Abstract
Background
The risk of infectious disease transmission in public washrooms causes concern particularly in the context of the COVID-19 pandemic. This systematic review aims to assess the risk of transmission of viral or bacterial infections through inhalation, surface contact, and faecal-oral routes in public washrooms in healthcare and non-healthcare environments.
Methods
We systematically reviewed environmental sampling, laboratory, and epidemiological studies on viral and bacterial infection transmission in washrooms using PubMed and Scopus. The review focused on indoor, publicly accessible washrooms.
Results
Thirty-eight studies from 13 countries were identified, including 14 studies carried out in healthcare settings, 10 in laboratories or experimental chambers, and 14 studies in restaurants, workplaces, commercial and academic environments. Thirty-three studies involved surface sampling, 15 air sampling, 8 water sampling, and 5 studies were risk assessments or outbreak investigations. Infectious disease transmission was studied in relation with: (a) toilets with flushing mechanisms; (b) hand drying systems; and (c) water taps, sinks and drains. A wide range of enteric, skin and soil bacteria and enteric and respiratory viruses were identified in public washrooms, potentially posing a risk of infection transmission. Studies on COVID-19 transmission only examined washroom contamination in healthcare settings.
Conclusion
Open-lid toilet flushing, ineffective handwashing or hand drying, substandard or infrequent surface cleaning, blocked drains, and uncovered rubbish bins can result in widespread bacterial and/or viral contamination in washrooms. However, only a few cases of infectious diseases mostly related to faecal-oral transmission originating from washrooms in restaurants were reported. Although there is a risk of microbial aerosolisation from toilet flushing and the use of hand drying systems, we found no evidence of airborne transmission of enteric or respiratory pathogens, including COVID-19, in public washrooms. Appropriate hand hygiene, surface cleaning and disinfection, and washroom maintenance and ventilation are likely to minimise the risk of infectious disease transmission.
Keywords: Bacterial and viral contamination, Environmental exposure, SARS-CoV-2, Aerosol, Hand drying, Public toilet
Graphical abstract
1. Introduction
The COVID-19 pandemic has raised concerns about the potential risk of disease transmission in public washrooms (toilets) via direct inhalation of aerosolised viruses or contact with surfaces contaminated by respiratory droplets or faecal waste. Faecal shedding seems to occur in COVID-19 patients with or without gastrointestinal symptoms (Gu et al., 2020), which could enable asymptomatic individuals with no respiratory symptoms to be a potential source of faecal transmission (McDermott et al., 2020). This has been indicated as a possible risk in both healthcare (Lane et al., 2020) and non-healthcare (Luo et al., 2020; Wan et al., 2021) settings. Anecdotal evidence suggests that public washrooms have been avoided by users due to the real or perceived risk of COVID-19 transmission in these environments during the pandemic (e.g. Calechman, 2020).
In general, routine use of washrooms may result in the dispersal of urine- and faecal-derived microbiota, including pathogens and opportunistic pathogens (i.e. microorganisms that do not usually infect healthy hosts but may produce infections in immunocompromised persons or in those with certain underlying diseases), and surface contamination is typically found to be higher in public toilets compared to domestic toilets (Flores et al., 2011; Gerhardts et al., 2012). Washrooms in public settings such as commercial sites, workplaces, and healthcare environments, can be unhygienic if subject to high use, and infrequent or substandard cleaning and maintenance. This, combined with the greater number of individual washroom visitors in a public setting compared to a private one, likely increases the microbial diversity in the public washroom environment, and possibly the risk of infection, particularly via hand-to-mouth transmission of bacteria and viruses, as frequently touched surfaces may be contaminated with pathogens of faecal or urine origin (Flores et al., 2011). Metal, plastic, wood, ceramic and textile surfaces in public washrooms can all serve as pathogen reservoirs (Gerhardts et al., 2012). Studies investigating this in non-healthcare environments focused primarily on surface contamination with bacteria of faecal or skin origin, or from vomiting (Flores et al., 2011; Gerhardts et al., 2012; Mkrtchyan et al., 2013). Other studies, mainly in healthcare settings, examined water and wastewater contamination (Breathnach et al., 2012; Halabi et al., 2001), and potential aerosolisation of pathogens through toilet flushing (Knowlton et al., 2018), showering (Feazel et al., 2009) and hand drying (Gammon and Hunt, 2019). It has been suggested that the presence of pathogens (e.g., Escherichia coli, Enterovirus, Norovirus, and Rotavirus) may be associated with increased risk of infection in settings where aerosols are contaminated by sewage (Carducci et al., 2016).
The main aim of this systematic review is to assess the risk of transmission of infectious diseases, including COVID-19 and other viral or bacterial infections, through inhalation, surface contact, and faecal-oral routes in public washrooms. We examine in particular: (a) the risk of transmission of infectious diseases in washrooms by using electric hand dryers, paper towels, water taps, flushing toilets, or touching other surfaces; (b) the dominant route and potential range of transmission of infectious diseases in washrooms; and (c) the personal precautions, environmental hygiene and washroom design measures that can reduce transmission risk. We focus on real-world healthcare and non-healthcare settings in high and middle-income countries where indoor washrooms are publicly accessible by a wide range of users.
2. Methods
This systematic review and search strategy were prospectively registered in the PROSPERO database (CRD42020203238) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009).
2.1. Search strategy
We searched PubMed and Scopus databases for studies investigating transmission of viral or bacterial infectious diseases in indoor washrooms in public, workplace, healthcare, commercial, entertainment, sport, and educational establishments. Additional manual searches in the reference lists of relevant reviews and guidelines were carried out. We included studies of any experimental or epidemiological design (including laboratory-simulated washroom conditions), but excluded studies reporting exclusively on patient subgroups, mathematical modelling simulations, or based entirely on questionnaires. We also excluded studies reporting exclusively on transmission of non-human infectious diseases (apart from laboratory studies using model organisms), bacterial infections typically associated with food-borne pathogens, such as Salmonella or Campylobacter, parasitic infections associated with contaminated water, such as Cryptosporidium or Giardia, or fungal infections (see also Supplementary materials).
As this review focuses on publicly accessible indoor washrooms with common features such as toilets, sinks and hand dryers, we excluded data reported exclusively for: outdoor washrooms, toilets, or latrines such as those in rural settings, holiday camps, and informal or low income settings; transport microenvironments such as those in aircrafts, trains, coaches or ships; and private washrooms, such as those in domestic environments and hotel rooms, or in healthcare settings not accessible by the general public (e.g. patient rooms, operating theatres, intensive care units, and isolation units). We also excluded public washrooms and shower rooms (e.g. in sports centres) without toilet facilities within the same space.
2.2. Screening and data extraction
All records were managed in EndNote version X7.1.1, and duplicates were removed using the in-built software function. All remaining records were independently double-screened by title, abstract, and full-text against eligibility criteria. To complement the online database searches, we also manually screened bibliographies of retrieved studies.
Data were double-extracted and any discrepancies were resolved by consensus between authors. We created an extraction spreadsheet and piloted it with a few studies before starting full-text screening and data extraction (see Supplementary materials).
2.3. Quality assessment
Two authors independently assessed the quality of studies based on a modified version of the NIH (National Institutes of Health) Quality Assessment Tool for Observational Cohort and Cross-sectional Studies. We assessed studies using all the items of the NIH tool and additionally assessed whether studies had appropriate analytical or statistical methods, whether conditions of experimental studies were realistic, and whether quality assurance and/or quality control steps were verifiable to ensure data quality (see Supplementary materials).
3. Results
Overall, 3049 titles were identified through the bibliographic database searches and 19 through manual searches. After screening these records, 65 full-text articles were obtained and assessed for eligibility, with 38 of them included in the evidence synthesis (Fig. 1 ). The eligible studies were from 13 countries, with a relatively high number of studies from the UK and USA (Supplementary materials, Fig. S1).
Fig. 1.
Numbers of papers at each stage of the review process (PRISMA diagram).
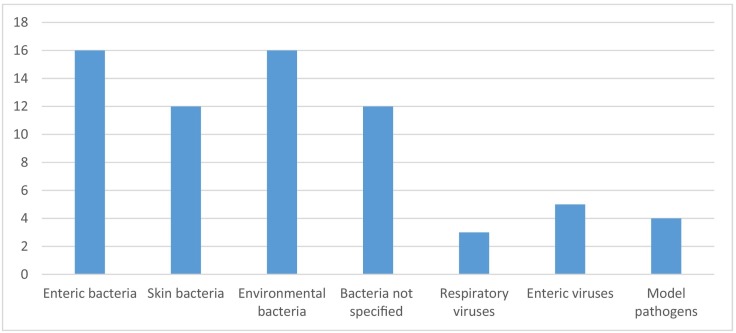
A wide range of bacteria and viruses were targeted and/or identified in these studies, with the most studied species being enteric bacteria (e.g. Escherichia coli), skin bacteria (e.g. Staphylococcus spp.), and common environmental spore-forming bacteria (e.g. Bacillus spp.) (Fig. 2 ).
Fig. 2.
Number of eligible studies examining different categories of microorganisms.
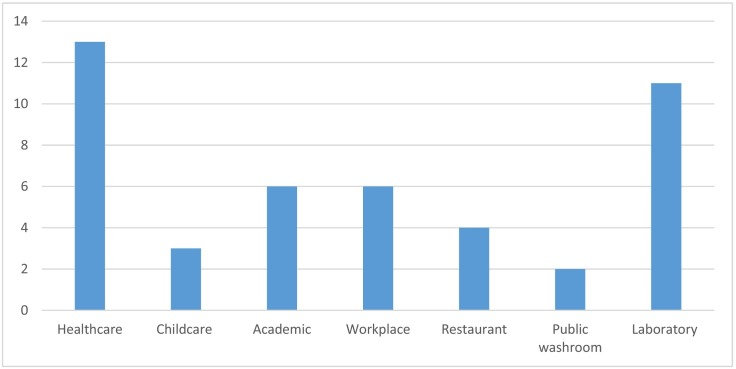
Of the 38 eligible studies, 13 were carried out in shared toilets in healthcare settings, 11 were laboratory experiments, and the rest were conducted in a range of workplace, commercial, academic (i.e., universities, schools), restaurant, or other public washroom environments (Fig. 3 ).
Fig. 3.
Study settings in eligible studies (four studies included multiple settings).
Most studies (n = 33) involved surface sampling with swabs, and/or air sampling (n = 15) using wet/dry active samplers or settle plates, with fewer studies (n = 8) conducting water sampling (including tap, sink and toilet bowl water). A smaller number of studies (n = 5) involved risk assessment or outbreak investigation.
A full list of the extracted information can be found in the Supplementary materials, while a summary of the studies is included in Table 1 .
Table 1.
