Abstract
One of major causes of cervical central stenosis (CCS) is thickened change of cervical ligament flavum (CLF). The association of a morphological parameter called cervical ligament flavum thickness (CLFT) with CCS has not been reported yet. Thus, the purpose of this research was to investigate the relationship between CCS and CFJT.
Data were obtained from 88 patients with CCS. A total of 87 normal controls also underwent cervical spine magnetic resonance imaging (CSMRI). All subjects underwent axial T2-weighted CSMRI. Using our picture archiving and communications system, thickness of ligament flavum of the cervical spine at C6/7 level was analyzed.
The mean CLFT was 1.41 ± 0.24 mm in normal subjects and 2.09 ± 0.39 mm in patients with CCS. The CCS group was found to have significantly (P < .001) higher rate of CLFT than normal subjects. ROC curves were used to assess the usefulness of CLFT as a predictor of CCS. In the CCS group, the best practical cut off-point of CLFT was 1.71 mm (sensitivity = 90.9%; specificity = 90.8%), with AUC of 0.94 (95% confidence interval: 0.90--0.98).
Greater CLFT values were associated with greater possibility of CCS. Thus, treating physician should carefully examine CLFT, as it can help diagnose CCS.
Keywords: cervical central stenosis, cervical ligament flavum, cervical ligament flavum thickness
1. Introduction
Cervical central stenosis (CCS) results from degeneration of cervical disc space, uncovertebral joint degradation, and hypertrophy of cervical facet, which dehydrate and collapse, thus increasing mechanical friction at the edge of the vertebral bodies at cartilaginous end plates.[1–3] This repeated mechanical stress results in osteophyte formation and subperiosteal bone formation. Stenotic symptoms gradually arise during repeated cervical nerve root or spinal cord compression. They can manifest as myelopathy, radiculopathy, or neck pain syndromes.[4–6] Cervical spine magnetic resonance imaging (CSMRI) is the most effective image modality for analyzing the cervical vertebrae such as cervical facet, ligamentum flavum, and intervertebral disk.[7] An accurate CSMRI diagnosis of CCS is important to determine appropriate management.[8] Previous researches have indicated that morphologic parameters such as cervical pedicles, cervical dural sac-thickness, and cervical lateral masses are associated with aging, disc degeneration, and CCS.[9]
Anatomical research of cervical ligamentum flavum (CLF) is also critical to understand CCS. CLF height progressively increases from C2/3 to C6/7. It steadily decreases from medial to lateral within each cervical vertebra. CLF in cervical spine does not enter the cervical neural foramen.[10] Both thickness and width are comparatively constant from caudal to cranial. The laminar surface area covered by CLF progressively increases from 33.0% in para midline at C2 level to 70.0% in para midline at C6 level.[11]
However, previous studies did not assess the role of the thickness of CLF as a morphological determinant of CCS. To analyze the relationship between CCS and thickness of the CLF, we made a new morphological determinant called CLF thickness (CLFT). We hypothesize that CLFT is an important morphologic determinant in the diagnosis of CCS. Therefore, the objective of this study was to use axial T2-weighted CSMRI to analyze CLFT in CCS patients and controls.
2. Methods
2.1. Patients
The Independent Ethics Committee of Catholic Kwandong University approved the current study (IRB protocol number: IS18RISI0016). We investigated patients who underwent CSMRI between May 2017 and July 2019 and who were diagnosed with CCS. We included patients over age 50 years if patients had clinical symptoms compatible with CCS (weakness of fingers or hand and loss of sensation or tingling in the upper extremities, cervicalgia), the most stenotic level at C6/7, and CSMRI performed within 1 year of CCS diagnosis that was available for retrospective chart review. Our exclusion criteria were a history of previous cervical spinal cord damage or cervical spine surgery, congenital cervical spine defects, or cervical space occupying disease such as cysts or tumors, stroke, and syringomyelia.
We recruited 88 CCS patients who were diagnosed by 2 experienced neuroradiologists. In the CCS group, there were 51 (57.9%) males and 37 (42.1%) females with an average age of 59.09 ± 7.37 years (range: 50–81 years) (Table 1). To investigate CLFT in CCS patients and normal subjects, we recruited normal subjects who underwent CSMRI as part of medical check-up. These normal subjects consisted of 87 participants [38 males (43.7%) and 49 females (56.3%)] with an average age of 57.09 ± 6.52 years (range: 50–79 years) (Table 1). We analyzed CLFT in these normal subjects at C6/7 facet joint level.
