Abstract
Background
This study applies an umbrella review approach to summarise the global evidence on the risk of severe COVID-19 outcomes in patients with pre-existing health conditions.
Methods
Systematic reviews (SRs) were identified in PubMed, Embase/Medline and seven pre-print servers until December 11, 2020. Due to the absence of age-adjusted risk effects stratified by geographical regions, a re-analysis of the evidence was conducted. Primary studies were extracted from SRs and evaluated for inclusion in the re-analysis. Studies were included if they reported risk estimates (odds ratio (OR), hazard ratio (HR), relative risk (RR)) for hospitalisation, intensive care unit admission, intubation or death. Estimated associations were extracted from the primary studies for reported pre-existing conditions. Meta-analyses were performed stratified for each outcome by regions of the World Health Organization. The evidence certainty was assessed using GRADE. Registration number CRD42020215846.
Results
In total, 160 primary studies from 120 SRs contributed 464 estimates for 42 pre-existing conditions. Most studies were conducted in North America, European, and Western Pacific regions. Evidence from Africa, South/Latin America, and the Eastern Mediterranean region was scarce. No evidence was available from the South-East Asia region. Diabetes (HR range 1.2–2.0 (CI range 1.1–2.8)), obesity (OR range 1.5–1.75 (CI range 1.1–2.3)), heart failure (HR range 1.3–3.3 (CI range 0.9–8.2)), COPD (HR range 1.12–2.2 (CI range 1.1–3.2)) and dementia (HR range 1.4–7.7 (CI range 1.2–39.6)) were associated with fatal COVID-19 in different regions, although the estimates varied. Evidence from Europe and North America showed that liver cirrhosis (OR range 3.2–5.9 (CI range 0.9–27.7)) and active cancer (OR range 1.6–4.7 (CI range 0.5–14.9)) were also associated with increased risk of death. Association between HIV and undesirable COVID-19 outcomes showed regional heterogeneity, with an increased risk of death in Africa (HR 1.7 (CI 1.3–2.2)). GRADE certainty was moderate to high for most associations.
Conclusion
Risk of undesirable COVID-19 health outcomes is consistently increased in certain patient subgroups across geographical regions, showing high variability in others. The results can be used to inform COVID-19 vaccine prioritisation or other intervention strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-021-02058-6.
Keywords: Umbrella review, Pre-existing health conditions, Comorbidities, COVID-19, SARS-CoV-2, Hospitalisation, Death
Background
Early in 2020, the World Health Organization (WHO) declared the ongoing outbreak of Coronavirus Disease 2019 (COVID-19) to be a public health emergency of international concern [1]. Having spread around the world, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes rising numbers of COVID-19 cases and deaths. As of February 2021, the total number of confirmed cases has reached over 103 million, with over 2 million deaths worldwide [2]. With the ongoing pandemic, older people and people with chronic pre-existing conditions have been reported to be at higher risk of severe COVID-19 leading to hospitalisation, admission to intensive care, and death. Identified pre-existing conditions include hypertension, cardiovascular diseases, chronic kidney and liver diseases, cancer [3, 4], obesity, and immunosuppressed states [5, 6]. Around 22% of the global adult population is estimated to have at least one of the underlying health conditions, which can lead to severe COVID-19 if infected [7].
Since the start of the pandemic, multiple studies, which explore the association between pre-existing conditions and COVID-19 outcomes, have been conducted in different countries. Many literature reviews and meta-analyses further systematically examined the association for various health conditions. However, an overwhelming number of systematic reviews (SRs) with differences in outcomes definitions, selection criteria, synthesis and reporting complicate the interpretation of the overall body of evidence. It is unclear what pre-existing health conditions are associated with worse COVID-19 outcomes and whether the pre-existing conditions and the strength of associations differ between the geographical regions.
In this work, we aimed to summarise the evidence on age-adjusted associations between multiple pre-existing health conditions and clearly defined COVID-19 outcomes separating the effects between the geographical regions. To do it effectively and time-sparing, we conducted this study using an umbrella review approach.
Umbrella review is a systematic collection and assessment of evidence reported in systematic literature reviews. The methods of umbrella review allow for analysis of a large body of evidence on the strength of associations and the confidence in the estimates using the findings of SRs [8–12]. As previously discussed [13], umbrella reviews have been more frequently used to synthesise available evidence and inform clinical practice and public health policies. The methodology is valuable for compiling the findings for broad subject areas as it provides a uniform approach to evidence synthesis, increases statistical power and ensures confidence in the evidence. We applied the umbrella review methodology for this study because it allowed us to systematically and efficiently compile the published evidence on associations between a broad spectrum of pre-existing conditions and several COVID-19 outcomes across geographical regions into a single informative review.
Methods
Study design
We identified and analysed currently available SRs on pre-existing health conditions and risk of severe COVID-19, and the primary studies included therein using an umbrella review approach. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD42020215846).
Systematic reviews: search, selection, data extraction and quality assessment
We performed a systematic search for SRs in PubMed and Embase (including Medline), supplemented by hand searches on ArRvix, BioRvix, ChemRvix, MedRvix, Preprints.org, ResearchSquare and SSRN. All searches were done using the COVID-19 literature database constructed by the Robert Koch Institute library (date of the last search: 11 December 2020; search strings: Additional file 1 section 1.1).
To be eligible, an SR had to investigate the association between at least one pre-existing health condition (including but not limited to asthma, chronic obstructive pulmonary disease (COPD), cancer, diabetes mellitus, cardiovascular diseases, chronic kidney diseases, chronic liver diseases, chronic diseases of the digestive system, hypertension, obesity and immunocompromising conditions), and at least one of the following severe health outcomes due to COVID-19: hospitalisation, admission to ICU, ventilation (intubation) or death (Additional file 1: section 1.2). The titles and abstracts of identified SRs were screened independently by two investigators (MTS, SR). Data extraction (Additional file 1: section 1.3) and quality assessment were divided between the reviewers (AB, KK, SR, LH, AP, MTS) following the four-eyes principle, meaning that one reviewer performed the task and a second reviewer confirmed the results. All disagreements were resolved by discussion or by a third reviewer (TH). The methodological quality of included systematic reviews was assessed using the AMSTAR-2 (Assessment of Multiple Systematic Reviews-2) tool (Additional file 1 section 1.4) [14].
Primary studies: search, selection, data extraction and risk of bias assessment
The analysed systematic reviews did not provide evidence on age-adjusted risk estimates across geographical regions. Therefore, we evaluated the primary studies included in SRs for a re-analysis. Primary studies reported in the included SRs were included in the re-analysis if they (i) reported at least one quantitative measure of association (risk ratio (RR), odds ratio (OR), or hazard ratio (HR)) in patients with a pre-existing health condition, compared to patients without this condition; (ii) reported age-adjusted estimates (multivariate models, cohort matching or age stratification) for at least one pre-existing condition and at least one health outcome (hospitalisation, admission to ICU, mechanical ventilation, or intubation, in-hospital mortality, mortality among SARS-CoV2-positively tested persons (case mortality)). Primary studies of all designs were eligible. Study selection and data extraction were performed as for SRs (Additional file 1: section 1.5). To be consistent, when several models were reported, we chose those that adjusted for age, sex and pre-existing conditions and avoided (if possible) adjustment for laboratory values, vital signs or socio-economic factors. For evaluation of the risk of bias in the primary studies, the results of the assessments performed by the authors of the respective SRs that reported the primary studies were used. In case a primary study was not evaluated in any included SR, we assessed the risk of bias using the Newcastle-Ottawa Scale (NOS) [15].
Data analysis
If more than one estimate for an association between a pre-existing health condition and an outcome was reported, we used I2 (%) statistics to test whether a meta-analysis was appropriate to combine the estimates. If I2 was ≤ 40%, we decided that heterogeneity was low enough to allow a meta-analysis. Random-effects models (generic inverse variance method) were used to combine estimates by health outcome, the measure of association, and risk factor in R using the metaphor package, which allows the application of generic inverse variance methods for adjusted risk estimates for which confidence interval, but no standard error is reported. To consider the possible influence of setting-specific factors (geographical, healthcare and resources), we stratified meta-analyses by WHO regions. Countries were grouped into the WHO regions as follows. South Africa—the African Region (AFR). Kuwait and Iran—the Eastern Mediterranean Region (EMR). Denmark, France, Italy, Israel, Spain, Switzerland and the UK—the European Region (EUR). Republic of Korea, China and the Philippines—the Western Pacific Region (WPR). Regions of America (AMR) were divided into North America (the USA), and South/Latin America (Brazil, Mexico, Bolivia). There were no included studies from countries of the South-East Asia Region (SEAR). If I2 was > 40%, ranges (min–max) of the estimates were reported instead of the pooled results. If ten or more estimates for a given association and outcome were available, the likelihood of publication bias was assessed by inspection of funnel plots, followed by Egger’s test and Begg’s test. Due to the differences in methods of association estimation, the measures of associations were treated separately as odds ratio (OR), hazard ratio (HR) and risk ratio (RR).
Analysis of subgroups
We conducted analyses of subgroups for those primary studies that used age stratification to control the confounding effect of age and the studies that selected a specific study population, e.g. patients with cancer. Meta-analyses were not performed here because of the limited number of studies and high heterogeneity in reporting. Instead, the evidence was narratively synthesised and presented.
Certainty of the evidence (evidence quality)
Certainty of the evidence was assessed for each outcome using the methodology proposed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group for risk factor studies [16], as described in PRECEPT (Project on a Framework for Rating Evidence in Public Health) [17]. In data extraction, we looked at the covariates used in the multivariate regression models of the included studies. Observed variations in the number and nature of the included factors suggested different residual confounding of the resulted risk estimates. In the certainty assessment, for each estimate, we evaluated inconsistency, imprecision and bias. Downgrading factors included serious inconsistency defined as I2 > 40% heterogeneity, serious imprecision defined as confidence interval including 1.0, serious risk of bias judged based on the quality assessment extracted from SRs and risk of publication bias. For the estimates obtained from single studies, inconsistency was not graded.
Results
Characteristics of included systematic reviews
The systematic search identified a total of 3417 entries. After title/abstract and full-text screening, 120 reviews [3–6, 18–133] were found to be eligible (Additional file 1: Figure 1, Table 1). The majority of SRs explored a variety of risk factors for severe COVID-19 outcomes, including pre-existing conditions, vital signs and socio-economic factors. Other reviews focused on a specific pre-existing health condition such as obesity, diabetes, cancer or hypertension. The number of included primary studies varied between 3 and 212 (Additional file 1: Figure 3A). Only three reviews conducted literature searches up to September and October 2020, while the majority included data from primary studies published up to 31 May 2020 (Additional file 1: Table 1 and Figure 3B). The methodological quality of reviews was low to critically low, mostly due to the lack of a registered protocol and reporting on excluded studies or language restrictions (Additional file 1: Table 2 and Figure 3C).
Characteristics of included primary studies
Based on included SRs, a total of 356 primary studies were assessed for eligibility. After the exclusion of 196 papers, 160 remained for data extraction [134–293] (Additional file 1: Figure 2). Of those, 59 studies were single-centre studies, 77 studies followed a multi-centre approach, while the remaining studies came from diverse settings (e.g. used electronic databases) (Additional file 1: Table 3). All but 15 studies had a cohort design. The number of participants in the primary studies varied between 36 and 89,756. To minimise methodological heterogeneity, we excluded nine [294–302] studies from the main analysis since they reported community-based estimates only (Additional file 1: section 2.2, Table 4). For each study, we provided information regarding the COVID-19 case definition (Additional file 1: Table 3). In over 90% of the articles polymerase chain reaction (PCR) and/or viral sequencing was used. In 5% of the studies, PCR was used in at least 82% of the included cases or PCR was used alongside with / or was substituted by a blood test for SARS-Cov-2 IgG/IgM for antibodies or radiologic findings. In 2.5% of the studies, ICD-10 codes or a mix of PCR, clinical diagnosis or ICD-10 codes were used; however, the proportion of each method was not stated. Only in 1.8% of the articles, the authors did not explicitly state how COVID-19 cases were diagnosed. We decided to include the latter studies to obtain a complete overview over analyses conducted regarding our research question.
Additionally, the definitions of the pre-existing studies were extracted from the studies which reported them (Additional file 1: Table 3 and section 2.3). In the meta-analyses, we did not conduct additional grouping into broader disease categories to avoid increased heterogeneity of the results stemming from the differences in the disease definitions. Instead, we addressed each health condition as it was reported in the primary studies. Similar descriptions of the diseases were grouped as described in the Additional file 1 (section 2.3).
Data analyses
We extracted 1321 estimates for risk factors indicating pre-existing health conditions. Risk estimates that used comorbidity indexes (e.g. Charlson score) and age-stratified estimates were excluded from the meta-analyses (see below for separate reporting of age-stratified estimates). In total, 1019 extracted estimates for 54 pre-existing health conditions from 133 [134–143, 145–150, 152–170, 172–177, 180–182, 184–196, 198–200, 202–205, 207, 210–213, 216, 218–221, 225–230, 233–235, 237–242, 244–246, 248, 249, 251–281, 283, 284, 287–292] primary studies were included in the meta-analysis. The meta-analysis resulted in 521 estimates, of which 464 estimates for 42 pre-existing conditions for the primary outcomes were selected for further analysis, GRADE assessment and reporting (Table 1, excluded are listed in Additional file 1: section 2.4). For 281 associations, only single-study estimates were available. In total, 111 associations are reported as pooled estimates from at least two studies with low between-study heterogeneity. For 72 associations, heterogeneity was considerable (I2 > 40%). For the latter, the risk estimates were illustrated as ranges (min–max) (Additional file 1: section 2.5, Figures 4-6), and both the pooled estimates and the ranges are reported. All estimates are reported in Additional file 1 (section 2.5, Tables 5.1-9.7). The risk of bias was low in the majority of studies (Additional file 1: Table 3). Potential publication bias was detected only in one meta-analysis (risk of intubation for people with obesity) (Additional file 1: Table 9.4). Certainty of the evidence was high for 179 of the estimates, moderate for 234 and low for 51. None of the analysed bodies of evidence was assessed to have very low certainty. Studies that selected populations based on a pre-existing condition were summarised in Additional file 1 (section 2.8).
Table 1.
