Abstract
Background
The microbiota in the cecum of laying hens is crucial for host digestion, metabolism, and odor gas production. The results of recent studies have suggested that host microRNAs (miRNAs) can regulate gene expression of the gut microbiota. In the present study, the expression profiles of host-derived miRNAs in the cecal content of two laying hen breeds; Hy-line Gray and Lohmann Pink, which have dissimilar H2S production, were characterized; and their effects on H2S production by regulating the expression of gut microbiota-associated genes were demonstrated.
Results
The differential expression of microbial serine O-acetyltransferase, methionine synthase, aspartate aminotransferase, methionine-gamma-lyase, and adenylylsulfate kinase between the two hen breeds resulted in lower H2S production in the Hy-line hens. The results also revealed the presence of miRNA exosomes in the cecal content of laying hens, and an analysis of potential miRNA-target relationships between 9 differentially expressed miRNAs and 9 differentially expressed microbial genes related to H2S production identified two methionine synthase genes, Odosp_3416 and BF9343_2953, that are targeted by gga-miR-222a. Interestingly, in vitro fermentation results showed that gga-miR-222a upregulates the expression of these genes, which increased methionine concentrations but decreased H2S production and soluble sulfide concentrations, indicating the potential of host-derived gga-miR-222a to reduce H2S emission in laying hens.
Conclusion
The findings of the present study reveal both a physiological role by which miRNAs shape the cecal microbiota of laying hens and a strategy to use host miRNAs to manipulate the microbiome and actively express key microbial genes to reduce H2S emissions and breed environmentally friendly laying hens.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-021-01098-7.
Keywords: miRNA, Metatranscriptome, gga-miR-222a, Hydrogen sulfide, Laying hens
Background
The laying hen industry is an important livestock sector that produces eggs, a common source of human nutrition [1]. Nutrient digestion in laying hens is characterized by inadequate enzymatic hydrolysis in the foregut followed by further microbial fermentation in the cecum. An increasing number of studies have demonstrated that this ‘bacterial organ’ plays a crucial role in host metabolism, immunity, and disease [2–4]. Bacteria ferment undigested feed components to generate volatile fatty acids (VFAs), amino acids, ammonia (NH3), hydrogen sulfide (H2S), and other metabolites [5, 6]. Previous studies elucidated that NH3 and H2S are the two primary odorous gasses in poultry houses and represent a great loss in nutrients and cause environmental pollution that is a public concern [7, 8]. Although NH3, followed by H2S, accounts for the largest proportion of odor gas in livestock and poultry facilities, the odor threshold of H2S is significantly lower than that of NH3 and also contributes to the poor smell in the farm environment [9, 10]. In addition to its adverse effects on air quality, an early study reported that H2S is released in the intestines of humans, where it causes a number of intestinal diseases [11]. In a recent study, H2S inhalation was shown to induce pneumonia in chickens [12], revealing the potential for H2S to damage the health of both workers and animals. Therefore, in addition to mitigating environmental problems, reducing H2S levels will have a positive effect on animal health. Several recent studies have explored the effects of nutritional manipulation on H2S emission in animals. For example, probiotic inclusion or protein reduction in the diet has been used to regulate the gut microbiota in animals [13, 14]. However, there are a few disadvantages to nutritional manipulations, including the need for the continuous supplementation of probiotics to guarantee the sustained reduction in H2S emissions. Thus, the development of breeding laying hens with a low-H2S emission “cecal microbiota structure” may represent a better and more permanent solution to promote H2S reduction and an environmentally friendly culture in the poultry industry. However, to breed low-H2S emission laying hens, it is first important to understand the regulatory relationship between the host and its cecal microbiota.
Numerous factors influence the composition and function of the gut microbiota, such as the genetic background of the host, and to some extent, these factors can be transiently altered by diet, the environment, and disease states [15, 16]. MicroRNAs (miRNAs) are a group of noncoding RNAs ~ 22 nucleotides (nt) in length that are known for their sequence-specific regulatory function that involves targeting the 3′ untranslated region of mRNAs in the cytoplasm [17]. Increasing evidence has demonstrated that miRNAs also exist extracellularly and circulate in body fluids within exosomes and microvesicles. Secreted miRNAs have been isolated from blood, milk, and even stool and urine [18–22], with some studies having characterized miRNAs as potential markers of tumorigenesis in human stool [23–25]. Interestingly, recent studies have also revealed that host-derived miRNAs can serve as an important crosstalk channel between the host and the intestinal bacterial population. Liu et al. demonstrated that the host can modulate the gut microbiota through intestinal epithelial cell-secreted miRNAs, which enter gut bacteria and directly regulate bacterial gene expression [26]. Other studies have reported that plant-derived miRNAs can be taken up by gut bacteria and shape the gut microbiota [27, 28]. Therefore, we hypothesized that cross-regulation may occur between host-derived miRNAs and the cecal microbiota in laying hens. In addition, it remains unknown whether host-derived miRNAs regulate microbial abundance or the expression of bacterial functional genes, changes in which influence the structure and metabolic functions of the microbiota, and potentially lead to H2S production differences in different breeds of laying hens.
In our previous study, we observed a significantly higher daily H2S production in Lohmann laying hens than in Hy-line Gray laying hens (daily H2S production per kg average daily feed intake was 7.75 and 4.17 mg, respectively) [29]. However, whether this difference in H2S emissions was due to host miRNA regulation of the gut microbiota remained unelucidated. Therefore, in the present study, we determined the expression profiles of host-derived miRNAs in the cecal contents of these two breeds of laying hens to identify miRNAs showing significantly different expression and then predicted the target relationships between differentially expressed miRNAs and microbial genes related to H2S production. Finally, the effects of selected targeted miRNAs on H2S production in laying hens were verified through in vitro experiments. The results of the present study should provide a better understanding of the interkingdom regulatory relationships among miRNAs, cecal microbiota and H2S production in laying hens, providing a reference for the breeding of environmentally friendly laying hens. At the same time, miRNAs, which have been proven to regulate the production of H2S in the cecum of laying hens, could be used as a safe and clean additive to promote reductions in H2S emissions in the future.
Results
Determination of miRNA profiles in the cecum of laying hens
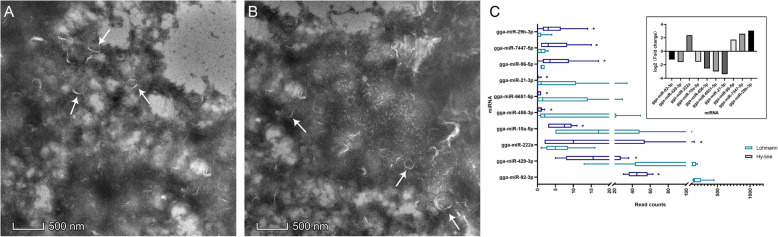
The morphology of exosomes derived from laying hen cecal contents was observed by transmission electron microscopy (TEM). Exosome-sized (approximately 50–200 nm in diameter) extracellular vesicles were present in the cecal contents of Lohmann and Hy-line hens, but their morphological characteristics were largely the same in the two breeds (Fig. 1A, B). Based on the observed presence of exosomes, miRNA sequencing was performed to investigate the miRNA differences between the two breeds.
Fig. 1.
Morphological characteristics of exosomes and significantly expressed miRNAs. Morphological characteristics of exosomes in Lohmann cecal contents (A) and in Hy-line cecal contents (B). White arrows indicate exosomes. C Ten differentially expressed miRNAs between the two breeds. Asterisk indicates a significant difference in an miRNA between the two breeds, and significant differences between the means were determined by Tukey’s test. Differences were considered significant at P < 0.05, and the bar graph in C shows the expression level of these 10 miRNAs. The bars on the “0” X axis indicate upregulation in the Hy-line hens compared to the Lohmann hens, and the bars under the “0” X axis indicate downregulation in the Hy-line hens compared to the Lohmann hens
From the high-throughput sequencing results, we identified 288 annotated miRNAs using miRBase, the associated information for which is presented in Table S1. Subsequently, a DEGSeq analysis using the R software environment revealed 10 miRNAs as being significantly differentially expressed between the two breeds, where the log2 (fold change) > 1 results identified four miRNAs (gga-miR-222a, gga-miR-96-5p, gga-miR-7447-5p, and gga-miR-29b-3p) with significantly higher expression levels and six miRNAs (gga-miR-92-3p, gga-miR-429-3p, gga-miR-10a-5p, gga-miR-456-3p, gga-miR-6651-5p, and gga-miR-21-3p) showing significantly lower expression levels in the Hy-line hens than in the Lohmann hens (P < 0.05). In addition, the top four highly expressed miRNAs were gga-miR-92-3p (Hy-line: 46 ± 11.47; Lohmann, 196 ± 113.67), gga-miR-429-3p (Hy-line, 17.5±10.5; Lohmann, 110.167 ± 61.50), gga-miR-222a (Hy-line, 32.167 ± 51.547; Lohmann, 6 ± 4.80), and gga-miR-10a-5p (Hy-line, 6.833 ± 3.08; Lohmann, 30 ± 35.81) in Hy-line and Lohmann (Fig. 1C). Thus, the results indicated that these four miRNAs should be considered for further analysis.