Table 1.
Comparison of characteristics of control and CCS groups.
| Variable | Control Group n = 87 | CCS Group n = 88 | Statistical significance |
| Gender (male/female) | 38/49 | 51/37 | NS |
| Age, yrs | 57.09 ± 6.52 | 59.09 ± 7.37 | NS |
| CLFT, mm | 1.41 ± 0.24 | 2.09 ± 0.39 | P < .001 |
Data represent the mean ± standard deviation (SD) or the numbers of patients.
CCS = central cervical stenosis; CLFT = cervical ligament flavum thickness; NS = not statistically significant (P > .05).
2.2. Imaging parameters
CSMRI examinations were performed with 3T Avanto (Siemens, Erlangen, Germany) with 3 T scanners (Philips Healthcare, The Netherlands). Cervical spine axial T2-W images with 4.00 mm thick slices were obtained using the following parameters: zoom of 100.42%, 0.4 mm intersection gap, 512 ms/18 ms repetition time/echo time, 458 × 318 cm field of view, 815 × 253 matrix, and 15 echo train length.
2.3. Image analysis
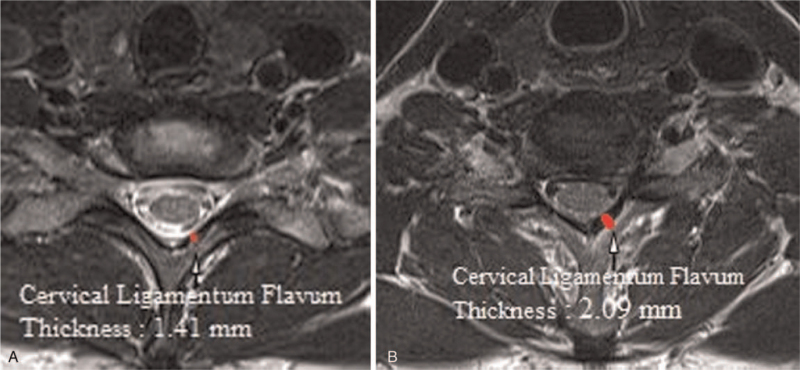
T2-weighted axial CSMR images were obtained at the cervical facet joint location for all subjects. To measure CLFT at cervical facet joint on CSMRI, we used multimodality PACS network (INFINITT med-health Co., South Korea). CLFT was measured by drawing a linear line along the side of the cervical ligament facing the cervical spinal canal and along the cervical facet side. The thickest level at C6/7 point was recorded (Fig. 1).
Figure 1.
Measurement of the cervical ligament flavum thickness on cervical spine MRI. (A) Control group. (B) Central cervical stenosis group.
2.4. Statistical analysis
Differences in demographic data between the normal and CCS groups were calculated using independent t tests. The ROC curve analysis was performed to calculate sensitivity, specificity, and the AUC for the validity of the CLFT. P-values < .05 were considered statistically significant. The relationship between age-related changes and CLFT was analyzed using 1-way analysis of variance (ANOVA). Data were entered and analyzed using Statistical Package for Social Science for Windows version 22 (SPSS Inc, Chicago, IL).