Characteristics of analysed primary studies per pre-existing condition
| Disease group | Pre-existing condition | Outcomes included | Number of systematic reviews reporting primary studies | Number of resulted estimates | Number of primary studies | Countries | Sample sizes (min – max) | GRADE confidence in estimate (min – max) |
|---|---|---|---|---|---|---|---|---|
| Circulatory diseases | Arrhythmia | Hospitalisation; case mortality; hospital mortality; intubation | 13 [35, 39, 42, 54, 57, 70, 78, 90, 96, 97, 114, 124, 133] | 9 | 7 [198, 210, 233, 240, 245, 262, 272] | Italy; UK; Denmark; Spain; USA; China | 322–11,122 | moderate - high |
| Circulatory diseases | Cardiovascular disease | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 57 [3, 4, 6, 18, 19, 23, 24, 26–29, 31, 34, 35, 37–42, 44, 46, 48, 53, 54, 57–60, 64, 66, 68–70, 74, 76, 78, 88, 90–92, 95–97, 103, 105, 111, 114, 116, 117, 120, 122, 124, 126, 127, 132, 133] | 21 | 32 [135, 139, 140, 156, 157, 159, 160, 175, 182, 187, 190, 196, 199, 203, 216, 218, 220, 228, 235, 238, 241, 242, 245, 255, 256, 262, 264–266, 274, 276, 290] | Iran; France; Spain; Italy; Israel; USA; Brazil; Mexico; China; Korea | 103–89,756 | low - high |
| Circulatory diseases | Coronary artery disease | Hospital mortality; hospitalisation; case mortality; ICU admission; intubation | 70 [4, 5, 18, 20, 21, 24, 25, 27–29, 31, 34, 35, 38–42, 47, 48, 50–52, 54, 55, 57–60, 63–66, 68, 70, 74, 77, 78, 80, 81, 83–85, 87–91, 94–97, 102–105, 111, 114, 116, 117, 120, 122, 124–126, 129–133] | 17 | 26 [63, 138, 153, 157, 158, 161, 163, 176, 186, 193, 198, 205, 207, 210, 213, 230, 233, 234, 240, 245, 252, 267, 272, 283, 290, 291] | UK; Italy; Europe; Denmark; USA; China; Korea | 112–11,210 | low - high |
| Circulatory diseases | Heart disease | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 17 [21, 24, 31, 37, 38, 42, 54, 57, 64, 74, 78, 81, 93, 104, 114, 120, 122] | 7 | 6 [149, 170, 192, 200, 254, 275] | Spain; UK; USA; China | 103–15,194 | low - high |
| Circulatory diseases | Heart failure | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 29 [4, 5, 21, 25, 28, 29, 35, 37–39, 42, 54, 57, 58, 64, 68, 70, 78, 81, 90, 96, 97, 111, 114, 117, 120, 124, 126, 133] | 15 | 22 [138, 139, 162, 169, 173, 177, 191, 198, 205, 213, 230, 233, 234, 240, 245, 248, 251, 252, 267, 271, 272, 283] | Italy; UK; Denmark; USA; China | 191–31,461 | low - high |
| Circulatory diseases | Hypertension | ICU admission; hospital mortality; hospitalisation; case mortality; intubation | 71 [3–6, 18, 19, 21, 23–31, 33–35, 37–42, 44, 46, 48, 50, 53–55, 57–59, 62–66, 68–70, 72, 74, 78, 81, 88, 90–93, 95–97, 99, 103–105, 107, 111, 114, 117, 120, 122–124, 127, 128, 132, 133] | 37 | 71 [134, 137–139, 143, 145, 148, 149, 152, 153, 155, 156, 158–160, 163, 164, 167–169, 173–177, 182, 192–194, 198–200, 203, 205, 207, 210, 213, 216, 219–221, 226–228, 233–235, 239–242, 245, 246, 248, 251, 252, 255, 258, 262, 265, 267, 268, 271, 272, 274, 275, 278, 281, 284, 287, 290] | Kuwait; Spain; UK; Italy; Europe; Denmark; Israel; Switzerland; France; USA; Mexico; Bolivia; China; Korea; South Africa | 103–89,756 | low - high |
| Circulatory diseases | Infarction | Hospital mortality; case mortality | 4 [37, 57, 68, 97] | 4 | 4 [169, 177, 191, 271] | Italy; USA; China | 2877 – 31,461 | moderate - high |
| Circulatory diseases | Peripheral vascular disease | Hospital mortality; hospitalisation; intubation; case mortality | 4 [37, 54, 97, 133] | 5 | 3 [191, 271, 272] | USA | 2015 – 31,461 | moderate - moderate |
| Circulatory diseases | Venous thromboembolism | Hospital mortality; hospitalisation; intubation | 9 [29, 39, 54, 70, 78, 97, 114, 120, 133] | 4 | 3 [233, 235, 272] | UK; USA | 238–3703 | low - moderate |
| Immunodeficiency | Autoimmune condition | Hospital mortality; hospitalisation; case mortality | 4 [54, 78, 90, 97] | 7 | 4 [149, 187, 228, 262] | Spain; USA | 322–9437 | moderate - high |
| Immunodeficiency | HIV | Hospital mortality; ICU admission; case mortality; intubation | 17 [5, 28, 35, 37–39, 42, 62, 64, 70, 90, 96, 97, 111, 114, 120, 124] | 10 | 8 [138, 148, 180, 191, 199, 229, 233, 257] | UK; USA; South Africa | 251–47,539 | low - high |
| Immunodeficiency | Inflammatory bowel disease | Hospital mortality; intubation; hospitalisation | 14 [5, 28, 35, 38, 39, 42, 90, 92, 96, 97, 111, 114, 120, 124] | 3 | 2 [138, 261] | USA | 464–841 | moderate - moderate |
| Immunodeficiency | Immunosuppression | Hospital mortality; intubation; hospitalisation; ICU admission; case mortality | 19 [5, 28, 29, 35, 38, 39, 42, 44, 57, 64, 68, 78, 90, 95–97, 111, 120, 124] | 12 | 12 [138, 140, 142, 147, 152, 158, 182, 203, 226, 252, 265, 271] | Italy; USA; Brazil; Mexico | 302–89,756 | moderate - high |
| Immunodeficiency | Organ transplant recipients | Hospital mortality; hospitalisation; case mortality; intubation | 13 [5, 28, 35, 38, 39, 42, 78, 90, 96, 97, 111, 120, 124] | 6 | 3 [138, 240, 271] | Denmark; USA | 841–11,122 | moderate - high |
| Immunodeficiency | Rheumatological disease | Hospital mortality; hospitalisation; case mortality; intubation | 15 [5, 28, 35, 37–39, 42, 78, 90, 96, 97, 111, 114, 120, 124] | 8 | 5 [138, 166, 191, 203, 240] | Denmark; USA | 156–31,461 | moderate - high |
| Liver & Metabolic diseases | Chronic kidney disease | Hospital mortality; hospitalisation; case mortality; ICU admission; intubation | 37 [4–6, 19, 21, 25, 28, 29, 35, 37–39, 42, 44, 46, 54, 55, 57, 58, 62, 64, 68, 70, 78, 81, 90, 95–97, 104, 111, 114, 117, 120, 124, 126, 133] | 30 | 55 [137, 138, 140, 142, 148, 149, 152, 153, 156, 159, 160, 162, 163, 169, 170, 176, 177, 182, 186, 187, 190, 191, 193, 198, 199, 203, 205, 207, 218, 219, 226, 228, 230, 233–235, 240–242, 245, 246, 248, 251, 252, 256, 262, 264–267, 271–273, 283, 290] | Spain; UK; Italy; Europe; Denmark; USA; Brazil; Mexico; China; Korea; South Africa | 112–89,756 | low - high |
| Liver & Metabolic diseases | Chronic liver disease | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 16 [21, 24, 25, 37, 42, 57, 64, 78, 81, 90, 97, 104, 114, 117, 120, 124] | 15 | 13 [140, 170, 187, 191, 192, 199, 228, 240, 251, 252, 260, 262, 290] | UK; Denmark; Spain; USA; Brazil; China | 322–31,461 | low - high |
| Liver & Metabolic diseases | Chronic liver disease/Cirrhosis | Hospital mortality; intubation | 17 [5, 28, 35, 38, 39, 42, 57, 64, 70, 90, 96, 97, 111, 114, 120, 124, 133] | 6 | 5 [138, 143, 192, 233, 248] | Spain; UK; USA | 242–4035 | moderate - high |
| Liver & Metabolic diseases | Chronic liver disease /Non-cirrhotic | Hospital mortality | 4 [70, 97, 114, 120] | 2 | 2 [192, 233] | UK; USA | 363–614 | moderate - moderate |
| Liver & Metabolic diseases | Diabetes | ICU admission; hospital mortality; hospitalisation; case mortality; intubation | 69 [3–6, 18, 19, 21, 23–31, 33–35, 37–42, 44, 46, 48, 50, 53–55, 57–59, 62–64, 68–70, 72, 78, 81, 88, 90, 92, 93, 95–97, 99, 103–105, 107, 111, 114, 117, 120, 123, 124, 126–128, 132, 133] | 38 | 80 [134, 135, 138–140, 142, 145, 148–150, 152, 153, 158–160, 163, 164, 167–170, 173, 175, 176, 182, 187, 191–193, 195, 196, 198–200, 202–205, 207, 210, 216, 218–221, 225–228, 230, 233–235, 238, 240–242, 245, 246, 248, 251, 252, 255, 256, 258, 262–267, 271, 272, 280, 283, 284, 289, 290, 292] | Kuwait; Iran; France; Spain; UK; Italy; Europe; Denmark; Israel; USA; Brazil; Mexico; China; Korea; South Africa | 92–89,756 | low - high |
| Liver & Metabolic diseases | Dyslipidemia or hyperlipidemia | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 32 [4–6, 21, 25, 27–29, 31, 33, 35, 39, 42, 54, 63, 64, 68, 70, 78, 81, 90, 93, 96, 97, 99, 111, 114, 120, 124, 126, 132, 133] | 12 | 12 [149, 192, 205, 218, 230, 234, 235, 245, 258, 262, 263, 271] | Spain; Italy; France; USA | 124–9437 | low - high |
| Liver & Metabolic diseases | Hepatitis | Hospital mortality; intubation | 13 [5, 28, 35, 38, 39, 42, 55, 90, 96, 97, 111, 120, 124] | 3 | 2 [138, 219] | USA; China | 841–2665 | moderate - moderate |
| Neurological diseases & Mental health | Cerebrovascular/Stroke | Hospital mortality; ICU admission; hospitalisation; case mortality; intubation | 44 [4–6, 19, 24, 27–29, 31, 34, 35, 37–42, 44, 48, 54, 55, 57, 58, 60, 64, 66, 68, 70, 74, 76, 78, 88, 90, 91, 95–97, 105, 111, 114, 116, 117, 120, 124, 133] | 17 | 20 [138, 145, 155, 156, 186, 191, 196, 207, 210, 213, 219, 233, 235, 240, 246, 256, 262, 271, 272, 276] | Italy; UK; Denmark; Spain; USA; China; Korea | 103–31,461 | low - high |
