Supplemental Digital Content is available in the text
Keywords: neutrophil-to-lymphocyte ratio, NLR, nonmetastatic nasopharyngeal carcinoma, pretreatment, prognostic factor, tumor-promoting inflammation
Abstract
Neutrophil-to-lymphocyte ratio (NLR) was reported as an independent prognostic factor in many studies, but its cutoff point was not yet concluded. We set forth to prove and validate cutoff point of NLR as a poor prognostic factor for overall survival (OS) in nonmetastatic nasopharyngeal carcinoma (NPC) patients.
Retrospective cohort of nonmetastatic NPC adult patients treated with intensity-modulated radiotherapy with curative aim at Siriraj hospital during 2007 to 2014 was enrolled. NLR was defined as absolute neutrophil count divided by absolute lymphocyte count. OS was the primary outcome. We explored our cutoff value by maximum concordance index (C-index) method, and we validated our cutoff and previously reported cutoff values by categorizing patients as NLR ≤ 3 or >3. Internal validation was done by bootstrapping method.
Four hundred sixty-three patients were included. The median follow-up time was 70.8 months. By the end of June 2019, 211 patients had died. In univariable analysis of OS by Cox model, an NLR value of 3 showed the highest C-index (0.548) with an HR of 1.43 (95% CI: 1.08–1.89). After adjustment for body mass index, overall staging, age, gender, and histology in multivariable analysis, an NLR >3 was still an independent prognostic factor of poor OS (HR = 1.34, 95% CI = 1.01–1.79). After internal validation, the resampling method shows no overfitting condition and corrected C-index was 0.547 for univariable analysis.
A cutoff point of NLR of 3 from routine blood test was found to be an independent poor prognostic factor among patients with nonmetastatic NPC. This prognostic factor could be included in clinical prediction model of NPC and this further prediction model would select high risk patients for intensive treatment.
1. Introduction
Nasopharyngeal carcinoma (NPC) is a common head and neck cancer in eastern China and Southeast Asia. The pathogenesis of NPC relates to previous Epstein–Barr virus (EBV) infection, which is a latent infection; however, not all persons infected with EBV develop invasive cancer cells.[1] Even though EBV DNA is a potential prognostic factor of NPC, the tumor microenvironment is one of the pathways that supports tumorigenesis and tumor progression. Hanahan et al[2] published ‘Hallmarks of cancer: the next generation’ in 2010, which includes tumor-promoting inflammation and avoiding immune destruction. Leukocytes play a role in both inflammatory process and the immune system, and the neutrophil-to-lymphocyte ratio (NLR) is recognized as a hematologic inflammatory marker in solid cancer. This ratio is repeatedly reported in many cancer sites, including NPC, as a poor prognostic factor relative to patient survival; however, some reported aspects of the use of the NLR remain controversial, such as publication bias and the NLR cutoff value.
In meta-analysis[3] of NLR from 100 studies with 40,559 patients and 21 solid tumors, an NLR higher than the cutoff showed a statistically significant hazard ratio (HR) of 1.81 (95% CI: 1.67–1.97) for overall survival (OS). However, funnel plot analysis revealed publication bias in that meta-analysis study. The 3 most common primary tumors in that study were colorectal carcinoma, gastroesophageal carcinoma, and hepatocellular carcinoma, consecutively, and there were only 2 studies in NPC. NLR was found to be a poor prognostic factor for OS in most NPC studies.[4–12] Takenaka et al[13] conducted a meta-analysis of non-nasopharyngeal head and neck cancer and reported association between a high NLR and poor OS. Similarly, Yang et al[14] found poor survival among high NLR patients in a meta-analysis of NPC. Alternatively, Chua et al[15] found and reported conflicting results from a randomized controlled trial database of locally-advanced NPC.
Although many studies included in meta-analysis found NLR to be an independent prognostic factor in many solid tumors, its cutoff point was not yet concluded (median cutoff: 4.0, range: 1.9–7.2).[3] The current lack of an established cutoff in this clinical setting can adversely affect both treatment decisions and patient outcomes. Accordingly, the aim of this study was to prove and validate cutoff point of NLR as a poor prognostic factor for OS in nonmetastatic NPC patients.
2. Material and methods
Retrospective cohort followed of nonmetastatic NPC adult patients treated with intensity-modulated radiotherapy with curative aim the Division of Radiation Oncology, Department of Radiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand during January 2007 to December 2014. Patients with previous history of other malignancy, previous radiotherapy, or having no complete blood count before any treatment in the medical record were excluded. All patients were newly reviewed for both T stage and N stage according to the 8th edition of the Union for International Cancer Control/ American Joint Committee on Cancer staging system by a qualified diagnostic radiologist.