3. Results
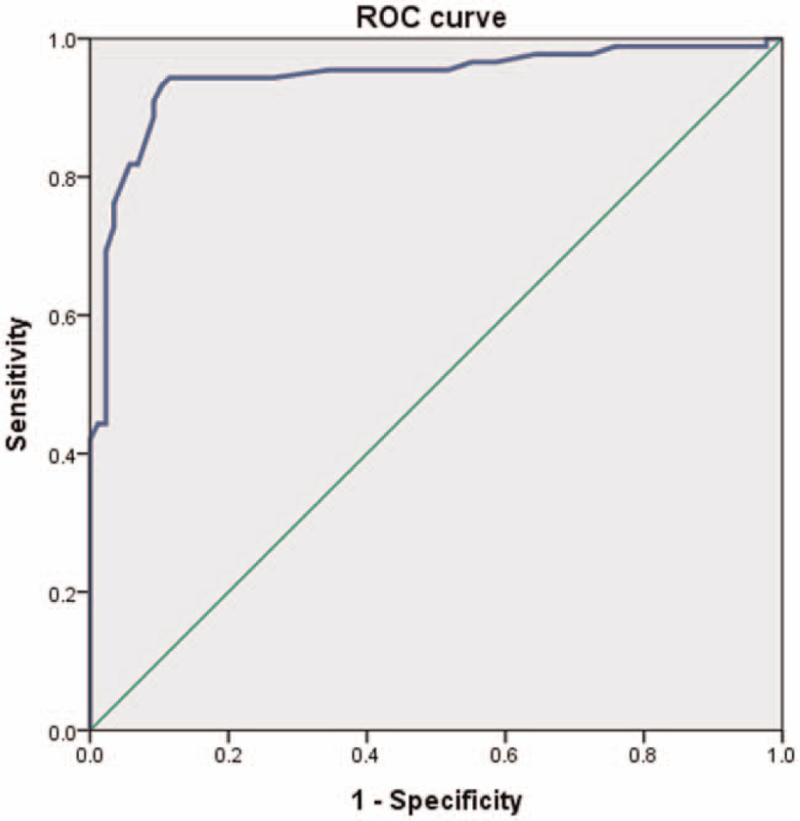
Demographic factors were not significantly different between CCS and normal groups (Table 1). The mean CLFT was 1.41 ± 0.24 mm in the normal group and 2.09 ± 0.39 mm in the CCS group. The CCS group was found to have significantly (P < .001) higher rate of CLFT than the control group (Table 1). The mean CLFT of the normal group was 1.41 ± 0.24 mm in subjects aged 50 to 59 years, 1.39 ± 0.21 mm in subjects aged 60 to 69 years, and 1.56 ± 0.39 mm in subjected aged 70 to 79 years (Table 2). In the normal group, there was no significant correlation between age-related changes and CLFT in 1-way ANOVA (F = 0.861; df = 2; P = .426). The mean CLFT of the CCS group was measured to be 2.09 ± 0.45 mm in patients aged 50 to 59 years, 2.10 ± 0.29 mm in patients aged 60 to 69 years, and 2.10 ± 0.23 mm in patients aged 70 to 81 years (Table 3). In the CCS group, we did not find any statistically significant correlation between CLFT and age-related changes either (F = 0.006; df = 2; P = .994). ROC curves were prepared to assess the usefulness of CLFT as a predictor of CCS. In the CCS group, the best practical cut off-point was 1.71 mm (sensitivity = 90.9%; specificity = 90.8%), with AUC of 0.94 [95% confidence interval (95% CI): 0.90--0.98] (Table 4, Fig. 2).
Table 2.
Age distribution and mean CLFT of the control group.
| Age distribution, yrs | Total (N) (87) |
| 50–59 | 1.41 ± 0.24 mm (63) |
| 60–69 | 1.39 ± 0.21 mm (20) |
| 70–79 | 1.56 ± 0.39 mm (4) |
CLFT = cervical ligament flavum thickness.
Table 3.
Age distribution and mean CLFT of the CCS group.
| Age distribution, yrs | Total (N) (88) |
| 50–59 | 2.09 ± 0.45 mm (56) |
| 60–69 | 2.10 ± 0.29 mm (22) |
| 70–81 | 2.10 ± 0.23 mm (10) |
CLFT = cervical ligament flavum thickness; CS = cervical central stenosis.
Table 4.
Sensitivity and specificity of each cut-off point of CLFT.
| CLFT, mm | Sensitivity (%) | Specificity (%) |
| 0.95 | 100 | 2.3 |
| 1.41 | 95.5 | 48.3 |
| 1.54 | 94.3 | 73.6 |
| 1.71∗ | 90.9 | 90.8 |
| 1.77 | 85.2 | 92.0 |
| 2.02 | 52.3 | 97.7 |
CLFT = cervical ligament flavum thickness.
The best cut-off point on the receiver operating characteristic (ROC) curve.
Figure 2.
Receiver operating characteristic curve of cervical ligament flavum thickness for prediction of central cervical stenosis. The best cut off point of cervical ligament flavum thickness was 1.71 mm, with sensitivity of 90.9%, specificity of 90.8%, and AUC of 0.94. AUC = area under the curve.
