 1986Dec1;154(6):1003–11. doi: 10.1093/infdis/154.6.1003 [DOI] [PubMed] [Google Scholar]
- 4.Fisa R, Gállego M, Castillejo S, Aisa MJ, Serra T, Riera C, et al. Epidemiology of canine leishmaniosis in Catalonia (Spain): The example of the Priorat focus. Vet Parasitol. 1999Jun15;83(2):87–97. doi: 10.1016/s0304-4017(99)00074-6 [DOI] [PubMed] [Google Scholar]
- 5.Carrillo E, Moreno J. Cytokine profiles in canine visceral leishmaniasis. Vet Immunol Immunopathol. 2009;128(1–3):67–70. doi: 10.1016/j.vetimm.2008.10.310 [DOI] [PubMed] [Google Scholar]
- 6.Nascimento MSL, Albuquerque TDR, Nascimento AFS, Caldas IS, Do-Valle-Matta MA, Souto JT, et al. Impairment of Interleukin-17A Expression in Canine Visceral Leishmaniosis is Correlated with Reduced Interferon-γ and Inducible Nitric Oxide Synthase Expression. J Comp Pathol. 2015;153(4):197–205. doi: 10.1016/j.jcpa.2015.10.174 [DOI] [PubMed] [Google Scholar]
- 7.Gonçalves-de-Albuquerque S da C, Pessoa-e-Silva R, Trajano-Silva LAM, de Goes TC, de Morais RCS, Oliveira CN d. C, et al. The equivocal role of Th17 cells and neutrophils on immunopathogenesis of leishmaniasis. Front Immunol. 2017Oct30;8:1437. doi: 10.3389/fimmu.2017.01437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacallar O, Faria D, Nascimento M, Cardoso TM, Gollob KJ, Dutra WO, et al. Interleukin 17 production among patients with American cutaneous leishmaniasis. J Infect Dis. 2009Jul1;200(1):75–8. doi: 10.1086/599380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Cortés A, Carrillo E, Martorell S, Todolí F, Ojeda A, Martínez-Flórez A, et al. Compartmentalized Immune Response in Leishmaniasis: Changing Patterns throughout the Disease. Stäger S, editor. PLoS One. 2016May12;11(5):1–10. doi: 10.1371/journal.pone.0155224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nylén S, Maurya R, Eidsmo L, Das Manandhar K, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4 +CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007Apr16;204(4):805–17. doi: 10.1084/jem.20061141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya P, Dey R, Dagur PK, Kruhlak M, Ismail N, Debrabant A, et al. Genetically modified live attenuated Leishmania donovani parasites induce innate immunity through classical activation of macrophages that direct the Th1 response in mice. Infect Immun. 2015;83(10):3800–15. doi: 10.1128/IAI.00184-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: Modulator of the immune system. Curr Opin Pharmacol. 2010Aug;10(4):482–96. doi: 10.1016/j.coph.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, et al. Vitamin D Induces Innate Antibacterial Responses in Human Trophoblasts via an Intracrine Pathway1. Biol Reprod. 2009Mar1;80(3):398–406. doi: 10.1095/biolreprod.108.073577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004Sep1;173(5):2909–12. doi: 10.4049/jimmunol.173.5.2909 [DOI] [PubMed] [Google Scholar]
- 15.Jäpelt RB, Jakobsen J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013May13;4:136. doi: 10.3389/fpls.2013.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabufetti A, Milani GP, Lava SAG, Edefonti V, Bianchetti MG, Stettbacher A, et al. Vitamin D status among male late adolescents living in Southern Switzerland: Role of body composition and lifestyle. Nutrients. 2019Nov1;11(11). doi: 10.3390/nu11112727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.How KL, Hazewinkel HAW, Mol JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol. 1994Oct1;96(1):12–8. doi: 10.1006/gcen.1994.1154 [DOI] [PubMed] [Google Scholar]
- 18.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat Rev Immunol. 2011Sep19;11(9):584–96. doi: 10.1038/nri3045 [DOI] [PubMed] [Google Scholar]