The KEGG pathway annotations of target genes in the chicken genome for these ten significantly expressed miRNAs are shown in Fig. S1 and were mostly enriched in metabolic pathways, neuroactive ligand-receptor interaction, focal adhesion, endocytosis and purine metabolism.
Expression of microbial genes related to H2S production
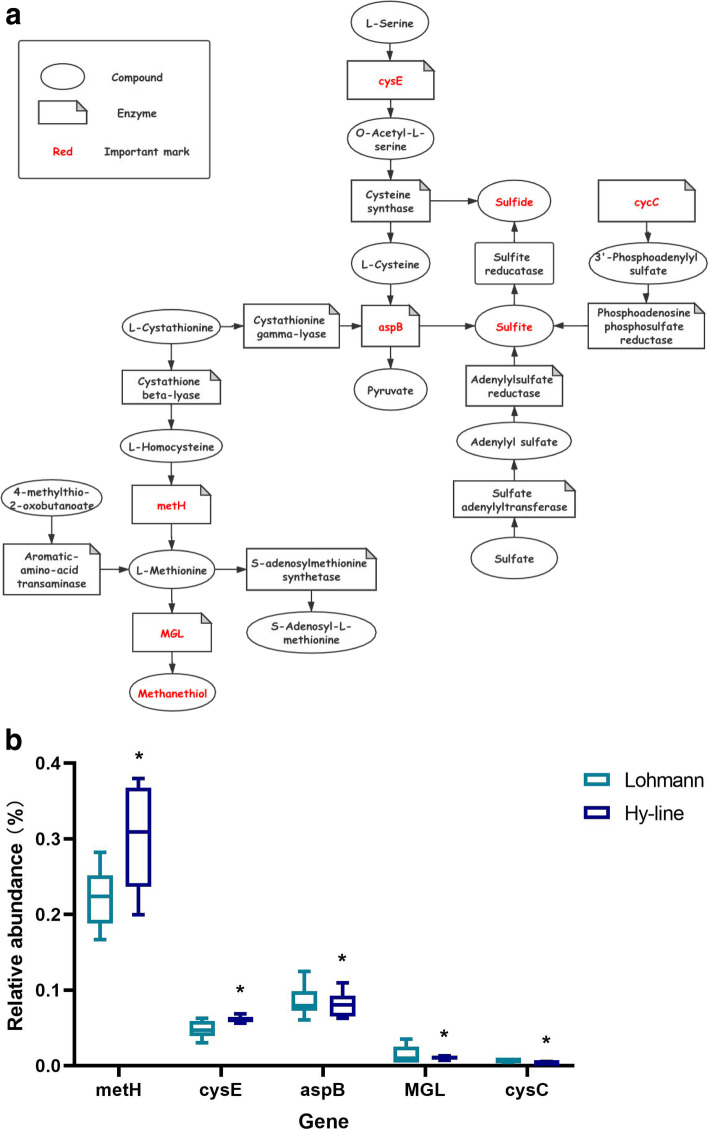
The sequencing information for the metatranscriptome is listed in Table S2, and the top 30 KEGG microbial function enrichment pathways are shown in Fig. S2. The 5 pathways showed significant differences in gene enrichment between the Lohmann and Hy-line hens, where the abundances of microbial genes associated with the two-component system were significantly higher in the Hy-line hens than in the Lohmann hens (P < 0.05), and the abundances of microbial genes enriched in the amino sugar and nucleotide sugar metabolism, cysteine and methionine metabolism, alanine aspartate and glutamate metabolism, and RNA degradation pathways were significantly higher in Lohmann hens than in the Hy-line hens (P < 0.05). The transcriptomics results also showed that the abundances of 22,237 genes were significantly different between the Lohmann and Hy-line hens (P < 0.05). In addition, based on the combined analysis of the microbial gene enrichment pathways and significantly differentially expressed genes, we focused on two metabolic pathways involved in H2S production, the cysteine and methionine metabolism pathway (map 00270) and the sulfur metabolism pathway (map 00920). Based on the KEGG database, the degradation of sulfur-containing amino acids, cysteine, and methionine, can result in the production of H2S. As shown in Fig. 2A, the synthesis of L-cysteine from L-serine and sulfide is catalyzed by serine O-acetyltransferase and cysteine synthase, and L-cysteine can also be synthesized from L-cystathionine by cystathionine gamma-lyase. L-cysteine is degraded into pyruvate and sulfite by aspartate aminotransferase, and sulfite is used as a raw material for H2S production in the sulfur metabolism pathway. In addition, methionine can be degraded into methanethiol by methionine-gamma-lyase, which can then be converted into H2S in subsequent processes. Another pathway for methionine degradation involves the formation of S-adenosyl-L-methionine by S-adenosylmethionine synthetase without H2S production. Sulfur metabolism is an essential pathway in the cecum of hens and is involved in the production of H2S. As the assimilatory reduction and dissimilatory reduction of sulfate promotes the production of sulfite and its eventual conversion into sulfide (Fig. 2A), adenylylsulfate kinase is a key enzyme for the production of sulfide from sulfate and was further evaluated to elucidate H2S production in hens.
Fig. 2.
Expression of genes encoding enzymes in the cysteine and methionine metabolism and sulfate metabolism pathways. A Enzymes and intermediates involved in the cysteine and methionine metabolic pathway and that of sulfate. B Differentially expressed genes that are marked in red in metabolic pathways. Asterisk indicates a significant difference in relative gene abundance between the two breeds, where significant differences between the means were determined with Tukey’s test. Differences were considered significant at P < 0.05
By analyzing the annotation information in the KEGG database, the results indicated that O-acetyltransferase (cysE, EC:2.3.1.30), methionine synthase (metH, EC:2.1.1.13), aspartate aminotransferase (aspB, EC:2.6.1.1), methionine-gamma-lyase (MGL, EC:4.4.1.11) and adenylylsulfate kinase (cysC, EC: 2.7.1.25) are the key enzymes for the production of H2S. Thus, the gene expression levels of these key enzymes were next tested. The expression levels of both cysE and metH were significantly higher in the Hy-line hens than in the Lohmann hens (P < 0.05); the expression level of cysE was 0.061 ± 0.004 and 0.048 ± 0.010 in the Hy-line and Lohmann hens, respectively; and the expression level of metH was 0.302 ± 0.062 and 0.222 ± 0.036 in the Hy-line and Lohmann hens, respectively. In contrast, the expression levels of both aspB and MGL were lower in the Hy-line hens than in the Lohmann hens (P < 0.05); the expression level of aspB was 0.081 ± 0.015 and 0.085 ± 0.020 in the Hy-line and Lohmann hens, respectively; and the expression level of MGL was 0.011 ± 0.002 and 0.014 ± 0.011 in the Hy-line and Lohmann hens, respectively. These results indicated that there was a higher tendency for the synthesis of cysteine and methionine but a lower tendency for their degradation and H2S production in the cecum of Hy-line hens (Fig. 2B). In addition, we also observed significantly lower cysC expression levels in the cecum of Hy-line hens than in Lohmann hens (P < 0.05; 0.004 ± 0.001 and 0.007 ± 0.001, respectively). This result also indicated that less sulfide is produced in Hy-line hens than in Lohmann hens (Fig. 2B).
Based on an analysis of the regulatory enzymes involved in H2S production, thirteen differentially expressed microbial genes involved in encoding these five enzymes were identified between the two breeds. MGL and cysC were assigned to only one gene and one genus, and the other three enzymes were assigned to several genes and genera. In addition, most of the genes were assigned to the genus Bacteroides (Table 1).
Table 1.
