Abstract
Background
Previous studies have shown that children and adolescents with COVID-19 generally have mild disease. Children and adolescents with cancer, however, can have severe disease when infected with respiratory viruses. In this study, we aimed to understand the clinical course and outcomes of SARS-CoV-2 infection in children and adolescents with cancer.
Methods
We did a cohort study with data from 131 institutions in 45 countries. We created the Global Registry of COVID-19 in Childhood Cancer to capture de-identified data pertaining to laboratory-confirmed SARS-CoV-2 infections in children and adolescents (<19 years) with cancer or having received a haematopoietic stem-cell transplantation. There were no centre-specific exclusion criteria. The registry was disseminated through professional networks through email and conferences and health-care providers were invited to submit all qualifying cases. Data for demographics, oncological diagnosis, clinical course, and cancer therapy details were collected. Primary outcomes were disease severity and modification to cancer-directed therapy. The registry remains open to data collection.
Findings
Of 1520 submitted episodes, 1500 patients were included in the study between April 15, 2020, and Feb 1, 2021. 1319 patients had complete 30-day follow-up. 259 (19·9%) of 1301 patients had a severe or critical infection, and 50 (3·8%) of 1319 died with the cause attributed to COVID-19 infection. Modifications to cancer-directed therapy occurred in 609 (55·8%) of 1092 patients receiving active oncological treatment. Multivariable analysis revealed several factors associated with severe or critical illness, including World Bank low-income or lower-middle-income (odds ratio [OR] 5·8 [95% CI 3·8–8·8]; p<0·0001) and upper-middle-income (1·6 [1·2–2·2]; p=0·0024) country status; age 15–18 years (1·6 [1·1–2·2]; p=0·013); absolute lymphocyte count of 300 or less cells per mm3 (2·5 [1·8–3·4]; p<0·0001), absolute neutrophil count of 500 or less cells per mm3 (1·8 [1·3–2·4]; p=0·0001), and intensive treatment (1·8 [1·3–2·3]; p=0·0005). Factors associated with treatment modification included upper-middle-income country status (OR 0·5 [95% CI 0·3–0·7]; p=0·0004), primary diagnosis of other haematological malignancies (0·5 [0·3–0·8]; p=0·0088), the presence of one of more COVID-19 symptoms at the time of presentation (1·8 [1·3–2·4]; p=0·0002), and the presence of one or more comorbidities (1·6 [1·1–2·3]; p=0·020).
Interpretation
In this global cohort of children and adolescents with cancer and COVID-19, severe and critical illness occurred in one fifth of patients and deaths occurred in a higher proportion than is reported in the literature in the general paediatric population. Additionally, we found that variables associated with treatment modification were not the same as those associated with greater disease severity. These data could inform clinical practice guidelines and raise awareness globally that children and adolescents with cancer are at high-risk of developing severe COVID-19 illness.
Funding
American Lebanese Syrian Associated Charities and the National Cancer Institute.
Introduction
Although the COVID-19 pandemic has left no population unscathed, children overall have had mild disease courses with low mortality.1, 2, 3 Measures of clinical status and service delivery differ between studies; however, severe disease has been reported in 1–6% of paediatric cases, even among hospitalised cohorts, compared with 14% in adults.1, 4, 5 Although children appear to do comparatively well, a small proportion still develop respiratory or multiorgan failure. Children and adolescents with cancer or who have received a haematopoietic stem-cell transplantation represent a potentially high-risk population with known risk of morbidity and mortality when infected by other respiratory viruses.6, 7, 8 In paediatric cohorts in the USA, influenza-attributable mortality was calculated to be 1% in patients with acute lymphoblastic leukaemia, whereas attributable case fatality due to any respiratory viral infection was 5·4% in the first year after haematopoietic stem-cell transplantation.7, 8 To support clinical and health policy decision making, appraisal of the clinical manifestations and outcomes of SARS-CoV-2 infection in this unique population is warranted, particularly given global resource constraints and current population-level treatment and vaccination prioritisation efforts.
Research in context.
Evidence before this study
Healthy children and adolescents infected by SARS-CoV-2 have a milder disease course than adults; however, children with cancer have a higher frequency of severe disease during infection by other respiratory viruses than do healthy children and thus they might be at higher risk for complications due to COVID-19. We searched PubMed from Dec 1, 2019, to Feb 1, 2021, for English language publications using search terms “pediatric”, “children”, “cancer”, “malignancy”, “COVID-19”, and “SARS-CoV-2”. To date, reports in paediatric oncology patients have been limited to case reports and single country observational studies, mostly in high-income countries. Analyses of large cohorts of adult patients with cancer suggest that SARS-CoV-2 could cause severe illness among those receiving treatment for malignancy; however, data suggest that various disease and lifestyle factors could drive these poor outcomes. Several of these factors, such as advanced age, presence of comorbidities, and poor performance status differ in children. None of the previous reports account for global differences in supportive care infrastructure that might affect outcomes.
Added value of this study
This study is the first multinational cohort spanning all World Bank income groups to report COVID-19 outcomes for children and adolescents with cancer. By creating a large, global, hospital-based registry of paediatric oncology patients with laboratory confirmed SARS-CoV-2 infections, we were able to study the effect of the disease across heterogeneous supportive care protocols and demographic backgrounds. Within our cohort, we found that although most children and adolescents with cancer generally had a mild illness during COVID-19 infection, 19·9% developed severe or critical illness. Mortality due to COVID-19 within our cohort was 3·8% (50 of 1319), and although low, this figure is more than four times greater than that reported in published cohorts of general paediatric patients. We were also able to identify several clinical patient factors, including lymphopenia and neutropenia, which were associated with more severe disease. Finally, we documented health disparities across World Bank income groups, with low-income and lower-middle-income countries contributing a greater proportion of cases with severe or critical illness.
Implications of all the available evidence
Children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection. These findings suggest that, in most cases, cancer-directed therapy can proceed and no antiviral therapy for SARS-CoV-2 is required for COVID-19 disease resolution; however, we did identify several clinical and laboratory factors associated with increased disease severity. The finding of worse outcomes associated with lymphopenia and neutropenia suggests that it might be prudent to delay myelosuppressive and lymphocyte-depleting therapy during active infection if this is feasible in patients with other risk factors for severe disease. Additionally, patients receiving or anticipated to receive intensive cancer-directed therapies should be prioritised for early access to vaccination, when appropriate, and for other supportive care interventions when resources are limited.
A multinational registry documented death in 13% of adult patients with cancer and a COVID-19 diagnosis, confirming early concerns that the COVID-19 death rate in adult oncology patients exceeded that in the general population.9, 10 A pooled meta-analysis showed mortality in 25·6% of adult patients with cancer.11 By contrast, data from a large cohort in the UK and a multinational European cohort showed that poor outcomes in these patients appear to be driven by age, sex, and comorbidities, with the European cohort showing no effect of cancer diagnosis when comparing patients with cancer to control individuals without cancer.12, 13 Data are scarce for children with cancer; small national series using different inclusion criteria, severity classifications, and testing strategies suggest disparities between countries with severe or critical infections ranging from 6·6% to 21% of included patients and mortality between zero and 10%.14, 15, 16, 17, 18, 19 The clinical risk factors associated with COVID-19 disease severity among these patients are not well established.20 In addition, as 90% of children and adolescents at risk of developing cancer live in low-income and middle-income countries, evaluation of differences in infection-related treatment and outcomes is necessary to address global inequities in COVID-19 management.21
Recognising that children and adolescents with cancer would constitute a small proportion of a sampled population within any individual institution, the St Jude Global programme of St Jude Children's Research Hospital and the International Society of Paediatric Oncology created the Global COVID-19 Observatory and Resource Center for Childhood Cancer and promoted centralised, hospital-based data collection from the global community. The primary goal was to facilitate collaboration and inform care decisions using real-time data and provide an interactive visualisation and online discussion community.22 In this study, we aimed to describe disease severity among a large global cohort of children and adolescents with cancer or haematopoietic stem-cell transplantation, and identify actionable risk factors associated with disease severity.