- 19.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Wu K, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (80-). 2006;311(5768):1770–3. doi: 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 20.Jo E-K. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010Aug1;12(8):1026–35. doi: 10.1111/j.1462-5822.2010.01491.x [DOI] [PubMed] [Google Scholar]
- 21.Zughaier SM, Shafer WM, Stephens DS. Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell Microbiol. 2005Sep;7(9):1251–62. doi: 10.1111/j.1462-5822.2005.00549.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nurminen V, Neme A, Ryynänen J, Heikkinen S, Seuter S, Carlberg C. The transcriptional regulator BCL6 participates in the secondary gene regulatory response to vitamin D. Biochim Biophys Acta—Gene Regul Mech. 2015Mar1;1849(3):300–8. doi: 10.1016/j.bbagrm.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D 3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J Immunol. 2009Nov1;183(9):5458–67. doi: 10.4049/jimmunol.0803217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, et al. Calcitriol Suppresses Antiretinal Autoimmunity through Inhibitory Effects on the Th17 Effector Response. J Immunol. 2009Apr15;182(8):4624–32. doi: 10.4049/jimmunol.0801543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory Effects of 1,25-Dihydroxyvitamin D 3 on Human B Cell Differentiation. J Immunol. 2007Aug1;179(3):1634–47. doi: 10.4049/jimmunol.179.3.1634 [DOI] [PubMed] [Google Scholar]
- 26.Feldman D, Krishnan A V., Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–57. doi: 10.1038/nrc3691 [DOI] [PubMed] [Google Scholar]
- 27.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and Diabetes. Endocrinol Metab Clin North Am. 2010Jun1;39(2):419–46. doi: 10.1016/j.ecl.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 28.Ponsonby A-L, Lucas RM, van der Mei IAF. UVR, Vitamin D and Three Autoimmune Diseases—Multiple Sclerosis, Type 1 Diabetes, Rheumatoid Arthritis. Photochem Photobiol. 2005Nov1;81(6):1267. doi: 10.1562/2005-02-15-IR-441 [DOI] [PubMed] [Google Scholar]
- 29.Rajapakse R, Uring-Lambert B, Andarawewa KL, Rajapakse RP, Abou-Bacar A, Marcellin L, et al. 1,25(OH)2D3 inhibits in vitro and in vivo intracellular growth of apicomplexan parasite Toxoplasma gondii. J Steroid Biochem Mol Biol. 2007Mar;103(3–5):811–4. doi: 10.1016/j.jsbmb.2006.12.058 [DOI] [PubMed] [Google Scholar]
- 30.Coussens AK, Naude CE, Goliath R, Chaplin G, Wilkinson RJ, Jablonski NG. High-dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV-1 replication in urban Southern Africans. Proc Natl Acad Sci U S A. 2015Jun30;112(26):8052–7. doi: 10.1073/pnas.1500909112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris SK, Pell LG, Rahman MZ, Dimitris MC, Mahmud A, Islam MM, et al. Maternal vitamin D supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): Protocol for a prospective cohort study nested within a randomized controlled trial. BMC Pregnancy Childbirth. 2016Oct13;16(1). doi: 10.1186/s12884-016-1103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cusick SE, Opoka RO, Lund TC, John CC, Polgreen LE. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. PLoS One. 2014;9(12):1–8. doi: 10.1371/journal.pone.0113185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das S, Sardar AH, Abhishek K, Kumar A, Rabidas VN, Das P. Cathelicidin augments VDR-dependent anti-leishmanial immune response in Indian Post-Kala-Azar Dermal Leishmaniasis. Int Immunopharmacol. 2017Sep1;50:130–8. doi: 10.1016/j.intimp.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay D, Mukherjee S, Roy S, Dalton JE, Kundu S, Sarkar A, et al. M2 Polarization of Monocytes-Macrophages Is a Hallmark of Indian Post Kala-Azar Dermal Leishmaniasis. McMahon-Pratt D, editor. PLoS Negl Trop Dis. 2015Oct23;9(10):e0004145. doi: 10.1371/journal.pntd.0004145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos-Martínez E, Villaseñor-Cardoso MI, López-Vancell MR, García-Vázquez FJ, Pérez-Torres A, Salaiza-Suazo N, et al. Effect of 1,25(OH)2D3 on BALB/c mice infected with Leishmania mexicana. Exp Parasitol. 