Abstract
Purpose
To evaluate the antiviral potential of five multipurpose disinfecting solutions against coronavirus (mouse hepatitis virus, a surrogate for SARS-CoV-2 human corona virus).
Methods
Test solutions (Biotrue, renu Advanced [Bausch and Lomb], ACUVUE RevitaLens [Johnson and Johnson Vision], cleadew [Ophtecs corp.] or AOSept Plus [Alcon]) were mixed with the coronavirus mouse hepatitis virus at 104 plaque forming units (PFU)/mL as the final concentration and incubated at room temperature for the specified disinfection time. Surviving virus from each sample was then quantified by standard plaque forming unit assay and the reduction of PFU for each disinfectant was compared to the phosphate buffer saline (PBS) treated negative control. A regimen test was also conducted using Biotrue.
Results
The three multipurpose disinfecting solutions Biotrue (containing PHMB and polyquaternium-1), renu Advanced (PHMB, polyquaternium-1 and alexidine) and ACUVUE RevitaLens (polyquaternium-1 and alexidine) did not kill the coronavirus at the manufacturers recommended disinfection time in the stand alone test. After treatment, the virus’s titer (3.8 ± 0.2 log10 for Biotrue, 3.7 ± 0.1 log10 for renu and 3.7 ± 0.2 log10 for RevitaLens) was similar to the negative control (3.7 ± 0.1 log10; p ≥ 0.996). AOSept Plus (hydrogen peroxide) and cleadew (povidone iodine) significantly (p < 0.001) reduced the numbers of coronaviruses to below the detection limit (i.e. killed 3.7 ± 0.1 log10 viruses compared to control). However, there was a significant reduction (p = 0.028) in numbers of coronaviruses attached to lenses when using the regimen test with Biotrue.
Conclusions
This study shows that oxidative contact lens disinfecting solutions (i.e. those containing povidone-iodine or hydrogen peroxide) provide superior antiviral activity against a coronavirus surrogate of SARS-CoV-2, unless the full regimen test (rub, rinse, disinfect) is used.
Keywords: Contact lens disinfection, Coronavirus, Hydrogen peroxide, Iodine, Quaternary ammonium
1. Introduction
The coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus (CoV) called Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) [1]. Due to rapid spread around the world, the World Health Organization (WHO) declared COVID-19 a pandemic on 11th March 2020 [2].
During COVID-19 it has been proposed that one portal of entry of SARS-CoV-2 is the eye. Some reports have reported red or sore eyes a few days prior to symptoms of COVID-19 developing [3], [4], although ocular signs are relatively unusual during the disease [5]. SARS-CoV-2 RNA has been found in tears or ocular swabs in some, albeit usually a small minority of COVID-19 patients [6]. Studies have suggested that people wearing spectacles are at a lower risk of developing COVID-19 than non-spectacle wearers [7], [8], although appropriate case control or similar studies have not yet been reported.
SARS-CoV-2 may enter eyes after a person’s hands have touched contaminated surfaces or via aerosols directly hitting the ocular surface. Hence, the worldwide emphasis on hand hygiene and avoidance of touching face, mouth and eye to contain the spread of the virus [1]. It has been assumed that CL wearers touch their eyes more than non-wearers as they need to insert and remove contact lenses, usually on a daily basis. Worldwide, the most common wear schedule for contact lenses remains daily wear [9], during which lenses are disinfected when not being worn. This disinfection provides an opportunity to kill any microbes, including coronavirus, that may be contaminating the lenses after wear and prior to re-wear. Contact lenses soaked in multipurpose disinfecting solutions can take up excipients and impart antibacterial activity [10], [11].
According to the Centres for Disease Control and Prevention, hydrogen peroxide-based systems are effective against the COVID-19 (https://www.cdc.gov/contactlenses/care-systems.html), and a study has reported that a 0.5% solution of hydrogen peroxide, much less than the 3% used in contact lens disinfecting solutions, can cause a > 4 log10 reduction in coronavirus infectivity within one minute [12], [13]. However, hydrogen peroxide is neutralised prior to the lenses being worn and so there is likely to be no residual antimicrobial activity with these types of solutions. Povidone-iodine, at a concentration of 0.23%, caused a > 4 log10 reduction in coronavirus infectivity within fifteen seconds [12].