Included studies (JAD: jet air dryer, WAD: warm air dryer, PT: paper towel, CFU: colony forming units, PFU: plaque forming units, GC: genomic copies, SD: standard deviation).
| Reference | Study characteristics | Microorganisms analysed/identified | Aerosol and droplet sampling (incl. air and deposition samples) | Other environmental sampling (incl. surface swabs and water samples) |
|---|---|---|---|---|
| Aithinne et al., 2019 |
Country: USA Setting: Laboratory Design: Toilet bowl water inoculated with Clostridium difficile; culture-based analysis Activity: Toilet flushing (seat down with open lid) Contaminated area/medium: Air, toilet bowl water, floor |
Clostridium difficile |
First 3 flushes: ~8, 3, 2.5 CFU counts, respectively; equivalent airborne droplet nuclei spore aerosol ~10.9, 3.8, 3.4 CFU/m3, respectively. Considering 5 m3toilet chamber size: droplet nuclei bioaerosol generation rate was ~54, 19, 17 CFU/flush, respectively. |
Water (>24 flushes): Spores captured in all trials, indicating persistent contamination. Approximate bowl water concentrations:
Floor: usually 1 or 2 CFU (max 4 CFU). All plates around toilet had at least 1 positive sample. Cumulative area density for all plates was 533 CFU/m2 after 24 flushes; 75% of this level attained after 4 flushes, 90% attained after 9 flushes. |
| Alharbi et al., 2016 |
Country: Saudi Arabia Setting: Academic Design: Culture-based analysis of ambient microbiome in university washrooms; Isolates identified using VITEK2R Activity: Hand drying (WAD) Contaminated area/medium: Airflow from warm air dryer |
Staphylococcus haemolyticus, Micrococcus luteus, Pseudomonas alcaligenes, Bacillus cereus and Brevundimonas diminuta/vesicularis |
Bacteria isolated per sampled dryer after exposure to airflow for 30 s:
|
N/A |
| Best et al., 2018 |
Country: UK, France, Italy Setting: Healthcare Design: 2 washrooms tested at 3 hospitals (1 each in the UK, France, Italy); 20 L air sample; culture-based analysis using both non-selective and selective media Activity: Hand drying (JAD, PT) Contaminated area/medium: Air, jet air dryer unit, paper towel dispenser, sink, door, floor, dust |
Total aerobic count; enterococci and vancomycin resistant enterococci (VRE); enterobacteria incl. Escherichia coli, Klebsiella spp., and extended spectrum β-lactamase (ESBL)-producing enterobacteria; Staphylococcus aureus and methicillin resistant S. aureus (MRSA); C. difficile |
Median counts in UK, France, and Italy, respectively:
|
Median counts in UK, France, and Italy, respectively:
Enterococci: greater recovery in floor and dust for JAD vs PT in UK; very low recovery in France and Italy. Enterobacteria: greater recovery in unit and floor for JAD vs PT in UK; greater recovery in dust for JAD vs PT in France; very low recovery in Italy. S. aureus: greater recovery in unit and floor for JAD vs PT in UK; very low recovery in France; no recovery in Italy. MRSA: very low recovery, but greater on floor in JAD vs PT in UK; no recovery in France and Italy. ESBL-producing bacteria: greater recovery on floor in JAD vs PT in UK; low recovery in France and Italy. C. difficile was not recovered from any samples in any country. |
| Best and Redway, 2015 |
Country: UK Setting: Not described Design: Gloved hands contaminated with Saccharomyces cerevisiae, or volunteers' hands naturally contaminated following toileting; culture-based analysis Activity: Hand drying (JAD, WAD, PT, textile roller towel) Contaminated area/medium: Wall and floor around hand drying unit |
Saccharomyces cerevisiae | N/A | JAD dispersed more bacteria than WAD, PT and textile roller towel. Vertical dispersal (height) during hand drying:
|
| Best et al., 2014 |
Country: UK Setting: Experimental setting not identified, 65 m3 room Design: Gloved hands contaminated with lactobacilli or black paint; culture-based analysis with lactobacillus-selective agar plates Activity: Hand drying (JAD, WAD, PT) Contaminated area/medium: Air and floor around hand drying unit |
Lactobacillus |
Mean counts after 15min; for 10 cm and 1 m away, respectively:
|
Mean counts under dryer,and 1 m and 2 m away, respectively:
|
| Best et al., 2012 |
Country: UK Setting: Healthcare, controlled experiment Design:C. difficile spiked faecal suspensions poured into toilet bowls to mimic diarrhoea; culture-based analysis Activity: Toilet flushing (seat down with open and closed lid) Contaminated area/medium: Air, toilet cistern, toilet seat, floor |
C. difficile |
Mean counts 0–30, 30–60, and 60–90 min after flush:
|
Droplets of varying size were ejected to the height of the seat upon flushing. Lid closed: No Clostridium difficile recovered on any surface. Lid open:Clostridium difficile recovered at all locations (mean 1–3 CFU), except floor on left-hand side. |
| Boone and Gerba, 2005 |
Country: USA Setting: Childcare and household Design: Fomites (e.g. door handles, light switches, children's toys) sampled in homes and day care centres, periodic sampling over a 2.5-year period; environmental swabs and RT-PCR analysis Activity: General toilet/station use Contaminated area/medium: Toilet seat, floor, faucet, diaper changing station |
Influenza A virus | N/A |
Seasonal Influenza A virus positive samples: 53% spring vs 23% fall. Influenza A virus positive samples on surfaces:
|
| Boxman et al., 2009a |
Country: Netherlands Setting: Restaurant Design: Outbreak investigation; clinical and environmental swabs and RT-PCR analysis Activity: General toilet use, vomiting in toilet, food handling Contaminated area/medium: Toilet seat |
Norovirus | N/A | Norovirus present in 4 of 9 samples: male and female toilet seats, grip of the knife used to cut bread, and hands of ill food handler cutting bread for restaurant guests. |
| Boxman et al., 2009b |
Country: Netherlands Setting: Restaurant Design: Outbreak investigations; clinical and environmental swabs and RT-PCR analysis Activity: General toilet use, food handling Contaminated area/medium: Toilet seat, toilet handle or tap |
Norovirus | N/A | Norovirus present in 48 of 119 (40%) samples from 14 of 27 (52%) outbreaks. Norovirus RNA was most often found on swabs taken in bathrooms (64%), with 10/18 samples positive (excl. cruise ship and summer camp). |
| Breathnach et al., 2012 |
Country: UK Setting: Healthcare Design: Outbreak investigations; clinical and environmental swabs; culture-based testing, antimicrobial susceptibility testing, and molecular typing (serotyping, PFGE, VNTR) Activity: General hospital activities Contaminated area/medium: Toilet, faucet, sink drain trap (U-bend), shower head, shower drain, water, ward sluice room, toilet brush |
Multidrug-resistant Pseudomonas aeruginosa | N/A |
Outbreak 1: Waste outlets on intensive care and haematology positive for outbreak strain indicated reservoir of organism in waste pipe system; sewer water sample yielded organism, but not known clinical case of Pseudomonas aeruginosa for several months at time of testing. Mean 391 notifications of blocked sinks, toilets or sluices in the hospital each year (2005–2010). Outbreak 2: Shower drain, toilet bowl, and toilet brush positive for outbreak strain; incoming water for drinking, hand washing, and showering negative. Pseudomonas aeruginosa not isolated from cleaning equipment, soap, and skin antiseptic preparations. Blockages partly due to paper towels and clinical wipes down toilet. |
| Carducci et al., 2016 |
Country: Italy Setting: Healthcare, workplace Design: Used previously published/collected data to develop a preliminary quantitative microbial risk assessment (QMRA) model for Human Adenovirus contaminated workplace environments. Activity: N/R Contaminated area/medium: Air |
Human Adenovirus | Human Adenovirus detected in all settings, with highest concentration in indoor environments. Average concentration range: 2 log10 GC/m3 outside landfill to 8 log10 GC/m3 in hospital toilets. Human Adenovirus concentration in toilets:
|
N/A |
| Cooper et al., 2016 |
Country: Canada Setting: Healthcare Design: Air and surface samples, culture-based analysis Activity: Toilet flushing Contaminated area/medium: Air, toilet seat, sink counter |
Anaerobic and aerobic bacteria |
Aerobic bacterial concentration (GM):
Anaerobic bacterial concentration (GM):
|
Bacterial concentration UVC-treated vs control (GM):
|
| Flores et al., 2011 |
Country: USA Setting: Academic Design: Surface sampling in public washrooms, culture-independent analysis; 16S rRNA sequencing Activity: General toilet use Contaminated area/medium: Door handle, stall handle, faucet handle, soap dispenser, toilet seat, toilet handle, floor |
Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria dominated the 19 phyla identified | N/A | 19 phyla observed across all surfaces, most sequences (<92%) as Actinobacteria, Bacteriodetes, Firmicutes, or Proteobacteria. Environmental source of bacteria on surfaces:
|
| Gerhardts et al., 2012 |
Country: Germany Setting: Laboratory Design: Transmission experiments; hand > non-porous surface > hand (x4); culture-based analysis Activity: Surface contact transmission model Contaminated area/medium: Toilet brush, plastic tube (representing door handle), acrylic glass rod (representing faucet handle) |
Escherichia coli, Bacillus subtilis (atrophaeus), MS2 bacteriophage | N/A |
Amount of bacteria transferred onto the toilet brush, door handle, handle, and hand of person 4, respectively:
|
| Gormley et al., 2017 |
Country: UK Setting: Laboratory Design: Model organism inoculated into a pilot test rig to investigate within-building transmission potential due to defective plumbing (dry U-traps); culture-base analysis Activity: Toilet flushing Contaminated area/medium: Air, toilet bowl, toilet seat |
Pseudomonas putida |
Passive airsampling: Cross-transmission of viable bacteria can occur between adjacent floors of a sanitary plumbing system: toilet flushing with wastewater on a lower floor contaminated the room on the upper floor with aerosols. This occurred both with an induced upward airflow and without; however, cross-contamination was less severe in absence of airflow. Active airsampling: Cross-transmission of bacteria through entire sanitary plumbing test-rig: from flushing contaminated wastewater at lower floor, into test chamber, and then into extract ventilation system. |
With upward airflow: Bacterial CFU on top, right and front walls of test chamber. With no induced airflow: Bacterial CFU only on bottom surface; thus cross-transmission even in absence of applied airflow. With partially-filled U-trap: Bacterial CFU on toilet, test chamber surfaces, and duct connected to extract fan; toilet contamination higher than test chamber, and focused towards front of toilet bowl probably influenced by draw of extract fan. |
| Halabi et al., 2001 |
Country: Austria Setting: Healthcare Design: water quality analysis; membrane filtration method; culture-based analysis; total CFU and selective media Activity: N/R Contaminated area/medium: Water from conventional and non-touch faucets |
Pseudomonas aeruginosa, Legionella spp. | N/A |
Faucet water samples contaminated withPseudomonas aeruginosa:
Faucet water samples contaminated with Legionella spp.:
|
| Harrison et al., 2003 |
Country: UK Setting: Laboratory Activity: Hand drying (PT) Contaminated area/medium: Paper towel dispenser, used paper towel |
Micrococcus luteus, Serratia marcescens | N/A |
Average bacterial transfer from contaminated hand to dispenser:
Average transfer rate from contaminated dispenser to hand and towel:
|
| Huesca-Espitia et al., 2018 |
Country: USA Setting: Academic Design: Investigated the effect of retrofitting HEPA filters to warm air hand dryers; agar plates exposed to hand dryer air in bathroom settings; culture-based analysis with isolate identification by MALDI-TOF Biotyper Activity: Hand drying (WAD) Contaminated area/medium: Airflow from dryer, washroom air, inner surface of warm air drier nozzle |
Bacterial CFU, Acinetobacter baumannii, Acinetobacter radioresistens, Bacillus cereus, Bacillus infantis, Bacillus licheniformis, Bacillus marisflavi, Bacillus megaterium, Bacillus pumilus, Bacillus simplex, Bacillus subtilis, kanamycin-resistant Bacillus subtilis, Erwinia sp., Exiguobacterium aurantiacum, Kocuria rhizophila, Micrococcus luteus, Pantoea septica, Paracoccus yeei, Pseudomonas luteola, Roseomonas mucosa, Staphylococcus aureus, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus pasteuri, Staphylococcus simulans |
Bacterial counts from hand dryer vs environmental air:
|
Hand dryer nozzles with and without HEPA filters:
Bacterial deposition by hand dryers:
Bacteria recovered following exposure to hand dryers or washroom air:
|
| Inkinen et al., 2017 |
Country: Finland Setting: Healthcare, childcare, workplace, retirement home Design: Investigated bacterial loads on copper surfaces vs chromed, plastic or wooden surfaces; surface swabs and culture-based analysis; selective plating for indicator bacteria Activity: N/R Contaminated area/medium: Toilet support rail, toilet flush button, door handle, floor drain |
Bacterial CFU, Enterobacteriaceae, coagulase positive Staphylococcus, Staphylococcus aureus | N/A |
Total bacterial counts across different types of materials:
Enterobacteriaceaeand Gram-negative rods positive samples across different types of materials:
Enterococcipositive samples across different types of materials:
Staphylococcus aureuspositive samples across different types of materials:
|
| Kanayama Katsuse et al., 2017 |
Country: Japan Setting: Healthcare Design: surface swabs and culture-based analysis to investigate bacterial loads on toilet seat and bidet nozzles; antimicrobial susceptibility testing; PCR antimicrobial resistance gene screening; PFGE; and sequencing Activity: General bidet-toilet use Contaminated area/medium: Toilet seat, warm-water bidet nozzle |
Enterobacteriaceae, Enterobacter spp., Enterococcus spp., Streptococcus spp., Klebsiella spp., Citrobacter spp., Acinetobacter spp., non-glucose fermenting rods, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, extended-spectrum β-lactamase (ESBL)-Escherichia coli, Stenotrophomonas maltophilia, Pseudomonas aeruginosa | N/A |
Number of toilets sampled positive for bacteria on bidet nozzle and toilet seat:
|
| Katano et al., 2014 |
