Introduction
The Cystic Fibrosis (CF) Foundation chronic care guidelines recommend monitoring spirometry during quarterly multidisciplinary visits to identify early lung function decline. During the COVID-19 pandemic, the CF adult clinic at University of Virginia (UVA) transitioned from the classic CF care model to a model that included quarterly multidisciplinary telemedicine visits. While using telemedicine, CF care needed to include spirometry monitoring. Only a fraction of adult CF patients at UVA owned and used home spirometers (HS) in March 2020.
Aim
The specific aims of this quality improvement (QI) project were to increase the percentage of eligible adult CF patients who owned an HSs from 37% to 85% and to increase the percentage of adult CF patients seen at UVA with available spirometry in telemedicine from 50% to 95% by 31 December 2020.
Methods
Following the Model for Improvement QI methodology, a standardised process was developed for monitoring forced expiratory volume in 1 s with HS during multidisciplinary telemedicine visits during the COVID-19 pandemic.
Intervention
(1) HSs were distributed to eligible patients and (2) Home spirometry was monitored in eligible patients with each telemedicine visit and results were used for clinical care decisions.
Results
Both specific aims were achieved ahead of expected date. In March 2020, the beginning of the pandemic, 37% (49/131) of patients owned an HS and 50% (9/18) of patients seen via telemedicine performed spirometry at home. By September 2020, 97% (127/131) of adult patients at UVA owned an HS and by October 2020, 96% (24/25) of patients provided spirometry results during their telemedicine encounters.
Conclusion
Employing QI tools to standardise the process of monitoring spirometry data with home devices via telemedicine is reliable and sustainable and can be replicated across centres that provide care for patients with CF.
Keywords: Quality improvement, COVID-19, Failure Modes and Effects Analysis (FMEA)
Background
Cystic fibrosis (CF) is a systemic genetic disorder affecting approximately 30 000 people in the USA and at least 70 000 people worldwide.1 2 CF is caused by abnormal function of the CF transmembrane conductance regulator leading to increased mucus viscosity and multiple organ dysfunction: lung, pancreas and liver.3 Lung involvement is characterised by chronic infection and inflammation and results in progressive lung disease and premature death due to respiratory failure. Early identification of acute lung infections or CF exacerbation can restore lung function and improve lung function decline.4 CF Foundation (CFF) care guidelines recommend close monitoring of forced expiratory volume in 1 s (FEV1) in clinic every 3 months.5 Prior to the COVID-19 pandemic, the adult University of Virginia (UVA) CF team provided standardised CF care and used routinely quality improvement (QI) tools in improving patient outcomes. One of these QI projects was to adhere to the CFF guidelines for chronic care, which included providing quarterly, multidisciplinary visits in the CF clinic for monitoring early lung function decline and for identifying other comorbidities related to poor CF outcomes.5 In addition, the UVA QI team participates in CF Learning Network (CFLN), a network of CF accredited centres committed to collaboration, innovation, and partnering to improve patient outcomes. The Model for Improvement is the QI methodology employed by the CFLN.6 One of the core principles of CFLN, is coproduction of care. Coproduction of care involves close collaboration between the CF care team and the CF patients and empowers the patients to be active participants in their care. Patient utilisation of home spirometry, when access to home spirometers (HS), coaching, instructions for HSs utilisation during telemedicine visits and appropriate education regarding the use of FEV1 results in the treatment decision process, are available, is a perfect example of coproduction between the CF interdisciplinary team and the patient.
Aim
Global aim
We aimed to provide the same high-quality care via telemedicine as we provided with in person visits prior to the COVID-19 pandemic. This was defined by access to quarterly multidisciplinary visits which are co-produced and include pulmonary function testing (PFT) measurement. Coproduced visits are defined as visits in which the patient is involved in the visit plan by setting a visit agenda with the team.
Specific aims
We aimed to increase the percentage of patients with HSs from 37% to 85% by 31 December 2020.
We aimed to increase the percentage of eligible patients with spirometry results in telemedicine visits from 50% to 95% by 31 December 2020.