| Neurological diseases & Mental health | Dementia | Hospital mortality; hospitalisation; case mortality; intubation | 22 [21, 29, 35, 37, 39, 42, 54, 57, 64, 68, 70, 78, 81, 90, 96, 97, 104, 114, 117, 120, 124, 133] | 12 | 14 [143, 146, 149, 150, 170, 191, 196, 199, 220, 233, 240, 245, 262, 272] | Spain; UK; Italy; Denmark; Israel; USA; Korea | 92–31,461 | low - high |
| Neurological diseases & Mental health | Depression | Hospitalisation | 4 [78, 90, 97, 114] | 2 | 3 [139, 220, 262] | Israel; Spain; USA | 322–1052 | low - moderate |
| Neurological diseases & Mental health | Neurological disease | Hospital mortality; ICU admission; case mortality | 15 [21, 37, 39, 42, 57, 64, 68, 78, 81, 97, 104, 114, 120, 126, 133] | 5 | 5 [140, 143, 170, 203, 266] | Spain; UK; USA; Brazil | 2070–15,194 | moderate - high |
| Neurological diseases & Mental health | Psychiatric disorder | Hospital mortality; hospitalisation; case mortality | 2 [78, 124] | 3 | 2 [220, 240] | Denmark; Israel | 782–11,122 | high - high |
| Oncological diseases | Cancer | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 38 [4–6, 19, 21, 24, 28–30, 35, 37, 39, 42, 44, 54, 55, 57, 58, 64, 68, 70, 72, 78, 81, 90, 96, 97, 104, 107, 111, 114, 117, 120, 123, 124, 126, 128, 133] | 19 | 30 [139, 149, 156, 167, 169, 170, 187, 191, 198, 199, 205, 207, 210, 211, 218, 219, 221, 225, 228, 234, 235, 240, 245, 252, 256, 262, 267, 271, 272, 283, 290] | Spain; Italy; UK; Denmark; USA; China; Korea | 238–31,461 | low - high |
| Oncological diseases | Cancer/Active | Hospital mortality; ICU admission; intubation | 18 [5, 25, 28, 35, 38, 39, 42, 57, 64, 78, 90, 96, 97, 104, 111, 120, 124, 133] | 8 | 6 [138, 141, 143, 161, 176, 251] | Spain; Italy; UK; USA | 407–4035 | moderate - high |
| Oncological diseases | Cancer/ Haematological | Hospital mortality | 5 [70, 97, 108, 114, 123] | 2 | 3 [233, 249, 253] | UK; Spain | 92–1183 | low - high |
| Oncological diseases | Cancer/Solid | Hospital mortality; case mortality | 4 [37, 70, 97, 114] | 2 | 2 [191, 233] | UK; USA | 614–31,461 | moderate - high |
| Overweight, obesity, underweight | Obesity/BMI > 30 | ICU admission; hospital mortality; hospitalisation; case mortality; intubation | 39 [4–6, 21, 25, 27–29, 31, 33, 35, 37–39, 42, 44, 54, 57, 63, 64, 68, 70, 78, 81, 90, 93, 95–97, 99, 104, 111, 114, 117, 120, 124, 126, 132, 133] | 27 | 53 [134, 137, 140–143, 149, 152, 154, 158, 163, 164, 168–170, 173, 181, 182, 184, 187, 192, 195, 198–200, 202, 203, 205, 213, 216, 218, 220, 226–228, 230, 234, 235, 237, 240, 245, 246, 251, 252, 258, 259, 262, 264–266, 271, 272, 283] | Kuwait; Spain; Italy; UK; France; Denmark; Israel; USA; Brazil; Mexico | 103–89,756 | low - high |
| Overweight, obesity, underweight | Obesity/BMI > 40 | ICU admission; hospital mortality; hospitalisation; intubation; case mortality | 19 [5, 21, 28, 29, 35, 39, 57, 58, 64, 68, 78, 81, 96, 97, 111, 114, 120, 124, 126] | 7 | 8 [134, 184, 195, 205, 234, 252, 267, 271] | Kuwait; USA | 463–6916 | high - high |
| Overweight, obesity, underweight | Overweight | ICU admission; intubation; hospital mortality; hospitalisation; case mortality | 25 [5, 6, 21, 25, 27, 29, 31, 33, 35, 39, 42, 54, 63, 78, 81, 93, 96, 97, 99, 114, 120, 124, 126, 132, 133] | 11 | 11 [134, 184, 187, 200, 227, 228, 234, 251, 258, 271, 272] | Kuwait; France; USA | 103–6916 | low - high |
| Overweight, obesity, underweight | Underweight | Hospital mortality; hospitalisation; intubation; case mortality | 12 [4, 21, 25, 28, 29, 37, 70, 78, 81, 97, 114, 120] | 7 | 6 [184, 187, 199, 228, 230, 271] | USA | 200–6916 | low - moderate |
| Respiratory diseases | Asthma | Hospital mortality; hospitalisation; intubation; case mortality | 19 [19, 29, 39, 42–44, 54, 57, 64, 70, 78, 90, 95, 97, 111, 114, 120, 126, 133] | 12 | 16 [139, 140, 158, 182, 212, 218, 226, 228, 233, 252, 262, 265, 271–273, 283] | UK; Spain; USA; Brazil; Mexico | 322–89,756 | low - high |
| Respiratory diseases | COPD | Hospital mortality; hospitalisation; case mortality; intubation; ICU admission | 54 [4, 6, 19, 21, 24, 25, 27–31, 34, 35, 38–42, 44, 46, 48, 54, 55, 57, 58, 60, 62, 64, 66, 68, 70, 72, 74, 76, 78, 81, 88, 90, 91, 95–97, 105, 107, 111, 114, 116, 117, 120, 123, 124, 126, 128, 133] | 21 | 34 [137, 139, 142, 148, 149, 152, 156, 158, 160, 167, 182, 186, 207, 213, 218, 219, 226, 228, 230, 233, 239, 241, 245, 252, 256, 257, 262, 263, 265, 272, 276, 283, 288, 290] | Spain; Italy; UK; Switzerland; USA; Mexico; China; Korea | 145–89,756 | low - high |
| Respiratory diseases | COPD or Asthma | Hospital mortality; hospitalisation; intubation; ICU admission | 17 [5, 21, 25, 29, 35, 38, 39, 64, 78, 81, 96, 97, 111, 114, 120, 124, 126] | 5 | 5 [173, 198, 234, 248, 251] | USA | 214–5279 | moderate - moderate |
| Respiratory diseases | Interstitial lung disease | Hospital mortality | 1 [97] | 1 | 1 [228] | USA | 5776 | high - high |
| Respiratory diseases | Obstructive sleep apnea | Intubation; hospitalisation | 11 [4, 21, 25, 28, 29, 70, 78, 81, 95, 111, 114] | 2 | 2 [158, 230] | USA | 200–1526 | moderate - high |
| Respiratory diseases | Respiratory disease | Hospital mortality; ICU admission; hospitalisation; case mortality; intubation | 33 [4, 5, 21, 28, 29, 31, 35, 37–39, 41, 42, 54, 57, 62, 64, 66, 68, 78, 81, 90, 93, 96, 97, 104, 111, 114, 117, 120, 124, 126, 133] | 24 | 26 [138, 140, 142, 145, 148, 150, 169, 170, 176, 187, 191, 192, 196, 199, 200, 203, 216, 220, 235, 240, 242, 255, 264, 266, 268, 271] | Italy; UK; Spain; Denmark; Israel; USA; Brazil; Mexico; China; Korea; South Africa | 92–51,633 | low - high |
| Respiratory diseases | Tuberculosis | Hospital mortality; hospitalisation; case mortality | 4 [55, 62, 68, 100] | 6 | 3 [148, 219, 269] | China; Philippines; South Africa | 330–22,308 | moderate - high |
No number, ICU intensive care unit
Risk estimates
Figures 1, 2 and 3 show all estimates (RR, OR or HR) with 95% CI for risk of hospitalisation, ICU admission and death (separately for case mortality and in-hospital mortality) due to COVID-19 in patients with a pre-existing health condition, as compared to patients without the respective condition. Figure 4 illustrates a summary of the GRADE assessment for these estimates. Figures 5 and 6 show associations supported by evidence of high certainty based on the GRADE assessment (also see Additional file 1: Table 10).
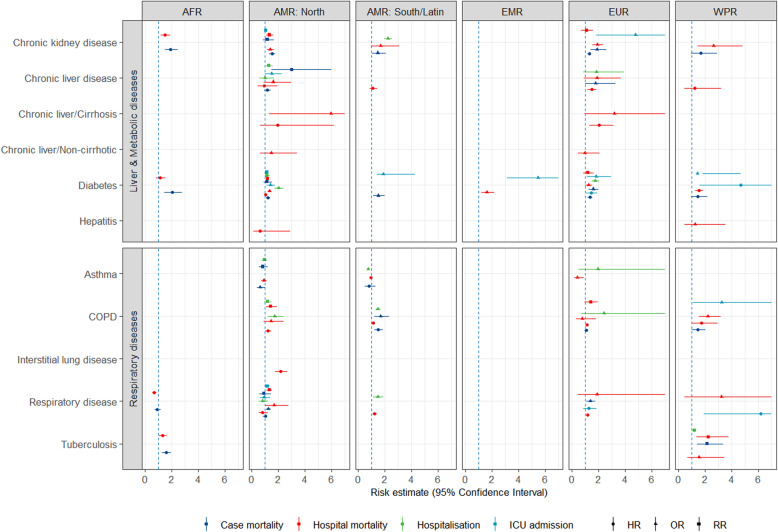
Fig. 1.
Results of the meta-analyses for pre-existing conditions: Liver and metabolic diseases (the upper panel) and respiratory diseases (the lower panel), by WHO region (excluding SEAR). The error bars represent 95% confidence intervals. The dashed line indicates 1.0 value. The estimates with error bars crossing the 1.0-line lack statistical significance
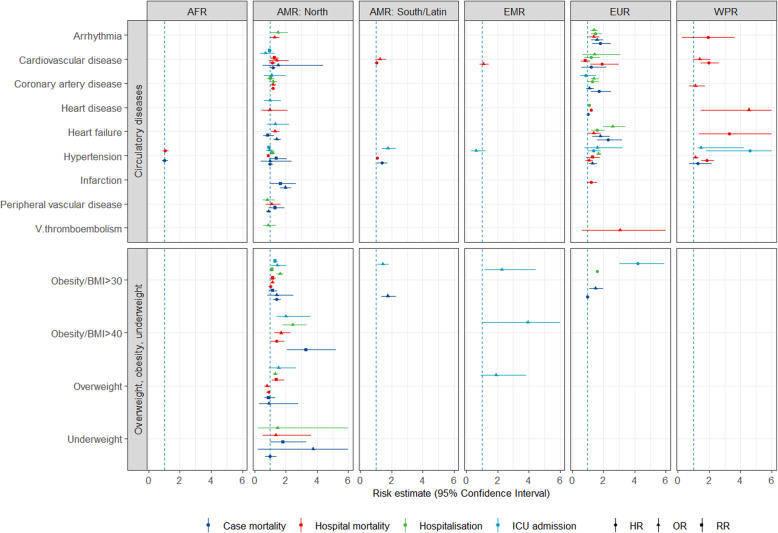
Fig. 2.
Results of the meta-analyses for pre-existing conditions: Circulatory diseases (the upper panel) and overweight/obesity/underweight (the lower panel), by WHO region (excluding SEAR). The error bars represent 95% confidence intervals. The dashed line indicates 1.0 value. The estimates with error bars crossing the 1.0-line lack statistical significance
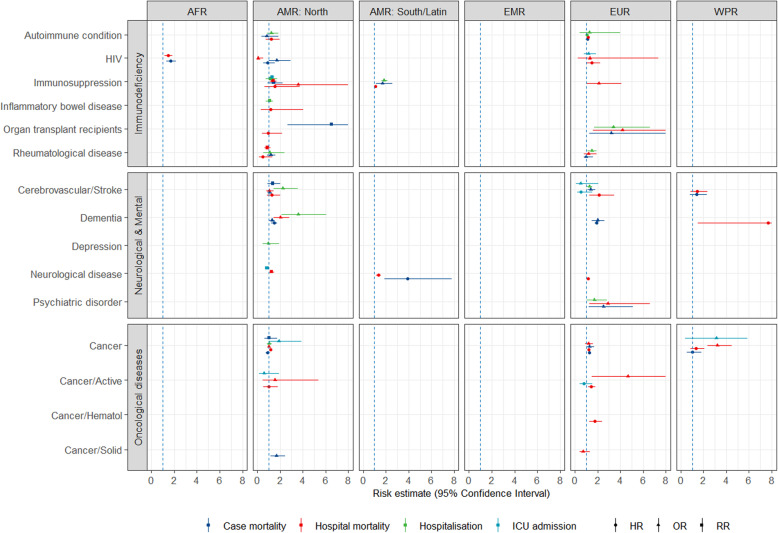
Fig. 3.
Results of the meta-analyses for pre-existing conditions: Immunodeficiency (the upper panel), neurological diseases and mental health (the middle panel) and oncological diseases (lower panel), by WHO region (excluding SEAR). The error bars represent 95% confidence intervals. The dashed line indicates 1.0 value. The estimates with error bars crossing the 1.0-line lack statistical significance
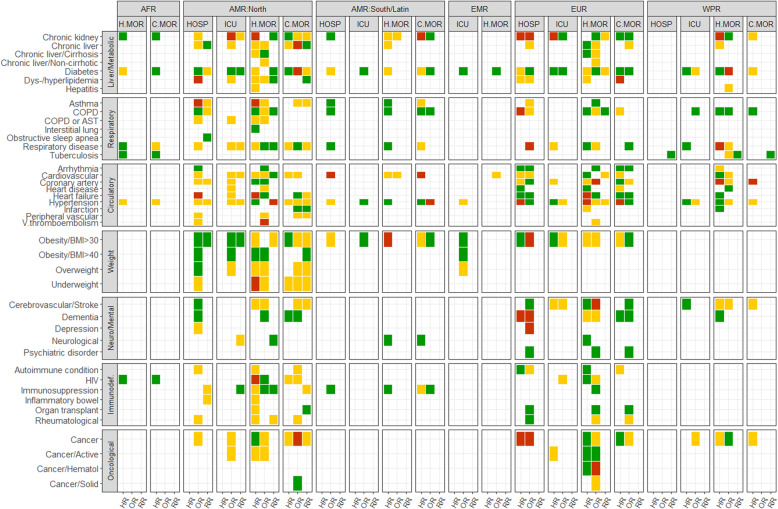
Fig. 4.
Summary of the GRADE assessment for each pre-existing condition, by health outcome and WHO region (red color - low,yellow color - moderate, green color - high). Outcomes include hospitalisation (HOSP), intensive care unit (ICU), in-hospital mortality (H.MOR), case mortality (C.MOR)
Fig. 5.
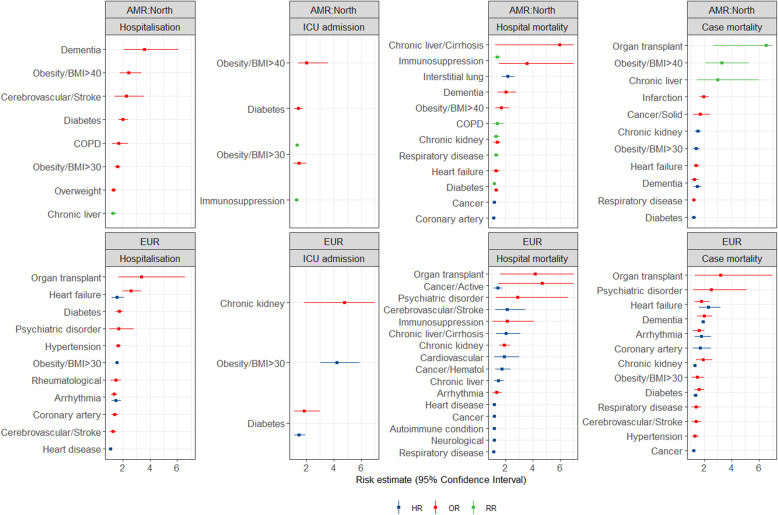
Estimated associations supported by high certainty of evidence (GRADE) presented for each pre-existing condition and outcome for the European region and North America. The estimated associations are arranged by ascending value of measures of effect. However, the presented order should be considered in the context of differences between the statistics (OR, RR, HR)
Fig. 6.