Differentially expressed genes (DEGs) and source bacteria of differentially expressed enzymes
| Enzymes | DEGs (log2 Fold change) | Source bacteria |
|---|---|---|
| CysE | Ddes_0279 (6.35) | Desulfovibrio desulfuricans |
| CK3_01000 (5.31) | Unclassified Clostridiales | |
| SELR_06150 (4.94) | Selenomonas ruminantium | |
| Bache_0784 (2.36) | Bacteroides helcogenes | |
| MetH | Odosp_3416 (8.48) | Odoribacter splanchnicus |
| Bacsa_0021 (5.67) | Bacteroides salanitronis | |
| BF9343_2953 (5.31) | Bacteroides fragilis NCTC9343 | |
| AspB | BVU_0144 (− 5.37) | Bacteroides vulgatus |
| Bache_2087 (− 4.20) | Bacteroides helcogenes | |
| PRU_1300 (− 3.66) | Prevotella ruminicola | |
| OBV_25710 (− 3.27) | Oscillibacter valericigenes | |
| MGL | GFO_2175 (− 5.68) | Gramella forsetii |
| CysC | Mmc1_2549 (− 4.76) | Magnetococcus marinus |
Target prediction of miRNAs
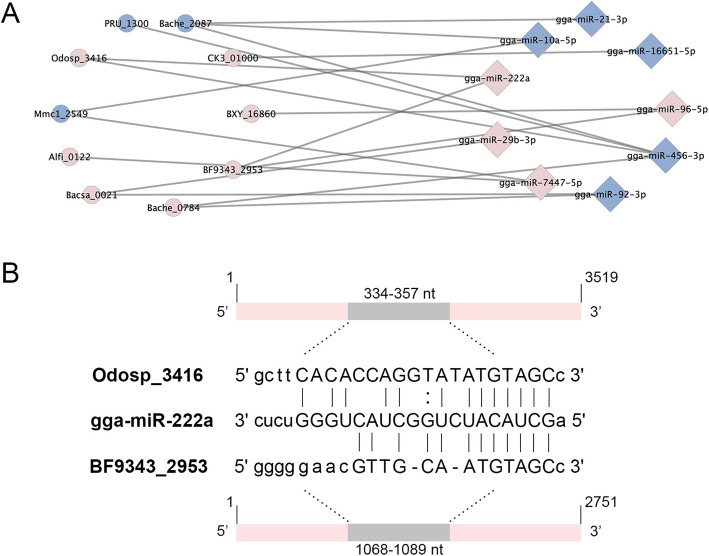
Through a combination analysis of differentially expressed miRNAs (Fig. 1C) and differentially expressed genes associated with H2S production (Table 1), nine miRNAs were identified as being able to target 9 genes through miRanda analysis (Fig. 3A). As mentioned above, gga-miR-222a and gga-miR-10a-5p were both highly expressed miRNAs in Hy-line and Lohmann hens, although the abundance of gga-miR-222a was significantly higher in Hy-line hens than in Lohmann hens, while the abundance of gga-miR-10a-5p was significantly lower in Hy-line hens than in Lohmann hens. In addition, the results of our previous study showed that the amount of H2S production was obviously higher in Lohmann hens than in Hy-line hens (daily H2S production per kg average daily feed intake of 7.75 and 4.17 mg, respectively) [29]. Therefore, we concluded that gga-miR-222a is a potential candidate additive for use in the reduction of H2S production in the cecum of laying hens and should be further investigated. We observed that gga-miR-222a could target two genes associated with methionine synthase, Odosp_3416 and BF9343_2953, expressed by the bacteria Odoribacter splanchnicus and Bacteroides fragilis NCTC 9343, respectively (Fig. 3B). The read counts for Odosp_3416 were 138 and 225 in the Hy-line and Lohmann hens, respectively, while those observed for BF9343_2953 were 75 and 137. Based on these results, we suspected that the regulatory effect of gga-miR-222a on the two genes may be notable. Therefore, functional verification of gga-miR-222a targeting Odosp_3416 and BF9343_2953 was performed.
Fig. 3.
Correlation and target site analysis between miRNAs and bacterial genes. A Significantly upregulated genes and miRNAs in the Hy-line hens compared to the Lohmann hens (pink); significantly downregulated genes and miRNAs in the Hy-line compared to the Lohmann hens (blue). Squares indicate bacterial genes, and circles indicate host miRNAs. B Target sites between gga-miR-222a and the genes Odosp_3416 and BF9343_2953
H2S production during fermentation
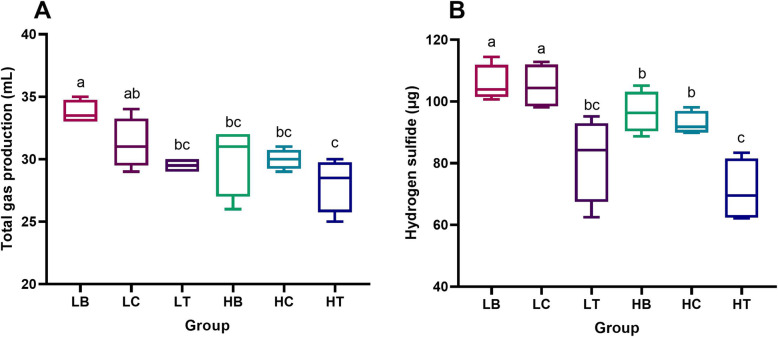
After 24 h of fermentation, the amount of total gas and H2S production in the different groups was tested, the results of which showed that the addition of gga-miR-222a could influence total gas and H2S production.
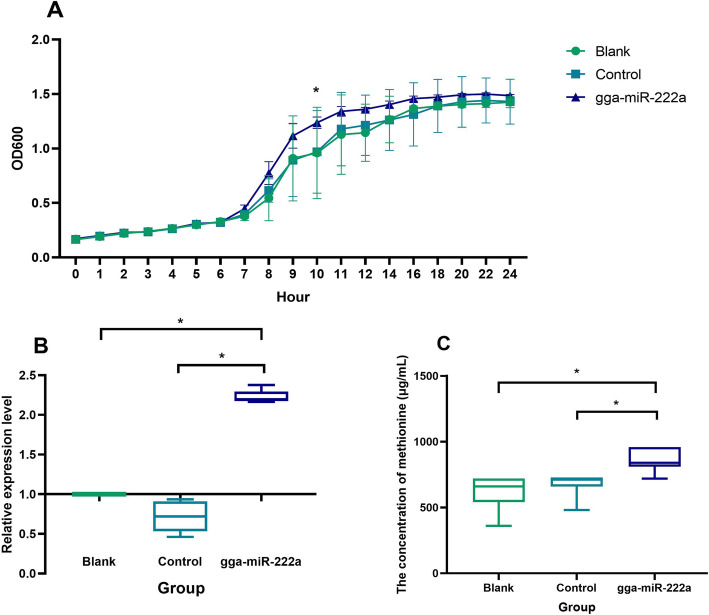
The total gas production was 33.75 ± 0.83 and 30 ± 0.71 mL in the Lohmann intestinal content broth with nothing added (LB) and Hy-line intestinal content broth with nothing added (HB), respectively. The statistical analysis showed that the total gas production in the LB group was significantly higher than that in the HB group (P < 0.05), potentially indicating that the total gas production ability of Lohmann hens was higher than that of Hy-line hens. In the present study, we observed that the amount of total gas production was significantly decreased in the Lohmann intestinal content broth supplemented with gga-miR-222a (LT; 29.5 ± 0.5 mL) compared to the LB group (P < 0.05); However, no significant difference was observed between the Hy-line intestinal content broth supplemented with gga-miR-222a (HT; 28 ± 1.87 mL) and HB groups. These results demonstrated that gga-miR-222a can effectively decrease the total gas production in Lohmann hens compared to Hy-line hens (Fig. 4A). In addition, no obvious difference was observed between the blank groups (intestinal content broth without the addition of miRNA, groups LB and HB) and the control groups (intestinal content broth with added miRNA control, groups LC and HC), suggesting that the result obtained with the commercially synthesized gga-miR-222a were credible.
Fig. 4.
Amount of total gas and H2S production in the fermentation experiment for each group. A Amount of total gas production and B H2S production. Different letters indicate significant differences in the amount of total gas and H2S production among the groups, and significant differences among the means were determined by Tukey’s test. Differences were considered significant at P < 0.05
The effect of gga-miR-222a on H2S production was investigated, the results of which are shown in Fig. 4B. The amount of H2S was 105.747 ± 5.22 and 96.592 ± 5.84 μg in the LB and HB groups, respectively, and the statistical analysis showed that the amount of H2S was significantly higher in the LB group than the HB group (P < 0.05), indicating that the ability of Lohmann hens to produce H2S gas was higher than that of the Hy-line hens. It is worth noting that gga-miR-222a addition significantly decreased the amount of H2S production in the fermentation broths of both breeds (P < 0.05), and the amount of H2S production was 81.553 ± 11 and 71.152 ± 8.94 μg in the LT and HT groups, respectively. Compared to the blank groups, gga-miR-222a addition decreased H2S production by 22.88 and 26.33% in the Lohmann and Hy-line intestinal content broths, respectively. These results suggest that gga-miR-222a has the potential to decrease H2S production in the intestine.