Whilst the disinfectants in often older versions of multipurpose disinfecting solutions such as benzalkonium chloride and chlorhexidine digluconate have been shown to have some activity against coronaviruses [12] there appear to be no studies examining the efficacy of current multipurpose disinfecting solutions. Therefore, the current study was designed to evaluate the anti-coronavirus potential of multipurpose disinfecting solutions in comparison with a hydrogen peroxide-based system against coronavirus.
2. Materials and methods
2.1. Cells and viruses
Mouse hepatitis virus (MHV) ATCC/VR261 stock was prepared prior to testing by growing in A9 ATCC/CCL 1.4 cells in Dulbecco’s minimum essential medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics (streptomycin sulphate and penicillin G). MHV titres were determined by plaque assay as described below.
2.2. Disinfecting solutions
Test solutions (Biotrue, renu Advanced [Bausch and Lomb, Rochester, NY, USA], ACUVUETM RevitaLens [Johnson and Johnson Vision, Jacksonville, FL, USA], cleadew soft [Ophtecs corp., Kobe, Japan] or AOSept Plus [Alcon, Fort Worth, TX, USA]) were purchased from the manufacturers. The components of the disinfecting solutions are given in Table 1 .
Table 1.
Components of the contact lens disinfecting solutions used in the current study.
| Disinfecting solution (Manufacturer; recommended disinfection time [hours]) | Disinfectants | Surfactants | Other |
|---|---|---|---|
| Biotrue (Bausch and Lomb; 4) | Polyaminopropyl biguanide 0.00013%; polyquaternium 0.0001%. | Sulfobetaine; poloxamine | Hyaluronan, boric acid, sodium borate, edetate disodium and sodium chloride |
| renu Advanced (Bausch and Lomb; 4) | Polyaminopropyl biguanide 0.00005%; polyquaternium 0.00015%; alexidine 0.0002% | Poloxamine; poloxamer 181 | Diglycine, sodium citrate, boric acid, sodium borate, edetate disodium and sodium chloride |
| ACUVUETM RevitaLens (Johnson and Johnson Vision; 6) | Alexidine dihydrochloride 0.00016%; polyquaternium-1 0.0003% | Tetronic 904 | Boric acid, sodium borate, edetate disodium and sodium chloride |
| cleadew soft (Ophtecs; 4) | Povidone–iodine (4.0 mg/tablet); hydrogen peroxide | Ascorbic acid, proteolytic enzyme, sodium borate, boric acid and sodium chloride | |
| AOSept Plus (Alcon; 6) | Hydrogen peroxide 3% | Poloxamer | Polyoxyethylene, polyoxybutylene, phosphonic acid, sodium chloride and phosphate buffer |
2.3. Exposure of viruses to disinfectant solutions
This used a modified stand alone assay from ISO 14729 “Ophthalmic Optics—Contact Lens Care Products—Microbiological Requirements and Test Methods for Products and Regimens for Hygienic Management of Contact Lenses”. Viruses, at 104 plaque forming units (PFU)/mL as the final concentration, were incubated with each disinfectant at ambient temperature for the specified disinfection time as recommended by each manufacturer (Table 1).
The lenses cases supplied for use with AOSept were used and as this contains a platinum disk, the hydrogen peroxide was neutralised during the test. Cleadew was used according to the manufacturers instructions and during use the povidone-iodine tablet dissolves releasing the iodine initially and then ascorbic acid to neutralise the iodine. Following incubation, Biotrue, renu Advance and ACUVUE RevitaLens were inactivated by serially diluting samples in Dey Engley broth (Becton, Dickinson and Company, Macquarie Park, NSW, Australia). Then, the number of viral cells from each sample was quantified by plaque forming assay. Controls for the assay were viruses incubated for six hours in phosphate buffered saline (8 g/L NaCl, 0.2 g/L KCl, 2.16 g/L Na2HPO4, 0.2 g/L KH2PO4, pH 7.4).
