Abstract
Background
Acute pancreatitis is a common and potentially lethal gastrointestinal disease, but literatures for the disease burden are scarce for many countries. Understanding the current burden of acute pancreatitis and the different trends across various countries is essential for formulating effective preventive intervenes. We aimed to report the incidence, mortality, and disability-adjusted life-years (DALYs) caused by acute pancreatitis in 204 countries and territories between 1990 and 2019.
Methods
Estimates from the Global Burden of Disease Study 2019 (GBD 2019) were used to analyze the epidemiology of acute pancreatitis at the global, regional, and national levels. We also reported the correlation between development status and acute pancreatitis’ age-standardized DALY rates, and calculated DALYs attributable to alcohol etiology that had evidence of causation with acute pancreatitis. All of the estimates were shown as counts and age-standardized rates per 100,000 person-years.
Results
There were 2,814,972.3 (95% UI 2,414,361.3–3,293,591.8) incident cases of acute pancreatitis occurred in 2019 globally; 1,273,955.2 (1,098,304.6–1,478,594.1) in women and 1,541,017.1 (1,307,264.4–1,814,454.3) in men. The global age-standardized incidence rate declined from 37.9/100,000 to 34.8/100,000 during 1990–2019, an annual decrease of 8.4% (5.9–10.4%). In 2019, there were 115,053.2 (104,304.4–128,173.4) deaths and 3,641,105.7 (3,282,952.5–4,026,948.1) DALYs due to acute pancreatitis. The global age-standardized mortality rate decreased by 17.2% (6.6–27.1%) annually from 1.7/100,000 in 1990 to 1.4/100,000 in 2019; over the same period, the age-standardized DALY rate declined by 17.6% (7.8–27.0%) annually. There were substantial differences in the incidence, mortality and DALYs across regions. Alcohol etiology attributed to a sizable fraction of acute pancreatitis-related deaths, especially in the high and high-middle SDI regions.
Conclusion
Substantial variation existed in the burden of acute pancreatitis worldwide, and the overall burden remains high with aging population. Geographically targeted considerations are needed to tailor future intervenes to relieve the burden of acute pancreatitis in specific countries, especially for Eastern Europe.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-021-01906-2.
Keywords: Acute pancreatitis, Epidemiology, Disease prevention, Global burden of disease study 2019
Background
Acute pancreatitis is a common and potentially lethal gastrointestinal disease. Previous studies have shown a variable incidence rate, ranging from 15.0 per 100,000 in Denmark [1] to 83.7 per 100,000 in Sweden [2]. In the USA, it accounts for about 275,000 hospital admissions and $2.5 billion health care costs each year [3]. Approximately 20% of patients develop moderate to severe acute pancreatitis, which lead to a mortality rate of 20–40% [4–6].
The global incidence of acute pancreatitis cited in previous publications was presented as a wide range of estimates, mainly because they were based on heterogeneous study populations and varying methodological quality. In a meta-analysis, Xiao et al [7] pooled the data from general population-based cohort studies, reported that the incidence and mortality rates of acute pancreatitis were 33.7 cases and 1.6 deaths per 100,000-person years. However, the original studies included in this systematic review were confined to only five countries or territories (Sweden, Denmark, Taiwan, USA and United Kingdom), all of which were high-income regions and may not be representative of the global population. In addition, the reported incidence and mortality rates were not age-standardized, which might skew the comparisons between different regions.
Global efforts using appropriate preventive and treatment approaches to reduce the morbidity and mortality of acute pancreatitis require timely information about the burden and their risk factors. However, the current analyses on the epidemiology of acute pancreatitis were based exclusively on limited local data [1, 8–15], which inevitably subjected to selection bias and could not describe the disease burden around the globe in a robust manner. The Global Burden of Disease study (GBD), with its broad collection of data sources and the state-of-the-art statistical modelling approaches [16–18] provides us a unique opportunity to deliver the most comprehensive estimates of acute pancreatitis’ burden to date. Since GBD 2017 [19], no comprehensive update of epidemiological levels and trends on acute pancreatitis have been released. In prior GBD analysis conducted by Ouyang et al. [20], the acute and chronic pancreatitis were modelled together; but in this study, we aimed to analyze acute pancreatitis separately based on GBD 2019. In this study, we summarized GBD 2019 findings on acute pancreatitis’ epidemiology in 204 countries and territories, presented the temporal and geographical trends in terms of incidence, mortality, disability-adjusted life-years (DALYs), and their age-standardized rates by sex and location during 1990–2019.
Methods
Data acquisition
Acute pancreatitis in GBD 2019 were identified according to the ICD-10 code K85 and ICD-9 code 577.0. For this study, we used GBD 2019 vital registration (19,618 site-years of data) and verbal autopsy (374 site-years) data sources that provided a representative partial or complete sample of incidence or mortality. Information about the data sources used for each location in this study can be found on the GBD 2019 Data Input Sources Tool website (http://ghdx.healthdata.org/gbd-2019/data-input-sources). The GBD 2019 database contains the statistical data of 369 diseases and 87 risk factors in 204 countries and territories [21, 22]. The data of acute pancreatitis in GBD 2019 including incidence, mortality, DALYs, and corresponding age-standardized rates by sex and location for each year from 1990 through 2019 were obtained publicly from the Global Health Data Exchange (GHDx) website (http://ghdx.healthdata.org/gbd-results-tool). More details on the case definition, input data, data processing, and modeling strategy, as well as the differences between GBD 2019 and GBD 2017 on acute pancreatitis are provided in Additional file 1.
Socio-demographic index (SDI)
The SDI for all the 204 countries and territories during 1990–2019 was downloaded from the GHDx website for the following correlation analysis. SDI is a compound indicator of development status, created according to a country’s total fertility rate for females younger than 25 years, mean education for those aged 15 years and older, and lag-distributed income per capita. Countries and territories were ranked into high, high-middle, middle, low-middle, and low SDI categories. The methods for SDI generation are detailed in previous GBD publications [23, 24].
Statistical analysis
The standardized methods of the GBD 2019 have been extensively reported [22]. Three main standardized tools—Cause of Death Ensemble model (CODEm), DisMod-MR 2.1 and spatiotemporal Gaussian process regression (ST-GPR)—were used to generate estimates for each quantity of interest by age, sex, location, and year. Briefly, CODEm was used to analyze death-related data. DisMod-MR 2.1 based on Bayesian meta-regression was applied to evaluate all available data on incidence and DALY. The expected relationship between SDI and age-standardized incidence, mortality and DALY rates were determined by fitting a ST-GPR for all locations from 1990 to 2019 [22]. The 95% uncertainty intervals (UIs) were determined for each parameter using the 25th and 975th of the 1000 ordered draws based on the posterior distribution.
The annual absolute number of incident cases, deaths, DALYs, and corresponding age-standardized rates were applied to delineate the burden of acute pancreatitis at the global, regional, and national levels. The age-standardized rates could exclude the impact from imbalance in population quantity and age distribution. DALYs was the summation of the years lived with disability (YLDs) and the years of life lost (YLLs). Moreover, the corresponding estimated annual percentage change (EAPC) values of age-standardized incidence/mortality rate (ASIR/ASMR) and age-standardized DALY rate per 100,000 people were employed to reflect the spatiotemporal trends of acute pancreatitis’ burden. In the formula Y = α + βX, Y refers to lg (age-standardized rate) while X means the calendar year. Then EAPC values were calculated by the formula EAPC = 100* (10β—1). In the case that both EAPC value and its 95% UI above zero, the corresponding age-standardized rate was in an upward trend and vice versa. Lastly, we searched the GBD 2019 database for potential risk factors that contributing to acute pancreatitis-related fatality. This study is compliant with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) [25].
