Abstract
Background
The SARS-CoV-2 virus is the cause of the latest pandemic of the 21st century; it is responsible for the development of COVID-19. Within the multiple study models for both the biology and the treatment of SARS-CoV-2, the use of stem cells has been proposed because of their ability to increase the immune response and to repair tissue. Therefore, the objective of this review is to evaluate the role of stem cells against SARS-CoV-2 and COVID-19 in order to identify their potential as a study model and as a possible therapeutic source against tissue damage caused by this virus. Therefore, the following research question was established: What is the role of stem cells in the study of SARS-CoV-2 and the treatment of COVID-19?
Materials and Methods
A search was carried out in the electronic databases of PUBMED, Scopus, and ScienceDirect. The following keywords were used: “SARS-CoV-2,” “COVID-19,” and “STEM CELL,” plus independent search strategies with the Boolean operators “OR” and “AND.” The identified reports were those whose main objective was the study of stem cells in relation to SARS-CoV-2 or COVID-19. For the development of this study, the following inclusion criteria were taken into account: studies whose main objective was the study of stem cells in relation to SARS-CoV-2 or COVID-19 and clinical case studies, case reports, clinical trials, pilot studies, in vitro, or in vivo studies. For assessment of the risk of bias for in vitro studies, the SciRAP tool was used. The data collected for each type of study, clinical or in vitro, were analyzed with descriptive statistics using the SPSS V.22 program.
Results
Of the total of studies included (n = 39), 22 corresponded to in vitro investigations and 17 to human studies (clinical cases (n = 9), case series (n = 2), pilot clinical trials (n = 5), clinical trials (n = 1)). In vitro studies that induced pluripotent stem cells were the most used (n = 12), and in clinical studies, the umbilical stem cells derived were the most reported (n = 11). The mean age of the study subjects was 58.3 years. After the application of stem cell therapy, the follow-up period was 8 days minimum and 90 days maximum. Discussion. The mechanism by which the virus enters the cell is through protein “S,” located on the surface of the membrane, by recognizing the ACE2 receptor located on the target cell. The evidence that the expression of ACE2 and TMPRSS2 in stem cells indicates that stem cells from bone marrow and amniotic fluid have very little expression. This shows that stem cell has a low risk of infection with SARS-CoV-2.
Conclusion
The use of stem cells is a highly relevant therapeutic option. It has been shown in both in vitro studies and clinical trials that it counteracts the excessive secretion of cytokines. There are even more studies that focus on long-term follow-up; thus, the potential for major side effects can be analyzed more clearly. Finally, the ethical use of stem cells from fetal or infant origin needs to be regulated. The study was registered in PROSPERO (no. CRD42021229038). The limitations of the study were because of the methodology employed, the sample was not very large, and the follow-up period of the clinical studies was relatively short.
1. Introduction
