Abstract
The relationship between chronic obstructive pulmonary disease (COPD) and reflux esophagitis (RE) was controversial. We investigated the factors influencing RE development in patients with COPD and evaluated the association between RE and AECOPD.
Patients with COPD who underwent esophagogastroduodenoscopy from January 2003 to December 2013 in St. Paul's Hospital, the Catholic University of Korea (Seoul, Korea) were enrolled retrospectively. The grade of RE was based on the Los Angeles classification and minimal change esophagitis. Body mass index, smoking history, medical history, AECOPD, pulmonary function test data, endoscopic findings, and comorbidities were reviewed.
Of a total of 218 patients with COPD, 111 (50.9%) were diagnosed with RE. None of age, sex, smoking history, or the severity of airflow limitation was associated with RE. AECOPD was not related to either the presence or severity of RE. There was no significant correlation between RE grade by Los Angeles classification and severity of airflow limitation (P = .625). Those who had RE used theophylline (P = .003) and long-acting muscarinic antagonists (P = .026) significantly more often than did controls. The use of theophylline (OR 2.05; 95% CI, 1.16–3.65, P = .014) was associated with an increased incidence of RE.
The use of theophylline might increase the risk of RE in COPD patients. RE may not be associated with airflow limitation or AECOPD.
Keywords: chronic obstructive pulmonary disease, endoscopy, gastroesophageal reflux, risk
1. Introduction
Gastroesophageal reflux disease (GERD) is a very common disease, affecting 9% to 28% of the Western population and 3% to 8% of those of East Asia.[1] GERD is a common cause of chronic cough and is a probable risk factor for other respiratory disorders, including chronic obstructive pulmonary disease (COPD).[2–4] Previous studies found that GERD might increase the prevalence of COPD, and also the frequency of COPD exacerbation.[2,5–8] Most of these studies defined GERD using diagnostic codes or surveys, however, many patients suffering from GERD do not have typical GERD symptoms such as heartburn or acid regurgitation.[9] Therefore, accurate diagnosis of GERD, such as esophagogastroduodenoscopy (EGD) or esophageal multichannel intraluminal impedance-pH monitoring, is needed to evaluate the relationship between GERD and COPD. EGD is helpful for diagnosing GERD, and also assessing GERD severity. As far as we know, several studies have described risk factors for GERD in patients with COPD, but few studies have evaluated reflux esophagitis (RE) via EGD. We defined factors influencing the development of RE in patients with COPD and evaluated the association between RE and exacerbation of COPD.
2. Materials and methods
2.1. Study subjects
Data were retrospectively collected from COPD patients who underwent EGD from January 2003 to December 2013 in St. Paul's Hospital, the Catholic University of Korea, Seoul, Republic of Korea. The inclusion criteria were based on the availability of EGD data among COPD patients diagnosed with the criteria of the Global Initiative for Obstructive Pulmonary Disease (GOLD).[10] We enrolled patients with COPD who underwent gastroscopy for 1 year before and after pulmonary function test (PFT). Patients with known esophagogastrointestinal cancers or who had undergone pneumonectomy or gastrectomy were excluded. In all, 13 patients had been diagnosed with esophagogastrointestinal cancers, 5 had undergone gastrectomy, and 1 patient had undergone pneumonectomy. Finally, 218 patients were enrolled in the present study. All of the patients were evaluated as follows: body mass index, smoking history, medical history, PFT, endoscopic findings, and comorbidities. All of the medications for COPD were reviewed; these included inhaled corticosteroids (ICS), long-acting β2 agonists, ICS/long-acting β2 agonists combinations, oral corticosteroids, short-acting β2 agonists, short-acting muscarinic antagonists, long-acting muscarinic antagonists (LAMA), oral β2 agonists, and theophylline. The study was approved by the institutional review board of St. Paul's Hospital, the Catholic University of Korea (PC09ZZZZ0060).
2.2. Pulmonary function test
PFTs were performed in all of the patients via Vmax 229 spirometry; the platform was from MIASYS Respiratory Care Inc. (CA). All of the following respiratory functions were recorded: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, peak expiratory flow, and forced expiratory flow at 25% and 75% of the vital capacity (FEF 25–75). All of the respiratory functions are reported as percentages of predicted values.