Methods
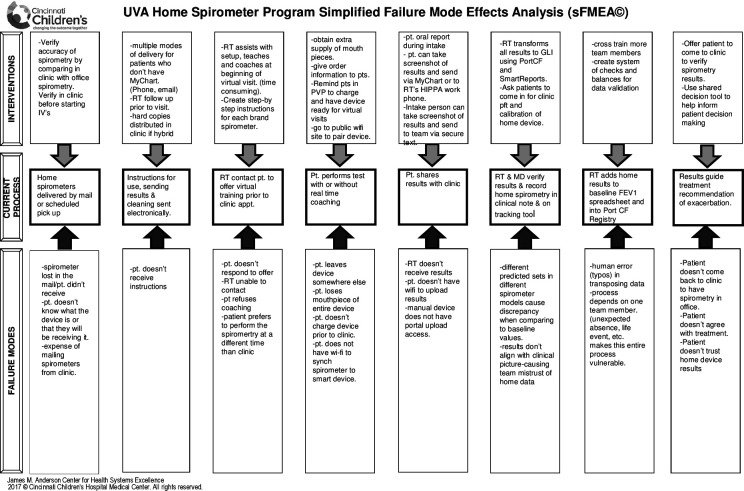
Following the model for improvement QI methodology, the UVA Adult CF programme developed a standardised process for monitoring FEV1 with HS during multidisciplinary telemedicine visits during the COVID-19 pandemic. A process map for distribution and use of HS (see online supplemental figure 1) and subsequently a simplified Failure Modes and Effects Analysis (figure 1) were created to identify possible barriers to HS utilisation.
Figure 1.
Simplified Failure Modes and Effects Analysis (sFMEA) for home spirometry utilisation in telemedicine clinic. The sFMEA was adapted from Cincinnati Children’s Hospital Medical Center. CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s; GLI, Global Lung Function Initiative; MD, medical doctor; HIPAA, Health Insurance Portability and Accountability Act; pt, patient; PVP, previsit planning; RT, respiratory therapist; UVA, University of Virginia.
bmjoq-2021-001529supp001.pdf (966KB, pdf)
Interventions
To achieve our global and specific aims multiple Plan Do Study Act (PDSA) cycles were created (online supplemental figure 2), the key driver diagram (KDD) (online supplemental figure 3) interventions were developed to test and standardise a reliable process for:
bmjoq-2021-001529supp002.pdf (124.2KB, pdf)
bmjoq-2021-001529supp003.pdf (1.1MB, pdf)
Providing spirometers to all eligible people with CF seen in the Adult CF clinic at UVA.
Monitoring FEV1 with each telemedicine visit conducted in the Adult CF clinic at UVA.
Telemedicine was defined as a clinic visit that occurred remotely and included a video and audio component. Hybrid clinic was defined as an in-person visit with a remote component. During a hybrid visit, the patient performed spirometry in clinic and some care team members visited with the patient remotely through a clinic provided tablet with video and audio capability.
Eligibility for HS
Adult patients seen in the outpatient UVA clinic were considered eligible for home spirometry if they owned an HS and/or a smart device compatible with the spirometer application as required by the manufacturer and could perform reliable spirometry at home independently or with respiratory therapist (RT) coaching.
Process
At the beginning of the COVID-19 pandemic, the adult UVA CF QI team met and discussed a strategy to transition in person visits to telemedicine to respect social distancing rules. A KDD ()was developed to focus the aims and track the improvement theories. In order to achieve the specific aim for spirometry, conditions needed to be created for patients to have access to HSs. With CFF centre grant support, HSs were purchased and distributed to all eligible patients.
The UVA adult CF team was in a uniquely favourable position to integrate home spirometry in the telemedince visits, having had significant experience with HSs utilisation prior to the COVID-19 pandemic. As part of a different project, 37% (49/131) of eligible patients had received an HS device along with coaching, and had proven the ability to perform spirometry at home with results that were comparable to those obtained in clinic. Coaching was provided in clinic: the RT directly observed and trained each eligible patient to perform spirometry with the provided HS, to obtain reproducible and reliable results.
