Abstract
Background
The full range of long-term health consequences of COVID-19 in patients who are discharged from hospital is largely unclear. The aim of our study was to comprehensively compare consequences between 6 months and 12 months after symptom onset among hospital survivors with COVID-19.
Methods
We undertook an ambidirectional cohort study of COVID-19 survivors who had been discharged from Jin Yin-tan Hospital (Wuhan, China) between Jan 7 and May 29, 2020. At 6-month and 12-month follow-up visit, survivors were interviewed with questionnaires on symptoms and health-related quality of life (HRQoL), and received a physical examination, a 6-min walking test, and laboratory tests. They were required to report their health-care use after discharge and work status at the 12-month visit. Survivors who had completed pulmonary function tests or had lung radiographic abnormality at 6 months were given the corresponding tests at 12 months. Non-COVID-19 participants (controls) matched for age, sex, and comorbidities were interviewed and completed questionnaires to assess prevalent symptoms and HRQoL. The primary outcomes were symptoms, modified British Medical Research Council (mMRC) score, HRQoL, and distance walked in 6 min (6MWD). Multivariable adjusted logistic regression models were used to evaluate the risk factors of 12-month outcomes.
Findings
1276 COVID-19 survivors completed both visits. The median age of patients was 59·0 years (IQR 49·0–67·0) and 681 (53%) were men. The median follow-up time was 185·0 days (IQR 175·0–198·0) for the 6-month visit and 349·0 days (337·0–361·0) for the 12-month visit after symptom onset. The proportion of patients with at least one sequelae symptom decreased from 68% (831/1227) at 6 months to 49% (620/1272) at 12 months (p<0·0001). The proportion of patients with dyspnoea, characterised by mMRC score of 1 or more, slightly increased from 26% (313/1185) at 6-month visit to 30% (380/1271) at 12-month visit (p=0·014). Additionally, more patients had anxiety or depression at 12-month visit (26% [331/1271] at 12-month visit vs 23% [274/1187] at 6-month visit; p=0·015). No significant difference on 6MWD was observed between 6 months and 12 months. 88% (422/479) of patients who were employed before COVID-19 had returned to their original work at 12 months. Compared with men, women had an odds ratio of 1·43 (95% CI 1·04–1·96) for fatigue or muscle weakness, 2·00 (1·48–2·69) for anxiety or depression, and 2·97 (1·50–5·88) for diffusion impairment. Matched COVID-19 survivors at 12 months had more problems with mobility, pain or discomfort, and anxiety or depression, and had more prevalent symptoms than did controls.
Interpretation
Most COVID-19 survivors had a good physical and functional recovery during 1-year follow-up, and had returned to their original work and life. The health status in our cohort of COVID-19 survivors at 12 months was still lower than that in the control population.
Funding
Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation.
Introduction
The continuing spread of SARS-CoV-2 remains a public health emergency of international concern, resulting in an enormous global disease burden. As of early August, 2021, more than 200 million COVID-19 cases have been confirmed globally, and more than 4·3 million people have died following SARS-CoV-2 infection.1
The sequelae after recovery from acute COVID-19 have been widely reported2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and have become an increasing concern. In our previous cohort study with a median follow-up time of 6 months after symptom onset, approximately three-quarters of COVID-19 survivors discharged from hospital still had persisting symptoms, and patients who were critically ill during hospital stay had higher risk of lung diffusion impairment and radiographic abnormality than did those who had lower disease severity.2 Up to now, only two small studies with no more than 120 COVID-19 survivors reported the 1-year outcomes after hospital discharge but were limited to respiratory outcomes, mainly including pulmonary function or lung imaging.10, 11 Hence, the full range of long-term health consequences of COVID-19 in patients who were discharged from hospital is largely unclear. A basic knowledge gap that urgently needs to be addressed is the pathophysiology of sequelae after COVID-19,13, 14 which is crucial for development of treatment and prevention of poor outcomes after acute SARS-CoV-2 infection. Additionally, information about health-care use and work status during the first year after hospital discharge among these patients is scare.
Research in context.
Evidence before this study
We searched PubMed and medRxiv for follow-up studies of long-term consequences of COVID-19 from Jan 1, 2020, to July 1, 2021, without language restriction. The search terms were: "(COVID-19 OR SARS-CoV-2 OR Coronavirus disease 2019 OR 2019-ncov) AND (survivor* OR recover* OR persistent OR follow up OR discharge* OR long term OR sequelae)". To our knowledge, only two small studies of COVID-19 survivors (≤120 patients) reported the 1-year outcomes after discharge but these studies were limited to respiratory outcomes. Additionally, the pathophysiology of persistent consequence after COVID-19, health-care use, and work status during the first year after discharge were still unknown. Urgent research is needed to address these knowledge gaps.
Added value of this study
To our knowledge, this is the largest longitudinal cohort study of hospital survivors with COVID-19 to describe the dynamic recovery of health consequences within 12 months after symptom onset. We reported that the proportion of patients with at least one sequelae symptom decreased significantly from 68% at 6 months to 49% at 12 months. Fatigue or muscle weakness was the most commonly reported symptom at both visits, but the proportion fell from 52% at 6 months to 20% at 12 months. The proportion of patients with modified British Medical Research Council score of 1 or more and anxiety or depression was slightly higher at 12 months than at 6 months. 88% of patients who were employed before COVID-19 had returned to their original work at 12 months. For up to 12 months, lung diffusion impairment was observed in about 20–30% of moderately ill patients, and as high as 54% in critically ill patients. 1 year after acute infection, COVID-19 survivors still had lower health status than did non-COVID-19 controls matched for age, sex, and comorbidities.
Implications of all the available evidence
Within 1 year after acute infection, patients with COVID-19 had a significant improvement of health status, including sequelae symptom, lung structure, and function. Most patients who were employed before COVID-19 had returned to their original work, and those with persisting severely impaired health status are rare. In view of the high prevalence of persistent lung diffusion impairment and imaging abnormality in patients who received critical care during acute infection, strategies for sequelae prevention or amelioration should be explored in this population. Our data suggest that a full recovery after 1 year is not possible for some patients, for whom it will take longer to attain their baseline health state before COVID-19.
The primary aim of this study was to comprehensively compare health consequences of COVID-19 patients who have been discharged from hospital between 6 months and 12 months after symptom onset. The secondary aim was to determine whether COVID-19 survivors had returned to a baseline health status 1 year after symptom onset compared with non-COVID-19 controls.
Methods
Study design and participants
This is an ambidirectional cohort study of COVID-19 survivors discharged from Jin Yin-tan Hospital (Wuhan, China) between Jan 7 and May 29, 2020. Inclusion and exclusion criteria of survivors have been described previously.2 Briefly, all patients with laboratory confirmed COVID-19 discharged from Jin Yin-tan Hospital between Jan 7 and May 29, 2020, were eligible for participation. Patients were excluded if they died after discharge; were living in a nursing or welfare home; had psychotic disorder, dementia, or osteoarthropathy; or were immobile. To determine whether COVID-19 patients completely recovered at 12 months, we recruited community-dwelling adults without SARS-CoV-2 infection (controls) from two districts of Wuhan city between Dec 24, 2020, and Jan 16, 2021. The inclusion and exclusion criteria are shown in the appendix (p 4). COVID-19 survivors and controls were further matched 1:1 by age, sex, and comorbidities including cardiovascular disease, chronic respiratory disease, chronic kidney disease, hypertension, and diabetes. The maximum allowed age difference between COVID-19 patients and their controls was 10 years.
