Abstract
Rationale and Objectives
To determine if, during the first wave of the COVID-19 pandemic, 1) the proportion of complicated appendicitis changed, and 2) if imaging strategies for appendicitis in children changed.
Materials and Methods
Retrospective cross-sectional study using administrative data from the Pediatric Health Information System, inclusive of pediatric patients diagnosed with appendicitis from March to May in 2017, 2018, 2019 and 2020. We compared trends during COVID-19 pandemic (March–May 2020) with corresponding pre-COVID-19 periods in 2017–201.9 Study outcomes were the proportion of complicated appendicitis and trends in imaging for appendicitis explained by patient-level variables.
Results
The proportion of complicated appendicitis cases increased by 4.4 percentage points, from 46.5% pre-COVID-19 (2017–2019) to 50.9% during COVID-19 (2020), p < 0.001. Mean count of uncomplicated acute appendicitis cases decreased from pre-COVID-19 to the 2020 COVID-19 period (2017: n = 2555; 2018: n = 2679; 2019: n = 2722; 2020: n = 2231). Mean count of complicated appendicitis was unchanged between study periods (2017: n = 2189; 2018: n = 2302, 2019: n = 2442; 2020: n = 2311). Imaging approaches were largely unchanged between study periods; ultrasound was the most utilized modality in both study periods (68.3%, 70.2%; p = 0.033).
Conclusion
During the first wave of the COVID-19 pandemic, the proportion of complicated appendicitis cases increased without an absolute increase in the number of complicated appendicitis cases, but instead a decrease in the number of uncomplicated acute appendicitis diagnoses.
Keywords: COVID-19, Pediatrics, Appendicitis, Outcomes
INTRODUCTION
In March 2020, the novel coronavirus SARS-CoV-2 (COVID-19) disease was classified as a global pandemic by the World Health Organization (WHO). As a mitigation response, many state and local governments enacted stay at home orders or shelter in place restrictions to reduce community spread (1). Though there were variations by state, in general, these policies closed schools and nonessential businesses, restricted in-restaurant dining, limited large gatherings, and temporarily restricted nontime-sensitive medical procedures (2,3).
In addition to imposed stay-at-home orders, individual perceived risk of exposure to COVID-19 may have led patients or patient families to delay medical care (4). This has been documented in terms of imaging utilization, with early reports in 2020 from academic medical systems demonstrating significant declines in medical imaging of up to 70% in the first 21 weeks of 2020 (5). More specifically, Davenport et al. (6) established that between January and September 2020, 3,689,874 fewer computed tomography (CT) examinations were performed than predicted, representing a 19% reduction in utilization.
In the context of a global pandemic, delaying elective procedures exemplifies appropriate public health practice without significant individual clinical impact, however, delays of necessary procedures can have meaningful outcomes both individually and at the population level. In pediatrics, acute appendicitis is the most common urgent abdominal surgical indication with an annual incidence of approximately 70,000 children per year in the United States (7, 8, 9). Appendicitis outcomes may be sensitive to delays in treatment, which can lead to complications including, but not limited to appendiceal rupture, abscess formation, and sepsis. We hypothesized that due to delays in care during the early phase of the COVID-19 pandemic there was a significant change in the proportion of complicated appendicitis compared to the proportion observed in previous years. Some data demonstrating this effect exists but is derived from single or limited institution cross-sectional studies and a national level investigation has not been performed to date (4,10, 11, 12, 13, 14, 15, 16, 17, 18).
On the provider side, imaging strategies for acute appendicitis may have changed during the global pandemic in an effort to prioritize resources while limiting staff and patient/family exposure. The purpose of the current study is to describe national trends of appendicitis and appendicitis imaging strategies across 47 pediatric hospitals in the early phase of the COVID-19 pandemic, depicted from March through May 2020, with trend comparison to corresponding time periods in 2017–2019.
METHODS AND MATERIALS
Data Source and Study Design
This was a cross-sectional retrospective study using administrative data from the Pediatric Health Information System (PHIS), managed by the Children's Hospital Association (19). The PHIS database contains information for patient encounters in the emergency department, inpatient, outpatient, observation, and ambulatory surgery settings, including imaging data, from 52 children's hospitals across the United States. Data included in the PHIS database are quality checked for accuracy and reliability by Children's Hospital Association (Overland Park, Kansas) and Truven Health Analytics (Ann Arbor, MI). The study was categorized as exempt by the institutional review board at, as the data did not contain patient identifying information.
