Abstract
Proteins in the SNF2/SWI2 family use ATP hydrolysis to catalyze rearrangements in diverse protein-DNA complexes. How ATP hydrolysis is coupled to these rearrangements is unknown, however. One attractive model is that these ATPases are ATP-dependent DNA-tracking enzymes. This idea was tested for the SNF2/SWI2 protein family member MOT1. MOT1 is an essential Saccharomyces cerevisiae transcription factor that uses ATP to dissociate TATA binding protein (TBP) from DNA. By using a series of DNA templates with one or two TATA boxes in combination with binding sites for heterologous DNA binding “roadblock” proteins, the ability of MOT1 to track along DNA was assayed. The results demonstrate that, following ATP-dependent TBP-DNA dissociation, MOT1 dissociates rapidly from the DNA by a mechanism that does not require a DNA end. Template commitment footprinting experiments support the conclusion that ATP-dependent DNA tracking by MOT1 does not occur. These results support a model in which MOT1 drives TBP-DNA dissociation by a mechanism that involves a transient, ATP-dependent interaction with TBP-DNA which does not involve ATP-dependent DNA tracking.
The SNF2/SWI2 protein family is a large group of evolutionarily conserved ATPases with diverse functions in transcriptional control, DNA repair, and chromosome segregation (10, 30). Genetic and biochemical approaches have revealed that several of these proteins function by using ATP hydrolysis to drive alterations in protein-DNA contacts. For example, SNF2/SWI2 and related proteins in Drosophila melanogaster and humans are components of large macromolecular complexes which can disrupt nucleosome structure in vitro in an ATP-dependent reaction (4, 5, 7, 18, 20, 24, 29, 43, 44). Another member of this family, ISWI (11), is a component of distinct complexes which can function both in nucleosome remodeling and in the ATP-dependent formation of closely spaced nucleosome arrays (19, 28, 42, 45). All SNF2/SWI2 protein family members which have been tested contain an ATPase which is essential for in vitro and in vivo function (2, 21, 25). The intrinsic ATPase activity of these proteins is low or undetectable but can be activated by DNA, proteins, or protein-DNA complexes with which these ATPases are known to interact (3, 6, 15, 25, 31, 39).
Based on these data, one hypothesis is that all SNF2/SWI2 family members participate in ATP-dependent reactions which result in alterations of protein-DNA contacts. ATP-dependent alterations of nucleosome structure can explain how SNF2/SWI2 and related complexes render the chromatin template accessible to the transcription machinery (43). Likewise, the essential transcriptional regulator MOT1 modulates transcription by catalyzing the ATP-dependent dissociation of TATA binding protein (TBP) from DNA (2). By extension, the roles of SNF2/SWI2 family members in DNA repair may reflect ATP-dependent dissociation or rearrangement of proteins on damaged DNA as an obligate part of certain repair pathways. Despite the apparently widespread utilization of the conserved SNF2/SWI2 ATPase domain, there is currently little mechanistic understanding of how ATP hydrolysis is coupled to these protein-DNA rearrangements (30). Initial sequence comparisons suggested that SNF2/SWI2 and related proteins fall within a larger family of DNA helicases (14). It was subsequently argued, however, that proteins within this group comprise a distinct family of proteins, none of which have been demonstrated to have helicase activity (16). If these ATPases do not appear to be helicases, an alternative model is that these proteins are ATP-dependent DNA-tracking enzymes (30). Such an enzyme might be targeted to a specific protein-DNA complex and then use ATP hydrolysis to translocate along DNA, disrupting protein-DNA contacts in its path. Disruption of specific protein-DNA contacts could then be a consequence of specific targeting of the ATPase (in an ATP-independent event) and specific interactions between the ATPase and the target protein-DNA complex which occur during ATP-dependent translocation along DNA. Alternatively, a specifically targeted ATPase might acquire the ability to translocate along DNA and disrupt protein-DNA contacts relatively nonspecifically (like a snowplow). This model is attractive because it can potentially explain how an ATPase might remodel the structure of a nucleosome which contains an extensive protein-DNA interface. Here we explicitly test the idea that the SNF2/SWI2 protein family member MOT1 is an ATP-dependent DNA-tracking enzyme. These experiments were possible because highly purified MOT1 catalyzes the dissociation of TBP-DNA complexes in a well-defined in vitro reaction (2, 3); as such, the MOT1 reaction lends itself to an explicit test of the tracking hypothesis. Using a variety of DNA templates with combinations of TATA boxes and heterologous “roadblock” proteins, we demonstrate that MOT1 is not an ATP-dependent DNA-tracking enzyme.
MATERIALS AND METHODS
Plasmid constructions and DNA probes.
The gel shift probes used (see Fig. 2) were made by phosphorylating oligonucleotides 2TATA-1 and 2TATA-3 (Table 1) by using T4 polynucleotide kinase and [γ-32P]ATP. 2TATA-1 was then annealed to 2TATA-2, and 2TATA-3 was annealed to 2TATA-4, by heating of the combined DNAs in TE (10 mM Tris-Cl [pH 8], 0.1 mM EDTA) plus 0.1 M NaCl and slow cooling to room temperature over a period of 30 to 60 min. The annealed DNAs were electrophoresed on a 6% polyacrylamide nondenaturing gel, excised, and eluted in TE plus 0.1 M NaCl. Following ethanol precipitation, the DNAs were resuspended in TE. Plasmid p2TATA5/6-1 was constructed by inserting the annealed 2TATA-5 and 2TATA-6 duplex into the SmaI site of pKSII+. The tandem TATA footprinting probe used in the experiment represented by Fig. 4 was obtained by digestion of p2TATA5/6-1 with ClaI and SacI followed by Klenow fill-in with [α-32P]dCTP and 0.6 mM unlabeled dGTP to uniquely label the top strand. This probe was purified on a nondenaturing gel as described above. Plasmid p2-2lac contains the oligonucleotide duplex 2TATA-1–2TATA-2 inserted into the SmaI site and the lac operator (selfcomplementary oligonucleotide lacO-Bam) (35, 36) inserted into the BamHI site of pKSII+. The TATA-containing insert is oriented such that the EcoRI site in the pKSII+ polylinker is upstream and the inserted lac operator is downstream of the TATA box. For footprinting and gel shift analysis to determine if MOT1 can dissociate from a template containing EcoRI-Gln 111 and/or lac repressor bound to flanking sites (see Fig. 1), p2-2lac DNA was digested with ClaI and SacI and labeled by Klenow fill-in with [α-32P]dCTP and unlabeled dGTP as described above. To determine the effect of EcoRI-Gln 111 bound downstream of a TATA box on MOT1 action (see Fig. 4E), we used a plasmid similar to p2-2lac, p15/16lac, in which the TATA-containing insert (oligonucleotide duplex 2TATA-15–2TATA-16) was ligated to the SmaI site of pKSII+ in the opposite orientation, thereby placing the EcoRI site in the pKSII+ polylinker downstream of the TATA box. A lac operator (lacO-Pst duplex) was also inserted into the PstI site of pKSII+ in p15/16lac in order to test the effect of lac repressor placed at a different position downstream of the TATA box (see Fig. 4D). p15/16lac without the lac operator is referred to as p2TATA15/16 below. The p15/16lac footprinting probe was generated by digestion with XbaI and KpnI, followed by labeling of the top strand by Klenow fill-in with [α-32P]dCTP and unlabeled dATP, dGTP, and dTTP. To test tracking in the 5′-to-3′ direction (with respect to the DNA strand containing the TATA sequence TATAAAAG) with the lac repressor reversible roadblock, plasmids p2TATAlac13 and p2TATAlac14 were constructed. These plasmids were constructed by inserting the lacO-TATA duplex with either the 9-bp spacer (lac14-lac15) or the 12-bp spacer (lac18-lac19) into the BamHI and PstI sites of p2TATA15/16 (described above) to generate p2TATAlac14 and p2TATAlac13, respectively. Footprinting probes were generated from these plasmids by digestion with XbaI and KpnI and labeling of the top strand by Klenow fill-in, as described above for the p15/16lac probe. To test for tracking by MOT1 in the 3′-to-5′ direction (with respect to the DNA strand containing the TATA sequence TATAAAAG), the lac16-lac17 duplex was inserted into the HindIII and ClaI sites and the lac18-lac19 duplex was inserted into the BamHI and PstI sites of pKSII+ to create p2TATAlac21. The 3′-to-5′ tracking footprinting probe was generated by digestion of p2TATAlac21 with XbaI and KpnI and labeling, as described above for the p15/16lac probe. All plasmid inserts were verified by sequencing.