2013Aug;134(4):413–21. doi: 10.1016/j.exppara.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 36.Bezerra IP da S, Oliveira-Silva G, Braga DSFS, de Mello MF, Pratti JES, Pereira JC, et al. Dietary Vitamin D3 Deficiency Increases Resistance to Leishmania (Leishmania) amazonensis Infection in Mice. Front Cell Infect Microbiol. 2019;9:88. doi: 10.3389/fcimb.2019.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Cortes A, Martori C, Martinez-Florez A, Clop A, Amills M, Kubejko J, et al. Canine Leishmaniasis Progression is Associated with Vitamin D Deficiency. Sci Rep. 2017Dec13;7(1):1–10. doi: 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Cortés A, Ojeda A, López-Fuertes L, Timón M, Altet L, Solano-Gallego L, et al. A long term experimental study of canine visceral leishmaniasis. Int J Parasitol. 2007;37(6):683–93. doi: 10.1016/j.ijpara.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez A, Solano-Gallego L, Ojeda A, Quintana J, Riera C, Gállego M, et al. Dynamics of Leishmania-specific immunoglobulin isotypes in dogs with clinical leishmaniasis before and after treatment. J Vet Intern Med. 2006May;20(3):495–8. doi: 10.1892/0891-6640(2006)20[495:doliii]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 40.Francino O, Altet L, Sánchez-Robert E, Rodriguez A, Solano-Gallego L, Alberola J, et al. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet Parasitol. 2006Apr;137(3–4):214–21. doi: 10.1016/j.vetpar.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001Dec;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 42.Rosa CT, Schoeman JP, Berry JL, Mellanby RJ, Dvir E. Hypovitaminosis D in dogs with spirocercosis. J Vet Intern Med. 2013Sep1;27(5):1159–64. doi: 10.1111/jvim.12161 [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira CL, Cureau FV, Cople-Rodrigues C dos S, Giannini DT, Bloch KV, Kuschnir MCC, et al. Prevalence and factors associated with hypovitaminosis D in adolescents from a sunny country: findings from the ERICA survey. J Steroid Biochem Mol Biol. 2020Jan1;199:105609. doi: 10.1016/j.jsbmb.2020.105609 [DOI] [PubMed] [Google Scholar]
- 44.Wheatley VR, Sher DW. Studies of the lipids of dog skin. I. The chemical composition of dog skin lipids. J Invest Dermatol. 1961;36(3):169–70. doi: 10.1038/jid.1961.29 [DOI] [PubMed] [Google Scholar]
- 45.Griffiths P, Fairney A. Vitamin D metabolism in polar vertebrates. Comp Biochem Physiol—Part B Biochem. 1988;91(3):511–6. doi: 10.1016/0305-0491(88)90014-4 [DOI] [PubMed] [Google Scholar]
- 46.Campos FA, Flores H, Underwood B A. Effect of an infection on vitamin A status of children as measured by the relative dose response (RDR)13. Am J Clin Nutr. 1987;46:91–4. doi: 10.1093/ajcn/46.1.91 [DOI] [PubMed] [Google Scholar]
- 47.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014Jan;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7 [DOI] [PubMed] [Google Scholar]
- 48.Mattin MJ, Solano-Gallego L, Dhollander S, Afonso A, Brodbelt DC. The frequency and distribution of canine leishmaniosis diagnosed by veterinary practitioners in Europe. Vet J. 2014;200(3):410–9. doi: 10.1016/j.tvjl.2014.03.033 [DOI] [PubMed] [Google Scholar]
- 49.Silva ME, Silva MEC, Silva ME, Nicoli JR, Bambirra EA, Vieira EC. Vitamin D overload and experimental Trypanosoma cruzi infection: Parasitological and histopathological aspects. Comp Biochem Physiol—Part A Physiol. 1993Jan1;104(1):175–81. doi: 10.1016/0300-9629(93)90026-z [DOI] [PubMed] [Google Scholar]
