Abstract
Backgrounds
The present study explored the viability of bovine milk macrophages, their intracellular production of reactive oxygen and nitrogen species (RONS), and their phagocytosis of Staphylococcus aureus, as well as the profile of lymphocytes, from healthy udder quarters and udder quarters infected by Corynebacterium bovis. The study included 28 healthy udder quarters from 12 dairy cows and 20 udder quarters infected by C. bovis from 10 dairy cows. The percentages of macrophages and lymphocytes were identified by flow cytometry using monoclonal antibodies. Macrophage viability, RONS production, and S. aureus phagocytosis were evaluated by flow cytometry.
Results
Milk samples from quarters infected with C. bovis showed a lower percentage of macrophages but an increased number of milk macrophages per mL and a higher percentage of macrophages that produced intracellular RONS and phagocytosed S. aureus. No effect of C. bovis infection on macrophage viability was found. Udder quarters infected by C. bovis showed a higher percentage of T cells and CD4+ T lymphocytes, but no effect was found on the percentage of CD8+ CD4− T, CD8− CD4− T, or B lymphocytes.
Conclusions
Thus, our results corroborate, at least in part, the finding that intramammary infections by C. bovis may offer protection against intramammary infections by major pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-021-02989-5.
Keywords: Immune response, Phagocytes, T lymphocyte, Mastitis, Milk, Dairy cow
Background
Corynebacterium bovis is one of the bacteria most commonly isolated from aseptically collected bovine milk samples worldwide that are subjected to microbiological examination to identify the pathogens that cause bovine mastitis [1–4]. Despite its high prevalence in the etiology of intramammary infections in cattle, C. bovis is considered a minor mastitis pathogen with limited clinical significance [5]. From another point of view, it has been considered part of the udder core microbiota with potential protective role against dysbiosis [6–8]. This bacterium colonizes the teat apices [9, 10], teat canal [11] but can also be isolated from the teat cistern, gland cistern, and mammary parenchyma [12]. Although the milk somatic cell count (SCC), which is an inflammatory indicator widely used in the diagnosis of bovine mastitis, is relatively low in milk samples from udder quarters from which C. bovis is isolated, their SCC value is still usually higher compared to healthy udder quarters [1, 13, 14]. No effect on milk production [4, 14] or on the percentage of fat, protein, casein, and total solids was described [4].
C. bovis is of interest to mastitis researchers because quarters infected with this bacterium are less likely to become infected with other, more pathogenic bacteria [6, 13, 15–19]. Previous studies conducted by our research group investigated the functions of milk neutrophils (e.g., viability, phagocytosis and intracellular reactive oxygen species production) in udder quarters infected with C. bovis [20, 21]. Thus, to deepen our understanding of the mammary gland immunity in C. bovis-infected udder quarters, this study aimed to investigate the lymphocyte profile of milk and the function of milk macrophages of healthy mammary glands compared to udder quarters naturally infected with C. bovis.
Results
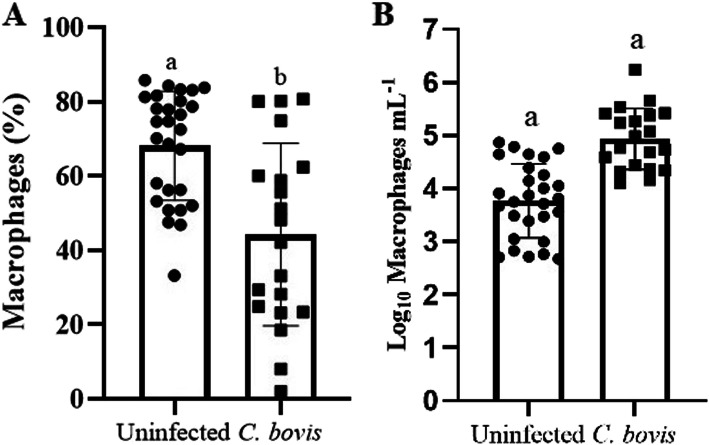
Here, no statistical difference on days in milk between uninfected (192.8 ± 19.8) and C. bovis-infected (223.6 ± 17.5) quarters was found (P = 0.53). The logarithmic milk SCC in C. bovis-infected quarters was higher than healthy ones (P < 0.0001). The percentage of milk macrophages was higher in healthy udder quarters (68.11 ± 2.78; P = 0.0008) than in quarters infected by C. bovis (44.25 ± 5.49; Fig. 1A), nonetheless the logarithmic number of macrophages per mL was higher in C. bovis-infected quarters (4.93 ± 0.13) than in healthy ones (3.77 ± 0.13, P < 0.0001; Fig. 1B). The percentages of viable milk macrophages (Annexin V−/PI−; Healthy quarters: 41.73 ± 3.30, C. bovis-infected quarters: 40.29 ± 4.33, P = 0.79) and apoptotic milk macrophages (Annexin V+/PI−; Healthy quarters: 39.20 ± 3.58, C. bovis-infected quarters = 31.86 ± 4.59, P = 0.17) did not differ by infection status.
Fig. 1.