FIG. 2.
Strategy for testing of models of MOT1 action by using a DNA template with tandem TATA boxes. (A) MOT1 interacts with TBP-DNA via interactions with both TBP and DNA upstream of the TBP-DNA complex. Under template commitment conditions, dissociation of the TBP molecule directly interacting with MOT1 but not the adjacent TBP molecule might occur by a mechanism involving a transient, ATP-driven power stroke. Alternatively, in the presence of ATP, interaction of MOT1 with TBP-DNA might be followed by engagement with the DNA template and dissociation of both TBP molecules by an ATP-dependent DNA-tracking mechanism. (B) One requirement of the tandem TATA experiment is that MOT1 is specifically targeted to only one of the two TBP-DNA complexes on the tandem TATA template. (C) One prediction is that MOT1 can disrupt TBP-DNA on this template only via interactions with DNA upstream of the 5′ TBP-DNA complex. Hence, upstream truncation of the DNA should permit TBP binding to both TATA boxes, but these complexes should be refractory to MOT1 action.
TABLE 1.
Oligonucleotides used
| Oligonucleotide | Sequence |
|---|---|
| 2TATA-1 | 5′-GTGTTCCTGAAGGGGGGCTGTAAAAGGGCCTCGTATAAAAGGGGGTGGGGGCGCGT-3′ |
| 2TATA-2 | 5′-ACGCGCCCCCACCCCCTTTTATACGAGGCCCTTTTACAGCCCCCCTTCAGGAACAC-3′ |
| 2TATA-3 | 5′-GGGGCTGTAAAAGGGCCTCGTATAAAAGGGGGTGGGGGCGCGT-3′ |
| 2TATA-4 | 5′-ACGCGCCCCCACCCCCTTTTATACGAGGCCCTTTTACAGCCCC-3′ |
| 2TATA-5 | 5′-GTGTTCCTGAAGGGGGGCTATAAAAGGGCCTCGTATAAAAGGGGGTGGGGGCGCGT-3′ |
| 2TATA-6 | 5′-ACGCGCCCCCACCCCCTTTTATACGAGGCCCTTTTATAGCCCCCCTTCAGGAACAC-3′ |
| 2TATA-15 | 5′-GTGTTCCTGAAGGGGGGCTATAAAAGGGCCTCGCGGGTGACAGCCCTCCGGGGGGT-3′ |
| 2TATA-16 | 5′-ACCCCCCGGAGGGCTGTCACCCGCGAGGCCCTTTTATAGCCCCCCTTCAGGAACAC-3′ |
| lac10 | 5′-AGCTTCCGAATTGTGAGCGCTCACAATTCTGAAGGGGGGCTATAAAAGGGCCTCGGGGGGCAT-3′ |
| lac11 | 5′-CGATGCCCCCCGAGGCCCTTTTATAGCCCCCCTTCAGAATTGTGAGCGCTCACAATTCGGA-3′ |
| lac12 | 5′-AGCTTCCGAATTGTGAGCGCTCACAATTCTGAAGGGGTATAAAAGGGCCTCGGGGGGCAT-3′ |
| lac13 | 5′-CGATGCCCCCCGAGGCCCTTTTATACCCCTTCAGAATTGTGAGCGCTCACAATTCGGA-3′ |
| lac14 | 5′-GATCCCGAATTGTGAGCGCTCACAATTCTGAAGGGGTATAAAAGGGCCTCGGGGGGCTGCA-3′ |
| lac15 | 5′-GCCCCCCGAGGCCCTTTTATACCCCTTCAGAATTGTGAGCGCTCACAATTCGG-3′ |
| lac16 | 5′-AGCTTGGCCTCGGGCCCACAGAGCCGTCCGGGGTGTTCCTGAAGGGGGGCTATAAAAGGAT-3′ |
| lac17 | 5′-CGATCCATTTATAGCCCCCCTTCAGGAACACCCCGGACGGCTCTGTGGGCCCGAGGCCA-3′ |
| lac18 | 5′-GATCCCGAATTGTGAGCGCTCACAATTCTGAAGGGGGGCTATAAAAGGGCCTCGGGGGGCTGCA-3′ |
| lac19 | 5′-GCCCCCCGAGGCCCTTTTATAGCCCCCCTTCAGAATTGTGAGCGCTCACAATTCGG-3′ |
| lacO-Bam | 5′-GATCCAATTGTGAGCGCTCACAATTG-3′ |
| lacO-Pst | 5′-GAATTGTGAGCGCTCACAATTCTGCA-3′ |
FIG. 4.
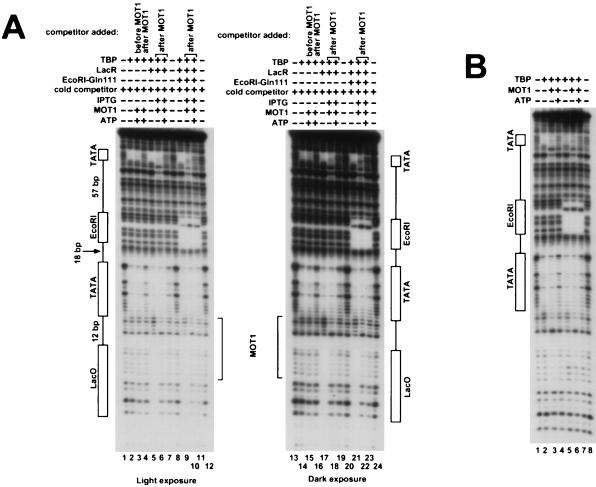
Template-committed MOT1 disrupts both TBPs bound to the tandem TATA template. (A) Experimental scheme. The radiolabeled DNA, shown on top (asterisk), was incubated with TBP (open saddle shapes) in one reaction, and a 10-fold molar excess of identical, unlabeled DNAs was incubated with TBP in a parallel reaction. Since the unlabeled reaction mixture contains a molar excess of DNA over TBP, this reaction mixture presumably contains unbound, singly bound, and doubly bound DNA molecules which, for simplicity, are not all shown. After 20 min, MOT1 (black oval) was added to the reaction mixture containing radiolabeled DNA, and then the two reaction mixtures were combined with ATP for 1 min. DNase was added for 1 min, and then the reactions were terminated and the reaction products were resolved on 8% sequencing gels (see Materials and Methods). (B) The indicated proteins were added as outlined for panel A with the exception that the competitor TBP-DNA was added at different points in the scheme, as indicated. No competitor was added to the reaction mixtures in lanes 1 to 4. (C) The template commitment footprinting experiment was performed as described for panel B except that the reactions were performed in duplicate and footprinting was performed with two different amounts of DNase I. Competitor TBP-DNA was not added to the reaction mixtures in lanes 1 and 2.
FIG. 1.
