Abstract
This study aimed to explore clinical significance of core needle biopsy (CNB) in pathological diagnosis of breast neoplasm.
Seventy one breast neoplasm samples were obtained from Tongzhou Maternal and Child Health Hospital of Beijing between the years of 2006 and 2014. Forty five specimens were obtained via CNB and cases offering 26 of them received neoadjuvant chemotherapy. Pathology, histology, and immunohistochemistry results were compared between CNB specimens and excisional biopsy.
Upward and downward tendencies could be observed in CNB specimens and excisional biopsy, respectively, in all items. Tumor proportion of CNB tissues was (33 + 2)/45 = 77.78%, when ductal carcinoma in situ detected by both CNB and excisional biopsy was 31/45 = 68.89%, with a consistency of (31 + 3)/45 = 75.56%. Tumor thrombus detected by both CNB and excisional biopsy was 2/45 = 4.44%. Among cases receiving neoadjuvant chemotherapy, CNB and excisional biopsy, in mitotic figure, cytological scoring and histological grading, showed a total change rate of >50% (50%–75%), while changes in duct and cellular heteromorphism were not distinct. Cases showing changes were up to 73.08%, with 8/26 = 30.77% for rise and 11/26 = 42.31% for descent.
CNB could be used for preoperative diagnosis of breast neoplasm, and help to determine proper treatment regimen, thus elevating the rate of breast conserving. However, this method still has several limitations, particularly in immunohistochemical tests of human epidermal receptor protein-2. Neoadjuvant chemotherapy may influence the accuracy of CNB diagnosis.
Keywords: breast tissues, core needle biopsy, excisional biopsy
1. Introduction
Breast cancer presents one of the most common tumors among women. According to domestic statistics, >1.6 million individuals are diagnosed with breast cancer every year, and its morbidity shows an upward tendency year by year.[1,2] Several techniques have been applied for breast cancer diagnosis, mainly including molybdenum palladium X-ray, breast ultrasound, and breast magnetic resonance imaging (MRI).[3–7] However, major defect of these techniques lies in their insufficiency in reaching pathological diagnosis, failing to determine the nature of the tumor.[8]
Core needle biopsy (CNB) is minimally invasive and intrusive, which could help doctors obtain sufficient breast tissues for pathological diagnosis without invasive surgery.[9] Tissues obtained through CNB could provide doctors with a series of information to establish feasible treatment regimens.[10–12] CNB also has been used in the diagnosis of different cancers.[13,14] Nonetheless, whatever examination approaches are excellent, they inevitably have some restrictions; and so does CNB. A variety of studies have compared pathological results between CNB specimens and excisional biopsy, but relevant analysis results varied across laboratories.[15,16] Clinical values of CNB specimens in the diagnosis of breast lesions require further verification.
The present study was designed to investigate clinical value of CNB specimens in pathological diagnosis of breast neoplasm. In this study, pathology, histology, and immunohistochemistry (IHC) diagnosis results were compared between CNB specimens and excisional biopsy. In addition, the values of CNB specimens in clinical diagnosis of breast lesions were investigated among cases receiving neoadjuvant chemotherapy.
2. Materials and methods
2.1. Sample collection
A total of 71 clinical breast neoplasm samples were collected from Tongzhou Maternal and Child Health Hospital of Beijing between the years of 2006 and 2014. The samples came from women aged between 29 and 73 years with an average age of 55.5 years. Of the breast neoplasm samples, 37 were from left breast while 34 from right. In addition, 45 specimens were collected via CNB, and cases offering 26 of them had received neoadjuvant chemotherapy, 9 from left breast and 17 from the right. This study was approved by the ethics committee of Tongzhou Maternal and Child Health Hospital of Beijing. All patients signed written informed consents.