4. Discussion
CCS is a multifactorial degenerative disease that can lead to neck pain and eventually spinal cord compression.[12–16] It is the result of a reactive hypertrophy of osteophytes in the endplate and ligament, uncovertebral structures in conjunction with degeneration and bulging of the disk area. The consequence is a compression of the cervical spinal cord and restriction of the anterior-posterior diameter of the spinal canal.[17–19] This process can lead to further nerve root or spinal cord injury and impingement.[7]
Previous studies have analyzed associations between cervical canal's medial–lateral diameter, cervical dural sac-thickness, cervical pedicles, and cervical lateral masses are associated with disc degeneration, aging, and CCS.[20,21] Freedman et al[21] have demonstrated that the lateral-medial diameter at the pedicle level of cervical canal is the most accurate and highly predictive. They also insisted that geometric characters of the cervical spinal canal were dangerous factors for a spinal cord injury.[21] Kwon et al[22] have announced anatomical differences in the thickness of dura mater with respect to cervical vertebral age and level. Prasad et al[23] have reported that the diameter of the cervical spinal canal is a threshold indicator or AP straight line distance for CCS. Chaput et al[24] have examined the lateral-medial diameter at the facet joint level of the cervical spinal canal in traumatic injury and found that the ratio of AP diameters is predictive of an acute spinal cord injury.
However, we frequently encounter discrepancies in CCS diagnosis according to previous reference imaging protocol. Previous grading system might have overlooked hypertrophy of CLF. Coughlin et al[25] have reported that calcified hypertrophic CLF is a known entity that causes myelopathy of the cervical spine. They also insisted that noncalcified hypertrophic CLF could cause progressive cervical myelopathy. Hartman et al[26] have shown mechanical play of posterior column segments in human cervical spine components. After analyzing interspinous/supraspinous ligaments, CLF, facet, and facets capsule using a robot-analysis system, they have concluded that CLF is very important mechanically in the cervical vertebrae. CLF contributes to moment resistance in flexion.[26] There are many previous researches of ligamentum flavum hypertrophy at thoracolumbar spine level. We thought that the same histopathologic and biomechanical changes could occur in the cervical vertebrae as well. Although the specific mechanism of hypertrophy of CLF is uncertain, Hur et al[27] have reported that the presence of angiogenic factors and alterations in mechanical stress might offer critical link. They insist that the pathophysiology of hypertrophy of CLF involves a significant change of angiogenic factors, including angiopoietin-1, vascular endothelial growth factor (VEGF), fibroblast growth factor, and vascular endothelial-cadherein. They concluded that elderly patients with thicker CLF and increased segmental movement had higher concentrations of CD34þ capillaries and VEGF than their normal subjects, suggesting that these factors might play an important role in the pathogenesis of hypertrophy of CLF.[27] However, no study has reported the optimal cut off point of CLFT to diagnose CCS clinically.
To analyze the relationship between CCS and hypertrophy of the CLF, we made a simple morphological diagnostic tool called CLFT. To the best of our knowledge, association CLFT with CCS has not been evaluated yet. We hypothesize that CLFT is a critical morphological diagnostic parameter in the diagnosis of CCS. We demonstrated a positive correlation between CCS and CLFT. In the current research, we found that cut off-point of CLFT at 1.71 mm had sensitivity of 90.9%, specificity of 90.8%, and AUC of 0.94 (95% CI: 0.90–0.98) to predict CCS. Our results suggest that CLFT is an objective and accurate morphological diagnostic parameter for CCS prediction. The current research included individuals aged above 50 years.
The cause of thickened CLF is that when CLF becomes harder and thicker, it loses tis elasticity, and becomes longer in CSS.[27,28] Thus, during repetitive motion of the cervical spine, harder and longer CLF may protrude into the cervical spinal canal, causing mechanical compression of the cervical spinal cord, which can be assessed on CSMRI as an increased diameter of CLFT. Thus, in patients with CSS, movement from extension to flexion causes significant spinal cord compression. In this study, we analyzed CLFT from CSMRI images. CSMRI studies are very important in the diagnosis of degenerative disorders of CCS and in highly detectable hypertrophy of CLF.[29,30]
This study has some limitations. First, although we measured CLFT in T2-weighted axial images at the most stenotic facet joint level, there might be some measurement errors on CSMRI because axial cervical images might be inhomogeneous due to differences in the cutting angle of CSMRI resulting from posture in patients and individual anatomic variations. Second, the diameter and volume of the spinal canal are irregular. In addition, the shape of the cervical spinal canal can also change from flexion to extension, as the diameter of the osseous canal widens during flexion and narrows during extension. Moreover, vertebral disks and CLF may change during motion of cervical vertebrae.[26] However, this study only investigated CLFT in conventional CSMRI. Finally, this research was retrospective in nature. In spite of these all limitations, this is the first research to report the association of CFLT with CCS.