COPD was considered present when the FEV1/FVC ratio was less than 0.7 upon postbronchodilator spirometry; this is the recognized criterion. Patients were classified based on airflow limitation using the GOLD criteria: GOLD 1 (mild, FEV1 ≥ 80%), GOLD 2 (moderate, 50% ≤ FEV1 ≤ 80%), GOLD 3 (severe, 30% ≤ FEV1 ≤ 50%), and GOLD 4 (very severe, FEV1 ≤ 30%).[10] Acute exacerbation of COPD (AECOPD) was defined as hospitalization or an emergency room visit because of sudden worsening of COPD symptoms in a 2-year interval running from 1 year before to 1 year after endoscopy was performed.
2.3. Esophagogastroduodenoscopy
All the patients were divided into 2 groups in terms of endoscopic findings: Patients with RE (the RE group) and patients without RE (the control group). RE was graded according to the Los Angeles (LA) classification[11] and minimal change esophagitis.[12] Based on LA classification, Grade A RE was associated with mucosal breaks no longer than 5 mm; Grade B with mucosal breaks longer than 5 mm; and Grade C with mucosal breaks that were continuous but involved less than 75% of the esophageal circumference. Grade D featured mucosal breaks that involved at least 75% of the esophageal circumference. In terms of minimal change esophagitis, Grade M patients had minimal endoscopic changes including excessive reddening of the cardia, erythema, friability, and blurring of the squamocolumnar junction, diffuse erythema, patchy erythema, increased vascularity of the distal esophagus and edema, and/or accentuation of the mucosal folds.
2.4. Statistical analysis
Statistical analyses were performed using SPSS Statistics 21.0 (IBM, Armonk, NY). Data are expressed as means ± SDs, as medians (with interquartile ranges), or as numbers (with percentages). The unpaired Student t test, the chi-squared test, and multiple logistic regression were used to compare data between groups, as appropriate. A P value <.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
The prevalence of RE in COPD patients was 50.9% (111/218); patients of all grades from M to D by LA classification were included. A total of 52 patients were of LA grade M, 39 grade A, 19 grade B, 0 grade C, and 1 grade D. A total of 111 patients in the RE group and 107 in the control group were categorized based on the severity of airflow limitation. A total of 33 (29.7%) patients in the RE group and 32 (29.9%) controls had mild airflow limitation (GOLD 1). A total of 40 (36.0%) RE patients and 46 (43.0%) controls had moderate airflow limitation (GOLD 2). A total of 30 (27.0%) RE patients and 25 (23.4%) controls had severe airflow limitation (GOLD 3). A total of 8 (7.2%) RE patients and 4 (3.7%) controls had very severe airflow limitation (GOLD 4). No significant between-group difference in airflow limitation was evident upon pulmonary function testing. No significant difference was evident between the 2 groups in terms of any of age, sex, or body mass index (Table 1).
Table 1.
Baseline characteristics of the study subjects.
| RE (n = 111) | Controls (n = 107) | P value | |
| Age (yr) | 73.1 ± 9.1 | 76.9 ± 9.8 | .004 |
| Sex | .123 | ||
| Male | 87 (78.4) | 73 (68.2) | |
| Female | 24 (21.6) | 34 (31.8) | |
| BMI (kg/m2) | 22.6 ± 3.6 | 23.1 ± 3.7 | .323 |
| FEV1 (%) | 62.9 ± 23.9 | 65.8 ± 23.8 | .370 |
| GOLD | .542 | ||
| GOLD 1 (mild) | 33 (29.7) | 32 (29.9) | |
| GOLD 2 (moderate) | 40 (36.0) | 46 (43.0) | |
| GOLD 3 (severe) | 30 (27.0) | 25 (23.4) | |
| GOLD 4 (very severe) | 8 (7.2) | 4 (3.7) | |
| FVC (%) | 82.0 ± 21.8 | 80.9 ± 20.8 | .708 |
| FEV1/FVC | 52.6 ± 12.2 | 54.8 ± 11.6 | .190 |
| Cigarette smoking | .973 | ||
| Never | 24 (21.6) | 26 (24.3) | |
| Ex-smoker | 50 (45.0) | 47 (43.9) | |
| Current smoker | 36 (32.4) | 33 (30.8) | |
| Unknown | 1 (0.9) | 1 (0.9) | |
| Cigarette smoking (pack-yr) | 32.4 ± 27.6 | 32.0 ± 27.9 | .917 |
Values are presented as means ± SDs or as numbers (with percentages).