The chemical indexes of the fermentation broth of each group are shown in Table 2. The concentration of soluble sulfide (S2−) was significantly lower in the HB group (12.32 ± 0.98 μg/g) than in the LB group (15.06 ± 0.94 μg/g) (P < 0.05). In addition, the S2− concentration was also significantly lower in the gga-miR-222a addition groups (LT, 12.50 ± 0.50 μg/g; HT, 9.84 ± 1.44 μg/g) than in the blank groups (LB and HB) (P < 0.05). The concentration of methionine in the fermentation broth was also evaluated, as methionine is the crucial amino acid that donates sulfur to form H2S through microbial metabolism. In the present study, the addition of gga-miR-222a also significantly increased the concentration of methionine in the LT (280.39 ± 5.78 μg/mL) and HT (299.12 ± 3.68 μg/mL) groups compared to the LB (246.21 ± 18.83 μg/mL) and HB (257.70 ± 19.30 μg/mL) groups, respectively (P < 0.05).
Table 2.
The comparison of fermentation incubation indexes. Significant differences between the means were determined by Tukey’s test. Differences were considered significant at P < 0.05
| Items | LB | LC | LT | HB | HC | HT |
|---|---|---|---|---|---|---|
| pH | 7.57 ± 0.08 | 7.49 ± 0.03 | 7.61 ± 0.10 | 7.59 ± 0.02 | 7.55 ± 0.05 | 7.48 ± 0.05 |
| S2−, μg/g | 15.06 ± 0.94a | 14.83 ± 1.12a | 12.50 ± 0.50b | 12.32 ± 0.98b | 12.99 ± 1.59ab | 9.84 ± 1.44c |
| SO42−, mg/g | 256.94 ± 29.93 | 268.52 ± 61.26 | 221.30 ± 79.04 | 257.87 ± 85.95 | 287.96 ± 46.39 | 243.98 ± 70.84 |
| Acetate, mmol/L | 31.53 ± 4.01 | 30.19 ± 0.96 | 32.81 ± 3.25 | 32.45 ± 1.85 | 34.63 ± 2.23 | 34.15 ± 2.70 |
| Propionate, mmol/L | 15.51 ± 1.54 | 15.34 ± 3.40 | 15.21 ± 1.35 | 16.00 ± 1.00 | 16.58 ± 0.91 | 15.59 ± 0.74 |
| Butyrate, mmol/L | 8.33 ± 0.54c | 8.16 ± 0.56c | 9.10 ± 0.73bc | 10.81 ± 1.44a | 10.67 ± 1.22a | 10.17 ± 0.60ab |
| Total VFAs, mmol/L | 55.37 ± 6.00 | 53.69 ± 3.18 | 57.11 ± 5.29 | 59.26 ± 2.94 | 61.88 ± 1.98 | 59.91 ± 3.70 |
| Methionine, μg/mL | 246.21 ± 18.83c | 245.88 ± 15.01c | 280.39 ± 5.78ab | 257.70 ± 19.30 | 258.92 ± 12.08 | 299.12 ± 3.68a |
Data are presented as means with their standard errors
Means within a row with different superscript letters differ (P < 0.05)
LB Lohmann intestinal content broth added nothing, LCs Lohmann intestinal content broth added miRNA control, LT Lohmann intestinal content broth added gga-miR-222a, HB Hy-line intestinal content broth added nothing, HC Hy-line intestinal content broth added miRNA control, HT Hy-line intestinal content broth added gga-miR-222a
Bacterial abundance and gene expression in fermentation broth
The methionine synthetase genes (metH) Odosp_3416 (expressed by the bacterium Odoribacter splanchnicus) and BF9343_2953 (expressed by the bacterium Bacteroides fragilis NCTC 9343) were predicted to be targeted by gga-miR-222a (Table 1). The abundances of Odoribacter splanchnicus and Bacteroides fragilis NCTC 9343 and the levels of Odosp_3416 and BF9343_2953 expression were quantified to assess the effect of gga-miR-222a on bacterial abundance and gene expression (Fig. 5). The addition of gga-miR-222a had no effect on the abundances of the two bacteria in the intestinal content broth of the two breeds when comparing the blank groups (Fig. 5A). In contrast, the expression of the genes Odosp_3416 and BF9343_2953 in the LT (Odosp_3416, 3.648 ± 0.72; BF9343_2953, 2.513 ± 0.14) and HT (Odosp_3416, 5.837 ± 1.04; BF9343_2953, 3.918 ± 0.42) groups was significantly higher than that observed in the LB (Odosp_3416, 1 ± 0; BF9343_2953, 1 ± 0) and HB (Odosp_3416, 3.353 ± 0.59; BF9343_2953, 2.199 ± 0.64) groups (P < 0.05) (Fig. 5B). These results suggest that gga-miR-222a increases metH (Odosp_3416 and BF9343_2953) expression in the intestinal content broths of the two breeds. However, the results also showed that gga-miR-222a did not significantly affect the abundances of target gene-related bacteria.
Fig. 5.
Relative bacterial abundances and gene expression levels in the fermentation experiment for each group. A Relative abundances of Odoribacter splanchnicus and Bacteroides fragilis NCTC9343 in each group. The relative abundances of the LC, LT, HB, HC, and HT groups are shown compared to that of the LB group (set as 1). B Relative expression levels of the genes Odosp_3416 and BF9343_2953 in each group. The relative abundances of the LC, LT, HB, HC, and HT groups are shown relative to that of LB group (set as 1). Different letters in different groups indicate a significant difference, and significant differences among the means were determined by Tukey’s test (P < 0.05).
Efficacy of gga-miR-222a treatment
To further understand whether the effects of gga-miR-222a truly influence the methionine synthetase genes-harboring bacteria, a culture medium experiment was performed. Because the relative abundance of Odoribacter splanchnicus was significantly lower than that of Bacteroides fragilis NCTC9343 in both hen breeds (Fig. S3), Bacteroides fragilis NCTC9343 was selected for the subsequent experiment. At 8 and 10 h, the bacterium Bacteroides fragilis NCTC9343 reached the logarithmic and plateau phases, respectively. In the gga-miR-222a addition group, this miRNA significantly improved (29.04%) the abundance of Bacteroides fragilis NCTC9343 at 10 h compared to the blank (P < 0.05) (Fig. 6A). In addition, gga-miR-222a addition also significantly enhanced the expression of the Bacteroides fragilis NCTC9343 gene BF9343_2953 by 2.23-fold compared to the control (P < 0.05) (Fig. 6B), and the concentration of methionine in the medium was also significantly increased (38.71%) with the addition of gga-miR-222a compared to the blank (P < 0.05) (Fig. 6C).
Fig. 6.
Effects of gga-miR-222a on the growth and metabolism of Bacteroides fragilis NCTC9343. A Growth curve of Bacteroides fragilis NCTC9343. B Expression of the BF9343_2953 gene in bacterial culture medium. The relative abundances of the control and gga-miR-222a groups are shown compared to that of the blank group (set as 1). C Methionine concentration in the bacterial culture medium. In the figures, asterisk indicates a significant difference among groups, and significant differences among the means were determined by Tukey’s test (P < 0.05)
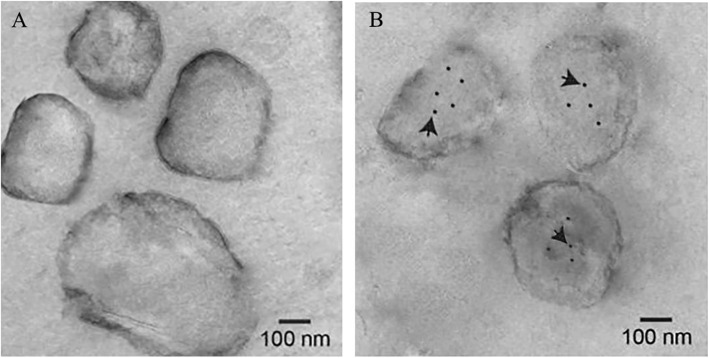
To determine whether gga-miR-222a could be taken up by and exhibit regulatory functions in Bacteroides fragilis NCTC9343, we measured the bacterial internalization of gga-miR-222a by in situ hybridization and TEM. Compared to the control (Fig. 7A), exogenous gga-miR-222a was selectively absorbed by Bacteroides fragilis NCTC9343 in the gga-miR-222a addition group (Fig. 7B).
Fig. 7.
In situ hybridization map of gga-miR-222a internalized in Bacteroides fragilis NCTC9343. In situ hybridization map, where A is the miRNA control group and B is the gga-miR-222a group. Black dots and arrows indicate the site of hybridization
Discussion
Although there is a known association among host genetic background, cecal microbiota structure, and odor production by laying hens [30, 31], the potential mediators of this relationship remain unclear. Recently, a number of findings have demonstrated that mammals such as mice secrete miRNAs that could regulate gene transcripts in bacteria (such as F. nucleatum and E. coli) and affect bacterial growth [26, 32]. In the present study, we presented the first characterization of miRNAs derived from the cecal contents of laying hens and observed that gga-miR-222a can reduce the production of H2S by regulating the expression of cecal microbial methionine synthetase genes in the cecum of laying hens (Fig. 8).