Results
Incidence of acute pancreatitis
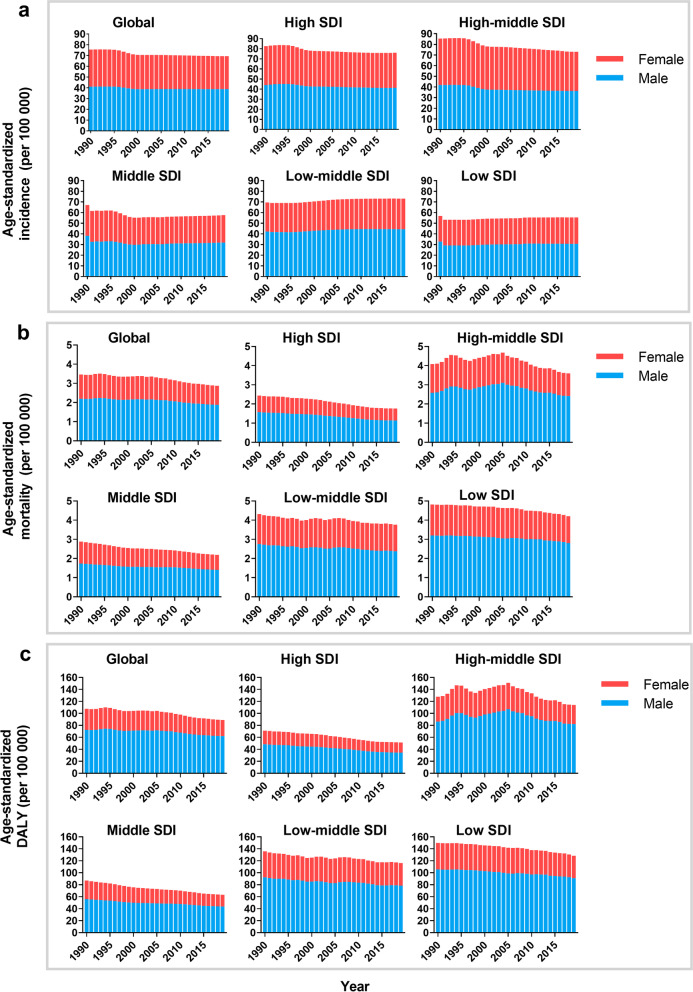
There were 1,727,789.3 (95% UI 1,452,132.4–2,059,695.3) acute pancreatitis occurred in 1990 and 2,814,972.3 (95% UI 2,414,361.3–3,293,591.8) occurred in 2019 in the globe, with an increase of 62.9% (Table 1). However, the ASIR declined by an average of 8.4% (95% UI 5.9–10.4%) annually during the same period, decreased from 37.9 to 34.8 per 100,000. Males were more likely to suffer from acute pancreatitis than females (41.0 vs 34.5 per 100,000 in 1990, and 38.8 vs 30.6 per 100,000 in 2019) (Table 1, Fig. 1a).
Table 1.
The incidence of acute pancreatitis in 1990/2019 and temporal trends
| Location | Incident cases (95% UI) | Age-standardized incidence rate (per 100,000) | EAPC of incidence rate | ||
|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | 1990–2019 (%) | |
| Global | 1,727,789.3 (1,452,132.4–2,059,695.3) | 2,814,972.3 (2,414,361.3–3,293,591.8) | 37.9 (32–44.6) | 34.8 (29.8–40.7) | – 8.4 (– 10.4 to – 5.9) |
| Sex | |||||
| Male | 929,028.7 (774,587.6–1,115,575.6) | 1,541,017.1 (1,307,264.4–1,814,454.3) | 41.0 (34.3–48.5) | 38.8 (33.1–45.5) | – 5.4 (– 7.5 to – 2.8) |
| Female | 798,760.6 (677,669.1–943,983.6) | 1,273,955.2 (1,098,304.6–1,478,594.1) | 34.5 (29.2–40.3) | 30.6 (26.4–35.6) | – 11.2 (– 13.2 to – 8.8) |
| SDI factor | |||||
| High SDI | 390,419.1 (335,676.7–452,156.8) | 533,633.2 (477,729.8–597,075.3) | 41.3 (35.4–48.0) | 38.1 (33.9–42.7) | – 7.9 (– 11.8 to – 3.1) |
| High-middle SDI | 482,857.1 (406,521.4–568,658.8) | 667,222.6 (569,618.5–769,408.6) | 43.2 (36.6–50.6) | 36.7 (31.5–42.6) | – 15.0 (– 16.6 to – 13.1) |
| Middle SDI | 455,054.8 (373,090.6–554,469.3) | 735,082.3 (616,164.7–867,454.5) | 33.7 (27.8–40.1) | 28.8 (24.4–34.2) | – 14.3 (– 16.7 to – 11.4) |
| Low-middle SDI | 297,240.1 (244,736.5–361,676.3) | 598,531.4 (497,030.5–720,521.9) | 34.9 (29.0–41.8) | 36.5 (30.7–43.7) | 4.6 (3.0–6.4) |
| Low SDI | 101,572 (83,136.7–123,390.1) | 224,224.5 (183,906.9–270,951.5) | 28.4 (23.8–33.7) | 27.7 (23.4–32.9) | – 2.4 (– 3.5 to – 1.3) |
| Region | |||||
| Andean Latin America | 12,751.6 (10,888.9–14,964.7) | 26,446.1 (23,242.3–30,101.7) | 45.8 (39.6–52.9) | 43.5 (38.4–49.3) | – 5.0 (– 7.3 to – 2.6) |
| Australasia | 8618.4(7250.0–10,167.5) | 14,394.8 (12,298.7–16,770.4) | 38.6 (32.5–45.3) | 37.6 (31.7–44) | – 2.8 (– 5.2 to – 0.3) |
| Caribbean | 8807.7 (7294.7–10,668.9) | 14,155.4 (11,772.9–16,692.7) | 28.8 (23.9–34.3) | 28.4 (23.5–33.6) | – 1.7 (– 2.9 to – 0.4) |
| Central Asia | 18,365.6 (15,467.5–21,700.0) | 29,035.3 (24,076.9–34,326.1) | 33.7 (28.4–39.4) | 32.9 (27.7–38.5) | – 2.4 (– 3.4 to – 1.1) |
| Central Europe | 67,402.6 (57,280.6–78,292.3) | 73,015.8 (64,542.3–82,156.6) | 49.4 (42–57.3) | 45.2 (40.1–50.9) | – 8.6 (– 12.5 to – 3.2) |
| Central Latin America | 46,647.8 (39,437.0–55,591.9) | 96,777.3 (83,302.4–112,767.0) | 37.6 (32.1–44) | 38.6 (33.3–44.7) | 2.6 (1.0–4.4) |
| Central Sub-Saharan Africa | 7427.2 (6052.5–9165.5) | 18,256.3 (14,929.3–22,374.1) | 21.1 (17.6–25) | 20.8 (17.5–24.7) | – 1.0 (– 2.9 to – 0.9) |
| East Asia | 396,687.7 (322,719.1–482,300.5) | 526,066.5 (444,786.6–615,193.3) | 38 (31.1–45.4) | 27.6 (23.5–32.2) | – 27.4 (– 30.4 to – 23.8) |
| Eastern Europe | 182,577.6 (155,056.7–211,692.2) | 221,945.2 (188,142.5–258,013.8) | 71.2 (60.8–82.9) | 79.6 (68.2–92.5) | 11.7 (10.8–12.7) |