Study Population
This study included pediatric patients (age range: 0–17 years) who either presented to the Emergency Department or transferred from another facility with an ultimate diagnosis of appendicitis between March and May during 2017, 2018, 2019, and 2020. To ensure a steady-state population, we limited included data to hospitals (n = 47) that submitted inpatient, emergency department, observation, ambulatory surgery, and imaging data for each year of the study period. Five institutions did not submit data pertinent to this study, including institutions in New York City, one of the epicenters of the COVID-119 pandemic during the study period. The time period of March through May was selected given that in 2020, mandated social distancing was implemented during these three months due to a variety of stay-at-home policies in many states throughout the US (2). Comparison was made to data during the same period in prior years to control for seasonality effects. Patients who had appendicitis were identified based on ICD-10 (International Classification of Diseases, 10th Revision) diagnosis codes K352x, K353x, K3580, and K3589x.
Variables and Outcome Measures
The data included in this study is administrative data from the PHIS database without clinical confirmation. The diagnosis codes were stratified into two different categories - complicated (perforated) appendicitis and uncomplicated acute appendicitis. Using the American Association for the Surgery of Trauma (AAST) Emergency Surgery Guidelines for acute appendicitis as a guide, the determination was made that acute appendicitis with generalized peritonitis with or without abscess would be categorized as complicated appendicitis (20). Following the methods described in previous reports (21,22), the study's primary outcome measure, proportion of complicated (perforated) appendicitis, was calculated as the number of cases of acute appendicitis with generalized peritonitis with or without abscess (ICD-10 K35.2, K35.20, K35.21) summed with the number of cases of acute appendicitis with localized peritonitis with or without abscess (ICD-10 K35.3, K35.30, K35.31, K35.32, K35.33) divided by the number of patients with any diagnosis of appendicitis. We identified patients with incidental appendectomies occurring during other additional surgical procedures using a method previously reported.
For analysis of imaging trends, only abdominal imaging reasonably assumed to be related to the diagnosis of appendicitis, including computed tomography (CT), ultrasound (US), magnetic resonance imaging (MRI) was included. Because the data is administrative, clinical details are absent and therefore certain assumptions were stipulated. For instance, all abdominal diagnostic imaging examinations performed for the cohort were considered to be for medical decision-making related to the diagnosis of appendicitis. Type(s) of imaging performed per patient were recorded. Imaging utilization trends were compiled by type of imaging examination by year (as counts).
The PHIS database contains data based on hospital city and we created a new variable that recoded hospital city to region based on U.S. Census Bureau categorization (Northeast, West, South and Midwest) (23). Inclusion of all other predictor variables was based on prior studies (24,25).
Statistical Analysis
Continuous data were summarized as means and standard deviations; categorical data were summarized as counts and percentages. Continuous variables were compared using Students t test. The X2 test was performed to examine differences between proportions. Multivariable logistic regression was used to predict the probability of complicated appendicitis based on predictor variables before and during COVID-19 with robust standard errors. The regression model was chosen for its efficiency in retaining the full sample as compared to matching and propensity scores (26,27). For ease of interpretation, average marginal effects were computed (using the margins command in STATA statistical software) and plotted using a forest plot. All statistical analyses were conducted using a statistical software package (STATA, version 15; StataCorp). A p value < 0.05 was considered significant for inference testing.
RESULTS
Characteristics of the Study Cohort
During the defined study period a total of 19,431 children met inclusion criteria (age range: 0–17 years; mean = 10.54, SD: 3.77; median = 11 years; IQR = 6), with 14,889 children included in the pre-COVID-19 pandemic years 2017–2019 and 4542 children included during the 2020 COVID-19 study period. The mean age and fraction of female patients in the two study periods (pre COVID-19 vs during COVID-19) were 10.5 (SD: 3.7) and 10.6 (SD: 3.8) years and 39.9% (5945/14,889) and 41.1% (1864/4542), respectively. Patient demographics, hospital region, insurance payer type, and length of stay are documented in each period (pre COVID-19 and during the COVID-19 pandemic) in Table 1 .
Table 1.