The SARS-CoV-2 virus was described for the first time in December 2019. It is the cause of the latest pandemic of the 21st century, and its spread throughout the world has been rapid, generating millions of infections and deaths. The affectation of this virus is responsible for the development of COVID-19, which is characterized from causing mild damage involving fever, cough, and dyspnea, to severe pneumonia, myocardial damage, arrhythmias, neurological damage, and multiple organ failure [1]. One of the main organs damaged is the liver, which, after infection with SARS-CoV-2, increases the levels of alanine aminotransferase, aspartate aminotransferase, lactic dehydrogenase, gamma glutamyl transferase, and total bilirubin given the cirrhotic poor immune function [2]. On the other hand, the microbiota plays a very relevant role in the severity of COVID-19 because coinfections by Candida albicans and Candida glabrata have been reported, contributing to the affectation of the immune system [3]. Due to these tragic effects, multiple research centers around the world have focused on understanding the biology of the virus as well as the treatment of the various side effects generated by SARS-CoV-2.
Stem cells are found within multiple study models for both the biology and treatment of SARS-CoV-2. These types of cells are unspecialized; they have the great advantage of being able to give rise to cells from specialized tissues and have the ability for self-renewal. According to their origin, these types of cells are found in fetuses, infants, and adults [4]. Another main characteristic is their ability to increase the immune response and repair tissue [5, 6], for which they have become a great source of relevant study of for the understanding and treatment of COVID-19. Therefore, the main objective of this work is to evaluate the role of stem cells against SARS-CoV-2 and COVID-19 in order to identify their potential as a study model and a possible therapeutic source against tissue damage caused by SARS-CoV-2.
To carry out this work, original studies were considered; clinical case reports, case series, and clinical trials were included, as well as basic science studies in vitro, in vivo, or in silico that covered the use of stem cells in relation to the SARS-CoV-2.
2. Materials and Methods
2.1. Research Question
What is the role of stem cells in the study of SARS-CoV-2 and the treatment of COVID-19?
2.2. Criteria Selection
2.2.1. Inclusion Criteria
Studies whose main objective was the study of stem cells in relation to SARS-CoV-2 or COVID-19
Clinical case studies, case reports, clinical trials, pilot studies, in vitro, or in vivo studies
2.2.2. Exclusion Criteria
Literature reviews, systematic reviews, or meta-analysis, as well as studies in a language other than English or Spanish
Studies were separated according to their focus: in vitro or clinical studies. All the studies that met the established criteria were placed in a bibliographic manager (Mendely) for further analysis.
2.3. Search Strategy
A search was carried out in the electronic databases of PUBMED, Scopus, and ScienceDirect, and the following keywords were used: “SARS-CoV-2,” “COVID-19,” and “STEM CELL” employee independent search strategies with the Boolean operators “OR” and “AND.” Due to the importance of the topic, no time limit was established for the search; the last was March 2020.
A further search of the references of the selected studies was performed to detect potential studies that met the selection criteria.
2.4. Study Selection