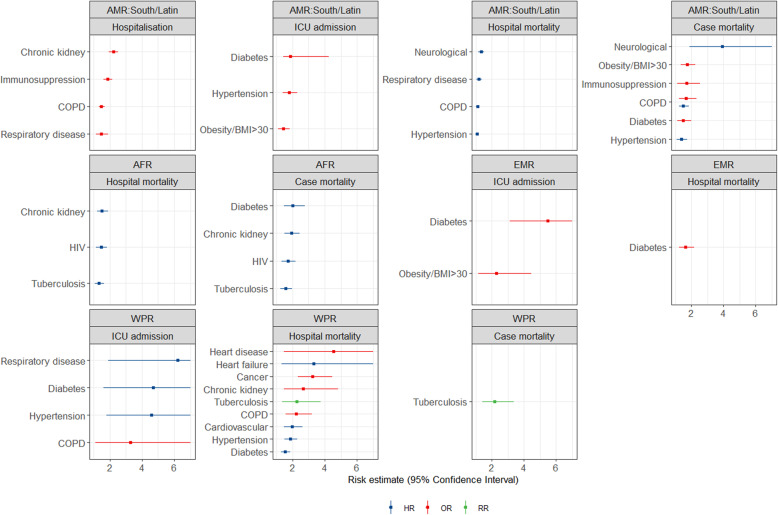
Estimated associations supported by high certainty of evidence (GRADE) presented for each pre-existing condition and outcome for the African, Eastern Mediterranean and Western Pacific regions. The estimated associations are arranged by ascending value of measures of effect. However, the presented order should be considered in the context of differences between the statistics (OR, RR, HR)
Results from meta-analyses and single-study estimates are stratified by seven disease groups and six regions (AFR, EMR, EUR, North America, South/Latin America and WPR). No data was available for SEAR. This section presents the effect estimates with high certainty of evidence based on GRADE (Additional file 1: section 2.6, Table 10). To facilitate reporting, we consider a relative association of 1.5–2.0 as increased risk and of > 2.0 as markedly increased risk regardless of outcome and measure of association. It is, however, essential to note that odds ratio, risk ratio and hazard ratio differ in the interpretation of the estimated association [303].
Liver and metabolic diseases
In liver and metabolic diseases (Fig. 1, the upper panel), the evidence was available for the following pre-existing conditions: chronic kidney disease, chronic liver disease (including cirrhosis and hepatitis), diabetes and dyslipidemia or hyperlipidemia. The highest number of estimates (38 estimates) was available for diabetes, including data from all regions. Diabetes was associated with an increased risk of hospitalisation in Europe and death in Europe and South/Latin America. A higher risk of death was observed in the study from the African region. Increased risk and markedly increased risk of ICU admission associated with diabetes were found for South/Latin America, EMR and WPR.
The estimates for chronic kidney disease showed heterogeneity between the studies across the regions and the outcomes. A markedly increased risk was shown for hospitalisation in South/Latin AMR, ICU admission in EUR, and for in-hospital death in WPR. An increased risk of death was observed in the studies from AFR, AMR North and EUR. Among chronic liver diseases, viral hepatitis was the condition with the lowest number of available estimates, with studies from WPR and North America showing no significant increase in the risk of in-hospital death. Regarding other liver diseases, the risk of in-hospital death was markedly increased in patients with liver cirrhosis in EUR and North America. Fewest estimates were available from EMR.
Respiratory diseases
Regarding lung diseases (Fig. 1, the lower panel), the highest number of estimates was available for the (unspecified) diagnosis group of respiratory diseases (24 estimates), followed by COPD (21 estimates). For COPD, all but two risk estimates (for ICU admission and in-hospital mortality in WPR) were either below 2.0 or not significant. COPD was associated with an increased risk of hospitalisation in North America and death in South/Latin America. In patients with asthma, risk estimates from three regions showed no significant effect on any of the outcomes but rather a tendency towards decreased risk. Increased risk of hospitalisation and death due to COVID-19 was reported in patients with tuberculosis in AFR and WPR. Only one risk estimate from North America was available for interstitial lung disease, showing a more than a twofold increased risk of in-hospital death. No estimates were available from EMR. The estimates for respiratory diseases as a generic condition varied greatly, possibly due to differences in the definitions among the studies.
Overweight, obesity or underweight
For overweight/obesity/underweight (Fig. 2, the lower panel), most estimates (34 out of 52) came from North America. Patients with severe obesity (BMI ≥ 40 kg/m2) had a particularly high risk of severe COVID-19 outcomes, including a more than threefold increased risk of ICU admission and death. Likewise, patients with BMI ≥ 30 kg/m2 had a more than two- to fourfold increased risk of COVID-19-related ICU admission in studies from EUR and EMR. Estimates were smaller and mostly non-significant for overweight and underweight, compared to normal weight.
Circulatory diseases
For nine circulatory and heart diseases, most of 119 estimates came from North America and Europe (53 and 38 respectively; the upper panel of Fig. 2). In particular, heart failure was associated with an increased risk of hospitalisation in EUR and death in EUR and WPR. For hypertension, the picture was more heterogeneous. The studies from WPR indicated an association with an increased risk of ICU admission and in-hospital death. Only sparse data with inconsistent results were available for infarction, peripheral vascular disease and venous thromboembolism. In patients with coronary artery disease, mostly small increases in risk or non-significant estimates were observed.
Immunodeficiency related conditions
For conditions related to immunodeficiency, data from four regions were available (Fig. 3, the upper panel). Two- to sixfold increased risk was estimated for hospitalisation and death in organ transplant recipients in the studies from EUR and AMR North. Geographically heterogeneous results were obtained for people living with HIV, with an increased risk of death in AFR, but mostly non-significant estimates in Europe and North America. The estimates for the generic definition of immunosuppression vary considerably due to differences in the conditions which compose this group among the studies.
Neurological diseases or mental health disorders
In the group of neurological diseases/conditions related to mental health (Fig. 3, the middle panel), patients with dementia had a markedly increased risk of hospitalisation and death in North America. However, other available estimates showed some variability. Only one estimate was available for patients with depression, showing no increased risk of hospitalisation due to COVID-19 in studies from North America. Results were heterogeneous for cerebrovascular disease and stroke, with the majority of estimates being not significant. Psychiatric disorders were associated with increased risk of hospitalisation and markedly increased risk of ICU admission and death in EUR.
Oncological diseases
Data for patients with oncological diseases were available only from three regions (Fig. 3, the lower panel). Active cancer was associated with increased hospital mortality due to COVID-19 in studies from Europe and North America. For haematological oncological conditions, only one estimate was available, showing an increased risk of death in hospital in EUR. For unspecified oncological diseases (any cancer or history of), results were heterogeneous.
Analyses of subgroups
Age-stratified estimates from 11 [142, 169, 205, 211, 214, 222–224, 227, 238, 285] (Additional file 1: Table 3) primary studies were used for an analysis of subgroups. The extracted estimates for 20 pre-existing conditions were illustrated across the original age strata reported in the primary studies (Fig. 7, the estimates are given in Additional file 1: section 2.7, Table 11). We did not conduct meta-analyses of age-stratified effects due to a small number of the estimates and heterogeneity of age groups used for stratification.
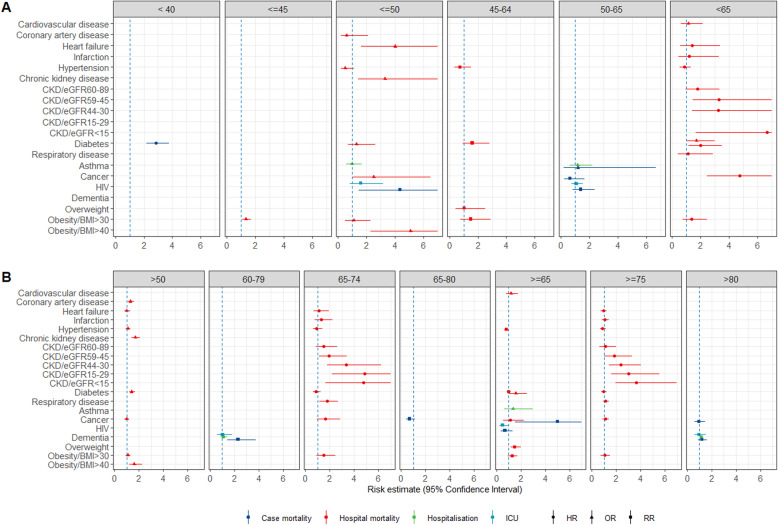
Fig. 7.
Age-stratified estimates for pre-existing conditions extracted from the single studies. Due to differences in the age groups, the estimates were not pooled. Age groups are illustrated in each column as reported in the primary studies. Panel A illustrates single-study estimates for younger age groups. Panel B gives the estimates for older age groups. The error bars represent 95% confidence intervals. The dashed line indicates 1.0 value. The estimates with error bars crossing the 1.0-line lack statistical significance
Fewer estimates were available for people younger than 65 years of age (Fig. 7A). In this group, markedly increased risk of death among < 50-year-olds was reported for heart failure (OR 4.0 (1.6–10.4)), chronic kidney disease (OR 3.3 (1.4–7.7)), severe obesity (BMI ≥ 40 kg/m2, OR 5.1 (2.3–11.1)) and HIV (RR 4.36 (1.43–13.3)) in the studies from North America [205, 222] (Fig. 7A, third column from the left). Statistically significant increased risk of death was also seen for diabetes (HR 2.0 (1.15–3.5)) and cancer (HR 4.76 (2.46–9.21)) in one study from the European region [169] (Fig. 7A, the last column). One study from South/Latin America also reported a markedly increased risk of death from diabetes (HR 2.86 (2.19–3.76)) [142] (Fig. 7A, the first column).
In older adults (> 65 years; Fig. 7B), obesity and heart failure were no longer an apparent risk factor for severe COVID-19 outcomes. Chronic kidney disease, diabetes and cancer showed weaker associations. However, hospital mortality due to COVID-19 showed a stepwise increase with decreasing glomerular filtration rate (i.e. all values of eGFR less than 59) in people with chronic kidney diseases in both age groups (Fig. 7, age groups < 65, 65–75, ≥ 75 [169].
Discussion
Available evidence
In the COVID-19 pandemic, individuals with chronic pre-existing health conditions are potentially at higher risk for disease progression to severe stages requiring hospitalisation and intensive care and leading to death. However, the occurrence of severe cases predominately in older age groups suggests that the effect of age on poor COVID-19 outcomes is more pronounced [304, 305]. In this umbrella review, we explored the estimated associations of various pre-existing health conditions with COVID-19 outcomes adjusted for the confounding effect of age. Because none of the analysed 120 SRs presented age-adjusted effects for different geographic regions, we evaluated the primary studies in SRs for inclusion and re-analysis.
Most of the evidence was derived from studies conducted in European countries, the USA and China. In contrast, only a few studies were available from EMR and South/Latin America, and there were nearly no studies from AFR and countries of WPR outside of China, and no studies from SEAR.
The results of this review show that heart failure, obesity, diabetes, liver cirrhosis, chronic kidney disease, active and haematological cancer, and history of organ transplantation are associated with an increased risk of poor COVID-19-related outcomes such as hospitalisation, need for intensive care and death. We did not aim to identify the causal effects of the pre-existing conditions but rather to summarise the evidence on the diseases associated with the worsening of COVID-19. Therefore, we could not elicit other underlying factors that could determine the detected associations. However, regional heterogeneity observed for multiple associations suggests an influence of other factors outside the scope of this review.
For example, the association between HIV and COVID-19-related mortality was stronger in the African region than in the European region and North America. Prevalent progressed stages of HIV, poor nutritional status and limited access to antiviral treatment are likely to strengthen the association between HIV and COVID-19-related morality in the African region. Further epidemiological studies conducted in different areas are needed to untangle the effects of potential confounding factors and to estimate the causal effects of pre-existing health conditions.
Heterogeneity of the effect estimates
Although we selected evidence for clearly defined outcomes and considered WHO regions separately, the strength of associations for the same pre-existing conditions was quite variable. This variability might have stemmed from differences in study designs, the included populations and methodological approaches. Regarding the type of included studies, nearly all studies had a retrospective cohort design. Also, most studies defined COVID-19 case based on PCR. Therefore, these factors are not likely to have influenced the results to a great extent.
Differences in study design and methods such as choice of model covariates, reference age group, age composition of the study population (younger and older groups) and definitions of pre-existing conditions might have driven the observed heterogeneity of the estimates. We placed only one restriction on the included primary studies, i.e. the adjustment for age. However, the majority of published models also adjusted for other factors (race, vital signs, socio-economic factors), which might have led to differences in residual confounding among the estimated effects. Whenever a choice was available, we avoided to include data from models with additional adjustment. However, most of the studies reported only one model structure. Therefore, a decision to conduct a meta-analysis presented a trade-off between reduction of the between-study variability and synthesis of evidence. We addressed the between-study variability by restricting acceptable heterogeneity to 40% and conducting the GRADE evaluation for each estimate.
Differences in the definitions of the pre-existing conditions and composition of the patient groups might have also contributed to the variability. For the meta-analyses, we did not compose larger disease groups and treated every individually defined chronic condition separately whenever possible. This approach allowed the identification of conditions, such as liver cirrhosis and interstitial lung disease, that are associated with a markedly increased risk of death due to COVID-19. Meta-analyses for associations between bodyweight, particularly obesity, and included COVID-19 outcomes presented a challenge due to differences in the definitions among the studies.
Most studies used bodyweight categories or BMI ranges to define underweight, overweight, obesity and severe obesity. A few included studies [135, 138, 179] used BMI as a numeric variable in their models, which were excluded from the meta-analyses of the bodyweight-related associations. We conducted meta-analyses using the bodyweight categories as reported in Additional file 1 (section 2.3). Pooled estimates for obesity showed higher heterogeneity than for other bodyweight categories (see Figs. 2 and 4). Among other bodyweight categories, obesity was mostly reported a pre-existing health condition; however, the definitions of obesity differed among the studies. The following deviations in the definition were observed: (1) Obesity was defined either using BMI metrics or reported as “obesity” without further detail. (2) For the categories built using BMI-based definitions, we included various BMI ranges that lie in BMI ≥ 30 area in the meta-analyses for obesity. If a study differentiated between BMI ≥ 30 and BMI ≥ 40, we included the estimate for BMI ≥ 40 in the respective meta-analysis for BMI ≥ 40. (3) The studies used different comparison categories, i.e. normal weight, BMI < 30 or not obese. Therefore, the associations between obesity and COVID-19 outcomes were likely to be stronger in the studies, which included all patients with obesity and severe obesity into the “obesity” category and compared to the patients with normal weight. These discrepancies in the definition of obesity and other differences in design and analyses likely contributed to the heterogeneity of the pooled estimates of associations for obesity.