| Eastern Sub-Saharan Africa | 25,232.9 (20,531.3–31,140.5) | 57,965.7 (46,838.7–71,275.5) | 21.1 (17.7–25.2) | 21.1 (17.7–25.2) | 0.1 (– 0.6 to – 0.7) |
| High-income Asia Pacific | 62,652.3 (52,382.8–74,360.9) | 78,997.7 (68,905.1–90,260.6) | 32.9 (27.6–39.1) | 31.5 (27.3–36.5) | – 4.3 (– 9.0 to – 1.3) |
| High-income North America | 200,836.4 (173,238.5–232,436.8) | 257,777.8 (236,252.7–284,187.5) | 62.4 (53.7–72.0) | 52.0 (47.5–56.9) | – 16.6 (– 22.3 to – 9.9) |
| North Africa and Middle East | 61,824.3 (50,782.1–75,020.1) | 140,637.9 (117,038.5–168,090.2) | 26.7 (22.3–31.5) | 26.6 (22.5–31.2) | – 0.2 (– 1.7 to – 1.6) |
| Oceania | 1078.6 (884.9–1322.0) | 2385.6 (1934.6–2925.5) | 24.9 (20.7–29.6) | 24.1 (20–28.7) | – 3.0 (– 5.0 to – 0.8) |
| South Asia | 328,618.8 (267,223.4–401,086.7) | 743,524.0 (611,176.0–901,906.4) | 38.1 (31.6–45.9) | 43.0 (35.9–51.7) | 12.8 (10.9–14.9) |
| Southeast Asia | 93,540.7 (76,473.3–113,689.3) | 174,246.5 (143,846.9–208,675.3) | 26.0 (21.6–30.9) | 25.3 (21.2–30.1) | – 2.6 (– 3.9 to – 1.5) |
| Southern Latin America | 15,849.3 (13,664.8–18,265.2) | 24,167.2 (20,962.5–27,835.3) | 33.6 (28.9–38.6) | 31.6 (27.4–36.6) | – 5.8 (– 7.9 to – 3.9) |
| Southern Sub-Saharan Africa | 8604.9 (7042.0–10,531.2) | 15,207.3 (12,560.8–18,528.5) | 22.1 (18.5–26.4) | 21.7 (18.1–25.9) | – 5.8 (– 7.9 to – 3.9) |
| Tropical Latin America | 23,704.1 (20,682.2–27,345.5) | 47,509.1 (41,862.2–53,770.0) | 20.1 (17.7–22.7) | 19.4 (17.1–21.9) | – 3.4 (– 4.7 to – 2.0) |
| Western Europe | 123,270.9 (107,562.6–140,276.4) | 170,344.2 (149,047.3–194,756.8) | 25.4 (22.1–29.2) | 26.3 (22.8–30.1) | 3.5 (1.5–5.3) |
| Western Sub-Saharan Africa | 33,289.8 (27,664.2–40,190.6) | 82,116.6 (68,165.6–98,901.6) | 26.0 (22.0–30.6) | 26.7 (22.7–31.4) | 3.0 (2.5–3.5) |
EAPC, estimated annual percentage change; UI, uncertainty interval
Fig. 1.
The change trends of age-standardized acute pancreatitis’ incidence (a), mortality (b), and DALY rates (c) per 100,000 person-years from 1990 to 2019. DALY: disability-adjusted life-year; SDI: social demographic index
In the SDI region level, the two highest quintiles of SDI regions had the highest acute pancreatitis burden in 2019, with ASIR of 38.1 and 36.7 per 100,000 respectively (Table 1). Subgroup analysis by geographical zone showed that Eastern Europe and High-income North America had the highest ASIR in both 1990 and 2019 (Eastern Europe: 71.2 in 1990 and 79.6 in 2019 per 100,000; High-income North America: 62.4 in 1990 and 52.0 in 2019 per 100,000). Most of the regions (13/21, 61.9%) observed a steadily annual decrease in ASIR during the last 30 years. However, South Asia and Eastern Europe showed a mushrooming rise in ASIR (EAPC of South Asia: 12.8%, 95% UI 10.9–14.9%; EAPC of Eastern Europe: 11.7%, 95% UI 10.8–12.7%) (Table 1).
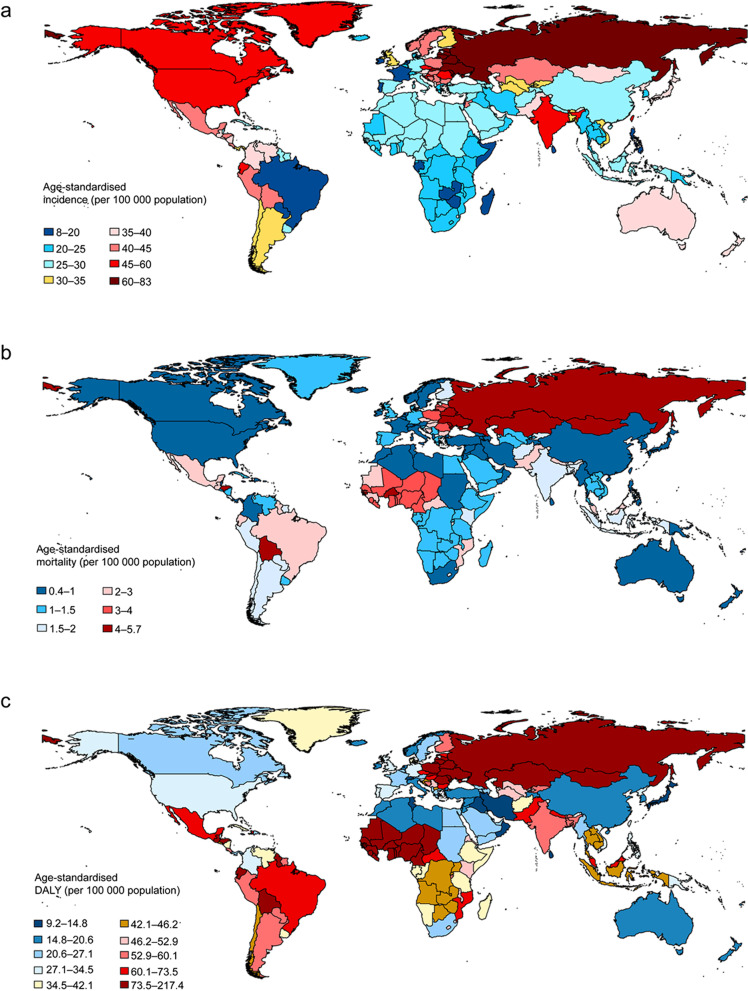
In 2019, countries with the greatest number of incident cases of acute pancreatitis were India (618,862.3), China (493,765.4), and USA (228,699.2) (Additional file 1: Table S1). The highest ASIR (more than 60 cases per 100,000 population) were observed in the Russia (82.0/100,000), Ukraine (77.0/100,000), Republic of Moldova (71.3/100,000), Belarus (69.7/100,000), Slovakia (68.4/100,000), Lithuania (64.8/100,000), Estonia (62.8/100,000) and Latvia (61.7/100,000) (Fig. 2a, Additional file 1: Table S1).