For the treatment protocol at our center, all patients were treated with simultaneous integrated boost technique of intensity-modulated radiotherapy with a radiation dose of 70 Gray in 33 fractions. Platinum-based chemotherapy was prescribed as either concurrent chemotherapy or as neoadjuvant/adjuvant chemotherapy. For concurrent chemotherapy, cisplatin every 3 weeks was the most common regimen; however, patients with renal insufficiency were instead prescribed carboplatin weekly. 5-FU was routinely combined with platinum-based chemotherapy for neoadjuvant/adjuvant treatment. The schedule of normal post-treatment evaluation was either computed tomography scan or magnetic resonance imaging scan and ear, nose, throat examination with biopsy at 3 months after treatment completion. Patients with no evidence of disease would be followed-up clinically every 3 to 6 months with or without imaging every 1 to 2 years.
Complete blood count, standard routine laboratory in clinical pathological department, was ordered 1 to 2 weeks before each cycle of concurrent and adjuvant chemotherapy. In this study, we used only the NLR before the start of treatment. NLR was defined as absolute neutrophil count divided by absolute lymphocyte count. We explored our own cutoff value, and we validated both our cutoff value and previously reported cutoff values by categorizing patients as NLR ≤3.0 (low NLR) or >3.0 (high NLR).
Sample size was calculated by two-sample comparison of survivor functions by HR of patients with high NLR vs low NLR as 1.4 and ratio of number of high NLR/ low NLR as 1:4. With type 1 error as 0.05 and power as 0.9, estimated sample size was 437.
The protocol for this study was approved by the Siriraj Institutional Review Board (COA no. 666/2560 (EC3)), and the requirement to obtain written informed approval was waived due to the retrospective nature of this study.
2.1. Statistical analysis
The primary outcome of this study was OS, which was defined as the time from initial treatment to death or censoring. First, we hypothesized the NLR to be a significant prognostic factor by exploring the NLR cutoff point. The NLR cutoff value was first determined as a median value. Second, univariable Cox proportional hazards (PH) regression model was used to vary the NLR cutoff point in order to determine the maximum concordance index (C-index). Third, we performed sensitivity analysis to identify a cutoff value as high sensitivity to 80%, and high specificity to 80%. Fourth and last, in order to validate cutoff points from other studies, we gathered cutoff values from the published literature.[4,6–8,10–12,15] To compare the characteristics all cutoff points, we reported C-index, sensitivity, specificity, percentage of dead patients in the low NLR group, percentage of dead patients in the high NLR group, and HR for death with its 95% confident interval and P value.
After exploration, we selected an NLR cutoff point with an appropriately high C-index with reasonable sensitivity, and specificity to include in and perform further statistical modeling. Univariable analysis (model 1) was performed using Cox PH regression model after the PH assumption test was determined not to be violated. Internal validation of univariable analysis of this NLR cutoff point was performed by bootstrap resampling method using R software version 3.6.2. Specifically, the model was refitted 2000 times with function ‘validate’ in the package ‘rms’ (regression modelling strategies by Professor Harrell) version 5.1-4 to correct overfitted coefficients, and to determine the robustness of the C-index value. Next, we compared patient characteristics between the low NLR and high NLR group to identify any potential confounders that affect survival in this dataset. Finally, to determine if the NLR remained a significant prognostic factor, we performed adjusted analysis of NLR (model 2). We used the same cutoff point as the 1 used in model 1, and we adjusted for selected known confounders from both the literature and from our dataset, including age, gender, histology, body mass index (BMI), and overall staging (8th AJCC staging system). Internal validation of adjusted analysis was also performed using the same technique.
Finally, we integrated the NLR into the standard staging system (model 3) by creating the low NLR and high NLR substages to see if improvement could be observed with the C-index compared to standard staging alone. We also showed the value of the NLR by demonstrating the number of deaths, the 5-year and 8-year OS rates, and also Kaplan–Meier survival curve in each stage with low and high NLR.
