Abstract
Background
Whether avoidable hospitalizations in community-dwelling persons with dementia have decreased during primary care reforms is unknown.
Methods
We described the prevalence and trends in avoidable hospitalizations in population-based repeated yearly cohorts of 192,144 community-dwelling persons with incident dementia (Quebec, 2000–2015) in the context of a province-wide primary care reform, using the provincial health administrative database.
Results
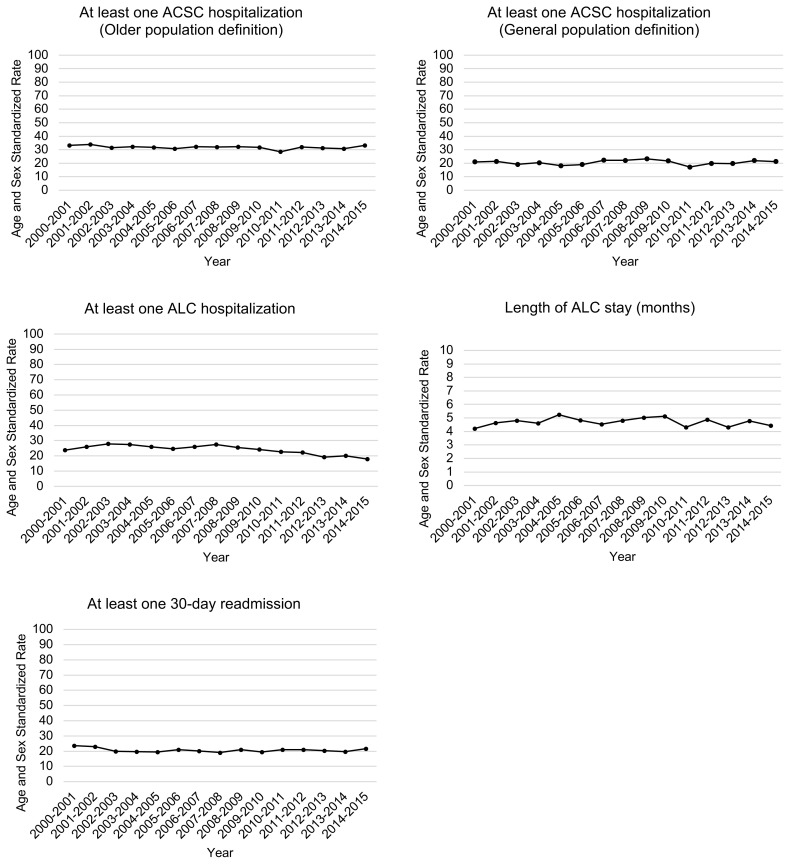
Trends in both types of Ambulatory Care Sensitive Condition (ACSC) hospitalization (general and older population) and 30-day readmission rates remained constant with average rates per 100 person-years: 20.5 (19.9–21.1), 31.7 (31.0–32.4), 20.6 (20.1–21.2), respectively. Rates of delayed hospital discharge (i.e., alternate level of care (ALC) hospitalizations) decreased from 23.8 (21.1–26.9) to 17.9 (16.1–20.1) (relative change −24.6%).
Conclusions
These figures shed light on the importance of the phenomenon, its lack of improvement for most outcomes over the years, and the need to develop evidence-based policies to prevent avoidable hospitalizations in this vulnerable population.
Keywords: primary care, interdisciplinary primary care teams, dementia, avoidable hospitalization, Ambulatory Care Sensitive Conditions
INTRODUCTION
Persons with dementia have twice as much hospital use (emergency department [ED] visits and hospital admissions) as older persons without dementia.(1) In 2015, 25% and 20% of persons with dementia in Canada visited the ED or were hospitalized at least once.(2) They stayed in hospital twice as long as persons without dementia and experienced one-and-a-half times more hospital harm than those without. A proportion of this hospital use might be avoidable with appropriate ambulatory care, including primary care.(1) For example, in the United States, between 20 and 40% of hospitalizations of persons with dementia are potentially avoidable, as measured by 30-day hospital readmissions, injuries, or Ambulatory Care Sensitive Conditions (ACSC) hospitalizations.(3,4)
Currently, there are more than 564,000 Canadians living with dementia, a figure projected to at least double by 2031 as Canadians continue to age.(5) Health-care costs for persons with dementia are estimated to be five-and-a-half times greater than for persons without dementia.(5) Reducing avoidable hospital use in persons with dementia and improving the quality of care they receive is a World Health Organization global health-care priority and one of the 2019 Canadian national dementia strategy objectives.(6–9)
Over the last few decades, in Canada and in many high-income countries, primary care delivery has transformed in order to improve the care delivered to persons with chronic diseases. Interdisciplinary primary care teams have been implemented in order to improve primary care quality, continuity, and accessibility. This may have impacted hospital use, especially potentially avoidable hospital use in persons with dementia. Optimal primary care could improve the management of chronic conditions and the detection and treatment of acute exacerbations, and thus reduce avoidable hospital use.
However, no study worldwide has examined hospital use trends in community-dwelling persons with dementia, in the context of a primary care reform. In Canada, there is little evidence on avoidable hospital use in persons with dementia,(10–20) and none specifically on the community-dwelling population. In order to develop evidence-informed policies and programs to prevent avoidable hospital use in community-dwelling persons with dementia, we need to measure the extent of the phenomenon and its trend over time. In this study, we describe the prevalence and trends in hospital use in community-dwelling persons with dementia in Quebec from 2000 to 2015, in the context of a province-wide primary care reform.
METHODS
We conducted a repeated cohort study of hospital use in community-dwelling persons with dementia in Quebec. Quebec accounts for 20% of Canadians with dementia,(21) with hospitalization rates among the highest of the Canadian population with dementia.(2) This study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) guidelines.(22,23)
Data Source
We analyzed the Quebec population-based health administrative database held at the Institut national de santé publique du Québec (The Quebec Public Health Institute). This database records most services provided via the public universal health insurance system (e.g., medical consultation, hospitalization, prescription drug use) and links these health-care services utilization data with individual level demographics and deaths.(24) This database covers 99% of the population 65+ in Quebec.(24) The database was accessed by LR, who performed the analyses, using SAS Enterprise Guide software, 7.15 version (SAS Institute, Inc., Cary, NC).
Individual-level data are not publicly available. This study is part of the continuous chronic disease surveillance mandate granted to the Institut national de santé publique du Québec by the provincial Minister of Health and Social Services and approved by the provincial Ethics Committee of Public Health, allowing surveillance activities without participant consent. In addition, it was approved by the McGill Faculty of Medicine Institutional Review Board (study # A03-E21-19B)
Study Design and Population
We analyzed repeated yearly cohorts from 2000–2001 to 2014–2015. We included community-dwelling older adults (aged 65+) with a new diagnosis of dementia between April 1st and March 31st of each year, inclusively (see details on included population in Appendix A). A dementia diagnosis was identified using a validated algorithm developed at ICES in Ontario and adopted by the Public Health Agency of Canada.(25) Persons were considered as diagnosed with dementia if they had at least one dementia diagnosis in the hospitalization dataset, or at least three dementia diagnoses in the medical consultation dataset (a non-archivist revised dataset), or at least one prescription of a dementia-specific drug in the drug dataset. (See details on the algorithm in Appendix A). The community-dwelling population was identified as persons with no evidence, in the linked health administrative database, of living in a long-term care facility.(26) The study population was restricted to persons with valid sex and birth date and enrolled in the public provincial health insurance plan for at least one day of the studied year.