2.2. Sample disposal and slide preparation
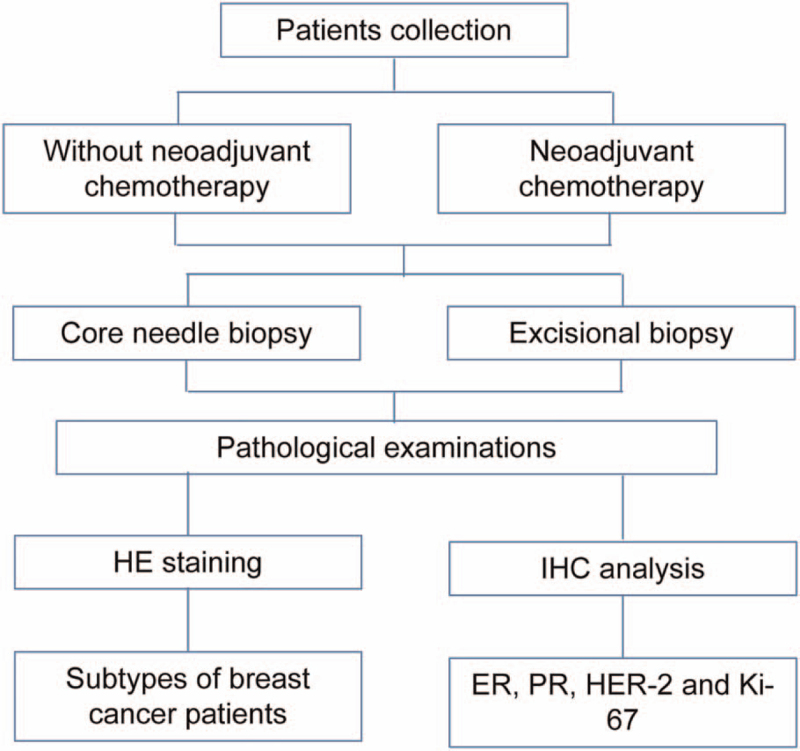
Samples were disposed according to methods recommended in ASCO/CAP guidance (2007).[17] CNB was performed through 14-core routine puncture on 3 to 7 tissues. Obtained samples were immediately sent to pathology department after collection. While excisional biopsy specimens, following general examination by pathologists, were cut into 5 mm slices and put into sufficient 4% neutral formalin. Then, the samples were embedded into paraffin, and cut into 4 μm slices. All CNB specimens and excisional biopsy ones were detected via hematoxylin-eosin staining and IHC analyses. Research flowchart was shown in Fig. 1.
Figure 1.
Flowchart for research process.
2.3. HE staining
Sections obtained for CNB specimens were detected adopting Hematoxylin and Eosin Staining Kit (Beyotime) following with the product specification.
2.4. IHC analysis
Sections were treated with sodium citrate buffer for antigen retrieval. 3% H2O2 was used to eliminate endogenous peroxidase activity. Then these sections were used for IHC which detected relative expressions of estrogen receptor, progesterone receptor, human epidermal receptor protein-2 (HER-2), and cell proliferation antigen Ki-67 (Ki-67) in breast neoplasm samples. The sections were cultured with anti-ER (abcam, ab32063), anti-PR (abcam, ab16661), anti-HER-2 (abcam, ab134182), and anti-Ki-67 (abcam, ab16667) antibodies at 4 °C overnight. After washed with phosphate buffered solution buffer for 10 minutes, the sections were cultured with second antibodies at room temperature for 1 hour. Finally, the sections were incubated with streptavidin-peroxidase complex for 20 minutes.
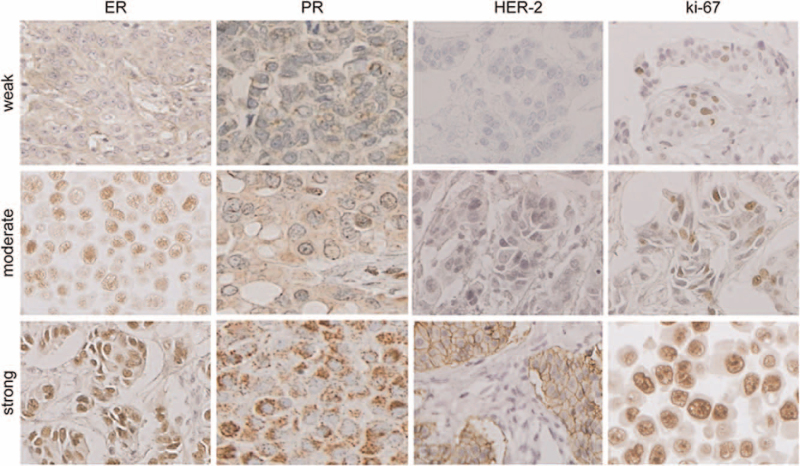
Staining intensity was defined as follows: weak, moderate, and strong. Staining area was defined to be 1, <10%; 2, 10% to 50%; and 3, >50%. Total staining score was the sum of staining intensity and area: 0 to 2 = negative expression and 3 to 6 = positive expression.