3. Results
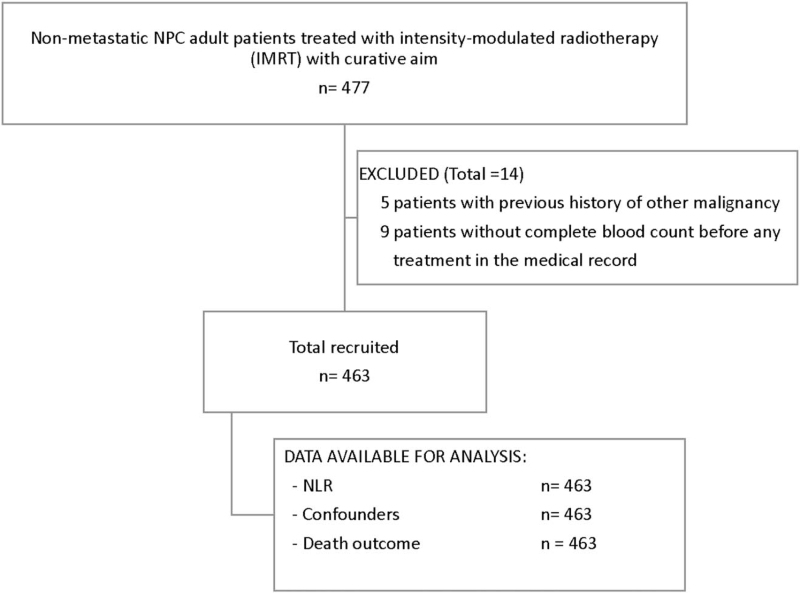
Four hundred seventy-seven NPC patients in this cohort were assessed for eligibility (Fig. 1). The main reasons for exclusion (n = 14, 3%) were the patients had history of previous malignancy (n = 5, 1%) and no available complete blood count (n = 9, 2%). A total of 463 patients were finally included in this analysis. The median follow-up time was 70.8 months. The average age of patients was 50 years, and 70% were male. Regarding histology classification, 64% of patients had undifferentiated type of nonkeratinizing carcinoma, and only 1% of patients had keratinizing squamous cell carcinoma. A total of 227 (49%) patients had stage IVA NPC, and 145 (31%), 84 (18%), and 7 (2%) patients were stage III, II, and I, respectively. A total of 237 (51%) patients underwent concurrent chemo-radiotherapy followed by adjuvant chemotherapy. The other treatment sequences were neoadjuvant chemotherapy followed by concurrent chemo-radiotherapy, and radiotherapy alone in 20% and 13% of patients, respectively. Complete course of radiotherapy was performed in 96% of patients. The median radiotherapy dose was 6996 cGy in patients who received a complete course of radiotherapy. The median dose among patients who did not complete their course of radiotherapy was 4664 cGy (interquartile range: 3604–6360). By the end of the follow-up period in June 2019, 211 patients had died.
Figure 1.
Flow diagram of number of patients. NLR = neutrophil-to-lymphocyte ratio, NPC = nasopharyngeal carcinoma.
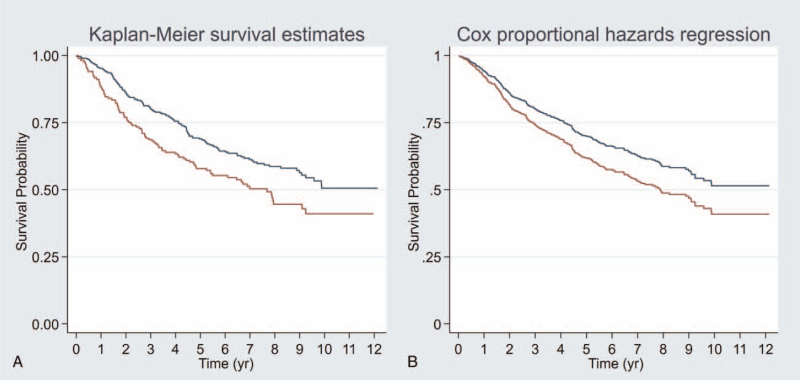
The average NLR among the entire study cohort was 3.18 ± 2.4 (range: 0.47–20.90). The median NLR was 2.59 (interquartile range: 1.81–3.67). Using the maximum C-index to identify the NLR cutoff point in univariable analysis of OS by univariable Cox PH regression model, an NLR value of 3.0 showed the highest C-index (0.548). Sensitivity analysis of all cutoff points, including that from our exploratory analysis and those from previous reports, showed that an NLR cutoff ranging from 2.6 to 4.2 yielded a statistically significant difference in OS between the high and low NLR groups (Table 1). A cutoff of NLR of 3.0 was also found in subgroups of stage III and stage IVA, and the cutoff point was 2.9 in stage II with the same method of the maximum C-index (Supplement 1, Supplement Digital Content, http://links.lww.com/MD2/A368). An NLR value of 3.0 was selected as the cutoff point in our study due to it having the highest C-index (0.548) and reasonable sensitivity and specificity (41.7%, and 67.9%, respectively). To identify potential confounders, we compared other prognostic factors and found that patients who had an NLR greater than 3.0 had significantly more T3 and T4 stage than patients with a low NLR (30% and 34% vs 22% and 25%, respectively). As a result of this clearly different T staging, overall staging in the high NLR group was higher than in the low NLR group – particularly for stage IVA (54% vs 46%, respectively) (Table 2). BMI showed significant difference between the high and low NLR groups. Moreover, underweight patients were more likely to have an NLR greater than 3.0 (20% vs 5%, respectively; Table 2). In univariable analysis (model 1), an NLR greater than 3.0 showed a HR of 1.43 (95% confidence interval [95% CI]: 1.08–1.89) (Fig. 2A and Table 3). After adjustment for BMI, overall staging, age, gender, and histology (model 2) revealed an NLR > 3.0 to be an independent prognostic factor of poorer OS in patients with NPC (HR: 1.34, 95% CI: 1.01–1.79) (Fig. 2B and Table 3). After internal validation, the resampling method showed no overfitting condition, so the corrected C-index was 0.547 for univariable analysis (model 1), and 0.663 for adjusted analysis (model 2) (Table 3).