Country: Japan Setting: Public restroom, residential Design: water sampling and culture-based analysis to investigate bacterial loads in bidet lavage water; selective media Activity: Bidet-toilet use Contaminated area/medium: Water from bidet lavage tanks |
Pseudomonas aeruginosa, Escherichia coli | N/A |
Mean total bacteria counts in different settings:
|
| Kimmitt and Redway, 2016 |
Country: UK Setting: Laboratory Design: Gloved hands contaminated with MS2 bacteriophage; culture-based analysis Activity: Hand drying (JAD, WAD, PT) Contaminated area/medium: Air, vertical board/wall |
MS2 bacteriophage |
Mean total viral plaques after 0–2.5, 2.5–5, 5–7.5, 7.5–10, 10–12.5, and 12.5–15 min of drying device use:
|
Mean total viral plaques across all heights (0.15–1.65 m) at 0.4m vs plaques set at 0.71m height across all distances (0–3 m) from drying units:
|
| Knowlton et al., 2018 |
Country: USA Setting: Healthcare Design: Bioaerosol sampling; culture-based analysis Activity: Toilet flushing (with and without waste) Contaminated area/medium: Air |
Bacterial CFU |
Mean bioaerosol concentration at different conditions:
|
N/A |
| Kouadri, 2020 |
Country: Saudi Arabia Setting: Academic Design: Bathroom handwashing conditions; Selective media; culture-based; antimicrobial susceptibility testing of 16 isolates Activity: Hand drying (WAD, PT) Contaminated area/medium: Airflow from dryer, air around warm air dryer, inner surface of dryer nozzle |
Bacterial CFU, multi-drug resistant Escherichia coli, Klebsiella spp., Staphylococcus aureus, Bacillus cereus, coagulase-negative Staphylococcus spp. |
Mean bacterial number recovered in different settings:
|
Swabs from WAD nozzle found 43 bacterial colonies. |
| Kurgat et al., 2019 |
Country: USA Setting: Workplace Design: Used viral tracers (MS2 phage) to identify office environment fomites and evaluate hygiene intervention; culture-based analysis Activity: General toilet use Contaminated area/medium: Soap dispenser, faucet handle, restroom door handle |
MS2 bacteriophage | N/A |
Mean concentration on surfaces across different conditions:
|
| Margas et al., 2013 |
Country: UK Setting: Laboratory Design: Settle plates, air sampling and surface swabs; culture-based analysis Activity: Hand drying (JAD, PT) Contaminated area/medium: Air, floor, wall, sink, soap dispenser, jet air dryer unit, paper towel dispenser |
Coliform bacteria CFU | No significant difference between drying method; however, bacterial level increased rapidly after starting hand washing and drying. Bacterial counts after 3 min vs end of trial by distance from device:
|
Mean bacterial counts on settle plates exposed to washroom air for 1 h by distance from device:
Mean bacterial counts on surfaces after 100 people washed and dryed hands according to JAD or PT use:
|
| Mkrtchyan et al., 2013 |
Country: UK Setting: Public restroom Design: Selective culture-based analysis; 16S rRNA sequencing and MALDI-TOF for identification; culture-based antimicrobial susceptibility testing; PCR for mec and ccr genes Activity: General toilet use Contaminated area/medium: Hand dryer unit, toilet seat, stall door surface, tap, soap dispenser, urinal floor |
Staphylococcus, Bacillus, Micrococcus, Escherichia, Proteus, Citrobacter, Morganella, Acinetobacter, Corynebacterium, Delftia, Sphingobacteria, Campylobacter, Pseudomonas, Korucia, Rothia, Arthrobacter, Anaerococcus, Rhodococcus | N/A | Most contaminated surfaces were hand dryer, toilet seat, inner door, tap, soap dispenser, and urinal floor. Number of isolates identified by genera:
|
| Mohamed et al., 2015 |
Country: USA Setting: Healthcare, restaurant, shopping centre, supermarket, public park, gas station Design: Culture-based; isolates subject to virulence genotyping, phylotyping, clonal typing, PFGE, and disc diffusion AST Activity: General toilet/station use Contaminated area/medium: Toilet seat, toilet surfaces, toilet water, floor near toilet, in-stall sanitary napkin receptacle, stall handle, sink drain, faucet tap, diaper changing station handle |
Escherichia coli, extraintestinal pathogenic Escherichia coli (ExPEC), antimicrobial-resistant Escherichia coli | N/A | 25/1120 (2.2%), or 14.9% of fluorescent cultures, from 18/56 (32%) washrooms had confirmed Escherichia coli isolates. 10/1120 (0.9%), or 40% of confirmed Escherichia coli samples, from 9/56 (16%) washrooms had presumptive ExPEC; 8 samples with confirmed ExPEC. Prevalence ofEscherichia coliand ExPC isolates by washroom category:
Prevalence ofEscherichia coliand ExPC isolates by washroom gender:
|
| Patrick et al., 2010 |
Country: New Zealand Setting: Childcare Design: Culture-based Activity: Hand drying (WAD, PT, cloth towel) Contaminated area/medium: Chamois cloth (representing skin), liquorice straps (representing food), pipette tip (representing toy) |
Bacterial CFU | N/A |
Mean bacterial counts across different surfaces at baseline, usual practice, and dual hand drying, respectively:
|
| Pitt et al., 2018 |
Country: UK Setting: Academic Design: Culture-based Activity: Hand drying (JAD, WAD, PT) Contaminated area/medium: Wall, floor under dryer or paper towel dispenser, trough of jet air dryer unit, side and underside of warm air dryer, paper towel dispenser knob |
Bacterial CFU, Staphylococcus spp., Staphylococcus aureus, Staphylococcus epidermis, Staphylococcus haemolyticus, Pantoea agglomerans, Bacillus spp. | N/A | Fewer organisms recovered underneath and to the right of PT dispenser; highest counts underneath WAD. Notable counts at 20 cm to the right of JAD in vertical line down the wall. Sampling of PT dispenser knobs, sides of WAD and trough of JAD yielded high bacterial counts (‘too many to count’) in all cases. Bacterial colonies: Most isolates either Staphylococcus spp. (Staphylococcus epidermidis or Staphylococcus aureus) or non-pathogenic Bacillus spp.; organisms from trough in JADs included Staphylococcus haemolyticus in female washroom and Pantoea agglomerans in male washroom. |
| Repp et al., 2013 |
Country: USA Setting: Workplace Design: Outbreak investigation; surface swabs, RNA extraction, and PCR Activity: General toilet use Contaminated area/medium: Diaper changing station |
Norovirus | N/A | Corporeal brown matter found inside and underneath diaper changing station; swabs were positive for Norovirus, although sequencing was not possible. |
| Sassi et al., 2018 |
Country: USA Setting: Laboratory Design: MS2 phage inoculation; culture-based analysis Activity: Toilet flushing Contaminated area/medium: Toilet handle, cistern, toilet seat, toilet bowl, toilet water, wall behind toilet, floor near toilet, toilet paper dispenser |
MS2 bacteriophage | N/A |
Geometric mean bacteriophage concentration on washroom surfaces after flushing:
Disinfectant evaluation on bacteriophage concentration: At 15 min contact time, all disinfectants showed reduction when compared with no disinfectant; at 30 min contact time, all disinfectants except hydrogen peroxide showed reduction when compared with no disinfectant. Chlorine bleach was the only treatment to show significant reduction between 15 and 30 min contact time. Peracetic acid showed greatest reduction of all treatments for all contact times; hydrogen peroxide exhibited the least reduction for all contact times. |
| Snelling et al., 2011 |
Country: UK Setting: Laboratory Design: Hands contaminated by handling chicken prior to washing and drying experiment; culture-based analysis Activity: Hand dying (JAD, WAD, PT) Contaminated area/medium: Aluminium foil (representing surfaces) |
Bacterial CFU | N/A |
Mean bacterial transfer to aluminium foil after hand drying procedure:
JAD vs WAD drying procedure: 21 instances of no bacteria transferred, most often (7 times) with JAD, followed by WAD Turbodry (5 times, at 35 s). Effect of rubbing hands when using WAD: Rubbing increased bacteria transferred in many instances. No statistical difference between any of the dryers when hands still, and bacterial reduction comparable to PT for middle of fingers. Rubbing with PT proved effective and to be the best means of reducing bacterial loading on fingertips. |
| Suen et al., 2019 |
Country: Hong Kong Setting: Healthcare, restaurant, food market, shopping centre, public library, sport centre, tourist spot, hotel, public housing state Design: Surface swabs, culture-based analysis; MALDI-TOF and disc diffusion AST of isolates Activity: General toilet use Contaminated area/medium: Paper towel dispenser, dryer unit, air outlet of air dryers, exit door handle, paper towel |
Bacterial CFU, Escherichia coli, Proteus mirabilis, Moraxella spp., Staphylococcus aureus, Staphylococcus epidermis, Staphylococcus saprophyticus, methicillin resistant Staphylococcus epidermis, methicillin resistant Staphylococcus saprophyticus | N/A |
Highest bacterial counts in washroom surfaces:
Bacterial colonies: Potentially pathogenic Escherichia coli, Proteus mirabilis, Moraxella spp., Staphylococcus aureus, and Staphylococcus saprophyticus isolated from outlets of PT dispenser, hand dryer, and/or door handle. Antibiotic susceptibility assay: Swabs from PT dispensers, JAD, WAD and internal door handles showed 87.1% (27/31) of Staphylococcus spp. samples resistant to at least one first-line antimicrobial agent; 23% (7/31) exhibited co-resistance to ≥3 antimicrobial agents, most common combination penicillin, erythromycin, and clindamycin. Methicillin-resistant Staphylococcus epidermidis found in PT dispenser and Methicillin-resistant Staphylococcus saprophyticus found in WAD. Both strains additionally resistant to erythromycin and clindamycin. |
| Taylor et al., 2000 |
Country: UK Setting: Laboratory, workplace Design: Selective culture-based analysis Activity: Hand drying (WAD, PT) Contaminated area/medium: Air, air from dryer inlet, air from dryer outlet nozzle, inside dryer inlet, inside/outside dryer outer nozzle, dryer sensor/switch, top of dryer unit, wall below dryer, faucet tap, restroom door handle, floor, wall away from dryer |
Bacterial CFU, Enterobacteriaceae, Pseudomonas aeruginosa, Staphylococcus aureus |
Production of airborne bacteria after hand drying: No significant difference when drying hands with WAD or PT. Bacterial recovery from WAD without and with heater, respectively:
Reduction in recovery was greater for Pseudomonas aeruginosa. Air emitted from the outlet of the driers contained significantly fewer microorganisms than air entering the driers. |
Mean bacterial counts on different surfaces:
Microbiological testing of paper towels: bacteria transferred from hand to towels; if disposal not managed correctly, paper towels could act as bacteriological reservoir. |
| Tsunoda et al., 2019 |
Country: Japan Setting: Healthcare Design: Surface swabs and water samples, selective media targeting extended-spectrum beta lactamase and metallo-beta-lactamase producing bacteria, and vancomycin resistant Enterococci; isolate identification by MALDI-TOF Activity: General bidet-toilet use Contaminated area/medium: Warm-water bidet nozzle, water from nozzle |
Klebsiella spp., Enterococcus spp., Staphylococcus spp., Acinetobacter spp., Sphingomonas spp., Escherichia coli, extended-spectrum β-lactamase (ESBL)-Escherichia coli, Stenotrophomonas maltophylia | N/A |
Bacterial contamination in bidet toilet:
Mean counts of thin colonies recovered:
Bacterial species identified:
Tap water assessment: 1/123 sample contaminated with Sphingomonas paucimobilis in toilet in inpatient ward. |
| Verani et al., 2014 |
Country: Italy Setting: Healthcare, workplace Design: Surface swabs, air and water samples; culture-based analysis for surface and air samples; water samples analysed by isolating DNA with QIAamp DNA mini Kit Activity: Toilet flushing Contaminated area/medium: Air, toilet seat, toilet lid, toilet handle/button, internal door handle, water from toilet |
Bacterial CFU, Norovirus, Torque teno virus, Human Adenovirus | Viruses were detected in 35 (81%) of total aerosol samples tested. Frequency positive samples of bacteria and virus in offices:
|
Viruses were detected on 135 surfaces (78%), and in 17 (89%) water samples tested. The surface total positivity was 71% in offices, and 82% in hospital. Frequency positive samples of bacteria and virus in offices:
Geometric mean concentration before vs after disinfection in offices:
|
| Zapka et al., 2011 |
Country: USA Setting: Laboratory, academic Design: Controlled studies to assess bacterial hand contamination and transfer post-hand washing with contaminated or uncontaminated soap; culture-based analysis Activity: Hand washing Contaminated area/medium: Soap dispenser, contaminated and un-contaminated soap |
Bacterial CFU, Serratia marcescens, Klebsiella pneumoniae | N/A | Bulk-soap-refillable dispensers: all (14/14) soap dispensers used in an elementary school were contaminated with bacteria, ranging from 6.0 to 7.0 log10 CFU/ml of soap. Gram-negative species included Citrobacter, Providencia, Pseudomonas, Serratia genera. |
3.1. Study quality
Based on the quality assessment criteria (Section 2.3), the studies included were deemed to be of mixed quality. A number of studies were conducted in laboratories simulating unrealistic washroom conditions, including drying of unwashed or gloved hands covered with model organism solutions (Best et al., 2014; Best and Redway, 2015). Eleven studies were assessed as good quality (Aithinne et al., 2019; Boone and Gerba, 2005; Inkinen et al., 2017; Knowlton et al., 2018; Margas et al., 2013; Mkrtchyan et al., 2013; Mohamed et al., 2015; Snelling et al., 2011; Suen et al., 2019; Verani et al., 2014; Zapka et al., 2011), 16 studies were assessed as fair quality (Boxman et al., 2009a; Breathnach et al., 2012; Carducci et al., 2016; Cooper et al., 2016; Flores et al., 2011; Gormley et al., 2017; Halabi et al., 2001; Harrison et al., 2003; Kanayama Katsuse et al., 2017; Katano et al., 2014; Kurgat et al., 2019; Patrick et al., 2010; Pitt et al., 2018; Sassi et al., 2018; Taylor et al., 2000; Tsunoda et al., 2019), and the remaining 11 studies were assessed as poor quality (Alharbi et al., 2016; Best et al., 2018; Best et al., 2014; Best and Redway, 2015; Best et al., 2012; Boxman et al., 2009a; Gerhardts et al., 2012; Huesca-Espitia et al., 2018; Kimmitt and Redway, 2016; Kouadri, 2020; Repp et al., 2013).