Meta-analyses based on unspecific generic definitions of disease groups such as respiratory or cardiovascular diseases can be less informative. Due to the broad definition of the risk factor, individual conditions which are potentially associated with a higher risk of poor COVID-19 outcomes are not identifiable. However, it has to be noted that the broader definitions of risk factors had to be made in the studies with the smaller sample sizes. Nonetheless, the evidence on the strength of effects for disease groups available in the studies provides direction for further investigation when more data are available. Also, it has to be considered that inherent predispositions do not drive the associations with the undesirable COVID-19 outcomes alone. Instead, other factors such as local medical standards, therapeutic decisions made in hospitals and self-preserving behaviours of certain patient groups during the pandemic, including social distancing and wearing face masks, might have influenced the outcomes. These factors may contribute to observed geographical variation and explain paradox findings, such as unexpectedly lower risk estimates for certain pre-existing conditions such as asthma. The influence of these factors, however, could not be untangled in this review.
Strengths and limitations
Our study has several strengths. To our knowledge, it is the first comprehensive global overview on this topic. It comprises information from 120 reviews conducted worldwide, providing a solid basis for decision-makers regarding the effects of pre-existing health conditions on severe and fatal COVID-19 outcomes. Applying a rigorous methodological approach regarding SRs and primary studies, the umbrella review also allowed for identifying gaps in the evidence and thus priorities for future research. The limitations of this umbrella review mainly stem from the limitations of the included SRs and primary studies. Lack of a registered protocol and reporting on excluded studies observed in the systematic review could have affected our initial pool of the primary studies.
Due to our approach, primary studies that were (for any reason) not included in the systematic reviews could also not be included in our analysis. Most primary studies included in SRs and analysed here were conducted in the first wave of the pandemic. In times of rapidly accumulating evidence, this might be seen as a weakness of our approach. To avoid additional heterogeneity, we excluded studies that used the rather unspecified outcome “severe COVID-19”. By further restricting our analysis to age-adjusted estimates, we might have excluded evidence from countries that are now not otherwise represented in our review. Although 14 [138, 145, 149, 171, 198, 207, 218, 232, 241, 259, 265, 273, 288, 293] of the primary studies in our selection have not yet been peer-reviewed (but were published on pre-print servers), they were included in our analyses to increase the evidence pool. These studies were reviewed and evaluated in the included systematic reviews; however, their inclusion might limit our work.
Implication of evidence for policy
This review provides a global overview of currently available evidence across disease groups and geographic regions. Thereby, our work can support decision-makers worldwide in the process of identifying those who are at particularly high risk of hospitalisation, admission to intensive care unit and death related to COVID-19. This might be important for prioritising certain patient groups for vaccination against COVID-19, given the global vaccine supply shortage. It is, however, outside the scope of this review to provide guidance on prioritisation of the patient groups because the decision-making would require setting threshold values for relative risk measures that would indicate higher or lower priority. The evidence collected in this review allows identification of pre-existing conditions, which increase the risk of COVID-19-related health outcomes relevant to public health, and can facilitate decision-making. Further, the risk estimates combined with the disease definitions extracted from the primary studies may help clinicians to identify patients at higher risk for poor COVID-19-related health outcomes.
It is important to note that, despite the abundance of evidence, identification of further high-risk groups remains a challenging issue. Individuals with rare diseases or exacerbated health conditions are likely to be underrepresented in the populations included in the primary studies possibly due to self-preserving behavioural changes in response to the COVID-19 pandemic. As a consequence, rare chronic conditions may be overlooked in decision-making. Also, we are aware of the fact that in a given country context, a variety of other factors, including advocacy groups, special population groups, media press and lobbying, influence the prioritisation procedure. Therefore, it is important to support NITAGs with evidence on risk groups to enable them to develop evidence-based vaccination recommendation and defend them adequately in public discussions.
Implication of evidence for research
The results of our review might be used to identify further research needs. The review shows a gap in evidence on age-adjusted effect estimates in the countries from the African and the South-East Asia regions and the countries of the Western Pacific region outside of China. As the profile of prevalent chronic pre-existing conditions and age structure in these populations differ from the European and American regions, further research would provide valuable information.
Currently, few studies conduct age-adjusted analysis using age-stratified data. Based on the current data, it is reasonable to suggest that age is also an effect modifier. Therefore, further investigation of the effects of pre-existing conditions on COVID-19 outcomes stratified by age groups is needed to unmask health condition effects existent in specific age bands.
Additionally, it can be informative to consider chronic conditions in greater granularity. For example, differentiation for severity for some pre-existing conditions (e.g. diabetes, HIV, depression, chronic kidney disease) can identify more specific target patient groups for policy actions or clinical practice. It is also helpful to explore the effects of multimorbidity and effect modifications among pre-existing health conditions. Furthermore, the standardisation or provision of complete definitions of COVID-19 disease and pre-existing conditions would allow for greater comparability across studies.
It is also desirable that future studies identify the reason for admission to hospital or intensive care. For severe episodes of pre-existing conditions such as active cancer, COPD, chronic kidney and liver disease, it was impossible to differentiate whether the admission was due to COVID-19 or due to worsening of the underlying health condition. The strength of association with COVID-19-related outcome might have been overestimated in the studies, which included patients admitted with an exacerbation of pre-existing condition and consequently found positive for the infection.
Also, an update and transformation of this review into a living review would be useful to add evidence that has emerged in the second and third waves of the COVID-19 pandemic.
Finally, this work summarises the evidence on the associations between the pre-existing health conditions and undesirable COVID-19 outcomes without looking into plausible biological factors that could provide an insight into the resulted estimates. However, it is known that SARS-CoV-2 targets epithelial cells of the nasal and bronchioalveolar tract where the spike-S-glycoprotein of the virus binds via the angiotensin-converting enzyme (ACE)-2 receptor [306]. High densities of ACE-2 receptors are present in the respiratory tract, the gut, vessels and kidneys. An upregulated expression of ACE-2 receptors is also associated with several pre-existing chronic health conditions: hypertension, COPD, diabetes, liver and kidney diseases [307]. Whether or not a strong expression of ACE-2 receptors offers a mechanistic explanation for the associations between chronic diseases and severe COVID-19 outcomes described here needs to be investigated.
Conclusions
In this review, a number of pre-existing conditions were associated with hospitalisation, ICU admission and death in hospitalised with COVID-19 and SARS-CoV-2-positive individuals when controlled for age. The strength of associations varied supposedly due to differences in definitions of pre-existing conditions and methodological approaches. The review shows multiple gaps in evidence, including a pressing need for evidence from the African, South-East-Asian and Western Pacific regions, exploration of effects of multimorbidity and rare diseases. The results may serve as an efficient starting point for policy-makers to prioritise patient groups for protection via vaccination and other health interventions against COVID-19.
Supplementary Information
Additional file 1. Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence”. This supplementary file provides details on study search and selection, the results of the searches, data extraction from reviews and primary studies, and meta-analyses. The tables give all obtained pooled estimates of the main analyses, including the GRADE assessment. Age-stratified estimates and analysis of specific population groups are presented. The file also provides results of the risk of bias evaluation conducted in this review and the lists of excluded SRs and primary studies.
Acknowledgements
Not applicable
Abbreviations
- ACE-2
Angiotensin-converting enzyme-2
- AFR
African region
- AMR
Regions of America
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- EMR
Eastern Mediterranean region
- EUR
European region
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HR
Hazard ratio
- ICU
Intensive care unit
- NITAGs
National Immunisation Technical Advisory Groups
- NOS
The Newcastle-Ottawa Scale
- OR
Odds ratio
- PRECEPT
Project on a Framework for Rating Evidence in Public Health
- RR
Relative risk
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus type 2
- SEAR
South-East Asia region
- SR
Systematic review
- WHO
The World Health Organization
- WHO SAGE
The World Health Organization’s Strategic Advisory Group of Experts on Immunisation
Authors’ contributions
All authors contributed to the conception of the study. MTS conducted searches, selecting the studies, data extraction, meta-analyses, interpretation of the results and drafting of the manuscript. LH performed data extraction, quality evaluation, interpretation of the results and drafting of the manuscript. SR conducted searches, selection and quality appraisals of SRs, data extraction, interpretation of the results and drafting of the manuscript. AP extracted the data and participated in the quality evaluation and drafting of the manuscript. AB and KK participated in the quality appraisal of SRs and partly in data extraction. TN participated in data extraction. VS contributed to the study selection and data extraction. TH substantially contributed to the study design, interpretation of the data and drafting of the manuscript. JK, SVB, STS and OW critically revised the manuscript and contributed to the final version. All authors read and approved the version of the manuscript to be published.
Funding
This study was funded by the Federal Ministry of Health of Germany (project ImVaCov). The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All data generated and analysed during this study are included in this published article and its supplementary information file (Additional file 1).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare no conflicts competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation. COVID 19 Public Health Emergency of International Concern (PHEIC). Global research and innovation forum: towards a research roadmap. 2020.
- 2.World Health Organisation. COVID-19 Dashboard [Internet]. [cited 01.02.2021]. Available from: https://covid19.who.int/.
- 3.Aggarwal G, Cheruiyot I, Aggarwal S, Wong J, Lippi G, Lavie CJ, et al. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Curr Problems Cardiol. 2020;45(8):100617. doi: 10.1016/j.cpcardiol.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirol. 2020:1–12. [DOI] [PMC free article] [PubMed]
- 5.Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):64. doi: 10.1186/s40001-020-00464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, et al. Predictors of adverse prognosis in Covid-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020:e13362. [DOI] [PubMed]
- 7.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1e17. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis J. Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med. 2017;51(20):1456–1458. doi: 10.1136/bjsports-2017-097621. [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181(8):488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papatheodorou S. Umbrella reviews: what they are and why we need them. Eur J Epidemiol. 2019;34(6):543–546. doi: 10.1007/s10654-019-00505-6. [DOI] [PubMed] [Google Scholar]
- 11.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthcare. 2015;13(3):132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100. doi: 10.1136/ebmental-2018-300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mentis AA, Dardiotis E, Efthymiou V, Chrousos GP. Non-genetic risk and protective factors and biomarkers for neurological disorders: a meta-umbrella systematic review of umbrella reviews. BMC Med. 2021;19(1):6. doi: 10.1186/s12916-020-01873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G, Shea B, O'Connell J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Res Inst Web site. 2014;7.
- 16.Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 17.Harder T, Takla A, Eckmanns T, Ellis S, Forland F, James R, et al. PRECEPT: an evidence assessment framework for infectious disease epidemiology, prevention and control. Euro Surveill. 2017;22(40):16–00620. doi: 10.2807/1560-7917.ES.2017.22.40.16-00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awortwe C, Cascorbi I. Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions. Pharmacol Res. 2020:105250. [DOI] [PMC free article] [PubMed]
- 19.Bellou V, Tzoulaki I, Evangelou E, Belbasis L. Risk factors for adverse clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 20.Biswas M. Effects of sex, age and comorbidities on the risk of infection and death associated with COVID-19: a meta-analysis of 47807 confirmed cases. Lancet. 2020.
- 21.Chang TH, Chou CC, Chang LY. Effect of obesity and body mass index on coronavirus disease 2019 severity: a systematic review and meta-analysis. Obes Rev. 2020. [DOI] [PubMed]
- 22.Chen Y, Gong X, Wang L, Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: a systematic review and meta-analysis. MedRxiv. 2020.
- 23.Cheruiyot I, Kipkorir V, Ngure B, Misiani M, Munguti J. Cancer is associated with coronavirus disease (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 24.Chidambaram V, Tun NL, Haque W, Majella MG, Sivakumar RK, Kumar A, et al. Factors associated with disease severity and mortality among patients with coronavirus disease 2019: a systematic review and meta-analysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 25.Das P, Samad N, Seidu A-A, Aboagye RG, Tetteh JK, Ahinkorah BO. Obesity as a predictor for adverse outcomes among COVID-19 patients: a meta-analysis. Res Square 2020.
- 26.de Almeida-Pititto B, Dualib PM, Zajdenverg L, Dantas JR, de Souza FD, Rodacki M, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndrome. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degarege A, Naveed Z, Kabayundo J, Brett-Major D. Risk factors for severe illness and death in COVID-19: a systematic review and meta-analysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 28.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. Plos One. 2020;15(12):e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of Body mass index (BMI) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metabolism. 2020:154373. [DOI] [PMC free article] [PubMed]
- 30.Elgohary G. The risk and prognosis of COVID-19 infection in cancer patients: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2020. [DOI] [PMC free article] [PubMed]
- 31.Fernandez Villalobos NV, Ott JJ, Klett-Tammen CJ, Bockey A, Vanella P, Krause G, et al. Quantification of the association between predisposing health conditions, demographic, and behavioural factors with hospitalisation, intensive care unit admission, and death from COVID-19: a systematic review and meta-analysis. medRxiv 2020.
- 32.Florez-Perdomo WA, Serrato-Vargas SA, Bosque-Varela P, Moscote-Salazar LR, Joaquim AF, Agrawal A, et al. Relationship between the history of cerebrovascular disease and mortality in COVID-19 patients: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2020:106183. [DOI] [PMC free article] [PubMed]
- 33.Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020. [DOI] [PMC free article] [PubMed]
- 34.Gao Y, Liu M, Shi S, Chen Y, Sun Y, Chen J, et al. Cancer is associated with the severity and mortality of patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020:2020.05.01.20087031.
- 35.Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Shi Z, Zhang Y, Wang C, Cristina Do Vale Moreira N, Zuo H, et al. Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: a meta-analysis. Diab Res Clin Pract. 2020:108346. [DOI] [PMC free article] [PubMed]
- 37.Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2020:104299. [DOI] [PMC free article] [PubMed]
- 38.Hessami A, Shamshirian A, Heydari K, Alizadeh-Navaei R, Moosazadeh M, Abrotan S. Cardiovascular diseases and COVID-19 mortality and intensive care unit admission: a systematic review and meta-analysis. MedRxiv. 2020.
- 39.Huang Y, Lu Y, Huang Y-M, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain S, Baxi H, Chand Jamali M, Nisar N, Hussain MS. Burden of diabetes mellitus and its impact on COVID-19 patients: a meta-analysis of real-world evidence. Diab Metab Syndrome 2020. [DOI] [PMC free article] [PubMed]
- 41.Islam MS, Barek MA, Aziz MA, Aka TD, Jakaria M. Association of age, sex, comorbidities, and clinical symptoms with the severity and mortality of COVID-19 cases: a meta-analysis with 85 studies and 67299 cases. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 42.Izcovich A, Ragusa M. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. SSRN. 2020. [DOI] [PMC free article] [PubMed]
- 43.Kahathuduwa C, Dhanasekara C, Chin S-H. Severity and case fatality rates of COVID-19: a systematic review, meta-analysis and an exploratory meta-regression of risk factors. Lancet. 2020.