Table 1.
Sensitivity analysis of cutoff point of NLR.
| Sensitivity analysis cutoff point in our exploratory study | ||||||||
| Method | Cutoff | C-index | Sensitivity | Specificity | Low NLR, no. of dead (%) | High NLR, no. of dead (%) | HR; 95%CI, P value | |
| Our study | Continuous | – | 0.535 | – | – | – | – | 1.07; 1.01 to 1.13, .019 |
| Median | 2.6 | 0.539 | 54.0% | 53.6% | 97/232 (42%) | 114/231 (49%) | 1.31; 1.00 to 1.72, .048 | |
| Maximum C-index∗ | 3.0 | 0.548 | 41.7% | 67.9% | 123/294 (42%) | 88/169 (52%) | 1.43; 1.09 to 1.89, .010 | |
| Mean | 3.2 | 0.539 | 35.6% | 73.0% | 136/320 (43%) | 75/143 (52%) | 1.39; 1.05 to 1.84, .023 | |
| High sensitivity | 1.6 | 0.510 | 80% | 16.7% | 40/80 (50%) | 171/383 (45%) | 0.89; 0.63 to 1.26, .519 | |
| High specificity | 3.8 | 0.529 | 27% | 80% | 154/357 (43%) | 57/106 (53%) | 1.38; 1.02 to 1.87, .037 | |
| Sensitivity analysis of cutoff point from previous report | ||||||||
| Lin et al[11] | AuROC | 2.2 | 0.510 | 63.5% | 37.3% | 77/171 (45%) | 134/292 (46%) | 1.10; 0.83 to 1.45, .523 |
| Lu et al[12] | AuROC | 2.3 | 0.517 | 61.1% | 41.67% | 82/187 (44%) | 129/276 (47%) | 1.15; 0.87 to 1.52, .325 |
| Li et al[8] | AuROC | 2.5 | 0.532 | 55.5% | 50.4% | 94/221 (43%) | 117/242 (48%) | 1.26; 0.96 to 1.65, .094 |
| Sun et al[6] | AuROC | 2.6 | 0.539 | 54.0% | 53.6% | 97/232 (42%) | 114/231 (49%) | 1.31; 1.00 to 1.72, .048 |
| Jiang et al[4] | AuROC | 2.7 | 0.536 | 49.8% | 57.9% | 106/252 (42%) | 105/211 (50%) | 1.32; 1.01 to 1.73, .046 |
| Liao et al[10] | Mean | 3.6 | 0.530 | 29.9% | 77.8% | 148/344 (43%) | 63/119 (53%) | 1.34; 1.00 to 1.80, .050 |
| An et al[7] | AuROC | 3.7 | 0.528 | 28.4% | 56.2% | 152/352 (43%) | 590111 (53%) | 1.35; 1.00 to 1.83, .049 |
| Chua et al[15] | Median | 3.0 | 0.548 | 41.7% | 67.9% | 123/294 (42%) | 88/169 (52%) | 1.43; 1.09 to 1.89, .010 |
| P80th | 4.2 | 0.537 | 23.7% | 84.9% | 161/375 (43%) | 50/88 (57%) | 1.57; 1.14 to 2.15, .006 | |
CI = confidence interval, C-index = concordance index, HR = hazard ratio, NLR = neutrophil-to-lymphocyte ratio, AuROC = area under the receiver operating characteristic curve.
Table 2.
Patient characteristics comparing the patients who had neutrophil to lymphocyte ratio (NLR) more than 3 to those with NLR equal or less than 3.
| NLR > 3 | NLR ≤ 3 | ||
| Characteristics | N = 169 | N = 294 | P value |
| Age: Mean (SD) | 51 (13) | 51 (11) | .47 |
| Gender: Men | 113 (67%) | 213 (72%) | .21 |
| Women | 56 (33%) | 81 (28%) | |
| BMI: < 18.5 | 33 (20%) | 16 (5%) | <.001 |
| 18.5 to 22.9 | 92 (54%) | 173 (59%) | |
| 23 to 24.9 | 35 (21%) | 84 (29%) | |
| ≥ 25 | 9 (5%) | 21 (7%) | |
| WHO classification: Type I | 4 (2%) | 2 (1%) | .206 |
| Type II | 63 (37%) | 99 (34%) | |
| Type III | 102 (61%) | 193 (65%) | |
| T stage: T1 | 22 (13%) | 43 (15%) | .005 |
| T2 | 40 (24%) | 113 (38%) | |
| T3 | 50 (30%) | 64 (22%) | |
| T4 | 57 (34%) | 74 (25%) | |
| N stage: N0 | 12 (7%) | 28 (10%) | .72 |
| N1 | 52 (31%) | 96 (33%) | |
| N2 | 58 (34%) | 98 (33%) | |
| N3 | 47 (28%) | 72 (24%) | |
| AJCC staging: I | 2 (1%) | 5 (2%) | .043 |
| II | 20 (12%) | 64 (22%) | |
| III | 55 (33%) | 90 (30%) | |
| IVA | 92 (54%) | 135 (46%) | |
| NLR: Mean (SD) | 5.3 (2.8) | 2.0 (0.6) | <.001 |
| Median (IQR) | 4.2 (3.5–6.4) | 2.0 (1.6–2.5) | <.001 |
IQR = interquartile range, SD = standard deviation.