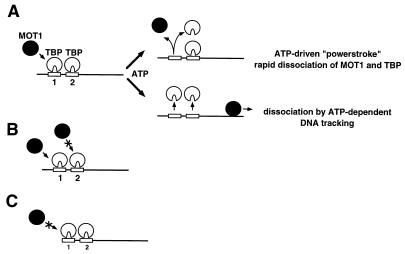
MOT1 and TBP dissociate rapidly from DNA in an ATP-dependent reaction that does not require an unobstructed DNA end. (A) Experimental scheme for the reactions analyzed in panels B and C. (B) Analysis of MOT1-catalyzed TBP-DNA dissociation on a “blocked” template by gel mobility shift. The template used is diagrammed above the autoradiogram. The total length of the probe was 154 bp, with 18 bp extending upstream of the EcoRI site and 36 bp downstream of the lac operator. The bands corresponding to various protein-DNA complexes are indicated by arrows. Note that addition of ATP to DNA preincubated with TBP, MOT1, lac repressor, and EcoRI-Gln 111 (lane 11) leads to rapid formation of a protein-DNA complex which comigrates with the lac repressor–EcoRI-Gln 111–DNA complex (lane 9). (C) Analysis of the fate of MOT1 and TBP on the same template as in panel B by DNase I footprinting. The footprints of proteins bound to EcoRI, TATA, and lac operator sites are indicated by rectangles. The bracket on the right denotes the DNA upstream of the TATA box protected from DNase I digestion by MOT1 when added to a reaction mixture containing TBP in the absence of ATP (lanes 3, 7, and 11). Note that addition of ATP for 1 min caused loss of the TBP and MOT1 footprints (lane 4), and this result is unaffected by EcoRI-Gln 111 bound upstream of the TATA box (lane 8) as well as by the binding of both EcoRI-Gln 111 and lac repressor to sites on either side of the TATA box (lane 12). (D) DNase I footprinting analysis of MOT1 activity on a DNA template containing a lac operator 36 bp downstream of a TATA box. The experiment was performed as described for panel C except that unlabeled lac operator DNA (“cold DNA”) was added as indicated to verify that lac repressor does not dissociate from the lac operator on the footprinting probe over the time course of the MOT1-catalyzed reaction. (E) DNase I footprinting analysis was performed as described for panel C by using a radiolabeled probe containing an EcoRI site 42 bp downstream from a TATA box. To verify that EcoRI-Gln 111 remains stably bound to the EcoRI site over the course of the MOT1-catalyzed reaction, a molar excess of unlabeled EcoRI-containing DNA (“cold DNA”) was added as indicated. (F) DNase footprinting experiments were performed as described for panels D and E by using the same footprinting probe as for panel C; unlabeled EcoRI-containing DNA was added as indicated to verify that EcoRI-Gln 111 is not dissociated from its site upstream of the TATA box over the course of the MOT1-catalyzed reaction.
Recombinant proteins.
The TBP used in these experiments consisted of the C-terminal 180 amino acids of yeast TBP (22), the minimal sequence required to provide full function in vivo (32). This TBP core domain was used because it gave more reproducible and complete occupancy of the TATA box than full-length TBP under the conditions described below. Furthermore, MOT1 appears to bind to the yeast TBP core domain more stably than to full-length TBP (1a), thus facilitating the template commitment footprinting experiments. The TBP core domain was purified from Escherichia coli as described previously (22) and was a gift from Jim Geiger. The “altered specificity” TBP, TBPm3 (37), was obtained by subcloning the TBPm3 open reading frame into an E. coli overexpression plasmid. TBPm3 was purified from E. coli as previously described for wild-type TBP (32). MOT1 was obtained from a yeast overexpression strain and purified as previously described (3). EcoRI-Gln 111 (23) was generously provided by Paul Modrich, and lac repressor was generously provided by Kathleen Matthews.
Gel mobility shift and DNase footprinting reactions.
Binding reactions were performed essentially as described previously (1a) in 20-μl reaction mixtures containing 5 ng of TBP, radiolabeled DNA (approximately 1,000 cpm for the gel shift reactions and 20,000 cpm for the footprinting reactions), and approximately 5 units of MOT1, 10 ng of lac repressor, and/or 2.5 nM EcoRI-Gln 111. In general, TBP was incubated with the DNA probe for 20 to 30 min, and EcoRI-Gln 111 and/or lac repressor was added for 5 min prior to addition of MOT1 and 10 μM ATP. All incubations were performed at room temperature. Incubation of TBP with the radiolabeled templates led to a 5- to 19-fold decrease in DNase cleavage in the TATA box; the magnitude of the decrease in band intensity depended on the particular band which was quantified and the particular TATA sequence. Binding of MOT1 to TBP-DNA was complete within 1 min, and ATP-dependent TBP-DNA dissociation was complete in under 1 min following addition of ATP, so the template commitment experiments were performed by incubating MOT1 with or without ATP with the preformed protein-DNA complexes for 15 s to 1 min (as indicated) prior to termination. Rapid dissociation of lac repressor was induced where indicated by the addition of 60 ng of unlabeled duplex lacO-Pst oligonucleotide and 350 μM IPTG (isopropyl-β-d-thiogalactopyranoside). Gel shift analyses were performed by loading the reaction mixtures onto 6% nondenaturing polyacrylamide gels, as previously described (1a). For template commitment experiments, reactions with unlabeled DNA (cold competitor reactions [see Results]) were set up in parallel with the reactions with radiolabeled probe. Cold competitor reaction mixtures contained 200 ng of annealed 2TATA-1–2TATA-2 and 100 ng of TBP in 5 μl containing the same salt and buffer conditions as in the standard reaction mixtures containing radiolabeled DNA. Following a preincubation of 20 to 40 min, the 5-μl cold competitor reaction mixtures were added to the reaction mixtures containing radiolabeled DNA, as indicated. DNase footprinting was performed by addition of 1 μl of 5 U of DNase I (Worthington) per ml for 1 min. Reactions were terminated by addition of an equal volume of 5 M ammonium acetate and 3 volumes of ethanol. Following ethanol precipitation, the DNase I-digested DNAs were analyzed by electrophoresis on 8% polyacrylamide sequencing gels.
RESULTS
Experimental strategy.
In previous work, MOT1-TBP-DNA complexes were detected in the absence of ATP by either a gel mobility shift assay or a DNase I footprinting assay (1a). In the presence of ATP, rapid dissociation of both TBP and MOT1 from the template was detected (1a, 2). Since all of these experiments were performed on radiolabeled linear templates, however, it was impossible to determine if TBP-DNA dissociation was catalyzed by a DNA-tracking mechanism or by a transient local interaction between MOT1 and TBP-DNA exclusively at the TATA box. To test for tracking, two kinds of experiments were performed. First, heterologous DNA binding proteins were used to trap a putatively tracking MOT1 molecule on DNA. This approach has been used previously to demonstrate DNA tracking by replication and transcription factors (40, 41). If MOT1 disrupts TBP-DNA complexes by using ATP hydrolysis to processively track along DNA, we reasoned that it might be possible to detect these putative ATP-dependent MOT1-DNA-tracking complexes with heterologous DNA binding proteins which might prevent MOT1 from translocating off the end of a linear DNA molecule. Alternatively, a hypothetical tracking complex might be able to disrupt heterologous DNA binding protein complexes as it moves along DNA. Both of these possibilities were tested. A second approach to test for DNA tracking by MOT1 involved performing template commitment DNase footprinting experiments on DNAs containing two TATA boxes. Using strategies to target MOT1 to just one of the two TBP-DNA complexes formed on these templates, we tested whether or not a template-committed MOT1 molecule can translocate along DNA from one TBP-DNA complex to another without first dissociating.
Rapid dissociation of TBP and MOT1 from DNA does not require a DNA end.
The first test of DNA tracking by MOT1 utilized templates with a lac operator and/or EcoRI site placed on either side of a TATA box (Fig. 1). The lac operator forms a stable complex with lac repressor (35), and the EcoRI site is tightly bound by a catalytically inactive form of EcoRI endonuclease, EcoRI-Gln 111 (23). TBP was incubated with the radiolabeled templates in the presence or absence of lac repressor and/or EcoRI-Gln 111; then, MOT1 was added, followed by ATP, and the reactions were analyzed by nondenaturing polyacrylamide gel electrophoresis or DNase footprinting (Fig. 1A). The DNA probe used in the experiments shown in Fig. 1B and C contained a lac operator 21 bp downstream of the TATA box and an EcoRI binding site 45 bp upstream of the TATA box. By gel mobility shift analysis, complexes with discrete and distinguishable mobilities could be identified when the probe was incubated with all combinations of the proteins used. The complex containing TBP, MOT1, EcoRI-Gln 111, and lac repressor migrated with the lowest mobility, as expected (Fig. 1B, lane 10), and the association of MOT1 with the template required the binding of TBP (lane 12). Following the addition of ATP, the supershifted complex containing all of the proteins collapsed to the position of the probe incubated with just EcoRI-Gln 111 and lac repressor (compare lanes 8 and 11 in Fig. 1B), indicating that MOT1-catalyzed TBP-DNA dissociation leads to the dissociation of both TBP and MOT1. This reaction was complete following incubation with ATP for 15 s and immediate loading onto the gel. The results in Fig. 1B are also consistent with the results shown in Fig. 1C, in which the fate of these proteins was monitored by DNase footprinting. Incubation of MOT1 with TBP and DNA led to an extension of the DNase footprint upstream of the TATA box (compare lanes 2 and 3 in Fig. 1C), and the addition of ATP led to virtually complete disruption of the footprint after 1 min (compare lanes 3 and 4 to lane 1 in Fig. 1C). Note that in contrast to bidirectional binding of TBP to DNA, which has previously been reported (8), based on the asymmetry of MOT1 binding to these TBP-DNA complexes, we conclude that binding of wild-type TBP to the DNA probes used in these experiments occurs unidirectionally, with a defined orientation. Incubation of the DNA with either EcoRI-Gln 111 (Fig. 1C, lanes 6 to 9) or EcoRI-Gln 111 and lac repressor (lanes 10 to 13) led to clear protection of the lac operator and EcoRI sites, and the occupancy of these sites did not detectably affect MOT-catalyzed disruption of TBP-DNA (compare lanes 8, 9, 12, and 13 to lanes 4 and 5 [Fig. 1C]). Furthermore, the DNase digestion patterns between the TATA box and the roadblocks are indistinguishable in reaction mixtures containing no MOT1 or MOT1 plus ATP, supporting the conclusion that MOT1 rapidly dissociates from DNA following ATP-dependent TBP-DNA dissociation.