- 44.Khan M, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: a systematic review and meta-analysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 45.Khunti K, Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, et al. The prevalence of comorbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Lancet Diab Endocrinol. 2020. [DOI] [PMC free article] [PubMed]
- 46.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020:1–9. [DOI] [PMC free article] [PubMed]
- 47.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Clinical Features of COVID-19 and Factors Associated with Severe Clinical Course: A Systematic Review and Meta-Analysis. Ssrn. 2020:3566166.
- 48.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diab Metab Syndrome. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: A Systematic Review and Meta-analysis of Clinical Characteristics, Risk factors and Outcomes. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 51.Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart (British Cardiac Society). 2020. [DOI] [PMC free article] [PubMed]
- 52.Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): A pooled analysis. Polish Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid Chronic Diseases are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11(3):668–678. doi: 10.14336/AD.2020.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H. The Impact of Dementia on the Clinical Outcome of COVID-19: A Systematic Review and Meta-Analysis. J Alzheimer’s Dis. 2020. [DOI] [PubMed]
- 55.Liu Y, Lu H, Wang W, Liu Q, Zhu C. Clinical risk factors for mortality in patients with cancer and COVID-19: a systematic review and meta-analysis of recent observational studies. Expert Rev Anticancer Ther. 2020. [DOI] [PubMed]
- 56.Liu Y-F, Zhang Z, Pan X-L, Xing G-L, Zhang Y, Liu Z-S, et al. The Chronic Kidney Disease and Acute Kidney Injury Involvement in COVID-19 Pandemic: A Systematic Review and Meta-analysis. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 57.Luo L, Fu M, Li Y, Hu S, Luo J, Chen Z, et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: A pooled analysis. Clin Cardiol. 2020;n/a(n/a). [DOI] [PMC free article] [PubMed]
- 58.Malik P, Patel U, Patel K, Martin M, Shah C, Mehta D, et al. Obesity a predictor of outcomes of COVID-19 hospitalized patients- A systematic Review and Meta-Analysis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 59.Mantovani A, Byrne CD, Zheng M-H, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed]
- 60.Matsushita K, Ding N, Kou M, Hu X, Chen M, Gao Y, et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: A systematic review and meta-analysis. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 61.Mehraeen E, Karimi A, Barzegary A, Vahedi F, Afsahi AM, Dadras O, et al. Predictors of mortality in patients with COVID-19 – a systematic review. Eur J Integr Med. 2020:101226. [DOI] [PMC free article] [PubMed]
- 62.Mellor M, Bast A, Jones N, Roberts N, Ordonez-Mena J, Reith A, et al. Risk of adverse COVID-19 outcomes for people living with HIV: a rapid review and meta-analysis. medRxiv. 2020.
- 63.Meng M, Zhao Q, Kumar R, Bai C, Deng Y, Wan B. Impact of cardiovascular and metabolic diseases on the severity of COVID-19: a systematic review and meta-analysis. Aging. 2020;12. [DOI] [PMC free article] [PubMed]
- 64.Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, Sarriá Cabrera MA, Maffei de Andrade S, Sequí-Dominguez I, et al. Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. Plos One. 2020;15(11):e0241742. doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Momtazmanesh S, Shobeiri P, Hanaei S, Mahmoud-Elsayed H, Dalvi B, Malakan RE. Cardiovascular disease in COVID-19: a systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt Heart J. 2020;72(1):41. doi: 10.1186/s43044-020-00075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moula AI, Micali LR, Matteucci F, Lucà F, Rao CM, Parise O, et al. Quantification of Death Risk in Relation to Sex, Pre-Existing Cardiovascular Diseases and Risk Factors in COVID-19 Patients: Let's Take Stock and See Where We Are. J Clin Med. 2020;9(9). [DOI] [PMC free article] [PubMed]
- 67.Nandy K, Salunke A, Pathak SK, Pandey A, Doctor C, Puj K, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diab Metab Syndrome. 2020. [DOI] [PMC free article] [PubMed]
- 68.Noor FM, Islam MM. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Commun Health. 2020. [DOI] [PMC free article] [PubMed]
- 69.Ofori-Asenso R, Ogundipe O, Agyeman AA, Chin KL, Mazidi M, Ademi Z, et al. Cancer is associated with severe disease in COVID-19 patients: a systematic review and meta-analysis. Ecancermedicalscience. 2020;14:1047. doi: 10.3332/ecancer.2020.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palaiodimos L, Chamorro-Pareja N, Karamanis D, Li W, Zavras PD, Mathias P, et al. Diabetes is associated with increased risk for in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis comprising 18,506 patients. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 71.Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: A meta-analysis. Dig Liver Dis. 2020. [DOI] [PMC free article] [PubMed]
- 72.Park R, Chidharla A, Mehta K, Sun W, Wulff-Burchfield E, Kasi A. Sex-bias in COVID-19-associated illness severity and mortality in cancer patients: A systematic review and meta-analysis. EClinicalMed. 2020:100519. [DOI] [PMC free article] [PubMed]
- 73.Park R, Lee SA, Kim SY, de Melo AC, Kasi A. Association of active oncologic treatment and risk of death in cancer patients with COVID-19: a systematic review and meta-analysis of patient data. Acta Oncol (Stockholm). 2020:1-7. [DOI] [PubMed]
- 74.Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male 2020:1-9. [DOI] [PubMed]
- 75.Parveen R, Sehar N, Bajpai R, Bharal Agarwal N. Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diab Res Clin Pract. 2020:108295. [DOI] [PMC free article] [PubMed]
- 76.Patel U, Malik P, Shah D, Patel A, Dhamoon M, Jani V. Pre-existing cerebrovascular disease and poor outcomes of COVID-19 hospitalized patients: a meta-analysis. J Neurol. 2020:1–8. [DOI] [PMC free article] [PubMed]
- 77.Patel U, Malik P, Usman MS, Mehta D, Sharma A, Malik FA, et al. Age-Adjusted Risk Factors Associated with Mortality and Mechanical Ventilation Utilization Amongst COVID-19 Hospitalizations—a Systematic Review and Meta-Analysis. SN Compr Clin Med. 2020. [DOI] [PMC free article] [PubMed]
- 78.Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020. [DOI] [PMC free article] [PubMed]
- 79.Pranata R, Huang I, Raharjo SB. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2020. [DOI] [PMC free article] [PubMed]
- 80.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. 2020;21(2):1470320320926899. doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, et al. Body Mass Index and Outcome in Patients with COVID-19: A Dose-Response Meta-Analysis. Diab Metab. 2020. [DOI] [PMC free article] [PubMed]
- 82.Pranata R, Soeroto AY, Huang I, Lim MA, Santoso P, Permana H, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020;24(8):838–843. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 83.Pranata R, Supriyadi R, Huang I, Permana H, Lim MA, Yonas E, et al. The Association Between Chronic Kidney Disease and New Onset Renal Replacement Therapy on the Outcome of COVID-19 Patients: A Meta-analysis. Clin Med Insights. 2020;14. [DOI] [PMC free article] [PubMed]
- 84.Rahman A, Sathi NJ. Risk Factors of the Severity of COVID-19: A Meta-Analysis. MedRxiv. 2020. [DOI] [PubMed]
- 85.Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabatino J, De Rosa S, Di Salvo G, Indolfi C. Impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. Plos One. 2020;15(8):e0237131. doi: 10.1371/journal.pone.0237131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salunke AA, Nandy K, Pathak SK, Shah J, Kamani M, Kotakotta V, et al. Impact of COVID -19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diab Metab Syndrome 2020. [DOI] [PMC free article] [PubMed]
- 88.Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: A systematic review and meta-analysis. Respir Med. 2020:106096. [DOI] [PMC free article] [PubMed]
- 89.Sepandi M, Taghdir M, Alimohamadi Y, Afrashteh S, Hosamirudsari H. Factors Associated with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Iran J Public Health. 2020;49(7):1211–1221. doi: 10.18502/ijph.v49i7.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shang L, Shao M, Guo Q, Shi J, Zhao Y, Xiaokereti J, et al. Diabetes Mellitus is Associated with Severe Infection and Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis. Arch Med Res. 2020. [DOI] [PMC free article] [PubMed]
- 91.Shi C, Wang L, Ye J, Gu Z, Wang S, Xia J, et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. Res Square 2020. [DOI] [PMC free article] [PubMed]
- 92.Singh AK, Jena A, Kumar MP, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease (COVID-19) in patients with inflammatory bowel disease: a systematic review and meta-analysis. United Eur Gastroenterol J. 2020:2050640620972602. [DOI] [PMC free article] [PubMed]
- 93.Soeroto AY, Soetedjo NN, Purwiga A, Santoso P, Kulsum ID, Suryadinata H, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: A systematic review and meta-analysis. Diab Metab Syndrome. 2020;14(6):1897–1904. doi: 10.1016/j.dsx.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sreenivasan J, Khan MS, Anker SD, Kaul R, Khan SU, Metra M, et al. Cardiovascular Risk Factors and Complications in Patients Infected with COVID-19: A Systematic Review. Lancet. .
- 95.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. Plos One. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su Q, Hu J-x, Lin H-s, Zhang Z, Zhu EC, Zhang C-g, et al. Prevalence and risks of severe events for cancer patients with COVID-19 infection: a systematic review and meta-analysis. medrxiv. 2020.
- 97.Sze S, Pan D, Nevill CR, Gray LJ, Martin CA, Nazareth J, et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMed. 2020:100630. [DOI] [PMC free article] [PubMed]
- 98.Tabrizi R, Lankarani KB, Nowrouzi-sohrabi P, Shabani-Borujeni M, Rezaei S, Hosseini-bensenjan M, et al. The role of comorbidities and clinical predictors of severe disease in COVID-19: a systematic review and meta-analysis. MedRxiv. 2020.
- 99.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diab Metab Syndrome 2020. [DOI] [PMC free article] [PubMed]
- 100.Tamuzi JL, Ayele BT, Shumba CS, Adetokunboh OO, Uwimana-Nicol J, Haile ZT, et al. Implications of COVID-19 in high burden countries for HIV/TB: A systematic review of evidence. BMC Infect Dis. 2020;20(1):744. doi: 10.1186/s12879-020-05450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tavan H, Shams M, Karimian M, Kalvandi G, Borji M. An evaluation on the frequencies of underlying diseases and symptoms along with the mortality rate of COVID-19, a systematic review and meta-analysis. Lancet. 2020.
- 102.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020;92(10):1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian Y, Qiu X, Wang C, Zhao J, Jiang X, Niu W, et al. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int J Cancer. 2020. [DOI] [PMC free article] [PubMed]
- 104.Tian Y, Wu Q, Li, Wu Q, Xie Y, Li L, et al. Distinct symptoms and underlying comorbidities with latitude and longitude in COVID-19: A systematic review and meta-analysis. Res Square 2020. [DOI] [PMC free article] [PubMed]
- 105.Toraih EA, Elshazli RM, Hussein MH, Elgaml A, Amin MN, El-Mowafy M, et al. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: A meta-regression and Decision tree analysis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 106.Varikasuvu SR, Dutt N, Thangappazham B, Varshney S. Diabetes and COVID-19: A pooled analysis related to disease severity and mortality. Prim Care Diab. 2020. [DOI] [PMC free article] [PubMed]
- 107.Venkatesulu BP, Chandrasekar VT, Girdhar P, Advani P, Sharma A, Elumalai T, et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 108.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood. 2020. [DOI] [PMC free article] [PubMed]
- 109.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12. [DOI] [PMC free article] [PubMed]
- 110.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, et al. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Research (Washington, DC) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, Ao G, Qi X, Xie B. The association between COVID-19 and asthma: a systematic review and meta-analysis. Clin Exp Allerg. 2020. [DOI] [PubMed]
- 112.Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, et al. Does Asthma Increase the Mortality of Patients with COVID-19?: A Systematic Review and Meta-Analysis. Int Arch Allerg Immunol. 2020:1–7. [DOI] [PMC free article] [PubMed]
- 113.Wang Z, Deng H, Ou C, Liang J, Wang Y, Jiang M, et al. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine. 2020;99(48):e23327. doi: 10.1097/MD.0000000000023327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wingert A, Pillay J, Gates M, Guitard S, Rahman S, Beck A, et al. Risk factors for severe outcomes of COVID-19: a rapid review. medRxiv. 2020:2020.08.27.20183434.
- 115.Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, et al. Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2 infection. Diab Obes Metab. 2020. [DOI] [PMC free article] [PubMed]
- 116.Wu ZH, Tang Y, Cheng Q. Diabetes increases the mortality of patients with COVID-19: a meta-analysis. Acta Diabetol. 2020. [DOI] [PMC free article] [PubMed]
- 117.Xu J, Xiao W, Liang X, Zhang P, Shi L, Wang Y, et al. The Association of Cerebrovascular Disease with Adverse Outcomes in COVID-19 Patients: A Meta-Analysis Based on Adjusted Effect Estimates. J Stroke Cerebrovasc Dis. 2020;29(11):105283. doi: 10.1016/j.jstrokecerebrovasdis.2020.105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging. 2020;12. [DOI] [PMC free article] [PubMed]
- 119.Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 120.Yang J, Tian C, Chen Y, Zhu C, Chi H, Li J. Obesity aggravates COVID-19: an updated systematic review and meta-analysis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 121.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 122.Yang S, Chen C, Yan D, Zhou Y, Tian G, Wu J, et al. Risk factors associated with fatal outcomes of novel coronavirus infection pneumonia (COVID-19): A systematic review and meta-analysis. Res Square. 2020.
- 123.Yekedüz E, Utkan G, Ürün Y. A Systematic Review and Meta-Analysis: The Effect of Active Cancer Treatment on Severity of COVID-19. Eur J Cancer. 2020. [DOI] [PMC free article] [PubMed]
- 124.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Gutierrez EJ, et al. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 125.Youssef M, Hussein M, Attia AS, Elshazli R, Omar M, Zora G, et al. COVID-19 and Liver Dysfunction: a systematic review and meta-analysis of retrospective studies. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 126.Yu JN, Wu BB, Yang J, Lei XL, Shen WQ. Cardio-Cerebrovascular Disease is Associated With Severity and Mortality of COVID-19: A Systematic Review and Meta-Analysis. Biol Res Nurs. 2020:1099800420951984. [DOI] [PMC free article] [PubMed]
- 127.Zaki N, Mohamed EA, Ibrahim S, Khan G. The influence of comorbidity on the severity of COVID-19 disease: A systematic review and analysis. Res Square. 2020.