Figure 2.
Overall survival between low NLR (equal or less than 3.0) in red color and high NLR (more than 3.0) in blue color. Figure A was Kaplan–Meier survival curve (crude survival curve). Figure B was postestimation survival curve after Cox-proportional hazards model of adjusted by age, gender, histology, BMI, and AJCC stage (adjusted survival curve). NLR = neutrophil-to-lymphocyte ratio, BMI = body mass index.
Table 3.
Overall survival of neutrophil-lymphocyte ratio (NLR) with cutoff value 3.0 in models 1 to 3 and internal validation with bootstrapping technique.
| Model 1∗ | Model 2† | Model 3‡ | ||||
| HR; 95% CI, P value | C-index (95% CI) | HR; 95% CI, P value | C-index (95% CI) | HR; 95% CI, P value | C-index (95% CI) | |
| NLR | 1.43; 1.09 to 1.89, .010 | 0.548 (0.512–0.583) | 1.34; 1.01 to 1.79, .040 | 0.674 (0.646–0.717) | 1.34; 1.01 to 1.76, .040 | 0.645 (0.608–0.679) |
| AJCC stage | ||||||
| Stage II | 1.73; 0.23 to 12.87, .590 | 2.20; 0.30 to 16.29, .439 | ||||
| Stage III | 2.25; 0.31 to 16.38, .422 | 2.75; 0.38 to 19.94, .316 | ||||
| Stage IVa | 5.16; 0.72 to 37.20, .103 | 6.21; 0.87 to 44.44, .069 | ||||
| Age | 1.03; 1.02 to 1.04, .001 | |||||
| Gender | 1.31; 0.96 to 1.80, .091 | |||||
| BMI | 0.99; 0.96 to 1.03, .740 | |||||
| Histology | ||||||
| NK, diff | 0.66; 0.21 to 2.09, .479 | |||||
| NK, undiff | 0.49; 0.16 to 1.56, .228 | |||||
| Internal validation§ | 0.547 (0.531–0.566) | 0.663 (0.651–0.686) | 0.642 (0.627–0.661) | |||
Model 1 included NLR by cutoff value 3.0.
Model 2 included NLR by cutoff value 3.0, AJCC stage, age, gender, body mass index (BMI), and histology.
Model 3 included NLR by cutoff value 3.0, and AJCC stage.
After adjusted slope to coefficient.
CI = confidence interval, C-index = concordance index, HR = hazard ratio.
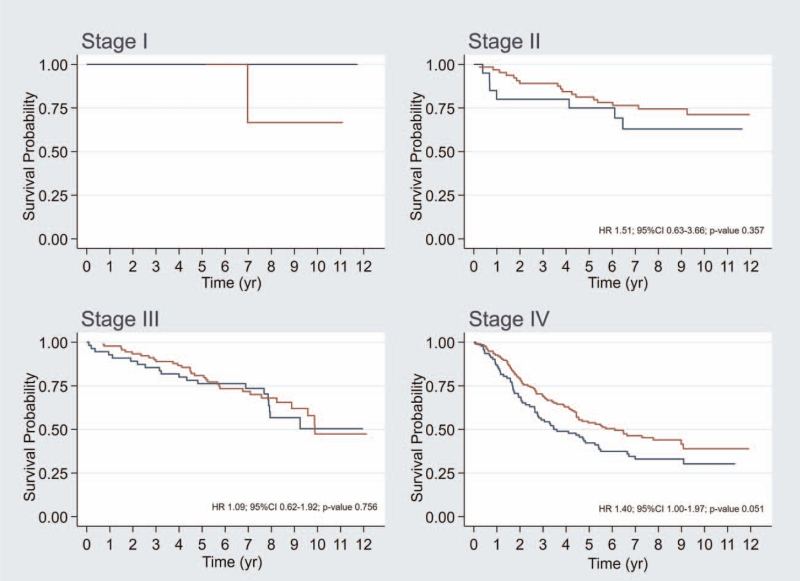
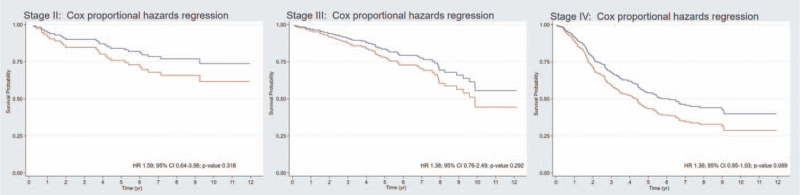
According to the 8th edition of the AJCC staging system, the 5-year OS rate was 100%, 80%, 79%, and 49% for stage I, II, III, and IVa, respectively. The 8-year OS rate was 75%, 71%, 64%, and 39% for stage I, II, III, and IVa, respectively. The C-index of this staging system was 0.629 (95% CI: 0.591–0.664). After adjustment for overall staging (model 3), an NLR >3.0 showed a HR of 1.34 (95% CI: 1.01–1.76) (Table 3). After integration of the NLR into the staging system, the C-index increased to 0.645 (95% CI: 0.608–0.679). There were few patients in stage I NPC, 5 patients in low NLR group and 2 patients in high NLR group (Table 4 and Fig. 3). There was only 1 patient had died in stage I of NPC patient. This patient was in high NLR group and his cause of death was noncancer death from heart disease. Thus, the adjusted analysis was not able to report in this stage. Five-year OS compared between low NLR (equal to or less than 3.0) and high NLR (more than 3.0) was not significantly different for stage II and III (81% vs 75% and 81% vs 76%, respectively) (Table 4 and Fig. 3). And, HR with 95% CI for adjusted analysis with age, gender, BMI, and histology was 1.59 (0.64 to 3.96) and 1.38 (0.76 to 2.49), respectively (Fig. 4). However, stage IVA NPC patients showed trend of the 5-year and 8-year OS rates difference between the low NLR and high NLR groups (54% vs 42% and 44% vs 33%, respectively; P = .050) (Table 4 and Fig. 3) as well as the HR with 95% CI for adjusted analysis with age, gender, BMI, and histology was 1.36 (95%CI: 0.95–1.93, P value .089) (Fig. 4).