2.4. Regimen testing
Biotrue was selected for this assay and the assays was performed according to ISO 14729 with slight modifications, which were the substitution of virus for the bacteria and fungi in the original procedure and the use of a control of phosphate buffered saline and Biotrue with no rub and rinse. The virus (50 µL at 104 PFU/mL) was added on both sides of contact lenses (etafilcon A; Acuvue 2; Johnson and Johnson Vision, Jacksonville, FL, USA) and allowed to adhere to the lenses for 10 min. Thereafter, each side of lens was rubbed and rinsed with Biotrue disinfectant for 5 sec. Then, lenses were incubated with 2 mL of Biotrue for 4 h at ambient temperature. Viruses were recovered from the lenses by vortexing in 2 mL of Dey Engley neutralising broth. Surviving viruses were then quantified by standard plaque forming unit assay. Lenses inoculated with virus but without rubbing and rinsing were treated in the same way and used as controls, as were lenses inoculated with virus, without rubbing and rinsing, but incubated for 4 h in phosphate buffered saline.
2.5. Plaque assay
For titration of infectious virus, A9 cells were seeded in 12-well tissue culture plates at 5–10 × 105 cells per well and allowed to adhere overnight at 37 °C in 5% CO2. After incubation, the culture medium was removed and cells were washed with 1X phosphate buffered saline. Thereafter, 100 µL of serially diluted (in Dey Engley neutralising broth) disinfecting solutions or the viruses removed from contact lenses after the regimen procedure were inoculated in each well and incubated for 1 h at ambient temperature. After incubation, the cells were overlaid with 1% agarose (Sigma-Aldrich, Castle Hill, NSW, Australia) and further incubated for 72 h. Following incubation, the cells were fixed with 4 % formaldehyde for 2–3 h. The number of viral PFU from each sample was quantified after staining cells with 1% crystal violet (Sigma-Aldrich). Reduction in PFU for each disinfectant compared to the negative control (PBS) was calculated [14].
2.6. Statistical analyses
Statistical analyses were performed using GraphPad Prism 7.02 software (GraphPad Software, La Jolla, CA, USA). The comparison on the activity of all the disinfectants compared to control was made using one way ANOVA with Tukey’s test setting statistical significance at p < 0.05. For the data from regimen testing, Mann-Whitney U test was performed with significance also set at p < 0.05.
3. Results
3.1. Stand alone testing
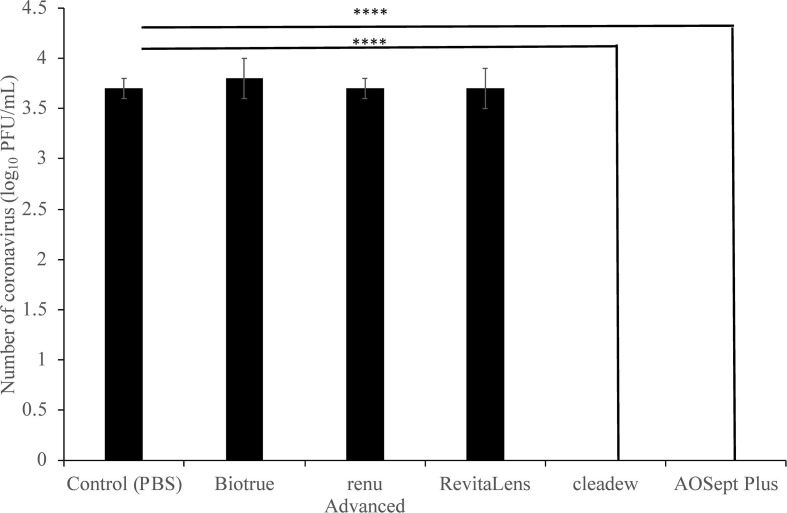
The three multipurpose disinfecting solutions Biotrue (containing PHMB and polyquaternium-1), renu Advanced (PHMB, polyquaternium-1 and alexidine) and ACUVUE RevitaLens (polyquaternium-1 and alexidine) were unable to reduce the number of PFU of the coronavirus at the manufacturers recommended disinfection time (p ≥ 0.996; Fig. 1 ). After treatment, the virus’s titre (3.8 ± 0.2 log10 p = 0.996 for Biotrue; 3.7 ± 0.1 log10 p > 0.996 for renu Advance; 3.7 ± 0.2 log10 p > 0.996 for RevitaLens) was almost identical to the negative control (3.7 ± 0.1 log10). However, those containing hydrogen peroxide (AOSept Plus) and povidone iodine (cleadew) were highly antiviral. At the manufacturer’s disinfection time, for both AOSept Plus (hydrogen peroxide) and cleadew (povidone iodine) the numbers of coronaviruses were reduced to below the detection limit (10 PFU/mL; p < 0.0001; Fig. 1).