Outcomes
Outcomes were measured during the follow-up period: one year after the diagnosis date, death, or admission to long-term care, whichever occurred first. We chose a one-year follow-up, as the year following diagnosis for persons with dementia is known to be especially at risk of service use and transitions.(27) We measured hospital use based on the following indicators: all-causes of ED visits, hospitalizations, and length of hospital stay (see details on operationalization of variables in Appendix B, Tables B1 and B2). Several measures of potentially avoidable hospital use have been used in populations with dementia.(10–19) In order to draw a comprehensive picture, we measured the four most commonly used indicators of potentially avoidable hospital use in persons with dementia: ACSC hospitalizations (general and older population definitions), 30-day readmission, and delayed hospital discharge (i.e., alternate level of care (ALC) hospitalizations). (See details on operationalization of variables in Appendix B, Tables B1 and B2).
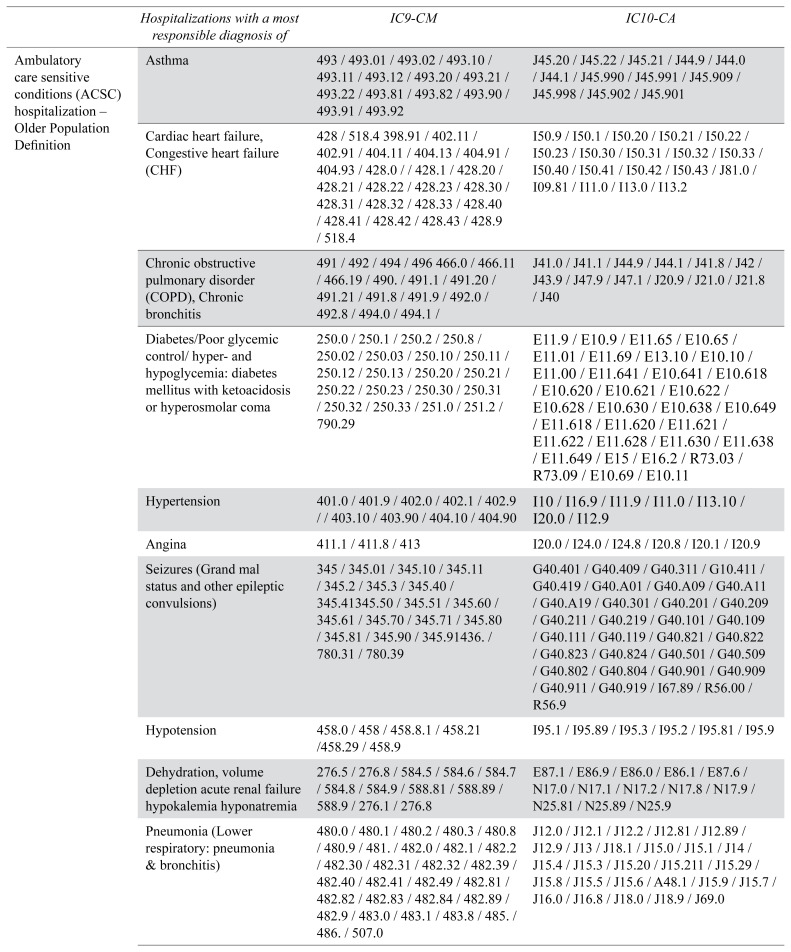
ACSC are conditions, “where appropriate ambulatory care may prevent or reduce the need for admission to hospital”.(28) We measured ACSC hospitalizations two ways. First, with a general population definition as defined by Canadian Institute for Health Information (CIHI).(28) Second, with an older population definition: the measure developed by Walsh and colleagues on older persons dually eligible for Medicare and Medicaid, previously used in a population with dementia by Feng et al.(14) This definition includes additional conditions that are more specific to an older population, such as: hypotension, constipation, skin ulcers, or nutritional deficiency. Details on lists of conditions and International Classification of Diseases (ICD) codes for each definition are presented in Appendix B, Tables B1 and B2. Delayed hospital discharge or ALC hospitalization is coded, by physicians or their delegates, in the administrative database as “a person who has completed the acute care phase of his or her treatment but remained in an acute care bed”.(29) These prolonged hospital stays correspond to persons who cannot be discharged from the hospital even though they do not require the level of care provided in hospitals; they are typically waiting for a long-term care admission.
Analysis
Indicators of hospital use in each study year were summarized as rates, adjusted for differential person-time. Rates were standardized for differences in the distribution of age and sex over time using direct standardization. The age and sex distribution of the 2011 Canada census data was used as the reference population for the standardization.(30)
We assessed the trends in indicators of hospital use based on graphical assessment, absolute, and relative changes from the last to first study year. Following this assessment, we classified the trends as constant, increasing, or decreasing. We interpreted the clinical and health-care significance of the trends based on the expert knowledge of over 100 researchers, clinicians, surveillance experts, decision-makers, managers, and knowledge users from the Canadian Consortium for Neurodegeneration and Aging, the Institut national de santé publique du Québec, the Quebec Ministry of Health, and the Quebec Alzheimer’s Plan implementation team.
RESULTS
Study Population
From 2000 to 2015, there were 192,144 community-dwelling 65+ persons with a new diagnosis of dementia in Quebec, on average 12,810 persons per year. They accounted for 1.0% to 1.2% of the community-dwelling 65+ persons in Quebec. Overall, their mean age increased by roughly one year over the study period (80.3 to 81.6), and the proportion of women decreased by five percentage points (66.2 to 61.0). The demographic characteristics of the cohorts are presented in Table 1.
TABLE 1.
Description of the cohorts of community-dwelling adults 65 years or older with a new diagnosis of dementia in Quebec, Canada, from 2000–2015
| Community-dwelling adults 65 years or older with a new diagnosis of dementia | Follow-up time | Age | Female | |
|---|---|---|---|---|
| Diagnostic Year | N | Person-year | mean (SD) | N (%) |
| 2000 | 9117 | 7595 | 80.3 (0.1) | 6031 (66.2) |
| 2001 | 9500 | 7846 | 80.5 (0.1) | 6272 (66.0) |
| 2002 | 10102 | 8424 | 80.7 (0.1) | 6581 (65.2) |
| 2003 | 10370 | 8714 | 80.8 (0.1) | 6696 (64.6) |
| 2004 | 10822 | 9102 | 80.7 (0.1) | 6962 (64.3) |
| 2005 | 11282 | 9660 | 80.9 (0.1) | 7163 (63.5) |
| 2006 | 12804 | 10894 | 81.0 (0.1) | 8110 (63.3) |
| 2007 | 13008 | 11191 | 81.1 (0.1) | 8232 (63.3) |
| 2008 | 13525 | 11685 | 81.2 (0.1) | 8574 (63.4) |
| 2009 | 14433 | 12466 | 81.4 (0.1) | 9263 (64.2) |
| 2010 | 14556 | 12560 | 81.4 (0.1) | 9288 (63.8) |
| 2011 | 15519 | 13505 | 81.5 (0.1) | 9636 (62.1) |
| 2012 | 15716 | 13651 | 81.6 (0.1) | 9634 (61.3) |
| 2013 | 15925 | 13857 | 81.7 (0.1) | 9747 (61.2) |
| 2014 | 15455* | 13424 | 81.6 (0.1) | 9430 (61.0) |
| Overall Population | 192144 | 164574 | 80.3 (0.0) | 121619 (63.3) |
SD = standard deviation
The 2014–2015 cohort is incomplete (See Appendix A).