The study was approved by the Research Ethics Commission of Jin Yin-tan Hospital (KY-2020-78.01, KY-2020-78.03). Written informed consent was obtained from controls and COVID-19 survivors who attended the follow-up visit.
Data collection of COVID-19 patients at acute phase
The definitions for the acute phase, collected data, and category of disease severity according to the highest seven-category scale during the hospital stay (termed the severity scale)15 are described in our previous study2 and appendix (p 4). We confirmed the data for demographic and self-reported comorbidity with participants, face to face, at the 12-month follow-up visit.
Follow-up assessment of COVID-19 survivors
Eligible COVID-19 survivors were invited to attend two follow-up visits at Jin Yin-tan Hospital at 6 and 12 months after symptom onset. The detailed 6-month follow-up procedures have been described previously.2 At each visit, patients underwent a detailed interview, physical examination, and a 6-min walking test; completed a series of questionnaires, including a self-reported symptom questionnaire, the modified British Medical Research Council (mMRC) dyspnoea scale,16 the EuroQol five-dimension five-level (EQ-5D-5L) questionnaire to assess health-related quality of life,17 the EuroQol Visual Analogue Scale (EQ-VAS) (scores range from 0–100; a higher score indicates a better health status),18 and an ischaemic stroke and cardiovascular event registration form;19 and received laboratory tests. Notably, at the 12-month visit, they were also asked to complete a questionnaire to record their health-care use after discharge and work status.
A stratified disproportional random sampling procedure according to severity scale was used to select patients to receive pulmonary function tests and chest high-resolution CT (HRCT) at 6-month follow-up visit.2 Of participants selected, 349 had completed the pulmonary function tests and 353 chest HRCT at the 6-month visit. The 349 participants who had completed the pulmonary function tests at 6-month visit were all invited to perform this test again at the 12-month visit. Of 353 participants who had completed chest HRCT at 6-month visit, the 186 who presented with abnormal CT were further invited to receive another HRCT scan at the 12-month visit.
COVID-19 patients whose plasma samples were all collected at the acute phase, discharge, 6-month visit, and 12-month visit received a cytokine test. They had previously been enrolled in the Lopinavir Trial for suppression of SARS-CoV-2 in China (LOTUS).15 Their plasma samples were screened with the Bio-Plex Pro Human Cytokine Screening Panel 27-plex (Bio-rad, Hercules, CA, USA) in Bio-Plex 200 System (Bio-rad). The concentrations of 27 cytokines were measured: interleukin (IL)-1ra, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, interferon (IFN)-γ-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, RANTES, fibroblast growth factors, platelet derived growth factor-BB, vascular endothelial growth factor, granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), IFN-γ, and tumor necrosis factor (TNF)-α.
Data collection of community-dwelling non-COVID-19 adults
Community-dwelling non-COVID-19 adults were interviewed face to face at their community centre by trained medical staff from Jin Yin-tan Hospital. Standard questionnaires were administered to collect information about demographic characteristics, personal medical history, and lifestyle information. They were also asked to undergo physical examination and completed a questionnaire to record prevalent symptoms, the mMRC dyspnoea scale,16 the EQ-5D-5L questionnaire,17 and EQ-VAS.18 Venous blood samples were collected for laboratory tests.
Outcome measures
All outcome measures and assessment tools are listed in the appendix pp 9–12. The primary outcomes were symptoms, mMRC score, health-related quality of life (pain or discomfort, anxiety or depression, mobility, personal care, and usual activity), and distance walked in 6 min (6MWD). The secondary outcomes were lung function, chest CT pattern, outpatient visit and hospital admission after discharge, and work status at follow-up.
Statistical analysis
Demographic characteristics and long-term health consequences of COVID-19 in patients are presented as median (IQR) for continuous variables and expressed as absolute values along with percentages for categorical variables. Participants were categorised into three groups according to their severity scale during their hospital stay (scale 3, not requiring supplemental oxygen; scale 4, requiring supplemental oxygen; or scale 5–6, requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation). Demographic and clinical characteristics and long-term consequences across participants with different categories of severity scale are shown. For the comparison of demographic and clinical characteristics among participants with different disease severity, Kruskal-Wallis test, χ2 test, Fisher's exact, or Mann-Whitney U test were used when appropriate. For the comparison of symptoms, exercise capacity, and health-related quality of life between 6-month and 12-month follow-up, we used Wilcoxon signed-rank test, or McNemar test when appropriate. The comparison of demographic and clinical characteristics, symptoms, health-related quality of life, and laboratory test results between COVID-19 patients and controls was done with Mann-Whitney U test, χ2 test, or Fisher's exact test when appropriate.
We used multivariable adjusted logistic regression analysis to explore risk factors associated with diffusion impairment, anxiety or depression, and fatigue or muscle weakness. For the association of disease severity with outcome, age, sex, cigarette smoking, education, comorbidity, corticosteroids, antivirals, and intravenous immunoglobulin were adjusted. For the association of factors including sex, corticosteroid, antiviral, and intravenous immunoglobulin with outcome, the aforementioned variables were all included in the model. When exploring the associations of education and smoking with outcome, the aforementioned variables except for comorbidity, and both comorbidity and disease severity (due to the potential mediation) were included, respectively. Only sex, smoking, and education were adjusted for the association between age and outcome due to the potential mediation of other factors. For the association of comorbidity with outcome, the aforementioned variables except for disease severity were all included. Additionally, a sensitivity analysis with inverse probability-weighted generalised estimating equations was done to reduce the effect of bias due to differences between patients who were included in these analyses and those who were not because of loss to follow-up.
For the comparison of cytokine concentrations at the acute phase, discharge, 6-month follow-up, and 12-month follow-up, Wilcoxon signed-rank test was used. Log10-transformation was done for each cytokine. Partial correlation coefficients between different cytokine pair in COVID-19 patients at discharge, 6-month follow-up, and 12-month follow-up were estimated with adjustment for age, disease severity, and sampling days after symptom onset. For the association of change in cytokine (at discharge until 6-month follow-up) with categorical outcomes at 12-month follow-up, multivariable adjusted logistic regression models were used to estimate the odds ratios (ORs) and 95% CIs per IQR change of log10-transformed cytokine concentration. For the association between change in cytokine concentrations and continuous outcomes, multivariable adjusted linear regression models were used to calculate the β estimates and 95% CIs per IQR change of log10-transformed cytokine concentration. The results following log-transformation were calculated on the basis of geometric mean ratio of cytokines. Age, sex, and corticosteroids were adjusted.