5. Conclusion
We demonstrated that higher CLFT values were associated with higher possibility of CCS. Thus, treating physician should carefully examine CLFT, as it can help diagnose CCS.
Acknowledgments
The all authors thank the International ST. Mary‘s Hospital.
Author contributions
Conceptualization: Young Uk Kim.
Data curation: Young Uk Kim.
Investigation: Hye-Won Jeong, Sukhee Park, Young Uk Kim.
Methodology: Young Uk Kim.
Writing – review & editing: Hye-Won Jeong, Jungmin Yi, Sooho Lee, Keum Nae Kang, Jonghyuk Lee, Hyung Rae Cho.
Footnotes
Abbreviations: AUC = area under the curve, CCS = cervical central stenosis, CLF = cervical ligament flavum, CLFT = cervical ligament flavum thickness, CSMRI = cervical spine magnetic resonance imaging, ROC curve = receiver operating characteristic curve.
How to cite this article: Jeong HW, Yi J, Lee S, Park S, Kang KN, Lee J, Cho HR, Kim YU. Prognostic value of cervical ligamentum flavum thickness as a morphological parameter to predict cervical stenosis. Medicine. 2021;100:34(e27084).
No funding was received.
No additional data are available.
This study conforms to the Declaration of Helsinki.
The authors declare no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files];
References
- [1].Fujimori T, Le H, Hu SS, et al. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine (Phila Pa 1976) 2015;40:E394–403. [DOI] [PubMed] [Google Scholar]
- [2].Kawaguchi Y, Nakano M, Yasuda T, et al. Characteristics of ossification of the spinal ligament; incidence of ossification of the ligamentum flavum in patients with cervical ossification of the posterior longitudinal ligament - Analysis of the whole spine using multidetector CT. J Orthop Sci 2016;21:439–45. [DOI] [PubMed] [Google Scholar]
- [3].Ohara Y. Ossification of the ligaments in the cervical spine, including ossification of the anterior longitudinal ligament, ossification of the posterior longitudinal ligament, and ossification of the ligamentum flavum. Neurosurg Clin N Am 2018;29:63–8. [DOI] [PubMed] [Google Scholar]
- [4].Mori K, Yoshii T, Hirai T, et al. Prevalence and distribution of ossification of the supra/interspinous ligaments in symptomatic patients with cervical ossification of the posterior longitudinal ligament of the spine: a CT-based multicenter cross-sectional study. BMC Musculoskelet Disord 2016;17:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sasaki E, Ono A, Yokoyama T, et al. Prevalence and symptom of ossification of posterior longitudinal ligaments in the Japanese general population. J Orthop Sci 2014;19:405–11. [DOI] [PubMed] [Google Scholar]
- [6].Wu D, Ba Z, Zhao W, Zhang Y, Liu J, Meng Y. Ossification of the posterior longitudinal and yellow ligaments on the lumbar spine. Orthopedics 2012;35:e298–301. [DOI] [PubMed] [Google Scholar]
- [7].Takahashi T, Hanakita J, Minami M. Pathophysiology of calcification and ossification of the ligamentum flavum in the cervical spine. Neurosurg Clin N Am 2018;29:47–54. [DOI] [PubMed] [Google Scholar]
- [8].Lee JE, Park HJ, Lee SY, et al. Interreader reliability and clinical validity of a magnetic resonance imaging grading system for cervical foraminal stenosis. J Comput Assist Tomogr 2017;41:926–30. [DOI] [PubMed] [Google Scholar]
- [9].Miyazaki M, Takita C, Yoshiiwa T, Itonaga I, Tsumura H. Morphological analysis of the cervical pedicles, lateral masses, and laminae in developmental canal stenosis. Spine (Phila Pa 1976) 2010;35:E1381–1385. [DOI] [PubMed] [Google Scholar]
- [10].Rahmani MS, Terai H, Akhgar J, et al. Anatomical analysis of human ligamentum flavum in the cervical spine: special consideration to the attachments, coverage, and lateral extent. J Orthop Sci 2017;22:994–1000. [DOI] [PubMed] [Google Scholar]