Fig. 2.
The age-standardized incidence (a), mortality (b) and DALY rates (c) of acute pancreatitis per 100,000 person-years by location for both sexes combined, 2019. DALY: disability-adjusted life-year
Mortality due to acute pancreatitis
Globally, there were 69,817.6 (95% UI 62,046.7–82,529.3) deaths in 1990 and 115,053.2 (95% UI 104,304.4–128,173.4) deaths in 2019 caused by acute pancreatitis, increasing by 64.8% (95% UI 55.3–68.1%). However, the global ASMR decreased from 1.7 deaths per 100,000 (95% UI 1.5–2.0) in 1990 to 1.4 (95% UI 1.3–1.6) deaths per 100,000 in 2019, with EAPC of − 17.2% (95% UI − 27.1% to − 6.6%) (Table 2). The ASMR in males was much higher than that in females (2.2 vs 1.3 per 100,000 in 1990; 1.9 vs 1.0 per 100,000 in 2019) (Fig. 1 b).
Table 2.
The death of acute pancreatitis in 1990/2019 and temporal trends
| Death cases (95% UI) | Age-standard mortality rate (per 100,000) | EAPC of mortality rate | |||
|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | 1990–2019 (%) | |
| Global | 69,817.6 (62,046.7–82,529.3) | 115,053.2 (104,304.4–128,173.4) | 1.7 (1.5–2.0) | 1.4 (1.3–1.6) | − 17.2 (− 27.1 to − 6.6) |
| Sex | |||||
| Male | 43,129.0 (37,615.8–51,561.2) | 71,983.2 (63,882.3–81,418.6) | 2.2 (1.9–2.6) | 1.9 (1.7–2.1) | − 14.5 (− 24.4 to − 3.1) |
| Female | 26,688.6(23,528.9–32,952.8) | 43,070 (36,592.6–50,773.7) | 1.3 (1.1–1.6) | 1.0 (0.8–1.2) | − 21.5 (− 36.2 to − 8.3) |
| SDI factor | |||||
| High SDI | 12,159.0 (11,441.0–13,388.5) | 16,160.0 (14,600.9–18,476.3) | 1.2 (1.1–1.3) | 0.9 (0.8–1.0) | − 26.9 (− 30.9 to − 21.5) |
| High-middle SDI | 21,366.4 (19,824.1–25,093.1) | 34,393.0 (31,194.4–37,307.4) | 2.0 (1.9–2.4) | 1.8 (1.6–1.9) | − 11.7(− 27.4 to − 3.0) |
| Middle SDI | 15,561.3 (13,213.7–20,288.1) | 25,776.2 (22,835.9–30,111.2) | 1.4 (1.2–1.9) | 1.1 (1.0–1.3) | − 24.7 (− 39.6 to − 7.6) |
| Low-middle SDI | 14,314.8 (11,855.7–18,507.1) | 26,440.8 (21,686.3–30,710.8) | 2.2 (1.8–2.8) | 1.9 (1.5–2.2) | − 13.7 (− 31.8 to − 13.1) |
| Low SDI | 6385.7 (4653.4–8612.9) | 12,232.2 (9722.6–15,560.5) | 2.4 (1.7–3.3) | 2.1 (1.7–2.7) | − 13.5 (− 27.8 to − 4.8) |
| Region | |||||
| Andean Latin America | 1076.8 (811.2–1299.4) | 1444.9 (1122.3–1971.2) | 4.6(3.4–5.6) | 2.5 (2.0–3.5) | − 44.2 (− 59.6 to − 15.1) |
| Australasia | 215.9 (198.6–233.7) | 339 (292.5–396.5) | 0.9 (0.9–1) | 0.7 (0.6–0.8) | − 29.7 (− 37.7 to − 17.5) |
| Caribbean | 384.3 (338.7–436.9) | 659.0(557.6–795.7) | 1.4 (1.3–1.6) | 1.3 (1.1–1.6) | − 9.6 (− 24.2 to 7.0) |
| Central Asia | 1179.1 (1016.3–1321.5) | 1653.3 (1381.5–1884.1) | 2.4 (2.1–2.8) | 2.1 (1.8–2.4) | − 12.1 (− 24.3 to 1.7) |
| Central Europe | 4476.7 (4274.1–5033.9) | 5140.4 (4513.4–5828.0) | 3.2 (3.1–3.6) | 2.7 (2.4–3.1) | − 15.6 (− 27.2 to − 4.2) |
| Central Latin America | 2018.5 (1909.5–2130.6) | 4332.6 (3728.8–5065.2) | 2.1 (1.9–2.2) | 1.8 (1.6–2.1) | − 12.4 (− 24.8 to 2.2) |
| Central Sub-Saharan Africa | 452.4 (302.2–763.6) | 911.5 (530.4–1648.7) | 1.8 (1.2–3.1) | 1.4 (0.8–2.7) | − 19.7 (v43 to 4.6) |
| East Asia | 9323.9 (7503.7–12,418.1) | 11,289.8 (8798.1–13,450.7) | 1.1 (0.9–1.5) | 0.6 (0.5–0.7) | − 44.3 (− 60.6 to − 26.3) |
| Eastern Europe | 7662.1 (6921.7–10,789) | 15,578.4 (13,366.9–17,734.7) | 2.9 (2.6–4.1) | 5.3 (4.5–6) | 83.2(20.1–115.4) |
| Eastern Sub-Saharan Africa | 1398.2(899.1–2104.9) | 2769.3 (1710.4–4739.2) | 1.7 (1.0–2.6) | 1.5 (0.9–2.7) | − 12.1 (− 34.6 to 9.5) |
| High-income Asia Pacific | 1792.4 (1602.4–2057.1) | 2248.0 (1886.8–2803.8) | 1.0 (0.9–1.1) | 0.5 (0.4–0.6) | − 49.5 (− 55.7 to − 39.5) |
| High-income North America | 3348.2 (3123.7–3579.4) | 5443.7 (4993.9–5955.3) | 1.0 (0.9–1) | 0.9 (0.9–1.0) | − 5.6 (− 9.6 to 1.3) |
| North Africa and Middle East | 1718.7 (1389–2350.2) | 3394.9 (2654.5–4069.2) | 1.1 (0.9–1.5) | 0.9 (0.7.0–1.1) | − 21.2 (− 42.6 to 2.9) |
| Oceania | 36.8 (25.7–52.2) | 76.7 (53.7–107.9) | 1.1 (0.7–1.5) | 0.9 (0.7–1.3) | − 11.9 (− 32 to 11) |
| South Asia | 14,050.3 (11,344.3–19,215) | 25,936.8 (20,085.4–31,351.7) | 2.3 (1.8–3.1) | 1.8 (1.4–2.2) | − 20.2 (− 42.4 to 11.9) |
| Southeast Asia | 4993.4 (3842.9–7457.8) | 7913.5 (6540.7–11,170.8) | 1.8 (1.4–2.6) | 1.3 (1.1–1.8) | − 25.1 (− 40.9 to − 2.5) |
| Southern Latin America | 1267.6 (1136.0–1374.1) | 1495.7 (1347.0–1732.5) | 2.8 (2.5–3.0) | 1.8 (1.7–2.1) | − 33.8 (− 42.5 to − 17.9) |
| Southern Sub-Saharan Africa | 325.4 (263.3–414.7) | 573.9 (453–675.4) | 1.0 (0.8–1.3) | 0.9 (0.7–1.1) | − 7.8 (− 33.5 to 10.6) |
| Tropical Latin America | 2329.6(2212.2–2485.9) | 5557.4 (4793.8–5987.2) | 2.3 (2.1–2.4) | 2.3 (2.0–2.5) | 1.0 (− 15.1 to 12) |
| Western Europe | 7986.7 (7447.9–8873.5) | 9984.5 (8925.7–11,455.5) | 1.4 (1.4–1.6) | 1.1 (1.0–1.2) | − 25.7 (− 30.9 to − 19.4) |