The main reasons for poor quality classification included poor or limited description of methods (Alharbi et al., 2016; Boxman et al., 2009a; Kouadri, 2020), sub-optimal sampling methods (Alharbi et al., 2016; Huesca-Espitia et al., 2018; Kouadri, 2020); lack of significance testing (Best et al., 2012); over-interpretation of limited experimental results (Best et al., 2018; Best et al., 2014; Best and Redway, 2015); and unrealistic experimental conditions, such as results based on gloved hands (Best et al., 2014; Best and Redway, 2015; Kimmitt and Redway, 2016) which could affect the interaction between resident and transient bacteria, washing procedure and drying time.
3.2. Microbial contamination
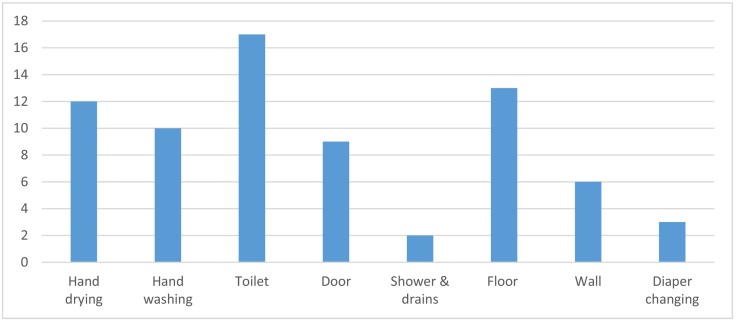
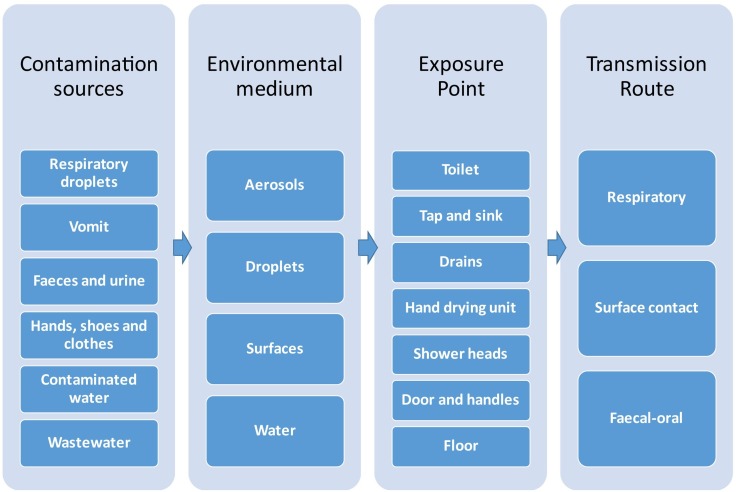
Most of the 38 studies included in the review investigated the potential for microbial contamination to occur in public washroom environments, and in specific areas, such as around the hand dryer or towel dispenser, the handwashing area (sink, tap, soap dispenser), toilet bowl (including the seat, toilet seat lid, bidet, and flushing system), doors and handles, and the floor and walls (Fig. 4 ). Seventeen studies documented the presence of microorganisms in and around toilets (Table 1). This is expected given that any non-sterile environment will host a microbiome; however multiple studies also specifically demonstrated the potential for washroom activities, such as toilet flushing, to contribute faecal derived microorganisms to the washroom microbiome. Toilet use, including flushing, produces droplets and aerosols that may contaminate users and the environment and create an infectious disease transmission risk. Aerosols (i.e. smaller particles typically generated during speech, coughing, sneezing, vomiting, or atomization of faecal waste) can remain suspended in indoor air for prolonged periods and be propagated over extended distance by airflows (Morawska, 2006; Ai and Melikov, 2018).
Fig. 4.
Washroom areas examined for microbial contamination in eligible studies.
In addition to viruses, such as Norovirus and Human Adenovirus, a variety of Gram-positive and Gram-negative opportunistic pathogens were identified in and around toilet bowls in these studies (see Table 1), including among others Staphylococcus aureus, Streptococcus spp., Enterococcus spp., Klebsiella spp., Acinetobacter spp., Pseudomonas aeruginosa, and Escherichia coli. As these bacteria are colonising opportunistic pathogens that are commonly found in environmental and human microbiomes (Price et al., 2017), additional risk of colonisation of a person through washroom use may occur, but this is highly unlikely to be the single source of exposure.
Twelve studies investigated microbial contamination following the use of paper towels, warm air dyers or jet air dryers (Table 1). These studies used a variety of designs, from controlled experimental designs, where volunteers' hands were inoculated with indicator microbes prior to washing/drying, to various forms of environmental sampling such as air and/or surface sampling.
Previous studies have suggested that warm air or jet air dryers introduce a potential risk of infection for those standing near them either through inhalation or deposition of pathogens (Gammon and Hunt, 2019; Huang et al., 2012; Dancer et al., 2021), but none of the studies meeting the inclusion criteria for this review directly assessed disease transmission. Several experimental studies found that warm air and jet air dryers dispersed more droplets into the environment when compared to paper towels (Best et al., 2018; Best et al., 2014; Best and Redway, 2015). However, two studies did not find statistically significant differences in the levels of environmental contamination when comparing paper towels and warm air or jet air dryers (Suen et al., 2019; Taylor et al., 2000), and one study reported that retrofitting warm air hand dryers with High Efficiency Particulate Air (HEPA) filters reduced bacterial deposition around the unit approximately 4-fold (Huesca-Espitia et al., 2018). Harrison et al. showed there is a potential risk of transmission if either the paper towel or the paper towel dispenser is contaminated (Harrison et al., 2003).
Surface contact was identified as potential transmission route for bacteria and viruses, including Influenza A and Norovirus in toilets in day care centres (Boone and Gerba, 2005; Patrick et al., 2010) or with diaper-changing stations (Repp et al., 2013), and in restaurants (Boxman et al., 2009b). Overall, the reviewed literature suggests that the type of microbial contamination may differ according to surface type. Skin-associated bacteria were found to dominate on surfaces that are routinely touched by hands and unlikely to come into direct contact with other body parts or fluids, while toilet flush handles and seats were typically enriched in pathogens of faecal origin, and bacteria commonly associated with soil were more abundant on toilet floors (Flores et al., 2011). Commonly contaminated surfaces included soap dispensers (Kurgat et al., 2019; Mkrtchyan et al., 2013), while Norovirus, Human Adenovirus and a variety of bacteria including multidrug-resistant opportunistic pathogens (e.g. Pseudomonas aeruginosa) were also detected on toilet bowls, seats, lids, flushing buttons, and brushes in hospitals, restaurants and offices (Boxman et al., 2009b; Breathnach et al., 2012; Cooper et al., 2016; Mkrtchyan et al., 2013; Verani et al., 2014). Enteric and skin bacteria including Escherichia coli and Staphylococcus spp. (including antimicrobial resistant strains) and viruses (including Human Adenovirus) were found on urinal floors, hand drying systems, inner door surfaces and handles, and water taps (Flores et al., 2011; Mkrtchyan et al., 2013; Suen et al., 2019; Verani et al., 2014).
3.3. Potential transmission pathways
Potential routes of infectious disease transmission in washrooms include: (a) the faecal-oral route, i.e. contaminated hands touching the face or food; (b) the respiratory route, i.e. personal exposure to droplets and aerosols carrying pathogens; and (c) transmission through contact with contaminated surfaces and fomites (Fig. 5 ).
Fig. 5.
Potential infectious disease transmission pathways in public washrooms.
3.3.1. Faecal-oral
Two studies identified faecal-oral transmission as the probable transmission route for Norovirus outbreaks in restaurants in the Netherlands (Boxman et al., 2009a; Boxman et al., 2009b), and one for a workplace Norovirus outbreak in USA (Repp et al., 2013). One of these Dutch studies highlighted that transmission could have occurred either directly (i.e. hand-to-mouth) or indirectly via contaminated surfaces, food or water (Boxman et al., 2009b).
Five studies showed distribution by hands to be a potential mechanism of transmission in washrooms, with poor hand washing and ineffective hand drying increasing the likelihood of transfer onto other surfaces (Boone and Gerba, 2005; Boxman et al., 2009b; Margas et al., 2013; Pitt et al., 2018; Snelling et al., 2011). Microbial identification in these studies was methodologically restricted due to the use of specific plating protocols, but collectively, these studies demonstrated the presence and transfer of coliform bacteria, skin-associated and environmental bacteria, and respiratory and enteric viruses (Influenza A and Norovirus). Boone and Gerba stressed the importance of contact with fomites as a potential mechanism of transmission (Boone and Gerba, 2005).