Hospital Use
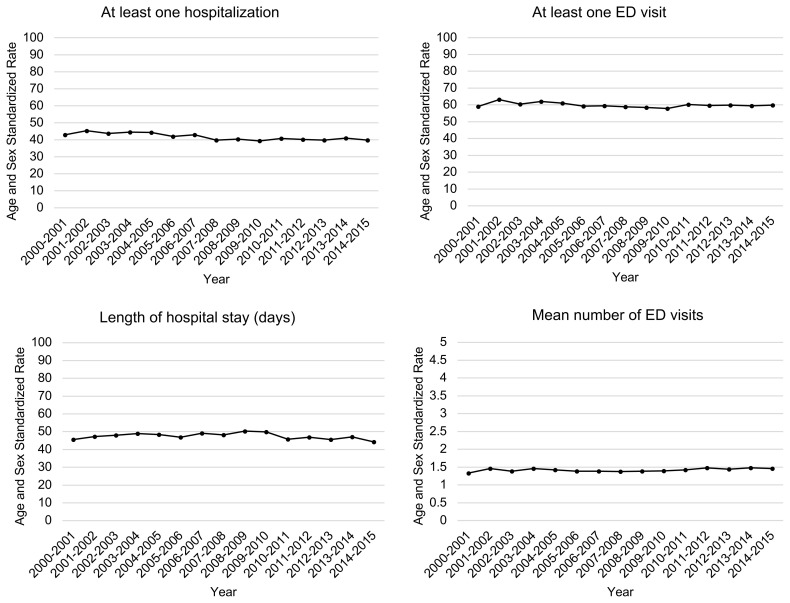
From 2000–2015, the rates of at least one ED visit per 100 person-years remained constant at an average of 59.8 (95% confidence interval (CI) [59.3–60.4]) (Table 2 and Figure 1a). The mean number of ED visits per 1 person-year remained constant at an average 1.4 visits (95%CI [1.4–1.4]).
TABLE 2.
Hospital use (total and potentially avoidable) in the year following diagnosis among community-dwelling adults 65 years or older with a new diagnosis of dementia in Quebec, Canada, from 2000–2015: age and sex standardized rates with 95% confidence intervals, absolute and relative changes
| Indicator | 2000–2001 Rate b (95% CI) | 2014–2015 Rate b (95% CI) | 2000–2015 Average Rate b (95% CI) | Absolute Change c (%) | Relative Change c (%) |
|---|---|---|---|---|---|
| At least one ED visit | 59.0 (56.6–61.6) | 60.0 (58.1–61.9) | 59.8 (59.3–60.4) | + 0.9 | + 1.6 |
| Mean number of ED visits | 1.3 (1.3–1.4) | 1.5 (1.4–1.5) | 1.4 (1.4–1.4) | + 0.1 | + 0.1 |
| At least one hospitalization | 42.9 (40.9–45.1) | 39.9 (38.4–41.5) | 41.5 (41.0–41.9) | − 3.1 | − 7.1 |
| Length of hospital stay (days)a | 45.7 (45.2–46.1) | 44.3(44.0–44.6) | 47.3 (47.2–47.4) | − 1.4 | − 3.0 |
| At least one ACSC hospitalization (General population)a | 21.0 (18.6–23.9) | 21.3 (19.3–23.5) | 20.5 (19.9–21.1) | + 0.2 | + 1.0 |
| At least one ACSC hospitalization (Older population)a | 33.3 (30.2–36.7) | 33.2 (30.7–36.0) | 31.7 (31.0–32.4) | − 0.1 | − 0.2 |
| At least one 30-day readmissiona | 23.7 (20.7–27.0) | 21.7 (19.7–24.0) | 20.6 (20.1–21.2) | − 1.9 | − 8.2 |
| At least one delayed hospital discharge (ALC hospitalization)a | 23.8 (21.1–26.9) | 17.9 (16.1–20.1) | 23.5 (22.9–24.1) | − 5.9 | − 24.6 |
| Length of delayed hospital discharge (ALC stay)a (months) | 4.2 (4.1–4.3) | 4.4 (4.4–4.5) | 4.7 (4.7–4.7) | + 0.2 | + 5.5 |
CI = confidence Interval; ED = emergency department; ACSC = Ambulatory Care Sensitive Conditions; ALC = alternate level of care
The length of hospital stay, Ambulatory Care Sensitive Conditions (ACSC) hospitalizations, alternate level of care (ALC) hospitalization were measured in the subset of persons with at least one hospital admission during the study period; 30-day readmission was measured in the subset of persons with at least one hospital discharge during the study period.
Rates are reported as per one person-year for length of hospital stay, mean number of ED visits, and length of ALC stay; the remaining indicators are per 100 person-years. The census population of Canada in 2011 was used as the standard population for age and sex standardized rates.
Absolute and relative change are calculated between the first and last year of the study.
FIGURE 1a.
Age and sex standardized rates of hospital use in the year following diagnosis among community-dwelling adults 65 years or older with a new diagnosis of dementia in Quebec, Canada, from 2000–2015.
The length of hospital stay was computed as the total number of hospital days during the follow-up period in persons with at least one hospitalization. The rate of at least one Emergency Department visit was computed on the entire cohorts. Rates are reported as per 1 person years for length of hospital stay, and mean number of emergency department visits. The remaining indicators are per 100 person years. Canadian census population of 2011 was used as the reference population for standardization. The 2014–2015 cohort is incomplete (See Appendix A).
ED: Emergency Department
The rate of at least one hospitalization per 100 person-years decreased from 42.9 (95%CI [40.9–45.1]) to 39.9 (95%CI [38.4–41.5]); relative change −7.1%). Among the persons hospitalized, the length of hospital stay in the year of diagnosis per one person-year remained constant at an average of 47.3 days (95%CI [47.2–47.4]).
Potentially Avoidable Hospital Use
From 2000–2015, the rates of at least one ACSC hospitalization (general and older population definition) and 30-day readmission per 100 person-years remained constant at averages of 20.5 (95%CI [19.9–21.1], 31.7 [31.0–32.4], and 20.6 [20.1–21.2]), respectively (Table 2 and Figure 1b).
FIGURE 1b.
Age and sex standardized rates of potentially avoidable hospital use in the year following diagnosis among community-dwelling adults 65 years or older with a new diagnosis of dementia in Quebec, Canada, from 2000–2015.
The rate of Ambulatory Care Sensitive Conditions (ACSC) hospitalizations, and ALC hospitalizations were measured in the subset of persons with at least one hospital admission during the study period. 30-day readmission was measured in the subset of persons with at least 1 hospital discharge during the study period. The length of ALC stay was computed as the total number of ALC hospital days during the follow-up period in persons with at least one ALC hospitalization. Rates are reported as per 1 person years for length of ALC stay. The remaining indicators are per 100 person years. Canadian census population of 2011 was used as the reference population for standardization. The 2014–2015 cohort is incomplete (See Appendix A).
ACSC: Ambulatory Care Sensitive Conditions; ALC: Alternate level of care
The rate of at least one delayed hospital discharge or ALC hospitalization per 100 person-years decreased from 23.8 (95%CI [21.1–26.9]) to 17.9 (95%CI [16.1–20.1]); relative change −24.6%). Among those with ALC hospitalization, the length of ALC stay in the year of diagnosis for one person-year remained constant at an average of 4.7 months (95%CI [4.7–4.7]).
DISCUSSION
In this descriptive study, around 40 and 60 per 100 person-years of community-dwelling persons with dementia had at least one hospitalization and one ED visit during the year of diagnosis, respectively. In those hospitalized, the average length of hospital stay in the year of diagnosis was around 1.5 months. Between 20% and 30% of those hospitalized, depending on the indicator, had a potentially avoidable hospital use, with an average length of delayed discharge or ALC stay of more than 4.5 months. Most indicators remained constant over the 15 years.