Characteristics of the Patient Population
| Characteristic | Pre-COVID-19 (2017–2019) (n = 14,889) | During COVID-19 (2020) (n = 4542) | p Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 10.5 (3.7) | 10.6 (3.8) | 0.59 |
| Females (%) | 5,945 (39.9) | 1,864 (41.0) | 0.18 |
| Males (%) | 8,944 (60.1) | 2,678 (59.0) | |
| Race/ethnicity | |||
| Non-Hispanic White (%) | 7,188 (48.3) | 2,166 (47.7) | 0.10 |
| Non-Hispanic Black (%) | 1,136 (7.6) | 317 (7.0) | |
| Hispanic any race (%) | 4,330 (29.1) | 1,399 (30.8) | |
| Other (%) | 2,235 (15.0) | 660 (14.5) | |
| Insurance Type | |||
| Private (%) | 6,851 (46.0) | 2,075 (45.7) | <0.001 |
| Public (%) | 7,086 (47.6) | 2,026 (44.6) | |
| Other (%) | 774 (5.2) | 287 (6.3) | |
| Unknown (%) | 178 (1.2) | 154 (3.4) | |
| Region | |||
| Midwest | 3,353 (22.5) | 953 (21.0) | <0.001 |
| Northeast | 1,584 (10.6) | 485 (10.7) | |
| South | 7,477 (50.2) | 2,196 (48.3) | |
| West | 2,475 (16.6) | 908 (20.0) | |
| Clinical | |||
| Length of stay, mean (SD), d | 2.7 (5.0) | 2.7 (3.8) | 0.15 |
D, days; SD, standard deviation; y, years.
Percentages may not add to 100% due to rounding.
Overall, there were fewer children with appendicitis in the COVID-19 study period (n = 4542) than the average number of cases in the pre-COVID-19 study period (mean = 4963), with an absolute average difference of 421 cases. Among children with appendicitis, the proportion that had an appendectomy during the COVID-19 study period slightly declined from the pre-COVID-19 study period (68.4%; 10,184/14,889 vs 64.2%; 2918/4542; p value < 0.001). Incidental appendectomies, defined as appendectomy performed during other surgery without presence of acute appendicitis, were infrequent in our cohort, representing less than 1% of appendectomies in each study period (0.51%; 76/14,889 vs 0.37%; 17/4542; p = 0.207).
Complicated Appendicitis
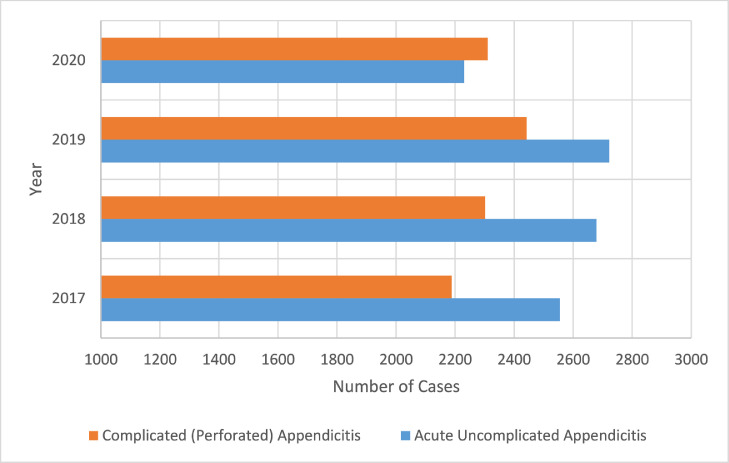
The proportion of complicated appendicitis cases increased by 4.4 percentage points, from 46.5% in the pre-COVID-19 (2017–2019) period compared to 50.9% during COVID-19 (2020), p < 0.001 (Fig 1 ). Overall, the count of uncomplicated acute appendicitis cases decreased from the pre-COVID-19 study period compared to the COVID-19 study period, with 2555 cases in 2017, 2679 cases in 2018, 2722 cases in 2019, and 2231 cases in 2020. Conversely, the count of complicated appendicitis cases was essentially unchanged between the pre-COVID-19 study period and the COVID-19 study period, with 2189 cases in 2017, 2302 cases in 2018, 2442 cases in 2019, and 2311 cases in 2020. The increase in the proportion of complicated appendicitis varied by geographic region with a 6.5% increase in the Midwest (pre-COVID-19 period: 48.2% vs COVID-19 period 54.7%, p > 0.001) and a 6.2% increase in the South region (pre-COVID-19 period: 47.4% vs COVID-19 period 53.6%, p > 0.001).