For study selection, an initial filter was performed by title and abstract, which mentioned the study of stem cells related to SARS-CoV-2 and/or COVID-19. The selected studies were placed in a database in which a second full-text filter was performed identifying a specific bibliography that met the established selection criteria. The selection of the studies was carried out independently by two examiners (Cuevas-Gonzalez JC and Cuevas Gonzalez MV); in case of discrepancy, a third evaluator participated (Donohue-Cornejo A).
2.5. Data Extraction and Analysis
The data recollected were specific for each type of study; clinical or in vitro, the information gathered from in vitro studies was author, country, study type, stem cell origin, objective of the study, and conclusion. Additionally, in clinical studies, the data were number of subjects, mean age (years), treatment plan, and follow-up time after treatment administration. The data were placed in a database, and statistics were made based measures of central tendency. Descriptive statistics of the data were performed using the SPSS V.22 program.
2.6. Risk of Bias
To determine the risk of bias, different tools were used according to the type of study. For in vitro studies, the SciRAP tool was used, which was adapted for its application in this type of approach. The JBI critical appraisal checklist was used to evaluate the risk of bias in clinical cases [7]. All evaluations were carried out by two evaluators independently; in the case of discrepancy, a third evaluator participated.
3. Results
The search strategy included characteristics of the studies, which initially analyzed the title and abstract; 696 studies were eliminated because they did not specify whether the research was on stem cells in COVID-19, leaving only 113 full-text studies. When these were analyzed, 72 were eliminated because they were review and systematic review studies, repeated studies, and others not specific to the topic of interest. After filtering according to the established search criteria, only 39 studies were included (PRISMA flow chart Figure 1).
Figure 1.
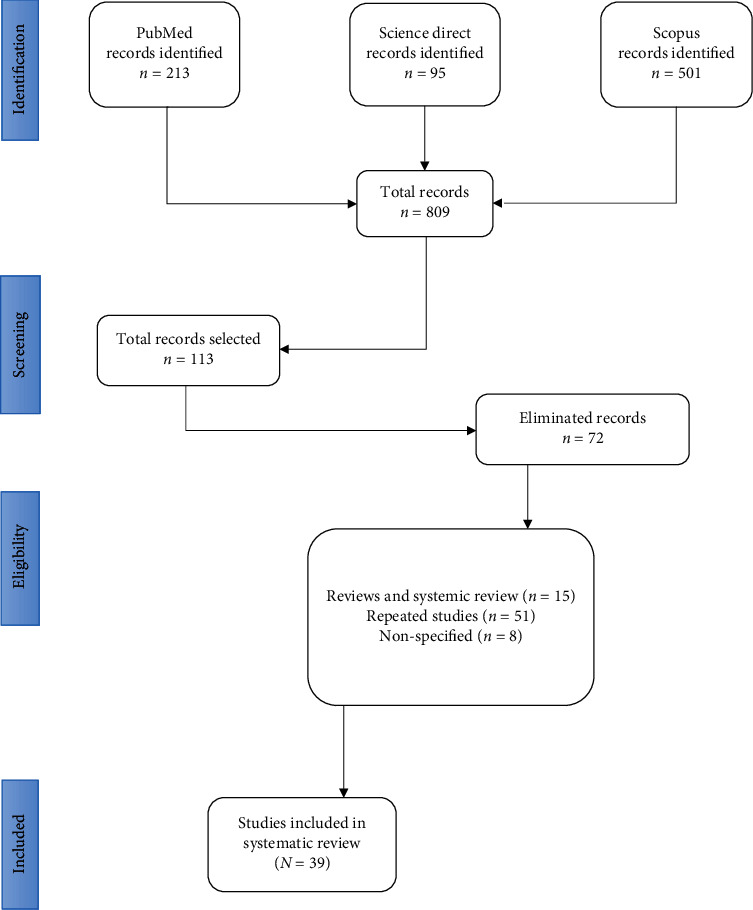
Search strategy used in the study, PRISMA flow chart.
3.1. Description of the Data
Of the total studies included (n = 39), 22 corresponded to in vitro investigations and 17 to human studies (clinical cases (n = 9), case series (n = 2), pilot clinical trials (n = 5), and clinical trials (n = 1)).
In vitro studies that induced pluripotent stem cells were the most used (n = 12). In clinical studies, stem cells derived from umbilical cord were the most reported (n = 11). The mean age of the study subjects was 58.3 years. After the application of stem cell therapy, the follow-up period was a minimum of 8 days and a maximum of 90 days (Supplementary) [8–38].
3.2. Risk of Bias
To determine the risk of bias, the SciRAP tool was used, which was adapted according to the studies to be evaluated; three fundamental aspects were evaluated: report quality, methodological quality, and study relevance. One hundred percent of the studies were identified as having low bias. The JBI critical appraisal checklist was used for case reports, which consists of eight questions focused on the description of the patient, their evaluation, the interventions carried out, and the adverse effects. All of the studies showed a low risk of bias (Table 1).
Table 1.
Risk of bias.
(a).
| In vitro studies | |||
|---|---|---|---|
| Author | Reporting quality | Methodological quality | Relevance |
| Huang J. |