To our knowledge, this is the first Canadian estimation of prevalence and trends in hospital use in community-dwelling persons with dementia. These findings are consistent with the literature on the prevalence of hospital use in community-dwelling persons with dementia.(27,31) In Canada, only two studies reported hospitalization rates specifically from the community-dwelling population. In Ontario (2012), among community-dwelling home care recipients with dementia, around 40 and 50% had a hospitalization or an ED visit during the year.(31) In British Columbia (2001), in the community-dwelling population newly diagnosed with dementia, more than 60% had a hospitalization in the year of diagnosis, with average length of stay in the year of diagnosis, for those hospitalized, of 42 days.(27)
Regarding potentially avoidable hospital use, the evidence is scarce in Canada; specifically, there is no study of ACSC hospitalizations or 30-day readmissions in persons with dementia. Our findings are consistent with US estimates. In a 2013 US study, 10% and 18% of the 65+ Medicare population with dementia had at least one ACSC hospitalization or one 30-day readmission during the year.(3) Regarding delayed hospital discharge or ALC hospitalization, our results are consistent with the CIHI Report on Dementia in Canada (2015–2016): “One in 5 seniors with dementia had an ALC component to their stay”.(2)
Overall, during the entire study period, we did not observe a decrease in most indicators (7/9) of hospital use. This absence of variation is in contrast with the several provincial policies, primary care reforms, and care recommendations for primary care physicians that occurred or were released over the 15-year period that could have influenced hospital use in Quebec. However, none of these policies, reforms, or care recommendations specifically focused on reducing avoidable hospital use, which might be essential to have a measurable impact. Since 2000, major reforms of primary care delivery occurred in Quebec, including the implementation of interdisciplinary primary care teams, called the Family Medicine Groups (FMGs), to enhance primary care access, continuity, and quality.(32,33) In contrast to other countries relying on specialist care and memory clinics, Canadian recommendations for dementia care emphasize the importance of primary care in the care of persons with dementia.(34) Recommendations emphasizing this importance were issued regularly over the last 15 years: the Quebec Alzheimer’s Plan (Qc AP) expert report in 2009, and the 3rd and 4th Canadian consensus conferences on the diagnosis and treatment of dementia (2006 and 2014).(35–37) The Qc AP implementation began in 2014, aiming at improving the quality of care offered in FMGs to persons living with dementia.(35) Interdisciplinary primary care teams are considered as the way forward to care for the complex and evolving cognitive, functional, emotional, and social needs of persons with dementia.(20,35,37) Indeed, they require care from an array of different services and health-care professionals to meet these needs. The implementation of these interdisciplinary primary care teams responsible for the care of persons with dementia, by enhancing primary care access, continuity, and quality, could have helped decrease avoidable hospital use through the improvement in the management of chronic conditions, and the detection and treatment of acute exacerbations.(38)
On the contrary, the rate of delayed hospital discharge or ALC hospitalizations decreased over the 15-year period. Since 2006, the Quebec Ministry of Health aimed specifically at reducing ALC hospitalizations. One of the main interventions was the implementation of the Relevé quotidien de la situation à l’urgence et en centre hospitalier (RQSUCH) in 2006, a daily measurement of the number of persons identified as ALC at the hospital level.(39) In addition to being closely monitored at the Ministry level, this measurement is sent daily to all health-care administrators and hospital managers in the province. The Ministry convenes regular ALC monitoring meetings with the different stakeholders involved in reducing ALC hospitalizations in each health-care region, from hospital administrators to administrators of long-term care and home care services, where ALC measurement, as well as the implementation of best practices to reduce ALC hospitalizations, are presented and discussed. Individualized follow-ups are held with institutions encountering difficulties. While the observed decrease in ALC is promising, we cannot infer causality from this observational study. Formal evaluation of ministerial policies to reduce ALC hospitalizations are needed to inform decision-makers.
Our results are a call for the design and implementation of policies and reforms to specifically target the reduction of avoidable hospital use in community-dwelling persons with dementia, especially during the year of diagnosis. One of the three objectives of the Canadian national dementia strategy is to improve the quality of life of those living with dementia and their caregivers, especially through an improvement of the quality of care they receive.(9) Reducing avoidable hospital use in persons with dementia is one way to improve the quality of life of persons with dementia and their caregivers, prevent adverse outcomes for persons with dementia, and minimize rising health-care costs.
To prevent avoidable hospital use, Gruneir and colleagues emphasized the importance of offering timely access to integrated, pro-active primary care that includes community and home care services.(40) Indeed, offering timely access to supportive care was shown to prevent a share of ALC hospitalizations.(41) In addition, offering timely primary care access was shown to prevent a share of avoidable hospitalizations, especially ACSC hospitalizations and 30-day readmissions.(38,42–47) Experts emphasize the importance of caring for the caregiver, as caregiver absence, burden, and stress are major drivers of crisis leading to avoidable hospitalizations.(48–52) Finally, palliative care approaches might be promising avenues, as they have been shown to prevent a large share of non-desired end-of-life hospitalizations in older adults.(14,53,54)
By working closely with decision-makers, specifically the Quebec Ministry of Health and the Alzheimer Plan design and implementation team, and sharing these results to policy-makers, primary care physicians, specialists, patients, caregivers, and patients and caregivers’ representatives, we stress the vulnerability of persons with dementia to avoidable hospital use in the year of diagnosis. These results can be used to support policies and care organization. They were already used to inform the third phase of the Quebec Alzheimer’s Plan, which focuses on persons trajectories, and care coordination among different care providers and organizations.
Our study has limits. We identified persons diagnosed with dementia with a validated algorithm.(25) Delayed diagnosis is highly prevalent in this population. In addition, being identified with this algorithm requires the use of the health-care system. Thus our findings might represent service use of a subsample, those who are diagnosed and use the health-care system, of the entire population with dementia. Our estimations are specific to the Quebec context. Further studies in other countries are needed to inform policy makers.
This study is the first Canadian population-based descriptive study of the prevalence and trends over 15 years of hospital use in community-dwelling persons with dementia. This study highlights the magnitude of the phenomenon, and for most indicators, their absence of improvement over the study period. As Canada is implementing its federal dementia strategy and several provinces are conducting Alzheimer’s Plans, these findings are particularly relevant and timely.(9) Health-care professionals know the adverse outcomes associated with hospital use in persons with dementia. These figures are a call for action to develop and implement evidence-based policies to prevent avoidable hospital use in this vulnerable population.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Mary Henein for creating and formatting all the tables and graphs in this manuscript, as well as editing final versions of the manuscript. The authors would like to thank the following for their valuable insights on the results: the researchers, clinicians, surveillance experts, decision-makers, managers, and knowledge users stemming from the Canadian Consortium for Neurodegeneration and Aging, the Institut national de santé publique du Québec, the Quebec Ministry of Health, and the Quebec Alzheimer’s Plan implementation team. The authors would like to thank Pierre Pluye, Howard Bergman, and Roxane Borgès Da Silva for their insightful comments on the design, analysis, and interpretation of the results. Claire Godard-Sebillotte and Nadia Sourial received support through a Doctoral Research Award from the Canadian Institutes of Health Research (CIHR): Vanier Canada Graduate Scholarship (Vanier CGS). Claire Godard-Sebillotte received support through a fellowship from the Canadian Institutes of Health Research (CIHR). Erin Strumpf and Isabelle Vedel were supported by a Chercheur boursier Junior 2 from the Fonds de la recherche du Québec−Santé. The work of Louis Rochette and Eric Pelletier was subsidized through a research grant from The Canadian Consortium for Neurodegeneration and Aging (CCNA): “Assessing care models implemented in primary health care for persons with Alzheimer’s disease and related disorders” 2014–2019 (CNA-137794). The Canadian Consortium on Neurodegeneration in Aging is supported by a grant from the Canadian Institutes of Health Research with funding from several partners.