The behavior of MOT1 in this assay was further analyzed by using additional templates in which either the lac operator was placed a different distance downstream of the TATA box (Fig. 1D) or the EcoRI binding site was placed downstream of the TATA box instead of the lac operator (Fig. 1E). Results of experiments with these templates performed similarly to the experiment shown in Fig. 1C support the conclusion that MOT1 is not blocked on the template by the presence of heterologous DNA binding proteins, and this conclusion does not depend on any particular placement or combination of EcoRI or lac operator sites.
If MOT1 tracks along DNA, one possibility was that an “engaged” tracking enzyme might acquire the ability to dissociate heterologous DNA binding proteins. In the experiments represented by Fig. 1D to F, this possibility was tested by adding unlabeled lac operator or EcoRI binding site DNA along with ATP during the disruption reaction. While these unlabeled DNAs can completely prevent the association of lac repressor or EcoRI-Gln 111 with DNA (Fig. 1D, lanes 6 and 7; Fig. 1E, lanes 8 and 9), the lac repressor and EcoRI-Gln 111 footprints are unaffected by MOT1 and ATP, indicating that MOT1 does not disrupt their binding (Fig. 1D, lane 11; Fig. 1E, lane 13; Fig. 1F, lane 12). These results demonstrate that TBP-DNA disruption by MOT1 leads to rapid dissociation of TBP and MOT1 from the template in a reaction that does not require an unobstructed DNA end.
Use of a template containing tandem TATA boxes to test for tracking by MOT1.
The results in Fig. 1 rule out models for MOT1 action in which the ATP-dependent reaction leads to a very tight and long-lived association of MOT1 with DNA following loading at a TBP-DNA complex. On the other hand, some DNA tracking proteins can diffuse along DNA over relatively long distances, and yet they are released from DNA in just a few seconds following loading (12, 40). To determine if MOT1 might function in this way, a series of template commitment DNase footprinting experiments was performed. The aim of these experiments was to determine if MOT1 loaded onto one TBP-DNA complex could translocate to a second TBP-DNA complex on the same template without prior dissociation from the DNA. The first strategy that was employed is shown in Fig. 2. It was previously shown that MOT1 requires DNA sequences upstream of the TATA box (with respect to the start site of transcription) in order to form MOT1-TBP-DNA ternary complexes and also to catalyze TBP-DNA dissociation in the presence of ATP (1a). Additional data demonstrate that 17 bp of DNA upstream of the TATA box is required, whereas DNA sequences downstream of the TATA box are not (46). The specific sequence of the DNA upstream of the TATA box also appears to be relatively unimportant (46). These results suggested that two TBP complexes placed in close apposition might create a situation in which MOT1 can recognize the TBP-DNA complex bound to the upstream TATA box but not TBP bound to the downstream TATA box, because the upstream TBP molecule would provide a steric block for recognition by MOT1 (Fig. 2B). One important prediction of this model is that two closely spaced TATA boxes located at the end of a template with a short upstream end should not be recognized by MOT1 (Fig. 2C).
Two suitable “tandem TATA” probes were examined by gel mobility shift analysis (Fig. 3). The DNAs contain one wild-type and one mutant TATA box separated by 7 bp. The tandem TATAs were placed either within a probe of sufficient length to support MOT1 action (long probe) or near the upstream end of a short probe. Different TATA sequences were used to differentially target different TBPs to different sites on each of the two probes. Wild-type TBP binds only to the wild-type TATA sequence TATAAAAG, whereas the altered-specificity TBP, TBPm3 (37), binds to both the wild-type site and TGTAAAAG. TBPm3 was chosen because it is recognized by MOT1 indistinguishably from wild-type TBP (1; also see below).
FIG. 3.
Gel mobility shift analysis of protein-DNA complexes formed on tandem TATA templates. The sequences of the two DNA probes used are shown at the top; the TBP binding sites are boxed. The upstream site, TGTAAAAG, is recognized by the altered-specificity TBP, TBPm3, but not by wild-type TBP. TBPm3 and wild-type TBP bind to the downstream TATA box. The free DNA as well as protein-DNA complexes formed on these probes are indicated by arrows. In this depiction, the open circles represent TBP or TBPm3, the black oval is MOT1, the DNA probe is represented by the black horizontal line, and the TATA boxes are shown as rectangles. The amounts of purified MOT1 added to the reaction mixtures are in microliters; this preparation of MOT1 had an activity of about 1 unit/μl (1a).
As shown in Fig. 3, incubation of the long probe with TBPm3 (lanes 1 to 5) results in the detection of TBPm3 bound to either one or both TATA boxes in the absence of MOT1 (lane 1). Supershifted species are formed when MOT1 is added (lanes 2 and 4) and, at steady state, the TBPm3-DNA complexes are largely disrupted when ATP is added (lanes 3 and 5). Note that the probe is not quantitatively shifted to the positions of full occupancy because TBP-DNA complexes formed with full-length TBP are unstable in the gel (17). As was seen with the doubly occupied TATA boxes formed on the long probe, binding of wild-type TBP to TATA box 2 on the long probe generates a complex which is also recognized and disrupted by MOT1 (Fig. 3, lanes 6 to 10). (The MOT1-TBP-DNA ternary complex is less obvious in lanes 7 and 9 in this particular experiment because less of the probe is shifted by wild-type TBP than in the adjacent lanes containing TBPm3.) In contrast, binding of TBPm3 to both TATA boxes formed on the short probe (Fig. 3, lanes 11 to 15) generates a protein-DNA complex which is only weakly bound by MOT1 in the absence of ATP (lanes 12 and 14). In the presence of ATP, MOT1 dissociates from TBPm3-DNA, but these TBP-DNA complexes are largely refractory to MOT1 action (compare lanes 13 and 15 to lanes 3 and 5). The small effect of MOT1 that we do observe could be due to a small fraction of TBPm3 which binds to TATA box 2 in the opposite orientation (8). These results indicate that tandem TBP-DNA complexes can be disrupted by MOT1, but this activity requires interaction with DNA upstream of TATA box 1. Furthermore, the results with the short probe show that TBP bound to TATA box 1 effectively blocks access by MOT1 to TBP bound to TATA box 2 as required to test the models shown in Fig. 2.
Based on the results shown in Fig. 3, template commitment footprinting experiments using the long tandem TATA probe were performed next. TBP was incubated with either the radiolabeled tandem TATA probe or, in a parallel reaction, with a 10-fold molar excess of unlabeled DNA (Fig. 4A). Following a 20-min preincubation, MOT1 was added to the reaction mixture containing the labeled DNA for 1 min. The binding of TBP to DNA is stable, and this 1-min incubation with MOT1 is sufficient to commit MOT1 to TBP-DNA but is sufficiently short that TBP does not appreciably dissociate from the template during this time. The reaction mixtures containing labeled and unlabeled DNA were then combined with ATP for 1 min and then DNase treated and terminated as described in Materials and Methods.