|

|

|
| Jacob F. |

|

|

|
| Bin Yu |

|

|

|
| Wong CK |

|

|

|
| Yang L |

|

|

|
| Youk J |

|

|

|
| Ropa J |

|

|

|
| Cao Y |

|

|

|
| Sharma A |

|

|

|
| Surendran H |

|

|

|
| Schäfer R |

|

|

|
| Katsura H |

|

|

|
| Purwati |

|

|

|
| Kase Y. |

|

|

|
| Ghazizadeh Z |

|

|

|
| Hekman R |

|

|

|
| Kwon Y |

|

|

|
| Duan F |

|

|

|
| Han Y |

|

|

|
(b).
| Clinical studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Quality |
| Tao J | y | y | y | y | y | y | y | y | High |
| Peng H | y | y | y | y | y | y | y | y | High |
| Liang B | Y | Y | Y | Y | Y | Y | Y | Y | High |
| Zengin R | y | y | y | y | y | y | y | y | High |
| Zhu Y | y | y | y | y | y | y | y | y | High |
| Zhang Y | y | y | y | y | y | y | y | y | High |
| Fisler G | y | y | y | y | y | y | y | y | High |
| Soler Rich R | y | y | y | y | y | y | y | y | High |
| Lázaro Del Campo P | y | y | y | y | y | y | y | y | High |
4. Discussion
One of the main characteristics of stem cells is their ability to modulate an innate and adaptive immune response due to the presence of proinflammatory receptors in their membrane, as well as their ability to synthesize IL-10, TGF-β, TSG-6, LIF, HGF, COX-2, prostaglandin-2, and others, in addition to promoting the regeneration and regulation of the expansion of immune cells [39]. This is why it has been a widely used model in the study of SARS-CoV-2.
The mechanism by which the virus enters the cell is through protein “S,” located on the surface of the membrane, by recognizing the ACE2 receptor located on the target cell [40]. Once recognition of the virus is carried out, transmembrane proteins (i.e., ADAM 17 or TMPRSS2) participate in the process of fusion and internalization of SARS-CoV-2 [41]. The evidence that exists relating to the expression of ACE2 and TMPRSS2 in stem cells indicates that stem cells from bone marrow and amniotic fluid have very little expression, and—in the case of adipose tissue stem cells—the expression decreases less than 10% without finding important changes in its expression when faced with inflammatory events [42], which indicates a low risk of infection of these cells to SARS-CoV-2. On the other hand, bioinformatics studies that compare the expression of ACE2 in stem cells of different origins have shown that the expression of ACE2 in adipose stem cells is higher compared to those of placental or umbilical cord origin. In addition, the low expression of ACE2 is related to a low expression of genes related to inflammasomes and immune regulation [43]. All of the above suggest that stem cells are a reliable therapeutic option due to the low expression of receptors for SARS-CoV-2 and that within the multiple sources of stem cells; those of fetal origin are the best therapeutic option. This is demonstrated in our review since the vast majority of clinical case studies focus on the effect of umbilical cord stem cells for the treatment of COVID-19 (n = 11).
In this study, pluripotent stem cells were identified as the cell type most used as a study model for SARS-CoV-2 (n = 12), specifically those that were induced. These derive from adult somatic cells that have been genetically reprogrammed into embryonic stem cells [44]. These cells have the capacity for self-renewal and differentiation to all cell types in the body; however, they have certain disadvantages depending on the technique used for their differentiation and the method based on the vector integration system. Despite being highly efficient, there is a risk that a cancer cell will develop [45]. By studying the susceptibility of different cell lines from induced stem cells, pancreatic and liver cells have been identified as being more susceptible to infection by SARS-CoV-2; on the other hand, cells that express ACE2—such as the endothelium, macrophages, and cortical neurons—show low or no permissiveness for the SARS-CoV-2 virus, suggesting that the nonlinear relationship between ACE2 and the permissiveness to SARS-CoV-2 infection highlights the importance of using pluripotent stem cells in place of cells that overexpress ACE2 to study the biology of SARS-CoV-2 [46].
As the lungs are the organ most affected by COVID-19, studies have been carried out that evaluate the response of pluripotent stem cells induced to an epithelial alveolar phenotype when infected with SARS-CoV-2, finding that they present a global transcriptomic change characterized by the expression of encoded cytokines by NF-κB genes and epithelial interferon late responses [47]. Another study revealed that changes in RNA processing reducing the translation of 5′ capped mRNA and identified inhibitors of the mTOR and MAPK pathways, suggesting that SARS-CoV-2 induces arrest of the cell cycle, apoptosis, and damage to the nuclear envelope [48].