Methods
Study design and participants
We did a cohort study with cases reported from 131 institutions in 45 countries (appendix p 2). Launched on April 15, 2020, the St Jude Global and International Society of Paediatric Oncology Global Registry of COVID-19 in Childhood Cancer (GRCCC) is housed in the REDCap application, which was created by Vanderbilt University Medical Center (Nashville, TN, USA) and hosted at St Jude Children's Research Hospital (Memphis, TN, USA). The registry is based on voluntary reporting of laboratory-confirmed cases of SARS-CoV-2 infection in children and adolescents (<19 years) who have a current or past diagnosis of cancer or who have received a haematopoietic stem-cell transplantation. Cases without laboratory confirmation are excluded to reduce confounding by other respiratory infections with similar clinical presentations. The registry has been publicised through conferences and targeted emails through St Jude Global and International Society of Paediatric Oncology networks of providers. There were no centre-specific exclusion criteria.
This study was reviewed by St Jude Children's Research Hospital institutional review board as not involving human participants as no identifiable private information or biospecimens are provided. This study was subject to approvals by local ethics committees according to local policy. Individual investigators were responsible for assuring that participation was compliant with local regulations.
Procedures
De-identified data were requested on a maximum of 80 variables (21 required responses) contained on three forms. No dates were collected beyond the date of data entry; day 0 was defined by the reporter as the day of onset of symptoms or the day of diagnosis of an asymptomatic infection. The reason for SARS-CoV-2 testing was not collected. Data fields include oncological diagnosis, treatment phase, non-oncological comorbid conditions, imaging findings, anatomical location of infection, and COVID-19-directed therapy. Outcomes collected include requirement for higher level of care, respiratory support requirements, laboratory and clinical status of the SARS-CoV-2 infection, vital status, and interruptions in cancer-directed treatment. One form is structured for initial intake, a second for outcomes at 30 days, and an additional form at 60 days for patients without documented clinical or laboratory resolution of infection at the 30-day timepoint. Data collection could be prospective or retrospective, but follow-up is prompted at 30 days after data entry or at intake if the reporter confirms that at least 30 days since symptoms or diagnosis have passed.
Data submitted as of Feb 1, 2021, are included in this report. Each institution that had reported at least a single case as of the time of the data cutoff, was requested to confirm that all institutional cases had been entered in an unbiased fashion as of the time of the final case entry by the institution. All cases corresponding to institutions that did not confirm unbiased data entry were excluded from analysis.
Statistical analysis
Descriptive statistics were used to summarise demographic and clinical characteristics and outcomes. Age in years was categorised as younger than 1 year, 1–9 years, 10–14 years, and 15–18 years based on known associations of age group with the prognosis of children with cancer. Neutropenia (absolute neutrophil count ≤500 cells per mm3) and lymphopenia (absolute lymphocyte count ≤300 cells per mm3) definitions were selected to be globally applicable and clinically relevant. World Bank designations for income groups—high-income countries, upper-middle-income countries, lower-middle-income countries, and low-income countries—were used to describe economic context.
Assuming outcomes such as mortality and admission to a higher level of care would be rare, a primary outcome measure of disease severity was defined to evaluate the association of potential risk factors with adverse outcomes. This is a composite measure incorporating anatomical level of respiratory tract involvement (upper versus lower), respiratory support level, requirement for higher level of care for any reason, and death attributed to COVID-19. To distinguish groups of patients with different support requirements, severity was collapsed into three levels: asymptomatic, mild or moderate, and severe or critical. Disease severity was classified as follows. Critical indicated evidence of organ dysfunction, intubation, or death due to COVID-19. Severe indicated requirement for a higher level of care for any reason or any oxygen support needed greater than regular nasal cannula or facemask, but less than intubation. Moderate indicated upper respiratory tract illness requiring nasal cannula or face mask, lower respiratory tract infection with support no greater than conventional nasal cannula or face mask, no need for higher support levels for any organ system, or no need for higher level of care. Mild indicated respiratory disease limited to upper respiratory tract illness on room air, any symptoms without need for higher support levels for any organ system, or no need for higher level of care. Asymptomatic indicated positive test for SARS-CoV-2, and no symptoms of a respiratory or non-respiratory nature. Another primary outcome was treatment modification, defined as one or more of the following: chemotherapy reduction, chemotherapy withheld, surgery delay, or radiotherapy delay; treatment on plan included treatment as planned or unknown treatment modification status.
Recognising the difficulty of aggregating data across centres using different treatment protocols, a treatment intensity variable was created to summarise anticipated effects of therapy on haematological parameters and organ systems. Treatment was categorised as intense if it would be expected to result in long-lasting myelosuppression and immunosuppression, including treatment for acute myeloid leukaemia, induction or re-induction therapy for acute lymphoblastic leukaemia, acute lymphoblastic lymphoma, or any other lymphoma except for Hodgkin lymphoma, as well as if the patient was less than 30 days from autologous or less than 100 days from allogeneic haematopoietic stem-cell transplantation.
Comparison of proportions between groups was made with χ2 or Fisher's exact tests. The Kruskal-Wallis test was used to compare medians between groups. Univariate logistic regression was used to examine the association between each outcome and patient characteristics. Multivariable logistic regression was used to explore the effect of factors that were significant (p<0·05) in univariate analyses for each outcome. All data analyses were done using SAS software, version 9.4 and R, version 4.0.4.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between April 15, 2020, and Feb 1, 2021, 1520 cases of SARS-CoV-2 infection were submitted to the GRCCC. Of the 1520 cases submitted, 20 were excluded from analyses. 12 were ineligible due to non-malignancy or no haematopoietic stem-cell transplantation, and eight due to inadequate data submission. There were 1500 qualifying episodes entered as of Feb 1, 2021 (appendix p 1), with 1319 having complete 30-day follow-up. Clinical characteristics of the included patients by World Bank country income level are summarised in table 1 . The median age of patients included was 8 years (IQR 4–13), with 42 (2·8%) of 1500 aged younger than 1 year and 250 (16·7%) aged 15–18 years. Most cases of SARS-CoV-2 infection occurred in patients with a diagnosis of acute lymphoblastic lymphoma or acute lymphoblastic leukaemia (737 [49·1%]), followed by extracranial solid tumours (363 [24·2%]). Comorbidities were documented in 256 (17·1%) patients, with receipt of high-dose steroids within the previous 14 days being the most common comorbidity (101 [6·7%]; appendix p 3). Most patients (824 [54·9%]) were reported from upper middle-income countries, with few (five [0·3%]) reported from low-income countries. Low-income countries and lower-middle income countries were pooled together for analyses due to the small amount of data.
Table 1.