BMI = body mass index, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, GOLD = Global Initiative for Chronic Obstructive Lung Disease, RE = reflux esophagitis.
In the present study, the presence of RE in COPD patients was not significantly correlated with smoking history. We divided patients into 4 groups; never-smokers, ex-smokers, current smokers, and unknown. There were no significant differences between the 4 groups. Mean pack-years of smoking did not significantly differ between the RE and control groups (Table 1).
3.2. Correlation between RE grade and severity of COPD
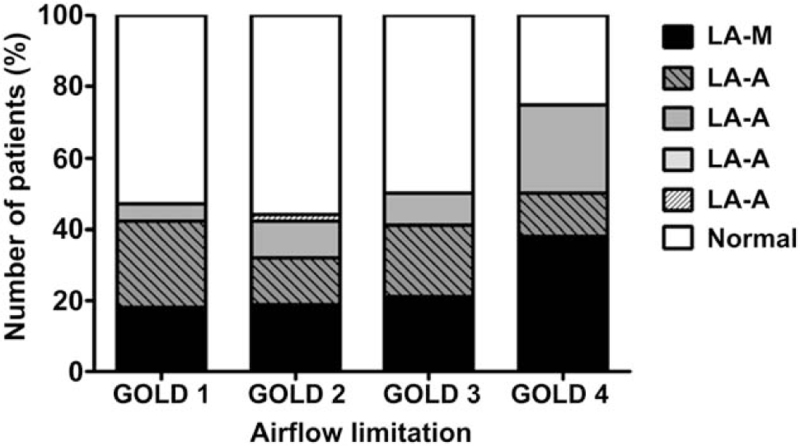
All the patients were classified in terms of RE severity using the LA classification. In addition, they were categorized based on airflow limitation, employing the GOLD criteria. Among patients with minimal changes in RE, 16 (30.8%) were GOLD 1, 19 (36.5%) GOLD 2, 13 (25.0%) GOLD 3, and 4 (7.7%) GOLD 4. Among those of LA Class A, 13 (33.3%) were GOLD 1, 12 (30.8%) GOLD 2, 13 (33.3%) GOLD 3, and 1 (2.6%) GOLD 4. Among those of LA Class B, 4 (21.1%) were GOLD 1, 8 (42.1%) GOLD 2, 4 (21.1%) GOLD 3, and 3 (15.8%) GOLD 4. No patient was of LA Class C, and the single patient of LA Class D was GOLD 2. No significant positive correlation was found between RE grade according to the LA classification and severity of airflow limitation (Fig. 1).
Figure 1.
Correlation between the grade of reflux esophagitis and the airflow limitation criteria of the GOLD. No significant correlation between GERD grade by the LA classification and severity of airflow limitation was evident (P = .625). LA = Los Angeles classification, GOLD = Global Initiative for Chronic Obstructive Lung Disease.
3.3. Correlation between development of AECOPD and RE
AECOPD was not significantly correlated with the presence of RE in the present study. The mean frequencies of hospitalization and emergency room visits triggered by AECOPD were 0.6 and 0.1 in the RE group, and 0.5 and 0.1 in the control group. No significant between-group difference was evident in terms of AECOPD levels (Table 2).
Table 2.
AECOPD in RE and control groups.
| RE (n = 111) | Controls (n = 107) | P value | |
| AECOPD (frequency) | 2.3 ± 4.0 | 3.1 ± 6.4 | .259 |
| Hospitalization because of AECOPD | 1.6 ± 3.1 | 2.0 ± 3.7 | .373 |
| ER visit because of AECOPD | 0.7 ± 1.4 | 1.1 ± 2.8 | .186 |
Values are presented as means ± SDs.