2.5. Statistics analysis
All data analyses were performed with SPSS 18.0 software (IBM Corporation, Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation (SD), while categorical ones as case number and percentage. The comparison of continuous data was performed via student t test, and chi-square test was adopted for categorical variables. All analyses were two-tailed, and P values <.05 were considered to be significant threshold.
3. Results
3.1. Comparisons on histological grading between CNB specimens and excisional biopsy ones
As shown in Table 1, upward and downward tendencies could be observed in CNB and excisional biopsy specimens, respectively, in all items (duct, cellular heteromorphism, mitotic figure, histological scoring, and histological grading),[18–20] with equivalency taking a dominant position in duct, cellular heteromorphism, and histological grading (Fig. 2). However, in mitotic figure and histological scoring, equivalent situation only accounted for 45.45% and 31.82%, respectively, while the proportions of significant alterations were up to 54.55% and 68.18%, respectively.
Table 1.
Comparisons on histological grading between CNB and excisional biopsy (cases/total number = %).
| Duct | Cellular heteromorphism | Mitotic figure | Histological scoring | Histological grading | |
| Upward | 2/44 = 4.55 | 8/44 = 18.18 | 20/44 = 45.45 | 23/44 = 52.27 | 13/44 = 29.55 |
| Downward | 3/44 = 6.82 | 5/44 = 11.36 | 4/44 = 9.09 | 7/44 = 15.91 | 3/44 = 6.82 |
| General alteration | (2 + 3)/44 = 11.36 | (8 + 5)/44 = 29.55 | (20 + 4)/44 = 54.55 | (23 + 7)/44 = 68.18 | (13 + 3)/44 = 36.36 |
| equivalent | 39/44 = 88.64 | 31/44 = 70.45 | 20/44 = 45.45 | 14/44 = 31.82 | 28/44 = 63.64 |
CNB = core needle biopsy.
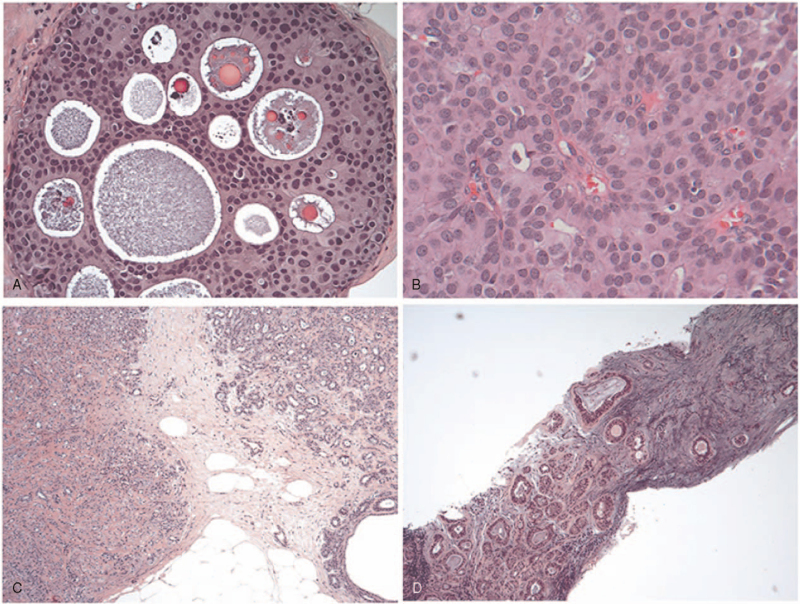
Figure 2.