Fig. 1.
Reduction in the number of plaque forming units of the coronavirus (mouse hepatitis virus) by various contact lens disinfecting solutions. **** represents p = 0.001 compared to negative control.
3.2. Regimen testing
Table 2 shows the effects of the regimen test incorporating a rub and rinse step.
Table 2.
The effect the regimen test on the numbers of coronavirus attached to etafilcon A contact lenses.
| Treatment of lenses | Number of viruses (log10 PFU/lens) | P-value (vs. lenses treated with Biotrue only) |
|---|---|---|
| Lenses treated with Biotrue only | 2.01 ± 0.05 | |
| Regimen test – lenses rubbed, rinsed and treated with Biotrue | ≤1.00 ± 0.001a | 0.028 |
| Lenses incubated in phosphate buffered saline only | 2.51 ± 0.02 | 0.029 |
a, no PFU were detected in the rub, rinse and Biotrue treated samples.
Lenses that were rubbed and rinsed before being treated with Biotrue showed a significant reduction (P = 0.028) in the number of coronaviruses that were able to form plaques compared to lenses incubated in Biotrue without rubbing and rinsing. Lenses that were incubated in Biotrue alone showed a small but significant (p = 0.029) 0.5log10 reduction in viral numbers (Table 2).
4. Discussion
This research has shown that a SARS-CoV-2 coronavirus surrogate (MHV) is unaffected when added directly to multipurpose disinfecting solutions containing various mixtures and concentrations of the disinfectants polyaminopropyl biguanide, polyquaternium-1 and alexidine as well as various surfactants. However, contact lens disinfecting solutions containing 3% hydrogen peroxide or 4.0 mg povidone-iodine (final concentration 0.5 mg/mL [or 0.05%]) can reduce the numbers of plaque forming units of this virus to below the limit of detection (10 PFU). The regimen test was able to reduce the number of viruses adherent to etafilcon A contact lenses with Biotrue.
MHV is recognised by the Therapeutic Goods Administration of Australia as an appropriate surrogate for SARS-CoV-2 for use to test the effectiveness of disinfectants (https://www.tga.gov.au/surrogate-viruses-use-disinfectant-efficacy-tests-justify-claims-against-covid-19). MHV and SARS-CoV-2, along with SARS and Middle Eastern Respiratory Syndrome virus (MERS), all belong to Group 2 coronaviruses [15] (also known as betacoronaviruses) as do other human coronaviruses such as OC43 and HKU1. On the other hand, human coronavirus 229E, also a recommended surrogate of SARS-CoV-2, and NL63 belong to Group 1 coronaviruses [15] (also known as alphacoronaviruses). MHV has a mechanism of infection very similar to SARS-CoV-2 and produces severe acute respiratory disease in mice [16], [17], [18], [19].
MHV is sensitive to some disinfecting agents. Seventy percent ethanol can reduce the PFU of MHV on stainless steel by 3.9 log10 within one minute, whereas 1:100 hypochlorite reduced the PFU by 0.62 log10 in one minute [20]. Household disinfectants or antiseptics containing 0.05% triclosan or 0.1% of the quaternary compound alkyl dimethyl benzyl ammonium saccharinate in combination with 79% of ethanol reduced the PFU of MHV by ≥ 3 log10 within 30 s [21]. Wipes containing n-alkyl [68% C12, 32% C14] dimethyl ethylbenzyl ammonium chloride (0.14%) and n-alkyl [60% C14, 30% C12, 5% C18] dimethyl benzyl ammonium chlorides (0.14%) reduced the number of PFU of either MHV or SARS-CoV-2 on face masks by ≥ 5 log10 after a single wipe and 5 min drying [22]. Exposing the masks to ionised 3% hydrogen peroxide for 15 min also reduced the viral loads on masks by a similar amount [22]. SARS-CoV-2 and MERS are sensitive to povidone-iodine at concentrations of 7.5% (surgical scrub) [23], [24] or 4% (hand wash) [24]. Hypochlorous acid (0.01%) reduced the numbers of adenovirus type 19/64 on contact lenses by ≥ 3.5 log10 but could only reduce Herpes simplex-1 by ≤ 0.6 log10 PFU [25].