All significance tests were two-sided, and a p value of less than 0·05 was considered statistically significant unless stated otherwise. To correct for multiple comparison of demographic and clinical characteristics between two groups of study participants with different severity scale, we used a Bonferroni corrected α-threshold of 0·0167. To correct for multiple comparison of cytokine concentrations at the acute phase, discharge, 6-month follow-up, and 12-month follow-up, we used a Bonferroni corrected α-threshold of 0·0083. A stringent Bonferroni correction was also used for testing correlation of 351 cytokine pairs, using an α-threshold of 1·4 × 10−4 to determine statistical significance. All statistical analyses were done with SAS (version 9.4). The partial correlation plot was generated in R (version 3.5.2).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
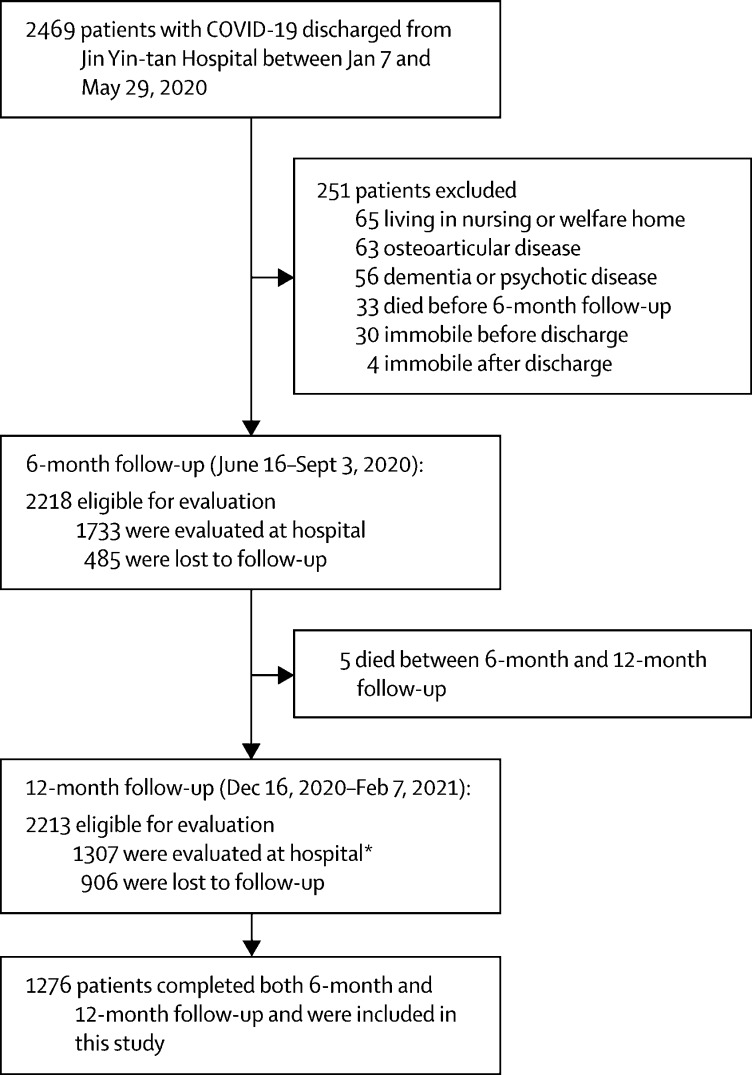
2469 patients with COVID-19 were discharged from Jin Yin-tan Hospital between Jan 7 and May 29, 2020. The 6-month follow-up visit was done between June 16 and Sept 3, 2020, and the 12-month follow-up visit between Dec 16, 2020, and Feb 7, 2021. 1276 (58%) participants who attended both visits were included in final analysis (figure 1 ). The proportion of men and participants who received oxygen therapy during hospital stay were slightly higher in patients who were included in final analysis than in those who were not (appendix p 20). There was no significant difference in age, smoking status, comorbidities, and intensive care unit (ICU) admission between both groups (appendix p 20).
Figure 1.
Flow chart of hospital survivors with COVID-19
*Of whom 31 patients did not participate in the 6-month follow-up.
Table 1 shows the demographic and clinical characteristics of 1276 patients who attended both visits. Median follow-up time was 185·0 days (IQR 175·0–198·0) after symptom onset for 6-month visit and 349·0 days (337·0–361·0) after symptom onset for 12-month visit. The median age of patients was 59·0 years (IQR 49·0–67·0), and 681 (53%) were men. 864 patients (68%) received oxygen via nasal cannulae and mask during hospitalisation, and 94 (7%) required high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation. 54 (4%) patients were admitted to ICU, with a median length of ICU stay of 18·0 days (IQR 7·0–30·0). 307 (24%) patients received corticosteroids therapy during hospital stay.
Table 1.
Characteristics of COVID-19 patients who completed both 6-month and 12-month follow-up
| Total (n=1276) | Scale 3: not requiring supplemental oxygen (n=318) | Scale 4: requiring supplemental oxygen (n=864) | Scale 5–6: requiring HFNC, NIV, or IMV (n=94) | p value | ||
|---|---|---|---|---|---|---|
| Age, years | 59·0 (49·0–67·0) | 59·0 (49·0–67·0) | 59·0 (50·0–67·0) | 58·0 (49·0–67·0) | 0·71 | |
| Sex | .. | .. | .. | .. | 0·011 | |
| Men | 681 (53%) | 157 (49%) | 461 (53%) | 63 (67%)*† | .. | |
| Women | 595 (47%) | 161 (51%) | 403 (47%) | 31 (33%)*† | .. | |
| Education | .. | .. | .. | .. | 0·013 | |
| College or higher | 348/1269 (27%) | 84 (26%) | 226/857 (26%) | 38 (40%)*† | .. | |
| Middle school or lower | 921/1269 (73%) | 234 (74%) | 631/857 (74%) | 56 (60%)*† | .. | |
| Cigarette smoking | .. | .. | .. | .. | 0·34 | |
| Never-smoker | 1047/1272 (82%) | 251 (79%) | 720/860 (84%) | 76 (81%) | .. | |
| Current smoker | 87/1272 (7%) | 24 (8%) | 57/860 (7%) | 6 (6%) | .. | |
| Former smoker | 138/1272 (11%) | 43 (14%) | 83/860 (10%) | 12 (13%) | .. | |
| Comorbidity | ||||||
| Hypertension | 451/1251 (36%) | 124/313 (40%) | 288/849 (34%) | 39/89 (44%) | 0·06 | |
| Diabetes | 188/1257 (15%) | 46/315 (15%) | 127/850 (15%) | 15/92 (16%) | 0·92 | |
| Coronary heart diseases | 96/1268 (8%) | 27/317 (9%) | 62/859 (7%) | 7/92 (8%) | 0·76 | |
| Cerebrovascular diseases | 51/1269 (4%) | 11 (3%) | 39/857 (5%) | 1 (1%) | 0·15 | |
| Malignancy | 40/1271 (3%) | 10 (3%) | 26/859 (3%) | 4 (4%) | 0·83 | |
| Chronic obstructive pulmonary disease | 12/1269 (1%) | 3/317 (1%) | 8/858 (1%) | 1 (1%) | 0·90 | |
| Chronic kidney disease | 49/1269 (4%) | 10 (3%) | 33/857 (4%) | 6 (6%) | 0·40 | |
| Highest seven-category scale during hospital stay | ||||||
| 3: hospitalisation, not requiring supplemental oxygen | 318 (25%) | 318 (100%) | 0 | 0 | .. | |
| 4: hospitalisation, requiring supplemental oxygen | 864 (68%) | 0 | 864 (100%) | 0 | .. | |
| 5: hospitalisation, requiring HFNC or non-IMV, or both | 86 (7%) | 0 | 0 | 86 (91%) | .. | |
| 6: hospitalisation, requiring ECMO or IMV, or both | 8 (1%) | 0 | 0 | 8 (9%) | .. | |
| Treatment received during hospital stay | ||||||
| Corticosteroids | 307 (24%) | 32 (10%) | 206 (24%)‡ | 69 (73%)*† | <0·0001 | |
| Antivirals | 705 (55%) | 164 (52%) | 481 (56%) | 60 (64%) | 0·10 | |
| Lopinavir–ritonavir | 173 (14%) | 27 (8%) | 120 (14%)‡ | 26 (28%)*† | <0·0001 | |
| Arbidol | 622 (49%) | 149 (47%) | 423 (49%) | 50 (53%) | 0·54 | |
| Chloroquine phosphate | 4 (0%) | 0 | 3 (0%) | 1 (1%) | 0·21 | |
| Hydroxychloroquine | 2 (0%) | 1 (0%) | 1 (0%) | 0 | 0·54 | |
| Antibiotics | 987 (77%) | 183 (58%) | 712 (82%)‡ | 92 (98%)*† | <0·0001 | |
| Thymosin | 204 (16%) | 42 (13%) | 146 (17%) | 16 (17%) | 0·30 | |