- 128.Zhang H, Han H, He T, Labbe KE, Hernandez AV, Chen H, et al. Clinical Characteristics and Outcomes of COVID-19-Infected Cancer Patients: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed]
- 129.Zhang J, Wu J, Sun X, Xue H, Shao J, Cai W, et al. Associations of hypertension with the severity and fatality of SARS-CoV-2 infection: A meta-analysis. Epidemiol Infect. 2020:1–19. [DOI] [PMC free article] [PubMed]
- 130.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, et al. The impact of COPD and smoking history on the severity of Covid-19: A systemic review and meta-analysis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 131.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020. [DOI] [PMC free article] [PubMed]
- 132.Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 133.Zuin M, Guasti P, Roncon L, Cervellati C, Zuliani G. Dementia and the risk of death in elderly patients with COVID-19 infection: Systematic review and meta-analysis. Int J Geriatr Psychiatry. 2020. [DOI] [PMC free article] [PubMed]
- 134.Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: Impact of obesity and diabetes on disease severity. Clin Obes. 2020;10(6):e12414. doi: 10.1111/cob.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Al-Salameh A, Lanoix JP, Bennis Y, Andrejak C, Brochot E, Deschasse G, et al. Characteristics and outcomes of COVID-19 in hospitalized patients with and without diabetes. Diabetes Metab Res Rev. 2020:e3388. [DOI] [PMC free article] [PubMed]
- 136.Amit M, Sorkin A, Chen J, Cohen B, Karol D, Tsur AM, et al. Clinical Course and Outcomes of Severe Covid-19: A National Scale Study. J Clin Med. 2020;9(7). [DOI] [PMC free article] [PubMed]
- 137.Antwi-Amoabeng D, Beutler B, Awad M, Kanji Z, Mahboob S, Ghuman J, et al. Sociodemographic predictors of outcomes in COVID-19: examining the impact of ethnic disparities in Northern Nevada. Cureus. 2020;13(2):e13128. doi: 10.7759/cureus.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Argenziano M, Bruce S, Slater C. Characterization and Clinical Course of 1000 Patients with COVID-19 in New York: retrospective case series. medrxiv. 2020:10.1101. [DOI] [PMC free article] [PubMed]
- 139.Azar KMJ, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S, et al. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Affairs (Project Hope). 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 140.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8(8):e1018–e1e26. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bellan M, Patti G, Hayden E, Azzolina D, Pirisi M, Acquaviva A, et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep. 2020;10(1):20731. doi: 10.1038/s41598-020-77698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):dgaa346. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Berenguer J, Ryan P, Rodríguez-Baño J, Jarrín I, Carratalà J, Pachón J, et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 145.Bi Q, Hong C, Meng J, Wu Z, Zhou P, Ye C, et al. Characterizing clinical progression of COVID-19 among patients in Shenzhen, China: an observational cohort study. medRxiv. 2020:2020.04.22.20076190.
- 146.Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, et al. Clinical Presentation of COVID19 in Dementia Patients. J Nutr Health Aging. 2020;24(6):560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Borghesi A, Zigliani A, Golemi S, Carapella N, Maculotti P, Farina D, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boulle A, Davies M-A, Hussey H, Ismail M, Morden E, Vundle Z, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2020:ciaa1198. [DOI] [PMC free article] [PubMed]
- 149.Burn E, Tebe C, Fernandez-Bertolin S, Aragon M, Recalde M, Roel E, et al. The natural history of symptomatic COVID-19 in Catalonia, Spain: a multi-state model including 109,367 outpatient diagnoses, 18,019 hospitalisations, and 5585 COVID-19 deaths among 5,627,520 people. medRxiv. 2020:2020.07.13.20152454.
- 150.Busetto L, Bettini S, Fabris R, Serra R, Dal Pra C, Maffei P, et al. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed]
- 151.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carrillo-Vega MF, Salinas-Escudero G, García-Peña C, Gutiérrez-Robledo LM, Parra-Rodríguez L. Early estimation of the risk factors for hospitalization and mortality by COVID-19 in Mexico. Plos One. 2020;15(9):e0238905. doi: 10.1371/journal.pone.0238905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carter B, Collins JT, Barlow-Pay F, Rickard F, Bruce E, Verduri A, et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople) J Hosp Infect. 2020;106(2):376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diab Endocrinol. 2020. [DOI] [PMC free article] [PubMed]
- 155.Chen F, Sun W, Sun S, Li Z, Wang Z, Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China. Clin Transl Med. 2020;10(2):e40. doi: 10.1002/ctm2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen J, Bai H, Liu J, Chen G, Liao Q, Yang J, et al. Distinct Clinical Characteristics and Risk Factors for Mortality in Female Inpatients With Coronavirus Disease 2019 (COVID-19): A Sex-stratified, Large-scale Cohort Study in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 157.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chhiba KD, Patel GB, Vu THT, Chen MM, Guo A, Kudlaty E, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–14.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, Tejada J, et al. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West J Emerg Med. 2020;21(4):779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ciardullo S, Zerbini F, Perra S, Muraca E, Cannistraci R, Lauriola M, et al. Impact of diabetes on COVID-19-related in-hospital mortality: a retrospective study from Northern Italy. J Endocrinol Invest. 2020. [DOI] [PMC free article] [PubMed]
- 161.Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, Galli L, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Conversano A, Melillo F, Napolano A, Fominskiy E, Spessot M, Ciceri F, et al. Renin-Angiotensin-Aldosterone System Inhibitors and Outcome in Patients With SARS-CoV-2 Pneumonia: A Case Series Study. Hypertension. 2020;76(2):e10–ee2. doi: 10.1161/HYPERTENSIONAHA.120.15312. [DOI] [PubMed] [Google Scholar]
- 163.Costa Monteiro AC, Suri R, Emeruwa IO, Stretch RJ, Cortes Lopez RY, Sherman A, et al. Obesity and Smoking as Risk Factors for Invasive Mechanical Ventilation in COVID-19: a Retrospective, Observational Cohort Study. Plos One. 2020;15(12):e0238552. doi: 10.1371/journal.pone.0238552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin Use Is Associated With Reduced Mortality in a Diverse Population With COVID-19 and Diabetes. Front Endocrinol. 2021;11(1081). [DOI] [PMC free article] [PubMed]
- 165.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020. [DOI] [PMC free article] [PubMed]
- 166.D’Silva KM, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79(9):1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Denova-Gutiérrez E, Lopez-Gatell H, Alomia-Zegarra JL, López-Ridaura R, Zaragoza-Jimenez CA, Dyer-Leal DD, et al. The Association of Obesity, Type 2 Diabetes, and Hypertension with Severe Coronavirus Disease 2019 on Admission Among Mexican Patients. Obesity (Silver Spring) 2020;28(10):1826–1832. doi: 10.1002/oby.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Di Castelnuovo A, Bonaccio M, Costanzo S, Gialluisi A, Antinori A, Berselli N, et al. Common cardiovascular risk factors and in-hospital mortality in 3894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30(11):1899–1913. doi: 10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Du R, Liang L, Yang C, Li M, Guo G, van Halm-Lutterodt N, et al. Patient Predisposition at Hospital Admission Indirectly Dictates Disease Severity, Clinical Course and Outcomes of COVID-19 Pneumonia Patients in Wuhan, China. SSRN. 2020.
- 172.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur Respir J. 2020. [DOI] [PMC free article] [PubMed]
- 173.Ebinger JE, Achamallah N, Ji H, Claggett BL, Sun N, Botting P, et al. Pre-existing traits associated with Covid-19 illness severity. Plos One. 2020;15(7):e0236240-e. doi: 10.1371/journal.pone.0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Escalera-Antezana JP, Lizon-Ferrufino NF, Maldonado-Alanoca A, Alarcon-De-la-Vega G, Alvarado-Arnez LE, Balderrama-Saavedra MA, et al. Risk factors for mortality in patients with Coronavirus Disease 2019 (COVID-19) in Bolivia: An analysis of the first 107 confirmed cases. Le infezioni Med. 2020;28(2):238–242. [PubMed] [Google Scholar]
- 175.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020. [DOI] [PMC free article] [PubMed]
- 176.Galloway JB, Norton S, Barker RD, Brookes A, Carey I, Clarke BD, et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: An observational cohort study. J Infect. 2020;81(2):282–288. doi: 10.1016/j.jinf.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Garassino M, Whisenant J, Huang L-C, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21. [DOI] [PMC free article] [PubMed]
- 179.Gayam V, Chobufo MD, Merghani MA, Lamichhane S, Garlapati PR, Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2020;n/a(n/a). [DOI] [PMC free article] [PubMed]
- 180.Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 181.Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Giannouchos TV, Sussman RA, Mier JM, Poulas K, Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases. Eur Respir J. 2020. [DOI] [PMC free article] [PubMed]
- 183.Goicoechea M, Sánchez Cámara LA, Macías N. Muñoz de Morales A, Rojas ÁG, Bascuñana A, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98(1):27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goyal P, Ringel JB, Rajan M, Choi JJ, Pinheiro LC, Li HA, et al. Obesity and COVID-19 in New York City: A Retrospective Cohort Study. Ann Intern Med. 2020;173(10):855–858. doi: 10.7326/M20-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gu T, Chu Q, Yu Z, Fa B, Li A, Xu L, et al. History of coronary heart disease increased the mortality rate of patients with COVID-19: a nested case–control study. BMJ Open. 2020;10(9):e038976. doi: 10.1136/bmjopen-2020-038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gu T, Mack JA, Salvatore M, Prabhu Sankar S, Valley TS, Singh K, et al. Characteristics Associated With Racial/Ethnic Disparities in COVID-19 Outcomes in an Academic Health Care System. JAMA Netw Open. 2020;3(10):e2025197-e. doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020;180(11):1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Halasz G, Leoni ML, Villani GQ, Nolli M, Villani M. Obesity, overweight and survival in critically ill patients with SARS-CoV-2 pneumonia: is there an obesity paradox? Preliminary results from Italy. Eur J Prev Cardiol. 2020:2047487320939675. [DOI] [PMC free article] [PubMed]
- 190.Harmouch F, Shah K, Hippen JT, Kumar A, Goel H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J Med Virol. 2020;n/a(n/a). [DOI] [PubMed]
- 191.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. Plos Med. 2020;17(9):e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40(10):2515–2521. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–ee51. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43(8):824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hur K, Price C, Gray E, Gulati R, Maksimoski M, Racette S, et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:019459982092964. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hwang J-M, Kim J-H, Park J-S, Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Imam Z, Odish F, Gill I, O'Connor D, Armstrong J, Vanood A, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jun T, Nirenberg S, Kovatch P, Huang K-l. Sex-specificity of mortality risk factors among hospitalized COVID-19 patients in New York City: prospective cohort study. medRxiv. 2020:2020.07.29.20164640.
- 199.Kabarriti R, Brodin NP, Maron MI, Guha C, Kalnicki S, Garg MK, et al. Association of Race and Ethnicity With Comorbidities and Survival Among Patients With COVID-19 at an Urban Medical Center in New York. JAMA Netw Open. 2020;3(9):e2019795-e. doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity. 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khalil K, Agbontaen K, McNally D, Love A, Mandalia S, Banya W, et al. Clinical characteristics and 28-day mortality of medical patients admitted with COVID-19 to a central London teaching hospital. J Infect. 2020;81(3):e85–ee9. doi: 10.1016/j.jinf.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Killerby M, Link-Gelles R, Haight S, Schrodt C, England L, Gomes D, et al. Characteristics Associated with Hospitalization Among Patients with COVID-19 — Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69. [DOI] [PMC free article] [PubMed]
- 203.Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 204.Kim MK, Jeon JH, Kim SW, Moon JS, Cho NH, Han E, et al. The Clinical Characteristics and Outcomes of Patients with Moderate-to-Severe Coronavirus Disease 2019 Infection and Diabetes in Daegu, South Korea. Diab Metab J. 2020;44(4):602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed]
- 206.Kuderer N, Choueiri T, Shah D, Shyr Y, Rubinstein S, Rivera D, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395. [DOI] [PMC free article] [PubMed]
- 207.Lee H-Y, Ahn J, Kang CK, Won S-H, Park J-H, Kang C, et al. Association of Angiotensin II Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors on COVID-19-Related Outcome. SSRN Electron J. 2020.
- 208.Lee LYW, Cazier J-B, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee LYW, Cazier J-B, Starkey T, Briggs SEW, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li J, Guo T, Dong D, Zhang X, Chen X, Feng Y, et al. Defining heart disease risk for death in COVID-19 infection. QJM. 2020. [DOI] [PMC free article] [PubMed]
- 211.Li Q, Chen L, Li Q, He W, Yu J, Chen L, et al. Cancer increases risk of in-hospital death from COVID-19 in persons < 65 years and those not in complete remission. Leukemia. 2020;34(9):2384–2391. doi: 10.1038/s41375-020-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lieberman-Cribbin W, Rapp J, Alpert N, Tuminello S, Taioli E. The Impact of Asthma on Mortality in Patients With COVID-19. Chest. 2020. [DOI] [PMC free article] [PubMed]
- 213.Magleby R, Westblade LF, Trzebucki A, Simon MS, Rajan M, Park J, et al. Impact of SARS-CoV-2 Viral Load on Risk of Intubation and Mortality Among Hospitalized Patients with Coronavirus Disease 2019. Clin Infect Dis. 2020:ciaa851. [DOI] [PMC free article] [PubMed]
- 214.Mahdavinia M, Foster KJ, Jauregui E, Moore D, Adnan D, Andy-Nweye AB, et al. Asthma prolongs intubation in COVID-19. J Allerg Clin Immunol Pract. 2020;8(7):2388–2391. doi: 10.1016/j.jaip.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McCarty TR, Hathorn KE, Redd WD, Rodriguez NJ, Zhou JC, Bazarbashi AN, et al. How Do Presenting Symptoms and Outcomes Differ by Race/Ethnicity Among Hospitalized Patients with COVID-19 Infection? Experience in Massachusetts. Clin Infect Dis. 2020:ciaa1245. [DOI] [PMC free article] [PubMed]
- 217.Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020. [DOI] [PMC free article] [PubMed]
- 218.Mendy A, Apewokin S, Wells A, Morrow A. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medrxiv. 2020:10.1101.