In April 2020, our team acquired 70 additional HSs with reallocated CFF grant funding. In May 2020, the CF Foundation began distributing HSs to all eligible people with CF as identified by their CF Centres throughout the country.
Distribution of HSs
A process to distribute the devices was created, consisting of three options:
Drive by pick up: Patients were given date and time options for drive by pick up of spirometers.
Prepurchased shipping label and shipment to home: Patients were given the option to purchase a shipping label and send it to the care team through patient portal (Mychart) or email. Once the label was received, the device was shipped to their homes.
Pick up during a clinic visit.
Instructions for HS use
A standardised letter was sent to patients via MyChart or email that included instructions related to brand specific HS use, download of the HS application and guidance on frequency of use and disinfection. Real-time virtual coaching sessions with the RT were offered and scheduled per patient request.
Reminders
Most patients did not choose to participate in a separate coaching session, preferring to wait until their next clinic appointment (virtual or in person) for real time coaching with the RT. Many did not have the spirometry application downloaded or the device paired with their phone prior to their virtual appointment, which created time delays in telemedicine clinic visits. Part of a new PDSA ramp, to alleviate increasing in telemedicine clinic visit time, the RT started sending messages prior to patient appointments reminding the patients to download the HS app and pair the spirometer with the smart phone prior to the visit.
Coaching for HS use
RT provided coaching prior to visit, using an asynchronous telemedicine visit for patients who agreed or requested coaching outside of their clinic visit. In addition, most patients received remote coaching through spirometry during the telemedicine visit if they had not already obtained spirometry results on their own prior to the visit.
If the patient was seen in clinic in person, an HS was offered. If they agreed to receive the device, they also received the user instructions and RT coaching on use on site, plus the results were compared with the in person FEV1 measurements.
Monitoring
Home Spirometry was monitored in eligible patients with each telemedicine visit and results were used for clinical care decisions. Patients shared their spirometry results with the team through multiple methods: via MyChart, screen share during their telemedicine encounter or through the HS device portal. The results were recorded in the patients’ electronic medical record and in the CF Foundation Patient Registry (CFFPR).
Recommended utilisation of HSs with telemedicine visits
Day prior to or during telemedicine visits.
Team utilisation of FEV1 measurements
When available, FEV1 was recorded in the electronic medical record and CFFPR with each telemedicine visit.
FEV1 values were discussed with the patient.
If patient felt unable to perform a reliable spirometry at home independently, coaching was provided.
If FEV1 measurements remained discordant with previous measurements even with coaching, as compared with baseline or in clinic measurements, the patient was advised to have repeat measures in the PFT lab. If this was a recurrent problem with subsequent telemedicine visits the patient was considered ineligible for HS/telemedicine and the recommendation was made for the patient to follow up for in person PFTs with each visit.
If FEV1 measurements were decreased compared with baseline in the absence of symptoms of exacerbation, there was a discussion with the patient regarding follow-up; the patient was offered the option to repeat measurement in the PFT lab in approximately a week from telemedicine visit to confirm the decline and to discuss further assessment and plan.
If FEV1 measurements were decreased compared with baseline in the presence of symptoms of CF exacerbation, a treatment plan for exacerbation was agreed on with the patient and close follow-up was scheduled, typically in clinic for an in-person evaluation; more frequent HS was advised to monitor for FEV1 improvement or further decline.
Other issues identified that decreased the HS utilisation
Patients were missing mouthpieces and devices; some spirometers were not charged and some had poor Wi-Fi connection. Of note, several patients left their devices behind while travelling for the holidays which caused the December and January home spirometry numbers to decline slightly.
Multiple interventions were employed concomitantly by our team to increase HS utilisation with telemedicine visits, depending on patients’ personal challenges, including: patients with poor Wi-Fi started driving to a different location for better Wi-Fi connection, such as outside of local businesses, churches and schools; mouthpieces were acquired with grant support and provided to patients to replace those that were lost; and for patients who had issues with using a specific device, in-clinic/in-person follow-up with face-to-face RT coaching and comparison of FEV1 results with the PFT lab FEV1 results was recommended. If this intervention did not produce better results, we replaced the spirometer with a different brand. If the patient could not use the new device, he/she was considered ineligible for telemedicine and recommended for in person visits only, to ensure equitable quality of care in concordance with CFF care guidelines.