| Intravenous immunoglobulin | 249 (20%) | 29 (9%) | 166 (19%)‡ | 54 (57%)*† | <0·0001 | |
| Length of hospital stay, days | 14·0 (10·0–20·0) | 11·0 (8·0–16·0) | 14·0 (11·0–19·0)‡ | 40·0 (25·0–52·0)*† | <0·0001 | |
| ICU admission | 54 (4%) | 0 | 20 (2%)‡ | 34 (36%)*† | <0·0001 | |
| Length of ICU stay, days | 18·0 (7·0–30·0) | NA | 7·0 (2·5–18·0) | 22·5 (10·0–43·0)† | 0·0001 | |
| Time from symptom onset to 6-month follow-up, days | 185·0 (175·0–198·0) | 187·0 (174·0–198·0) | 184·0 (175·0–196·0)‡ | 204·0 (183·0–217·0)*† | <0·0001 | |
| Time from symptom onset to 12-month follow-up, days | 349·0 (337·0–361·0) | 346·0 (335·0–357·0) | 349·0 (338·0–361·0)‡ | 360·0 (351·0–373·0)*† | <0·0001 | |
Data are median (IQR), n (%), or n/N (%) when data are missing. The differing denominators used indicate missing data. To correct for multiple comparison between two groups of study participants with different severity scale, a Bonferroni corrected α-threshold of 0.0167 was used. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. ECMO=extracorporeal membrane oxygenation. ICU=intensive care unit. NA=not applicable.
p<0·0167 for the comparison of scale 5–6 with scale 3 .
p<0·0167 for the comparison of scale 5–6 with scale 4.
p<0·0167 for the comparison of scale 4 with scale 3.
Table 2 shows the temporal trend in sequelae symptom, health-related quality of life, and exercise capacity. The proportion of patients with at least one sequelae symptom decreased from 68% (831/1227) at 6 months to 49% (620/1272) at 12 months (p<0·0001); the decrease was observed in all three subgroups of patients with different disease severity (all p<0·0001). Fatigue or muscle weakness was the most commonly reported symptom at both visits, but the proportion fell from 52% (636/1230) at 6 months to 20% (255/1272) at 12 months (p<0·0001). Many symptoms significantly resolved over time in the total cohort and in all three subgroups—eg, fatigue or muscle weakness, sleep difficulties, hair loss, smell disorder, and taste disorder (all p<0·05; table 2). The proportion of patients with dyspnoea, characterised by mMRC score of 1 or more, slightly increased from 26% (313/1185) at 6-month visit to 30% (380/1271) at 12-month visit (p=0·014). Additionally, more patients had anxiety or depression (23% [274/1187] at 6-month vs 26% [331/1271] at 12-month visit, p=0·015), among whom mild anxiety or depression was predominant (appendix p 22) and only one patient visited the psychological department after discharge. The proportion of patients with 6MWD less than lower limit of the normal range was 12% (147/1248) at 12 months, which was statistically lower than 14% (174/1254) at 6 months (p=0·033). We noted no significant difference in median 6MWD between 6 months and 12 months (table 2). Within 12 months after symptom onset, three of 1276 patients developed ischaemic stroke, and one patient newly developed stable angina pectoris.
Table 2.
Sequelae symptom, exercise capacity, and health-related quality of life among COVID-19 patients at 6-month and 12-month follow-up
|
Total (n=1276) |
Scale 3: not requiring supplemental oxygen (n=318) |
Scale 4: requiring supplemental oxygen (n=864) |
Scale 5–6: requiring HFNC, NIV, or IMV (n=94) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 month | 12 month | p value | 6 month | 12 month | p value | 6 month | 12 month | p value | 6 month | 12 month | p value | ||
| Sequelae symptom | |||||||||||||
| Any one of the following symptoms | 831/1227 (68%) | 620/1272 (49%) | <0·0001 | 211/307 (69%) | 151 (47%) | <0·0001 | 543/828 (66%) | 420/860 (49%) | <0·0001 | 77/92 (84%) | 49 (52%) | <0·0001 | |
| Fatigue or muscle weakness | 636/1230 (52%) | 255/1272 (20%) | <0·0001 | 158/307 (51%) | 65 (20%) | <0·0001 | 410/831 (49%) | 169/860 (20%) | <0·0001 | 68/92 (74%) | 21 (22%) | <0·0001 | |
| Sleep difficulties | 335/1230 (27%) | 215/1272 (17%) | <0·0001 | 84/307 (27%) | 49 (15%) | <0·0001 | 217/831 (26%) | 152/860 (18%) | <0·0001 | 34/92 (37%) | 14 (15%) | 0·0002 | |
| Hair loss | 268/1230 (22%) | 135/1272 (11%) | <0·0001 | 68/307 (22%) | 29 (9%) | <0·0001 | 177/831 (21%) | 98/860 (11%) | <0·0001 | 23/92 (25%) | 8 (9%) | 0·0003 | |
| Smell disorder | 135/1230 (11%) | 57/1272 (4%) | <0·0001 | 35/307 (11%) | 17 (5%) | 0·0030 | 86/831 (10%) | 34/860 (4%) | <0·0001 | 14/92 (15%) | 6 (6%) | 0·033 | |
| Palpitations | 118/1230 (10%) | 117/1272 (9%) | 0·88 | 32/307 (10%) | 23 (7%) | 0·12 | 72/831 (9%) | 87/860 (10%) | 0·17 | 14/92 (15%) | 7 (7%) | 0·09 | |
| Joint pain | 132/1225 (11%) | 157/1272 (12%) | 0·13 | 42/308 (14%) | 37 (12%) | 0·49 | 74/826 (9%) | 103/860 (12%) | 0·018 | 16/91 (18%) | 17 (18%) | 1·00 | |
| Decreased appetite | 97/1230 (8%) | 37/1272 (3%) | <0·0001 | 28/307 (9%) | 6 (2%) | <0·0001 | 58/831 (7%) | 27/860 (3%) | 0·0003 | 11/92 (12%) | 4 (4%) | 0·05 | |
| Taste disorder | 89/1230 (7%) | 37/1272 (3%) | <0·0001 | 22/307 (7%) | 6 (2%) | 0·0007 | 59/831 (7%) | 31/860 (4%) | 0·0007 | 8/92 (9%) | 0 | 0·0047 | |
| Dizziness | 69/1230 (6%) | 65/1272 (5%) | 0·56 | 22/307 (7%) | 16 (5%) | 0·24 | 41/831 (5%) | 40/860 (5%) | 0·71 | 6/92 (7%) | 9 (10%) | 0·41 | |