Fig. 8.
The involving pathway of gga-miR-222a reduce the production of H2S in the intestine of laying hens
Differential expression of cecal microbial genes led to dissimilar H2S production between the two hen breeds
In a previous study, we demonstrated that Hy-line hens exhibit lower H2S production than Lohmann hens due to different microbiota structures related to H2S production in the cecum [29]. However, due to the limitation of 16S rRNA sequencing, we did not annotate and identify pathways and genes related to bacterial sulfur metabolism. Transcriptomic sequencing can more accurately elucidate the functional makeup of a microbial community than other methods and allow potential miRNA interactions to be characterized across the microbiome and transcriptome. The gene expression of the cecal microbiota was characterized with respect to H2S production-related pathways through a metatranscriptome analysis. The synthesis of cysteine and methionine requires the participation of sulfur, and its related decomposition is accompanied by the release of sulfur [33]. Higher serine O-acetyltransferase and methionine synthase expression but lower of aspartate aminotransferase and methionine-gamma-lyase expression in the Hy-line cecal microbiota community indicated that the Hy-line hens had a stronger ability to utilize sulfur for the synthesis of cysteine and methionine than the Lohmann hens.
Dissimilatory sulfate reduction is the exclusive sulfate reduction pathway for most sulfate-reducing bacteria (SRB), but assimilatory sulfate reduction can be carried out by most bacteria in the gut [34, 35]. The metatranscriptome results showed that there were no significant differences in the expression of dissimilatory sulfate reduction pathway-related genes, possibly due to the abundance of SRB being low in the animal gut (approximately 0.028–0.097%) [14]. Low abundances of gut SRB led to low and unobvious differential expression of related genes. However, with respect to the assimilatory sulfate reduction pathway, the gene expression of adenylylsulfate kinase in the Hy-line hens was significantly lower than that observed in the Lohmann hens, indicating a greater transformation of sulfate to sulfide in the latter case.
In the present study, we observed that the differentially expressed microbial genes in sulfur-related metabolic pathways led to dissimilar H2S production between the Lohmann and Hy-line hens, but whether the host specifically regulates microbial genes by some factors involved in cross-regulation remains unclear. In the present study, we identified cecal miRNAs and showed that they can directly regulate specific bacterial gene expression and affect gut microbial growth to affect H2S production in laying hens.
Cecal content miRNAs differ between the two laying hen breeds
miRNAs have not been previously characterized in the cecal content of laying hens. First, we demonstrated that microvesicles exist in the cecal contents of Lohmann and Hy-line hens, but only 288 known miRNAs were sequenced. Because the bacterial RNA accounted for the primary proportion of total RNA in the cecal contents of laying hens, and since some miRNAs may be degraded by the high temperature and high uric acid environment of the cecum [36], the number of types and abundances of sequenced miRNAs was relatively low. Only 10 miRNAs were differentially expressed between the Lohmann and Hy-line hens. The highly conserved and homologous characteristics of miRNAs may lead to a high similarity in miRNA types and abundances between two breeds [37]. Most of the chicken genome targets of these miRNAs were enriched in metabolic pathways, neuroactive ligand-receptor interaction, focal adhesion, endocytosis, and purine metabolism but not pathways related to cancer occurrence and disease formation, indicating that these miRNAs did not have a potentially negative effect on the normal life activities of the host. These results indicate that the use of these miRNAs in odor reduction may be harmless to the host itself.
Host-derived miRNAs target the genes of the cecal microbiota of laying hens
miRNAs bind to mRNAs to perform their regulatory functions. We predicted the possible target relationships between differentially expressed miRNAs and differentially expressed microbial genes related to H2S production. The results revealed that gga-miR-222a targets the methionine synthetase genes Odosp_3416 and BF9343_2953 (expressed by Odoribacter splanchnicus and Bacteroides fragilis NCTC 9343, respectively). Therefore, gga-miR-222a may be a host regulator that affects H2S emission in laying hens by regulating the production of methionine, a sulfur-containing amino acid. In vitro fermentation and bacterial cultivation analysis showed that gga-miR-222a can upregulate the Odosp_3416 and BF9343_2953 expression and increase the abundance of Bacteroides fragilis NCTC 9343 at the logarithmic growth period (10 h), which resulted in a higher concentration of methionine but lower H2S production and soluble sulfide levels in fermentation broth and bacterial culture medium. In previous studies, small RNAs from plants, bacteria, and fungi within the order Hypocreales were shown to be ubiquitous in human plasma [38], and the microbiome was observed to play a role in the appropriate regulation of miRNA expression in brain regions implicated in anxiety-like behaviors [39]. The results of these studies demonstrated that miRNAs or small RNAs can function as “regulation messages” between different organisms, and the results of the present study showed that an miRNA of laying hens regulates the physiology of gut bacteria. The concentrations of gut soluble sulfide are positively correlated with the release of H2S [40], and observations of decreased H2S production and soluble sulfide levels showed that gga-miR-222a can reduce H2S emissions in laying hens.
The host-derived miRNA gga-miR-222a influences H2S emission in laying hens
Interestingly, we observed that gga-miR-222a has a positive role in regulating Odosp_3416 and BF9343_2953 expression, contrasting with the results of most studies [41, 42] that have indicated miRNAs always inhibit mRNA transcription or directly degrades mRNAs after binding [41, 42]. However, some studies have shown that miRNAs do not always negatively regulate mRNAs [26, 27, 43]. How miRNAs regulate the expression of genes and affect bacterial growth may be dependent on the function of the genes targeted by the miRNA and the site at which miRNAs binds mRNAs. Binding between miRNAs and bacterial transcripts of the 16S rRNA, yegH, RNaseP, and β-galactosidase genes upregulates their expression and promotes the bacterial growth [31, 32]. In the present study, we observed that gga-miR-222a plays a similar role in the regulation of methionine synthetase gene expression and promoted an increased abundance of bacteria in culture medium, especially in the logarithmic phase. After in situ hybridization, we observed that exogenous gga-miR-222a could be selectively taken up by Bacteroides fragilis NCTC 9343, indicating an intracellular cross-regulatory role of gga-miR-222a. However, the increase in bacterial abundance in fermentation broth was not significant except for a slight rise after gga-miR-222a treatment. The reason for this discrepancy may be that the intestinal environment is more complex and there are many interfering factors, such as interactions among various microorganisms, which is why conducting bacterial growth experiment in a pure culture environment was necessary.
Conclusions
In summary, the results of the present study led to the first identification of host-derived miRNAs in the cecum of laying hens, and the expression profiles of miRNAs were shown to be different between different breeds. It was also demonstrated for the first time that the methionine synthetase genes (metH) Odosp_3416 (expressed by the bacterium Odoribacter splanchnicus) and BF9343_2953 (expressed by the bacterium Bacteroides fragilis NCTC 9343) could be targeted by gga-miR-222a and affected the production of H2S in laying hens. Additionally, gga-miR-222a could enter Bacteroides fragilis NCTC 9343, which increased its abundance in the logarithmic period. Therefore, different profiles of host-derived miRNAs in different breeds of laying hens could affect the production of H2S through gene expression regulation in H2S production-related bacteria. The regulation of H2S production in the cecum of laying hens by host miRNAs such as gga-miR-222a provides the possibility that if these miRNAs could be incorporated into the breeding of laying hens, they could promote the selection of low odor yield and environmentally friendly laying hen breeds.
Methods
Animals and feeding
Approximately 100 Hy-line Gray laying hens and 100 Lohmann Pink laying hens were hatched and fed together at a local hatchery to reduce the differences that may be caused by diet, age, weight, and feeding environment. At 28 weeks of age, 30 Hy-line Gray and 30 Lohmann Pink laying hens with similar weights (1.70 ± 0.02 and 1.71 ± 0.02 kg, respectively) were selected from moved into twelve respiration chambers in an environmentally controlled room for daily H2S production measurements [29], where 6 replicates (chambers) were set, and five birds were fed in one replicate (chamber). The birds were provided water and the commercial egg-type laying hen diet ad libitum (Table S3), and a 12-h light cycle at 24 °C room temperature management schedule was used. At the end of the experiment, all birds were euthanized by cervical dislocation, and then the cecum was ligated at both sides and removed from the gastrointestinal tract. The contents were aseptically collected into an Eppendorf tube containing Bacterial Protect RNA reagent (Qiagen, Hilden, Germany) at an approximate 1:1 ratio (w/v), immediately frozen in liquid nitrogen and stored at − 80 °C until analysis.