HE staining results for breast cancer patients with different subtypes. A, Ductal carcinoma in situ; B, papillary carcinoma; C, atypical lobular hyperplasia; D, fibroadenoma. HE = hematoxylin-eosin.
Taking mitotic figure and histological scoring as examples, the former elevated 20/44 = 45.45% and descended 4/44 = 9.09%; while the upward and downward values for the latter were 23/44 = 52.27% and 7/44 = 15.91%, respectively.
3.2. Comparing pathology between CNB and excisional biopsy
According to data in Table 2, change in tumor proportion was (33 + 2)/45 = 77.78%, showing an upward value of 33/45 = 73.33% and a downward value of 2/45 = 4.44%.
Table 2.
Comparisons on pathology between CNB and excisional biopsy (cases/total number = %).
| Tumor proportion | DCIS | Tumor thrombus | Nerve invasion | ||
| Upward | 33/45 = 73.33 | All | 31/45 = 68.89 | 2/45 = 4.44 | 2/45 = 4.44 |
| Downward | 2/45 = 4.44 | None | 3/45 = 6.67 | 33/45 = 73.33 | 35/45 = 77.78 |
| General alteration | (33 + 2)/45 = 77.78 | Consistent | (31 + 3)45/75.56 | (2 + 33)/45 = 77.78 | (2 + 35)/45 = 82.22 |
| Equivalent | 10/45 = 22.22 | Existing only in CNB | 3/45 = 6.67 | 1/45 = 2.22 | 3/45 = 6.67 |
| Existing only in general group | 8/45 = 17.78 | 9/45 = 20 | 5/45 = 11.11 | ||
| Existing in CNB | 34/45 = 75.56 | 3/45 = 6.67 | 5/45 = 11.11 | ||
| Existing in general group | 39/45 = 86.67 | 11/45 = 24.44 | 7/45 = 15.56 |
CNB = core needle biopsy, DCIS = ductal carcinoma in situ.
3.2.1. Ductal carcinoma in situ (DCIS)
The proportion of DCIS detected by both CNB and excisional biopsy was 31/45 = 68.89%, and those not detected by either of the techniques accounted for 3/45 = 6.67%, with a consistency of (31 + 3)45 = 75.56%. The proportion of DCIS detected only by CNB was 3/45 = 6.67%, and that only by excisional biopsy was up to 8/45 = 17.78%, extremely higher in excisional biopsy than in CNB. Besides, cases detected by CNB accounted for 34/45 = 75.56% while the value for excisional biopsy was 39/45 = 86.67%.
3.2.2. Tumor thrombus
The proportion of tumor thrombus detected by both CNB and excisional biopsy was 2/45 = 4.44%, and those not detected by either of the techniques accounted for 33/45 = 73.33%, with a consistency of (2 + 33)/45 = 77.78%. The proportion of tumor thrombus detected only by CNB was 1/45 = 2.22%, and that only by excisional biopsy was up to 9/45 = 20%, extremely higher in excisional biopsy than in CNB as well. Besides, cases detected by CNB accounted for 3/45 = 6.67% while the value for excisional biopsy was 11/45 = 24.44%.
3.3. Comparisons on IHC results between CNB and excisional biopsy
According to statistics in Table 3, for all immunohistochemical items, CNB and excisional biopsy, in terms of both proportion and magnitude, showed a total change rate of >50% either in an upward or in a downward trend, ranging from 52.5% to 72.5% (Fig. 3).
Table 3.