Table 4.
Five-year and 8-yr OS between low NLR (equal or less than 3.0) and high NLR (more than 3.0) stratified by staging system.
| Overall survival (95% CI) | ||||
| 5-yr | 8-yr | P value | ||
| Stage I | Low NLR | 100% | 67% (5%–95%) | .564 |
| High NLR | 100% | 100% | ||
| Stage II | Low NLR | 81% (69%–9%) | 74% (61%–3%) | .354 |
| High NLR | 75% (50%–9%) | 62% (36%–0%) | ||
| Stage III | Low NLR | 81% (71%–8%) | 68% (56%–7%) | .756 |
| High NLR | 76% (63%–5%) | 58% (42%–2%) | ||
| Stage IV | Low NLR | 54% (45%–2%) | 44% (35%–2%) | .050 |
| High NLR | 42% (32%–2%) | 33% (24%–3%) | ||
CI = confidence interval.
Figure 3.
Kaplan–Meier overall survival curve (crude survival curve) with stratification by 8th edition of AJCC staging system between low NLR (equal or less than 3.0) in red color and high NLR (more than 3.0) in blue color. NLR = neutrophil-to-lymphocyte ratio.
Figure 4.
Postestimation survival curve after adjusted analysis with age, gender, body mass index, and histology using Cox proportional hazards regression (adjusted survival curve) with stratification by 8th edition of AJCC staging system between low NLR (equal or less than 3.0) in red color and high NLR (more than 3.0) in blue color. CI = confidence interval, HR = hazard ratio, NLR = neutrophil-to-lymphocyte ratio.
4. Discussion
The results of this retrospective cohort study with the NLR cutoff point set at 3.0 supported the key results of our exploratory analysis and validation analysis that revealed a high NLR to be a significant poor prognostic factor of OS in nonmetastatic NPC patients, even when varying the cutoff points from 2.6 to 4.2 (Table 1). Tumor-promoting inflammation is one of the enabling characteristics in ‘Hallmarks of cancer: the next generation’, and these factors play a role as microenvironmental factors in cancer development and progression.[2]
NLR is an indicator of systemic inflammatory response; however, prior to the present study, its effect as a prognostic factor in NPC had not been conclusively established. While most studies found positive association between NLR and a worse prognosis, Chua et al[15] reported conflicting results. This may be explained by the identified publication bias in that study. Most studies were retrospective cohort studies that retrieved consecutive patient data. However, Chua et al used data from 2 randomized controlled trials that were found to have inclusion and/or exclusion selection bias.[16] Regardless, we validated the 3.0 and 4.2 NLR cutoff values that were evaluated in the Chua et al study, and we found that high NLR patients had worse survival outcome than low NLR patients at both cutoff points (Table 1).