3.3.2. Respiratory
Sixteen studies identified droplets as the potential route of transmission for infectious diseases associated with bacteria (Alharbi et al., 2016; Best et al., 2018; Best et al., 2014; Best and Redway, 2015; Best et al., 2012; Carducci et al., 2016; Cooper et al., 2016; Gormley et al., 2017; Kanayama Katsuse et al., 2017; Katano et al., 2014; Kimmitt and Redway, 2016; Knowlton et al., 2018; Margas et al., 2013; Sassi et al., 2018; Taylor et al., 2000; Verani et al., 2014). Details of microbial species identified in these studies are provided in Table 1; they include a diverse range of opportunistic pathogens as well as viruses. Six studies examined droplet and/or aerosol dispersal for bacteria (Best et al., 2014; Cooper et al., 2016; Knowlton et al., 2018), including Pseudomonas putida (Gormley et al., 2017), and viruses including Human Adenovirus (Carducci et al., 2016). One study examined contamination by droplet and direct surface contact for coliform bacteria (Margas et al., 2013).
Mechanisms of aerosolisation considered in the literature included toilet flushing, showering, hand washing and drying, and vomiting. Eight studies identified toilet flushing as a potential transmission mechanism, as it may produce droplets and aerosols that can contaminate the washroom environment (Aithinne et al., 2019; Best et al., 2012; Carducci et al., 2016; Cooper et al., 2016; Gerhardts et al., 2012; Knowlton et al., 2018; Sassi et al., 2018; Verani et al., 2014). Dispersion of droplets and aerosols was reported as a result of toilet flushing with open lid (Best et al., 2012; Gormley et al., 2017; Knowlton et al., 2018; Verani et al., 2014), with droplet dispersion as the dominant route within a single cubicle/unit, and aerosol transmission as the probable route to other toilets within the same washroom (Gormley et al., 2017). Vomiting from an infected person was also reported as a mechanism of microbial aerosolisation (Gerhardts et al., 2012).
3.3.3. Surface contact
Fourteen studies identified contact with contaminated surfaces as a potential route of transmission for infectious diseases associated with bacteria and viruses (Boone and Gerba, 2005; Breathnach et al., 2012; Flores et al., 2011; Gerhardts et al., 2012; Harrison et al., 2003; Huesca-Espitia et al., 2018; Inkinen et al., 2017; Kurgat et al., 2019; Margas et al., 2013; Patrick et al., 2010; Snelling et al., 2011; Suen et al., 2019; Tsunoda et al., 2019; Zapka et al., 2011) (Table 1). Studies varied from ambient microbiome analysis (e.g. non-selective culturing and isolate identification by MALDI-TOF; environmental swabs and qPCR or 16S rRNA sequencing for identification) through to studies specifically targeting the analysis of antimicrobial resistant bacterial strains, and targeted bacterial/viral inoculation and tracing experiments.
Bacterial aerosolisation and deposition on inanimate surfaces (Huesca-Espitia et al., 2018), contact with contaminated surfaces and contaminated water (Tsunoda et al., 2019), and wet, contaminated hands (Harrison et al., 2003) were all identified as potential transmission pathways. Eight studies identified surface contamination and contact with fomites as the main exposure pathway in public washrooms (Best et al., 2018; Flores et al., 2011; Harrison et al., 2003; Inkinen et al., 2017; Kurgat et al., 2019; Mohamed et al., 2015; Patrick et al., 2010; Repp et al., 2013), with Flores et al. pointing out that routine use of public toilets results in dispersal of urine- and faecal-bacteria throughout the washroom (Flores et al., 2011). Microorganisms identified included a variety of Gram-positive and Gram-negative bacteria, including skin microbiota, opportunistic pathogens, enteric pathogens, and viruses (Table 1). Verani et al. mentioned surface to surface spreading by hands as an important transfer route given the high contamination of flushing buttons and door handles in public washrooms (Verani et al., 2014).
In 3.4, 3.5, 3.6, we examine in more detail the potential for infectious disease transmission in three frequently touched washroom areas which have been extensively studied, including: (a) toilets with flushing mechanisms; (b) areas with hand drying systems; and (c) water taps and sinks.
3.4. Toilet type and usage
Seven studies investigated dispersion of pathogens following toilet flushing (Aithinne et al., 2019; Best et al., 2012; Cooper et al., 2016; Gormley et al., 2017; Knowlton et al., 2018; Sassi et al., 2018; Verani et al., 2014), highlighting that the toilet plume is an important vector of pathogens (Cooper et al., 2016; Verani et al., 2014). Studies included deliberate inoculation and experimental designs to test the effects of flushing, as well as ambient environmental sampling in the toilet area (Table 1). Two of these studies indicate the presence of bioaerosols following multiple flushes (Aithinne et al., 2019; Knowlton et al., 2018): Aithinne et al. identified bioaerosols over at least 12 flushes, with spore contamination identifiable even after 24 flushes following seeding of a toilet with Clostridium difficile in a sealed chamber; while Knowlton et al. identified bioaerosols after flushing even when no faecal waste was present, suggesting that bacterial residues from previous users remained in the toilet water.
Gormley et al. showed that bioaerosols can potentially be transmitted to other building sections following flushing via plumbing airstreams and extraction fan systems, and contaminate room surfaces (Gormley et al., 2017). Using an experimental 2-story sanitary plumbing system to test aerosolisation and dispersal of a model organism (Pseudomonas putida) inoculated into the toilet bowl, they demonstrated that typical sanitary plumbing system airflows are sufficient to carry aerosolised particles between different floors of a building and noted that cross-transmission is a particular risk in the case of defective plumbing conditions. They noted that empty U-traps were not uncommon and suggested that greater consideration should be given to this possible mode of pathogen transmission, particularly in high risk environments such as hospitals, where sewer pathogen loads are high and populations are particularly vulnerable. A follow-up study using the same experimental setup indicated that the number of particles emitted from the sanitary plumbing system as a result of a toilet flush is equivalent to a person talking loudly for just over 6 and a half minutes (Gormley et al., 2021).
Three studies addressed toilet design (Best et al., 2012; Breathnach et al., 2012; Sassi et al., 2018), recommending the use of toilet lids (Best et al., 2012), toilets with low bowl volume and flush force (Sassi et al., 2018), and easy to clean toilet bowls, proper disposal of sanitary items, and weekly disposal of toilet brushes (Breathnach et al., 2012). Three Japanese studies investigated transmission from bidet toilets (Kanayama Katsuse et al., 2017; Katano et al., 2014; Tsunoda et al., 2019), and indicated risk of infection following the use of the warm-water nozzle to clean the genital and gluteal area following defaecation.
3.5. Hand drying methods
Six studies identified hand drying with warm air or jet air dryers as a potential mechanism of transmission associated with the production of droplets and aerosols (Alharbi et al., 2016; Best et al., 2014; Best and Redway, 2015; Huesca-Espitia et al., 2018; Suen et al., 2019; Taylor et al., 2000). Suen et al. reported that rubbish bins were frequently found to be uncovered in public washrooms and sometimes positioned underneath warm air dryers, which could further increase the spread of pathogens by dispersing rubbish via the airflow generated and by increasing the amount of aerosols in the washroom environment (Suen et al., 2019). Huesca-Espitia et al. suggested that it is unlikely that hand dryers are reservoirs of bacteria internally, but they may mobilise pathogens in the washroom air (Huesca-Espitia et al., 2018); therefore HEPA filters can reduce the amount of bacterial contamination from hand dryers.
Eight studies of varying quality (see Section 3.1) found that paper towels were potentially more effective in reducing the risk of transmission when compared to warm air and/or jet air dryers based on droplet dispersal experiments and surface contamination analyses following hand contact (Best et al., 2018; Best et al., 2014; Best and Redway, 2015; Huesca-Espitia et al., 2018; Kimmitt and Redway, 2016; Kouadri, 2020; Pitt et al., 2018; Snelling et al., 2011). Alharbi et al. found that warm air dryers can disperse bacteria into the environment and potentially deposit them on users (Alharbi et al., 2016). Contrary to these studies, Taylor et al. found no difference in the amount of bacteria left on hands following the use of warm air dryers or paper towels (Taylor et al., 2000). In addition, an intervention trial with combined cloth towel and warm air hand drying reported a substantial reduction in surface contamination (Patrick et al., 2010).
Drawbacks to the use of paper towels in washroom environments have also been noted. Harrison et al. found that the front of the paper towel dispenser can become contaminated due to general use and as a result of freeing jammed towels; this could lead to higher transmission risk if the unit is not routinely cleaned (Harrison et al., 2003). Taylor et al. found that paper towels can become highly contaminated (Taylor et al., 2000), which in turn can contaminate the washroom environment due to inappropriate disposal as a result of carelessness or rubbish bins that are full (Snelling et al., 2011). In addition, Breathnach et al. found that blockages were common due to incorrect disposal of paper towels into toilets (Breathnach et al., 2012). Finally, the paper towel supply may be exhausted, leaving users with damp hands and increasing the risk of transmission via door handles (Snelling et al., 2011). Retractable, single-serve, cloth towel dispensing units can present similar challenges, if not regularly serviced.
When comparing warm air and jet air dryers, assessment of transmission risk varied depending on the measures used. Specifically, Kimmitt and Redway argued that jet air dryers had a higher risk of transmission given the higher rate of particle dispersal and production of aerosols that remained airborne for more than 15 min compared to warm air dryers (Kimmitt and Redway, 2016). However, when comparing the amount of bacteria left on hands or surface contamination following hand contact, warm air dryers had higher risk of transmission due to rubbing hands while drying (Pitt et al., 2018; Snelling et al., 2011) and inappropriate drying leaving hands partially wet (Snelling et al., 2011). Using a jet air dryer could prevent hand rubbing and promote appropriate drying over a shorter time period (Snelling et al., 2011).
3.6. Water and wastewater systems
Three studies examined the potential contribution of water taps to infection transmission in public washrooms (Breathnach et al., 2012; Flores et al., 2011; Halabi et al., 2001), with one of these studies also addressing shower heads in the same environment (Breathnach et al., 2012). Regarding water tap design, Halabi et al. showed that conventional fittings were preferable compared to non-touch fittings in the hospital setting investigated, with the low water pressure and the standing column of warm water in non-touch taps leading to greater contamination with P. aeruginosa and Legionella (Halabi et al., 2001). Taps with hot/cold temperature selection were less contaminated. As a result, the hospital involved in this investigation removed all non-touch taps and replaced them with conventional taps (Halabi et al., 2001). However, a more recent study has shown reduced incidence of healthcare-associated infections in a long-term care facility by converting to automated touchless dispensing and closed-refill systems (Handley and Hessefort, 2020).
Breathnach et al. (2012) provided a general assessment of sink design in hospital settings, stating that water flowing directly into the plughole may lead to splash-back from the U-bend, resulting in greater risk of microbial transmission. They conducted outbreak investigations of multidrug resistant P. aeruginosa in two hospitals and demonstrated the potential for hospital wastewater systems to act as environmental reservoirs for this emerging nosocomial infection. They suggested a variety of measures for reducing transmission risks, including reduction of incoming water pressure and flow rate in showers to reduce flooding, changes in storage practices to physically distance clean items from sluices, cleaning protocol reviews, and additional staff training to reduce blockages (Breathnach et al., 2012).
In a non-health care environment, Flores et al. found that the risk of transmission from water and taps in public washrooms was minimal. Environmental sampling of the tap mouth and tap water revealed minor bacterial contributions of Actinobacteria, Bacteriodetes, Firmicutes, and Proteobacteria (Flores et al., 2011). Other potential routes of transmission included bacteria contaminated soap from bulk soap dispensers (Zapka et al., 2011), plumbing system with depleted U-traps and airflow systems that may promote transmission of aerosols (Gormley et al., 2017), and blockages due to paper towels and clinical wipes being disposed of down toilets (Breathnach et al., 2012).