Animal ethics statement
All animal experiments were approved by the Animal Experimental Committee of South China Agricultural University (SYXK2014-0136). All experimental steps were performed to decrease animal suffering as much as possible. After the experiment, the bodies of laying hens were incinerated.
The determination of exosomes in the cecum contents
The exosome purification method used in the present study was previously described by Liu et al. Briefly, cecal contents from laying hens were suspended in PBS to 30 mg/mL, centrifuged at 10,000×g for 5 min to remove debris and then filtered through a 0.2-μm filter, after which the filtrates were observed using a Thermo Fisher Talos L120C transmission electron microscope (Thermo Fisher Scientific, MA, USA) [26].
Extraction and analysis of miRNAs from the cecum of laying hens
Total miRNA was extracted using a mirVana™ miRNA Isolation kit (Austin, TX, USA) according to Liu et al. [26]. Briefly, approximately 100 mg of cecal contents were adequately mixed in 600 μL of 1× DPBS, incubated at room temperature for 30 min and then completely homogenized into suspension. Then, 600 μL of acid-phenol:chloroform was added, and the samples were vortexed for 60 s before being then centrifuged for 15 min at 10,000×g to separate the organic and aqueous phases. Subsequently, the aqueous phase was recovered, and 1.25 volumes of 100% ethanol were added for miRNA isolation. For each sample, a filter cartridge was placed into one of the collection tubes (supplied by the kit), the sample was pipetted onto a filter and centrifuged for 90 s at 10,000×g, and then the flow-through was discarded. Then, the filter was washed with 700 μL of miRNA Wash Solution 1 followed by three washes with 700/500/250 μL of Wash Solution 2/3 (supplied by the kit). Finally, the filter was transferred into a fresh collection tube, and 50 μL of nuclease-free water was applied to the center of the filter. Subsequently, the filter was incubated at room temperature for 10 min before being centrifuged for 5 min at 8000×g to recover miRNAs, with the eluate then stored at – 80 °C. Pooled miRNA was prepared by combining equal amounts of extracted miRNA from five birds of the same breed such that each breed was represented by six pooled miRNA samples. miRNA libraries were constructed according to the TruSeq Small RNA Sample Preparation protocol and sequenced with an Illumina HiSeq™ 2500 instrument (Illumina, San Diego, CA, USA). Subsequently, FastQC was used to obtain clean reads from the raw data by removing the joint sequences, low-quality fragments, and sequences < 18 nucleotides (nt) in length. Then, miRDeep2 was used to align the clean sequences to the miRBase database sequences (http://www.mirbase.org/).
Extraction and analysis of RNA of cecal microbiota
RNA was extracted from cecal content aliquots (200 mg) using an RNeasy® PowerMicrobiome kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Briefly, 200 mg of samples were transferred into PowerBead Tubes. Then, cells were lysed according to the kit manual by adding 650 μL of Solution PM1 with β-mercaptoethanol and 100 μL of phenol/chloroform/isoamyl alcohol and vortexing for 10 minutes at maximum speed using a 24-sample vortex adapter (Kelly Bell, Jiangsu, China). Subsequently, the samples were centrifuged at 13,000×g for 1 min at room temperature (15–25 °C), and the supernatants were then transferred to clean 2-mL collection tubes. RNA purification was performed according to the manufacturer’s protocol, and the integrity and quantity of the extracted RNA were measured with a NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Pooled RNA was prepared by homogenization of equal amounts of extracted RNA from five birds of the same breed such that each breed was represented by six pooled RNA samples. The RNA was subjected to standard Illumina library preparation with a TruSeq RNA Sample Prep kit (Illumina, San Diego, CA, USA), and rRNA was depleted using a Ribo-ZeroTM rRNA Removal kit (Epicenter Biotechnologies, Madison, WI, USA). Sequencing was performed on an Illumina HiSeq 2500 sequencer (Illumina, San Diego, CA, USA). Then, the sequences were quality filtered, and poor-quality bases were removed from the raw reads using Cutadapt (v1.9.1). Using a 10-bp sliding window, nucleotides with a mean quality score < 20 were removed, reads with “N” bases (> 10%) and lengths below 75 bp were discarded, and primer sequences and adaptor sequences were also removed. Next, rRNA, tRNA, and host reads were filtered using BWA (v 0.7.5). Putative mRNA reads were then assembled using the Trinity (v2.1.1) de novo assembler. Gene annotation was performed by searching against the protein nonredundant database (NR database), and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis was conducted for gene function classification. After comparing the transcript profiles between the two breeds, we focused on three pathways related to H2S production, including cysteine and methionine metabolism, sulfur metabolism and butyrate metabolism, and the expression of microbial genes associated with these pathways between the two breeds was compared.
Target prediction of differentially expressed miRNAs
After determining the miRNA profiles in the cecal contents of laying hens, the miRNAs exhibiting significantly different expression between the two breeds were used for target prediction analysis of microbial genes related to H2S production exhibiting significantly different expression. The relationships between target bacterial mRNAs and miRNAs were identified using miRanda (http://www.microrna.org). In addition, an analysis of host genome genes targeted by the 10 differentially expressed miRNAs was also conducted with miRanda, and all the target genes were determined to be enriched by KEGG analysis.
In vitro fermentation experiment
The in vitro fermentation experiment was performed as described by Menke and Steingass [44]. Briefly, thirty 28-week-old Hy-line Gray and thirty Lohmann Pink laying hens were sacrificed, and the ceca were immediately ligated. Then, the cecal contents in the same breed group were pooled and thoroughly mixed with fermentation buffer solution preheated at 39 °C. The fermentation buffer solution was homogenized with 474 mL of deionized water, 237 mL of macroelement solution (per 1000 mL, 5.7 g of Na2HPO4, 6.2 g of KH2PO4, and 0.6 g of MgSO4·7H2O), 237 mL of buffer solution (per 1000 mL, 35.0 g of NaHCO3, and 4.0 g of NH4HCO3), 0.12 mL trace element solution (per 100 mL, 13.2 g of CaCl2·2H2O, 10.0 g of MnCl2·4H2O, 1.0 g of CoCl2·6H2O, and 0.8 g of FeCl2·6H2O), and 1.22 mL of resazurin and 50 mL of reductant (per 50 mL, 2.0 mL of 1 mol/L NaOH and 335 mg of Na2S·9H2O). The intestinal content-buffer mixture was blended for 60 s in a blender after the solution was filtered through four layers of surgical gauze, mixed with the fermentation buffer solution at a 1:2 ratio (V/V) and then flushed with CO2 at 40 °C to eliminate all the O2 in the solution. A corn-soybean basal laying hen diet was used as a substrate for fermentation.
After the air in syringe was eliminated from the head space, approximately 10 mL of fermentation broth (FB) was added to a 100-mL gas syringe with 0.2 g of the substrate. Three different groups were designed for each breed, the blank group (10 mL FB + 0.2 g substrate + 1 mL pure water), the control group (10 mL FB + 0.2 g substrate + 1 mL control mimic at a final concentration of 2 μM) and the treatment group (10 mL FB + 0.2 g substrate + 1 mL gga-miR-222a mimic at a final concentration of 2 μM) (Table S4). The miRNAs used in the present study were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Then, these syringes were sealed with clips and placed in incubator set at 42 °C and rotated at 60 rpm for 24 h. At the end of incubation, the syringes were placed on ice to stop fermentation, the gas production was recorded as the volume of head space of the syringe, and the gas was also injected into a gas collection bag for H2S analysis. Ten milliliters of fermentation broth was sampled and stored at − 80 °C for chemical analysis. The quantity of H2S in the gas sample and the concentrations of soluble sulfide (S2−) in the fermentation broth were determined using the methylene blue colorimetric method. Briefly, the adsorption liquid (per 1000 mL, 4.3 g of CdSO4·8H2O, 0.3 g of NaOH, and 10 g of ammonium polyvinyl phosphate) was mixed with gas (10 mL of adsorption liquid) and fermentation broth (adsorption liquid:fermentation broth, V/V = 9:1), after which procedures were followed as described in previous studies [45, 46]. The pH value was determined using a pH meter (INESA Scientific Instrument, Shanghai, China) [29], the concentration of sulfate radicals (SO42−) was determined using the turbidimetric method [29], the concentrations of VFAs were determined using high-performance liquid chromatography [29], and the concentration of methionine in the fermentation broth as tested using an automatic amino acid analyzer (Sykam, Munich, Germany). For the latter analysis, 1 mL of fermentation broth was mixed with 10 mL of hydrochloric acid solution (6 mol/L) followed by 3–4 drops of phenol before being incubated on ice for 3–5 min. Subsequently, the sample was flushed with nitrogen to remove oxygen before incubated in an air oven at 110 °C for 22 h. After cooling at room temperature, the solution was brought to a final volume of 50 mL after filtering using filter paper. The 15 mL of filtered solution was dried at 40–50 °C, and the deposition was washed with deionized water twice and dried again. Then, the precipitate was resuspended in 1 mL of a 0.02-mol/L hydrochloric acid solution, and after filtering through a 0.22-μm membrane, the methionine concentration was determined with an automatic amino acid analyzer.