| Nausea or vomiting | 17/1229 (1%) | 11/1272 (1%) | 0·26 | 8/307 (3%) | 5 (2%) | 0·41 | 9/830 (1%) | 4/860 (0%) | 0·17 | 0/92 (0%) | 2 (2%) | 0·16 | |
| Chest pain | 57/1225 (5%) | 92/1272 (7%) | 0·0023 | 17/308 (6%) | 25 (8%) | 0·14 | 36/826 (4%) | 63/860 (7%) | 0·0055 | 4/91 (4%) | 4 (4%) | 1·00 | |
| Sore throat or difficult to swallow | 47/1230 (4%) | 44/1272 (3%) | 0·57 | 19/307 (6%) | 11 (3%) | 0·08 | 24/831 (3%) | 29/860 (3%) | 0·55 | 4/92 (4%) | 4 (4%) | 1·00 | |
| Skin rash | 39/1230 (3%) | 55/1272 (4%) | 0·10 | 12/307 (4%) | 15 (5%) | 0·53 | 23/831 (3%) | 38/860 (4%) | 0·05 | 4/92 (4%) | 2 (2%) | 0·41 | |
| Myalgia | 33/1225 (3%) | 54/1272 (4%) | 0·013 | 10/308 (3%) | 12 (4%) | 0·64 | 20/826 (2%) | 36/860 (4%) | 0·018 | 3/91 (3%) | 6 (6%) | 0·26 | |
| Headache | 25/1225 (2%) | 61/1272 (5%) | 0·0001 | 7/308 (2%) | 16 (5%) | 0·050 | 15/826 (2%) | 40/860 (5%) | 0·0010 | 3/91 (3%) | 5 (5%) | 0·48 | |
| mMRC score | .. | .. | 0·014 | .. | .. | 0·68 | .. | .. | 0·0015 | 0·83 | |||
| 0 | 872/1185 (74%) | 891/1271 (70%) | .. | 231/309 (75%) | 238/317 (75%) | .. | 591/792 (75%) | 596/860 (69%) | .. | 50/84 (60%) | 57 (61%) | .. | |
| ≥1 | 313/1185 (26%) | 380/1271 (30%) | .. | 78/309 (25%) | 79/317 (25%) | .. | 201/792 (25%) | 264/860 (31%) | .. | 34/84 (40%) | 37 (39%) | .. | |
| EQ-5D-5L questionnaire* | |||||||||||||
| Mobility: problems with walking around | 76/1191 (6%) | 115/1271 (9%) | 0·0058 | 17/310 (5%) | 24/317 (8%) | 0·37 | 45/796 (6%) | 83/860 (10%) | 0·0004 | 14/85 (16%) | 8 (9%) | 0·05 | |
| Personal care: problems with washing or dishing | 9/1191 (1%) | 20/1271 (2%) | 0·033 | 0/310 (0%) | 3/317 (1%) | 0·08 | 8/796 (1%) | 13/860 (2%) | 0·20 | 1/85 (1%) | 4 (4%) | 0·32 | |
| Usual activity: problems with usual activity | 18/1182 (2%) | 18/1271 (1%) | 0·86 | 3/309 (1%) | 2/317 (1%) | 0·56 | 11/789 (1%) | 13/860 (2%) | 0·68 | 4/84 (5%) | 3 (3%) | 0·32 | |
| Pain or discomfort | 321/1186 (27%) | 371/1271 (29%) | 0·13 | 84/307 (27%) | 84/317 (26%) | 0·76 | 201/794 (25%) | 255/860 (30%) | 0·020 | 36/85 (42%) | 32 (34%) | 0·37 | |
| Anxiety or depression | 274/1187 (23%) | 331/1271 (26%) | 0·015 | 74/309 (24%) | 78/317 (25%) | 0·83 | 170/794 (21%) | 226/860 (26%) | 0·0013 | 30/84 (36%) | 27 (29%) | 0·32 | |
| Quality of life† | 80·0 (75·0–90·0) | 80·0 (70·0–90·0) | 0·044 | 80·0 (70·0–90·0) | 80·0 (70·0–90·0) | 0·91 | 80·0 (75·0–90·0) | 80·0 (75·0–90·0) | 0·0058 | 80·0 (70·0–85·0) | 80·0 (70·0–85·0) | 0·38 | |
| Distance walked in 6 min, m | 495·0 (450·0–540·0) | 495·0 (443·0–544·0) | 0·26 | 491·0 (450·0–540·0) | 494·0 (438·5–540·5) | 0·59 | 496·0 (450·0–540·0) | 495·0 (442·0–544·0) | 0·76 | 495·0 (430·0–526·0) | 496·5 (455·0–552·0) | 0·023 | |
| Percentage of predicted value‡ | 88·0 (79·5–96·4) | 90·0 (81·3–98·6) | <0·0001 | 86·9 (78·5–95·2) | 89·1 (80·5–96·8) | 0·0028 | 88·6 (80·5–97·2) | 90·6 (81·8–99·8) | <0·0001 | 82·4 (75·2–92·3) | 87·9 (80·1–98·1) | 0·0006 | |
| Less than lower limit of the normal range§ | 174/1254 (14%) | 147/1248 (12%) | 0·033 | 51/313 (16%) | 40/308 (13%) | 0·09 | 100/851 (12%) | 91/850 (11%) | 0·28 | 23/90 (26%) | 16/90 (18%) | 0·20 | |
Data are median (IQR), n (%), or n/N (%) when data are missing. The differing denominators used indicate missing data. p value indicates the comparison of consequences between 6 months and 12 months in total or each category of scale. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. mMRC=modified British Medical Research Council. EQ-5D-5L=EuroQol five-dimension five-level questionnaire.
Detailed results of EQ-5D-5L questionnaire among COVID-19 patients are shown in the appendix (pp 21–22).
Quality of life was assessed using the EuroQol Visual Analogue Scale, ranging from 0 (worst imaginable health) to 100 (best imaginable health).
Predicted values were calculated according to the method of Enright and Sherrill.
The lower limit of the normal range was calculated by subtracting 153 m from the predicted value for men or by subtracting 139 m for women.
We recruited 3383 community-dwelling adults without SARS-CoV-2 infection. The appendix p 23 shows the characteristics of 1164 matched pairs. We recorded no significant differences in age, sex, and comorbidities between both groups. 764 of 1164 (66%) COVID-19 patients had at least one prevalent symptom, which was significantly higher than for controls (383/1164 [33%], p<0·0001; appendix p 24). The proportions of each prevalent symptom and mMRC score of 1 or greater were all significantly higher in COVID-19 patients than in controls (all p<0·05; appendix p 24). COVID-19 patients had more problems with mobility, pain or discomfort, and anxiety or depression and had lower self-assessment scores of quality of life than did controls (all p<0·0001; appendix p 24). 62 of 1160 (5%) COVID-19 survivors had a leucocyte count lower than 4 × 109 per L at 12 months, which was slightly higher than for controls (33/1091 [3%], p=0·023; appendix p 25). The proportion of patients with lymphocyte count lower than 0·8 × 109 per L or serum creatinine abnormality did not differ between COVID-19 survivors and controls. The appendix p 25 shows other results of laboratory tests. The appendix p 26 shows the detailed results of EQ-5D-5L questionnaire of matched COVID-19 survivors and controls.