The two disinfecting solutions that were active against MHV in the current study were both oxidising agents (hydrogen peroxide and iodine). Iodine can oxidise proteins, nucleotides and fatty acids [26] and hydrogen peroxide oxidises many cellular components including lipid membranes, nucleosides and proteins [27]. These multifaceted actions may be the reason iodine and hydrogen peroxide were superior to the quaternary ammonium compounds used in the other multipurpose disinfecting solutions. Quaternary ammonium compounds such as polyaminopropyl biguanide, polyquaternium and alexidine act primarily on microbial membranes [28]. The membranes of many microbes have an overall negative charge and this allows the positively charged quaternary ammonium compounds to bind and disrupt membrane function [28]. Coronaviruses are enveloped viruses, that is they possess a lipid bilayer that surrounds an inner protein capsid and this lipid bilayer is derived from the mammalian host cells. Mammalian cells asymmetrically distribute their membrane lipids, with negatively charged lipids such as phosphatidylserine being almost exclusively found on the inner leaflet of the membrane [29], [30]. Whilst the lipidome of the envelop of SARS-CoV-2 or MHV is not yet known, it is possible that the asymmetry of the lipids persist and this would make the interaction with the positively charged quaternary ammonium compounds unfavourable. Manufacturers of multipurpose disinfecting solutions need to balance antimicrobial efficacy with mammalian cell toxicity [31]. This means that the concentration of quaternary ammonium disinfectants in contact lens multipurpose disinfecting solutions (0.00005 to 0.0003%) is substantially less than in other disinfectants (≥0.1%), and this may be another reason for the lack of efficacy of the quaternary ammonium disinfectants in the current study compared to other studies [22].
Multipurpose contact lens disinfecting solutions do not need to be active against viruses for them to pass tests, such as the International Organisation for Standardization test ISO 14729, that regulatory authorities such as the Federal Drug Administration of the USA, Therapeutic Goods Administration of Australia or European Medicines Agency mandate. Although older versions of tests such as the U.S. Food and Drug Administration, “DRAFT testing guidelines for class III soft (hydrophilic) contact lens solutions,” FDA, Washington, D.C. (July 15, 1985) did include the need to test contact lens disinfectants against Herpes simplex type 1 virus [32], [33]. Contact lens disinfecting solutions have been tested for their efficacy against viruses other than coronaviruses in the past. Often, these tests were spurred by other viral epidemics such as acquired immunodeficiency syndrome (AIDS) caused by the human immunodeficiency virus (HIV). [34]. ReNu Multipurpose (containing 0.00005% polyaminopropyl biguanide; Bausch and Lomb) was able to reduce the numbers of HIV on lenses only when a rubbing procedure was used [35]. MeniCare Soft Multipurpose Solution (containing 0.0001% polyaminopropyl biguanide; Menicon) reduced the infective numbers of Herpes simplex-1 by 1.4 log10 PFU and adenovirus by approximately 0.9 log10 PFU but was unable to significantly reduce the PFU of poliovirus when used without a rub and rinse step [34]. However, when the rub and rinse regime was used, MeniCare Soft was able to reduce the numbers of Herpes simplex-1, adenovirus and poliovirus on lenses by ≥ 4.5 log10. The need for a rub and rinse procedure (i.e. the ISO 14,729 regimen test) to significantly reduce the numbers of viruses removed from lenses was confirmed in the current study. Whilst the current study only examined Biotrue with etafilcon A in this regimen test, it is likely that the same efficacy would be seen with other lenses and solutions as has been shown with bacteria, fungi and Acanthamoeba [36]. However, this should be tested in future studies.