Comparisons on immunohistochemistry between CNB and excisional biopsy (cases/total number = %).
| ER proportion | ER magnitude | PR proportion | PR magnitude | HER-2 magnitude | Ki-67 proportion | |
| Upward | 22/40 = 55 | 16/40 = 40 | 22/40 = 55 | 14/40 = 35 | 10/41 = 24.39 | 14/41 = 34.15 |
| Downward | 5/40 = 12.50 | 5/40 = 12.50 | 7/40 = 17.50 | 8/40 = 20 | 14/41 = 34.15 | 15/41 = 36.59 |
| General alteration | (22 + 5)/40 = 67.5 | (16 + 5)/40 = 52.5 | (22 + 7)/40 = 72.5 | (14 + 8)/40 = 55 | (10 + 14)/41 = 58.54 | (14 + 15)/41 = 70.73 |
| Equivalent | 13/40 = 32.50 | 19/40 = 47.50 | 11/40 = 27.50 | 18/40 = 45 | 17/41 = 41.46 | 12/41 = 29.27 |
CNB = core needle biopsy, ER = estrogen receptor, HER-2 = human epidermal receptor protein-2, Ki-67 = cell proliferation antigen Ki-67, PR = progesterone receptor.
Figure 3.
IHC analysis results for ER, PR, HER-2, and ki-67. ER = estrogen receptor, HER-2 = human epidermal receptor protein-2, Ki-67 = cell proliferation antigen Ki-67, PR = progesterone receptor.
3.4. Comparisons between CNB and excisional biopsy among cases receiving neoadjuvant chemotherapy
Twenty six cases had received neoadjuvant chemotherapy, and we estimated clinical value of tissues obtained by CNB among them. As shown in Table 4, CNB and excisional biopsy, in terms of mitotic figure, cytological scoring, and histological grading, showed a total change rate of >50% either in an upward or in a downward trend, ranging from 50% to 75%, while changes in duct and cellular heteromorphism were not distinct.
Table 4.
Comparisons on histology between CNB and excisional biopsy among cases receiving neoadjuvant chemotherapy (cases/total number%).
| Duct | Cellular heteromorphism | Mitotic figure | Histological scoring | Histological grading | |
| Upward | 2/24 = 8.33 | 6/24 = 25 | 7/24 = 29.17 | 10/24 = 41.67 | 8/24 = 33.33 |
| Downward | 0/24 = 0 | 3/24 = 12.5 | 5/24 = 20.83 | 8/24 = 33.33 | 5/24 = 20.83 |
| General alteration | (2 + 0)/24 = 8.33 | (6 + 3)/24 = 37.5 | (7 + 5)/24 = 50 | (10 + 8)/24 = 75 | (8 + 5)/24 = 54.17 |
| Equivalent | 22/24 = 91.67 | 15/24 = 62.5 | 12/24 = 50 | 6/24 = 25 | 11/24 = 45.83 |
CNB = core needle biopsy.
According to Table 5, the total of changes, regardless of their upward or downward tendencies, was up to 73.08%, and the proportion was 8/26 = 30.77% for rise and 11/26 = 42.31% for descent. Compared with those without chemotherapy, the number was significantly decreased in cases exhibiting upward trends while increased in those with downward trends, which made us wonder whether puncture sites happened to be located at chemotherapy sensitive-positions. However, chemotherapy wielded certain effects in the most of cases, and the proportion of leveling off was similar.
Table 5.
Comparisons on pathology between CNB and excisional biopsy among cases receiving neoadjuvant chemotherapy (cases/total number%).
| Tumor proportion | DCIS | Tumor thrombus | Nerve invasion | ||
| Upward | 8/26 = 30.77 | All | 13/26 = 50 | 2/26 = 7.69 | |
| Downward | 11/26 = 42.31 | None | 4/26 = 15.38 | 17/26 = 65.38 | |
| General alteration | (8 + 11)/26 = 73.08 | Consistent | (13 + 4)/26 = 65.38 | (2 + 17)/26 = 73.08 | |
| Equivalent | 7/26 = 26.92 | Existing only in CNB | 0/26 = 0 | 0/26 = 0 | |
| Existing only in general group | 9/26 = 34.62 | 7/26 = 26.92 | 7/26 = 26.92 | ||
| Existing in CNB | 13/26 = 50 | 2/26 = 7.69 | |||
| Existing in general group | 22/26 = 86.62 | 9/26 = 34.62 |
CNB = core needle biopsy, DCIS = ductal carcinoma in situ.