- 50.Ehrchen J, Helming L, Varga G, Pasche B, Loser K, Gunzer M, et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007Oct5;21(12):3208–18. doi: 10.1096/fj.06-7261com [DOI] [PubMed] [Google Scholar]
- 51.Whitcomb JP, DeAgostino M, Ballentine M, Fu J, Tenniswood M, Welsh J, et al. The Role of Vitamin D and Vitamin D Receptor in Immunity to Leishmania major Infection. J Parasitol Res. 2012;2012:1–10. doi: 10.1155/2012/134645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bezerra JAB, Oliveira IVP de M, Yamakawa AC, Nilsson MG, Tomaz KLR, Oliveira KDS de, et al. Serological and molecular investigation of Leishmania spp. infection in cats from an area endemic for canine and human leishmaniasis in Northeast Brazil. Rev Bras Parasitol Vet. 2019Nov4;28(4):790–6. doi: 10.1590/S1984-29612019082 [DOI] [PubMed] [Google Scholar]
- 53.Lehrer RI, Barton A, Daher KA, Harwig SSL, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989;84(2):553–61. doi: 10.1172/JCI114198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulkarni MM, Barbi J, McMaster WR, Gallo RL, Satoskar AR, McGwire BS. Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cell Microbiol. 2011Jun;13(6):913–23. doi: 10.1111/j.1462-5822.2011.01589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005Jul1;19(9):1067–77. doi: 10.1096/fj.04-3284com [DOI] [PubMed] [Google Scholar]
- 56.Nelson CD, Reinhardt TA, Thacker TC, Beitz DC, Lippolis JD. Modulation of the bovine innate immune response by production of 1α,25-dihydroxyvitamin D3 in bovine monocytes. J Dairy Sci. 2010Mar1;93(3):1041–9. doi: 10.3168/jds.2009-2663 [DOI] [PubMed] [Google Scholar]
- 57.Waters WR, Miller JM, Palmer M V., Stabel JR, Jones DE, Koistinen KA, et al. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect Immun. 2003Sep1;71(9):5130–8. doi: 10.1128/IAI.71.9.5130-5138.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, β-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003Jul1;71(7):3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J, Yang YL, Jang SH, Jang YS. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol J. 2018Aug8;15(1):124. doi: 10.1186/s12985-018-1035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, et al. Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009Jun 5;4(6):e5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Damme CMM, Willemse T, van Dijk A, Haagsman HP, Veldhuizen EJA. Altered cutaneous expression of β-defensins in dogs with atopic dermatitis. Mol Immunol. 2009;46(13):2449–55. doi: 10.1016/j.molimm.2009.05.028 [DOI] [PubMed] [Google Scholar]
- 62.Da Silva LG, Costa-Júnior CRL, Figueiredo-Júnior CAS, Leal-Balbino TC, Crovella S, Otranto D, et al. Canine β-defensin-1 (CBD1) gene as a possible marker for Leishmania infantum infection in dogs. Parasites and Vectors. 2017;10(1):1–7. doi: 10.1186/s13071-016-1943-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.dos Santos JC, Heinhuis B, Gomes RS, Damen MSMA, Real F, Mortara RA, et al. Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species. PLoS Negl Trop Dis. 2017Feb27;11(2). doi: 10.1371/journal.pntd.0005413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erles K, Brownlie J. Expression of β-defensins in the canine respiratory tract and antimicrobial activity against Bordetella bronchiseptica. Vet Immunol Immunopathol. 2010May15;135(1–2):12–9. doi: 10.1016/j.vetimm.2009.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merriman KE, Kweh MF, Powell JL, Lippolis JD, Nelson CD. Multiple β-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol. 2015;154:120–9. doi: 10.1016/j.jsbmb.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 66.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (80-). 2006Mar24;311(5768):1770–3. doi: 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.