The overall role of NLR as a prognostic factor and the appropriate NLR cutoff point are both in question in clinical practice and in prognostic prediction model study. The accepted statistical technique for calculating the optimal cutoff point is area under the receiver operating characteristic curve (AuROC) curve analysis, which has its established basis in diagnostic study. There are, however, some limitations of AuROC analysis in prognostic prediction model study among cancer patients. The outcome is time-to-event analysis, which is different from binary outcome in diagnostic study; thus, Harrell C-statistic derived from Cox PH regression model is used instead of AuROC. Similarly, Harrell C-index demonstrates discrimination performance in prognostic study for distinguishing good prognosis patients from those with a poor prognosis. It is for this reason that we used Harrell maximum C-index to identify the optimal NLR cutoff value instead of AuROC analysis in this study.
Using the highest C-index (0.548) observed in this study, we determined the NLR cutoff to 3.0. Previous studies reported NLR cutoff values ranging from 2.6 to 4.2. Importantly, our analysis revealed significantly worse survival in high NLR compared to low NLR for all evaluated NLR cutoff points. Wongkrajang et al[17] reported complete blood count reference values for healthy Thai adults from Siriraj Hospital. Using their data, we analyzed the NLR value in healthy Thai adults. The mean NLR from healthy Thai adults was 1.74 ± 0.7 (range: 0.78–4.69) (unpublished data). In other words, an NLR value of 3.0 is equal to the mean plus 2 standard deviations in healthy Thai population. After multivariable analysis, this 3.0 cutoff was still an independent poor prognostic factor of OS in nonmetastatic NPC in our study. Furthermore, the C-index from internal validation by bootstrap resampling was similar to that from the exploratory model in univariable analysis (0.548 vs 0.547, respectively). Internal validation by adjusted analysis revealed a slight decrease in the C-index from 0.674 to 0.663. Taken together, the value of the C-index from the 3.0 cutoff point was found to be robust within our dataset; however, we urge external validation of our findings prior to the implementation of this cutoff in routine practice.
After stratification by cancer stage, the effect of NLR showed a borderline statistically significant difference between NLR groups in stage IVA NPC during the duration of follow-up; however, no significant effect of NLR was observed in stages II to III, and only limited effect was observed during the early short-term follow-up (Fig. 3). To explain this phenomenon, according to ‘Hallmarks of cancer: the next generation’,[2] the tumor microenvironment continuously changes during the course of tumorigenesis and progression in precancerous tumor, invasive tumor, and metastatic tumor. Therefore, the NLR could have different effect in different stages of NPC. Furthermore, neutrophils have both protumor and antitumor roles. Studies in tumor-associated neutrophils (TANs) reported conflicting results relative to the role of TANs in early-stage cancer, with some studies[18,19] reporting TANs to be associated with good prognosis in early-stage colorectal cancers, while other studies[20–22] showed TANs to be associated with worse prognosis in early-stage colorectal cancers, melanoma, and cervical squamous cell carcinoma. However, in late-stage cancer, most studies found TANs to be associated with worse prognosis. We found this same association in late-stage, but we need more sample size of stages II to III in order to prove this effect modification between NLR and tumor staging.
For clinical implication of this study, firstly, the NPC patient who had NLR > 3 should be advised of their sufficient calories and dietary intake to counteract their inflammatory process during the radio-chemotherapy course. Secondly, due to the controversial surveillance schedule in limited-resource countries, we are able to follow up the NPC patient who had pretreatment NLR > 3 after disease remission with intensive surveillance including routine ear, nose, throat examination and regular computer tomography of head and neck, chest, and upper abdomen. Finally, we are going to incorporate NLR with a cutoff point of 3 into the clinical prognostic model of in the future study and this further prediction model would select high risk patients for intensive treatment.
Interestingly, neutrophil-based therapeutic strategies, such as prevention of neutrophil exit from bone marrow and entry into tumor tissue, neutrophil depletion, inhibition of the T-cell suppression function of neutrophils, prevention of neutrophil capacity to foster tumor cell proliferation and migration, and promotion of the antitumor function of neutrophils, were investigated in many preclinical studies.[23] In addition to being a prognostic factor for cancer, NLR may have benefit as a predictive marker for cancer treatment in some neutrophil-based therapeutic strategies in the future.
The strength of this study is the robustness of NLR as a prognostic factor, even at varying cutoff points in both exploratory and validation analysis. The limitations of our study include its retrospective design, and the lack of availability of plasma EBV viral load data.
In conclusion, the results of this study showed a cutoff point of NLR of 3 from routine complete blood count to be an independent poor prognostic factor of OS among patients with nonmetastatic NPC. NLR with a cutoff point of 3 will be incorporated into the clinical prognostic model of NPC in the future study and this further prediction model would select high risk patients for intensive treatment.
Acknowledgments
The authors gratefully acknowledge Mr Kevin Jones for assistance with English language revision and also acknowledge Miss Nataya Somsanti for assistance with document preparation for Ethic Committee submission.
Author contributions
JS and KT initiated research question, designed methodology of the study, analyzed and interpreted data, and wrote the manuscript. WC and JP participated in data interpretation and manuscript revision. All authors agreed to be accountable for all aspects of the work and ensuring accuracy and integrity and approved the final version of this manuscript.