COVID-19 has affected millions of people around the world. The mildest symptoms range between fever and cough and/or digestive problems such as diarrhea and vomiting. In severe cases, there are pulmonary alterations with a respiratory rate of 30 per min and lower oxygen saturation below to 93% in addition to organ failure [49]; severe damage of this disease is characterized by an increase in the levels of inflammation mediators, such as cytokines and chemokines (IL-2, IL-7, IL-10; tumor necrosis factor) among others [ 50]. One of the markers of inflammation related to high mortality is IL-6; this suggests that high rates of mortality are related to the development of cytokine elevation syndrome induced by a cytokine storm [51]. In order to develop an immunological therapy that helps contain the side effects of SARS-CoV-2, clinical trials have been developed based on stem cell therapy.
It has been reported that the use of stem cells as immune therapy is related to the development of fever, without disclosing significant adverse effects [52]. When evaluating the safety and efficacy of stem cells from umbilical cord, it has been confirmed that patients do not develop side effects of consideration at a follow-up of 28 days. In addition, a decrease in the expression of IL-6 was observed [53], which is related to severe symptoms of COVID-19. On the other hand, when analyzing the clinical efficacy of the application of umbilical cord stem cells, it was observed that in patients with severe symptoms, the sensation of chest tightness, shortness of breath, and fatigue were improved. In the same way, the level of IL-6 and C-reactive proteins decreased from the third day of umbilical stem cell application, and a significant improvement from the seventh day of applying the therapy was reported [54]. Similarly, Guo et al. reported that when administering umbilical stem cells at a dose of 1 × 106 cells per kilogram of weight, oxygenation levels are restored in patients with severe disease without reporting secondary reactions [55].
Umbilical stem cells have the characteristic that their isolation is safe and risk-free for the donor; their long-term cryopreservation does not affect their function or viability, and these types of cells carry a low possibility of transmission of viral infections. They present excellent immunological properties due to the fact that they exhibit HLA class I and II antigens related to the interferon-gamma response, which is a central regulator of the defense system that mediates the innate and adaptive immune response [56, 57].
Although positive effects have been reported on the use of stem cells as a therapeutic option for COVID-19, the reported absence of side effects after its application has been for relatively short periods of time, which is why it is necessary to carry out long-term follow-up to advance the processes of establishing stem cells as a therapeutic option.
The two main limitations of this study are as follows: (1) because of the methodology employed, the sample was not very large, and (2) the follow-up period of the clinical studies was relatively short. New studies with longer observation periods are emerging every day because of the importance of this current issue.
5. Conclusion
The SARS-CoV-2 infection develops intense inflammation due to the cytokine storm that develops. In addition to the sequelae that it generates in the tissues, prolonged inflammatory states can trigger the loss of partial function of the affected organ. The use of stem cells is a highly relevant therapeutic option because in vitro studies and clinical trials have shown that it counteracts the excessive secretion of cytokines.
Acknowledgments
We appreciate the support provided by the CONACYT through its scholarship (number: 326127; CVU 537149) to Maria Veronica Cuevas Gonzalez during her Doctoral studies in the Programa de Maestría y Doctorado en Ciencias Médicas, Odontológicas y de la Salud at UNAM. We appreciate the support provided by the CONACYT (FONDO MIXTO) Government of the State of Chihuahua, Mexico (Grant Number CHIH-2018-02-01-1167).
Data Availability
All data obtained from this study can be found in the Institute of Biomedical Sciences of the Autonomous University of Ciudad Juarez, Chihuahua, and can be requested through the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