Figure 1.
Counts of acute appendicitis and complicated appendicitis cases from 47 children's hospitals from March through May in each year of 2017–2020. (Color version of figure is available online.)
The logit model demonstrated that, on average and while keeping all other variables constant, children with appendicitis in the COVID-19 study period had a 4.4 percentage point increase in the probability of having complicated appendicitis compared to the pre-COVID-19 study period (Fig 2 ). Other significant variables predictive of complicated appendicitis in the overall logit regression model (not stipulating study period) included: younger age, males compared to females, and country region (Southern and Midwestern regions compared to the Northeast as the reference).
Figure 2.
Logit regression with average marginal effects (AME) for outcome complicated appendicitis from 47 children's hospitals from March through May in each year of 2017–2020. AME are the average effects of changes in the explanatory variables on the change in the probability of the outcome. Diamonds reflect the AME value and error bars reflect the confidence intervals. The middle line represents the line of no effect.
Imaging Trends
Imaging approaches for appendicitis was largely unchanged between study periods (Fig 3 ). From the pre-COVID-19 study period to the COVID-19 study period, the percentage of patients undergoing a CT examination did not significantly change from 16.7% to 15.9% (p = 0.26). There was an increase between the study periods in the percentage of patients undergoing an US examination 68.3% to 70.2% (p = 0.033). MRI use remained low over both study periods at 1.8% and 2.4% (p = 0.036). Throughout both study periods, the use of CT was higher in those children with complicated appendicitis compared to children with uncomplicated appendicitis. For complicated appendicitis, the percentage of patients undergoing a CT was 20.9% during the pre-COVID-19 study period compared to 19.8% in the COVID-19 study period (p = 0.245). For uncomplicated appendicitis, the percentage of patients undergoing a CT was 12.2% during the pre-COVID-19 study period compared to 10.9% during the COVID-19 study period (p = 0.161).
Figure 3.
Imaging utilization trends from 47 children's hospitals for children with appendicitis from March through May in each year of 2017–2020.
DISCUSSION
Implications of public health and individual responses in the early phase of the COVID-19 pandemic, including the various national and local social distancing orders, had the potential to impact health outcomes through delays in care (28, 29, 30). We hypothesized the existence of an observable effect in trends of pediatric acute appendicitis, with a higher proportion of complicated appendicitis due to delayed presentation and/or delayed intervention during this period. Based on analysis of PHIS data from 47 children's hospitals, this study confirms part of our hypothesis with an observed 4.4 percentage point increase in the proportion of complicated appendicitis cases in children from March through May 2020, compared to the same period in the prior three years. Interestingly, this finding reflects not an increase in the absolute number of complicated appendicitis cases, but instead a decrease in the number of uncomplicated acute appendicitis diagnoses.
Possible explanations for the observed decrease in cases of uncomplicated acute appendicitis include shifts in consumption of medical care with patients receiving care at facilities not reported to PHIS, spontaneous resolution of mild appendicitis facilitated by delays in seeking care, or an overall decrease in the incidence of appendicitis during the COVID-19 pandemic. It is possible that social distancing and mask wearing resulted in decreased transmission of other viruses that infect the gastrointestinal tract and stimulate lymphoid hyperplasia of the appendix causing appendiceal obstruction and resultant appendicitis (31, 32, 33, 34).
Prior single institution reports have documented similar increases in the proportion of complicated appendicitis in children (1,10, 11, 12,15, 16, 17). One single institution study by Finkelstein et al showed a decrease in perforated appendicitis during the study period of March–May 2020, however the results were limited by a small sample size (35). Bhambhvani et al. (10) analyzed hospital admissions for medical emergencies in New York and California in both adults and children, which included appendicitis during the pandemic (March 1, 2020 through May 22, 2020), and compared admission rates to previous years using an interrupted time series. The study found significant differences in hospital emergency department visits for appendicitis by city. At Stanford University Medical Center, there was no statistical difference in pediatric and adult appendicitis emergency department visits during COVID-19 period compared to prior years (10). However, the New York site, New York Presbyterian/Weill Cornell Medical Center, had a significant 42% reduction in emergency department visits for appendicitis (10). Similar to Bhambhvani et al.’s study, our results demonstrated regional differences. In our study, the proportion of complicated appendicitis significantly increased in the Southern and Midwestern regions, with no statistically significant change in the Northeast and West. It is theoretically plausible that these regional differences may relate to local differences in stay-at-home orders and COVID-19 incidence rates, though this is out of the scope of this study. Alternatively, this finding could be attributed to study design, as certain major Northeast institutions, including areas disproportionately affected in the early phase of the COVID-19 pandemic, were not included in our study due to incomplete data.