3.7. Range or potential transmission
Six studies comparing the dispersal of droplets and/or aerosols following the use of jet air dyers, warm air dryers, and/or paper tower measured the range of spread using a variety of experimental designs (Best et al., 2018; Best et al., 2014; Best and Redway, 2015; Kimmitt and Redway, 2016; Margas et al., 2013; Taylor et al., 2000). The greatest dispersal of model organisms was found with jet air dryers, spreading over distances as far as 3.0 m (Kimmitt and Redway, 2016). Droplet dispersal from the sides of the unit ranged from 1.0 m (Best and Redway, 2015) to 2.24 m (Margas et al., 2013), dispersal diagonally from the unit was 2.44 m (Margas et al., 2013), dispersal from the front of the unit ranged from 50 cm (Best and Redway, 2015) to 1.5 m (Margas et al., 2013), and upward dispersal vertical from the unit ranged from 0.6–1.2 m (Best and Redway, 2015) to 0.75–1.25 m (Kimmitt and Redway, 2016). For paper towel dispensers, vertical dispersal on the wall next to the unit ranged from 0.9 to 1.2 m (Best and Redway, 2015) and 1.74 m to the side, 2.0 m diagonally, and 1.5 m in front of the unit (Margas et al., 2013); and for continuous roller towel vertical dispersal ranged from 1.2 to 1.5 m (Best and Redway, 2015). Finally, vertical dispersal from warm air dyers was found to range between 0.0 and 0.3 m (Best and Redway, 2015). In general, the main areas of contamination when using paper towels or dyers were the floor under the towel dispenser and jet air dryer unit (Best et al., 2018; Margas et al., 2013), and the wall below the warm air dryer (Taylor et al., 2000), possibly because water droplets were shaken onto the wall in the process of drying the hands.
Three studies investigating the distribution of droplets and aerosols following toilet flushing also provided measures for range of dispersal (Best et al., 2012; Cooper et al., 2016; Knowlton et al., 2018). The height of dispersal ranged from the toilet seat up to 0.25 m above the seat (approx. toilet handle height) (Best et al., 2012), and to distances up to 1.0 m (Knowlton et al., 2018) and 1.5 m (Cooper et al., 2016) away from the toilet. Knowlton et al. reported that the aerosol plume resulting from toilet flushing may extend beyond the 1.0 m distance and remain in the air for longer than 30 min post flush (Knowlton et al., 2018). Mitigating strategies such as closing the toilet lid when flushing may help prevent spread of aerosols (Best et al., 2012).
3.8. Pathogen infectivity and antimicrobial resistance
The infectious dose of a pathogen depends on the species or strain, but faecal pathogens with a low infectious dose that can potentially be transmitted via washroom surfaces include Rotavirus, Norovirus, Caliciviruses, and Enterohemorrhagic Escherichia coli (Boxman et al., 2009a; Gerhardts et al., 2012). Outbreak investigations included in this review demonstrated the role of fomites and contaminated surfaces as possible Norovirus transmission pathways (Boxman et al., 2009a, Boxman et al., 2009b). As only a few particles are sufficient to cause infection, low infectious dose pathogens pose a significant transmission risk in public washrooms, if an infected person has been present and cleaning and sanitation have been inadequate. By contrast, the risk of becoming infected with pathogens that require a high infectious dose is much lower, although appropriate hygiene practices are paramount to risk mitigation in both cases.
A number of studies identified viable opportunistic pathogens in washroom environmental samples (Cooper et al., 2016; Inkinen et al., 2017). Bacteria identified included Staphylococcus spp. (Inkinen et al., 2017), Klebsiella pneumoniae (Zapka et al., 2011), Klebsiella spp. and Enterococci spp. (Best et al., 2018), Escherichia coli (Best et al., 2018; Mohamed et al., 2015); Legionella spp. and Pseudomonas aeruginosa (Halabi et al., 2001), Pseudomonas putida (Gormley et al., 2017); Micrococcus luteus (Harrison et al., 2003), and Serratia marcescens (Harrison et al., 2003; Zapka et al., 2011) (Table 1). Some studies also documented the presence of antibiotic-resistant strains in and around toilets including extra-intestinal pathogenic and antimicrobial-resistant Escherichia coli (Mohamed et al., 2015; Suen et al., 2019) and methicillin-resistant Staphylococcus aureus (Best et al., 2018). Two Japanese studies investigating transmission risk from bidet toilets (Kanayama Katsuse et al., 2017; Tsunoda et al., 2019). Tsunoda et al. (2019) cultured extended-spectrum-β-lactamase producing Enterobacteriaceae from bidet toilets. These bacteria can cause a variety of infections including urinary tract and bloodstream infections and are particularly serious for immunocompromised individuals in healthcare settings (Kanayama Katsuse et al., 2017; Tsunoda et al., 2019).
3.9. Environmental conditions and toilet design
Four studies examined environmental factors and potential infection transmission in public washrooms (Boone and Gerba, 2005; Gormley et al., 2017; Inkinen et al., 2017; Katano et al., 2014). Based on controlled experiments with Pseudomonas putida, Gormley et al. identified ventilation and U-trap depletion in toilets to be a major source of cross-contamination of airstreams. These conditions were promoted by poor toilet design and system overload, which are prominent in high-rise buildings and can be exacerbated by external factors such as wind shear (Gormley et al., 2017). Katano et al. identified lack of chlorine in lavage tanks to be a major source of infection in bidets with Pseudomonas aeruginosa and Escherichia coli. Heating and long retention time of the tank water led to inactivation and evaporation of chlorine, which in turn enabled bacterial proliferation and subsequent infection in users (Katano et al., 2014). Also regarding toilet design, lidless toilets, which are common in disabled and hospital washrooms, may pose a risk particularly to immunocompromised patients due to the possible dispersal of pathogens from toilet flushing.
In relation to microorganism survival on surfaces, Boone and Gerba found no significant difference in the survival of Influenza A detected on moist and dry washroom surfaces (Boone and Gerba, 2005). Inkinen et al. (2017) found that bacterial survival of Staphylococcus, Enterobacteriaceae and other Gram-negative rods can be significantly reduced on surfaces made of copper compared to reference materials. Under dry-hand contamination, the antimicrobial effect is fast and works within a few minutes, but under wet-hand conditions the effect is slower and can be as long as hours (Inkinen et al., 2017).
3.10. Personal precautions
Eight studies identified appropriate hand washing as the most effective measure to prevent the spread of infectious diseases in washrooms (Best et al., 2018; Best et al., 2012; Boxman et al., 2009a; Boxman et al., 2009b; Flores et al., 2011; Gerhardts et al., 2012; Mohamed et al., 2015; Patrick et al., 2010). Two of these studies also suggested complementing hand washing with hand sanitisers (Gerhardts et al., 2012; Mohamed et al., 2015), and three suggested complementing with effective environmental disinfection (Boxman et al., 2009b; Gerhardts et al., 2012; Mohamed et al., 2015). These recommendations are supported by the intervention study conducted by Kurgat et al., which found that regardless of quality of hand washing, environmental disinfection and hand sanitiser use were effective at preventing the spread of infection in a workplace environment (Kurgat et al., 2019).
Following hand washing, three studies suggested that the use of paper towels is potentially more effective than the use of warm air or jet air dryers in reducing bacterial contamination in washrooms (Kimmitt and Redway, 2016; Kouadri, 2020; Pitt et al., 2018). Snelling et al. suggested that using a warm air dryer for at least 30 s with no rubbing of hands produced similar results to the 10 s drying time of a jet air dryer (Snelling et al., 2011). However, regardless of the method used, Taylor et al. argued that the best measure of prevention is fully dried hands (Taylor et al., 2000). Furthermore, two studies suggested the use of physical barriers to prevent the spread of infection, with one of these promoting a direct barrier in the toilet seat (Mohamed et al., 2015), and the other promoting the use of gloves in healthcare settings while caring for patients and cleaning toilets (Katano et al., 2014).
3.11. Environmental hygiene measures
Sixteen studies indicated frequent and effective cleaning practices to be an important measure to reduce transmission of pathogens in washrooms (Aithinne et al., 2019; Best et al., 2012; Boone and Gerba, 2005; Boxman et al., 2009a; Boxman et al., 2009b; Breathnach et al., 2012; Kanayama Katsuse et al., 2017; Kurgat et al., 2019; Margas et al., 2013; Mohamed et al., 2015; Repp et al., 2013; Sassi et al., 2018; Suen et al., 2019; Taylor et al., 2000; Tsunoda et al., 2019; Verani et al., 2014). They recommended the use of appropriate disinfectant for viruses and contact time (≥15 min) (Sassi et al., 2018), and targeted cleaning of frequently touched surfaces. Two of these studies investigated bidet toilets, recommending frequent and effective cleaning of the warm-water nozzle in particular (Kanayama Katsuse et al., 2017; Tsunoda et al., 2019). Properly covering, appropriately locating (e.g. away from hand dryers) and emptying rubbish bins is also important for reducing microbial contamination in washrooms (Suen et al., 2019). Incorporating adequate ventilation in the design and operation of public washrooms reduces the risk of airborne transmission of pathogens in high occupancy areas (Carducci et al., 2016; Schreck et al., 2021).
Other suggested environmental hygiene measures were refurbishment or replacement of inadequate taps, sinks, toilets and sluice (Breathnach et al., 2012), improved design of front and back panels of the jet air dryer (Margas et al., 2013), provision of a section to put belongings during handwashing, and increased visibility of hand sanitisers and paper towels (Suen et al., 2019), installation of UVC lights (Cooper et al., 2016), and use of sealed soap refills instead of open bulk soap refillable dispensers (Zapka et al., 2011).
3.12. COVID-19 transmission risk
There are a limited number of studies, mainly from China, reporting on surface or air sampling of SARS-CoV-2 in washrooms. However, all identified studies were conducted in toilets inside hospital respiratory isolation wards or intensive care units, or in patients' homes, therefore they did not meet the public washroom inclusion criteria for this review. Nevertheless, we briefly discuss key findings from these studies here, as they can potentially inform COVID-19 transmission prevention in public washroom settings.
Isolation of infectious SARS-CoV-2 in faeces of COVID-19 patients indicates the possibility of faecal-oral transmission though contaminated surfaces or faecal-respiratory transmission through aerosolised faeces (Xiao et al., 2020; Yong et al., 2020). Studies that analysed environmental samples from toilets in COVID-19 isolation wards in Singapore, China and Italy found evidence of SARS-CoV-2 presence on surfaces (toilet bowl and lid, sink, tap and drain, and toilet door handle) (D'Accolti et al., 2020; Ding et al., 2021; Ong et al., 2020; Wei et al., 2020). Bathroom door handles in COVID-19 patient designated healthcare units in England and China were identified as posing a SARS-CoV-2 contamination risk (Moore et al., 2021; Wan et al., 2021). Surface samples taken in private toilets used by COVID-19 patients in Guangzhou, China, also showed significant levels (23.8%) of SARS-CoV-2 contamination (Luo et al., 2020).
These studies provide evidence of potential for SARS-CoV-2 transmission through contamination of environmental surfaces in hospital or private toilets used extensively by COVID-19 patients. Air samples collected in washrooms in the examined healthcare settings were all negative for SARS-CoV-2, except for those reported from a study of two hospitals in Wuhan, China, which found high concentration of SARS-CoV-2 positive aerosols in a bathroom (Liu et al., 2020). However, this was a temporary, single-toilet room with no ventilation. To our knowledge, there have been no reports of faecal-oral transmission of SARS-CoV-2 (Vardoulakis et al., 2020) and no COVID-19 clusters have yet been linked to public washroom use (Nicol, 2020). However, faecal-respiratory transmission is suspected to have played a role in a COVID-19 community outbreak in a high-rise residential building in Guangzhou, China, via vertical spread of virus-laden aerosols in drainage systems (Kang et al., 2020).