Baseline characteristics by World Bank income group
| Overall (n=1500) | Low-income or lower-middle-income country (n=318) | Upper-middle-income country (n=824) | High-income country (n=358) | p value | ||
|---|---|---|---|---|---|---|
| WHO Region | ||||||
| African Region | 41 (2·7%) | 8 (2·5%) | 33 (4·0%) | 0 | .. | |
| Region of the Americas | 847 (56·5%) | 11 (3·5%) | 675 (81·9%) | 161 (45·0%) | .. | |
| Eastern Mediterranean | 211 (14·1%) | 211 (66·4%) | 0 | 0 | .. | |
| European Region | 322 (21·5%) | 10 (3·1%) | 115 (14·0%) | 197 (55·0%) | .. | |
| South-East Asian Region | 63 (4·2%) | 63 (19·8%) | 0 | 0 | .. | |
| Western Pacific Region | 16 (1·1%) | 15 (4·7%) | 1 (0·1%) | 0 | .. | |
| Age, years | 0·041 | |||||
| Median | 8 (4–13) | 8 (4–13) | 7 (4–12) | 9 (4–14) | ||
| Age, years (category) | 0·022 | |||||
| <1 | 42 (2·8%) | 6 (1·9%) | 24 (2·9%) | 12 (3·4%) | .. | |
| 1–9 | 823 (54·9%) | 181 (56·9%) | 471 (57·2%) | 171 (47·8%) | .. | |
| 10–14 | 385 (25·7%) | 69 (21·7%) | 207 (25·1%) | 109 (30·4%) | .. | |
| 15–18 | 250 (16·7%) | 62 (19·5%) | 122 (14·8%) | 66 (18·4%) | .. | |
| Sex | 0·17 | |||||
| Male | 891 (59·4%) | 199 (62·6%) | 494 (60·0%) | 198 (55·3%) | .. | |
| Female | 607 (40·5%) | 119 (37·4%) | 330 (40·0%) | 158 (44·1%) | .. | |
| Other | 2 (0·1%) | 0 | 0 | 2 (0·6%) | .. | |
| Cancer type | <0·0001 | |||||
| Acute lymphoblastic leukaemia or acute lymphoblastic lymphoma | 737 (49·1%) | 164 (51·6%) | 413 (50·1%) | 160 (44·7%) | .. | |
| Other haematological malignancies | 266 (17·7%) | 75 (23·6%) | 141 (17·1%) | 50 (14·0%) | .. | |
| Solid tumours | 363 (24·2%) | 68 (21·4%) | 204 (24·8%) | 91 (25·4%) | .. | |
| CNS tumours | 126 (8·4%) | 11 (3·5%) | 64 (7·8%) | 51 (14·2%) | .. | |
| Post-haematopoietic stem-cell transplantation, non-malignancy | 8 (0·5%) | 0 | 2 (0·2%) | 6 (1·7%) | .. | |
| Treatment type | <0·0001 | |||||
| Cancer-directed therapy | 1243 (82·9%) | 258 (81·1%) | 719 (87·3%) | 266 (74·3%) | .. | |
| Palliative therapy | 53 (3·5%) | 14 (4·4%) | 32 (3·9%) | 7 (2·0%) | .. | |
| Treatment completed | 127 (8·5%) | 17 (5·3%) | 53 (6·4%) | 57 (15·9%) | .. | |
| No active treatment | 69 (4·6%) | 28 (8·8%) | 13 (1·6%) | 28 (7·8%) | .. | |
| Unknown | 8 (0·5%) | 1 (0·3%) | 7 (0·8%) | 0 | .. | |
| Time since last chemotherapy, days | 0·58 | |||||
| ≤30 | 1171/1203 (97·3%) | 246/255 (96·5%) | 690/708 (97·5%) | 235/240 (97·9%) | .. | |
| >30 | 32/1203 (2·7%) | 9/255 (3·5%) | 18/708 (2·5%) | 5/240 (2·1%) | .. | |
| Received haematopoietic stem-cell transplantation | <0·0001 | |||||
| Yes | 81 (5·4%) | 9 (2·8%) | 31 (3·8%) | 41 (11·5%) | .. | |
| No | 1414 (94·3%) | 309 (97·2%) | 789 (95·8%) | 316 (88·3%) | .. | |
| Unknown | 5 (0·3%) | 0 | 4 (0·5%) | 1 (0·3%) | .. | |
| Time since transplantation, days | 0·51 | |||||
| <30 | 6/81 (7·4%) | 0 | 4/31 (12·9%) | 2/41 (4·9%) | .. | |
| 31–99 | 13/81 (16·0%) | 1/9 (11·1%) | 4/31 (12·9%) | 8/41 (19·5%) | .. | |
| 100–300 | 20/81 (24·7%) | 1/9 (11·1%) | 9/31 (29·0%) | 10/41 (24·4%) | .. | |
| >300 | 31/81 (38·3%) | 6/9 (66·7%) | 9/31 (29·0%) | 16/41 (39·0%) | .. | |
| Unknown or missing* | 11/81 (13·6%) | 1/9 (11·1%) | 5/31 (16·1%) | 5/41 (12·2%) | .. | |
| Received radiotherapy | <0·0001 | |||||
| Yes | 156/1453 (10·7%) | 25/279 (9·0%) | 73/819 (8·9%) | 58/355 (16·3%) | .. | |
| No | 1249/1453 (86·0%) | 253/279 (90·7%) | 730/819 (89·1%) | 266/355 (74·9%) | .. | |
| Unknown | 48/1453 (3·3%) | 1/279 (0·4%) | 16/819 (2·0%) | 31/355 (8·7%) | .. | |
| Absolute neutrophil count, cells per mm3 | <0·0001 | |||||
| ≤500 | 375/1212 (30·9%) | 109/243 (44·9%) | 202/716 (28·2%) | 64/253 (25·3%) | .. | |
| >500 | 837/1212 (69·1%) | 134/243 (55·1%) | 514/716 (71·8%) | 189/253 (74·7%) | .. | |
| Absolute lymphocyte count, cells per mm3 | <0·0001 | |||||
| ≤300 | 273/1188 (23·0%) | 82/237 (34·6%) | 128/702 (18·2%) | 63/249 (25·3%) | .. | |
| >300 | 915/1188 (77·0%) | 155/237 (65·4%) | 574/702 (81·8%) | 186/249 (74·7%) | .. | |
| Comorbidities† | 0·71 | |||||
| At least one comorbidity | 256 (17·1%) | 51 (16·0%) | 150 (18·2%) | 55 (15·4%) | .. | |
| None | 1173 (78·2%) | 259 (81·4%) | 659 (80·0%) | 255 (71·2%) | .. | |
| Unknown | 71 (4·7%) | 8 (2·5%) | 15 (1·8%) | 48 (13·4%) | .. | |
| Intensive treatment | <0·0001 | |||||
| Yes | 478 (31·9%) | 104 (32·7%) | 301 (36·5%) | 73 (20·4%) | .. | |
| No | 1022 (68·1%) | 214 (67·3%) | 523 (63·5%) | 285 (79·6%) | .. | |
p values were calculated using the χ2 test, Fisher's exact test, or the Kruskal-Wallis test.
Missing refers to patients who did not answer the question regarding time since transplantation.
Comorbidities included history of high-dose steroid within 14 days before diagnosis or illness, pre-existing pulmonary disease, pre-existing cardiac insufficiency, and a free text option for other conditions (eg, obesity, graft-versus-host disease, trisomy 21, pre-existing renal disease, among others).
Most patients (1243 [82·9%] of 1500) were receiving cancer-directed therapy at the time of SARS-CoV-2 infection. Of the 1203 respondents providing a response, most patients (1171 [97·3%]) had received chemotherapy within 30 days of the episode. 81 (5·4%) patients were haematopoietic stem-cell transplantation recipients; in these patients, most infections (31 [42·5%] of 73) occurred more than 300 days post-transplant (table 1). Age, primary diagnosis, treatment type, haematopoietic stem-cell transplantation receipt, radiotherapy, absolute lymphocyte count at SARS-CoV-2 diagnosis (≤300 cells per mm3 vs >300 cells per mm3) absolute neutrophil count at SARS-CoV-2 diagnosis (≤500 cells per mm3 vs >500 cells per mm3), and treatment intensity were all significantly associated with World Bank income group, whereas sex, time since last chemotherapy, time since transplant, and having at least one comorbidity were not (table 1).