- 219.Meng Y, Lu W, Guo E, Liu J, Yang B, Wu P, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13(1):75. doi: 10.1186/s13045-020-00907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020:10.1111/febs.15495. [DOI] [PMC free article] [PubMed]
- 221.Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, et al. Risk Factors for Mortality in Patients with COVID-19 in New York City. J Gen Intern Med. 2020:1–10. [DOI] [PMC free article] [PubMed]
- 222.Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med. 2020. [DOI] [PMC free article] [PubMed]
- 223.Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do Patients with Cancer Have a Poorer Prognosis of COVID-19? An Experience in New York City. Ann Oncol. 2020. [DOI] [PMC free article] [PubMed]
- 224.Miyashita S, Yamada T, Mikami T, Miyashita H, Chopra N, Rizk D. Impact of dementia on clinical outcomes in elderly patients with coronavirus 2019 (COVID-19): an experience in New York. Geriatr Gerontol Int. 2020;20(7):732–734. doi: 10.1111/ggi.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Moon S-S, Lee K, Park J, Yun S, Lee YS, Lee DS. Clinical Characteristics and Mortality Predictors of COVID-19 Patients Hospitalized at Nationally-Designated Treatment Hospitals. J Korean Med Sci. 2020;35(36). [DOI] [PMC free article] [PubMed]
- 226.Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult inpatients with COVID-19. Public Health. 2020. [DOI] [PMC free article] [PubMed]
- 227.Nakeshbandi M, Maini R, Daniel P, Rosengarten S, Parmar P, Wilson C, et al. The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obes. (2005) 2020;44(9):1832–1837. doi: 10.1038/s41366-020-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative Survival Analysis of Immunomodulatory Therapy for Coronavirus Disease 2019 Cytokine Storm. Chest. 2020:S0012-3692(20)34901-1. [DOI] [PMC free article] [PubMed]
- 229.Okoh AK, Sossou C, Dangayach NS, Meledathu S, Phillips O, Raczek C, et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19(1):93. doi: 10.1186/s12939-020-01208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx. New York. Metab. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7. [DOI] [PMC free article] [PubMed]
- 232.Patel NG, Bhasin A, Feinglass JM, Belknap SM, Angarone MP, Cohen ER, et al. Clinical Outcomes of Hospitalized Patients with COVID-19 on Therapeutic Anticoagulants. medRxiv. 2020:2020.08.22.20179911.
- 233.Perez-Guzman PN, Daunt A, Mukherjee S, Crook P, Forlano R, Kont MD, et al. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 234.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pettit NN, MacKenzie EL, Ridgway J, Pursell K, Ash D, Patel B, et al. Obesity is Associated with Increased Risk for Mortality Among Hospitalized Patients with COVID-19. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed]
- 236.Pinato DJ, Zambelli A, Aguilar-Company J, Bower M, Sng C, Salazar R, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10(10):1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 238.Rastad H, Karim H, Ejtahed H-S, Tajbakhsh R, Noorisepehr M, Babaei M, et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diab Metab Syndrome. 2020;12(1):57. doi: 10.1186/s13098-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Regina J, Papadimitriou-Olivgeris M, Burger R, Le Pogam M-A, Niemi T, Filippidis P, et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: An observational retrospective study. Plos One. 2020;15(11):e0240781. doi: 10.1371/journal.pone.0240781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Reilev M, Kristensen KB, Pottegård A, Lund LC, Hallas J, Ernst MT, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020:dyaa140. [DOI] [PMC free article] [PubMed]
- 241.Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid-19 Testing, Hospital Admission, and Intensive Care Among 2,026,227 United States Veterans Aged 54-75 Years. medRxiv. 2020:2020.04.09.20059964.
- 242.Rivera-Izquierdo M, Del Carmen V-UM, R-delAmo JL, Fernández-García MÁ, Martínez-Diz S, Tahery-Mahmoud A, et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. Plos One. 2020;15(6):e0235107-e. doi: 10.1371/journal.pone.0235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rossi A, Gottin L, Donadello K. Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by Covid-19 related respiratory failure. Res Square. 2020. [DOI] [PMC free article] [PubMed]
- 245.Rossi PG, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia, Italy. Plos One. 2020;15(8):e0238281. doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rottoli M, Bernante P, Belvedere A, Balsamo F, Garelli S, Giannella M, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol. 2020. [DOI] [PMC free article] [PubMed]
- 247.Russell B, Moss C, Papa S, Irshad S, Ross P, Spicer J, et al. Factors Affecting COVID-19 Outcomes in Cancer Patients: A First Report From Guy's Cancer Center in London. Front Oncol. 2020;10(1279). [DOI] [PMC free article] [PubMed]
- 248.Salacup G, Lo KB, Gul F, Peterson E, De Joy R, Bhargav R, et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: A single tertiary center cohort. J Med Virol. 2020;n/a(n/a). [DOI] [PMC free article] [PubMed]
- 249.Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, Gil Manso R, Colmenares R, Gil Alos D, et al. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 2020;105(5):597–607. doi: 10.1111/ejh.13493. [DOI] [PubMed] [Google Scholar]
- 250.Sapey E, Gallier S, Mainey C, Nightingale P, McNulty D, Crothers H, et al. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: an observational cohort study in an urban catchment area. BMJ Open Respir Res. 2020;7(1):e000644. doi: 10.1136/bmjresp-2020-000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Seiglie J, Platt J, Cromer SJ, Bunda B, Foulkes AS, Bassett IV, et al. Diabetes as a Risk Factor for Poor Early Outcomes in Patients Hospitalized With COVID-19. Diab Care. 2020;43(12):2938–2944. doi: 10.2337/dc20-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shah P, Owens J, Franklin J, Mehta A, Heymann W, Sewell W, et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52(7):354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shah V, Ko Ko T, Zuckerman M, Vidler J, Sharif S, Mehra V, et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King's College Hospital experience. Br J Haematol. 2020;190(5):e279–ee82. doi: 10.1111/bjh.16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shang Y, Liu T, Wei Y, Li J, Shao L, Liu M, et al. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMed. 2020;24:100426. doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diab Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 256.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed]
- 257.Sigel K, Swartz T, Golden E, Paranjpe I, Somani S, Richter F, et al. Covid-19 and People with HIV Infection: Outcomes for Hospitalized Patients in New York City. Clin Infect Dis. 2020:ciaa880. [DOI] [PMC free article] [PubMed]
- 258.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obes (Silver Spring). 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Singh S, Bilal M, Khan A, Chowdhry M, Sánchez-Luna S, Kochhar G, et al. Outcomes of COVID-19 in Patients with Obesity in United States: A Large Research Network Study. SSRN Electron J. 2020.
- 260.Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159(2):768–71.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(4):1575–8.e4. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sisó-Almirall A, Kostov B, Mas-Heredia M, Vilanova-Rotllan S, Sequeira-Aymar E, Sans-Corrales M, et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. Plos One. 2020;15(8):e0237960-e. doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Smith AA, Fridling J, Ibrahim D, Porter PS., Jr Identifying Patients at Greatest Risk of Mortality due to COVID-19: A New England Perspective. West J Emerg Med. 2020;21(4):785–789. doi: 10.5811/westjem.2020.6.47957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Soares RCM, Mattos LR, Raposo LM. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184–1190. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Solís P, Carreňo H. COVID-19 Fatality and Comorbidity Risk Factors among Diagnosed Patients in Mexico. MedRxiv. 2020.
- 266.Sousa GJB, Garces TS, Cestari VRF, Florêncio RS, Moreira TMM, Pereira MLD. Mortality and survival of COVID-19. Epidemiol Infect. 2020;148:e123. doi: 10.1017/S0950268820001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270-e. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sun H, Ning R, Tao Y, Yu C, Deng X, Zhao C, et al. Risk Factors for Mortality in 244 Older Adults With COVID-19 in Wuhan, China: A Retrospective Study. J Am Geriatr Soc. 2020;68(6):E19–e23. doi: 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (London) 2020;52(12):902–907. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 270.Tai S, Tang J, Yu B, Tang L, Wang Y, Zhang H, et al. Association between Cardiovascular Burden and Requirement of Intensive Care among Patients with Mild COVID-19. Cardiovasc Ther. 2020;2020:9059562. doi: 10.1155/2020/9059562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann Intern Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.van Gerwen M, Alsen M, Little C, Barlow J, Genden E, Naymagon L, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 273.Wang A-L, Zhong X, Hurd Y. Comorbidity and Sociodemographic determinants in COVID-19 Mortality in an US Urban Healthcare System. medRxiv. 2020:2020.06.11.20128926.
- 274.Wang D, Yin Y, Hu C, Liu X, Zhang X, Zhou S, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care (London) 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang K, Zuo P, Liu Y, Zhang M, Zhao X, Xie S, et al. Clinical and Laboratory Predictors of In-hospital Mortality in Patients With Coronavirus Disease-2019: A Cohort Study in Wuhan, China. Clin Infect Dis. 2020;71(16):2079–2088. doi: 10.1093/cid/ciaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. The Journal of infection. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wendel Garcia P, Fumeaux T, Guerci P, Heuberger D, Montomoli J, Roche-Campo F, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMed. 2020;25:100449. doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clinic Proc. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Xu J, Yang X, Yang L, Zou X, Wang Y, Wu Y, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care (London) 2020;24(1):394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diab Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yang Q, Zhou Y, Wang X, Gao S, Xiao Y, Zhang W, et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: a propensity score-matching analysis. Respir Res. 2020;21(1):172. doi: 10.1186/s12931-020-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yarza R, Bover M, Paredes D, López-López F, Jara-Casas D, Castelo-Loureiro A, et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yehia BR, Winegar A, Fogel R, Fakih M, Ottenbacher A, Jesser C, et al. Association of Race With Mortality Among Patients Hospitalized With Coronavirus Disease 2019 (COVID-19) at 92 US Hospitals. JAMA Netw Open. 2020;3(8):e2018039-e. doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yu C, Lei Q, Li W, Wang X, Liu W, Fan X, et al. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan. China. Am J Prev Med. 2020;59(2):168–175. doi: 10.1016/j.amepre.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 286.Zhang H, Wang L, Chen Y, Wu Q, Chen G, Shen X, et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;126. [DOI] [PMC free article] [PubMed]
- 287.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020. [DOI] [PMC free article] [PubMed]
- 288.Zhang X, Guo W, Hua J, Luo Z, Gao S, Ran L, et al. The Incidence, Risk Factors and Clinical Outcomes of Acute Kidney Injury in Critically Ill Patients with COVID-19: A Multicenter Study. SSRN. 2020.
- 289.Zhang Y, Cui Y, Shen M, Zhang J, Liu B, Dai M, et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diab Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhao M, Wang M, Zhang J, Gu J, Zhang P, Xu Y, et al. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. 2020;12(11):10070–10086. doi: 10.18632/aging.103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(6):1068–77.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zimmerman P, Stroever S, Burton T, Hester K, Kim M, Fahy R, et al. Mortality associated with intubation and mechanical ventilation in patients with COVID-19. medRxiv. 2020:2020.08.13.20174524.
- 294.Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo C-L, Kuchel GA, et al. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J Gerontol A. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bhaskaran K, Rentsch C, MacKenna B, Schultz A, Mehrkar A, Bates C, et al. HIV infection and COVID-19 death: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. medRxiv. 2020:2020.08.07.20169490. [DOI] [PMC free article] [PubMed]
- 296.Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study. Ann Intern Med. 2020;173(7):536–541. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hamer M, Gale CR, Kivimäki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci. 2020:202011086. [DOI] [PMC free article] [PubMed]
- 298.Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Khawaja AP, Warwick AN, Hysi PG, Kastner A, Dick A, Khaw PT, et al. Associations with covid-19 hospitalisation amongst 406,793 adults: the UK Biobank prospective cohort study. medRxiv. 2020:2020.05.06.20092957.
- 300.Lassale C, Gaye B, Hamer M, Gale CR, Batty GD. Ethnic disparities in hospitalisation for COVID-19 in England: the role of socioeconomic factors, mental health, and inflammatory and pro-inflammatory factors in a community-based cohort study. Brain Behav Immun. 2020;88:44–49. doi: 10.1016/j.bbi.2020.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Patel AP, Paranjpe MD, Kathiresan NP, Rivas MA, Khera AV. Race, socioeconomic deprivation, and hospitalization for COVID-19 in English participants of a National Biobank. medRxiv. 2020:2020.04.27.20082107. [DOI] [PMC free article] [PubMed]
- 302.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.George A, Stead TS, Ganti L. What's the risk: differentiating risk ratios, odds ratios, and hazard ratios? Cureus. 2020;12(8):e10047-e. doi: 10.7759/cureus.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cohen JF, Korevaar DA, Matczak S, Chalumeau M, Allali S, Toubiana J. COVID-19–related fatalities and intensive-care-unit admissions by age groups in Europe: a meta-analysis. Front Med. 2021;7(1097). [DOI] [PMC free article] [PubMed]
- 305.Romero Starke K, Petereit-Haack G, Schubert M, Kämpf D, Schliebner A, Hegewald J, et al. The age-related risk of severe outcomes due to COVID-19 infection: a rapid review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2020;17(16):5974. [DOI] [PMC free article] [PubMed]
- 306.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80.e8. [DOI] [PMC free article] [PubMed]
- 307.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. Journal of infection and public health. 2020;13(12):1833-9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence”. This supplementary file provides details on study search and selection, the results of the searches, data extraction from reviews and primary studies, and meta-analyses. The tables give all obtained pooled estimates of the main analyses, including the GRADE assessment. Age-stratified estimates and analysis of specific population groups are presented. The file also provides results of the risk of bias evaluation conducted in this review and the lists of excluded SRs and primary studies.
Data Availability Statement
All data generated and analysed during this study are included in this published article and its supplementary information file (Additional file 1).