The antidromic trend of the percentage and number of milk macrophages in Corynebacterium bovis-infected quarters. Percentage (mean ± standard error) of milk macrophages (A) and the number of milk macrophages per mL (B) in healthy mammary quarters vs. mammary quarters infected with C. bovis. Different letters indicated P ≤ 0.05
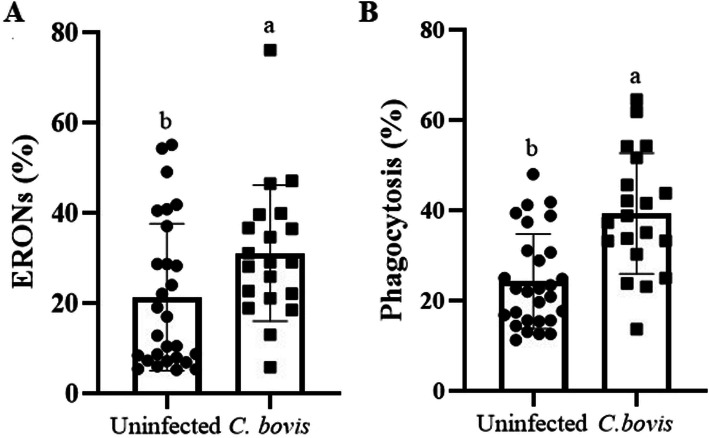
The percentage of milk macrophages that produced RONS was higher in udder quarters infected by C. bovis (31.12 ± 3.37; P = 0.035) than in those considered healthy (21.34 ± 3.082; Fig. 2A). However, the intensity of RONS production by milk macrophages did not differ between the udder quarters infected with C. bovis (1168 ± 177.3; P = 0.28) and healthy udder quarters (978.5 ± 72.39; Supplemental Material 2). Similarly, the percentage of macrophages that phagocytosed S. aureus was higher in udder quarters infected with C. bovis (39.36 ± 2.99; Fig. 2B) than in those considered healthy (24.32 ± 1.98; P = 0.0001). However, the intensity of phagocytosis did not differ between the healthy udder quarters (84.30 ± 6.89) and those infected with C. bovis (71.79 ± 8.00; P = 0.22; Supplemental Material 2).
Fig. 2.
Corynebacterium bovis-infected quarters was associated with a higher phagocytosis and intracellular RONS by milk macrophages. Percentage (mean ± standard error) of milk macrophages that produced RONS (A) and phagocytized S. aureus (B) in healthy mammary quarters vs. mammary quarters infected with C. bovis. RONS: reactive oxygen and nitrogen species. Different letters indicated P ≤ 0.05
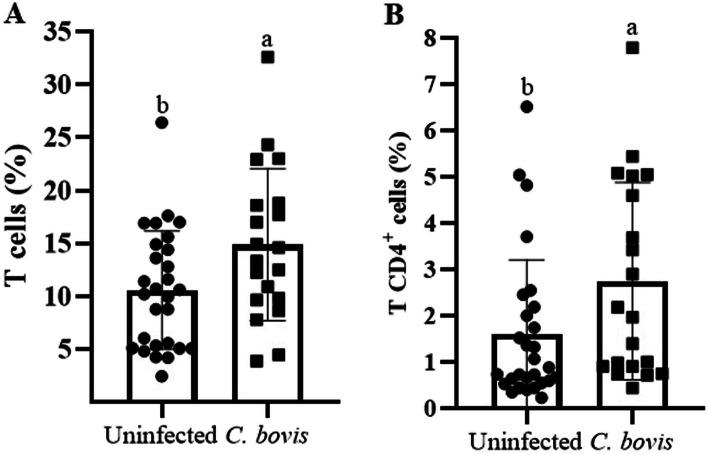
Corynebacterium bovis-infected udder quarters had higher percentages of milk T cells (CD3+; P = 0.02, Fig. 3A) and T CD4+ lymphocytes (P = 0.02, Fig. 3B) than uninfected ones, but no effect on the percentage of CD8+ CD4- T cells, CD8- CD4- T lymphocytes, or B cells (CD21+) was found (Supplemental Material 1).
Fig. 3.
Corynebacterium bovis-infected quarters had a higher percentage of T cells, especially T CD4+ lymphocytes. Percentages (mean ± standard error) of T lymphocytes (A) and CD4+ T lymphocytes (B) in healthy mammary quarters and mammary quarters infected with C. bovis. Different letters indicated P ≤ 0.05
Discussion
Here, we used milk samples from healthy and C. bovis-infected udder quarters from different dairy cows as well as from the same cow. In this regard, we should highlight that the immune response against C. bovis is primarily restricted to the C. bovis-infected quarter [5, 6, 20], and the interdependence of udder quarters was also regarded in the statistical analysis of the present study, as previously suggested [22, 23].
Although it is difficult to draw consistent conclusions about the role of C. bovis on bovine udder health, there is an increasing evidence that C. bovis is part of udder microbiota [7, 11], which could modulate the local immune response [7, 24]. Thus, a recent study published by Porcellato et al. [7] using milk microbiome analysis associate the presence of the genera Corynebacterium in milk samples with a protective role against bovine mammary gland dysbiosis. In another study that investigated the association of teat canal microbiome analysis and mastitis susceptibility and udder inflammation, Corynebacterium identification was negatively correlated with milk SCC [11]. Additionally, the Corynebacterium genera dominate the bacterial populations of teat apices from healthy quarters [9]. Altogether, although the mechanisms behind these phenomena remain to be fully determined, our findings indicated that C. bovis could positively impact udder health by optimizing mammary gland immunity. In this scenario, for the first time, we observed an augment in the percentage of T cells, especially T CD4+ cells, as well as a higher percentage of milk macrophages that phagocytosed S. aureus and produced RONS in C. bovis-infected quarters.