The information collected in the selected studies is shown in the following table; the information was divided according to the type of study, in vitro or clinical study. For in vitro studies, there is information about the main author, country, origin of stem cells, type of study, objective of the study, and conclusion. The clinical studies contain information related to the author, country, type of study, whether it is a clinical report, clinical series, or pilot study, origin of the stem cells, objective of the study, number of subjects included in the study, treatment plan, follow-up time to the established treatment plan, and the results obtained from the study.
References
- 1.Wang M.-Y., Zhao R., Gao L.-J., Gao X.-F., Wang D.-P., Cao J.-M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Frontiers in Cellular and Infection Microbiology. 2020;10:p. 587269. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini P., Afzali S., Karimi M., et al. The coronavirus disease 2019 and effect on liver function. Reviews in Medical Microbiology. 2021;Publish Ahead of Print doi: 10.1097/MRM.0000000000000267. [DOI] [Google Scholar]
- 3.Soltani S., Zakeri A., Zandi M., et al. The role of bacterial and fungal human respiratory microbiota in COVID-19 patients. BioMed Research International. 2021;2021:13. doi: 10.1155/2021/6670798.6670798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivakumar M., Dineshshankar J., Sunil P. M., et al. Stem cells: an insight into the therapeutic aspects from medical and dental perspectives. Journal of Pharmacy & Bioallied Sciences. 2015;7(6):S361–S371. doi: 10.4103/0975-7406.163453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naik S., Larsen S. B., Cowley C. J., Fuchs E. Two to tango: dialog between immunity and stem cells in health and disease. Cell. 2018;175(4):908–920. doi: 10.1016/j.cell.2018.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleem A., Saleh F. Mesenchymal stem cells in the fight against viruses: face to face with the invisible enemy. Curr Res Transl Med. 2020;68(3):105–110. doi: 10.1016/j.retram.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Joanna Briggs Institute. Reviewers’ Manual. Methodolgy for JBI Umbrella Reviews. The Joanna Briggs Institute: Adelaide, Australia; 2014. [Google Scholar]
- 8.Valyaeva A. A., Zharikova A. A., Kasianov A. S., Vassetzky Y. S., Sheval E. V. Expression of SARS-CoV-2 entry factors in lung epithelial stem cells and its potential implications for COVID-19. Scientific Reports. 2020;10(1):p. 17772. doi: 10.1038/s41598-020-74598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob F., Pather S. R., Huang W.-K., et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27(6):937–950.e9. doi: 10.1016/j.stem.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu B., Ikhlas S., Ruan C., Zhong X., Cai D. Innate and adaptive immunity of murine neural stem cell-derived piRNA exosomes/microvesicles against pseudotyped SARS-CoV-2 and HIV-based lentivirus. iScience. 2020;23(12):p. 101806. doi: 10.1016/j.isci.2020.101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong C.-K., Luk H. K.-H., Lai W.-H., et al. Human-induced pluripotent stem cell-derived cardiomyocytes platform to study SARS-CoV-2 related myocardial injury. Circulation Journal. 2020;84(11):2027–2031. doi: 10.1253/circj.CJ-20-0881. [DOI] [PubMed] [Google Scholar]
- 12.Youk J., Kim T., Evans K. V., et al. Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell. 2020;27(6):905–919.e10. doi: 10.1016/j.stem.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ropa J., Cooper S., Capitano M. L. M. L., Van’t Hof W., Broxmeyer H. E. Human hematopoietic stem, progenitor, and immune cells respond ex vivo to SARS-CoV-2 spike protein. Stem Cell Reviews and Reports. 2021;17(1):253–265. doi: 10.1007/s12015-020-10056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y., Wu H., Zhai W., et al. A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem Cell Research. 2020;49:p. 102066. doi: 10.1016/j.scr.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A., Garcia G., Wang Y., et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Reports Medicine. 2020;1(4):p. 100052. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surendran H., Nandakumar S., Pal R. Human induced pluripotent stem cell-derived lung epithelial system for SARS-CoV-2 infection modeling and its potential in drug repurposing. Stem Cells and Development. 2020;29(21):1365–1369. doi: 10.1089/scd.2020.0152. [DOI] [PubMed] [Google Scholar]
- 17.Katsura H., Sontake V., Tata A., et al. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27(6):890–904.e8. doi: 10.1016/j.stem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purwati M. A., Karsari N., Dinaryanti D., Hendrianto A., Ihsan E. The potency of hematopoietic stem cells (HSCs) and natural killer (NK) cells as a therapeutic of SARS-CoV-2 Indonesia isolates infection by viral inactivation (in vitro study) Sys Rev Pharm. 2020;11(5):772–777. [Google Scholar]