APPENDIX A. POPULATION
We included community-dwelling older adults (aged 65+) with a new diagnosis of dementia between April 1st and March 31st of each year inclusive. A dementia diagnosis was identified through a validated algorithm developed in Ontario by ICES and adopted by the Public Health Agency of Canada.(1)
The definition of dementia used to develop the algorithm includes Alzheimer’s disease, vascular dementia, dementia in other diseases classified elsewhere (frontotemporal dementia, idiopathic normal pressure hydrocephalus), and unspecified dementia (senile dementia, presenile dementia). The International Classification of Diseases (ICD)-9 and 10 codes used are: ICD-9 (46.1, 290.0, 290.1, 290.2, 290.3, 290.4, 294.x, 331.0, 331.1, 331.5, 331.82); ICD-10 (F00.x, F01.x, F02.x, F03.x, G30.x). The following characteristics were found in a validation study using as reference diagnoses recorded in electronic medical records: sensitivity 79.3% (confidence interval (CI) 72.9–85.8%), specificity 99.1% (CI 98.8–99.4%), positive predictive value 80.4% (CI 74.0–86.8%), and negative predictive value 99.0% (CI 98.7–99.4%).(1)
Persons were considered as diagnosed with dementia if they had at least one of the following conditions:
One dementia diagnosis (primary or secondary) in the hospitalization dataset, since the age of 40; or
At least 3 dementia diagnoses at least 30 days apart in a two-year period in the medical consultation dataset, since the age of 40; or
One prescription of a dementia related drug in the prescription drug use dataset, since the age of 40 (drugs are recorded in the database as of age 65 for every person, and for around one third of the population aged 40–64).
The date of diagnosis was the date on which the first of the three criteria became positive. For persons whose algorithm became positive through medical claims, the first medical claim was considered as the date of diagnosis.
It is to be noted, that the last available year of data, as of when we performed the analysis, was 2015–2016. Since the algorithm identifying dementia requires two years of data to identify every incident diagnosis, the 2014–2015 cohort is incomplete. Since around 75% of the diagnoses are identified within one year, the 2014–2015 cohort might be missing around 25% of incident diagnoses.
APPENDIX B. OUTCOMES: DETAILED DEFINITIONS
Hospital Use
We measured ED visits (probability of having at least one and mean number), hospitalization (probability of having at least one) and length of hospital stay. All causes were considered. A validated algorithm was used to identify distinct visits to the ED.(2) Day surgeries were excluded from the computation of hospitalizations. The rates of ED visits (at least one and mean number) and hospitalization were computed on the entire cohorts. The length of hospital stay was computed as the total number of hospital days during the follow-up period in persons with at least one hospitalization.
Potentially Avoidable Hospitalization
There are several definitions and measures of potentially avoidable hospitalization used in populations with dementia.(3–12) In order to draw a comprehensive picture, we measured the four most common indicators of potentially avoidable hospital use in persons with dementia: Ambulatory Care Sensitive Conditions (ACSC) hospitalizations (general and older population definitions), 30-day readmission, and delayed hospital discharge (i.e., alternate level of care (ALC) hospitalizations).
TABLE B1.
International Classification of Diseases (ICD) codes for the measures of Ambulatory Care Sensitive Conditions (ACSC) hospitalizations (general population definition)
| Hospitalizations with a most responsible diagnosis of | IC9-CM | IC10-CA | |
|---|---|---|---|
| Ambulatory care sensitive conditions (ACSC) hospitalization: general population definition | Asthma | 493 | J45 |
| Cardiac heart failure | 428 / 518.4 | I50 / J81 | |
| Chronic obstructive pulmonary disorder (COPD) | 491 / 492 / 494 / 496 | J41 / J42 / J43 / J44 / J47 | |
| Diabetes | 250.0 / 250.1 / 250.2 / 250.8 | E10.0 / E10.1 / E10.63 / E10.64 / E10.9 / E11.0 / E11.1 / E11.63 / E11.64 / E11.9 / E13.0 / E13.1 / E13.63 / E13.64 / E13.9 / E14.0 / E14.1 / E14.63 / E14.64 / E14.9 | |
| Hypertension | 401.0 / 401.9 / 402.0 / 402.1 / 402.9 | I10.0 / I10.1 / I11 | |
| Angina | 411.1 / 411.8 / 413 | I20 / I23.82 / I24.0 / I24.8 / I24.9 | |
| Seizures (Grand mal status and other epileptic convulsions) | 345 | G40 / G41 | |
| Hypotension | 458.0 / 458 / 458.8.1 / 458.21 /458.29 / 458.9 | I95.1 / I95.89 / I95.3 / I95.2 / I95.81 / I95.9 | |
| Dehydration, volume depletion acute renal failure hypokalemia hyponatremia | 276.5 / 276.8 / 584.5 / 584.6 / 584.7 / 584.8 / 584.9 / 588.81 / 588.89 / 588.9 / 276.1 / 276.8 | E87.1 / E86.9 / E86.0 / E86.1 / E87.6 / N17.0 / N17.1 / N17.2 / N17.8 / N17.9 / N25.81 / N25.89 / N25.9 |
TABLE B2.