The ability of cows to resist the establishment of new intramammary infections or to overcome existing intramammary infections depends on the efficiency the mammary gland immunity. In this context, macrophages play a critical role in the initiation of the innate immune response of the host in case of bacterial (i.e., Streptotoccus uberis) invasion into the mammary gland [25, 26], beyond their role on adaptative immunity, such as antigen processing and presentation [27]. Similarly, T CD4+ lymphocytes have been associated with clearance activity against several bacterial pathogens [28–30]. Furthermore, cows having higher frequency of T CD4+ lymphocytes than T CD8+ lymphocytes in their mammary gland secretions appear to be more resistant to mastitis [31]. Overall, these findings support, at least in part, the potential protective role of C. bovis in the bovine udder.
In the present study, no perturbation on CD8+ T lymphocytes was found in quarters in which C. bovis was isolated, although the percentage of this lymphocyte subpopulation increased during staphylococcal and streptococcal intramammary infections [32]and may play a suppressive and cytotoxic role in the mammary gland [27, 33, 34]. In addition, the percentage of CD4− CD8− T lymphocytes, mainly represented by γδ T cells [35] was not affected by C. bovis. Furthermore, the macrophage apoptosis did not appear to be involved in the inflammatory response during C. bovis-infection.
Thus, our results corroborate other studies that found a high correlation between isolation of C. bovis from aseptically collected milk samples and the reduction in the occurrence of intramammary infection by other pathogenic bacteria [6, 13, 16–18], although there is no consensus on this assertion [36–39]. In addition to our data regarding the lymphocytic and functional profile of milk macrophages, other factors may be related to the potential protective effect of intramammary infection by C. bovis, such as inhibition of growth by competition, bacterial antagonism, increased activity of milk neutrophils, and the presence of plasmocytes in the parenchymal tissue and the teat apices of quarters colonized with C. bovis [6, 16, 21, 40].
Furthermore, macrophages represent the first line of defense of the mammary gland against invasive pathogens because they make up the predominant leukocyte population in healthy udder quarters [27, 30, 41]. Although the proportion of macrophages usually decreases during intramammary infections due to the rapid and massive influx of neutrophils into the inflammatory site [19, 42], macrophages still represented the predominant leukocyte population in quarters infected by C. bovis in the present study. Besides that, an increase in the number of macrophages per mL was observed in C. bovis-infected quarters.
Conclusions
The present study by evaluating the phagocytosis of milk macrophages and the percentage of T lymphocytes corroborates with previous findings indicating that isolation of C. bovis from aseptically collected milk samples could be associated with a protection against the major mastitis pathogens, such as S. aureus. However, the mechanisms behind these findings need to be further in-depth investigated, which also consider the role of C. bovis in the complexity of mammary gland microbiota and how exactly this microorganism acts to promote udder health.
Methods
Animals and sampling
The present study used 48 udder quarters from 18 clinically healthy Holstein dairy cows (daily milk yield = 24.02 ± 1.97) on their second and third lactation, collected at different lactation stages from a commercial herd. Immediately postpartum dairy cows were not included. From these dairy cows, we selected 20 C. bovis-infected quarters from 10 dairy cows and 28 culture-negative control quarters from 12 dairy cows with no abnormal secretions in the strip cup test and a quarter SCC lower than 1 × 105 cells/mL, as the threshold for SCC described by Bansal et al. [43] for uninfected quarters.
First, the strip cup test was carried out to detect potential clinical mastitis cases. Then, a single milk quarter sample (about 4 mL) was aseptically collected for bacteriological analysis as recommended by National Mastitis Council [44]. Furthermore, quarter milk samples for SCC measurements (40 mL) were taken in sterile tubes containing micropellets of Bronopol (2-bromo-2-nitroprane-1,3-diol). Finally, 1 L of milk samples for the evaluation of monocyte/macrophage function and lymphocyte profile were collected. Until milk samples arrived at the laboratory, they were maintained at 4 °C. Subsequently, quarter milk samples for bacteriological examination were retained at -20 °C until the analysis.
Afterwards, all samples were randomized and codified, and milk analyses were carried out without knowledge of the status of the udder quarter. All methods were carried out in accordance with relevant guidelines and regulations.
Bacteriological culture
The bacterial analysis was carried out by culturing 0.01 mL of each milk quarter sample on 5 % ovine blood agar plates. The plates were incubated for 72 h at 37 °C, followed by Gram staining, observation of colony morphology, and biochemical testing [45]. A milk sample was considered culture positive when the growth of ≥ 4 pure C. bovis colonies was detected [20]. A sample was considered culture negative if there was no growth (no colony from a 0.01 mL sample; < 100 colony-forming units per mL).
Determination of the somatic cell count
The SCC was determined with the automated somatic cell counter Somacount 300 (Bentley Instruments, Chaska, MN, USA).