Most diagnostic tests were nasopharyngeal swabs (1212 [80·8%] of 1500), followed by nasal swabs (262 [17·5%]) and oropharyngeal swabs (126 [8·4%]; appendix p 4). 854 (56·9%) of 1500 patients were symptomatic at the time of COVID-19 testing; median time from onset of symptoms to testing was 2 days (IQR 1–4) in patients for whom this information was available (n=703). The most commonly reported symptoms at presentation were fever (619 [41·3%]) and cough (356 [23·7%]; appendix p 5).
Of 1319 patients with 30-day follow-up data, 889 (67·4%) were hospitalised, and 231 (17·5%) required admission or transfer to a higher level of care (table 2 ). When considering non-respiratory pathology, the most common single organ system with documented dysfunction was cardiac (33 [2·5%]), and 43 (3·3%) patients had multiorgan dysfunction (appendix p 7). 386 (29·3%) patients received COVID-19 directed therapy, with 275 (71·2%) of those receiving azithromycin and 197 (51·0%) steroids. Antivirals were rarely used, and remdesivir was administered in 65 (16·8%) patients receiving therapy (appendix p 6). There were 83 (6·3%) deaths in this cohort and 60·2% (50 of 83) of those deaths (3·8% of cohort) were attributed to COVID-19. Median time to death attributed to COVID-19 was 8 days (IQR 3–14; table 2).
Table 2.
Patient outcomes by World Bank income group
| Overall (n=1319) | Low-income or lower-middle-income country (n=296) | Upper-middle-income country (n=709) | High-income country (n=314) | ||
|---|---|---|---|---|---|
| Disease severity | |||||
| Asymptomatic | 456/1301 (35·0%) | 87/288 (30·2%) | 233/702 (33·2%) | 136/311 (43·7%) | |
| Mild or moderate | 586/1301 (45·0%) | 81/288 (28·1%) | 353/702 (50·3%) | 152/311 (48·9%) | |
| Severe or critical | 259/1301 (19·9%) | 120/288 (41·7%) | 116/702 (16·5%) | 23/311 (7·4%) | |
| Treatment modification* | |||||
| Treatment modified | 609/1092 (55·8%) | 163/240 (67·9%) | 299/622 (48·1%) | 147/230 (63·9%) | |
| Chemotherapy reduced | 80/609 (13·1%) | 34/163 (20·9%) | 41/299 (13·7%) | 5/147 (3·4%) | |
| Chemotherapy withheld | 487/609 (80·0%) | 125/163 (76·7%) | 234/299 (78·3%) | 128/147 (87·1%) | |
| Radiotherapy delayed | 25/609 (4·1%) | 3/163 (1·8%) | 14/299 (4·7%) | 8/147 (5·4%) | |
| Surgery delayed | 41/609 (6·7%) | 10/163 (6·1%) | 20/299 (6·7%) | 11/147 (7·5%) | |
| Treatment on plan (includes unknown) | 483/1092 (44·2%) | 77/240 (32·1%) | 323/622 (51·9%) | 83/230 (36·1%) | |
| Death | |||||
| Due to COVID-19 | 50/83 (60·2%) | 20/33 (60·6%) | 26/43 (60·5%) | 4/7 (57·1%) | |
| Median time to death, days | 8 (3–14) | 9 (4–13) | 7 (3–13) | 20 (11–25) | |
| Due to other causes | 33/83 (39·8%) | 13/33 (39·4%) | 17/43 (39·5%) | 3/7 (42·9%) | |
| Median time to death, days | 16 (8–29) | 16 (8–29) | 10 (4–21) | 26 (24–32) | |
| Hospitalisation | |||||
| Hospitalised, ward status | 658 (49·9%) | 61 (20·6%) | 472 (66·6%) | 125 (39·8%) | |
| Hospitalised, higher level of care† | 231 (17·5%) | 117 (39·5%) | 95 (13·4%) | 19 (6·1%) | |
| Not hospitalised | 430 (32·6%) | 118 (39·9%) | 142 (20·0%) | 170 (54·1%) | |
Data are n, n/N (%), n (%), or median (IQR).
Treatment modification corresponds to a “select all that apply” question in the survey, thus sum of count numbers could exceed total number.
Higher level of care included intensive care unit, intermediate care unit, high dependency unit, and emergency room.
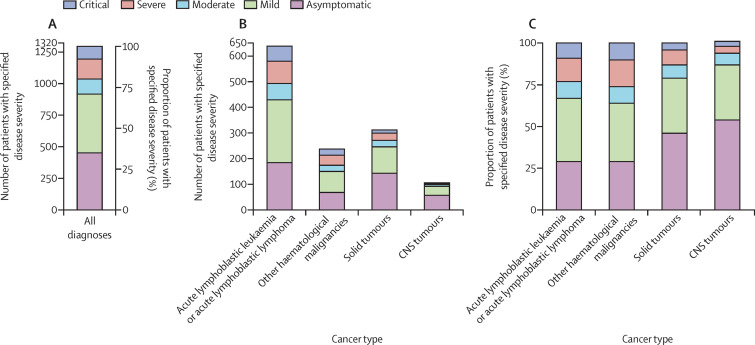
Most patients with follow-up data either remained asymptomatic (456 [35·0%] of 1301) or had mild or moderate infection (586 [45·0%] of 1301); however, a substantial percentage (259 [19·9%] of 1301) developed either severe or critical COVID-19-related illness (table 2). Chemotherapy was withheld in 44·6% (487 of 1092) of patients receiving active therapy and some modification to therapy occurred in 609 (55·8%) patients on active therapy. Treatment modifications were least common in patients from upper-middle-income countries compared with other income groups (p<0·0001), where treatment according to plan was more common than for other income groups (table 2). A higher proportion of severe or critical outcomes was seen in patients in low-income and lower-middle-income countries (120 [41·7%] of 288) relative to patients in other income groups. 330 (31·3%) of 1055 (the number of patients for whom data were available) infections were in patients with an absolute neutrophil count of 500 or less cells per mm3, who then made up 124 (50·2%) of 247 of those with severe or critical illness. Similarly, although patients with an absolute lymphocyte count of 300 or less cells per mm3 contributed only 240 (23·1%) of 1040 (the number of patients for whom data were available) infections in the total sample, this group contributed 103 (42·7%) of 241 infections in the severe or critical group (table 3 ). Patients with haematological malignancies contributed the largest proportion (208 [80·3%] of 259) of either severe or critical disease by diagnosis type (figure ). In patients with acute lymphoblastic leukaemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy, relapse or refractory therapy, and the maintenance or continuation phase of therapy (table 3).
Table 3.