AECOPD = acute exacerbation of chronic obstructive pulmonary disease, RE = reflux esophagitis.
3.4. Medications for COPD
Among COPD medications, RE patients used theophylline (P = .003) and LAMA (P = .026) significantly more often than did controls. No other medication for COPD was significantly associated with RE in COPD patients (Table 3).
Table 3.
Chronic obstructive pulmonary disease medications used in RE and control groups.
| RE (n = 111) | Controls (n = 107) | P value | |
| ICS | 2 (1.8) | 2 (1.9) | .970 |
| ICS + LABA | 71 (64.0) | 70 (65.4) | .822 |
| OCS | 24 (21.6) | 31 (29.0) | .212 |
| SABA | 18 (16.2) | 25 (23.4) | .185 |
| LABA | 7 (6.3) | 2 (1.9) | .100 |
| LAMA | 56 (50.5) | 38 (35.5) | .026 |
| Oral β2 agonist | 32 (28.8) | 39 (36.4) | .230 |
| Theophylline | 76 (68.5) | 52 (48.6) | .003 |
Values are presented as numbers (with percentages).
ICS = inhaled corticosteroid, LABA = long-acting β2 agonist, LAMA = long-acting muscarinic antagonist, OCS = oral corticosteroid, RE = reflux esophagitis, SABA = short-acting β2 agonist.
3.5. Factors associated with GERD in COPD patients
Multiple logistic regression analysis showed that theophylline use (OR 2.05; 95% CI, 1.16–3.65, P = .014) was independently associated with RE in COPD patients. Unlike the conclusion of univariate analysis, no significant increase in RE was associated with LAMA use (Table 4).
Table 4.
Risk factors for reflux esophagitis in patients with chronic obstructive pulmonary disease as revealed by multiple logistic regression analyses.
| Variable | Odds ratio | 95% CI | P value |
| Theophylline use | 2.05 | 1.16 to 3.65 | .014 |
| LAMA use | 1.54 | 0.87 to 2.72 | .139 |
LAMA = long-acting muscarinic antagonist.
4. Discussion
In the present study, the prevalence of RE in COPD patients was 50.9%. One previous large cohort study reported that the prevalence of GERD in Korean patients with COPD was about 28%.[8] This is very high compared to other Asian populations and the general Korean population, where the prevalence of GERD is about 3% to 12%.[13] Previous studies have also shown a high prevalence of GERD in COPD patients; the figures were 26.8% in Japan, 32% to 37% in the United States, and 53.6% in Iran.[2,3,5,14] Some studies reported a higher prevalence in COPD patients compared to controls.[3,5] Although we only investigated a small number of patients with COPD, our result was consistent with those of other studies, and suggests that RE may be a common comorbidity in patients with COPD.
In the present study, use of theophylline appeared to increase the incidence of RE. It has been demonstrated that theophylline worsens GERD by decreasing lower esophageal sphincter pressure.[15] Besides, previous cohort studies have shown that the relative risk of GERD was increased upon use of theophylline.[7,8] GERD may cause aspiration of gastrointestinal contents, in turn inducing bronchospasm via reflex mechanisms[2,16]; therefore, theophylline may trigger a vicious cycle. The GOLD guidelines indicate that theophylline exerts a modest bronchodilator effect compared to placebo in patients with stable COPD.[10] However, the effect of theophylline on COPD patients with GERD has not been largely discussed, and thus requires further study.