| Western Sub-Saharan Africa | 3781.0 (2498.4–5769.9) | 8310.1 (5927.1–11,883.0) | 3.8 (2.5–5.8) | 3.6 (2.7–5.1) | − 3.9 (− 30.4 to 28.5) |
EAPC, estimated annual percentage change; UI, uncertainty interval
Subgroup analysis by SDI indicated that the high-middle SDI region had the most deaths in both 1990 (21,366.4) and 2019 (34,393.0). As for a specific geographical zone, South Asia, Eastern Europe and East Asia were the top 3 regions with the most acute pancreatitis-related deaths in 2019 (Table 2). India, Russia and China were the top 3 countries that had the most deaths in 2019, with 20,455.9, 11,615.3 and 10,663.6 deaths respectively (Additional file 1: Table S1). Russia also had the highest ASMR in 2019, with 5.7 (95% UI 4.8–6.7) deaths per 100,000. Even though had a relatively large number of death cases, China had a very low ASMR in 2019, with 0.6 deaths per 100,000 and ranking eleventh from the bottom (Fig. 2b). The top five countries with the highest ASMR in 2019 were Russia (5.7/100,000), Kazakhstan (5.0/100,000), Guinea-Bissau (4.8/100,000), Ukraine (4.7/100,000), and Burkina Faso (4.6/100,000) (Additional file 1: Table S1).
Summary measures of health by DALYs
The number of DALYs increased from 2,437,815.7 in 1990 to 3,641,105.7 in 2019 in the globe, but the age-standardized DALY rate improved from 59.3 in 1990 to 44.4 in 2019 per 100,000, with EAPC of − 17.6% (95% UI − 27.0% to − 7.8%) (Table 3). Males were the main contributor to the age-standardized DALY rate compared with females (Fig. 1c). Subgroup analysis by socio-demographic factor demonstrated that although the high-middle SDI region had the most DALYs from 1990 to 2019 (720,516.0 in 1990 and 1,057,814.6 in 2019), the age-standardized DALY rate was highest in the low SDI region.
Table 3.
The DALYs of acute pancreatitis in 1990/2019 and temporal trends
| DALYs (95% UI) | Age-standardized DALY rate (per 100,000) | EAPC of DALY | |||
|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | 1990–2019 (%) | |
| Global | 2,437,815.7 (2,179,992.9–2,885,021.2) | 3,641,105.7 (3,282,952.5–4,026,948.1) | 53.9 (48.2–63.3) | 44.4 (40.1–49.1) | − 17.6 (− 27.0 to − 7.8) |
| Male | 1,633,012.8 (1,426,941.6–1,942,851.5) | 2,502,438.2 (2,224,864.1–842,383.8) | 72.4 (63.4–85.9) | 62.0 (55.2–70.4) | − 14.4 (− 23.8 to − 3.4) |
| Female | 804,802.9 (691,735.0–1,038,584.8) | 1,138,667.5 (969,571.1–1,334,422.8) | 35.2 (30.6–44.8) | 27.0 (22.9–31.5) | − 23.5 (− 38.2 to − 11.9) |
| SDI factor | |||||
| High SDI | 341,420.8 (321,211.0–370,387.3) | 389,932.5 (360,177.7–429,354.3) | 35.3 (33.2–38.3) | 25.7 (23.8–28.4) | − 27.1 (− 31.8 to − 22.7) |
| High-middle SDI | 720,516.0 (667,787.2–839,145.4) | 1,057,814.6 (962,196.3–1,156,821.3) | 63.7 (59.1–74.2) | 57.1 (51.9–62.4) | − 10.5 (− 24.7 to − 1.7) |
| Middle SDI | 580,739.7 (498,484.7–739,252.5) | 813,217.3 (727,039.6–960,672.8) | 43.7 (37.3–56.4) | 31.6 (28.2–37.2) | − 27.8 (− 40.3 to − 14.2) |
| Low-middle SDI | 549,795.0(462,013.9–726,880.2) | 919,232.5 (751,488.8–1,066,251.6) | 68.4 (57.0–88.3) | 58.0(47.3–66.8) | − 15.3 (− 32.5 to 8.6) |
| Low SDI | 244,325.0 (185,104.9–330,618.6) | 459,380.2 (367,333.8–583,539.5) | 75.1 (55.2–00.8) | 64.0 (50.8–81.3) | − 14.9 (− 29.9 to 3.3) |
| Region | |||||
| Andean Latin America | 41,681.2 (49,404.7–30,752.3) | 43,654.9 (34,042.4–57,110.3) | 144.8 (108.3–173.2) | 72.5 (56.6–96.0) | − 49.9 (− 63.0 to − 25.6) |
| Australasia | 5562.6 (6030.6–5108.7) | 7137.4 (6312.5–8100.2) | 24.4 (22.4–26.5) | 16.6 (14.7–18.8) | − 32.2 (− 39.4.2 to − 21.6) |
| Caribbean | 13,236.4 (15,634.5–11,659.6) | 20,254.6 (16,880.2–24,620.4) | 44.5 (39.3–51.6) | 40.1 (33.3–48.7) | − 9.9 (− 23.8.0 to 5.7) |
| Central Asia | 41,215.8 (45,305.5–36,642.2) | 60,311.2 (51,431.9–69,088.5) | 77.1 (68.3–85.3) | 67.4 (57–76.9) | − 12.5 (− 24.4 to 1.0) |
| Central Europe | 147,739.5 (161,428.9–141,054.8) | 140,578.0 (123,451.4–158,896) | 106.0 (101.2–115.9) | 84.4 (74–95.6) | − 20.4 (− 30.9.0 to − 9.5) |
| Central Latin America | 80,017.9 (84,085.9–74,360) | 141,733.4 (121,885.5–165,140.4) | 67.0 (63.3–70.8) | 56.5 (48.7–65.7) | − 15.7 (− 27.4.0 to − 0.9) |
| Central Sub-Saharan Africa | 17,413.0 (29,045.2–11,986.3) | 35,823.5 (21,454.6–62,656.8) | 54.4 (36.7–90.9) | 44.6 (26.5–80.0) | − 18.0 (− 42.1.1 to 10.6) |
| East Asia | 333,276.6(431,225.3–266,027.9) | 319,973 (255,865.5–381,413.7) | 31.7 (25.7–41.5) | 16.5 (13.2–19.6) | − 47.9 (− 62.1.3 to − 32.1) |
| Eastern Europe | 283,386.3 (383,366.6–254,907.8) | 558,129.2 (481,178.6–640,436.3) | 109.2 (98.2–146.8) | 206.7 (178.3–237.9) | − 89.2 (− 29.5.2 to 120.3) |