Measures
Primary process measures
Percentage of patients who own an HS.
Percentage of patients seen in the UVA adult telemedicine clinic who provided FEV1 measurements obtained on HS with the goal to achieve 95% within 10 months.
Results
At the beginning of the COVID-19 pandemic in 2020, 37% (49/131) of CF adult patients seen in the pulmonary clinic at UVA owned and used appropriately an HS and 50% (9/18) of telemedicine visits were associated with measurements of FEV1.
The first aim of this process was achieved early, as 97% (127/131) of adult CF patients followed at UVA owned an HS by September 2020.
The proportion of patients who provided FEV1 measurements with telemedicine visits each month was tracked. (figure 2).
Figure 2.
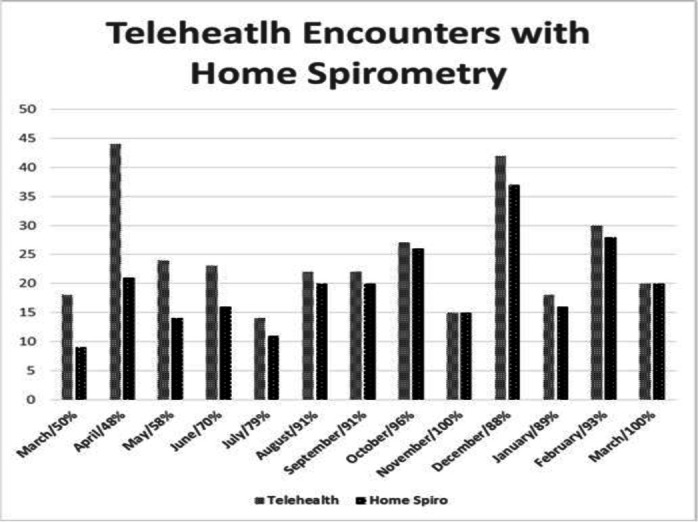
Monthly percentage of telemedicine encounters with forced expiratory volume in 1 s results obtained with home spirometers.
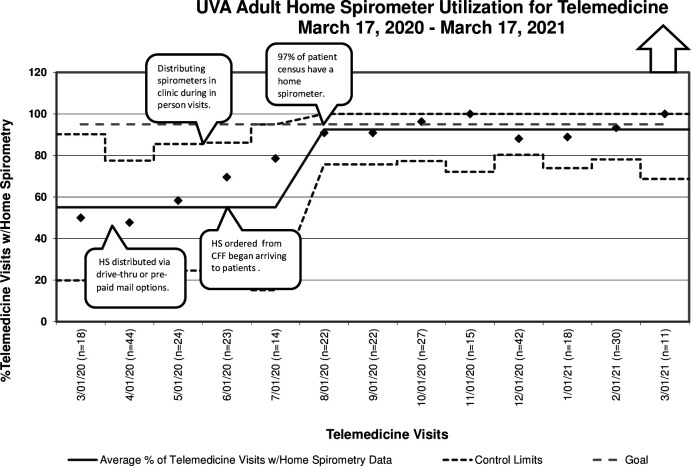
We achieved our second aim of monitoring FEV1 with home spirometry by October 2020; during October, 96% (24/25) of telemedicine visits conducted in the adult CF clinic at UVA were associated with FEV1 measurements. In addition, by October 2020, there was a data shift in HS utilisation and reliability of the data was achieved by March 2021 (figure 3), with more than 88% of telemedicine encounters being associated with a spirometry measurement.
Figure 3.
UVA adult programme spirometry utilisation for telemedicine March 2020 through March 2021. CFF, Cystic Fibrosis Foundation; HS, home spirometry; UVA, University of Virginia.