Baseline characteristics by disease severity
| Asymptomatic (n=456) | Mild or moderate (n=586) | Severe or critical (n=259) | Total (n=1301) | p value | ||
|---|---|---|---|---|---|---|
| Income group* | <0·0001 | |||||
| Low-income or lower-middle-income country | 87/288 (30·2%) | 81/288 (28·1%) | 120/288 (41·7%) | 288 | .. | |
| Upper-middle-income country | 233/702 (33·2%) | 353/702 (50·3%) | 116/702 (16·5%) | 702 | .. | |
| High-income country | 136/311 (43·7%) | 152/311 (48·9%) | 23/311 (7·4%) | 311 | .. | |
| Age, years* | 0·013 | |||||
| <1 | 13/31 (41·9%) | 15/31 (48·4%) | 3/31 (9·7%) | 31 | .. | |
| 1–9 | 264/707 (37·3%) | 315/707 (44·6%) | 128/707 (18·1%) | 707 | .. | |
| 10–14 | 120/336 (35·7%) | 150/336 (44·6%) | 66/336 (19·6%) | 336 | .. | |
| 15–18 | 59/227 (26·0%) | 106/227 (46·7%) | 62/227 (27·3%) | 227 | .. | |
| Sex* | 0·32 | |||||
| Male | 285/778 (36·6%) | 345/778 (44·3%) | 148/778 (19·0%) | 778 | .. | |
| Female | 171/521 (32·8%) | 239/521 (45·9%) | 111/521 (21·3%) | 521 | .. | |
| Other† | 0 | 2/2 (100·0%) | 0 | 2 | .. | |
| Cancer type | <0·0001 | |||||
| Acute lymphoblastic leukaemia or acute lymphoblastic lymphoma | 184 (40·4%) | 309 (52·7%) | 145 (56·0%) | 638 (49·0%) | .. | |
| Other haematological malignancies | 68 (14·9%) | 106 (18·1%) | 63 (24·3%) | 237 (18·2%) | .. | |
| Solid tumours | 143 (31·4%) | 128 (21·8%) | 41 (15·8%) | 312 (24·0%) | .. | |
| CNS tumours | 57 (12·5%) | 42 (7·2%) | 7 (2·7%) | 106 (8·1%) | .. | |
| Post-haematopoietic stem-cell transplantation, non-malignancy† | 4 (0·9%) | 1 (0·2%) | 3 (1·2%) | 8 (0·6%) | .. | |
| Phase of treatment*‡ | 0·0001 | |||||
| Induction | 37/168 (22·0%) | 80/168 (47·6%) | 51/168 (30·4%) | 168 | .. | |
| Consolidation | 48/106 (45·3%) | 46/106 (43·4%) | 12/106 (11·3%) | 106 | .. | |
| Reinduction or interim maintenance | 24/62 (38·7%) | 27/62 (43·5%) | 11/62 (17·7%) | 62 | .. | |
| Maintenance or continuation | 44/172 (25·6%) | 95/172 (55·2%) | 33/172 (19·2%) | 172 | .. | |
| Relapse or refractory therapy | 15/57 (26·3%) | 25/57 (43·9%) | 17/57 (29·8%) | 57 | .. | |
| Immunotherapy or cell therapy† | 2/3 (66·7%) | 1/3 (33·3%) | 0 | 3 | .. | |
| Received haematopoietic stem-cell transplantation* | 0·58 | |||||
| Yes | 30/76 (39·5%) | 30/76 (39·5%) | 16/76 (21·1%) | 76 | .. | |
| No | 425/1224 (34·7%) | 556/1224 (45·4%) | 243/1224 (19·9%) | 1224 | .. | |
| Unknown† | 1/1 (100·0%) | 0 | 0 | 1 | .. | |
| Time since transplant, days* | 0·61 | |||||
| <30 | 1/6 (16·7%) | 3/6 (50·0%) | 2/6 (33·3%) | 6 | .. | |
| 31–99 | 5/13 (38·5%) | 3/13 (23·1%) | 5/13 (38·5%) | 13 | .. | |
| 100–300 | 5/17 (29·4%) | 9/17 (52·9%) | 3/17 (17·6%) | 17 | .. | |
| >300 | 12/30 (40·0%) | 12/30 (40·0%) | 6/30 (20·0%) | 30 | .. | |
| Unknown† | 1/3 (33·3%) | 2/3 (66·7%) | 0 | 3 | .. | |
| Absolute neutrophil count, cells per mm3 | <0·0001 | |||||
| ≤500 | 42/299 (14·0%) | 164/509 (32·2%) | 124/247 (50·2%) | 330/1055 (31·3%) | .. | |
| >500 | 257/299 (86·0%) | 345/509 (67·8%) | 123/247 (49·8%) | 725/1055 (68·7%) | .. | |
| Absolute lymphocyte count, cells per mm3 | <0·0001 | |||||
| ≤300 | 30/297 (10·1%) | 107/502 (21·3%) | 103/241 (42·7%) | 240/1040 (23·1%) | .. | |
| >300 | 267/297 (89·9%) | 395/502 (78·7%) | 138/241 (57·3%) | 800/1040 (76·9%) | .. | |
| Comorbidities* | 0·0007 | |||||
| At least one comorbidity | 376/1016 (37·0%) | 449/1016 (44·2%) | 191/1016 (18·8%) | 1016 | .. | |
| None | 55/223 (24·7%) | 108/223 (48·4%) | 60/223 (26·9%) | 223 | .. | |
| Unknown† | 25/62 (40·3%) | 29/62 (46·8%) | 8/62 (12·9%) | 62 | .. | |
| Intensive treatment* | <0·0001 | |||||
| Yes | 105/422 (24·9%) | 183/422 (43·4%) | 134/422 (31·8%) | 422 | .. | |
| No | 351/879 (39·9%) | 403/879 (45·8%) | 125/879 (14·2%) | 879 | .. | |
p values were calculated using the χ2 test or Fisher's exact test.
Data based on row totals rather than column totals.
Excluded from significance testing.
Phase of treatment refers to acute lymphoblastic leukaemia or acute lymphoblastic lymphoma.
Figure.
Disease severity by cancer type
(A) Number and proportion of patients with specified disease severity in all diagnoses. (B) Number of patients with specified disease severity by cancer type. (C) Proportion of patients with specified disease severity by cancer type.
Income group, cancer type, age, absolute lymphocyte count, absolute neutrophil count, presence of comorbidities, and treatment intensity were significantly associated with severe or critical illness in univariate logistic regression analyses, whereas sex was not. In multivariable analysis, low-income or lower-middle-income (OR 5·8 [95% CI 3·8–8·8]; p<0·0001) and upper-middle-income group (1·6 [1·2–2·2]; p=0·0024), age 15–18 years (1·6 [1·1–2·2]; p=0·013), absolute lymphocyte count of 300 or less cells per mm3 (2·5 [1·8–3·4]; p<0·0001), absolute neutrophil count of 500 or less cells per mm3 (1·8 [1·3–2·4]; p=0·0001), and intensive treatment (1·8 [1·3–2·3]; p=0·0005) were associated with increased severity of infection, whereas other variables were not (table 4 ).
Table 4.