| Eastern Sub-Saharan Africa | 51,548.1 (78,409.9–34,736.6) | 103,187.0 (65,327.8–170,638.8) | 49.6 (32.1–74.6) | 43.3 (27.1–73.7) | − 12.7 (− 34.4.1 to 14.2) |
| High-income Asia Pacific | 54,433.0 (66,219.4–47,665.2) | 48,064.3 (41,966.4–56,862.1) | 27.6 (24.1–33.4) | 14.7 (12.7–17.4) | − 46.6 (− 57.3.3 to − 37) |
| High-income North America | 100,034.4 (109,713.5–92,703.1) | 145,495.4 (134,413.4–161,035.3) | 30.8(28.5–33.9) | 28.6 (26.4–31.6) | − 7.4 (− 10.7.0 to − 1.9) |
| North Africa and Middle East | 50,486.0 (69,094.5–42,546.5) | 91,833.5 (74,033.1–111,078.4) | 25.8 (21.5–35.0) | 19.5 (15.7–23.3) | − 24.6 (− 41.2.0 to − 4.6) |
| Oceania | 1540.1 (2186.2–1100.1) | 3181.1 (2194.1–4459.1) | 33.9 (23.9–47.8) | 30.3 (21.4–42.0) | − 10.7 (− 32.7.1 to 16.6) |
| South Asia | 548,424.8 (759,731.1–448,431.7) | 909,993.5 (707,700.0–1,093,715.6) | 69.9 (56.5–95.3) | 55.3 (43.1–66.4) | − 20.9 (− 43.6.0 to 8.2) |
| Southeast Asia | 187,984.2 (286,963–145,388) | 254,592.2 (204,677.0–376,978.7) | 54.5 (42.3–81.1) | 37.9 (30.8–54.8) | − 30.5 (− 43.6.1 to − 12.7) |
| Southern Latin America | 37,055.6 (40,031.4–33,771.4) | 40,292.4 (36,539.8–45,858.7) | 78.6 (71.7–85.0) | 51.6 (46.8–58.8) | − 34.3 (− 42.4.2 to − 21.1) |
| Southern Sub-Saharan Africa | 12,983.4 (16,365.4–10,471.3) | 21,220.6 (16,850.1–25,384.5) | 34.6 (28.1–43.7) | 30.0 (23.8–35.7) | − 13.3 (− 38.3.0 to 5.8) |
| Tropical Latin America | 90,244.6 (95,308.1–85,172.8) | 175,860.2 (155,755.4–189,220.5) | 75.4 (71.0–79.9) | 70.6 (62.5–76.0) | − 6.3 (− 17.3.0 to 2.0) |
| Western Europe | 196,013.5(212,945.9–184,807.6) | 199,700.1 (183,864.2–224,326.9) | 38.8 (36.6–41.9) | 27.1 (25.2–30.4) | − 30.1 (− 35.2.2 to − 24.1) |
| Western Sub-Saharan Africa | 143,538.8 (213,834.4–95,218.6) | 320,090.3 (226,679.1–458,546.1) | 122.5 (80.9–189.4) | 115.5 (82.3–165.9) | − 5.7 (− 33.1 to 26.2) |
EAPC, estimated annual percentage change; UI, uncertainty interval; DALY, disability-adjusted life-year
In the subgroup analysis by geographical zone, we found that Russia, Ukraine, and Republic of Moldova were the top 3 countries that had the highest age-standardized DALY rate in 2019 (with 217.3, 196.2 and 173.1 per 100,000 respectively) (Fig. 2c). Republic of Korea had the fastest decrease in age standard DALY rate during the past 30 years (dropped from 47.3 to 17.7 per 100,000, EAPC = − 62.6%, 95% UI − 79.9% to − 33.6%). By contrast, Russia showed a sharply increase in age-standardized DALY rate from 1990 to 2019, increased from 103.1 to 217.3 per 100,000 (EAPC = 110.9%, 95% UI 26.3–155.3%) (Additional file 1: Table S1).
The correlation between SDI and the burden of acute pancreatitis
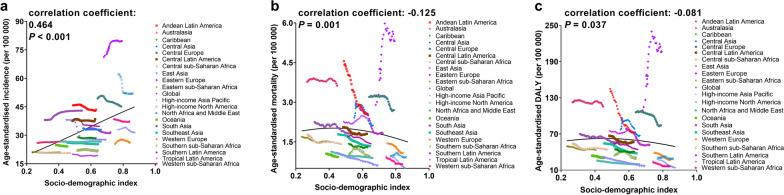
We investigated the correlation between SDI and ASIR, ASMR, and age-standardized DALY rate in 21 regions around the globe from 1990 to 2019. The results revealed that the ASIR was positively correlated with SDI (P < 0.001). In the contrary, both the ASMR (P = 0.001) and age-standardized DALY rate (P = 0.037) were negatively correlated with SDI (Fig. 3). Notably, despite gains in SDI over time, Eastern Europe had much higher age-standardized incidence, mortality and DALY rates than expected values based on SDI for nearly all years between 1990 and 2019.
Fig. 3.
The change trends and correlation analyses of acute pancreatitis’ burden and SDI for 21 world regions from 1990 to 2019. The age-standardized incidence (a), mortality (b) and DALY (c) rates per 100,000 person-years is shown. The solid black line is a mixed-effects and spatiotemporal Gaussian process regression, and represents the expected values across the spectrum of the SDI. DALY: disability-adjusted life-year; SDI: socio-demographic index
The burden of acute pancreatitis and age structure
We analyzed the incidence rate in five age groups: under 5 years, 5–14 years, 15–49 years, 50–69 years, and over 70 years in the globe and different SDI regions. The results demonstrated that the incidence rate (per 100,000) in people above 70 years was the highest across all regions from 1990 to 2019 (Additional file 1: Fig. S1). Based on data of 1990 and 2019, all the incidence, mortality and DALY rates rose with advancing age; of note, the mortality rate rose sharply in patients aged 70 years and older (Additional file 1: Fig. S2).
The mortality of acute pancreatitis due to alcohol etiology
In 1990, there were 0.8 males died per 100,000 due to alcohol-related pancreatitis in the globe, accounted for 36.1% (0.8/2.1) of all cause pancreatitis-related ASMR; among females, this percentage was 15.4% (0.2/1.3) (Additional file 1: Fig. S3a). The attributable fraction of alcohol etiology on mortality was highest among people in the high SDI region for both males and females.