Conceptualization: Jiraporn Setakornnukul, Waipoj Chanvimalueng, Jayanton Patumanond, kullathorn thephamongkhol.
Data curation: Jiraporn Setakornnukul.
Formal analysis: Jiraporn Setakornnukul, kullathorn thephamongkhol.
Investigation: Jiraporn Setakornnukul.
Methodology: Jiraporn Setakornnukul, Jayanton Patumanond, kullathorn thephamongkhol.
Supervision: Waipoj Chanvimalueng, Jayanton Patumanond, kullathorn thephamongkhol.
Validation: Waipoj Chanvimalueng, Jayanton Patumanond, kullathorn thephamongkhol.
Writing – original draft: Jiraporn Setakornnukul, kullathorn thephamongkhol.
Writing – review & editing: Jiraporn Setakornnukul, Waipoj Chanvimalueng, Jayanton Patumanond, kullathorn thephamongkhol.
Footnotes
Abbreviations: AuROC = area under the receiver operating characteristic curve, BMI = body mass index, CI = confidence interval, C-index = concordance index, EBV = Epstein–Barr virus, HR = hazard ratio, NLR = neutrophil-to-lymphocyte ratio, NPC = nasopharyngeal carcinoma, OS = overall survival, PH = proportional hazards, TANs = tumor-associated neutrophils.
How to cite this article: Setakornnukul J, Chanvimalueng W, Patumanond J, Thephamongkhol K. Cutoff point of neutrophil-to-lymphocyte ratio for predicting survival in nasopharyngeal carcinoma. Medicine. 2021;100:34(e27095).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet 2016;387:1012–24. [DOI] [PubMed] [Google Scholar]
- [2].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [3].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [4].Jiang Y, Qu S, Pan X, Huang S, Zhu X. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in intensity modulated radiation therapy for nasopharyngeal carcinoma. Oncotarget 2018;9:9992–10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jin Y, Ye X, He C, Zhang B, Zhang Y. Pretreatment neutrophil-to-lymphocyte ratio as predictor of survival for patients with metastatic nasopharyngeal carcinoma. Head Neck 2015;37:69–75. [DOI] [PubMed] [Google Scholar]
- [6].Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016;38: (Suppl 1): E1332–1340. [DOI] [PubMed] [Google Scholar]
- [7].An X, Ding PR, Wang FH, Jiang WQ, Li YH. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in nasopharyngeal carcinoma. Tumour Biol 2011;32:317–24. [DOI] [PubMed] [Google Scholar]
- [8].Li JP, Chen SL, Liu XM, et al. A novel inflammation-based stage (I stage) predicts overall survival of patients with nasopharyngeal carcinoma. Int J Mol Sci 2016;17:1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li J, Jiang R, Liu WS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One 2013;8:e83069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liao LJ, Hsu WL, Wang CT, et al. Prognostic impact of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in nasopharyngeal carcinoma: a retrospective study of 180 Taiwanese patients. Clin Otolaryngol 2018;43:463–9. [DOI] [PubMed] [Google Scholar]
- [11].Lin YH, Chang KP, Lin YS, Chang TS. Pretreatment combination of platelet counts and neutrophil-lymphocyte ratio predicts survival of nasopharyngeal cancer patients receiving intensity-modulated radiotherapy. Onco Targets Ther 2017;10:2751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lu A, Li H, Zheng Y, et al. Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed Res Int 2017;2017:3047802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takenaka Y, Oya R, Kitamiura T, et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: a meta-analysis. Head Neck 2018;40:647–55. [DOI] [PubMed] [Google Scholar]
- [14].Yang S, Zhao K, Ding X, Jiang H, Lu H. Prognostic significance of hematological markers for patients with nasopharyngeal carcinoma: a meta-analysis. J Cancer 2019;10:2568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chua ML, Tan SH, Kusumawidjaja G, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: a pooled analysis of two randomised controlled trials. Eur J Cancer 2016;67:119–29. [DOI] [PubMed] [Google Scholar]
- [16].Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- [17].Wongkrajang P, Chinswangwatanakul W, Mokkhamakkun C, et al. Establishment of new complete blood count reference values for healthy Thai adults. Int J Lab Hematol 2018;40:478–83. [DOI] [PubMed] [Google Scholar]
- [18].Wikberg ML, Ling A, Li X, Oberg A, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol 2017;68:193–202. [DOI] [PubMed] [Google Scholar]
- [19].Berry RS, Xiong MJ, Greenbaum A, et al. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS One 2017;12:e0188799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rao HL, Chen JW, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One 2012;7:e30806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jensen TO, Schmidt H, Moller HJ, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 2012;118:2476–85. [DOI] [PubMed] [Google Scholar]
- [22].Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer 2013;108:2116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lecot P, Sarabi M, Pereira Abrantes M, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol 2019;10:2155. [DOI] [PMC free article] [PubMed] [Google Scholar]