Results of univariate and multivariable analysis for disease severity
|
Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|
| p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | ||
| Income group | <0·0001 | .. | <0·0001 | .. | |
| Low-income or lower-middle-income country | .. | 3·5 (2·5–4·9) | <0·0001 | 5·8 (3·8–8·8) | |
| Upper-middle-income country | .. | 1·6 (1·3–2·1) | 0·0024 | 1·6 (1·2–2·2) | |
| High-income country | .. | 1 (ref) | .. | 1 (ref) | |
| Cancer type | <0·0001 | .. | 0·14 | .. | |
| Acute lymphoblastic leukaemia or acute lymphoblastic lymphoma | .. | 2·0 (1·6–2·6) | 0·086 | 1·4 (1·0–2·0) | |
| Other haematological malignancies | .. | 2·3 (1·6–3·1) | 0·55 | 1·2 (0·8–1·9) | |
| Solid tumours | .. | 1 (ref) | .. | 1 (ref) | |
| CNS tumours | .. | 0·7 (0·5–1·0) | 0·53 | 0·9 (0·5–1·5) | |
| Age, years | 0·0015 | .. | 0·046 | .. | |
| <1 | .. | 0·7 (0·4–1·4) | 0·99 | 1·0 (0·5–2·4) | |
| 1–9 | .. | 1 (ref) | .. | 1 (ref) | |
| 10–14 | .. | 1·1 (0·8–1·4) | 0·53 | 0·9 (0·7–1·3) | |
| 15–18 | .. | 1·7 (1·3–2·3) | 0·013 | 1·6 (1·1–2·2) | |
| Sex | 0·14 | .. | .. | .. | |
| Male | .. | 1 (ref) | .. | .. | |
| Female | .. | 1·2 (1·0–1·4) | .. | .. | |
| Absolute lymphocyte count, cells per mm3 | <0·0001 | .. | <0·0001 | .. | |
| ≤300 | .. | 3·6 (2·7–4·8) | <0·0001 | 2·5 (1·8–3·4) | |
| >300 | .. | 1 (ref) | .. | 1 (ref) | |
| Absolute neutrophil count, cells per mm3 | <0·0001 | .. | 0·0001 | .. | |
| ≤500 | .. | 3·2 (2·5–4·2) | 0·0001 | 1·8 (1·3–2·4) | |
| >500 | .. | 1 (ref) | .. | 1 (ref) | |
| Comorbidities | 0·0001 | .. | 0·074 | .. | |
| Yes (one or more) | .. | 1·7 (1·3–2·2) | 0·074 | 1·4 (1·0–1·9) | |
| None | .. | 1 (ref) | .. | 1 (ref) | |
| Intensive treatment | <0·0001 | .. | 0·0005 | .. | |
| Yes | .. | 2·3 (1·9–2·9) | 0·0005 | 1·8 (1·3–2·3) | |
| No | .. | 1 (ref) | .. | 1 (ref) | |
Data shown for 1301 participants.
To investigate the effect of COVID-19 diagnosis on cancer treatment, univariate and multivariable logistic regression analyses were done. Income group, cancer type, absolute lymphocyte count, presence of comorbidities, and presence of COVID-19 symptoms at presentation, were significantly associated with treatment modification in univariate logistic regression analyses, whereas other variables were not (table 5 ). In multivariable analysis, treatment modification was associated with the upper-middle income group (OR 0·5 [95% CI 0·3–0·7]; p=0·0004), other haematological malignancies (0·5 [0·3–0·8]; p=0·0088), the presence of COVID-19 symptoms (1·8 [1·3–2·4]; p=0·0002), and the presence of comorbidities (1·6 [1·1–2·3]; p=0·020; table 5).
Table 5.
Results of univariate and multivariable analysis for treatment modification
|
Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|
| p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | ||
| Income group | <0·0001 | .. | <0·0001 | .. | |
| Low-income or lower-middle-income country | .. | 1·2 (0·8–1·8) | 0·80 | 1·0 (0·6–1·6) | |
| Upper-middle-income country | .. | 0·5 (0·4–0·7) | 0·0004 | 0·5 (0·3–0·7) | |
| High-income country | .. | 1 (ref) | .. | 1 (ref) | |
| Cancer type | <0·0001 | .. | <0·0001 | .. | |
| Acute lymphoblastic leukaemia or acute lymphoblastic lymphoma | .. | 1·4 (1·1–1·9) | 0·089 | 1·3 (0·9–1·9) | |
| Other haematological malignancies | .. | 0·7 (0·5–1·0) | 0·0088 | 0·5 (0·3–0·8) | |
| Solid tumours | .. | 1 (ref) | .. | 1 (ref) | |
| CNS tumours | .. | 0·9 (0·5–1·5) | 0·069 | 0·5 (0·3–1·0) | |
| Age, years | 0·85 | .. | .. | .. | |
| <1 | .. | 1·0 (0·4–2·1) | .. | .. | |
| 1–9 | .. | 1 (ref) | .. | .. | |
| 10–14 | .. | 1·0 (0·8–1·4) | .. | .. | |
| 15–18 | .. | 1·2 (0·8–1·6) | .. | .. | |
| Sex | 0·86 | .. | .. | .. | |
| Male | .. | 1 (ref) | .. | .. | |
| Female | .. | 1·0 (0·8–1·2) | .. | .. | |
| Absolute lymphocyte count, cells per mm3 | 0·049 | .. | 0·58 | .. | |
| ≤300 | .. | 1·4 (1·0–1·9) | 0·58 | 1·1 (0·8–1·6) | |
| >300 | .. | 1 (ref) | .. | 1 (ref) | |
| Absolute neutrophil count, cells per mm3 | 0·23 | .. | .. | .. | |
| ≤500 | .. | 1·2 (0·9–1·6) | .. | .. | |
| >500 | .. | 1 (ref) | .. | .. | |
| Comorbidities | 0·016 | .. | 0·020 | .. | |
| Yes (one or more) | .. | 1·5 (1·1–2·1) | 0·020 | 1·6 (1·1–2·3) | |
| None | .. | 1 (ref) | .. | 1 (ref) | |
| Intensive treatment | 0·18 | .. | .. | .. | |
| Yes | .. | 0·8 (0·7–1·1) | .. | .. | |
| No | .. | 1 (ref) | .. | .. | |
| COVID-19 symptoms | 0·0007 | .. | 0·0002 | .. | |
| Yes (one or more) | .. | 1·5 (1·2–1·9) | 0·0002 | 1·8 (1·3–2·4) | |
| No symptoms | .. | 1 (ref) | .. | 1 (ref) | |
Data shown for 1092 participants.
Discussion
To our knowledge, this is the first international study and largest cohort to date to report COVID-19 outcomes for children and adolescents with cancer and those who have received a haematopoietic stem-cell transplantation. We have shown that although infection outcomes are generally favourable, severe disease can occur, particularly in patients receiving intensive chemotherapy, and patients with lymphopenia and neutropenia. Mortality is lower than reported in adults with cancer and SARS-CoV-2 infection; however, the proportion within our cohort is still more than four times that reported in the general paediatric population. This report provides important data to support frontline clinicians making data-driven decisions about COVID-19 management, governments making prioritisation decisions, and health-care societies and organisations developing evidence-based guidelines.
In terms of outcomes observed, although a fifth of children with cancer developed severe or critical disease after SARS-CoV-2 infection, most patients recovered without advanced support needs. The large proportion of asymptomatic patients underscores the need for aggressive infection control measures as these patients can pose infection risks to other patients and health-care providers. The GRCCC is restricted to laboratory-confirmed cases, so deaths could have been underestimated, particularly in low-income countries and lower-middle-income countries where testing capacity is limited by availability of testing supplies and infrastructure. Death related to COVID-19 was reported in 3·8% (50 of 1319) of all patients with registry follow-up data. This result is considerably lower than the 13–28% reported in adults with cancer; however, it is disproportionately high compared with 0·01–0·70% mortality in cohorts of general paediatric patients.9, 13, 23, 24
Given the urgency of a new infectious threat, we also developed an innovative dissemination mechanism for global data processing and reporting in a rare disease population. Quantifying the disease burden associated with SARS-CoV-2 in childhood cancer would take years using a standard population-based cancer registry approach. Additionally, with limited clinical information available in most population-based registries, key risk factors would be missed. Using a hospital-based approach, backed by strong leadership within the paediatric cancer community, we were able to coordinate a multi-institutional collaboration and provide detailed, near real-time data summaries within 6 months from the start of the pandemic as a free and interactive data visualisation.