Separation of milk cells
Milk cells were separated as previously described by Blagitz et al. [20]. Briefly, 1 L of milk was diluted in 1 L of phosphate-buffered saline solution (PBS). Centrifugation at 1000×g was performed for 15 min, and the fat layer and the supernatant were discarded. The cell pellet was then washed again with 30 mL of PBS solution and centrifuged at 400 × g for 10 min. This cell pellet was resuspended in 1 mL of RPMI-1640 cell culture medium (R7638, Sigma Aldrich, USA) supplemented with 10 % fetal bovine serum (Cultilab, Brazil), and then the cells were counted in a Neubauer chamber. Cell viability was initially assessed by exclusion using Trypan blue. The cells present in the milk were then resuspended in cell culture medium containing 10 % fetal bovine serum at a concentration of 2 × 106 mL− 1 viable cells.
Enumeration of lymphocyte subpopulations
The enumeration of lymphocyte subsets was performed as previously described [30] with some slight modifications. Briefly, the cells were washed with PBS and stained with a combination of CD3, CD4, and CD8 (tube 1) and for CD21 (tube 2) for 30 min at room temperature. The lymphocyte subpopulations were identified based upon their cytoplasmatic granularity and mean fluorescence intensity following two-step fluorescence immunolabeling with primary anti-bovine monoclonal antibodies (mAbs) and the secondary antibody (Ab) coupled to the long-wavelength fluorescent probes (Supplemental Material 3). After washing with PBS, the cells were incubated for 30 min at room temperature with the secondary Abs. The cells were subsequently washed with PBS and quickly evaluated by flow cytometry (FACSCalibur, BD Bioscience, San Jose, CA, USA). Here, 20,000 milk cells, excluding most of the cell debris, were examined in each quarter milk sample. A single-stained, fluorochrome-conjugated secondary Ab control and unstained control milk samples were also prepared as compensation controls. FlowJo software (TreeStar Inc., Ashland, OR, USA) was used to analyze the data.
Identification of milk macrophages
The identification of macrophages was carried out as previously described [46]. Initially, the cells were incubated with 1 µL of mouse IgG1 mAb against bovine CD14 (cat. n. MM61A, VMRD, Pullman, WA, USA) for 30 min at room temperature. Immediately after, 1 mL of PBS was added to the specific cytometry tube, and the samples were centrifuged for 8 min at 400 × g. Next, 1 µL of allophycocyanin-conjugated goat anti-mouse IgG1 secondary antibody (cat. n. A10541, Invitrogen, Carlsbad, CA, USA) was added to the samples, which were incubated for 30 min at room temperature. Then, PBS solution (1 mL) was added to the cell suspension, which was centrifuged for 8 min at 400 × g. Lastly, PBS (300 µL) was added to the samples, which were examined by flow cytometry (FACSCalibur, BD Bioscience, San Jose, CA, USA). A single-stained, fluorochrome-conjugated secondary Ab control and unstained control milk samples were also prepared as compensation controls. FlowJo software (TreeStar Inc., Ashland, OR, USA) was used to analyze the data.
Preparation of Staphylococcus aureus stained with propidium iodide
The staining of Staphylococcus aureus (ATCC 25,923) with propidium iodide (PI) was done as proposed [47, 48].
Intracellular production of reactive oxygen and nitrogen species
The intracellular production of RONS was measured by flow cytometry as described [30, 41, 48]. Briefly, 2 × 105 viable milk cells were incubated with 200 µL of 2′,7′-dichlorofluorescein diacetate (DCFH2-DA, 0.3 mM, cat. n. D6883, Sigma Aldrich, St. Louis, USA) for 30 min at 37°C. Various types of RONS (hydrogen peroxide, peroxynitrite, nitric oxide, hydroxyl radicals, and peroxyl) oxidize DCFH2-DA into 2’,7’-dichlorofluorescein (DCF), which is fluorescent and can be detected in a flow cytometer equipped with a set of standard filters for fluorescein green [49]. After the incubation in DCFH2-DA, 2 mL of 3 mM EDTA was added. Macrophages were identified using the CD14 mAb as described above. Finally, the samples were centrifuged at 400×g for 10 min, and the leukocytes were resuspended in 300 µL of PBS and analyzed by flow cytometry.
In the present study, 20,000 cells per sample were examined – most cellular debris was excluded. The readings of the samples were performed in a flow cytometer with argon (excitation 488 nm) and diode lasers (excitation 635 nm) (FACSCalibur, BD Bioscience, San Jose, CA, USA). FlowJo software (TreeStar Inc., Ashland, OR, USA) was used to examine the data. The data are presented as the percentage of macrophages (CD14+ cells) that produced RONS (percentage of fluorescent cells), and the geometric mean fluorescence intensity (GMFI) indicated the intensity of RONS production of each cell. The results were corrected for autofluorescence content using nonstained milk cells from milk samples from the same udder quarter.
Phagocytosis
The phagocytosis assay was performed by flow cytometry using S. aureus conjugated with propidium iodide (PI) as previously described [41, 46, 48]. Briefly, 2 × 105 viable milk cells were incubated with 100 µL of PI-conjugated S. aureus for 30 min at 37 °C and 900 µL of PBS. Then 2 mL of 3 mM EDTA was added to drastically reduce the number of bacteria adhering to the cell membrane that could be mistakenly considered phagocytized [47, 50]. The macrophages were identified using the CD14 mAb as described above. Finally, the samples were centrifuged at 400×g for 10 min, and the leukocytes were resuspended in 300 µL of PBS and analyzed by flow cytometry.