International Classification of Diseases (ICD) codes for the measures of Ambulatory Care Sensitive Conditions (ACSC) hospitalizations (older population definition)
| Hospitalizations with a most responsible diagnosis of | IC9-CM | IC10-CA | |
|---|---|---|---|
| Ambulatory care sensitive conditions (ACSC) hospitalization – Older Population Definition | Asthma | 493 / 493.01 / 493.02 / 493.10 / 493.11 / 493.12 / 493.20 / 493.21 / 493.22 / 493.81 / 493.82 / 493.90 / 493.91 / 493.92 | J45.20 / J45.22 / J45.21 / J44.9 / J44.0 / J44.1 / J45.990 / J45.991 / J45.909 / J45.998 / J45.902 / J45.901 |
| Cardiac heart failure, Congestive heart failure (CHF) | 428 / 518.4 398.91 / 402.11 / 402.91 / 404.11 / 404.13 / 404.91 / 404.93 / 428.0 / / 428.1 / 428.20 / 428.21 / 428.22 / 428.23 / 428.30 / 428.31 / 428.32 / 428.33 / 428.40 / 428.41 / 428.42 / 428.43 / 428.9 / 518.4 | I50.9 / I50.1 / I50.20 / I50.21 / I50.22 / I50.23 / I50.30 / I50.31 / I50.32 / I50.33 / I50.40 / I50.41 / I50.42 / I50.43 / J81.0 / I09.81 / I11.0 / I13.0 / I13.2 | |
| Chronic obstructive pulmonary disorder (COPD), Chronic bronchitis | 491 / 492 / 494 / 496 466.0 / 466.11 / 466.19 / 490. / 491.1 / 491.20 / 491.21 / 491.8 / 491.9 / 492.0 / 492.8 / 494.0 / 494.1 / | J41.0 / J41.1 / J44.9 / J44.1 / J41.8 / J42 / J43.9 / J47.9 / J47.1 / J20.9 / J21.0 / J21.8 / J40 | |
| Diabetes/Poor glycemic control/ hyper-and hypoglycemia: diabetes mellitus with ketoacidosis or hyperosmolar coma | 250.0 / 250.1 / 250.2 / 250.8 / 250.02 / 250.03 / 250.10 / 250.11 / 250.12 / 250.13 / 250.20 / 250.21 / 250.22 / 250.23 / 250.30 / 250.31 / 250.32 / 250.33 / 251.0 / 251.2 / 790.29 | E11.9 / E10.9 / E11.65 / E10.65 / E11.01 / E11.69 / E13.10 / E10.10 / E11.00 / E11.641 / E10.641 / E10.618 / E10.620 / E10.621 / E10.622 / E10.628 / E10.630 / E10.638 / E10.649 / E11.618 / E11.620 / E11.621 / E11.622 / E11.628 / E11.630 / E11.638 / E11.649 / E15 / E16.2 / R73.03 / R73.09 / E10.69 / E10.11 | |
| Hypertension | 401.0 / 401.9 / 402.0 / 402.1 / 402.9 / / 403.10 / 403.90 / 404.10 / 404.90 | I10 / I16.9 / I11.9 / I11.0 / I13.10 / I20.0 / I12.9 | |
| Angina | 411.1 / 411.8 / 413 | I20.0 / I24.0 / I24.8 / I20.8 / I20.1 / I20.9 | |
| Seizures (Grand mal status and other epileptic convulsions) | 345 / 345.01 / 345.10 / 345.11 / 345.2 / 345.3 / 345.40 / 345.41345.50 / 345.51 / 345.60 / 345.61 / 345.70 / 345.71 / 345.80 / 345.81 / 345.90 / 345.91436. / 780.31 / 780.39 | G40.401 / G40.409 / G40.311 / G10.411 / G40.419 / G40.A01 / G40.A09 / G40.A11 / G40.A19 / G40.301 / G40.201 / G40.209 / G40.211 / G40.219 / G40.101 / G40.109 / G40.111 / G40.119 / G40.821 / G40.822 / G40.823 / G40.824 / G40.501 / G40.509 / G40.802 / G40.804 / G40.901 / G40.909 / G40.911 / G40.919 / I67.89 / R56.00 / R56.9 | |
| Hypotension | 458.0 / 458 / 458.8.1 / 458.21 /458.29 / 458.9 | I95.1 / I95.89 / I95.3 / I95.2 / I95.81 / I95.9 | |
| Dehydration, volume depletion acute renal failure hypokalemia hyponatremia | 276.5 / 276.8 / 584.5 / 584.6 / 584.7 / 584.8 / 584.9 / 588.81 / 588.89 / 588.9 / 276.1 / 276.8 | E87.1 / E86.9 / E86.0 / E86.1 / E87.6 / N17.0 / N17.1 / N17.2 / N17.8 / N17.9 / N25.81 / N25.89 / N25.9 | |
| Pneumonia (Lower respiratory: pneumonia & bronchitis) | 480.0 / 480.1 / 480.2 / 480.3 / 480.8 / 480.9 / 481. / 482.0 / 482.1 / 482.2 / 482.30 / 482.31 / 482.32 / 482.39 / 482.40 / 482.41 / 482.49 / 482.81 / 482.82 / 482.83 / 482.84 / 482.89 / 482.9 / 483.0 / 483.1 / 483.8 / 485. / 486. / 507.0 | J12.0 / J12.1 / J12.2 / J12.81 / J12.89 / J12.9 / J13 / J18.1 / J15.0 / J15.1 / J14 / J15.4 / J15.3 / J15.20 / J15.211 / J15.29 / J15.8 / J15.5 / J15.6 / A48.1 / J15.9 / J15.7 / J16.0 / J16.8 / J18.0 / J18.9 / J69.0 | |
| Urinary Tract Infection | 590.10 / 590.11 / 590.80 / 590.81 / 590.9 / 595.0 / 595.1 / 595.2 / 595.4 / 595.89 / 595.9 / 597.0 / 598.00 / 598.01 / 599.0 / 601.0 / 601.1 / 601.2 / 601.3 / 601.4 / 601.8 / 601.9 | N10 / N12 / N16 / N15.9 / N30.00 / N30.01 / N30.10 / N30.11 / N30.20 / N20.21 / N30.80 / N30.81 / N30.90 / N30.91 / N34.0 / N35.111 / N37 / N39.0 / N41.0 / N41.1 / N41.2 / N41.3 / N51 / N41.4 / N41.8 / N41.9 | |
| Constipation /fecal impaction/obstipation | 560.39 / 564.00 / 564.01 / 564.09 | K56.49 / K59.00 / K59.01 / K59.03 / K59.04 / K59.09 | |
| Skin ulcers | 707.00 / 707.01 / 707.02 / 707.03 / 707.04 / 707.05707.06 / 707.07 / 707.09 / 707.10 / 707.11 / 707.12 / 707.13 / 707.14 / / 707.9 / 707.15 / 707.19 / 707.8 | L89.90 / L89.009 / L89.119 / L89.129 / L89.139 / L89.149 / L89.159 / L89.209 / L89.309 / L89.509 / L89.609 / L89.819 / L89.899 / L97.909 / L97.109 / L97.209 / L97.309 / L97.409 / L97.509 / L97.809 / L98.419 / L98.429 / L98.499 | |
| Weight loss adult failure to thrive | 783.21 / 783.22 / 783.3 / 783.7 | R63.4 / R63.6 / R63.3 / R62.7 | |
| Nutritional deficiency | 260. / 261. / 262 / 263.0. / 263.1 / 263.2 / 263.8 / 263.9 / 268.0 / 268.1 | E40 / E41 / E43 / E44.0 / E44.1 / E45 / E46 / E55.0 / E64.3 |
ACSC are conditions, “where appropriate ambulatory care may prevent or reduce the need for admission to hospital”.(13) We measured ACSC hospitalizations two ways. First, with a general population definition as defined by Canadian Institute for Health Information (CIHI). Second, with an older population definition: the measure developed by Walsh and colleagues on older persons dually eligible for Medicare and Medicaid, previously used in a population with dementia by Feng et al.(7,14) This definition includes additional conditions that are more specific to an older population such as: hypotension, constipation, skin ulcers, or nutritional deficiency. See details on lists of conditions and ICD codes for each definition below.
The list of conditions included in the CIHI measure (general population definition) are asthma, cardiac heart failure, chronic obstructive pulmonary disorder (COPD), diabetes, hypertension, angina, seizures (see Table B1). The list of conditions included in the older population definition are asthma, cardiac heart failure, Chronic obstructive pulmonary disorder (COPD), diabetes, hypertension, hypotension, dehydration, pneumonia, urinary tract infection (UTI), constipation, skin ulcers, weight loss, nutritional deficiency, adult failure to thrive, seizures (see Table B2).
Thirty-day readmission included any all cause hospital readmission within 30 days after any hospital discharge. Day surgeries were excluded from the computation of 30-day readmission. Thirty-day readmission was measured in the subset of persons with at least one hospital discharge during the study period, while the other indicators of potentially avoidable hospital use were measured in the subset of persons with at least one hospital admission.