A recent multi-institutional study conducted in Germany by Willms et al. analyzed appendicitis in both adults and children during the pandemic compared to pre-COVID-19 (18). The study included 1915 appendectomies in adults from 41 sites across Germany and analyzed complicated appendicitis cases in 2019 compared to 2020 (18). In keeping with Willms et al. paper, our data demonstrate the proportion of complicated appendicitis increased, however in our study, the count of complicated appendicitis remained steady from the previous years, while Willms et al. demonstrated a slight decrease in the count of complicated appendicitis cases (18). The Willms et al. study also concluded that the frequency and count of uncomplicated acute appendicitis cases decreased during COVID-19, which they attribute to a decrease in negative appendectomies and incidental appendectomies (18). This does not explain the results of our study as incidental appendectomies accounted for less than 1% of included appendectomies. A similar study in the United States conducted by Neufield et al. evaluated 956 patients with appendicitis at 14 different institutions during COVID 19 showed the same outcome trends as our data, with an overall decrease in uncomplicated appendicitis and no significant change in complicated appendicitis (13).
Our results do show a small, but statistically significant, decrease in the overall number of appendectomies performed during the COVID-19 time period in 2020 compared to prior years. This may reflect a combination of intentional efforts to conserve resources and limit viral spread by restricting surgeries during the early phase of the COVID-19 pandemic and increased use of nonoperative management (36).
We also looked at the use of diagnostic imaging during the COVID-19 pandemic. During the pandemic, staff exposure to COVID-19 positive patients and patients under investigation was intentionally limited. As a result, one might hypothesize that the use of ultrasound, which requires close patient contact, would have decreased. However, our results show that diagnostic imaging strategies did not grossly change during COVID-19 when compared to the pre-COVID-19 study period. Over the study time periods, US remained the dominant imaging modality for children with suspected appendicitis with CT more commonly used in the context of complications.
LIMITATIONS
While our study provides insight into trends in pediatric appendicitis during the early phase of the COVID-19 pandemic, our study has limitations. First, because we were using administrative data, we relied on ICD-10 coding to categorize uncomplicated vs complicated appendicitis, instead of primary source documents such as operative notes and pathology reports. Thus, there could be misclassification due to coding errors, however prior studies have utilized this approach and have noted the complication screening method can be applied to administrative data (21,22,37). Given this is an observational study, various confounding variables could have been affecting the results. Second, we assume that the abdominal imaging conducted during the patient's encounter is for appendicitis diagnosis. Finally, we excluded several (n = 3) hospitals in cities, such as New York City, that that had high COVID-19 incidence rates, as they did not report data throughout the study period. Two single institution experiences from New York City evaluating trends of complicated appendicitis during this time period, compared to prior years, showed an increase in complicated appendicitis in 2020 (38). As a result, it is possible that our study underestimates the true change in complicated appendicitis during the period of interest (leading to negative bias).
CONCLUSION
During the early phase of the COVID-19 pandemic, the proportion of complicated appendicitis presentations at children's hospitals increased compared to the same time in prior years, driven by a decrease in uncomplicated appendicitis cases. Despite social distancing guidelines, there was little change in imaging strategies for the evaluation of pediatric appendicitis, as ultrasound remained the dominant modality during both study periods. The results of our study provide insight into how societal stresses and behavior changes can impact medical care, elucidating that not all diseases are susceptible to delays in medical care due to societal changes. Future studies evaluating other disease processes during the study period, as well as long-term implications from the early phases of the COVID-19 pandemic, can provide additional information as guidance for potential future crises.