As above, 20,000 cells per sample were examined, and most cellular debris was excluded. FlowJo software (TreeStar Inc., Ashland, OR, USA) was used to examine the data. The data are presented as the percentage of macrophages (CD14+ cells) that phagocytized PI-stained bacteria (percentage of fluorescent cells), and the GMFI indicates the number of bacteria phagocytized by macrophages that phagocytosed S. aureus by measuring the fluorescence intensity, which was correlated with the number of phagocytized bacteria per cell. The results were corrected for autofluorescence content using nonstained milk cells from milk samples from the same udder quarter.
Detection of apoptosis by flow cytometry
Apoptosis of milk macrophages was determined by double staining with annexin-V conjugated to fluorescein isothiocyanate (FITC) and propidium iodide (PI) by flow cytometry analysis using a commercial kit (cat. n. K2350, APOPTEST-FITC, DakoCytomation, Netherlands), as previously described [30, 41, 48]. Initially, 2 × 105 milk cells were resuspended in 100 µL of binding buffer (10 mM HEPES, 150 mM NaCl, 1 mM MgCl2 and 1.8 mM CaCl2) containing annexin-V FITC and incubated at room temperature for 20 min in the dark. The macrophages were identified using the CD14 mAb as described above. Immediately before the flow cytometry analysis, 5 µL of a PI solution (250 µg mL− 1) was added. Cells negative for FITC-stained annexin-V and for PI were considered alive. Cells that were reactive to FITC-stained annexin-V but negative to PI were classified as apoptotic. Again 20,000 cells were examined per sample, and most cellular debris were excluded. FlowJo software (TreeStar Inc., Ashland, OR, USA) was used to examine the data.
Statistical analysis
The distributions of all variables were analyzed using normal probability plots obtained from the Shapiro-Wilk test. As all data presented high coefficient of variation, we carried out a logarithmic transformation (Log10). First, interclass correlation at the cow and quarter levels was calculated to determine the strength of clustering, as previously described by McGraw and Wong [51]. The data were analyzed ANOVA following by the pos-hoc Student-Newnan-Keuls test was applied. The model of mammary quarters and cows nested within cows was considered [23]. Statistical analyses were performed using the statistical software InfoStat (Cordoba, Argentina). The results are presented as the mean ± standard error. The level of significance was set at P ≤ 0.05.
Supplementary Information
Additional file 1: Supplemental Table 1. Percentage (mean ± standard error) of B and T lymphocytes in milk samples from healthy mammary quarters infected with Corynebacterium bovis.
Additional file 2: Supplemental Figure 1.
Additional file 3: Supplemental Table 2. Monoclonal antibodies used for immunophenotyping bovine milk lymphocytes by flow cytometry.
Acknowledgements
FNS thanks FAPESP for the scholarship awarded (Process no. 2014/23189-4). AMMPDL thanks the National Council for Scientific and Technological Development (CNPq) for the scholarship awarded.
Abbreviations
- SCC
Somatic cell count
- PBS
Phosphate-buffered saline solution
- mAb
Monoclonal antibody
- ab
Antibody
- PI
Propidium iodide
- RONS
Reactive oxygen and nitrogen species
- DCFH2-DA
2′,7′-dichlorofluorescein diacetate
- DCF
2’,7’-dichlorofluorescein
- GMFI
Geometric mean fluorescence intensity
- FITC
Fluorescein isothiocyanate
Authors’ contributions
VMS and MTS drafted and edited the manuscript. MGB performed the experiments and designed the studies. CFB participated in flow cytometry analysis. AJA, ACCF, EMRS, COR and LC provided technical help and edited the manuscript. AMMPDL designed and supervised the studies. FNS performed the analysis, designed the studies, and edited the manuscript. All authors have read and agreed to the published version of this manuscript. VMS, MTS and MGB have contributed equally to this work and share co-first authors. The author(s) read and approved the final manuscript.