Up to 2019, alcohol etiology still responsible for a sizable fraction of mortality rate in males than that in females (40.2% vs 12.3%) globally, and was the dominant cause for acute pancreatitis’ ASMR in males in the high (50.2%) and high-middle SDI (52.4%) regions (Additional file 1: Fig. S3b). The five highest alcohol etiology caused ASMR were seen in Russia (2.7/100,000), Ukraine (2.3/100,000), Republic of Moldova (2.3/100,000), Belarus (2.2/100,000) and Lithuania (2.1/100,000) (Additional file 1: Fig. S4).
Discussion
This study provides a systematic analysis of acute pancreatitis’ incidence, mortality, DALY and corresponding trends across all 204 countries and territories over a 30-year period. We estimated that in 2019, 2,814,972.3 acute pancreatitis occurred, with 115,053.2 (4.1%) person died globally. Between 1990 and 2019, the age-standardized incidence of acute pancreatitis declined in most countries, but the condition persisted severe in some of the regions, especially in Eastern Europe and High-income North America.
Our global estimate of acute pancreatitis’ incidence in 2019 is close to the estimation in previous meta-analysis (33.7/100,000); in addition, the GBD estimation of 1.4 deaths per 100,000 is also similar to the meta-analytical result (1.6 deaths per 100,000) [7]. Of note, the previous analyses failed to make the combined best use of different types of data on incidence, mortality, DALY, and risk factors reported in literature sources, claims databases, and vital registration systems. GBD estimates the attributable disease burden using a comparative risk assessment strategy through all countries and territories around the world [26]. The global analysis and cross-region comparisons could improve the comprehensive understanding of the burden of acute pancreatitis.
Although our results revealed an increase in acute pancreatitis’ incident cases during the 30-year study period, the mortality rate declined continuously. We have also observed a decreasing mortality rate over the last decade [8, 27–30]. These outcomes are justifiable, for patients with acute pancreatitis are becoming easier to identify with better testing approaches, and the complications can be detected at an earlier stage in the disease course [31]. Furthermore, improvements on the treatment of severe cases in intensive care units, combined with multidisciplinary management strategies have all contributed to the decrease in the acute pancreatitis-related death [32, 33].
However, it should be pointed out that patients in low SDI region had more than twofold higher mortality of acute pancreatitis than people in the high SDI region. Socioeconomic factors are important variables in acute pancreatitis’ epidemiology, especially for the mortality. There were indications that the size of hospital was closely associated with the risk of acute pancreatitis-related fatality. Compared with small hospitals, patients admitted into the large hospitals had a lower risk of death [34–36]. Better outcomes have also been observed for hospitals and surgeons with high volumes of cases [37, 38]. By contrast, the prognosis of acute pancreatitis in low-income regions or socially deprived areas was poorer [39, 40]. The morbidity and mortality of acute pancreatitis would be expected to correlate with the national health system infrastructure as regards the existence or not of specialist tertiary pancreatitis units, and improved outcomes might be achieved in countries with improved access to clinical resources such as specialist tertiary pancreatitis services.
Differences also existed between different age groups. We found that the incidence rate was significantly higher in population over 70 years old. Actually, aging is an important factor that contributing to acute pancreatitis. The incidence of acute pancreatitis attributable to gallstone increased sharply with age for both men and women [40]. In addition, Floyd et al. [13] found that the increase in the drug consumption, such as azathioprine, was also correlated with the higher incidence of acute pancreatitis in the elderly. Moreover, strong association between old age and mortality rate was also reported previously [13], and this was in line with our finding that mortality rate increased with aging and rose sharply in those aged 70 years and older. This is to be expected in relation to the more numerous and more severe coexisting conditions in the elderly people [41, 42].
In GBD 2019, we found alcohol etiology was an important risk factor for acute pancreatitis-related death. Males were much more likely to suffer from fatality due to alcohol-induced acute pancreatitis. In pace with the drinking prevalence increased towards higher levels of SDI [43], the fraction of mortality attributed to alcoholic acute pancreatitis also had a mushrooming rise towards high SDI region for both men and women. Notably, there were evidence indicated that the type of alcohol consumption would affect the acute pancreatitis’ risk. Roberts et al. [40] revealed that alcoholic acute pancreatitis was positively correlated with spirits and beer, but negatively with wine. Other studies showed that the risk of acute pancreatitis had a dose–response association with the number of units of spirits consumption, but not associated with beer or wine [44]. Therefore, public health policies that focus on reducing population-level alcohol intake might be effective in reducing the morbidity and mortality of acute pancreatitis.
This study has some limitations. First, apart from alcohol etiology, gallstone is another major cause of acute pancreatitis in most countries [45], so are the other less common but meaningful causes such as hypertriglyceridemia. However, the quantitative effect of gallstone and other causes on acute pancreatitis could not be assessed owing to unavailable data in the current round of GBD. Second, in order to formulate more effective preventive measures, proper stratification of severity grades and analysis for subtypes of acute pancreatitis according to aetiology is necessary. However, based on the data of GBD 2019, we couldn’t obtain such information. In addition, GBD faces several challenges for estimating cause-specific non-fatal and fatal burden of acute pancreatitis. Even though it employs spatiotemporal methods to inform estimates for locations with sparse data, these strategies cannot completely resolve issues when data are not available for some regions. As vital statistic systems and civil registration provide important information for disease preventions and public health policies, strengthening of these systems is essential for public health. An ongoing effort of adding new sources of data to the GBD should be taken to overcome the limitations.
Conclusions
In summary, the age-standardized incidence, mortality and DALY rates of acute pancreatitis decreased gradually in the globe. However, the overall burden remains high with aging population, and will not decrease without effective strategies to address associated risk factors. The attributable burden is higher in males and elderly, and alcohol etiology is an important driver that contribute to the morbidity and mortality. There were substantial differences in the burden of acute pancreatitis across regions, geographically targeted considerations are needed to tailor future interventions to relieve the burden of acute pancreatitis in specific countries, especially for Eastern Europe.
Supplementary Information
Additional file 1. Supplementary methods, tables, and figures.
Acknowledgements
We appreciate the works by the Global Burden of Disease study 2019 collaborators. We would like to thank American Journal Experts (https://www.aje.com) for editing this manuscript.
Abbreviations
- CODEm
Cause of death ensemble model
- DALYs
Disability-adjusted life year
- GBD
Global burden of diseases
- EAPC
Estimated annual percentage change
- ASIR
Age-standardized incidence rate
- ASMR
Age-standardized mortality rate
- GHDx
Global health data exchange
- ICD
International classification of diseases
- SDI
Socio-demographic Index
- ST-GPR
Spatiotemporal Gaussian process regression
- UIs
Uncertainty intervals
- YLDs
Years lived with disability
- YLLs
Years of life lost
Authors' contributions
CL and MJ conceptualized the study. MJ devised the design of the study. CP, JL and LX collected the data. CL and MJ contributed to the data analysing. MJ wrote the first draft of the manuscript. CP, JL and LX edited the paper. CL revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the Project funded by China Postdoctoral Science Foundation (2020M682422).
Availability of data and materials
The datasets generated for this study can be found in the GBD at http://ghdx.healthdata.org/gbd-results-tool.