Although LAMA use did not significantly increase the risk of RE upon multiple logistic regression analysis, the Chi-squared test revealed that LAMA increased the incidence of RE. This finding is similar to that of an earlier large cohort study conducted in the United Kingdom.[7] It is well known that anticholinergics increase the risk of GERD by diminishing lower esophageal sphincter pressure.[17] In addition, most Korean patients use tiotropium as an inhaled anticholinergic, and it has been reported that tiotropium significantly increased the risk of GERD.[18] However, any association between inhaled anticholinergics and GERD remains controversial. In a mouse model of chronic GERD, tiotropium reduced lung inflammation,[19] and inhaled anticholinergics may not have increased the risk of GERD in a national cross-sectional cohort study in Korea.[8]
Our findings differ in several respects from those of previous studies. One interesting finding was that smoking was not related with the presence of RE. In many previous cohort studies, smoking was correlated with the presence of GERD in COPD patients.[7,8,20] Several explanations are possible. Previous studies found that smoking possibly exacerbated GERD by directly provoking acid reflux, and perhaps by reducing lower esophageal sphincter pressure.[21,22] However, data on the impact of smoking on GERD is still doubtful. Furthermore, stopping smoking immediately did not affect esophageal total acid exposure.[23] Our results support these data.
A further difference was that ICS did not increase the risk of RE in our study, whereas previous studies found the opposite.[7,8] However, earlier works did not use EGD to diagnose GERD, and the condition may have been over diagnosed in patients using ICS, because chronic steroid inhaler use can trigger laryngeal inflammation that can mimic laryngopharyngeal reflux and GERD.[24]
We acknowledged several limitations of this study. First, our data were derived from a single-center, and only a limited number of subjects were enrolled. Second, we only examined RE which is part of GERD. This study was a retrospective study, no esophageal multichannel intraluminal impedance-pH monitoring and symptom-specific questionnaires were used to diagnose GERD. Third, since the number of serious cases such as LA classification C and D is very low in Korea, information on severe RE was insufficient. Fourth, there is a gap between the time of endoscopy and the time of receiving the PFT, so the relationship between the exact COPD deterioration and the RE may not be clear. In other words, it is not known whether enrolled patients have reflux symptoms or not. Therefore, since this is a retrospective study, the incidence of GERD in COPD patients is unknown exactly due to lack of data. Lastly, we should have investigated risk factors that exacerbated RE such as alcohol and meal related factors. Nevertheless, the strength of the study was that we evaluated the relationship between GERD and COPD through EGD, unlike previous cohort studies. Although GERD is common in COPD patients, the cause-and-effect relationship is lacking.[25,26] We suggested that theophylline and long-acting inhaled anticholinergics might increase the risk of RE in patients with COPD. Further studies are necessary that these medications can really increase the risk of GERD.
5. Conclusion
RE is common in patients with COPD. The use of theophylline might increase the risk of RE in COPD patients. The medications should be reviewed in COPD patients with reflux symptoms. RE may not be associated with airflow limitation or AECOPD.
Acknowledgments
None.
Author contributions
Minji Seo and Jongmin Lee collected the cases and analyzed and interpreted the data. Minji Seo, Hyeon Hui Kang, and Jung Hwan Oh designed the present study and carried out manuscript drafting and revising. So-Young Ha and Sang Haak Lee participated as a rater. All authors read and approved the final manuscript.
Conceptualization: Jung Hwan Oh.
Data curation: Minji Seo, Jongmin Lee.
Supervision: Sang Haak Lee.
Validation: So Young Ha.
Writing – original draft: Minji Seo.
Writing – review & editing: Hyeon Hui Kang, Jung Hwan Oh.
Footnotes
Abbreviations: AECOPD = Acute exacerbation of COPD, COPD = chronic obstructive pulmonary disease, EGD = esophagogastroduodenoscopy, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, GERD = gastroesophageal reflux disease, GOLD = Global Initiative for Obstructive Pulmonary Disease, ICS = inhaled corticosteroids, LA = Los Angeles, LAMA = long-acting muscarinic antagonists, PFT = pulmonary function test, RE = reflux esophagitis, the control group = patients without RE, the RE group = Patients with RE.
How to cite this article: Kang HH, Seo M, Lee J, Ha SY, Oh JH, Lee SH. Reflux esophagitis in patients with chronic obstructive pulmonary disease. Medicine. 2021;100:34(e27091).