Funding
The authors are thankful for the financial support from the São Paulo Research Foundation (FAPESP Project No. 2009/50672-0) and the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES). FAPESP and CAPES had no role in the study design; collection, analysis, or interpretation of data; or decision to submit the article for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study complied with the Ethical Principles in Animal Research and was approved by the Bioethics Commission of the School of Veterinary Medicine and Animal Science, University of São Paulo (Protocol n. 1685/2009). All methods were carried out in accordance with relevant guidelines and regulations. In this regard, the authors state that this study was performed in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson DJ, Gonzalez RN, Das HH. Bovine mastitis pathogens in the New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J. Dairy Sci. 1997;80:2592–2598. doi: 10.3168/jds.S0022-0302(97)76215-5. [DOI] [PubMed] [Google Scholar]
- 2.Langoni H, Camargo da Silva CP, Troncarelli MZ, Tata A, Belaz KRA, Eberlin MN, Joaquim SF, Guimarães FF, Pardo RB, Gomes EN. Short communication: Identification of Corynebacterium bovis by MALDI-mass spectrometry. J. Dairy Sci. 2017;100:4287–4289. doi: 10.3168/jds.2016-11922. [DOI] [PubMed] [Google Scholar]
- 3.Dalen G, Rachah A, Nørstebø H, Schukken YH, Reksen O. Dynamics of somatic cell count patterns as a proxy for transmission of mastitis pathogens. J Dairy Sci. 2019;102(12):11349–11358. doi: 10.3168/jds.2019-16847. [DOI] [PubMed] [Google Scholar]
- 4.Gonçalves JL, Tomazi T, Barreiro JR, Beuron DC, Arcari MA, Lee SH, Martins CM, Araújo Junior JP, dos Santos MV. Effects of bovine subclinical mastitis caused by Corynebacterium spp. on somatic cell count, milk yield and composition by comparing contralateral quarters. Vet J. 2016;209:87–92. doi: 10.1016/j.tvjl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Huxley JN, Helps CR, Bradley AJ. Identification of Corynebacterium bovis by endonuclease restriction analysis of the 16S rRNA gene sequence. J Dairy Sci. 2004;87:38–45. doi: 10.3168/jds.S0022-0302(04)73139-2. [DOI] [PubMed] [Google Scholar]
- 6.Brooks BW, Barnum DA. Experimental colonization of the bovine teat duct with Corynebacterium bovis and the effect on milk somatic cell counts. Can J Comp Med. 1984;48:141–145. [PMC free article] [PubMed] [Google Scholar]
- 7.Porcellato D, Meisal R, Bombelli A, Narvhus JA. A core microbiota dominates a rich microbial diversity in the bovine udder and may indicate presence of dysbiosis. Sci Rep. 2020;10(1):21608. doi: 10.1038/s41598-020-77054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rault L, Lévêque PA, Barbey S, Launay F, Larroque H, Le Loir Y, Germon P, Guinard-Flament J, Even S. Bovine teat cistern microbiota composition and richness are associated with the immune and microbial responses during transition to once-daily milking. Front Microbiol. 2020;11:602404. doi: 10.3389/fmicb.2020.602404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braem G, De Vliegher S, Verbist B, Heyndrickx M, Leroy F, De Vuyst L. Culture-independent exploration of the teat apex microbiota of dairy cows reveals a wide bacterial species diversity. Vet Microbiol. 2012;157(3–4):383–90. doi: 10.1016/j.vetmic.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Braem G, De Vliegher S, Verbist B, Piessens V, Van Coillie E, De Vuyst L, Leroy F. Unraveling the microbiota of teat apices of clinically healthy lactating dairy cows, with special emphasis on coagulase-negative staphylococci. J Dairy Sci. 2013;96(3):1499–510. doi: 10.3168/jds.2012-5493. [DOI] [PubMed] [Google Scholar]
- 11.Derakhshani H, Plaizier JC, De Buck J, Barkema HW, Khafipour E. Composition and co-occurrence patterns of the microbiota of different niches of the bovine mammary gland: potential associations with mastitis susceptibility, udder inflammation, and teat-end hyperkeratosis. Anim Microbiome. 2020;2:11. doi: 10.1186/s42523-020-00028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benites NR, Melville PA, Costa EO. Evaluation of the microbiological status of milk and various structures in mammary glands from naturally infected dairy cows. Trop Anim Health Prod. 2003;35:301–307. doi: 10.1023/A:1025137220425. [DOI] [PubMed] [Google Scholar]
- 13.Pankey JW, Nickerson SC, Boddie RL, Hogan JS. Effects of Corynebacterium bovis infection on susceptibility to major mastitis pathogens. J Dairy Sci. 1985;68:2684–2693. doi: 10.3168/jds.S0022-0302(85)81153-X. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves JL, Kamphuis C, Vernooij H, Araújo JP, Jr, Grenfell RC, Juliano L, Anderson KL, Hogeveen H, Dos Santos MV. Pathogen effects on milk yield and composition in chronic subclinical mastitis in dairy cows. Vet J. 2020;262:105473. doi: 10.1016/j.tvjl.2020.105473. [DOI] [PubMed] [Google Scholar]
- 15.Rainard P, Poutrel B. Effect of naturally occurring intramammary infections: by minor pathogens on new infections by major pathogens in cattle. Am J Vet Res. 1988;49:327–329. [PubMed] [Google Scholar]