4. Discussion
Upward and downward tendencies could be observed in CNB and excisional biospsy in all items (duct, cellular heteromorphism, mitotic figure, histological scoring, and histological grading).[21,22] Tumor thrombus was more frequently detected by excisional biopsy than CNB. There was no extremely distinct difference between CNB and excisional biopsy. CNB is operated with the assistance of B ultrasound, but dose not obtain samples directly guided by eyes, so such sampling could be regarded to be accomplished blindly.[23,24] Consequently, without any knowledge about tissue features, purposeful puncture could not be realized; besides, tissue ribbons obtained by CNB are slimsy, and after dehydration, embedding and slicing, only several banded tissues could be observed under a microscope. On the contrary, naked eyes can see the whole and sections of neoplasms in excisional biopsy, and targeted sampling could be performed according to their color and luster, texture, and shape and properties.[25–27] Therefore, the number of tissue blocks obtained in excisional biopsy could be determined based on visual inspection; and the size of each tissue block is significantly larger than that of tissue ribbons, so microscopic observational area is bigger as well, making pathological observation more sufficient and thus getting a relatively full view of lesions. Reportedly, CNB could not obtain sufficient samples, and fail to effectively reflect pathological changes in lesions.[28,29] In addition, for some lesions, it is hard to accomplish diagnosis only based on HE staining results due to small and little samples.[28]
In our study, excisional biopsy tissues showed dramatically higher proportions of carcinoma in situ and tumor thrombus than CNB specimens. It indicated again that CNB frequently fails to represent whole lesions, which easily led to under-diagnosis due to limited samples.[30] This conclusion was in accordance with that in a previous study.[31] The earlier research reported that it was more common for CNB to miss the components of invasive carcinoma, causing underestimation in diagnosis. This phenomenon suggested that CNB for breast had major deficiency in the diagnosis of carcinoma in situ and invasive carcinoma.[32] In a relevant study, 2 cases diagnosed as ductal intraepithelial neoplasia by CNB were conformed to be invasive ductal carcinoma in postoperative pathological diagnosis, demonstrating certain discrepancy between 2 approaches.[33] Huo et al[34] reported that 20% of ductal carcinomas in situ declared by results from CNB were conformed to be invasive carcinoma according to postoperative diagnosis, while such figure in China was supposed to be 30% in some literature, without difference. With regard to immunohistochemical results, different degrees of elevation, descent and leveling off could be found in terms of ER, PR, HER-2, and Ki-67. Since these results, especially those for HER-2, are directly related to the development of postoperative chemotherapy regimens, they would be better to be reached using tissue blocks, and fluorescence in situ hybridization test would be adopted if necessary, with the expectation of getting more accurate results.
Difference between CNB and excisional biopsy was not obvious, with observational value slightly higher for the latter technique than for CNB. Such situation might be explained by the small number of the cases and morbidity rate of nerve invasion, and further reasons still should be explored on the basis of larger sample size. Mitotic figure, cytological scoring, and histological grading indicated that changes in duct and cellular heteromorphism were not distinct. Puncture site happened to be associated with chemotherapy sensitive-position. However, chemotherapy wielded certain effects in the most of cases, and the proportion of leveling off was similar. In the analysis of leveling off, high consistency was found between CNB and excisional biopsy, according to observations on duct and cellular heteromorphism.
5. Conclusion
In conclusion, the appearance of CNB has solved many clinical problems, with the advantages of simple operation and tiny injury. Besides, this technique could be used for preoperative diagnosis, and after obtaining essential information on tumor type, grading and staging, help to determine proper treatment regimen, thus elevating the rate of breast conserving. That being said, CNB has its own defects, just like any other examination approaches as we discussed before. Therefore, when operating this technique, special attentions should be paid to some respects, such as the sufficiency of sampling and the fineness of slide preparation. In addition, when mitotic figure, histological scoring, and IHC (especially those items on HER-2) are involved, tissue blocks are recommended for examinations; besides, fluorescence in situ hybridization staining could also be employed, if necessary, for more precise results. Consequently, the combination of CNB with other examinations is necessary.