In conclusion, this study shows that oxidative contact lens disinfecting solutions (i.e. those containing povidone-iodine or hydrogen peroxide) provide superior antiviral activity against a coronavirus surrogate of SARS-CoV-2. However, the application of a rub and rinse procedure prior to disinfection for the manufacturers recommended disinfection time could reduce the numbers of coronaviruses removed from lenses. Contact lens prescribers should reinforce the need to rub and rinse lenses with multipurpose disinfecting solutions, as this is likely to reduce the numbers of any coronaviruses on the lenses.
Declaration of Competing Interest
The authors MY and AKV declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. One author, MW, is a member of the Scientific Advisory Board of Ophtecs corp. whose cleadew disinfectant was tested in this study.
References
- 1.Jones L., Walsh K., Willcox M., Morgan P., Nichols J. The COVID-19 pandemic: Important considerations for contact lens practitioners. Cont Lens Anterior Eye. 2020;43(3):196–203. doi: 10.1016/j.clae.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C.-W., Liu X.-F., Jia Z.-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Chen X., Chen L., Deng C., Zou X., Liu W., et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18(3):360–362. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal K, Agarwal A, Jaiswal N, Dahiya N, Ahuja A, Mahajan S, et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS One 2020;15(11):e0241661. 10.1371/journal.pone.0241661. [DOI] [PMC free article] [PubMed]
- 6.Peng M., Dai J., Sugali C.K., Rayana N.P., Mao W. The role of the ocular tissue in SARS-CoV-2 transmission. Clin Ophthalmol. 2020;14:3017–3024. doi: 10.2147/OPTH.S269868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senior AKS. Risk of corona virus disease 2019 (COVID-19) among spectacles wearing population of northern India. medRxiv 2021;February 13th. 10.1101/2021.02.12.21249710.
- 8.Zeng W., Wang X., Li J., Yang Y., Qiu X., Song P., et al. Association of daily wear of eyeglasses with susceptibility to coronavirus disease 2019 infection. JAMAOphthalmol. 2020;138(11):1196. doi: 10.1001/jamaophthalmol.2020.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan P., Woods C., Tranoudis I.G., Efron N., Jones L., Grupcheva C.N., et al. International contact lens prescribing In 2020. Contact Lens. Spectrum. 2021 January https://www.clspectrum.com/issues/2021/january-2021/international-contact-lens-prescribing-in-2020 [Google Scholar]
- 10.Dutta D, Zhu H, Willcox M. Antimicrobial activity of multipurpose disinfection solution soaked contact lenses. In: 7th International Conference on the Tear Film and Ocular Surface: Basic Science and Clinical Relevance. Taormina, Sicily, Italy; 2013.
- 11.Morris CA, Maltseva IA, Rogers VA, Ni J, Khong KT, Derringer CB, et al. Consequences of preservative uptake and release by contact lenses. Eye Contact Lens 2018;44 Suppl 2:S247-S55. 10.1097/ICL.0000000000000480. [DOI] [PubMed]
- 12.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampf G, Todt D, Pfaender S, Steinmann E. Corrigendum to “Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents” [J Hosp Infect 104 (2020) 246-251]. J Hosp Infect 2020. 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed]
- 14.Mendoza EJ, Manguiat K, Wood H, Drebot M. Two detailed plaque assay protocols for the quantification of infectious SARS-CoV-2. Curr Protoc Microbiol 2020;57(1):ecpmc105. 10.1002/cpmc.105. [DOI] [PMC free article] [PubMed]
- 15.Singh D., Joshi K., Samuel A., Patra J., Mahindroo N. Alcohol-based hand sanitisers as first line of defence against SARS-CoV-2: a review of biology, chemistry and formulations. Epidemiol Infect. 2020;148:e229. doi: 10.1017/S0950268820002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Albuquerque Nadine, Baig Ehtesham, Ma Xuezhong, Zhang Jianhua, He William, Rowe Andrea, et al. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J Virol. 2006;80(21):10382–10394. doi: 10.1128/JVI.