In December 2020 and January 2021, we noticed a slight decline in HS patient utilisation with telemedicine; this was due partially to patient travel and inability to transport devices or forgetting devices at home. For the months of February and March 2021, we noticed an increased uptake of HS use with telemedicine visits and in March 2021 100% (20/20) of patient seen in telemedicine visits provided FEV1 measurements.
Our process included a follow-up in person visit, or only in person visits for patients who either did not have an HS device, could not perform home spirometry, did not trust the HS and/or did not have symptoms compatible with the decline in FEV1 noticed on HS. We identified 11 patients who were not eligible for HS. In addition, five patients were asked to come to clinic for follow-up due to a decline in FEV1 in absence of symptoms, to confirm FEV1 indicated exacerbation signal, one of them was found to be in exacerbation and received treatment appropriately; two patients were found to be in exacerbation by symptoms and home spirometry results and were admitted to the hospital for intravenous antibiotic treatment without a subsequent clinic visit.
Reliability of FEV1 measurements with the HS was obtained for 46 patients, for 34 HSs provided to the patients prior to the pandemic and 12 HS provided during the COVID-19 pandemic. The mean difference between the FEV1 obtained with the HS when compared with in office spirometer, during the same clinic visit was 0.141 L. The patients received information regarding the FEV1 HS values compared with the in-office values. Most spirometers were either shipped or delivered via drive through during pandemic, to minimise staff–patient and patient–patient interaction, so direct comparison with the in-office spirometers could not be performed.
Discussion
Employing model for improvement QI methodology, the UVA adult CF team created a standardised process for providing HSs and monitoring FEV1 measurements with telemedicine visits. This process achieved reliability and sustainability and can be reproduced in other CF clinics, as well as other patient populations that require spirometry monitoring. Telemedicine has been proven to be a feasible method to provide outpatient care for patients with CF7 8; however, spirometry monitoring can be a prohibiting factor for telemedicine utilisation in this patient population as it is paramount for guiding CF care decisions.
Historically, FEV1 has been measured in the clinic setting. During the COVID-19 pandemic, multiple clinics employed telemedicine visits in lieu of in person care. In context of COVID-19 social distancing, long-distance travel to CF clinic, and prohibiting device and shipping cost, access to HSs during the pandemic was scarce. In addition, CF patient utilisation of HSs in general is variable: patients who feel well are less likely to reach out for free spirometers or perform spirometry outside of clinic visits, some patients prefer not to know what their FEV1 levels are, and performing spirometry at home requires training and coaching that can be time consuming. Patients who received the devices outside of clinic are likely to question the FEV1, as they had not previously correlated their HSs results to clinic/PFT lab spirometry. In some instances, there can be deviations in results related to variation in devices, technique and effort, or user or technology errors. Such deviations often impair patient trust in their device. In order to achieve reliable results, we created interventions that addressed these barriers and diligently worked with our CF patients to gain trust and proficiency in their HS devices. Emphasising the importance of FEV1 in clinical decisions is also paramount.
The increase in spirometry use with telemedicine visits was due to employment of multiple interventions (see the Process section) to overcome utilisation barriers: patient education on importance of FEV1 measurements for formulating a care plan, patient training and coaching on HS use and increased patient buy in regarding device utility. In addition, there was an increased uptake in telemedicine visits (figures 2 and 3) during the COVID-19 pandemic and some patients built trust in the devices using them more routinely with each telemedicine visit, as the pandemic progressed and the patients were seen multiple times through telemedicine. Other interventions employed: RT coaching during telemedicine visit, comparing HS results to previous HS results or in person results, comparing FEV1 results during subsequent trials. Some patients found the results more reliable with the RT observing their technique.
Our study was conducted by a proficient QI team, with expertise in telemedicine; in addition, the home spirometry process was led by a senior RT with extensive experience in CF care. In order for this process to be employed successfully at other centres, it is recommended that the team implementing the process of HS use is proficient in QI work and has an experienced RT to lead the process.
Our study is limited by the fact that it is a single-centre study. Multicentre implementation of this process could be employed to evaluate the reliability of the process and the results.