4. Discussion
Public washrooms are considered by many as high risk environments for infectious disease transmission. Certain toilet designs and practices, such as open-lid toilet flushing, ineffective handwashing and/or hand drying, substandard or infrequent surface cleaning, blocked drains, and improperly located or open rubbish bins can result in bacterial and/or viral contamination in washrooms. However, very few cases of disease originating from public washrooms have been reported in the scientific literature. Reported cases mainly refer to intestinal diseases involving hand-to-mouth transfer of pathogens as a result of faecal contamination of hands, surfaces or food (Boxman et al., 2009b); therefore correct handwashing greatly minimises this risk. In addition, appropriate disinfectant in the toilet bowl prior to flushing reduces the level of contamination in the washroom environment after flushing (Sassi et al., 2018).
There is increasing recognition of the importance of hand drying in the process of hand hygiene, suggesting that the efficacy of hand drying is a critical factor in the prevention of the transfer of pathogens and cross-infection particularly in healthcare settings (Gammon and Hunt, 2019). It has been suggested that numbers of bacteria translocating on touch contact decrease progressively as drying removes residual moisture from hands (Patrick et al., 1997). Methods for hand drying in public washrooms vary considerably and include cloth or paper towels and warm air or jet air dryers. These methods may differ in their ability to dry hands (e.g. warm air dryers are often slow and inefficient (Alharbi et al., 2016)) as well as to act as pathogen reservoirs or aerosolise pathogens, and thus in their potential to transmit infectious diseases (Huesca-Espitia et al., 2018). Retrofitting warm air hand dryers with HEPA filters is likely to reduce bacterial deposition around hand drying units and hence the risk of transmission.
It is unclear what the risk of infection is depending on hand drying methods in real-world settings. We did not find published evidence of outbreaks or epidemiological studies characterising this risk. However, there is potentially a risk of infection through surface contamination (such as drying unit box, floor and wall around the unit). A number of other considerations, such as cost, noise and environmental sustainability, also need to be taken into account in addition to hygiene and the risk of infection transmission when comparing hand drying methods (Huang et al., 2012).
The probability of airborne transmission depends on the infectivity of the pathogen, its concentration in the air, and exposure time (Carducci et al., 2016). Although there is a potential risk of aerosolisation of bacteria and viruses through toilet flushing, vomiting, and the use of electric hand dryers, we found no evidence of airborne transmission of enteric or respiratory pathogens, including COVID-19, in public washrooms. This may be for a number of reasons: (a) toilet flushing would mainly generate a plume of aerosols from the user's own faeces (if a pathogen was present, that person would already be infected); (b) good adherence to handwashing which reduces the risk of pathogen transmission; (c) the limited exposure time (typically only a few minutes (Baillie et al., 2009)) in a washroom environment; and (d) the relatively small number of concurrent users and limited close face-to-face interaction.
It is generally difficult to establish the risk of infection in public washrooms considering that the persistence and initial dose of pathogen required are widely variable, and dependent on the type of pathogen and overall transmission mechanism (Gerhardts et al., 2012). The empirical evidence examined showed a limited number of studies where transmission of enteric pathogens may have originated from surface contamination in restaurant or workplace washrooms (Boxman et al., 2009b; Repp et al., 2013).
COVID-19 is mainly transmitted through the inhalation of respiratory droplets and aerosols, direct contact with infected individuals, and potentially via contact with contaminated surfaces (Vardoulakis et al., 2020). Indoor environments that promote close contact for longer periods are the most likely to facilitate respiratory transmission (Morawska et al., 2020). Faecal-oral transmission is theoretically possible as the SARS-CoV-2 virus is continually shed by infected and convalescent individuals (Xiao et al., 2020), although it is not entirely clear whether the viral particles in faeces remain infectious and for how long (Nicol, 2020). Computational Fluid Dynamic (CFD) modelling simulations have indicated that urinal and open-lid toilet flushing may result in significant spread of bioaerosols in washrooms (Li et al., 2020; Wang et al., 2020). In most cases, however, adequate surface disinfection and room ventilation is expected to limit the virus's concentration in the washroom environment.
Although the risk of airborne transmission of bacterial or viral infections in a public washroom is low, it is recommended as a precaution to limit the time spent in a public washroom in a single visit, maintain at least 1.5 m distance from other users and wear a facemask in washrooms within high risk settings. Regular disinfection of toilet surfaces is also an important COVID-19 precautionary intervention (Ding et al., 2021), and will reduce the risk of transmission of other viral and bacterial infectious diseases in washroom environments. Plumbing design and standing water volumes are key considerations for hospital and aged care facility water quality, particularly where retrofitting and extensions are involved, and management should thus be tailored on a case-by-case basis. Personal precautions, environmental hygiene and washroom design recommendations from the examined studies are summarised in Box 1 . Assessing the efficacy of these measures was beyond the scope of the present review.
Box 1. Personal precautions, environmental hygiene and washroom design recommendations.
- Personal precautions:
-
•Appropriate hand washing with water and soap (for at least 20 s) followed by drying until hands are fully dry.
-
•Carry hand sanitiser and disinfectant wipes in case facilities lack soap or running water.
-
•Limit time spent in a public washroom in a single visit (to less than 15 min).
-
•Close the toilet lid before flushing; leave cubicle immediately after activating the flush button.
-
•Wear a facemask in settings with significant risk of COVID-19 transmission.
-
•Maintain physical distance from other users and avoid crowded washrooms.
-
•Avoid touching the exit door handle (instead open door using elbow) or other surfaces in the washroom after washing hands and before leaving the area.
-
•Avoid eating, smoking, drinking or using a mobile phone within the washroom.
-
•
- Environmental hygiene:
-
•Regular and effective surface cleaning, particularly of frequently touched surfaces such as door and stall handles, water taps, sink counters, soap and towel dispensers, hand drying units, toilet lids, seats, roll holders, and flush buttons.
-
•Proper disposal of sanitary items and weekly disposal of toilet brushes.
-
•Chlorine residual should be maintained in toilet lavage tanks.
-
•Drains should be regularly cleaned and unblocked to avoid overflow.
-
•Use of sealed soap refills instead of open bulk soap refillable dispensers.
-
•Installation of UVC lights may be a useful supplementary decontamination method in healthcare settings.
-
•
- Washroom design:
-
•Provision of adequate ventilation, including mechanical ventilation with air filtration where possible.
-
•Hand sanitisers should be available and visible in washroom entrance/exit.
-
•Rubbish bins properly covered and regularly emptied. Bins located away from electric hand dryers.
-
•Easy to clean toilet bowls, with low volume and flush force.
-
•Hand drying units regularly cleaned; electric hand dryers equipped with HEPA filters where possible; paper towel dispensers regularly stocked.
-
•Use copper products in small frequently touched locations such as toilet flush buttons, light switches and door handles.
-
•Use sink designs that reduce the risk of splash-back from plugholes or U-bends in plumbing systems.
-
•Reduce incoming water pressure and flow rate in showers to reduce the risk of flooding.
-
•Avoid use of warm-water bidet toilets.
-
•Non-touch flush buttons and other sensor-operated fittings for hand dryers, soap and paper towel dispensers and sinks (but avoid low water pressure in non-touch taps).
-
•Automatic doors or doorless entry ways into public washrooms reduce the risk of cross-contamination.
-
•
Alt-text: Box 1
5. Conclusions
Public washroom surfaces can become contaminated with bacteria and viruses particularly when heavily used and not frequently cleaned or correctly maintained. Many of the bacteria identified in the reviewed studies were part of the normal skin flora or environmentally ubiquitous, so the risk of novel infection is low in healthy individuals. However, there was a number of pathogens and colonising opportunistic pathogens identified that may pose an infection risk to immunocompromised individuals in healthcare settings. Faecal-oral transmission and washroom surface contamination were implicated in a number of intestinal disease outbreaks mainly in restaurants. We found no evidence of airborne transmission of pathogens, including COVID-19, in public washrooms.
The key to health protection from toilet associated pathogens is correct hand washing and drying, which can prevent direct transmission via the faecal-oral route, as well as contamination of other people and surfaces. Thoroughly washing and drying hands after toilet use greatly reduces the risk of any pathogen transmission irrespective of the drying method. Good air ventilation and frequent cleaning of surfaces, particularly of those frequently touched (e.g., door handles), are strongly recommended.
More high-quality environmental sampling studies assessing the risk of COVID-19 and other infectious disease transmission via all possible exposure routes in public washrooms, and the efficacy of preventive measures, are urgently needed. The role and frequency of defective plumbing in high risk settings should also be further evaluated.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: SV is member of the Dyson Scientific Advisory Board and has received research funding and honoraria from Dyson.
Acknowledgment
This work was supported by Dyson Technology Ltd. We thank Amelia Joshy (ANU) for her assistance with the preparation of tables and figures.
Editor: Lidia Morawska
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.149932.