- 19.Kase Y., Okano H. Expression of ACE2 and a viral virulence-regulating factor CCN family member 1 in human iPSC-derived neural cells: implications for COVID-19-related CNS disorders. Inflammation and Regeneration. 2020;40(1):p. 32. doi: 10.1186/s41232-020-00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel R. M., Majd H., Richter M. N., et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27(6):876–889. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon Y., Nukala S. B., Srivastava S., et al. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Research & Therapy. 2020;11(1):p. 514. doi: 10.1186/s13287-020-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan F., Guo L., Yang L., et al. Modeling COVID-19 with human pluripotent stem cell-derived cells reveals synergistic effects of anti-inflammatory macrophages with ACE2 inhibition against SARS-CoV-2. Res Sq. 2020 doi: 10.21203/rs.3.rs-62758/v1. [DOI] [Google Scholar]
- 23.Li Y., Li H., Zhou L. EZH2-mediated H3K27me3 inhibits ACE2 expression. Biochemical and Biophysical Research Communications. 2020;526(4):947–952. doi: 10.1016/j.bbrc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han Y., Duan X., Yang L., et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589(7841):270–275. doi: 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao J., Nie Y., Wu H., et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. Journal of Infection in Developing Countries. 2020;14(10):1138–1145. doi: 10.3855/jidc.13081. [DOI] [PubMed] [Google Scholar]
- 26.Peng H., Gong T., Huang X., et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Research & Therapy. 2020;11(1):p. 291. doi: 10.1186/s13287-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang B., Chen J., Li T., et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore) 2020;99(31, article e21429) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang L., Jiang Y., Zhu M., et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Frontiers in Medicine. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zengin R., Beyaz O., Koc E. S., et al. Mesenchymal stem cell treatment in a critically ill COVID-19 patient: a case report. Stem Cell Investigation. 2020;7:17–17. doi: 10.21037/sci-2020-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y., Zhu R., Liu K., et al. Human umbilical cord mesenchymal stem cells for adjuvant treatment of a critically ill COVID-19 patient: a case report. Infect Drug Resist. 2020;Volume 13:3295–3300. doi: 10.2147/IDR.S272645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Ding J., Ren S., et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Research & Therapy. 2020;11(1):p. 207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y., Huang J., Wu J., et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Proliferation. 2020;53(12):p. e12947. doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2− mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Guijo F., García-Arranz M., López-Parra M., et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. A proof of concept study. EClinicalMedicine. 2020;25:p. 100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisler G., Haimed A., Levy C. F., et al. Severe coronavirus disease 2019 infection in an adolescent patient after hematopoietic stem cell transplantation. Chest. 2020;158(4):e139–e142. doi: 10.1016/j.chest.2020.05.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soler Rich R., Rius Tarruella J., Melgosa Camarero M. T. Expanded Mesenchymal Stem Cells: a novel therapeutic approach of SARS-CoV-2 pneumonia (COVID-19). Concepts regarding a first case in Spain. Medicina Clínica. 2020;155(7):318–319. doi: 10.1016/j.medcli.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra A., Ernest D., Rogers B. A., et al. Umbilical cord blood therapy to prevent progression of COVID-19 related pneumonia: a structured summary of a study protocol for a pilot randomised controlled trial. Trials. 2020;21(1):p. 474. doi: 10.1186/s13063-020-04387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lázaro del Campo P., de Paz Arias R., Ramírez López A., de la Cruz Benito B., Humala Barbier K., Sánchez Vadillo I. No transmission of SARS-CoV-2 in a patient undergoing allogeneic hematopoietic cell transplantation from a matched-related donor with unknown COVID-19. Transfusion and Apheresis Science. 2020;59(6):p. 102921. doi: 10.1016/j.transci.2020.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha J. L. M., de Oliveira W. C. F., Noronha N. C., et al. Mesenchymal stromal cells in viral infections: implications for COVID-19. Stem Cell Reviews and Reports. 