Telemedicine could be a very reliable form of CF care delivery for the future, not just in a pandemic, but for sick visits, for patients who live a distance from the CF care enter, to decrease lodging and transportation cost as well as work absenteeism, for infection control and patient convenience. In order to replace current CF care model that recommends in person, interdisciplinary care visits, four times a year, which include in office spirometry monitoring, blood tests, weight assessments and vital signs, future studies need to be employed to demonstrate non inferiority of the new care model that includes telemedicine visits based on patient outcomes. In this context, a reliable and sustainable process that ensures FEV1 monitoring with HSs is desirable.
Conclusion
Using QI tools to standardise the processes for providing access, training and monitoring of spirometry data with home devices via telemedicine is reliable and sustainable. Employing similar QI methods and tools, other CF care centres could adapt these processes to their context and obtain similar results.
Footnotes
Twitter: @LindsSomerville
Contributors: MC: primary author of original manuscript, primary analysis and interpretation of data, visualisation, project administration including methodology and investigation, final approval of manuscript. RL: project conceptualisation, project administration including methodology and investigation, acquisition of the data, primary analysis and interpretation of data, writing and revisions of intellectual content. ES: project conceptualisation, project administration including methodology, revisions for intellectual content, approval of final manuscript. LS: project conceptualisation, project administration including methodology, revisions for intellectual content, approval of final manuscript. LW: project conceptualisation, revisions for intellectual content, approval of final manuscript. RM: project conceptualisation, revisions for intellectual content, approval of final manuscript. DJ: project conceptualisation, revisions for intellectual content, approval of final manuscript. HB: project conceptualisation, revisions for intellectual content, approval of final manuscript. DA: principal investigator, primary conceptualisation, project administration including methodology, data acquisition and investigation, critical revision for intellectual content, final approval of manuscript.
Funding: Cystic Fibrosis Foundation Care Center Grant (CFF CC043-AD): supported salaries for our Quality Improvement coordinators and principal investigator. Cystic Fibrosis Learning Network Grant (CFLN SEID16AB0): supported salaries for our Quality Improvement coordinators. Vertex Circle of Care Grant (CG-2016-105531): supported spirometers for home use in our CF clinic for our patients prior to the pandemic.
Disclaimer: The funding organisations had no role in the design, implementation, interpretation, and reporting of these data.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethics approval statements that refer to your institution: Being a Quality Improvement Project no IRB approval is required.
References
- 1.Cystic fibrosis Foundation patient Registry report, 2018
- 2.Heltshe SL, Cogen J, Ramos KJ. Cystic fibrosis: the dawn of a new therapeutic era. Am J Respir Crit Care Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elborn JS. Cystic fibrosis. Lancet 2016;388:2519–31. 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- 4.Mogayzel PJ, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013;187:680–9. 10.1164/rccm.201207-1160oe [DOI] [PubMed] [Google Scholar]
- 5.Berlinski A, Chambers MJ, Willis L, et al. Redesigning care to meet national recommendation of four or more yearly clinic visits in patients with cystic fibrosis. BMJ Qual Saf 2014;23 Suppl 1:i42–9. 10.1136/bmjqs-2013-002345 [DOI] [PubMed] [Google Scholar]
- 6.Langley N, Norman P. The improvement guide: a practical approach to enhancing organizational performance. New York: Jossey-Bass Inc, 1996. [Google Scholar]
- 7.Compton M, Soper M, Reilly B, et al. A feasibility study of urgent implementation of cystic fibrosis multidisciplinary telemedicine clinic in the face of COVID-19 pandemic: single-center experience. Telemed J E Health 2020;26:978–84. 10.1089/tmj.2020.0091 [DOI] [PubMed] [Google Scholar]
- 8.List R, Compton M, Soper M, et al. Preserving multidisciplinary care model and patient safety during reopening of ambulatory cystic fibrosis clinic for Nonurgent care: a hybrid telehealth model. Telemed J E Health 2021;27:193–9. 10.1089/tmj.2020.0247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2021-001529supp001.pdf (966KB, pdf)
bmjoq-2021-001529supp002.pdf (124.2KB, pdf)
bmjoq-2021-001529supp003.pdf (1.1MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.