Bacterial abundance and gene expression in fermentation broth
Following the manufacturer’s instructions, a QIAamp PowerFecal DNA kit (Qiagen, Hilden, Germany) was used to extract DNA from the precipitate (approximately 200 mg) collected from 1 mL of fermentation broth centrifuged at 20,000×g for 1 min at 4 °C. Following the manufacturer’s instructions, an RNeasy® PowerMicrobiome™ kit (Qiagen, Hilden, Germany) was used to extract RNA from the precipitate (approximately 200–250 mg) collected from 1–2 mL of fermentation broth centrifuged at 20,000×g for 1 min at 4 °C.
DNA was used to quantify the relative abundances of Odoribacter splanchnicus and Bacteroides fragilis NCTC 9343. Briefly, primers for these two bacteria were designed using Primer 3, and the genome sequences of the two bacteria were referenced for 16S rRNA sequencing on the NCBI website. The details of the primers are shown in Table S5, and the bacterial 16S rRNA primers were referenced in a previous study [47]. Q-PCR was used to confirm the relative abundances of the two bacteria, and the q-PCR reaction steps followed the protocol for the SYBR® Green PCR kit (SYBR, Japan) (Table S6). The relative abundances of the two bacteria were calculated using the 2ΔCt method, where ΔCt represents the difference in the Ct value for the 16S rRNA gene minus that for the reference genes [48].
Extracted RNA was reverse transcribed into cDNA using a PrimeScript™ RT reagent kit (TaKaRa, Kusatsu, Japan), and the resulting cDNA was used to quantify the expression of Odosp_3416 and BF9343_2953. The primers were designed using the NCBI website using the complete bacterial 16S rRNA gene as the reference gene (Table S5). Q-PCR was used to confirm the relative expression levels of the two genes, and the q-PCR reaction steps followed the protocol for the SYBR® Green PCR kit (SYBR, Japan) with slight modification (Table S7). The relative expression levels of the two genes were calculated using the 2△Ct method, where ΔCt represents the difference in the Ct value for the 16S rRNA gene minus that for the reference genes [48].
In vitro bacterial growth measurements
The anaerobic bacterium Bacteroides fragilis NCTC9343 was cultured at 37 °C by inoculating 40-mL aliquots of anaerobic basal medium (Becton Dickinson and Company, Lincoln Park, IL, USA) and then grown anaerobically in an anaerobic chamber (Mitsubishi Gas Chemical Company, Inc. Tokyo, Japan). gga-miR-222a and the control mimic were added to the culture at a concentration of 2 μM (RiboBio, Guangzhou, China). Growth was monitored as absorbance at 600 nm once per hour for up to 24 h with a spectrophotometer. The cultured bacterial cells were collected after 10 h and used to assess BF9343_2953 expression using the Bacteroides fragilis 16S rRNA gene as the reference gene. The concentrations of methionine in culture medium at 10 h were tested as described above.
In situ hybridization detection of gga-miR-222a uptake
Bacteroides fragilis NCTC9343 cells were centrifuged at 12,000×g and washed twice with ice-cold PBS. Then, the cells were fixed in 4% PFA/0.25% glutaraldehyde. A 5′-DIG and 3′-DIG dual-labeled probe for gga-miR-222a was used for in situ hybridization, and detection of gga-miR-222a uptake by bacteria was assessed using a Thermo Fisher Talos L120C transmission electron microscope Thermo (Fisher Scientific, MA, USA).
Statistical analysis
Comparisons of fermentation incubation indexes; the relative abundances of miRNA, bacteria, and genes; gas production levels; H2S production; and growth curves were examined by analysis of variance (ANOVA) with Statistical Package for the Social Sciences (SPSS), version 22.0. Significant differences between the means were determined by Tukey’s test.
Differences were considered significant at P < 0.05
The metatranscriptomic results of each sample were analyzed using HTSeq using the union model, and the number of genes at different expression levels and the expression levels of individual genes were statistically analyzed. In general, an FPKM value of 0.1 or 1 was used as the threshold for determining whether genes are expressed. DESeq was used to normalize the read counts from analysis of gene expression levels [49].
miRNA expression levels were calculated with the TPM formula [normalization read counts = ((readCount × 1,000,000)/libsize)], where libsize is the sum of the read count of all miRNAs.
Supplementary Information
Additional file 1: Table S1. miRNA sequencing information. Table S2 Metatranscriptomic sequencing information, where (A) shows the metatranscriptomic sequencing information and (B) shows the total transcript length after splicing. Table S3 Diet composition and nutrient levels. Table S4 Group details. Table S5 Primer information for bacterial abundance and gene expression. Table S6 Quantitative PCR thermal cycle programs for bacteria. Table S7 Quantitative PCR thermal cycle programs for genes.
Additional file 2: Fig. S1. KEGG pathway annotation of chicken genome target genes of 10 significantly expressed miRNAs.
Additional file 3: Fig. S2. Metatranscriptomic annotation of the top 30 KEGG metabolic pathways.
Additional file 4: Fig. S3. Relative abundances of Odoribacter splanchnicus and Bacteroides fragilis NCTC9343 in the intestines of Lohmann and Hy-line hens. * Indicates a significant difference between different bacteria, and significant differences between the means were determined by Tukey’s test (P < 0.05).
Acknowledgements
The authors greatly acknowledge the excellent support of the staff of the Zengcheng Experimental Animal Center during sample collection in Guangzhou.
Abbreviations
- H2S
Hydrogen sulfide
- SO42−
Sulfate radical
- S2−
Soluble sulfide
- SRB
Sulfate-reducing bacteria
- VFAs
Volatile fatty acids
Authors’ contributions
SX and CH conducted experiments and manuscript writing. CH, SX, and XL were involved in the acquisition of funding, experimental design and review of the manuscript. RW analyzed the data, and JC and YY performed sample collection. SX, JM, YW, and YBW made manuscript corrections. The authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Natural Science Foundation of China (No. 31872993) and the earmarked fund from the Modern Agroindustry Technology Research System (CARS-41). The funders had no role in the design, analysis, or interpretation of these experiments.
Availability of data and materials
The miRNA and metatranscriptomic sequence data are deposited in the NCBI Sequence Read Archive (SRA) database (PRJNA665380 and PRJNA666118)
Declarations
Ethics approval and consent to participate
The protocol for this experiment was approved by the Animal Experimental Committee of South China Agricultural University (SYXK2014-0136).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Si-Cheng Xing and Chun-Bo Huang contributed equally as first authors.
Contributor Information
Si-Cheng Xing, Email: 472776374@qq.com.
Chun-Bo Huang, Email: 384233521@qq.com.
Yi-Wen Yang, Email: 775999552@qq.com.
Jing-Yuan Chen, Email: 929904035@qq.com.
Jian-Dui Mi, Email: mijiandui@163.com.
Yin-Bao Wu, Email: wuyinbao@scau.edu.cn.
Yan Wang, Email: 39169401@qq.com.
Xin-Di Liao, Email: xdliao@scau.edu.cn.