- [11].Bae SH, Son DW, Kwon OI, Lee SH, Lee JS, Song GS. Thickening ligamentum flavum mimicking tumor in the epidural space of the cervical spine. Korean J Neurotrauma 2018;14:43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baran O, Kir B, Ates I, Sahin A, Uzturk A. Combined supraclavicular and superficial cervical plexus block for clavicle surgery. Korean J Anesthesiol 2020;73:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Choi EJ, Go G, Han WK, Lee PB. Radiation exposure to the eyes and thyroid during C-arm fluoroscopy-guided cervical epidural injections is far below the safety limit. Korean J Pain 2020;33:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Diwan S, Sethi D, Gaikwad A, Sancheti P, Nair A. Subcoracoid tunnel block as an alternative infraclavicular brachial plexus approach: a case series. Korean J Anesthesiol 2020;73:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heo Y, Cho N, Cho H, Won HS, Yang M, Kim YD. New insights into pathways of the accessory nerve and transverse cervical artery for distal selective accessory nerve blockade. Korean J Pain 2020;33:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kannan S, Surhonne NS, CK R, BK D, DR RSR. Effects of bilateral superficial cervical plexus block on sevoflurane consumption during thyroid surgery under entropy-guided general anesthesia: a prospective randomized study. Korean J Anesthesiol 2018;71:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim JS, Ko JS, Bang S, Kim H, Lee SY. Cervical plexus block. Korean J Anesthesiol 2018;71:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Manchikanti L, Malla Y, Cash KA, Pampati V, Hirsch JA. Comparison of effectiveness for fluoroscopic cervical interlaminar epidural injections with or without steroid in cervical post-surgery syndrome. Korean J Pain 2018;31:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC. The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 2009;18:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sureka B, Mittal A, Mittal MK, Agarwal K, Sinha M, Thukral BB. Morphometric analysis of cervical spinal canal diameter, transverse foramen, and pedicle width using computed tomography in Indian population. Neurol India 2018;66:454–8. [DOI] [PubMed] [Google Scholar]
- [21].Freedman BA, Hoffler CE, 2nd, Cameron BM, et al. A comparison of computed tomography measures for diagnosing cervical spinal stenosis associated with myelopathy: a case-control study. Asian Spine J 2015;9:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kwon S, Suh SW, Kim D, et al. Analysis of dural sac thickness in the human cervical spine. Anat Sci Int 2018;93:284–90. [DOI] [PubMed] [Google Scholar]
- [23].Prasad SS, O’Malley M, Caplan M, Shackleford IM, Pydisetty RK. MRI measurements of the cervical spine and their correlation to Pavlov's ratio. Spine (Phila Pa 1976) 2003;28:1263–8. [DOI] [PubMed] [Google Scholar]
- [24].Chaput CD, Allred JJ, Pandorf JJ, Song J, Rahm MD. The significance of facet joint cross-sectional area on magnetic resonance imaging in relationship to cervical degenerative spondylolisthesis. Spine J 2013;13:856–61. [DOI] [PubMed] [Google Scholar]
- [25].Coughlin DJ, Rymarczuk GN, Dirks MS. Noncalcified hypertrophic ligamentum flavum causing severe cervical stenosis and myelopathy: case report and review of the literature. World Neurosurg 2016;95:618.e621-618 e626. [DOI] [PubMed] [Google Scholar]
- [26].Hartman RA, Tisherman RE, Wang C, et al. Mechanical role of the posterior column components in the cervical spine. Eur Spine J 2016;25:2129–38. [DOI] [PubMed] [Google Scholar]
- [27].Hur JW, Kim BJ, Park JH, et al. The mechanism of ligamentum flavum hypertrophy: introducing angiogenesis as a critical link that couples mechanical stress and hypertrophy. Neurosurgery 2015;77:274–81. discussion 281-282. [DOI] [PubMed] [Google Scholar]
- [28].Zeng C, Xiong J, Wang JC, et al. The evaluation and observation of “Hidden” hypertrophy of cervical ligamentum flavum, cervical canal, and related factors using kinetic magnetic resonance imaging. Global Spine J 2016;6:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Verma R, Virdi JK, Singh N, Jaggi AS. Animals models of spinal cord contusion injury. Korean J Pain 2019;32:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang L, Shen J, Das S, Yang H. Diffusion tensor imaging of the C1-C3 dorsal root ganglia and greater occipital nerve for cervicogenic headache. Korean J Pain 2020;33:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]