HHK, MS contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The clinical data are available from the corresponding author upon request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest 2006;130:1096–101. [DOI] [PubMed] [Google Scholar]
- [3].Terada K, Muro S, Sato S, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax 2008;63:951–5. [DOI] [PubMed] [Google Scholar]
- [4].Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–38. [DOI] [PubMed] [Google Scholar]
- [5].Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest 2001;119:1043–8. [DOI] [PubMed] [Google Scholar]
- [6].Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J 2004;23:841–5. [DOI] [PubMed] [Google Scholar]
- [7].García Rodríguez LA, Ruigómez A, Martín-Merino E, Johansson S, Wallander MA. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest 2008;134:1223–30. [DOI] [PubMed] [Google Scholar]
- [8].Kim J, Lee JH, Kim Y, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med 2013;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med 2000;162:34–9. [DOI] [PubMed] [Google Scholar]
- [10].Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. [DOI] [PubMed] [Google Scholar]
- [11].Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakamura T, Shirakawa K, Masuyama H, Sugaya H, Hiraishi H, Terano A. Minimal change oesophagitis: a disease with characteristic differences to erosive oesophagitis. Aliment Pharmacol Ther 2005;21: (Suppl 2): 19–26. [DOI] [PubMed] [Google Scholar]
- [13].Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil 2011;17:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rogha M, Behravesh B, Pourmoghaddas Z. Association of gastroesophageal reflux disease symptoms with exacerbations of chronic obstructive pulmonary disease. J Gastrointestin Liver Dis 2010;19:253–6. [PubMed] [Google Scholar]
- [15].Ruzkowski CJ, Sanowski RA, Austin J, Rohwedder JJ, Waring JP. The effects of inhaled albuterol and oral theophylline on gastroesophageal reflux in patients with gastroesophageal reflux disease and obstructive lung disease. Arch Intern Med 1992;152:783–5. [PubMed] [Google Scholar]
- [16].Özdemir P, Erdinc M, Vardar R, et al. The role of microaspiration in the pathogenesis of gastroesophageal reflux-related chronic cough. J Neurogastroenterol Motil 2017;23:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van Soest EM, Dieleman JP, Kuipers EJ. The effect of anticholinergic agents on gastro-oesophageal reflux and related disorders. Expert Opin Drug Saf 2008;7:173–80. [DOI] [PubMed] [Google Scholar]
- [18].Kesten S, Celli B, Decramer M, Leimer I, Tashkin D. Tiotropium HandiHaler in the treatment of COPD: a safety review. Int J Chron Obstruct Pulmon Dis 2009;4:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cui Y, Devillier P, Kuang X, et al. Tiotropium reduction of lung inflammation in a model of chronic gastro-oesophageal reflux. Eur Respir J 2010;35:1370–6. [DOI] [PubMed] [Google Scholar]
- [20].Kim SW, Lee JH, Sim YS, Ryu YJ, Chang JH. Prevalence and risk factors for reflux esophagitis in patients with chronic obstructive pulmonary disease. Korean J Intern Med 2014;29:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut 1990;31:04–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kadakia SC, Kikendall JW, Maydonovitch C, Johnson LF. Effect of cigarette smoking on gastroesophageal reflux measured by 24-h ambulatory esophageal pH monitoring. Am J Gastroenterol 1995;90:1785–90. [PubMed] [Google Scholar]
- [23].Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989;84:1076–8. [PubMed] [Google Scholar]
- [24].DelGaudio JM. Steroid inhaler laryngitis: dysphonia caused by inhaled fluticasone therapy. Arch Otolaryngol Head Neck Surg 2002;128:677–81. [DOI] [PubMed] [Google Scholar]
- [25].Broers C, Tack J, Pauwels A. Review article: gastro-oesophageal reflux disease in asthma and chronic obstructive pulmonary disease. Aliment Pharmacol Ther 2018;47:176–91. [DOI] [PubMed] [Google Scholar]
- [26].Lee AL, Goldstein RS. Gastroesophageal reflux disease in COPD: links and risks. Int J Chron Obstruct Pulmon Dis 2015;10:1935–49. [DOI] [PMC free article] [PubMed] [Google Scholar]