The addition of TBP to the tandem TATA probe results in full protection of both TATA boxes from DNase digestion (Fig. 4B and C, lanes 2). Addition of MOT1 causes an extension of the footprint upstream of the TATA boxes, as expected (Fig. 4B, lane 3), and the footprints are disrupted in the presence of MOT1 and ATP (Fig. 4B, lane 4). Competitor TBP-DNA prevents the interaction between TBP and the labeled probe (Fig. 4B, lane 5; Fig. 4C, lanes 3 and 4), but over the time course of the reaction the competitor has no effect on TBP-DNA complexes preformed on the radiolabeled probe (Fig. 4B, lane 6; Fig. 4C, lanes 5 and 6). Addition of the competitor TBP-DNA reaction mixture prior to the addition of MOT1 and ATP prevents MOT1 activity towards TBP-DNA complexes formed on the radiolabeled probe (Fig. 4B and C, lanes 7). Combining the preformed complexes under template commitment conditions, as shown in Fig. 4A, results in uniform disruption of both TBP-DNA complexes (Fig. 4B, lane 7 versus lane 8; Fig. 4C, lane 7 versus lane 9). Under these conditions, phosphorimager quantitation of the band intensities demonstrates that 30 to 60% of the radiolabeled templates retain a committed molecule of MOT1 when challenged with competitor TBP-DNA and ATP. (In the experiment represented by Fig. 4B, 30% of the TBP-DNA complexes were disrupted by MOT1.) This efficiency of template commitment reflects the fact that in the absence of ATP, MOT1 dissociates rapidly from TBP-DNA complexes under these conditions (1), and so even rapid mixing of these reaction mixtures gives rise to some dissociation of MOT1 from the template prior to ATP-dependent TBP-DNA disruption. This degree of template commitment may also be related to the efficiency with which MOT1 successfully dissociates a TBP-DNA complex in the presence of ATP prior to dissociation from the template.
The uniform disruption of TBPs bound to both TATA boxes by template-committed MOT1 is consistent with a model in which MOT1 functions by an ATP-dependent DNA-tracking mechanism. Due to the close proximity of the tandem TATA boxes, however, there are other explanations for these results (see Discussion). To permit a more direct test of tracking by MOT1, a system was required to determine the effect of template-committed MOT1 on a TBP-DNA complex located more remotely from the site at which it is loaded.
Targeting of MOT1 to one of two TBP-DNA complexes on the same template by using a reversible steric block.
The strategy that was employed utilized DNA templates with two TATA boxes placed between 80 and 90 bp apart (Fig. 5A). The results shown in Fig. 4 demonstrate that access to a TBP-DNA complex can be blocked by the binding of a second TBP molecule just upstream. To test for DNA tracking from one TBP-DNA complex to another on the same template, a reversible steric block that prevented interaction between MOT1 and one of the TBP-DNA complexes was required. lac repressor is a suitable reversible steric block because it binds with high affinity and stability to its binding site, and the binding of lac repressor can be rapidly reversed by the addition of the inducer IPTG (26, 33, 34, 40, 47). In the presence of IPTG and a molar excess of unlabeled lac operator DNA, the quantitative dissociation of lac repressor from DNA was found to occur in just a few seconds (1). The template commitment experiment using templates with the reversible lac repressor block were performed similarly to the tandem TATA box template commitment experiments, with the exception that the unlabeled competitor TBP-DNA complex was combined with IPTG and unlabeled lac operator to induce the dissociation of lac repressor from the labeled template (Fig. 5A).
FIG. 5.
Strategy for testing for DNA tracking by MOT1 with lac repressor as a reversible steric block. (A) DNA containing two TATA boxes (open rectangles) separated by 80 to 100 bp is incubated with TBP (open saddle shapes) and lac repressor (gray circle), which binds to a lac operator (gray rectangle) precisely positioned upstream of one TATA box to interfere with MOT1 (black circle) binding to its proximally positioned TBP. The other TBP-DNA complex remains available for MOT1 interaction. One configuration of these sites is shown, but an analogous DNA containing a lac operator positioned just upstream of the 5′ TATA box was also tested (see text). Template commitment footprinting experiments were then performed in a fashion analogous to those in Fig. 4, with the exception that IPTG and unlabeled lac operator DNA were added along with unlabeled competitor TBP-DNA and ATP to induce the rapid dissociation of lac repressor from the operator on the footprinting probe. Following DNase treatment, the reactions were analyzed as described for Fig. 4 to determine if MOT1 bound to one TBP-DNA complex can influence the rate of dissociation of a second TBP-DNA complex on the same template without prior dissociation. (B) lac repressor bound to a lac operator positioned 12 bp (lanes 1 to 10) or 9 bp (lanes 11 to 18) upstream of a TATA box can block MOT1 action. TBP and lac repressor were incubated with radiolabeled DNA as indicated, and then MOT1 in the presence or absence of ATP and/or IPTG plus unlabeled lac operator was added for 1 min followed by addition of DNase I and analysis of the reaction products, as described for Fig. 4. The regions of DNA protected by TBP and lac repressor are indicated (rectangles), and the upstream extension of the TBP footprint induced by MOT1 in the absence of ATP is indicated by a bracket.
Several versions of the lac operator-TATA template were constructed. First, the lac operator was placed either 9 or 12 bp upstream of a TATA box. A second TATA box was then placed either upstream or downstream in order to test for tracking in both directions. A test of the efficiency of the lac repressor block to MOT1 function is shown in Fig. 5B. Lanes 1 to 4 demonstrate that TBP binds to this TATA box and that MOT1 recognizes the TBP-DNA complex and disrupts it in the presence of ATP. In contrast, occupancy of the lac operator by lac repressor in DNAs with either the 12-bp spacing (lanes 5 to 10) or 9-bp spacing (lanes 11 to 18) resulted in virtually complete inhibition of MOT1 activity (compare DNase sensitivity in the TATA box in lanes 3 and 4 with lanes 7, 8, 14, and 15). Addition of IPTG and unlabeled lac operator resulted in the appearance, again, of the upstream protection characteristic of the MOT1-TBP-DNA ternary complex (lanes 9 and 16), and the footprint was disrupted in the presence of ATP (lanes 10 and 17). Comparison of lanes 6 to 8 and 13 to 15 reveals that two positions in the 9- and 12-bp spacers (just above arrow) are weakly protected by MOT1, suggesting that MOT1 can bind weakly to TBP-DNA even when access to DNA upstream of the TATA box is blocked by the binding of lac repressor. Nonetheless, tracking by MOT1 to this TBP-DNA complex could still be assessed by directly comparing the activity of MOT1 on templates with one TATA box versus two TATA boxes (see below).
The results in Fig. 5 demonstrate that lac repressor can be used to reversibly block ATP-dependent removal of TBP from DNA by MOT1. This made it possible to test for translocation of MOT1 from one TBP-DNA complex to another, as shown in Fig. 5A. As an additional feature, the DNA templates used for these experiments contained an EcoRI site placed between the two TATA boxes at a position which does not sterically interfere with MOT1 action (see below and Fig. 6 and 7). If the effect of template-committed MOT1 bound to one TBP-DNA complex on TBP bound to a second site could be blocked by EcoRI-Gln 111 bound to the EcoRI site, then this would suggest that intramolecular transfer of MOT1 involves tracking or DNA looping.
FIG. 6.
Testing for ATP-dependent DNA tracking by MOT1 in the 5′-to-3′ direction by DNase footprinting. (A) The footprinted regions centered over the indicated sites are shown as open rectangles, and the distances between the sites are shown in base pairs. The indicated proteins were added to the probe as schematized in Fig. 5A, with the exception that the unlabeled competitor TBP-DNA was added in the order indicated at the top of the figure. For the reactions in lanes 1, 2, 8, 9, and 12, the competitor reaction mixture was added after preincubation with the indicated proteins and immediately prior to the addition of DNase. The unmarked arrow indicates a position upstream of the 5′ TATA box which is protected by MOT1 in the absence of ATP (lanes 6 and 11). (B) Comparison of the effects of MOT1 on TBP-DNA complexes subject to the reversible lac repressor steric block on templates with (lanes 1 to 14) or without (lanes 15 to 18) the second 5′ TATA box. Experiments were performed as described for panel A; the unlabeled TBP-DNA competitor was added to the reaction mixtures in lanes 1, 2, 5, 8, 11, 14, 15, and 16 after preincubation with the indicated proteins and immediately prior to addition of DNase.
FIG. 7.