Delayed hospital discharge or ALC hospitalization is coded, by physicians or their delegates, in the administrative database as “a person who has completed the acute care phase of his or her treatment but remained in an acute care bed”.(27) These prolonged hospital stays correspond to persons who cannot be discharged from the hospital even though they do not require the level of care provided in hospitals, mostly waiting for a long-term care admission. The length of delayed hospital discharge or ALC stay was computed as the total number of ALC hospital days during the follow-up period in persons with at least one ALC hospitalization.(22)
APPENDIX REFERENCES
- 1.Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54(1):337–49. doi: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 2.Belzile E, Sanche S, McCusker J, et al. Technical Report: A measure of emergency department use based on Quebec’s Administrative Data. Montreal, QC: St Mary’s Research Center; 2011. [Google Scholar]
- 3.Amjad H, Carmichael D, Austin AM, et al. Continuity of care and health care utilization in older adults with dementia in fee-for-service Medicare. JAMA Intern Med. 2016;176(9):1371–78. doi: 10.1001/jamainternmed.2016.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pimouguet C, Rizzuto D, Fastbom J, et al. Influence of incipient dementia on hospitalization for primary care sensitive conditions: a population-based cohort study. J Alzheimers Dis. 2016;52(1):213–22. doi: 10.3233/JAD-150853. [DOI] [PubMed] [Google Scholar]
- 5.Zhu CW, Cosentino S, Ornstein K, et al. Use and cost of hospitalization in dementia: longitudinal results from a community-based study. Int J Geriatr Psychiatry. 2015;30(8):833–41. doi: 10.1002/gps.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasteridis P, Mason AR, Goddard MK, et al. The influence of primary care quality on hospital admissions for people with dementia in England: a regression analysis. PLoS One. 2015;10(3):e0121506. doi: 10.1371/journal.pone.0121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Z, Coots LA, Kaganova Y, et al. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff. 2014;33(4):683–90. doi: 10.1377/hlthaff.2013.1179. [DOI] [PubMed] [Google Scholar]
- 8.Ennis SK, Larson EB, Grothaus L, et al. Association of living alone and hospitalization among community-dwelling elders with and without dementia. J Gen Intern Med. 2014;29(11):1451–59. doi: 10.1007/s11606-014-2904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davydow DS, Zivin K, Katon WJ, et al. Neuropsychiatric disorders and potentially preventable hospitalizations in a prospective cohort study of older Americans. J Gen Intern Med. 2014;29(10):1362–71. doi: 10.1007/s11606-014-2916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin P-J, Fillit HM, Cohen JT, et al. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 2013;9(1):30–38. doi: 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Phelan EA, Borson S, Grothaus L, et al. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–72. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bynum JPW, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–94. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 13.CIHI. Ambulatory care sensitive conditions [Internet] North York, ON: CIHI; n.d. [cited 2019 Oct 21]. Available from: http://indicatorlibrary.cihi.ca/display/HSPIL/Ambulatory+Care+Sensitive+Conditions. [Google Scholar]
- 14.Jutan N, Langlois L, Damiano N. Seniors and alternate level of care: building on our knowledge [Internet] Healthc Q. 2013;16(3):7–10. doi: 10.12927/hcq.2013.23503. [cited 2019 Jun 18] [DOI] [PubMed] [Google Scholar]
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors declare that no conflicts of interest exist.
REFERENCES
- 1.Prince M, Comas-Herrera A, Knapp M, et al. World Alzheimer Report 2016: Improving healthcare for people living with dementia: coverage, quality and costs now and in the future [Internet] London, UK: Alzheimer’s Disease International; 2016. Available from: https://www.alz.co.uk/research/world-report-2016. [Google Scholar]
- 2.CIHI. Dementia in Canada [Internet] North York, ON: CIHI; 2018. [cited 2019 Oct 31]. Available from: https://www.cihi.ca/en/dementia-in-canada. [Google Scholar]
- 3.Lin P-J, Zhong Y, Fillit HM, et al. Hospitalizations for ambulatory care sensitive conditions and unplanned readmissions among Medicare beneficiaries with Alzheimer’s disease. Alzheimers Dement. 2017 Oct;13(10):1174–78. doi: 10.1016/j.jalz.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TS, Marcantonio ER, McCarthy EP, et al. National trends in potentially preventable hospitalizations of older adults with dementia. J Am Geriatr Soc. 2020;68(10):2240–48. doi: 10.1111/jgs.16636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzheimer Society of Canada. Report summary: Prevalence and monetary costs of dementia in Canada (2016): a report by the Alzheimer Society of Canada. Health Promot Chronic Dis Prev Can. 2016;36(10):231–32. doi: 10.24095/hpcdp.36.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. The epidemiology and impact of dementia: current state and future trends 2015 [Internet] Geneva, Switzerland: World Health Organization; 2015. Available from: http://www.who.int/mental_health/neurology/dementia/dementia_thematicbrief_epidemiology.pdf?ua=1. [Google Scholar]
- 7.World Health Organization. Dementia: a public health priority [Internet] Geneva, Switzerland: World Health Organization; 2012. Available from: https://extranet.who.int/agefriendlyworld/wp-content/uploads/2014/06/WHO-Dementia-English.pdf. [Google Scholar]
- 8.World Health Organization. WHO | Global action plan on the public health response to dementia 2017–2025 [Internet] Geneva, Switzerland: WHO; 2017. [cited 2018 Feb 6]. Available from: https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025. [Google Scholar]
- 9.Public Health Agency of Canada. A dementia strategy for Canada: together we aspire [Internet] Ottawa, ON: The Agency; 2019. [cited 2019 Jun 18]. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/dementia-strategy.html. [Google Scholar]
- 10.Amjad H, Carmichael D, Austin AM, et al. Continuity of care and health care utilization in older adults with dementia in fee-for-service Medicare. JAMA Intern Med. 2016;176(9):1371–78. doi: 10.1001/jamainternmed.2016.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pimouguet C, Rizzuto D, Fastbom J, et al. Influence of incipient dementia on hospitalization for primary care sensitive conditions: a population-based cohort study. J Alzheimers Dis. 2016;52(1):213–22. doi: 10.3233/JAD-150853. [DOI] [PubMed] [Google Scholar]
- 12.Zhu CW, Cosentino S, Ornstein K, et al. Use and cost of hospitalization in dementia: longitudinal results from a community-based study. Int J Geriatr Psychiatry. 2015;30(8):833–41. doi: 10.1002/gps.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasteridis P, Mason AR, Goddard MK, et al. The influence of primary care quality on hospital admissions for people with dementia in England: a regression analysis. PLoS One. 2015;10(3):e0121506. doi: 10.1371/journal.pone.0121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Z, Coots LA, Kaganova Y, et al. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff. 2014;33(4):683–90. doi: 10.1377/hlthaff.2013.1179. [DOI] [PubMed] [Google Scholar]
- 15.Ennis SK, Larson EB, Grothaus L, et al. Association of living alone and hospitalization among community-dwelling elders with and without dementia. J Gen Intern Med. 2014;29(11):1451–59. doi: 10.1007/s11606-014-2904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davydow DS, Zivin K, Katon WJ, et al. Neuropsychiatric disorders and potentially preventable hospitalizations in a prospective cohort study of older Americans. J Gen Intern Med. 2014;29(10):1362–71. doi: 10.1007/s11606-014-2916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin P-J, Fillit HM, Cohen JT, et al. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 2013;9(1):30–38. doi: 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Phelan EA, Borson S, Grothaus L, et al. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–72. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bynum JPW, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–94. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 20.Sourial N, Vedel I, Godard-Sebillotte C, et al. Sex differences in dementia primary care performance and health service use: a population-based study. J Am Geriatr Soc. 2020;68(5):1056–63. doi: 10.1111/jgs.16347. [DOI] [PubMed] [Google Scholar]