References
- 1.Sparks NHC. The hen's egg-is its role in human nutrition changing? Worlds Poultry Sci J. 2006;62(2):308–315. doi: 10.1079/WPS200599. [DOI] [Google Scholar]
- 2.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson ME, Jakobsson HE, Holmen-Larsson J, Schutte A, Ermund A, Rodriguez-Pineiro AM, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18(5):582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson JL, Erickson JM, Hess JM, Gould TJ, Slavin JL. Prebiotic dietary fiber and gut health: comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients. 2017;9(12):1361. doi: 10.3390/nu9121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. 2014;360(2):100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- 7.Stokstad E. Air pollution. Ammonia pollution from farming may exact hefty health costs. Science. 2014;343(6168):238. doi: 10.1126/science.343.6168.238. [DOI] [PubMed] [Google Scholar]
- 8.Xin H, Gates RS, Green AR, Mitloehner FM, Moore PJ, Wathes CM. Environmental impacts and sustainability of egg production systems. Poult Sci. 2011;90(1):263–277. doi: 10.3382/ps.2010-00877. [DOI] [PubMed] [Google Scholar]
- 9.Gemert LJV. Compilations of odour threshold values in air, water and other media. 2003. [Google Scholar]
- 10.Hunde A, Patterson P, Ricke S, Kim WK. Supplementation of poultry feeds with dietary zinc and other minerals and compounds to mitigate nitrogen emissions—a review. Biol Trace Elem Res. 2012;147(1-3):386–394. doi: 10.1007/s12011-011-9310-8. [DOI] [PubMed] [Google Scholar]
- 11.Loubinoux J, Bronowicki JP, Pereira IA, Mougenel JL, Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 2002;40(2):107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Chen M, Jin X, Li X, Yang Z, Lin H, et al. H2S induces Th1/Th2 imbalance with triggered NF-κb pathway to exacerbate lps-induce chicken pneumonia response. Chemosphere. 2018;208(OCT):241–246. doi: 10.1016/j.chemosphere.2018.05.152. [DOI] [PubMed] [Google Scholar]
- 13.Wu-Haan W, Powers WJ, Angel CR, Hale CR, Applegate TJ. Effect of an acidifying diet combined with zeolite and slight protein reduction on air emissions from laying hens of different ages. Poult Sci. 2007;86(1):182–190. doi: 10.1093/ps/86.1.182. [DOI] [PubMed] [Google Scholar]
- 14.Deng YF, Liu YY, Zhang YT, Wang Y, Liang JB, Tufarelli V, et al. Efficacy and role of inulin in mitigation of enteric sulfur-containing odor in pigs. J Sci Food Agric. 2017;97(8):2382–2391. doi: 10.1002/jsfa.8050. [DOI] [PubMed] [Google Scholar]
- 15.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343r–382r. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmody RN, Gerber GK, Luevano JJ, Gatti DM, Somes L, Svenson KL, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301(5631):336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 18.Chen T, Xi QY, Ye RS, Cheng X, Qi QE, Wang SB, et al. Exploration of microRNAs in porcine milk exosomes. BMC Genomics. 2014;15(100):100. doi: 10.1186/1471-2164-15-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modepalli V, Kumar A, Hinds LA, Sharp JA, Nicholas KR, Lefevre C. Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii) BMC Genomics. 2014;15(1012):1012. doi: 10.1186/1471-2164-15-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Title AC, Denzler R, Stoffel M. Uptake and function studies of maternal milk-derived MicroRNAs. J Biol Chem. 2015;290(39):23680–23691. doi: 10.1074/jbc.M115.676734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witwer KW, McAlexander MA, Queen SE, Adams RJ. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: Limited evidence for general uptake of dietary plant xenomiRs. RNA Biol. 2013;10(7):1080–1086. doi: 10.4161/rna.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Y, Li X, Zhu Y, Li N, Zhang N, Niu M. Faecal microRNA as a biomarker of the activity and prognosis of inflammatory bowel diseases. Biochem Biophys Res Commun. 2018;503(4):2443–2450. doi: 10.1016/j.bbrc.2018.06.174. [DOI] [PubMed] [Google Scholar]
- 24.Link A, Balaguer F, Shen Y, Nagasaka T, Lozano JJ, Boland CR, et al. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomark Prev. 2010;19(7):1766–1774. doi: 10.1158/1055-9965.EPI-10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y, Gao J, Liu JQ, Wang XW, Gu JJ, Huang HJ, et al. Differential signature of fecal microRNAs in patients with pancreatic cancer. Mol Med Rep. 2012;6(1):201–209. doi: 10.3892/mmr.2020.11132. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Da CA, Rezende RM, Cialic R, Wei Z, Bry L, et al. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe. 2016;19(1):32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, et al. Plant-Derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu S, Zhao Z, Xu X, Li M, Li P. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 2019;272:372–378. doi: 10.1016/j.foodchem.2018.08.059. [DOI] [PubMed] [Google Scholar]
- 29.Huang CB, Xiao L, Xing SC, Chen JY, Yang YW, Zhou Y, et al. The microbiota structure in the cecum of laying hens contributes to dissimilar H2S production. BMC Genomics. 2019;20(1):770. doi: 10.1186/s12864-019-6115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kers JG, Velkers FC, Fischer E, Hermes G, Stegeman JA, Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman S. Effects of laying-hen strain on manure properties and ammonia emission. T Asabe. 2012;55(3):1059–1065. doi: 10.13031/2013.41510. [DOI] [Google Scholar]
- 32.Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26(6): 779-794.e8 [DOI] [PMC free article] [PubMed]
- 33.Kondoh M, Hirasawa T. L-Cysteine production by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2019;103(6):2609–2619. doi: 10.1007/s00253-019-09663-9. [DOI] [PubMed] [Google Scholar]
- 34.Brunner B, Bernasconi SM. A revised isotope fractionation model for dissimilatory sulfate reduction in sulfate reducing bacteria. 2005;69(20):4759–71.
- 35.Marietou A, Roy H, Jorgensen BB, Kjeldsen KU. Sulfate transporters in dissimilatory sulfate reducing microorganisms: a comparative genomics analysis. Front Microbiol. 2018;9:309. doi: 10.3389/fmicb.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Y, Underwood A, Gielbert A, Woodward MJ, Petrovska L. Metaproteomics analysis reveals the adaptation process for the chicken gut microbiota. Appl Environ Microbiol. 2014;80(2):478–485. doi: 10.1128/AEM.02472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibanez-Ventoso C, Vora M, Driscoll M. Sequence relationships among C. Elegans, D. Melanogaster and human microRNAs highlight the extensive conservation of microRNAs in biology. PLoS One. 2008;3(7):e2818. doi: 10.1371/journal.pone.0002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beatty M, Guduric-Fuchs J, Brown E, Bridgett S, Chakravarthy U, Hogg RE, et al. Small RNAs from plants, bacteria and fungi within the order Hypocreales are ubiquitous in human plasma. BMC Genomics. 2014;15(1):1–12. doi: 10.1186/1471-2164-15-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoban AE, Stilling RM, Moloney GM, Moloney RD, Shanahan F, Dinan TG, et al. Microbial regulation of microrna expression in the amygdala and prefrontal cortex. Microbiome. 2017;5:102. doi: 10.1186/s40168-017-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muezzinoglu A. A study of volatile organic sulfur emissions causing urban odors. Chemosphere. 2003;51(4):245–252. doi: 10.1016/S0045-6535(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 41.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19(6):586–593. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- 42.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304(5670):594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 43.Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30(4):460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Wang A, Wang Y, Liao X, Wu Y, Liang JB, Laudadio V, et al. Sodium butyrate mitigates in vitro ammonia generation in cecal content of laying hens. Environ Sci Pollut Res Int. 2016;23(16):16272–16279. doi: 10.1007/s11356-016-6777-z. [DOI] [PubMed] [Google Scholar]
- 45.Strocchi A, Furne JK, Levitt MD. A modification of the methylene blue method to measure bacterial sulfide production in feces. J Microbiol Methods. 1992;15(2):75–82. doi: 10.1016/0167-7012(92)90071-B. [DOI] [Google Scholar]
- 46.Sands AE. The determination of low concentrations of hydrogen sulfide in gas by the methylene blue method: US Department of the Interior, Bureau of Mines; 1949.
- 47.Denman SE, Mcsweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006;58(3):572–582. doi: 10.1111/j.1574-6941.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, et al. Diverse and abundant antibiotic resistance genes in chinese swine farms. Pna. 2013;110(9):3435–3440. doi: 10.1073/pnas.1222743110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing SC, Mi JD, Chen JY, Xiao L, Wu YB, Liang JB, et al. The metabolism and morphology mutation response of probiotic bacillus coagulans for lead stress. Sci Total Environ. 2019;693:133490. doi: 10.1016/j.scitotenv.2019.07.296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. miRNA sequencing information. Table S2 Metatranscriptomic sequencing information, where (A) shows the metatranscriptomic sequencing information and (B) shows the total transcript length after splicing. Table S3 Diet composition and nutrient levels. Table S4 Group details. Table S5 Primer information for bacterial abundance and gene expression. Table S6 Quantitative PCR thermal cycle programs for bacteria. Table S7 Quantitative PCR thermal cycle programs for genes.
Additional file 2: Fig. S1. KEGG pathway annotation of chicken genome target genes of 10 significantly expressed miRNAs.
Additional file 3: Fig. S2. Metatranscriptomic annotation of the top 30 KEGG metabolic pathways.
Additional file 4: Fig. S3. Relative abundances of Odoribacter splanchnicus and Bacteroides fragilis NCTC9343 in the intestines of Lohmann and Hy-line hens. * Indicates a significant difference between different bacteria, and significant differences between the means were determined by Tukey’s test (P < 0.05).
Data Availability Statement
The miRNA and metatranscriptomic sequence data are deposited in the NCBI Sequence Read Archive (SRA) database (PRJNA665380 and PRJNA666118)