Testing for ATP-dependent DNA tracking by MOT1 in the 3′-to-5′ direction by DNase footprinting. (A) The binding sites and footprinted regions on DNA are indicated as in Fig. 6. The brackets indicate the region on DNA protected by MOT1 in the MOT1-TBP-DNA ternary complex formed in the absence of ATP. The experiment was performed as described for Fig. 5A and 6. Unlabeled TBP-DNA competitor was added to the reaction mixtures in lanes 1, 2, 5, 8, 9, and 12 after preincubation with the indicated proteins and immediately prior to the addition of DNase. The results shown in lanes 13 to 24 are from the same experiment as for lanes 1 to 12, but the film was exposed longer in order to better visualize the partial DNase protection upstream of the 5′ TATA box (brackets) afforded by MOT1, which has translocated along DNA from its loading site at the downstream TATA box (see text). (B) Control DNase footprinting experiment for panel A which demonstrates that EcoRI-Gln 111 binding to the EcoRI site (lanes 5 to 7) does not interfere with MOT1 action at either of the TBP-DNA complexes formed at the two TATA boxes in the absence of lac repressor. The template used in this experiment was the same as that used for panel A.
Figure 6 shows the results of experiments to test if MOT1 can translocate along DNA in the 5′-to-3′ direction (with respect to the DNA strand encoding the TATA sequence TATAAAAG). The radiolabeled template contains an upstream TATA box which is accessible to MOT1 and then 39 bp of downstream flanking DNA followed by an EcoRI site, 15 additional base pairs of 3′ flanking DNA, and then a lac operator separated from the 3′ TATA box by either 12 bp (Fig. 6A) or 9 bp (Fig. 6B, lanes 1 to 14). For comparison, the effects of MOT1 on TBP bound to a template with an appropriately positioned lac operator and only one TATA box were also determined (Fig. 6B, lanes 15 to 18). Addition of the competitor TBP-DNA reaction mixtures prior to MOT1 completely blocked MOT1 action on the radiolabeled template (Fig. 6A and B, lanes 2 versus lanes 3) whereas addition of the competitor reaction mixture following incubation of MOT1 with TBP-DNA complexes formed on the radiolabeled probe led to disruption of 30 to 50% of the TBP-DNA complexes (Fig. 6A and B, lanes 3 versus lanes 4), as was seen in the tandem TATA experiments described above. lac repressor protected the lac operator on both templates, as expected (Fig. 6A and B, lanes 5). On the template containing the 12-bp spacing between the downstream TATA and the lac operator (Fig. 6A), addition of MOT1 to TBP-lac repressor-DNA complexes followed by addition of IPTG and the competitor reaction mixture led to the loss of the lac repressor footprint and partial protection of DNA upstream of the 5′ TATA box (Fig. 6A, lane 6). Since template commitment by MOT1 was not complete, the degree of protection by MOT1 at the available TBP-DNA complex was difficult to detect in some experiments. No protection of the DNA upstream of the 3′ TATA box was observed, consistent with the expectation that lac repressor prevents association of MOT1 with this TBP-DNA complex and that MOT1 cannot translocate from the upstream TATA box to the downstream TATA box on this template. Performance of the template commitment experiment in the presence of ATP (Fig. 6A, lane 7) demonstrates that MOT1 catalyzes disruption of TBP-DNA complexes at the upstream TATA box equivalently to that seen in the absence of lac repressor (Fig. 6A; compare lanes 4 and 7). In contrast, there is very little effect of MOT1 on TBP-DNA disruption at the downstream TATA box (Fig. 6A, lanes 4 and 7). Phosphorimager analysis was used to quantitate the changes in band intensities in the footprinted regions. These results demonstrate that the small effects (less than twofold) of MOT1 on TBP-DNA complexes formed at the downstream TATA box are the same whether or not the upstream TATA box is present on the same DNA template. The low level of MOT1-catalyzed disruption of TBP-DNA at the downstream TATA box therefore reflects the slight “leakiness” of the lac repressor block and is not due to tracking by MOT1 in the 5′-to-3′ direction. In agreement with this conclusion, the results obtained with this probe are the same when EcoRI-Gln 111 is bound to its site between the two TATA boxes (Fig. 6A, lanes 9 to 12), demonstrating that tracking by MOT1 is not detectable.
By using the probe with the 9-bp spacing between the 3′ TATA box and the lac operator, similar results were obtained (Fig. 6B). In this configuration, the lac repressor-blocked TATA box is slightly more leaky, and release of lac repressor by IPTG gives rise to a low level of MOT1-catalyzed TBP-DNA disruption on the template containing one TATA box (Fig. 6B, lane 17 versus lane 18). This degree of disruption is equivalent to that seen on the template containing two TATA boxes (Fig. 6B, lanes 6 and 7; also phosphorimager analysis not shown). Also in support of the observation that MOT1 loaded at the upstream TBP-DNA complex does not effect disruption at the downstream TBP-DNA complex is the observation that EcoRI-Gln 111 binding between the two TATA boxes has no detectable effect on the results (Fig. 6B, lanes 9 and 10). These results indicate that tracking by MOT1 protein in the 5′-to-3′ direction is not detectable.
An analogous experiment to determine if tracking by MOT1 in the 3′-to-5′ direction (with respect to the DNA strand containing the TATA sequence TATAAAAG) could be detected is represented by Fig. 7. This experiment utilized a radiolabeled DNA fragment containing a downstream TATA box for formation of an accessible TBP-DNA complex. A second TATA box is located 81 bp upstream. The upstream TATA box is appropriately positioned to a lac operator on its upstream side for reversible blocking by lac repressor. An EcoRI binding site is located between the two TATA boxes, as was described for the 5′-to-3′ tracking probes used for the experiments depicted in Fig. 6. The autoradiograms shown in Fig. 7A represent two different exposures of the same gel. As was observed for the other tracking probes, incubation with TBP resulted in protection of both TATA boxes (Fig. 7A, lane 2). Addition of the unlabeled competitor reaction mixture and ATP prior to the addition of MOT1 resulted in no detectable change in the TBP footprints (Fig. 7A, lane 3), whereas addition of MOT1 to preformed TBP-DNA complexes prior to the addition of the competitor reaction led to partial disruption of both TBP-DNA complexes (Fig. 7A, lane 4), as required for the template commitment experiment. lac repressor protected the lac operator (Fig. 7A, lane 5 or 17), and addition of MOT1 in the absence of ATP led to an upstream extension of the TBP footprint at the downstream TATA box. Surprisingly, dissociation of lac repressor by IPTG in the absence of ATP led to the appearance of the characteristic MOT1 footprint at the upstream TATA box as well (Fig. 7A, lane 6 or 18). MOT1 bound to the upstream TBP-DNA complex was not loaded onto this site directly, since a single TATA box separated from a lac operator by 12 bp does not display this behavior (not shown), nor was this behavior seen with the analogous tracking probe shown in Fig. 6. The appearance of MOT1 at the upstream TBP-DNA complex appears to depend on an unobstructed path between the two TBP-DNA complexes, since binding of EcoRI-Gln 111 to the site between them prevents the appearance of the MOT1 footprint at the upstream but not the downstream TBP-DNA complex (Fig. 7A, lane 9 versus 10 or lane 21 versus 22). Since EcoRI-Gln 111 binding per se does not affect MOT1 activity at the upstream TBP-DNA complex (Fig. 7B, lanes 3 and 4 versus lanes 6 and 7), we suggest that MOT1 loaded at the downstream TBP-DNA complex might slide to the upstream TBP-DNA complex in the absence of ATP. Alternatively, MOT1 might be transferred to the upstream TBP-DNA complex by another mechanism, such as DNA looping. Additional experiments will be required to determine the mechanism of this ATP-independent behavior of MOT1. In the presence of ATP, disruption of TBP-DNA complexes formed at both TATA boxes was observed (Fig. 7A, lanes 7 and 19), as expected, since MOT1 is associated with TBP molecules bound to both TATAs. These results support a model in which MOT1 can be transferred from one TBP-DNA complex to another on the same DNA molecule, but this reaction is not ATP dependent and therefore does not represent the mechanism by which MOT1 catalyzes TBP-DNA dissociation.
DISCUSSION
Rapid TBP-DNA dissociation by MOT1.