Among 349 patients who had completed pulmonary function tests at 6-month visit, 254 attended the 12-month visit but ten were not able to complete the test (table 3 ). Spirometric and lung volume parameters of most patients were within normal limits at 12-month visit. Lung diffusion impairment, defined as diffusion capacity for carbon monoxide less than 80% of predicted, did not improve from 6 months to 12 months in the three groups of patients with variable severity at acute phase (all p>0·05; table 3). At 12 months, lung diffusion impairment was found in 23% (13/56) of patients in the severity scale 3 group, 31% (36/117) in the scale 4 group, and 54% (38/70) in the scale 5–6 group. The proportion of total lung capacity less than 80% of predicted in patients with scale 5–6 severity decreased significantly from 39% (27/69) at 6 months to 29% (20/70) at 12 months (p=0·021).
Table 3.
Lung function and chest CT among COVID-19 patients at 6-month and 12-month follow-up according to severity scale
|
Scale 3: not requiring supplemental oxygen |
Scale 4: requiring supplemental oxygen |
Scale 5–6: requiring HFNC, NIV, or IMV |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 month | 12 month | p value | 6 month | 12 month | p value | 6 month | 12 month | p value | |
| Lung function | |||||||||
| Number of patients | 59 | 56 | .. | 125 | 118 | .. | 70 | 70 | .. |
| FEV1 <80%, % of predicted | 4 (7%) | 2 (4%) | 0·32 | 2 (2%) | 3 (3%) | 0·56 | 10 (14%) | 4 (6%) | 0·014 |
| FVC <80%, % of predicted | 3 (5%) | 2 (4%) | <0·0001 | 0 (0%) | 2 (2%) | 0·16 | 9 (13%) | 6 (9%) | 0·08 |
| FEV1/FVC <70% | 5 (8%) | 4 (7%) | 0·32 | 11 (9%) | 6 (5%) | 0·10 | 2 (3%) | 0 (0%) | 0·16 |
| TLC <80%, % of predicted | 6/57 (11%) | 3 (5%) | 0·18 | 12/124 (10%) | 8/117 (7%) | 0·65 | 27/69 (39%) | 20 (29%) | 0·021 |
| FRC <80%, % of predicted | 4/57 (7%) | 6 (11%) | 0·32 | 5/124 (4%) | 5/116 (4%) | 1·00 | 14/67 (21%) | 16 (23%) | 1·00 |
| RV <80%, % of predicted | 12/57 (21%) | 15 (27%) | 1·00 | 18/124 (15%) | 26/117 (22%) | 0·050 | 34/69 (49%) | 44 (63%) | 0·11 |
| DLCO <80%, % of predicted* | 12/57 (21%) | 13 (23%) | 0·53 | 32/124 (26%) | 36/117 (31%) | 0·13 | 39/69 (57%) | 38 (54%) | 0·53 |
| Chest CT | |||||||||
| Number of patients | 33 | 28 | .. | 56 | 52 | .. | 39 | 38 | .. |
| At least one abnormal CT pattern | 33 (100%) | 11 (39%) | <0·0001 | 56 (100%) | 21 (40%) | <0·0001 | 39 (100%) | 33 (87%) | 0·025 |
| GGO | 28 (85%) | 11 (39%) | 0·0047 | 52 (93%) | 14 (27%) | <0·0001 | 32 (82%) | 29 (76%) | 0·56 |
| Irregular lines | 6 (18%) | 6 (21%) | 1·00 | 13 (23%) | 12 (23%) | 1·00 | 18 (46%) | 23 (61%) | 0·22 |
| Subpleural line | 5 (15%) | 1 (4%) | 0·10 | 1 (2%) | 2 (4%) | 0·56 | 3 (8%) | 8 (21%) | 0·06 |
| Interlobular septal thickening | 1 (3%) | 0 (0%) | 0·32 | 2 (4%) | 1 (2%) | 0·56 | 0 (0%) | 4 (11%) | 0·046 |
| Reticular pattern | 0 (0%) | 0 (0%) | NA | 0 (0%) | 1 (2%) | 0·32 | 1 (3%) | 3 (8%) | 0·16 |
| Consolidation | 0 (0%) | 0 (0%) | NA | 4 (7%) | 0 (0%) | 0·08 | 0 (0%) | 1 (3%) | 0·32 |
Data are absolute values, n (%), or n/N (%) when data are missing. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. FEV1=forced expiratory volume in 1 s. FVC=forced vital capacity. TLC=total lung capacity. FRC=functional residual capacity. RV=residual volume. DLCO=diffusion capacity for carbon monoxide. GGO=ground glass opacity. NA=not applicable.
Carbon monoxide diffusion capacity was not corrected for haemoglobin.
Of 186 patients with abnormal lung CT at 6-month visit, 128 attended the 12-month visit but ten refused to do the test (table 3). The lung imaging abnormality gradually recovered during 1-year follow-up. The proportion of patients with abnormal CT decreased significantly from 6 months to 12 months in all three groups (table 3; all p<0·05). The proportions of ground glass opacity (GGO) in the three groups all decreased over time, and 76% (29/38) of patients with severity scale 5–6 still had GGO at 12 months. The proportion of patients in the scale 5–6 group with interlobular septal thickening significantly increased over time (0 at 6 months vs 11% [four of 38] at 12 months, p=0·046). 105 patients completed both pulmonary function tests and CT at 12 months. The appendix p 27 shows the association between lung imaging pattern and lung diffusion impairment. When adjusting for age, sex, cigarette smoking, education, comorbidity, and disease severity, GGO and irregular lines were positively associated with risk of lung diffusion impairment. After further adjustment for corticosteroids, antivirals, and intravenous immunoglobulin, the point estimate of ORs did not change substantially although the statistically significant associations did not remain (appendix p 27).
1252 COVID-19 patients at the 12-month visit reported their health-care use after discharge and work status (table 4 ). After COVID-19 discharge from hospital, 228 (18%) patients visited the outpatient clinic, 13 (1%) visited the emergency department, and 161 (13%) were admitted to hospital, but no one was admitted to ICU. 11 patients visited the rehabilitation department and five participated in the professional rehabilitation programme because of physical dysfunction.
Table 4.
Health-care use after discharge until 12-month follow-up, and work status at 12-month follow-up among COVID-19 patients
| COVID-19 patients (%) (N=1252)* | ||
|---|---|---|
| Health-care use | ||
| Outpatient clinic visit | 228/1252 (18%) | |
| Cardiology | 40/1252 (3%) | |
| Pulmonology | 31/1252 (2%) | |
| Gastroenterology | 22/1252 (2%) | |
| Endocrinology | 19/1252 (2%) | |
| Rehabilitation | 11/1252 (1%) | |
| Nephrology | 12/1252 (1%) | |
| Neurology | 10/1252 (1%) | |
| Psychology | 1/1252 (0%) | |
| Others | 108/1252 (9%) | |
| Hospitalisation | 161/1252 (13%) | |
| Respiratory disease | 21/1252 (2%) | |
| Cardiac disease | 14/1252 (1%) | |
| Hypertension | 9/1252 (1%) | |
| Diabetes | 9/1252 (1%) | |
| Neurological disorders | 8/1252 (1%) | |
| Physical examination | 5/1252 (0%) | |
| Kidney disease | 4/1252 (0%) | |
| Others | 100/1252 (8%) | |
| Emergency department visit | 13/1252 (1%) | |
| Professional rehabilitation programme | 5/1252 (0%) | |
| ICU admission | 0 | |
| Work status before COVID-19 | ||
| Retired | 658/1252 (53%) | |
| Full-time or part-time job | 479/1252 (38%) | |
| Jobless | 70/1252 (6%) | |
| Homemaker | 41/1252 (3%) | |
| Full-time student | 4/1252 (0%) | |
| Work status at 12-month follow-up | ||
| Returned to original work | 422/479 (88%) | |
| Returned to pre-COVID-19 level of work | 321/422 (76%) | |
| Not returned to pre-COVID-19 level of work | 101/422 (24%) | |
| Not returned to original work | 57/479 (12%) | |
| Due to decreased physical function | 18/57 (32%) | |
| Unwilling to return to original work | 14/57 (25%) | |
| Unemployment | 10/57 (18%) | |
| Others | 15/57 (26%) | |
Data are n or n/N (%). ICU=intensive care unit.