Declarations
Ethics approval and consent to participate
This study did not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nøjgaard C. Prognosis of acute and chronic pancreatitis—a 30-year follow-up of a Danish cohort. Dan Med Bull. 2010;57:B4228. [PubMed] [Google Scholar]
- 2.Ekbom A, McLaughlin JK, Karlsson BM, Nyrén O, Gridley G, Adami HO, et al. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86:625–627. doi: 10.1093/jnci/86.8.625. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149:1731–1741. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schepers NJ, Bakker OJ, Besselink MG, Ahmed AU, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 5.van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed AU, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263. doi: 10.1053/j.gastro.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 6.Bang JY, Wilcox CM, Arnoletti JP, Varadarajulu S. Superiority of endoscopic interventions over minimally invasive surgery for infected necrotizing pancreatitis: meta-analysis of randomized trials. Dig Endosc. 2020;32:298–308. doi: 10.1111/den.13470. [DOI] [PubMed] [Google Scholar]
- 7.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 8.Bilal M, Kline KT, Trieu JA, Saraireh H, Desai M, Parupudi S, et al. Trends in same-admission cholecystectomy and endoscopic retrograde cholangiopancreatography for acute gallstone pancreatitis: a nationwide analysis across a decade. Pancreatology. 2019;19:524–530. doi: 10.1016/j.pan.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The changing epidemiology of acute pancreatitis hospitalizations. Pancreas. 2017;46:482–488. doi: 10.1097/MPA.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts SE, Thorne K, Evans PA, Akbari A, Samuel DG, Williams JG. Mortality following acute pancreatitis: social deprivation, hospital size and time of admission: record linkage study. BMC Gastroenterol. 2014;14:153. doi: 10.1186/1471-230X-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung S, Chen K, Xirasagar S, Tsai M, Lin H. More than 9-times increased risk for pancreatic cancer among patients with acute pancreatitis in Chinese population. Pancreas. 2012;41:142–146. doi: 10.1097/MPA.0b013e31822363c3. [DOI] [PubMed] [Google Scholar]
- 12.Sadr-Azodi O, Andrén-Sandberg Å, Orsini N, Wolk A. Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut. 2011;61:262–267. doi: 10.1136/gutjnl-2011-300566. [DOI] [PubMed] [Google Scholar]
- 13.Floyd A, Pederson L, Nielsen GL, Thorlacius-Ussing O, Sorensen HT. Secular trends in incidence and 30-day case fatality of acute pancreatitis in North Jutland County, Denmark: a register-based study from 1981–2000. Scand J Gastroenterol. 2009;37:1461–1465. doi: 10.1080/003655202762671369. [DOI] [PubMed] [Google Scholar]
- 14.Blomgren KJ, Sundström A, Steineck G, Wiholm B. Interviewer variability—quality aspects in a case-control study. Eur J Epidemiol. 2006;21:267–277. doi: 10.1007/s10654-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 15.Lindkvist B, Appelros S, Manjer J, Borgström A. Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol H. 2004;2:831. doi: 10.1016/S1542-3565(04)00355-6. [DOI] [PubMed] [Google Scholar]
- 16.Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 19.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392:1736–88. [DOI] [PMC free article] [PubMed]
- 20.Ouyang G, Pan G, Liu Q, Wu Y, Liu Z, Lu W, et al. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020; 18. [DOI] [PMC free article] [PubMed]
- 21.Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vos T, Abbasi M, Abbasifard M, Abbastabar H, Abd-Allah F, Abdelalim A, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaborators GPAF. Population and fertility by age and sex for 195 countries and territories, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1995–2051. doi: 10.1016/S0140-6736(18)32278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Cao X, Guo M, Xie M, Liu X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ. 2020; 368:m234. [DOI] [PMC free article] [PubMed]
- 25.Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 26.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175–184. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNabb-Baltar J, Ravi P, Isabwe GA, Suleiman SL, Yaghoobi M, Trinh QD, et al. A population-based assessment of the burden of acute pancreatitis in the United States. Pancreas. 2014;43:687–691. doi: 10.1097/MPA.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 28.Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CJ. Increasing United States hospital admissions for acute pancreatitis, 1988–2003. Ann Epidemiol. 2007;17:491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Singla A, Simons J, Li Y, Csikesz NG, Ng SC, Tseng JF, et al. Admission volume determines outcome for patients with acute pancreatitis. Gastroenterology. 2009;137:1995–2001. doi: 10.1053/j.gastro.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Brown A, Young B, Morton J, Behrns K. N Shaheen (2008) Are health related outcomes in acute pancreatitis improving? An analysis of national trends in the US from 1997 to 2003. JOP. 2008;9:408–414. [PubMed] [Google Scholar]
- 31.Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 32.McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg. 1999;86:1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- 33.Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:890–893. doi: 10.1002/bjs.1800810632. [DOI] [PubMed] [Google Scholar]
- 34.Andrén-Sandberg A. Organization of care for pancreatic cancer. N Am J Med Sci. 2011;3:400–405. doi: 10.4297/najms.2011.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata A, Matsuda S, Mayumi T, Yokoe M, Kuwabara K, Ichimiya Y, et al. Effect of hospital volume on clinical outcome in patients with acute pancreatitis, based on a national administrative database. Pancreas. 2011;40:1018–1023. doi: 10.1097/MPA.0b013e31821bd233. [DOI] [PubMed] [Google Scholar]
- 36.Shen HN, Lu CL, Li CY. The effect of hospital volume on patient outcomes in severe acute pancreatitis. BMC Gastroenterol. 2012;12:112. doi: 10.1186/1471-230X-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg. 2007;94:145–161. doi: 10.1002/bjs.5714. [DOI] [PubMed] [Google Scholar]
- 38.McAteer JP, LaRiviere CA, Drugas GT, Abdullah F, Oldham KT, Goldin AB. Influence of surgeon experience, hospital volume, and specialty designation on outcomes in pediatric surgery: a systematic review. JAMA Pediatr. 2013;167:468–475. doi: 10.1001/jamapediatrics.2013.25. [DOI] [PubMed] [Google Scholar]
- 39.Roberts SE, Williams JG, Meddings D, Goldacre MJ. Incidence and case fatality for acute pancreatitis in England: geographical variation, social deprivation, alcohol consumption and aetiology—a record linkage study. Aliment Pharmacol Ther. 2008;28:931–941. doi: 10.1111/j.1365-2036.2008.03809.x. [DOI] [PubMed] [Google Scholar]
- 40.Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:539–548. doi: 10.1111/apt.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong S, Qiwen B, Ying J, Wei A, Chaoyang T. Body mass index and the risk and prognosis of acute pancreatitis: a meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:1136–1143. doi: 10.1097/MEG.0b013e32834b0e0e. [DOI] [PubMed] [Google Scholar]
- 42.Krishna SG, Hinton A, Oza V, Hart PA, Swei E, El-Dika S, et al. Morbid obesity is associated with adverse clinical outcomes in acute pancreatitis: a propensity-matched study. Am J Gastroenterol. 2015;110:1608–1619. doi: 10.1038/ajg.2015.343. [DOI] [PubMed] [Google Scholar]
- 43.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt DN. Apparent risk factors for chronic and acute pancreatitis in Stockholm county: spirits but not wine and beer. Int J Pancreatol. 1991;8:45–50. doi: 10.1007/BF02930222. [DOI] [PubMed] [Google Scholar]
- 45.Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155–165. doi: 10.1016/j.pan.2017.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary methods, tables, and figures.
Data Availability Statement
The datasets generated for this study can be found in the GBD at http://ghdx.healthdata.org/gbd-results-tool.