To test for ATP-dependent DNA tracking by MOT1, we first monitored the fate of MOT1 bound to a TBP-DNA complex assembled between two stable heterologous DNA binding proteins. By both gel mobility shift and DNase footprinting analyses, it was found that the ATP-dependent disruption of TBP-DNA complexes is fast and leads to release of both TBP and MOT1 from DNA in a reaction that does not require a DNA end. The results are identical regardless of the orientation or spacing of lac repressor or EcoRI-Gln 111 with respect to MOT1-TBP-DNA ternary complexes, indicating that these observations do not depend on the particular arrangement of proteins along the DNA. Since heterologous roadblock proteins are not disrupted by MOT1 and they do not detectably provide a barrier which traps MOT1 on DNA, we conclude that MOT1 activity leads to dissociation from internal sites on the DNA template in just a few seconds. These results rule out extreme versions of the ATP-dependent DNA-tracking model in which MOT1 binds very tightly to the DNA template and is stably associated with it for more than approximately 30 s. Some highly processive enzymes can be arrested by a heterologous roadblock protein. For instance, lac repressor can arrest elongating RNA polymerase II (9) and RNA polymerase III (38). There are examples of DNA-tracking proteins, however, which nonetheless can dissociate from DNA very rapidly. For instance, phage T4 gp45 protein is a well-established DNA-tracking protein that has been observed to quantitatively dissociate from DNA in just a few seconds; its movement along DNA can be readily detected, however, under conditions in which its continuous loading offsets rapid unloading from the template (12, 40). The β subunit of E. coli DNA polymerase III holoenzyme is an example of a DNA-tracking protein with intermediate stability on DNA. β can associate with DNA for several minutes prior to dissociation (12). Since known DNA-tracking proteins possess a wide range of half times for dissociation from DNA (13, 27), the rapid dissociation of MOT1 from the template with roadblocks at either end does not directly address the question of whether or not MOT1 tracks.
MOT1-catalyzed disruption of tandem TBP-DNA complexes.
Juxtaposition of two TATA boxes resulted in the differential targeting of MOT1 to only the upstream TBP-DNA complex. In the presence of ATP, this template-committed MOT1 then disrupted both TBP-DNA complexes equivalently. This result is consistent with a model in which MOT1 causes TBP-DNA dissociation by an ATP-dependent DNA-tracking mechanism. This result is also consistent, however, with a number of other possibilities. First, since MOT1 is much larger than TBP, the ATP-dependent reaction at the upstream TBP-DNA complex might result in a conformational change in MOT1 which leads to an incidental collision between MOT1 and the downstream TBP which triggers the release of both TBP molecules. In some experiments, we observed a DNase I-hypersensitive site between the tandem TBP-DNA complexes in the presence of MOT1 (Fig. 4B, lane 3), which might reflect an alteration of the DNA structure between the two TATA boxes, perhaps due to MOT1 bound to the upstream TBP-DNA complex incidentally bumping into the TBP molecule bound to the downstream TATA box. Alternatively, MOT1 might trigger TBP-DNA dissociation by propagating a structural change in DNA through the TATA box and into the downstream DNA. In this case, the disruption of both TBP-DNA complexes would result from the singular action of MOT1 bound to the upstream TBP-DNA complex. Another possibility is that the ejection of TBP from the upstream TATA box might precede dissociation of MOT1, and template-associated MOT1 might then preferentially disrupt the downstream TBP-DNA complex in a subsequent step simply because it is located proximally to this complex. Finally, MOT1 might use ATP to disrupt TBP-DNA complexes by a power stroke which involves a short translocation along DNA through the TATA box. In this case, template-committed MOT1 would behave as a DNA-tracking enzyme on the tandem TATA template but no tracking would be observed when the TBP-DNA complexes were placed further apart. It should be possible to discriminate among these possibilities by spectroscopic methods which can detect the very rapid changes in MOT1, TBP, and DNA which accompany this reaction.
ATP-independent transfer of MOT1 from one TBP-DNA complex to another.
Targeting of MOT1 to one of two TBP-DNA complexes separated by 80 to 100 bp demonstrated that MOT1 does not use ATP to track along DNA in the 5′-to-3′ direction. Surprisingly, MOT1 bound to a downstream TBP-DNA complex can be transferred to an upstream TBP-DNA complex in the absence of ATP. ATP-independent tracking by MOT1 is suggested by the observation that the appearance of MOT1 at the upstream TBP-DNA complex depends on loading at the downstream TBP-DNA complex. Translocation of MOT1 from one TBP-DNA complex to another also depends on an unobstructed DNA path between the two complexes, since EcoRI-Gln 111 can block the translocation without interfering directly with MOT1 action at either TBP-DNA complex. An alternative explanation is that ATP-independent transfer of MOT1 occurs by DNA looping. A rigorous test of MOT1’s ability to track along DNA in the absence of ATP will require additional experimental approaches including protein-DNA cross-linking and the use of longer DNA templates.
Implications for other SNF2/SWI2 protein family members?
ATPase activity of SNF2/SWI2 protein family members is generally very low, but the activity of several of these factors can be activated by DNA (see the introduction). In contrast, the MOT1 ATPase is activated by TBP but not by DNA (3). Likewise, a human homolog of MOT1, TAF-172, has an ATPase which is activated by TBP plus DNA but only weakly by DNA alone (6). The high degree of sequence conservation among the ATPases of all SNF2/SWI2 protein family members suggests that they undergo similar ATP-dependent conformational changes but that they simply respond to different allosteric effectors. How ATP hydrolysis by other SNF2/SWI2-related proteins drives rearrangements of other protein-DNA complexes is unknown; as more detailed mechanistic analyses proceed, it will be interesting to compare the ways in which this evolutionarily conserved ATPase is exploited to catalyze this diverse array of reactions.
ACKNOWLEDGMENTS
We are grateful to Kevin Struhl for the TBPm3 gene, Jim Geiger for yeast TBP core domain, Kathleen Matthews for lac repressor, and Paul Modrich for EcoRI-Gln 111. We appreciate the thoughtful comments, criticisms, and encouragement of David Levens, E. Peter Geiduschek, George Kassavetis, Blaine Bartholomew, Bob Kadner, and Jerry Workman. We are also grateful to Rong Li, Tamara Muldrow, Tom Thompson, Mitch Smith, and Mike Christman for suggestions and for critically reading the manuscript.
This work was supported by NIH grant GM55763 to D.T.A.
REFERENCES
- 1.Auble, D. T. Unpublished observations.