To identify outcome differences, we developed an objective measure of disease severity. Previously proposed schema included subjective data measurements, such as shortness of breath combined with findings from imaging, which might not always be assessed.1 Additionally, other criteria might require measures such as oxygen saturation, which are not reliably available globally. Our clinical GRCCC classification system closely resembles the WHO Clinical Progression Scale, and was designed to be feasible across multiple institutions and health-care delivery settings, as it relied on a large and heterogeneous group of voluntary reporters who received no training in data preparation.25 Using this measure, we observed a larger proportion of severe or critical illness compared with case series from high-income countries and cohorts of paediatric oncology patients.18, 26 This result might be due to the larger contribution of patients from low-income countries and lower-middle-income countries to our registry, as we observed an association between the two income groups combined and severe or critical illness.
Using the GRCCC severity score classification, we identified risk factors associated with the development of severe or critical disease. In particular, being aged 15–18 years, lymphopenia (absolute lymphocyte count ≤300 cells per mm3), neutropenia (absolute neutrophil count ≤500 cells per mm3), and intensive treatment were identified as significant on multivariable analyses. However, the effect of older age on outcomes needs further study due to the study limitations discussed below. Several variables associated with immune status were also independently associated with severe disease in our model. Our finding that lymphopenia was associated with more severe disease replicates findings for respiratory syncytial virus infections in paediatric patients with cancer, even though lymphopenia has not been associated with more severe disease in non-SARS-CoV-2 coronaviruses.27, 28 Neutropenia showed a weaker association but remains a clinically relevant marker for immunocompromise associated with infection risk. Treatment intensity, a modifiable non-patient associated variable, was also independently associated with disease severity and could inform practice. The observed relationship of severe illness with low-income and lower-middle income countries could be related to differences in supportive care infrastructure and delays in presentation. In addition, health-care system disruptions that affect all aspects of care delivery might be more pronounced in low-income and lower-middle-income countries. Lastly, although male sex is associated with more severe COVID-19 disease in adults, there was no effect of sex upon disease severity in our cohort.
From a clinical perspective, we have also identified how treatment practices appear to differ globally and do not correlate with the risk factors associated with severe illness. In our cohort, 55·8% (609 of 1092) of patients were reported to have an interruption in cancer-directed therapy. Our finding of frequent interruption to intended therapy agrees with a large multinational survey; however, the finding that treatment modifications are less frequent in upper-middle-income countries differs from that report.29 Instead, we observed that disruption in care was bimodal, occurring more frequently in high-income countries and low-income countries and lower-middle-income countries. Treatment modifications could occur for various reasons; a Latin American survey showed interruptions in supply of blood products and chemotherapy during the pandemic, which would result in treatment modification.30 Our study design did not allow us to assess the drivers of interruption or its effect on overall survival or other important metrics, which merit further study. However, as absolute lymphocyte count, absolute neutrophil count, and age are not significantly associated with treatment disruption in our models, our data suggest there might be opportunity to improve outcomes by tailoring treatment decisions to identified risk factors when provider decision making, rather than health system limitations, are driving disruptions.
Finally, there are several potential limitations to consider when interpreting our results. First, our findings used a hospital-based registry approach, which prohibits us from making population-level conclusions. It is possible that recall bias might have led to the inclusion of more severe cases. To mitigate this bias, we requested that all reporters attest that they submitted all institutional cases meeting inclusion criteria. Although we are not aware of a bias towards sicker patients or more advanced disease, we cannot confirm that contributing institutions are representative. Reporting cases to the GRCCC was an additional task for an overwhelmed workforce, so reporting institutions might have been ones with greater human resources and support. Second, our requirement for laboratory confirmation of SARS-CoV-2 infection probably contributed to under-representation by lower-income settings where there might have been poorer availability of diagnostic tests. Specifically, participation was rare from the African, South-East Asian, and Western Pacific Regions, and low-income countries in general. Third, our severity scores might include some misclassifications. However, as the severe and critical categories rely on service delivery interventions only, our aggregate analyses should not be affected. Fourth, due to global data privacy rules, we were unable to collect protected health information, including all elements relating to dates. As a result, we cannot comment on time trends in disease severity or treatments delivered. Fifth, due to the inability to capture race or ethnicity consistently across a global cohort, we were unable to ascertain the effects of these factors on infection severity. Race and ethnicity represent an important area for continued investigation as such factors are associated with disease severity in other cohorts. Finally, the data fields we used at the onset of the pandemic did not adequately capture multisystem inflammatory syndrome in children. These data fields were updated on Nov 23, 2020, to incorporate multisystem inflammatory syndrome in children and we expect that continued data field updates will yield additional information in subsequent analyses.
Although vaccination and treatment options are developing, disparities in vaccine access, vaccine hesitancy, and the evolution of viral variants mean that the pandemic will continue to affect health care for the foreseeable future. Several of these factors will also increase the burden in countries with limited resources, in which disease severity is already the most concerning. For these reasons, we plan to continue the GRCCC programme to inform global treatment and resource allocation decision making with current and relevant data.
Data sharing
Aggregate de-identified data are available as part of a public data visualisation in the Global COVID-19 Observatory and Resource Center for Childhood Cancer. Individual de-identified patient data with site identifiers removed and geographical region of patient residence limited to country will be made available upon request beginning 6 months after Article publication. Individuals can email the corresponding author with enquiries regarding the dataset.
Declaration of interests
EB participates on the data safety monitoring board or advisory board for Novartis and Bayer and reports institutional clinical trial support from Roche and Bristol Myers Squibb. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work has been supported in part by the American Lebanese Syrian Associated Charities and Cancer Center Support grant (number CA21765) from the National Cancer Institute through the National Institutes of Health. We thank Whitney Foster, Meghana Avula, Janet Middlekauff, Paula Naidu, Susanne Wollaert, Olga Kozhaeva, Tessie Laub, and Andrew Pappas for their technical support.