Author contributions
Conceptualization: Chunjie Sun.
Data curation: Chunjie Sun, Qun Lu, Shuai Jia.
Formal analysis: Qun Lu, Jing Wang, Zhongqiu Zhang.
Investigation: Xinrong Zhang, Jing Wang, Wen He.
Methodology: Xinrong Zhang, Shuai Jia.
Software: Yuehong Zhang, Hailun Zhu.
Supervision: Yuehong Zhang.
Writing – original draft: Zhongqiu Zhang.
Writing – review & editing: Wen He.
Footnotes
Abbreviations: CNB = core needle biopsy, DCIS = ductal carcinoma in situ, HER-2 = human epidermal receptor protein-2, IHC = immunohistochemistry, Ki-67 = cell proliferation antigen Ki-67.
How to cite this article: Sun C, Lu Q, Zhang X, Zhang Y, Jia S, Wang J, Zhu H, He W, Zhang Z. Comparison between core needle biopsy and excisional biopsy for breast neoplasm. Medicine. 2021;100:34(e26970).
Funding: None.
The authors declare that they have no competing interests.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [2].DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52–62. [DOI] [PubMed] [Google Scholar]
- [3].Gidcumb E, Gao B, Shan J, et al. Carbon nanotube electron field emitters for x-ray imaging of human breast cancer. Nanotechnology 2014;25:245704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Şendur HN, Cerit MN, Gültekin S, et al. Accuracy in tumor size measurements: comparison of digital mammography, digital breast tomosynthesis and synthetic mammography. Clin Imaging 2021;69:115–9. [DOI] [PubMed] [Google Scholar]
- [5].Kim Y, Sim SH, Park B, et al. Magnetic resonance imaging (MRI) assessment of residual breast cancer after neoadjuvant chemotherapy: relevance to tumor subtypes and MRI interpretation threshold. Clin Breast Cancer 2018;18:459.e1–67.e1. [DOI] [PubMed] [Google Scholar]
- [6].Avabratha K, Sweta S, Rilna CJ, et al. A study of maternal breast feeding issues during early postnatal days. SciMed J 2020;2:219–24. [Google Scholar]
- [7].Kosvyra A, Chouvarda C, Maramis I. Developing an integrated genomic profile for cancer patients with the use of NGS data. Emerg Sci J 2019;3:157–67. [Google Scholar]
- [8].Cedolini C, Bertozzi S, Londero AP, et al. Type of breast cancer diagnosis, screening, and survival. Clin Breast Cancer 2014;14:235–40. [DOI] [PubMed] [Google Scholar]
- [9].Zhou JY, Tang J, Wang ZL, et al. Accuracy of 16/18G core needle biopsy for ultrasound-visible breast lesions. World J Surg Oncol 2014;12:07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xie L, Li X, Wang Q, et al. Effects of core needle biopsy and subsequent neoadjuvant chemotherapy on molecular alterations and outcome in breast cancer. Onco Targets Ther 2018;11:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zare H. Effects of Salvia officinalis extract on the breast cancer cell line. SciMed J 2019;1:25–9. [Google Scholar]
- [12].Abdelaal AM, Attalla EM, Elshemey WM. Estimation of out-of-field dose variation using markus ionization chamber detector. SciMed J 2020;2:08–15. [Google Scholar]
- [13].Chen PT, Liu KL, Cheng TY, et al. Indirect percutaneous core needle biopsy of solid pancreatic or peripancreatic lesions. Abdom Radiol (NY) 2019;44:292–303. [DOI] [PubMed] [Google Scholar]
- [14].Pride RM, Jimenez RE, Hoskin TL, et al. Upgrade at excisional biopsy after a core needle biopsy diagnosis of classic lobular carcinoma in situ. Surgery 2021;169:644–8. [DOI] [PubMed] [Google Scholar]
- [15].Kombak FE, Şahin H, Mollamemişoğlu H, et al. Concordance of immunohistochemistry between core needle biopsy and surgical resection of breast cancer. Turk J Med Sci 2017;47:1791–6. [DOI] [PubMed] [Google Scholar]