Appendix A. Supplementary data
Supplementary material
References
- Ai Z.T., Melikov A.K. Airborne spread of expiratory droplet nuclei between the occupants of indoor environments: a review. Indoor Air. 2018;28:500–524. doi: 10.1111/ina.12465. [DOI] [PubMed] [Google Scholar]
- Aithinne K.A.N., Cooper C.W., Lynch R.A., Johnson D.L. Toilet plume aerosol generation rate and environmental contamination following bowl water inoculation with Clostridium difficile spores. Am. J. Infect. Control. 2019;47:515–520. doi: 10.1016/j.ajic.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Alharbi S.A., Salmen S.H., Chinnathambi A., Alharbi N.S., Zayed M.E., Al-Johny B.O., et al. Assessment of the bacterial contamination of hand air dryer in washrooms. Saudi J. Biol. Sci. 2016;23:268–271. doi: 10.1016/j.sjbs.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie M.A., Fraser S., Brown M.J. Do women spend more time in the restroom than men? Psychol. Rep. 2009;105:789–790. doi: 10.2466/PR0.105.3.789-790. [DOI] [PubMed] [Google Scholar]
- Best E., Parnell P., Couturier J., Barbut F., Le Bozec A., Arnoldo L., et al. Environmental contamination by bacteria in hospital washrooms according to hand-drying method: a multi-centre study. J. Hosp. Infect. 2018;100:469–475. doi: 10.1016/j.jhin.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Best E.L., Parnell P., Wilcox M.H. Microbiological comparison of hand-drying methods: the potential for contamination of the environment, user, and bystander. J. Hosp. Infect. 2014;88:199–206. doi: 10.1016/j.jhin.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Best E.L., Redway K. Comparison of different hand-drying methods: the potential for airborne microbe dispersal and contamination. J. Hosp. Infect. 2015;89:215–217. doi: 10.1016/j.jhin.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Best E.L., Sandoe J.A.T., Wilcox M.H. Potential for aerosolization of Clostridium difficile after flushing toilets: the role of toilet lids in reducing environmental contamination risk. J. Hosp. Infect. 2012;80:1–5. doi: 10.1016/j.jhin.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Boone S.A., Gerba C.P. The occurrence of influenza a virus on household and day care center fomites. J. Infect. 2005;51:103–109. doi: 10.1016/j.jinf.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Boxman I., Dijkman R., Verhoef L., Maat A., van Dijk G., Vennema H., et al. Norovirus on swabs taken from hands illustrate route of transmission: a case study. J. Food Prot. 2009;72:1753–1755. doi: 10.4315/0362-028x-72.8.1753. [DOI] [PubMed] [Google Scholar]
- Boxman I.L., Dijkman R., te Loeke N.A., Hägele G., Tilburg J.J., Vennema H., et al. Environmental swabs as a tool in norovirus outbreak investigation, including outbreaks on cruise ships. J. Food Prot. 2009;72:111–119. doi: 10.4315/0362-028x-72.1.111. [DOI] [PubMed] [Google Scholar]
- Breathnach A.S., Cubbon M.D., Karunaharan R.N., Pope C.F., Planche T.D. Multidrug-resistant Pseudomonas aeruginosa outbreaks in two hospitals: association with contaminated hospital waste-water systems. J. Hosp. Infect. 2012;82:19–24. doi: 10.1016/j.jhin.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Calechman S. Harvard Health Publishing; Boston, MA: 2020. How Risky is Using a Public Bathroom During the Pandemic? [Google Scholar]
- Carducci A., Donzelli G., Cioni L., Verani M. Quantitative microbial risk assessment in occupational settings applied to the airborne human adenovirus infection. Int. J. Environ. Res. Public Health. 2016;13 doi: 10.3390/ijerph13070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J., Bryce E., Astrakianakis G., Stefanovic A., Bartlett K. Efficacy of an automated ultraviolet C device in a shared hospital bathroom. Am. J. Infect. Control. 2016;44:1692–1694. doi: 10.1016/j.ajic.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Accolti M., Soffritti I., Passaro A., Zuliani G., Antonioli P., Mazzacane S., et al. SARS-CoV-2 RNA contamination on surfaces of a COVID-19 ward in a hospital of northern Italy: what risk of transmission? Eur. Rev. Med. Pharmacol. Sci. 2020;24:9202–9207. doi: 10.26355/eurrev_202009_22872. [DOI] [PubMed] [Google Scholar]
- Dancer S.J., Li Y., Hart A., Tang J.W., Jones D.L. What is the risk of acquiring SARS-CoV-2 from the use of public toilets? Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.-L., et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feazel L.M., Baumgartner L.K., Peterson K.L., Frank D.N., Harris J.K., Pace N.R. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G.E., Bates S.T., Knights D., Lauber C.L., Stombaugh J., Knight R., et al. Microbial biogeography of public restroom surfaces. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon J., Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods and clinical implication: a review. Infect. Prev. 2019;20:66–74. doi: 10.1177/1757177418815549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardts A., Hammer T.R., Balluff C., Mucha H., Hoefer D. A model of the transmission of micro-organisms in a public setting and its correlation to pathogen infection risks. J. Appl. Microbiol. 2012;112:614–621. doi: 10.1111/j.1365-2672.2012.05234.x. [DOI] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. Aerosol and bioaerosol particle size and dynamics from defective sanitary plumbing systems. Indoor Air. 2021;00:1–14. doi: 10.1111/ina.12797. [DOI] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A., Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi M., Wiesholzer-Pittl M., Schöberl J., Mittermayer H. Non-touch fittings in hospitals: a possible source of Pseudomonas aeruginosa and legionella spp. J. Hosp. Infect. 2001;49:117–121. doi: 10.1053/jhin.2001.1060. [DOI] [PubMed] [Google Scholar]
- Handley A.K., Hessefort Y.Z. Reduced incidence of healthcare-associated infections in a long-term care facility by converting to automated touchless dispensing and closed-refill systems. Am. J. Infect. Control. 2020;48:S28. [Google Scholar]
- Harrison W.A., Griffith C.J., Ayers T., Michaels B. Bacterial transfer and cross-contamination potential associated with paper-towel dispensing. Am. J. Infect. Control. 2003;31:387–391. doi: 10.1067/mic.2003.81. [DOI] [PubMed] [Google Scholar]
- Huang C., Ma W., Stack S. The hygienic efficacy of different hand-drying methods: a review of the evidence. Mayo Clin. Proc. 2012;87:791–798. doi: 10.1016/j.mayocp.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesca-Espitia L.D.C., Aslanzadeh J., Feinn R., Joseph G., Murray T.S., Setlow P. Deposition of bacteria and bacterial spores by bathroom hot-air hand dryers. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkinen J., Mäkinen R., Keinänen-Toivola M.M., Nordström K., Ahonen M. Copper as an antibacterial material in different facilities. Lett. Appl. Microbiol. 2017;64:19–26. doi: 10.1111/lam.12680. [DOI] [PubMed] [Google Scholar]
- Kanayama Katsuse A., Takahashi H., Yoshizawa S., Tateda K., Nakanishi Y., Kaneko A., et al. Public health and healthcare-associated risk of electric, warm-water bidet toilets. J. Hosp. Infect. 2017;97:296–300. doi: 10.1016/j.jhin.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Kang M., Wei J., Yuan J., Guo J., Zhang Y., Hang J., et al. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann. Intern. Med. 2020;173:974–980. doi: 10.7326/M20-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H., Yokoyama K., Takei Y., Tazume S., Tsukiji M., Matsuki H. A survey on bacterial contamination of lavage water in electric warm-water lavage toilet seats and of the gluteal cleft after lavage. J. UOEH. 2014;36:135–139. doi: 10.7888/juoeh.36.135. [DOI] [PubMed] [Google Scholar]
- Kimmitt P.T., Redway K.F. Evaluation of the potential for virus dispersal during hand drying: a comparison of three methods. J. Appl. Microbiol. 2016;120:478–486. doi: 10.1111/jam.13014. [DOI] [PubMed] [Google Scholar]
- Knowlton S.D., Boles C.L., Perencevich E.N., Diekema D.J., Nonnenmann M.W., Program C.D.C.E. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. antimicrob resist. Infect. Control. 2018;7 doi: 10.1186/s13756-018-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouadri F. Microbiological assessment of the different hand drying methods and washroom environment cross-contamination. Int. J. Microbiol. 2020;2020:8815147. [Google Scholar]
- Kurgat E.K., Sexton J.D., Garavito F., Reynolds A., Contreras R.D., Gerba C.P., et al. Impact of a hygiene intervention on virus spread in an office building. Int. J. Hyg. Environ. Health. 2019;222:479–485. doi: 10.1016/j.ijheh.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Lane M.A., Brownsword E.A., Morgan J.S., Babiker A., Vanairsdale S.A., Lyon G.M., et al. Bioaerosol sampling of a ventilated patient with COVID-19. Am. J. Infect. Control. 2020;48:1540–1542. doi: 10.1016/j.ajic.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-Y., Wang J.-X., Chen X. Can a toilet promote virus transmission? From a fluid dynamics perspective. Phys. Fluids. 2020;32 doi: 10.1063/5.0013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Luo L., Liu D., Zhang H., Li Z., Zhen R., Zhang X., et al. Air and surface contamination in non-health care settings among 641 environmental specimens of 39 COVID-19 cases. PLoS Negl. Trop. Dis. 2020;14:1–13. doi: 10.1371/journal.pntd.0008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margas E., Maguire E., Berland C.R., Welander F., Holah J.T. Assessment of the environmental microbiological cross contamination following hand drying with paper hand towels or an air blade dryer. J. Appl. Microbiol. 2013;115:572–582. doi: 10.1111/jam.12248. [DOI] [PubMed] [Google Scholar]
- McDermott C.V., Alicic R.Z., Harden N., Cox E.J., Scanlan J.M. Put a lid on it: are faecal bio-aerosols a route of transmission for SARS-CoV-2? J. Hosp. Infect. 2020;105:397–398. doi: 10.1016/j.jhin.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtchyan H.V., Russell C.A., Wang N., Cutler R.R. Could public restrooms be an environment for bacterial resistomes? PLoS One. 2013;8 doi: 10.1371/journal.pone.0054223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M., Owens K., Gajewski A., Clabots C., Johnston B., Thuras P. Extraintestinal pathogenic and antimicrobial-resistant Escherichia coli contamination of 56 public restrooms in the greater Minneapolis-St. Paul metropolitan area. Appl. Environ. Microbiol. 2015;81:4498–4506. doi: 10.1128/AEM.00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G., Rickard H., Stevenson D., Aranega-Bou P., Pitman J., Crook A., et al. Detection of sars-cov-2 within the healthcare environment: a multi-centre study conducted during the first wave of the covid-19 outbreak in England. J. Hosp. Infect. 2021;108:189–196. doi: 10.1016/j.jhin.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Study Quality Assessment Tools: Quality Assessment Tool for Observational Cohort and Cross-sectional Studies. National Institutes of Health: National Heart, Lung, and Blood Institute.
- Nicol A.-M. National Collaborating Centre for Environmental Health; Vancouver, BC: 2020. Public Washrooms in the Time of COVID-19: Facility Features and User Behaviours can Influence Safety. 2021. [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) fom a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D., Miller T., Ormrod D. Reduction of microbial transmission in childcare using an improved hand drying protocol. Healthc. Infect. 2010;15:15–19. [Google Scholar]
- Patrick D.R., Findon G., Miller T.E. Residual moisture determines the level of touch-contact-associated bacterial transfer following hand washing. Epidemiol. Infect. 1997;119:319–325. doi: 10.1017/s0950268897008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt S.J., Crockett S.L., Andreou G.M. The contribution of hand drying in prevention of transmission of microorganisms: comparison of the efficacy of three hand drying methods in the removal and distribution of microorganisms. J. Infect. Prev. 2018;19:310–317. doi: 10.1177/1757177418789485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L.B., Hungate B.A., Koch B.J., Davis G.S., Liu C.M. Colonizing opportunistic pathogens (COPs): the beasts in all of us. PLoS Pathog. 2017;13:e1006369. doi: 10.1371/journal.ppat.1006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp K.K., Hostetler T.P., Keene W.E. A norovirus outbreak related to contaminated surfaces. J. Infect. Dis. 2013;208:295–298. doi: 10.1093/infdis/jit148. [DOI] [PubMed] [Google Scholar]
- Sassi H.P., Reynolds K.A., Pepper I.L., Gerba C.P. Evaluation of hospital-grade disinfectants on viral deposition on surfaces after toilet flushing. Am. J. Infect. Control. 2018;46:507–511. doi: 10.1016/j.ajic.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Schreck J.H., Lashaki M.J., Hashemi J., Dhanak M., Verma S. Aerosol generation in public restrooms. Phys. Fluids. 2021;33 doi: 10.1063/5.0040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling A.M., Saville T., Stevens D., Beggs C.B. Comparative evaluation of the hygienic efficacy of an ultra-rapid hand dryer vs conventional warm air hand dryers. J. Appl. Microbiol. 2011;110:19–26. doi: 10.1111/j.1365-2672.2010.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen L.K.P., Siu G.K.H., Guo Y.P., Yeung S.K.W., Lo K.Y.K., O'Donoghue M. The public washroom - friend or foe? An observational study of washroom cleanliness combined with microbiological investigation of hand hygiene facilities. Antimicrob. Resist. Infect. Control. 2019;8:47. doi: 10.1186/s13756-019-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.H., Brown K.L., Toivenen J., Holah J.T. A microbiological evaluation of warm air hand driers with respect to hand hygiene and the washroom environment. J. Appl. Microbiol. 2000;89:910–919. doi: 10.1046/j.1365-2672.2000.01122.x. [DOI] [PubMed] [Google Scholar]
- Tsunoda A., Otsuka Y., Toguchi A., Watanabe K., Nishino R., Takahashi T. Survey on bacterial contamination of bidet toilets and relation to the interval of scrubbing these units. J. Water Health. 2019;17:863–869. doi: 10.2166/wh.2019.234. [DOI] [PubMed] [Google Scholar]
- Vardoulakis S., Sheel M., Lal A., Gray D. COVID-19 environmental transmission and preventive public health measures. Aust. N. Z. J. Public Health. 2020;44:333–335. doi: 10.1111/1753-6405.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani M., Bigazzi R., Carducci A. Viral contamination of aerosol and surfaces through toilet use in health care and other settings. Am. J. Infect. Control. 2014;42:758–762. doi: 10.1016/j.ajic.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan B., Zhang X., Luo D., Zhang T., Chen X., Yao Y., et al. On-site analysis of COVID-19 on the surfaces in wards. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-X., Li Y.-Y., Liu X.-D., Cao X. Virus transmission from urinals. Phys. Fluids. 2020;32 doi: 10.1063/5.0021450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Huang W., Lu X., Wang Y., Cheng L., Deng R., et al. Contamination of SARS-CoV-2 in patient surroundings and on personal protective equipment in a non-ICU isolation ward for COVID-19 patients with prolonged PCR positive status. Antimicrob. Resist. Infect. Control. 2020;9:167. doi: 10.1186/s13756-020-00839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Z., Cao C., Shuangli Z., Chang S., Dongyan W., Jingdong S., et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Wkly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- Zapka C.A., Campbell E.J., Maxwell S.L., Gerba C.P., Dolan M.J., Arbogast J.W., et al. Bacterial hand contamination and transfer after use of contaminated bulk-soap-refillable dispensers. Appl. Environ. Microbiol. 2011;77:2898–2904. doi: 10.1128/AEM.02632-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material