Of 1276 patients who completed both 6-month and 12-month follow-up visits, the results of 1252 patients were available because 24 were unwilling to complete the questionnaire.
Before COVID-19, 53% (658/1252) of patients had retired and 38% (479/1252) had a full-time or part-time job. At 12-month visit, of those 479 patients who had a job before COVID-19, 422 (88%) had returned to their original work and most of these patients (321/422, 76%) had returned to their level of work before COVID-19. 57 (12%) of 479 patients did not return to their original work: 32% (18/57) because of decreased physical function, 25% (14/57) because they were unwilling to do the previous work, and 18% (ten of 57) because of unemployment (table 4).
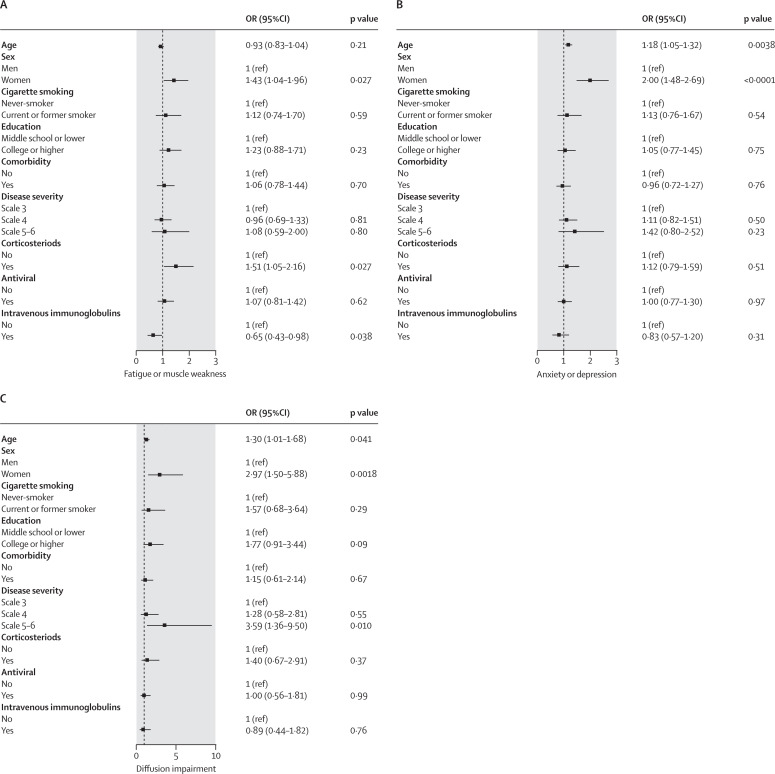
After multivariable adjustment, participants with severity scale 5–6 had higher risk of diffusion impairment at 12 months than did those with scale 3 (OR 3·59, 95% CI 1·36–9·50), but no significant difference in fatigue or muscle weakness (1·08, 0·59–2·00) and anxiety or depression (1·42, 0·80–2·52; figure 2 ). Compared with men, women had an OR of 1·43 (95% CI 1·04–1·96) for fatigue or muscle weakness, 2·00 (1·48–2·69) for anxiety or depression, and 2·97 (1·50–5·88) for diffusion impairment (figure 2). Corticosteroids therapy at acute phase was associated with increased risk of fatigue or muscle weakness (OR 1·51, 95% CI 1·05–2·16). Intravenous immunoglobulin therapy at acute phase decreased the risk of fatigue or muscle weakness (OR 0·65, 95% CI 0·43–0·98). We observed no significant association of corticosteroids therapy and intravenous immunoglobulin therapy with anxiety or depression, or diffusion impairment (figure 2). Age was positively associated with anxiety or depression and diffusion impairment, with the risk of anxiety or depression 18% higher (OR 1·18, 95% CI 1·05–1·32) and risk of diffusion impairment 30% higher (1·30, 1·01–1·68) per 10-year increase of age. There was no significant association between age and fatigue or muscle weakness (figure 2).
Figure 2.
Risk factors associated with fatigue or muscle weakness (A), anxiety or depression (B), and diffusion impairment (C) at 12-month follow-up
For the association of disease severity with outcome, age, sex, cigarette smoking, education, comorbidity, corticosteroids, antivirals, and intravenous immunoglobulin were adjusted. For the association of factors including sex, corticosteroid, antiviral, and intravenous immunoglobulin with outcome, the aforementioned variables were all included in the model. When exploring the associations of education and smoking with outcome, the aforementioned variables except for comorbidity, and both comorbidity and disease severity (due to the potential mediation) were included, respectively. Only sex, smoking, and education were adjusted for the association between age and outcome due to the potential mediation of other factors. For the association of comorbidity with outcome, the aforementioned variables except for disease severity were all included. OR (95% CI) for age indicates the risk of fatigue or muscle weakness, anxiety or depression, and diffusion impairment per 10-year age increase. OR=odds ratio.
Sensitivity analysis with inverse probability-weighted generalised estimating equations for risk factors with fatigue or muscle weakness, anxiety or depression, and diffusion impairment are shown in the appendix p 28. The associations of sex with three outcomes—age with diffusion impairment, corticosteroids with fatigue or muscle weakness, and disease severity with diffusion impairment—remained statistically significant. No significant effect of intravenous immunoglobulin therapy on fatigue or muscle weakness was observed, although the effect of age on fatigue or muscle weakness became statistically significant. Participants with scale 5–6 showed a higher risk of fatigue or muscle weakness and anxiety or depression than did those with scale 3 (appendix p 28).
We measured the plasma samples of 73 COVID-19 patients collected at the acute phase, discharge, 6-month visit, and 12-month visit. The concentrations of pro-inflammatory cytokines (IL-1β, IL-6, IL-12, GM-CSF, IFN-γ and TNF-α), anti-inflammatory cytokine (IL-10), and chemokines (IP-10, MCP-1, and MIP-1α) gradually decreased over time from onset of symptom until 12 months (appendix p 29). After adjusting for age, disease severity, and the sampling time after days of disease onset, we noted two intercorrelated cytokine clusters (IL-9, MIP-1β, and TNF-α in cluster one; IL-7, IL-17, and IFN-γ in cluster two; appendix p 33). The association of reduction of cytokines at discharge until 6 months with 12-month consequences is shown in the appendix pp 34–35. The greater reduction of IL-2, IL-5, IL-7, IL-12, and G-CSF was associated with lower risk of lung radiographic abnormality at 12 months (appendix p 34).