2021;17(1):71–93. doi: 10.1007/s12015-020-10032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. International Journal of Antimicrobial Agents. 2020;55(6):p. 105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni W., Yang X., Yang D., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Critical Care. 2020;24(1):p. 422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer R., Spohn G., Bechtel M., et al. Human mesenchymal stromal cells are resistant to SARS-CoV-2 infection under steady-state, inflammatory conditions and in the presence of SARS-CoV-2-infected cells. Stem Cell Reports. 2020;16(3) doi: 10.1016/j.stemcr.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desterke C., Griscelli F., Imeri J., et al. Molecular investigation of adequate sources of mesenchymal stem cells for cell therapy of COVID-19-associated organ failure. Stem Cells Translational Medicine. 2021;10(4) doi: 10.1002/sctm.20-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye L., Swingen C., Zhang J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Current Cardiology Reviews. 2013;9(1):63–72. doi: 10.2174/157340313805076278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh V. K., Kalsan M., Kumar N., Saini A., Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Development Biology. 2015;3 doi: 10.3389/fcell.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L., Han Y., Nilsson-Payant B. E., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125–136.e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J., Hume A. J., Abo K. M., et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27(6):962–973.e7. doi: 10.1016/j.stem.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hekman R. M., Hume A. J., Goel R. K., et al. Actionable cytopathogenic host responses of human alveolar type 2 cells to SARS-CoV-2. Molecular Cell. 2020;80(6):1104–1122.e9. doi: 10.1016/j.molcel.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouassou H., Kharchoufa L., Bouhrim M., et al. The pathogenesis of coronavirus disease 2019 (COVID-19): evaluation and prevention. Journal of Immunology Research. 2020;2020 doi: 10.1155/2020/1357983.1357983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh B., Mal G., Verma V., et al. Stem cell therapies and benefaction of somatic cell nuclear transfer cloning in COVID-19 era. Stem Cell Research & Therapy. 2021;12(1):p. 283. doi: 10.1186/s13287-021-02334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hojyo S., Uchida M., Tanaka K., et al. How COVID-19 induces cytokine storm with high mortality. Inflammation and Regeneration. 2020;40(1):p. 37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson M., Mei S. H. J., Wolfe D., et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:p. 100249. doi: 10.1016/j.eclinm.2019.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng F., Xu R., Wang S., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduction and Targeted Therapy. 2020;5(1):p. 172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shu L., Niu C., Li R., et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy. 2020;11(1):p. 361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo Z., Chen Y., Luo X., He X., Zhang Y., Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Critical Care. 2020;24(1):p. 420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roura S., Pujal J. M., Gálvez-Montón C., Bayes-Genis A. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Research & Therapy. 2015;6(1):p. 123. doi: 10.1186/s13287-015-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhat M. Y., Solanki H. S., Advani J., et al. Comprehensive network map of interferon gamma signaling. J Cell Commun Signal. 2018;12(4):745–751. doi: 10.1007/s12079-018-0486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information collected in the selected studies is shown in the following table; the information was divided according to the type of study, in vitro or clinical study. For in vitro studies, there is information about the main author, country, origin of stem cells, type of study, objective of the study, and conclusion. The clinical studies contain information related to the author, country, type of study, whether it is a clinical report, clinical series, or pilot study, origin of the stem cells, objective of the study, number of subjects included in the study, treatment plan, follow-up time to the established treatment plan, and the results obtained from the study.
Data Availability Statement
All data obtained from this study can be found in the Institute of Biomedical Sciences of the Autonomous University of Ciudad Juarez, Chihuahua, and can be requested through the corresponding author.