- [16].Cha YJ, Ahn SG, Bae SJ, et al. Comparison of tumor-infiltrating lymphocytes of breast cancer in core needle biopsies and resected specimens: a retrospective analysis. Breast Cancer Res Treat 2018;171:295–302. [DOI] [PubMed] [Google Scholar]
- [17].Yaziji H, Taylor CR. Begin at the beginning, with the tissue! The key message underlying the ASCO/CAP Task-force Guideline Recommendations for HER2 testing. Appl Immunohistochem Mol Morphol 2007;15:239–41. [DOI] [PubMed] [Google Scholar]
- [18].Kim M, Park JM, Lee SJ, et al. [Pancreatic neuroendocrine tumor presenting as acute pancreatitis]. Korean J Gastroenterol 2018;71:98–102. [DOI] [PubMed] [Google Scholar]
- [19].Sakellariou M, Dellaportas D, Grapsa E, et al. Primary adrenal leiomyosarcoma: a case report and review of the literature. Mol Clin Oncol 2020;12:317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Samantaray S, Panda N, Besra K, et al. Utility of Tru-Cut biopsy of breast lesions - an experience in a regional cancer center of a developing country. J Clin Diagn Res 2017;11:EC36–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014;89:536–47. [DOI] [PubMed] [Google Scholar]
- [22].Bulte JP, Halilovic A, Kalkman S, et al. Assessment of HER2 status in breast cancer biopsies is not affected by accelerated tissue processing. Histopathology 2018;73:81–9. [DOI] [PubMed] [Google Scholar]
- [23].Volk GF, Guntinas-Lichius O, Geissler K. [Core needle biopsy]. Laryngorhinootologie 2015;94:658–9. [DOI] [PubMed] [Google Scholar]
- [24].Ding J, Hu P, Chen J, et al. The importance of tissue confirmation of metastatic disease in patients with breast cancer: lesson from a brain metastasis case. Oncoscience 2016;3:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patel AR, Jones JS. The prostate needle biopsy gun: busting a myth. J Urol 2007;178:683–5. [DOI] [PubMed] [Google Scholar]
- [26].Chen J, Chen H, Zhong Z, et al. Investigating rectal toxicity associated dosimetric features with deformable accumulated rectal surface dose maps for cervical cancer radiotherapy. Radiat Oncol 2018;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tang Q, Liu W, Zhang Q, et al. Dynamin-related protein 1-mediated mitochondrial fission contributes to IR-783-induced apoptosis in human breast cancer cells. J Cell Mol Med 2018;22:4474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen X, Yuan Y, Gu Z, et al. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat 2012;134:957–67. [DOI] [PubMed] [Google Scholar]
- [29].Mathenge EG, Dean CA, Clements D, et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 2014;16:950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ha EJ, Suh CH, Baek JH. Complications following ultrasound-guided core needle biopsy of thyroid nodules: a systematic review and meta-analysis. Eur Radiol 2018;28:3848–60. [DOI] [PubMed] [Google Scholar]
- [31].Rosa M, Agosto-Arroyo E. Core needle biopsy of benign, borderline and in-situ problematic lesions of the breast: Diagnosis, differential diagnosis and immunohistochemistry. Ann Diagn Pathol 2019;43:151407. [DOI] [PubMed] [Google Scholar]
- [32].Chand JT, Sharma MM, Dharmarajan JP, et al. Digital breast tomosynthesis as a tool in confirming negative surgical margins in non-palpable breast lesions. Ind J Surg Oncol 2019;10:624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Riedl CC, Pfarl G, Memarsadeghi M, et al. Lesion miss rates and false-negative rates for 1115 consecutive cases of stereotactically guided needle-localized open breast biopsy with long-term follow-up. Radiology 2005;237:847–53. [DOI] [PubMed] [Google Scholar]
- [34].Huo L, Sneige N, Hunt KK, et al. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer 2006;107:1760–8. [DOI] [PubMed] [Google Scholar]