Discussion
To our knowledge, this is the largest longitudinal cohort study of hospital survivors with COVID-19 so far to describe the dynamic recovery of health consequences within 12 months after symptom onset. We found that most patients had a good physical and functional recovery during follow-up, and the majority of study participants who were employed before COVID-19 had returned to their original work. However, sequelae symptoms, lung diffusion impairment, and radiographic abnormalities persisted to 12 months in some patients, especially in patients who were critically ill during hospital stay. The current health status in the COVID-19 cohort was still lower than that in the control population.
A previous study of SARS has showed that the health status of survivors at 1 year after symptom onset was significantly lower than that of the general population,20 and lasted to 2 years.21 Fatigue was the most commonly reported symptom of patients with SARS, which could last as long as 4 years.22 We found that female sex and corticosteroid therapy at acute phase were risk factors for fatigue or muscle weakness at 12 months. The cause and pathogenesis of fatigue and muscle weakness after COVID-19 are unclear, but on the basis of previous evidence in SARS, lung diffusion capacity impairment and some extrapulmonary causes, including viral-induced myositis at initial presentation, cytokine disturbance, muscle wasting and deconditioning, or corticosteroids myopathy, or a combination of these factors, could have contributed to the condition.20, 21, 23, 24, 25
That dyspnoea and anxiety or depression were more frequently reported at 12 months than 6 months is worrying, although the increased proportion in our cohort is relatively low. COVID-19 survivors are at increased risk of psychiatric outcomes, and new-onset respiratory and cardiovascular disease during convalescence.26, 27 Al-Aly and colleagues28 reported that COVID-19 survivors had a high burden of incident use of bronchodilators, antitussives, expectorants, antidepressants, and anxiolytics after COVID-19. The chronic or late-onset psychological symptoms after COVID-19 could be driven by a direct effect of virus infection and might be explained by several hypotheses including aberrant immune response, hyperactivation of the immune system, or autoimmunity.28, 29 Additionally, indirect effects including reduced social contact, loneliness, incomplete recovery of physical health, and loss of employment could affect psychiatric symptoms.
At 12 months, we recorded high prevalence of lung diffusion impairment in patients with varying disease severity. Lung diffusion impairment could be attributable to lung epithelial damage, or interstitial or pulmonary vascular abnormalities.30, 31, 32 Lung structural abnormality during late recovery of SARS was associated with the lung diffusion impairment;33 however, the association during convalescence after COVID-19 was unclear. We undertook an initial exploratory analysis on the basis of a small group of patients and found that lung imaging patterns at 12 months might be associated with lung diffusion impairment, which should be confirmed in a larger sample study. Previous SARS follow-up studies have shown that persistent lung diffusion impairment could last for months or even years.20, 21, 23, 34 Hence, a longitudinal study is needed to describe the natural history of lung structural and functional abnormality after COVID-19, and to explore the effect of these persistent abnormalities on physical function and quality of life.
Our study had several limitations. First, the moderate response rate could have introduced bias to our study. Fortunately, we recorded no significant difference in most baseline characteristics between COVID-19 patients who were included in final analysis and those who were not. The sensitivity analyses for risk factors associated with primary outcomes that used generalised estimating equations to reduce the effect of bias also showed similar results. Second, this is a single centre study focused on previously hospitalised COVID-19 patients in the early stage of the pandemic, which limits the representativeness of this cohort. Moreover, a low proportion of patients with ICU admission in our cohort limits the generalisability of the study findings to this particular population. Future large sample studies are needed to evaluate the long-term consequences of COVID-19 in patients with varying severity, including outpatients, inpatients, and patients requiring admission to ICU. Third, we did not have the health status of COVID-19 survivors before acute infection. However, the health status of matched non-COVID-19 controls could represent the baseline state of COVID-19 patients, although residual confounders cannot be excluded. The comparison between COVID-19 patients and controls indicated whether COVID-19 patients completely recovered at 12 months. Finally, the small sample size of participants with cytokines tests could have affected the reliability of the association between change in cytokines concentrations and 12-month outcomes. These findings should be interpreted as exploratory and need to be validated in a future study with a lager sample.
Within 1 year after acute infection, most hospital survivors with COVID-19 had a good physical and functional recovery over time, and had returned to their original work and life, but current health status was still lower than that in the control population. Lung diffusion impairment and radiographic abnormalities were still common in critically ill patients at 12 months. Ongoing longitudinal follow-up is needed to better characterise the natural history and pathogenesis of long-term health consequences of COVID-19.
Data sharing
Restrictions apply to the availability of these data and so they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003 and 2020-I2M-CoV19-005); the National Natural Science Foundation of China (82041011/H0104); the National Key Research and Development Program of China (2018YFC1200102); and Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis (2020ZX09201001). This work was also supported by the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. We acknowledge all patients who participated in this study and their families. We thank community workers at Dongxihu district and Jiangan district, Wuhan City, for helping to recruit community-dwelling non-COVID-19 participants. We would also like to thank all staff of this follow-up study team at Jin Yin-tan Hospital.
Contributors
BC, XW, and JW had the idea for and designed the study. They had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. LH, BC, XG, YW, JXu, XZ, LR, and LG drafted the paper. BC, XG, LH, LR, and LG did the analysis, and all authors critically revised the manuscript for important intellectual content and agreed to submit the final version for publication. QY, QW, PH, YQ, YF, XL, CL, TY, JXia, MW, LC, YL, FX, DL, XG, and LH completed the follow-up work. YQ, YF, XL, CL, TY, JXia, MW, LC, YL, FX, DL, XG, LH, LG, LR, and ML collected and verified the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Material
References
- 1.WHO WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
- 2.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur Respir J. 2021;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinkmann F, Müller M, Reif A, et al. Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection. Eur Respir J. 2021;57 doi: 10.1183/13993003.03002-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco JR, Cobos-Ceballos MJ, Navarro F, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect. 2021;27:892–896. doi: 10.1016/j.cmi.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan X, Huang H, Wang C, et al. Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J Infect. 2021 doi: 10.1016/j.jinf.2021.05.034. published online May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021 doi: 10.1038/s41591-021-01433-3. published online June 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerner AM, Robinson DA, Yang L, et al. Toward understanding COVID-19 recovery: National Institutes of Health workshop on postacute COVID-19. Ann Intern Med. 2021;174:999–1003. doi: 10.7326/M21-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Lancet Respiratory Medicine COVID-19 pathophysiology: looking beyond acute disease. Lancet Respir Med. 2021;9:545. doi: 10.1016/S2213-2600(21)00242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 17.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 19.Xie W, Wu Y, Wang W, et al. A longitudinal study of carotid plaque and risk of ischemic cardiovascular disease in the Chinese population. J Am Soc Echocardiogr. 2011;24:729–737. doi: 10.1016/j.echo.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 23.Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su MC, Hsieh YT, Wang YH, Lin AS, Chung YH, Lin MC. Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome. Respiration. 2007;74:511–516. doi: 10.1159/000095673. [DOI] [PubMed] [Google Scholar]
- 25.Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 26.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 29.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanidziar D, Robson SC. Hyperoxia and modulation of pulmonary vascular and immune responses in COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;320:L12–L16. doi: 10.1152/ajplung.00304.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel BV, Arachchillage DJ, Ridge CA, et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20:1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu HH, Tzao C, Wu CP, et al. Correlation of high-resolution CT, symptoms, and pulmonary function in patients during recovery from severe acute respiratory syndrome. Chest. 2004;126:149–158. doi: 10.1378/chest.126.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of these data and so they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.