Contributors
SM provided an overall lead to the registry development, implementation, and analysis, with the operational support of MRH. SM, NB, GLC, VMS, MAC, MD, and CRG designed the study, with additional input from DCM, MS, EB, and KPJ. KPJ, GLC, EB, MS, RD, and LH supported and oversaw the dissemination of the registry through the International Society of Paediatric Oncology. NB, PF, SJ, MM, CL, and AA supported and oversaw the dissemination of the registry through the St Jude Global Alliance. LF, HMT, YC, YV, and MD oversaw data collection. YC, YV, MD, and RR were responsible for all the data summaries and analyses, and data visualisations, with input from SM and NB. SM wrote the first draft of the manuscript with input from NB and CRG. All authors reviewed and approved the final manuscript. YC and YV verified the raw data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
Global Registry of COVID-19 in Childhood Cancer:
A Juan Ribelles, Adriana Balduzzi, Alaa Elhaddad, Alejandra Casanovas, Alejandra Garcia Velazquez, Aliaksandra Laptsevich, Alicia Chang, Alessandra Lamenha F. Sampaio, Almudena González Prieto, Alvaro Lassaletta, Amaranto Suarez M, Ana Patricia Alcasabas, Anca Colita, Andres Morales La Madrid, Angélica Samudio, Annalisa Tondo, Antonella Colombini, Antonis Kattamis, N Araceli Lopez Facundo, Arpita Bhattacharyya, Aurélia Alimi, Aurélie Phulpin, Barbora Vakrmanova, Basak A Aksoy, Benoit Brethon, Jator Brian Kobuin, Carla Nolasco Monteiro, Catherine Paillard, Catherine Vezina, Bozkurt Ceyhun, Cristiana Hentea, Cristina Meazza, Daniel Ortiz-Morales, Roque Daniel Solorzano, Daniela Arce Cabrera, Daniele Zama, Debjani Ghosh, Diana Ramírez-Rivera, Doris A Calle Jara, Dragana Janic, Elianneth Rey Helo, Elodie Gouache, Enmanuel Guerrero Quiroz, Enrique Lopez, Eric Thebault, Essy Maradiegue, Eva de Berranger, Fatma S E Ebeid, Federica Galaverna, Federico Antillon-Klussmann, Felipe Espinoza Chacur, Fernando Daniel Negro, Francesca Carraro, Francesca Compagno, Francisco Barriga, Gabriela Tamayo Pedraza, Gissela Sanchez Fernandez, Gita Naidu, Gülnur Tokuc, Hamidah Alias, Hannah Grace B Segocio, Houda Boudiaf, Imelda Asetre Luna, Iris Maia, Itziar Astigarraga, Ivan Maza, Jacqueline E Montoya Vásquez, Janez Jazbec, Jelena Lazic, Jeniffer Beck Dean, Jeremie Rouger-Gaudichon, Johanny Carolina Contreras González, Jorge Huerta Aragonés, José L Fuster, Juan Quintana, Julia Palma, Karel Svojgr, Karina Quintero, Karolina Malic Tudor, Kleopatra Georgantzi, Kris Ann P Schultz, Laura Ureña Horno, Lidia Fraquelli, Linda Meneghello, Lobna Shalaby, Lola L Macias Mora, Lorna A Renner, Luciana Nunes Silva, Luisa Sisinni, Mahmoud Hammad, M Fernández Sanmartín, C Marcela Zubieta A, María Constanza Drozdowski, Maria Kourti, Marcela María Palladino, Maria R Miranda Madrazo, Marilyne Poiree, Marina Popova, Mario Melgar, Marta Baragaño, Martha J Avilés-Robles, Massimo Provenzi, Mecneide Mendes Lins, Mehmet Fatih Orhan, Milena Villarroel, Mónica Jerónimo, Mónica Varas Palma, Muhammad Rafie Raza, Mulindwa M Justin, Najma Shaheen, Nerea Domínguez-Pinilla, Nicholas S Whipple, Nicolas André, Ondrej Hrusak, Pablo Velasco Puyó, Pamela Zacasa Vargas, Paola Olate Mellado, Pascale Yola Gassant, Paulina Diaz Romero, Raffaella De Santis, Rejin Kebudi, Riza Boranbayeva, Roberto Vasquez, Romel A. Segura, Roy Enrique Rosado, Sandra Gómez, Sandra Raimbault, Sanjeeva Gunasekera, Sara M Makkeyah, Sema Buyukkapu Bay, Sergio M Gómez, Séverine Bouttefroy, Shahnoor Islam, Sherif Abouelnaga, Silvio Fabio Torres, Simone Cesaro, Sofia Nunes, Soraia Rouxinol, Sucharita Bhaumik, Symbat Saliyeva, Tamara Inostroza, Thelma Velasquez, Tint Myo Hnin, Ulrika Norén-Nyström, Valentina Baretta, Yajaira Valentine Jimenez-Antolinez, Vanesa Pérez Alonso, Vanessa Ayer Miller, Virginie Gandemer, Viviana Lotero, Volha Mishkova, Wendy Gómez-García, Yeva Margaryan, and Yumna Syed
Supplementary Material
References
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2.Mehta NS, Mytton OT, Mullins EWS, et al. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Bellino S, Punzo O, Rota MC, et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146 doi: 10.1542/peds.2020-009399. [DOI] [PubMed] [Google Scholar]
- 6.Hakim H, Dallas R, Zhou Y, et al. Acute respiratory infections in children and adolescents with acute lymphoblastic leukemia. Cancer. 2016;122:798–805. doi: 10.1002/cncr.29833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GE, Fisher BT, Xiao R, et al. Burden of influenza-related hospitalizations and attributable mortality in pediatric acute lymphoblastic leukemia. J Pediatric Infect Dis Soc. 2015;4:290–296. doi: 10.1093/jpids/piu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher BT, Danziger-Isakov L, Sweet LR, et al. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatric Infect Dis Soc. 2018;7:275–282. doi: 10.1093/jpids/pix051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rüthrich MM, Giessen-Jung C, Borgmann S, et al. COVID-19 in cancer patients: clinical characteristics and outcome-an analysis of the LEOSS registry. Ann Hematol. 2021;100:383–393. doi: 10.1007/s00277-020-04328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisogno G, Provenzi M, Zama D, et al. Clinical characteristics and outcome of severe acute respiratory syndrome coronavirus 2 infection in Italian pediatric oncology patients: a study from the Infectious Diseases Working Group of the Associazione Italiana di Oncologia e Ematologia Pediatrica. J Pediatric Infect Dis Soc. 2020;9:530–534. doi: 10.1093/jpids/piaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montoya J, Ugaz C, Alarcon S, et al. COVID-19 in pediatric cancer patients in a resource-limited setting: national data from Peru. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouger-Gaudichon J, Thébault E, Félix A, et al. Impact of the first wave of COVID-19 on pediatric oncology and hematology: a report from the French Society of Pediatric Oncology. Cancers. 2020;12 doi: 10.3390/cancers12113398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebudi R, Kurucu N, Tuğcu D, et al. COVID-19 infection in children with cancer and stem cell transplant recipients in Turkey: a nationwide study. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millen GC, Arnold R, Cazier JB, et al. Severity of COVID-19 in children with cancer: report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br J Cancer. 2021;124:754–759. doi: 10.1038/s41416-020-01181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.André N, Rouger-Gaudichon J, Brethon B, et al. COVID-19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc. 2021;10:34–48. doi: 10.1093/jpids/piaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20:483–493. doi: 10.1016/S1470-2045(18)30909-4. [DOI] [PubMed] [Google Scholar]
- 22.Moreira DC, Sniderman E, Mukkada S, et al. The Global COVID-19 Observatory and Resource Center for Childhood Cancer: a response for the pediatric oncology community by SIOP and St. Jude Global. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisk B, Cull W, Harris JM, Rothenburger A, Olson L. National trends of cases of COVID-19 in children based on US state health department data. Pediatrics. 2020;146 doi: 10.1542/peds.2020-027425. [DOI] [PubMed] [Google Scholar]
- 25.Working WHO. Group on the characterization and management of COVID-19 infection, a minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulad F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020;6:1459–1460. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Saleeby CM, Somes GW, DeVincenzo JP, Gaur AH. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121:235–243. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 28.Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc. 2019;8:21–28. doi: 10.1093/jpids/pix093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graetz D, Agulnik A, Ranadive R, et al. Global effect of the COVID-19 pandemic on paediatric cancer care: a cross-sectional study. Lancet Child Adolesc Health. 2021;5:332–340. doi: 10.1016/S2352-4642(21)00031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasquez L, Sampor C, Villanueva G, et al. Early impact of the COVID-19 pandemic on paediatric cancer care in Latin America. Lancet Oncol. 2020;21:753–755. doi: 10.1016/S1470-2045(20)30280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregate de-identified data are available as part of a public data visualisation in the Global COVID-19 Observatory and Resource Center for Childhood Cancer. Individual de-identified patient data with site identifiers removed and geographical region of patient residence limited to country will be made available upon request beginning 6 months after Article publication. Individuals can email the corresponding author with enquiries regarding the dataset.