- 16.Sordillo LM, Oliver SP, Guidry AJ, Dermody JT. Humoral immune response of bovine mammary glands colonized with Corynebacterium bovis. Enumeration of plasma cell populations in tissue and immunoglobulin concentrations in milk. J Vet Med. 1988;35:617–627. doi: 10.1111/j.1439-0450.1988.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 17.Lam TJ, van Vliet JH, Schukken YH, Grommers FJ, van Velden-Russcher A, Barkema HW, Brand A. The effect of discontinuation of postmilking teat disinfection in low somatic cell count herds. I. Incidence of clinical mastitis. Vet J. 1997;19:41–47. doi: 10.1080/01652176.1997.9694738. [DOI] [PubMed] [Google Scholar]
- 18.Schukken YH, Leslie KE, Barnum DA, Mallard BA, Lumsden JH, Dick GH, Vessie ME, Kehrli ME. Experimental Staphylococcus aureus intramammary challenge in late lactation dairy cows: quarter and cow effects determining the probability of infection. J Dairy Sci. 1999;82:2393–2401. doi: 10.3168/jds.S0022-0302(99)75490-1. [DOI] [PubMed] [Google Scholar]
- 19.Rainard P, Riollet C. Mobilization of neutrophils and defense of the bovine mammary gland. Reprod Nutr Dev. 2003;43:439–457. doi: 10.1051/rnd:2003031. [DOI] [PubMed] [Google Scholar]
- 20.Blagitz MG, Souza FN, Santos BP, Batista CF, Parra AC, Azevedo LF, Melville PA, Benites NR, Della Libera AM. Function of milk polymorphonuclear neutrophil leukocytes in bovine mammary glands infected with Corynebacterium bovis. J Dairy Sci. 2013;96:3750–3757. doi: 10.3168/jds.2012-6370. [DOI] [PubMed] [Google Scholar]
- 21.Blagitz MG, Souza FN, Batista CF, Santos BP, Parra AC, Azevedo LFF, Della Libera AMMP. Expression of CD14 and toll-like receptors 2 and 4 by milk neutrophils in bovine mammary glands infected with Corynebacterium bovis. Pesq Vet Bras. 2015;35:1–5. doi: 10.1590/S0100-736X2015000100001. [DOI] [Google Scholar]
- 22.Barkema HW, Schukken YH, Lam TJ, Galligan DT, Beiboer ML, Brand A. Estimation of interdependence among quarters of the bovine udder with subclinical mastitis and implications for analysis. J Dairy Sci. 1997;80(8):1592–1599. doi: 10.3168/jds.S0022-0302(97)76089-2. [DOI] [PubMed] [Google Scholar]
- 23.Blagitz MG, Souza FN, Batista CF, Diniz SA, Azevedo LF, Silva MX, Haddad JP, Heinemann MB, Cerqueira MM, Della Libera AM. Flow cytometric analysis: Interdependence of healthy and infected udder quarters. J Dairy Sci. 2015;98(4):2401–2408. doi: 10.3168/jds.2014-8727. [DOI] [PubMed] [Google Scholar]
- 24.Bronzo V, Lopreiato V, Riva F, Amadori M, Curone G, Addis MF, Cremonesi P, Moroni P, Trevisi E, Castiglioni B. The role of innate immune response and microbiome in resilience of dairy cattle to disease: the mastitis model. Animals (Basel) 2020;10(8):1397. doi: 10.3390/ani10081397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Archer N, Egan SA, Coffey TJ, Emes RD, Addis MF, Ward PN, Blanchard AM, Leigh JA. A paradox in bacterial pathogenesis: activation of the local macrophage inflammasome is required for virulence of Streptococcus uberis. Pathogens. 2020;9(12):997. doi: 10.3390/pathogens9120997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Günther J, Czabanska A, Bauer I, Leigh JA, Holst O, Seyfert HM. Streptococcus uberis strains isolated from the bovine mammary gland evade immune recognition by mammary epithelial cells, but not of macrophages. Vet Res. 2016;47:13. doi: 10.1186/s13567-015-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alnakip ME, Quintela-Baluja M, Böhme K, Fernández-No I, Caamaño-Antelo S, Calo-Mata P, Barros-Velázquez J. The immunology of mammary gland of dairy ruminants between healthy and inflammatory conditions. J Vet Med. 2014;31:659801. doi: 10.1155/2014/659801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunkley ML, Clancy RL, Cripps AW. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83(3):362–369. [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas AL, Quimby FW, Coksaygan O, Olmstead L, Lein DH. Longitudinal evaluation of CD4+ and CD8+ peripheral blood and mammary gland lymphocytes in cows experimentally inoculated with Staphylococcus aureus. Can J Vet Res. 2000;64(4):232–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Souza FN, Blagitz MG, Batista CF, Takano PV, Gargano RG, Diniz SA, Silva MX, Ferronatto JA, Santos KR, Heinemann MB, De Vliegher S, Della Libera AMMP. Immune response in nonspecific mastitis: what can it tell us? J Dairy Sci. 2020;103:5376–5386. doi: 10.3168/jds.2019-17022. [DOI] [PubMed] [Google Scholar]
- 31.Park YH, Joo YS, Park JY, Moon JS, Kim SH, Kwon NH, Ahn JS, Davis WC, Davies CJ. Characterization of lymphocyte subpopulations and major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J Vet Sci. 2004;5(1):29–39. doi: 10.4142/jvs.2004.5.1.29. [DOI] [PubMed] [Google Scholar]
- 32.Soltys J, Quinn MT. Selective recruitment of T-cell subsets to the udder during staphylococcal and streptococcal mastitis: analysis of lymphocyte subsets and adhesion molecule expression. Infect Immun. 1999;67:293–302. doi: 10.1128/iai.67.12.6293-6302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park YH, Fox LK, Hamilton MJ, Davis WC. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet Immunol Immunopathol. 1993;36:137–151. doi: 10.1016/0165-2427(93)90103-B. [DOI] [PubMed] [Google Scholar]