- 1a.Auble D T, Hahn S. An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev. 1993;7:844–856. doi: 10.1101/gad.7.5.844. [DOI] [PubMed] [Google Scholar]
- 2.Auble D T, Hansen K E, Mueller C G F, Lane W S, Thorner J, Hahn S. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8:1920–1934. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- 3.Auble D T, Wang D, Post K W, Hahn S. Molecular analysis of the SNF2/SWI2 protein family member MOT1, an ATP-driven enzyme that dissociates TATA-binding protein from DNA. Mol Cell Biol. 1997;17:4842–4851. doi: 10.1128/mcb.17.8.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown S A, Kingston R E. Disruption of downstream chromatin by a transcriptional activator. Genes Dev. 1997;11:3116–3121. doi: 10.1101/gad.11.23.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns B R, Lorch Y, Li Y, Zhang M, Lacomis L, Erdjument-Bromage H, Tempst P, Du J, Laurent B, Kornberg R D. RSC, an essential, abundant chromatin-remodeling complex. Cell. 1996;87:1249–1260. doi: 10.1016/s0092-8674(00)81820-6. [DOI] [PubMed] [Google Scholar]
- 6.Chicca J J I, Auble D T, Pugh B F. Cloning and biochemical characterization of TAF-172, a human homolog of yeast MOT1. Mol Cell Biol. 1998;18:1701–1710. doi: 10.1128/mcb.18.3.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote J, Quinn J, Workman J L, Peterson C L. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science. 1994;265:53–60. doi: 10.1126/science.8016655. [DOI] [PubMed] [Google Scholar]
- 8.Cox J M, Hayward M M, Sanchez J F, Gegnas L D, van der Zee S, Dennis J H, Sigler P B, Schepartz A. Bidirectional binding of the TATA box binding protein to the TATA box. Proc Natl Acad Sci USA. 1997;94:13475–13480. doi: 10.1073/pnas.94.25.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuschle U, Hipskind R A, Bujard H. RNA polymerase II transcription blocked by Escherichia coli lac repressor. Science. 1990;248:480–483. doi: 10.1126/science.2158670. [DOI] [PubMed] [Google Scholar]
- 10.Eisen J A, Sweder K S, Hanawalt P C. Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res. 1995;23:2715–2723. doi: 10.1093/nar/23.14.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elfring L K, Deuring R, McCallum C M, Peterson C L, Tamkun J W. Identification and characterization of Drosophila relatives of yeast transcriptional activator SNF2/SWI2. Mol Cell Biol. 1994;14:2225–2234. doi: 10.1128/mcb.14.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu T-J, Sanders G M, O’Donnell M, Geiduschek E P. Dynamics of DNA-tracking by two sliding-clamp proteins. EMBO J. 1996;15:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 13.Gelles J, Landick R. RNA polymerase as a molecular motor. Cell. 1998;93:13–16. doi: 10.1016/s0092-8674(00)81140-x. [DOI] [PubMed] [Google Scholar]
- 14.Gorbalenya E G, Koonin E V, Donchenko A P, Blinov V M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzder S N, Sung P, Prakash L, Prakash S. Yeast Rad7-Rad16 complex, specific for the nucleotide excision repair of the nontranscribed DNA strand, is an ATP-dependent DNA damage sensor. J Biol Chem. 1997;272:21665–21668. doi: 10.1074/jbc.272.35.21665. [DOI] [PubMed] [Google Scholar]
- 16.Henikoff S. Transcriptional activator components and poxvirus DNA-dependent ATPases comprise a single family. Trends Biochem Sci. 1993;18:291–292. doi: 10.1016/0968-0004(93)90037-n. [DOI] [PubMed] [Google Scholar]
- 17.Hoopes B C, LeBlanc J F, Hawley D K. Kinetic analysis of yeast TFIID-TATA box complex formation suggests a multi-step pathway. J Biol Chem. 1992;267:11539–11547. [PubMed] [Google Scholar]
- 18.Imbalzano A N, Kwon H, Green M R, Kingston R E. Facilitated binding of TATA-binding protein to nucleosomal DNA. Nature. 1994;370:481–485. doi: 10.1038/370481a0. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Bulger M, Pazin M J, Kobayashi R, Kadonaga J T. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell. 1997;90:145–155. doi: 10.1016/s0092-8674(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 20.Kadonaga J T. Eukaryotic transcription: an interlaced network of transcription factors and chromatin-modifying machines. Cell. 1998;92:307–313. doi: 10.1016/s0092-8674(00)80924-1. [DOI] [PubMed] [Google Scholar]
- 21.Khavari P A, Peterson C L, Tamkun J W, Mendel D B, Crabtree G R. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature. 1993;366:170–174. doi: 10.1038/366170a0. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Geiger J H, Hahn S, Sigler P B. Crystal structure of a yeast TBP/TATA-box complex. Nature. 1993;365:512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- 23.King K, Benkovic S J, Modrich P. Glu-111 is required for activation of the DNA cleavage center of EcoRI endonuclease. J Biol Chem. 1989;264:11807–11815. [PubMed] [Google Scholar]
- 24.Kwon H, Imbalzano A N, Khavari P A, Kingston R E, Green M R. Nucleosome disruption and enhancement of activator binding by a human SWI/SNF complex. Nature. 1994;370:477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- 25.Laurent B C, Treich I, Carlson M. The yeast SNF2/SWI2 protein has DNA-stimulated ATPase activity required for transcriptional activation. Genes Dev. 1993;7:583–591. doi: 10.1101/gad.7.4.583. [DOI] [PubMed] [Google Scholar]
- 26.Lewis M, Chang G, Horton N C, Kercher M A, Pace H C, Schumacher M A, Brennan R G, Lu P. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- 27.Lohman T M, Thorn K, Vale R D. Staying on track: common features of DNA helicases and microtubule motors. Cell. 1998;93:9–12. doi: 10.1016/s0092-8674(00)81139-3. [DOI] [PubMed] [Google Scholar]
- 28.Mizuguchi G, Tsukiyama T, Wisniewski J, Wu C. Role of nucleosome remodeling factor NURF in transcriptional activation of chromatin. Mol Cell. 1997;1:141–150. doi: 10.1016/s1097-2765(00)80015-5. [DOI] [PubMed] [Google Scholar]
- 29.Owen-Hughes T, Utley R T, Peterson C L, Workman J L. Persistent site-specific remodeling of a nucleosome array by transient action of the SWI/SNF complex. Science. 1996;273:513–516. doi: 10.1126/science.273.5274.513. [DOI] [PubMed] [Google Scholar]
- 30.Pazin M J, Kadonaga J T. SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions? Cell. 1997;88:737–740. doi: 10.1016/s0092-8674(00)81918-2. [DOI] [PubMed] [Google Scholar]
- 31.Petukhova G, Stratton S, Sung P. Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature. 1998;393:91–94. doi: 10.1038/30037. [DOI] [PubMed] [Google Scholar]
- 32.Reddy P, Hahn S. Dominant negative mutations in yeast TFIID define a bipartite DNA-binding region. Cell. 1991;65:349–357. doi: 10.1016/0092-8674(91)90168-x. [DOI] [PubMed] [Google Scholar]
- 33.Riggs A D, Bourgeois S, Cohn M. lac repressor-operator interaction. III. Kinetic studies. J Mol Biol. 1970;53:401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- 34.Riggs A D, Suzuki H, Bourgeois S. lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970;48:67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- 35.Sadler J R, Sasmor H, Betz J L. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc Natl Acad Sci USA. 1983;80:6785–6789. doi: 10.1073/pnas.80.22.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons A, Tils D, von Wilcken-Bergmann B, Muller-Hill B. Possible ideal lac operator: Escherichia coli lac operator-like sequences from eukaryotic genomes lack the central G-C pair. Proc Natl Acad Sci USA. 1984;81:1624–1628. doi: 10.1073/pnas.81.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strubin M, Struhl K. Yeast and human TFIID with altered DNA-binding specificity for TATA elements. Cell. 1992;68:721–730. doi: 10.1016/0092-8674(92)90147-5. [DOI] [PubMed] [Google Scholar]
- 38.Syroid D E, Capone J P. RNA chain elongation and termination by mammalian RNA polymerase III. Analysis of tRNA gene transcription by imposing a reversible factor-mediated block to elongation using a sequence-specific DNA binding protein. J Mol Biol. 1994;244:482–493. doi: 10.1006/jmbi.1994.1747. [DOI] [PubMed] [Google Scholar]
- 39.Tantin D, Kansal A, Carey M. Recruitment of the putative transcription-repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol Cell Biol. 1997;17:6803–6814. doi: 10.1128/mcb.17.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinker R L, Kassavetis G A, Geiduschek E P. Detecting the ability of viral, bacterial and eukaryotic replication proteins to track along DNA. EMBO J. 1994;13:5330–5337. doi: 10.1002/j.1460-2075.1994.tb06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinker-Kulberg R L, Fu T-J, Geiduschek E P, Kassavetis G A. A direct interaction between a DNA-tracking protein and a promoter recognition protein: implications for searching DNA sequence. EMBO J. 1996;15:5032–5039. [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukiyama T, Daniel C, Tamkun J, Wu C. ISWI, a member of the SWI2/SNF2 ATPase family, encodes the 140 kDa subunit of the nucleosome remodeling factor. Cell. 1995;83:1021–1026. doi: 10.1016/0092-8674(95)90217-1. [DOI] [PubMed] [Google Scholar]
- 43.Tsukiyama T, Wu C. Chromatin remodeling and transcription. Curr Opin Genet Dev. 1997;7:182–191. doi: 10.1016/s0959-437x(97)80127-x. [DOI] [PubMed] [Google Scholar]
- 44.Utley R T, Cote J, Owen-Hughes T, Workman J. SWI/SNF stimulates the formation of disparate activator-nucleosome complexes but is partially redundant with cooperative binding. J Biol Chem. 1997;272:12642–12649. doi: 10.1074/jbc.272.19.12642. [DOI] [PubMed] [Google Scholar]
- 45.Varga-Weisz P D, Wilm M, Bonte E, Dumas K, Mann M, Becker P B. Chromatin-remodeling factor CHRAC contains the ATPases ISWI and topoisomerase II. Nature. 1997;388:598–602. doi: 10.1038/41587. [DOI] [PubMed] [Google Scholar]
- 46.Wang, D., and D. T. Auble. Unpublished observations.
- 47.Winter R B, Berg O G, von Hippel P H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 3. The Escherichia coli lac repressor-operator interaction: kinetic measurements and conclusions. Biochemistry. 1981;20:6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]