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Zhangsheng, Du Jun, Chen Gang, Zhao Jie, Yang Xuanming, Su Lishan, et al. Coronavirus MHV-A59 infects the lung and causes severe pneumonia in C57BL/6 mice. Virol Sin. 2014;29(6):393–402. doi: 10.1007/s12250-014-3530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Körner Robert, Majjouti Mohamed, Alcazar Miguel, Mahabir Esther. Of mice and men: The coronavirus MHV and mouse models as a translational approach to understand SARS-CoV-2. Viruses. 2020;12(8):880. doi: 10.3390/v12080880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan X., Cao D., Kong L., Zhang X. Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein. Nat Commun. 2020;11(1):3618. doi: 10.1038/s41467-020-17371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulkower R.L., Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Am J Infect Control. 2011;39(5):401–407. doi: 10.1016/j.ajic.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellanno C., Vega Q., Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am J Infect Control. 2009;37(8):649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch Jennifer L., Xiang Jinhua, Mackin Samantha R., Perlman Stanley, Thorne Peter, O’Shaughnessy Patrick, et al. Inactivation of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) and diverse RNA and DNA viruses on three-dimensionally printed surgical mask materials. Infect Control Hosp Epidemiol. 2021;42(3):253–260. doi: 10.1017/ice:2020.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin Alex W H, Chu Julie T S, Perera Mahen R A, Hui Kenrie P Y, Yen Hui-Ling, Chan Michael C W, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA) Infect Dis Ther. 2015;4(4):491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanowski E.G., Yates K.A., Romanowski J.E., Mammen A., Dhaliwal D.K., Jhanji V., et al. The use of hypochlorous acid to disinfect bacteria, fungi and virus from contact lenses and cases. JSM Ophthalmol. 2020;7:1075. [Google Scholar]
- 26.Lepelletier D., Maillard J.Y., Pozzetto B., Simon A. Povidone iodine: Properties, mechanisms of action, and role in infection control and Staphylococcus aureus decolonization. Antimicrob Agents Chemother. 2020;64(9) doi: 10.1128/AAC.00682-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finnegan M., Linley E., Denyer S.P., McDonnell G., Simons C., Maillard J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother. 2010;65(10):2108–2115. doi: 10.1093/jac/dkq308. [DOI] [PubMed] [Google Scholar]
- 28.Gerba Charles P., Müller V. Quaternary ammonium biocides: efficacy in application. Appl Environ Microbiol. 2015;81(2):464–469. doi: 10.1128/AEM.02633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Op den Kamp J A F. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48(1):47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 30.Devaux P.F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 31.Willcox Mark, Keir Nancy, Maseedupally Vinod, Masoudi Simin, McDermott Alison, Mobeen Rabia, et al. CLEAR - contact lens wettability, cleaning, disinfection and interactions with tears. Cont Lens Anterior Eye. 2021;44(2):157–191. doi: 10.1016/j.clae.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal R.A., Sutton S.V., Schlech B.A. Review of standard for evaluating the effectiveness of contact lens disinfectants. PDA J Pharm Sci Technol. 2002;56(1):37–50. https://www.ncbi.nlm.nih.gov/pubmed/11865782 [PubMed] [Google Scholar]
- 33.Pepose J.S. Contact lens disinfection to prevent transmission of viral disease. CLAO J. 1988;14(3):165–168. https://www.ncbi.nlm.nih.gov/pubmed/2850120 [PubMed] [Google Scholar]
- 34.Heaselgrave W., Lonnen J., Kilvington S., Santodomingo-Rubido J., Mori O. The disinfection efficacy of MeniCare soft multipurpose solution against Acanthamoeba and viruses using stand-alone biocidal and regimen testing. Eye Contact Lens. 2010;36(2):90–95. doi: 10.1097/ICL.0b013e3181d13c2d. [DOI] [PubMed] [Google Scholar]
- 35.Amin R.M., Dean M.T., Zaumetzer L.E., Poiesz B.J. Virucidal efficacy of various lens cleaning and disinfecting solutions on HIV-I contaminated contact lenses. AIDS Res Hum Retroviruses. 1991;7(4):403–408. doi: 10.1089/aid.1991.7.403. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H., Bandara M.B., Vijay A.K., Masoudi S., Wu D., Willcox M.D. Importance of rub and rinse in use of multipurpose contact lens solution. Optom Vis Sci. 2011;88(8):967–972. doi: 10.1097/OPX.0b013e31821bf976. [DOI] [PubMed] [Google Scholar]