- 34.Shafer-Weaver KA, Sordillo LM. Bovine CD8+ suppressor lymphocytes alter immune responsiveness during the postpartum period. Vet Immunol Immunopathol. 1997;56:53–64. doi: 10.1016/S0165-2427(96)05725-X. [DOI] [PubMed] [Google Scholar]
- 35.Nieto Farias MV, Souza FN, Lendez PA, Martínez-Cuesta L, Santos KR, Della Libera AMMP, Ceriani MC, Dolcini GL. Lymphocyte proliferation and apoptosis of lymphocyte subpopulations in bovine leukemia virus-infected dairy cows with high and low proviral load. Vet Immunol Immunopathol. 2018;206:41–48. doi: 10.1016/j.vetimm.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Rainard P, Poutrel B. Dynamics of nonclinical bovine intramammary infections with major and minor pathogens. Am J Vet Res. 1982;43:2143–2146. [PubMed] [Google Scholar]
- 37.Brooks BW, Barnum DA, Meek AH. An observational study of Corynebacterium bovis in selected Ontario dairy herds. Can J Comp Med. 1983;47:73–78. [PMC free article] [PubMed] [Google Scholar]
- 38.Zadoks RN, Allore HG, Barkema HW, Sampimon OC, Wellenberg GJ, Gröhn YT, Schukkent YH. Cow- and quarter-level risk factors for Streptococcus uberis and Staphylococcus aureus mastitis. J Dairy Sci. 2001;84:2649–2663. doi: 10.3168/jds.S0022-0302(01)74719-4. [DOI] [PubMed] [Google Scholar]
- 39.Reyher KK, Dohoo IR, Scholl DT, Keefe GP. Evaluation of minor pathogens intramammary infection, susceptibility parameters, and somatic cell counts on the development of new intramammary infections with major mastitis pathogens. J Dairy Sci. 2012;95:3766–3780. doi: 10.3168/jds.2011-5148. [DOI] [PubMed] [Google Scholar]
- 40.Woodward WD, Besser TE, Ward AC, Corbeil LB. In vitro growth inhibition of mastitis pathogens by bovine teat skin normal flora. Can J Vet Res. 1987;51(1):27–31. [PMC free article] [PubMed] [Google Scholar]
- 41.Blagitz MG, Souza FN, Batista CF, Azevedo LF, Benites NR, Melville PA, Diniz SA, Silva MX, Haddad JP, Heinnemann MB, Cerqueira MM, Della Libera AM. The neutrophil function and lymphocyte profile of milk from bovine mammary glands infected with Streptococcus dysgalactiae. J Dairy Res. 2015;82:460–469. doi: 10.1017/S0022029915000308. [DOI] [PubMed] [Google Scholar]
- 42.Souza FN, Ramos Sanchez EM, Heinemann MB, Gidlund MA, Reis LC, Blagitz MG, Della Libera AMMP, Cerqueira MMOP. The innate immunity in bovine mastitis: the role of pattern-recognition receptors. Am J Immunol. 2012;8:166–178. doi: 10.3844/ajisp.2012.166.178. [DOI] [Google Scholar]
- 43.Bansal BK, Hamann J, Grabowskit NT, Singh KB. Variation in the composition of selected milk fraction samples from healthy and mastitic quarters, and its significance for mastitis diagnosis. J Dairy Res. 2005;72:144–152. doi: 10.1017/S0022029905000798. [DOI] [PubMed] [Google Scholar]
- 44.National Mastitis Council . Laboratory Handbook on Bovine Mastitis. Madison: National Mastitis Council Inc.; 1999. [Google Scholar]
- 45.Oliver SP, González RN, Hogan JS, Jayarao BM, Owens WE. Microbiological procedures for the diagnosis of bovine udder infection and determination of milk quality. 4. Verona: National Mastitis Council; 2004. p. 47. [Google Scholar]
- 46.Lima ES, Blagitz MG, Batista CF, Alves AJ, Fernandes ACC, Ramos Sanchez EM, Torres HF, Diniz AS, Silva MX, Della Libera AMMP, Souza FN. Milk macrophages function in bovine leukemia virus-infected dairy cows. Front Vet Sci. 2021;8:650021. [DOI] [PMC free article] [PubMed]
- 47.Hasui M, Hirabayashi Y, Kobayashi Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in while blood. J Immunol Methods. 1989;117:53–58. doi: 10.1016/0022-1759(89)90118-X. [DOI] [PubMed] [Google Scholar]
- 48.Della Libera AM, de Souza FN, Batista CF, Santos BP, de Azevedo LF, Sanchez EM, Diniz SA, Silva MX, Haddad JP, Blagitz MG. Effects of bovine leukemia virus infection on milk neutrophil function and milk lymphocyte profile. Vet Res. 2015;46:1–8. doi: 10.1186/s13567-014-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shehat MG, Tigno-Aranjuez J. Flow Cytometric Measurement of ROS production in macrophages in response to FcγR cross-linking. J Vis Exp. 2019;145:1–15. doi: 10.3791/59167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batista CF, Souza FN, Santos KR, Ramos Sanchez EM, Reis LC, Bertagnon HG, et al. R-Phycoerythrin-labeled Mannheimia haemolytica for the simultaneous measurement of phagocytosis and intracellular reactive oxygen species production in bovine blood and bronchoalveolar lavage cells. Vet Immunol Immunopathol. 2018;196:53–59. doi: 10.1016/j.vetimm.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 51.McGraw KO, Wong SP. Forming Inferences about some intraclass correlation coefficients. Psych Metho. 1996;1:30–46. doi: 10.1037/1082-989X.1.1.30. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Percentage (mean ± standard error) of B and T lymphocytes in milk samples from healthy mammary quarters infected with Corynebacterium bovis.
Additional file 2: Supplemental Figure 1.
Additional file 3: Supplemental Table 2. Monoclonal antibodies used for immunophenotyping bovine milk lymphocytes by flow cytometry.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.