- 21.Government of Queèbec. Instutut national de santé publique. Monitoring of Alzheimer’s disease and related disorders: feasibility study based on health administrative databases [Internet] Quebec, QC: INSPQ; 1984. [cited 2018 Mar 30]. Available from: https://www.inspq.qc.ca/en/publications/1984. [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–77. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 23.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blais C, Jean S, Sirois C, et al. Quebec Integrated Chronic Disease Surveillance System (QICDSS), an innovative approach. Chronic Dis Inj Can. 2014;34(4):226–35. doi: 10.24095/hpcdp.34.4.06. [DOI] [PubMed] [Google Scholar]
- 25.Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54(1):337–49. doi: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 26.Godard-Sebillotte C, Sourial N, Hardouin M, et al. Development of two hierarchical algorithms identifying the 65+ community-dwelling population in the provincial administrative database in Quebec [poster presentation]. Annual CAHSPR Conference; 2019 May 27; Halifax, NS, Canada. Ottawa, ON: CAHSPR; 2019. [Google Scholar]
- 27.Sivananthan SN, McGrail KM. Diagnosis and disruption: population-level analysis identifying points of care at which transitions are highest for people with dementia and factors that contribute to them. J Am Geriatr Soc. 2016;64(3):569–77. doi: 10.1111/jgs.14033. [DOI] [PubMed] [Google Scholar]
- 28.CIHI. Ambulatory care sensitive conditions [Internet] North York, ON: CIHI; n.d. [cited 2019 Oct 21]. Available from: http://indicatorlibrary.cihi.ca/display/HSPIL/Ambulatory+Care+Sensitive+Conditions. [Google Scholar]
- 29.Jutan N, Langlois L, Damiano N. Seniors and alternate level of care: building on our knowledge [Internet] Healthc Q. 2013;16(3):7–10. doi: 10.12927/hcq.2013.23503. [cited 2019 Jun 18] [DOI] [PubMed] [Google Scholar]
- 30.Government of Canada Statistics Canada. Census of Population Program–data products [Internet] Ottawa, ON: Statistics Canada; 2011. 2016. [cited 2019 Oct 31]. Available from: https://www12.statcan.gc.ca/census-recensement/2011/dp-pd/index-eng.cfm. [Google Scholar]
- 31.Mondor L, Maxwell CJ, Hogan DB, et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: a retrospective analysis of a population-based cohort. PLoS Med. 2017;14(3):e1002249. doi: 10.1371/journal.pmed.1002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strumpf E, Levesque J-F, Coyle N, et al. Innovative and diverse strategies toward primary health care reform: lessons learned from the Canadian experience. J Am Board Fam Med. 2012;25(Suppl 1):S27–S33. doi: 10.3122/jabfm.2012.02.110215. [DOI] [PubMed] [Google Scholar]
- 33.Diop M, Fiset-Laniel J, Provost S, et al. Does enrollment in multidisciplinary team-based primary care practice improve adherence to guideline-recommended processes of care? Quebec’s Family Medicine Groups, 2002–2010. Health Policy. 2017;121(4):378–88. doi: 10.1016/j.healthpol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Teper MH, Godard-Sebillotte C, Vedel I. Achieving the goals of dementia plans: a review of evidence-informed implementation strategies. Healthc Policy. 2019;14(4):10–20. doi: 10.12927/hcpol.2019.25860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergman H. Report of the Committee of Experts for the Development of an Action Plan on Alzheimer’s Disease and Related Disorders. Quèbec, QC: Government of Quèbec, Ministry of Health & Social Services; 2009. Meeting the challenge of Alzheimer’s disease and related disorders: a vision focused on the individual, humanism, and excellence. [Google Scholar]
- 36.Chertkow H. Diagnosis and treatment of dementia: introduction. Introducing a series based on the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. CMAJ. 2008;178(3):316–21. doi: 10.1503/cmaj.070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore A, Patterson C, Lee L, Vedel I, Bergman H. Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia: recommendations for family physicians. Can Fam Physician. 2014;60(5):433–38. [PMC free article] [PubMed] [Google Scholar]
- 38.Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ. 2017;356:j84. doi: 10.1136/bmj.j84. [DOI] [PubMed] [Google Scholar]
- 39.Government of Quebec, Ministry of Health & Social Services. Répertoire des indicateurs de gestion en santé et services sociaux [Internet] Quebec, QC: the Minsitry; n.d. [cited 2019 Jun 18]. Available from: http://www.msss.gouv.qc.ca/repertoires/indicateurs-gestion/sources/rqsuch-j74. [Google Scholar]
- 40.Gruneir A, Silver MJ, Rochon PA. Emergency department use by older adults: a literature review on trends, appropriateness, and consequences of unmet health care needs. Med Care Res Rev. 2011;68(2):131–55. doi: 10.1177/1077558710379422. [DOI] [PubMed] [Google Scholar]
- 41.Béland F, Bergman H, Lebel P, et al. A system of integrated care for older persons with disabilities in Canada: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61(4):367–73. doi: 10.1093/gerona/61.4.367. [DOI] [PubMed] [Google Scholar]
- 42.van den Berg MJ, van Loenen T, Westert GP. Accessible and continuous primary care may help reduce rates of emergency department use. An international survey in 34 countries. Fam Pract. 2016;33(1):42–50. doi: 10.1093/fampra/cmv082. [DOI] [PubMed] [Google Scholar]
- 43.van Loenen T, van den Berg MJ, Westert GP, et al. Organizational aspects of primary care related to avoidable hospitalization: a systematic review. Fam Pract. 2014;31(5):502–16. doi: 10.1093/fampra/cmu053. [DOI] [PubMed] [Google Scholar]
- 44.Huntley A, Lasserson D, Wye L, et al. Which features of primary care affect unscheduled secondary care use? A systematic review. BMJ Open. 2014;4(5):e004746. doi: 10.1136/bmjopen-2013-004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riverin BD, Strumpf EC, Naimi AI, et al. Optimal timing of physician visits after hospital discharge to reduce readmission. Health Serv Res. 2018;53(6):4682–703. doi: 10.1111/1475-6773.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riverin BD, Li P, Naimi AI, et al. Team-based innovations in primary care delivery in Quebec and timely physician follow-up after hospital discharge: a population-based cohort study. CMAJ Open. 2017;5(1):E28–E35. doi: 10.9778/cmajo.20160059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riverin BD, Li P, Naimi AI, et al. Team-based versus traditional primary care models and short-term outcomes after hospital discharge. CMAJ. 2017;189(16):E585–E593. doi: 10.1503/cmaj.160427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toot S, Hoe J, Ledgerd R, et al. Causes of crises and appropriate interventions: the views of people with dementia, carers and healthcare professionals. Aging Ment Health. 2013;17(3):328–35. doi: 10.1080/13607863.2012.732037. [DOI] [PubMed] [Google Scholar]
- 49.Ledgerd R, Hoe J, Hoare Z, et al. Identifying the causes, prevention and management of crises in dementia. An online survey of stakeholders. Int J Geriatr Psychiatry. 2016;31(6):638–47. doi: 10.1002/gps.4371. [DOI] [PubMed] [Google Scholar]
- 50.Sloane PD, Schifeling CH, Beeber AS, et al. New or worsening symptoms and signs in community-dwelling persons with dementia: incidence and relation to use of acute medical services. J Am Geriatr Soc. 2017;65(4):808–14. doi: 10.1111/jgs.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toot S, Devine M, Akporobaro A, et al. Causes of hospital admission for people with dementia: a systematic review and meta-analysis. J Am Med Dir Assoc. 2013;14(7):463–70. doi: 10.1016/j.jamda.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Phelan EA, Debnam KJ, Anderson LA, et al. A systematic review of intervention studies to prevent hospitalizations of community-dwelling older adults with dementia. Med Care. 2015;53(2):207–13. doi: 10.1097/MLR.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;(6):CD007760. doi: 10.1002/14651858.CD007760.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanson LC, Zimmerman S, Song M-K, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017;177(1):24–31. doi: 10.1001/jamainternmed.2016.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]