References
- 1.Moreland A, Herlihy C, Tynan MA, et al. Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement—United States, March 1–May 31, 2020. Morb Mortal Wkly Rep. 2020;69:1198. doi: 10.15585/mmwr.mm6935a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foundation . KFF; San FranciscoCA: 2021. HJKF Stay at a home State Data and Policy Actions to Address Coronavirus. [Google Scholar]
- 3.Lyu W, Wehby GL. Shelter-in-place orders reduced COVID-19 mortality and reduced the rate of growth in hospitalizations: study examine effects of shelter-in-places orders on daily growth rates of COVID-19 deaths and hospitalizations using event study models. Health Aff. 2020;39:1615–1623. doi: 10.1377/hlthaff.2020.00719. [DOI] [PubMed] [Google Scholar]
- 4.Findling MG, Blendon RJ, Benson JM. Delayed care with harmful health consequences—reported experiences from national surveys during coronavirus disease 2019. JAMA Health Forum. 2020;1(12):e201463. doi: 10.1001/jamahealthforum.2020.1463. [DOI] [PubMed] [Google Scholar]
- 5.Norbash AM, Van Moore Jr A, Recht MP, et al. Early-stage radiology volume effects and considerations with the coronavirus disease 2019 (COVID-19) pandemic: adaptations, risks, and lessons learned. J Am Coll Radiol. 2020;17:1086–1095. doi: 10.1016/j.jacr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport MS, Fruscello T, Chatfield M, et al. CT volumes from 2,398 radiology practices in the united states: a real-time indicator of the effect of COVID-19 on routine care, January to September 2020. J Am Coll Radiol. 2020;18:380–387. doi: 10.1016/j.jacr.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass Charity C, Rangel Shawn J. Overview and diagnosis of acute appendicitis in children. Seminars in Pediatric Surgery. 2016;25(4):198–203. doi: 10.1053/j.sempedsurg.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Short HL, Sarda S, Travers C, et al. Trends in common surgical procedures at children's and nonchildren's hospitals between 2000 and 2009. J Pediatr Surg. 2018;53:1472–1477. doi: 10.1016/j.jpedsurg.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 9.Sømme S, Bronsert M, Morrato E, et al. Frequency and variety of inpatient pediatric surgical procedures in the United States. Pediatrics. 2013;132:e1466–e1472. doi: 10.1542/peds.2013-1243. [DOI] [PubMed] [Google Scholar]
- 10.Bhambhvani HP, Rodrigues AJ, SY Jonathan, et al. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern. Med. 2020;181(2):272–274. doi: 10.1001/jamainternmed.2020.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeJong C, Katz MH, Covinsky K. Deferral of care for serious non–COVID-19 conditions: a hidden harm of COVID-19. JAMA Intern. Med. 2020;181(2):274. doi: 10.1001/jamainternmed.2020.4016. [DOI] [PubMed] [Google Scholar]
- 12.Dreifuss N, Schlottmann F, Sadava E, et al. Acute appendicitis does not quarantine: surgical outcomes of laparoscopic appendectomy in COVID-19 times. Br J Surg. 2020;107:e368–e369. doi: 10.1002/bjs.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neufeld MY, Bauerle W, Eriksson E, et al. Changes in acute appendicitis presentation and severity of illness during the coronavirus disease 2019 pandemic: a retrospective cohort study. Surgery. 2021;169:808–815. doi: 10.1016/j.surg.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufeld MY, Sanchez SE, Drake FT. Handle with care: use of proportions to assess changes in acute appendicitis during the 2020 COVID-19 “surge”. J Am Coll Radiol. 2021;18(7):893–894. doi: 10.1016/j.jacr.2021.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien CM, Jung K, Dang W, et al. Collateral damage: the impact of the COVID-19 pandemic on acute abdominal emergency presentations. J Am Coll Radiol. 2020;17:1443–1449. doi: 10.1016/j.jacr.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Place R, Lee J, Howell J. Rate of pediatric appendiceal perforation at a children's hospital during the COVID-19 pandemic compared with the previous year. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.27948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero J, Valencia S, Guerrero A. Acute appendicitis during coronavirus disease 2019 (COVID-19): changes in clinical presentation and CT findings. J Am Coll Radiol. 2020;17:1011–1013. doi: 10.1016/j.jacr.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willms AG, Oldhafer KJ, Conze S, et al. Appendicitis during the COVID-19 lockdown: results of a multicenter analysis in Germany. Langenbeck’s Arch Surg. 2021;406(2):1–9. doi: 10.1007/s00423-021-02090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association CsH Pediatric Health Information System®. 2021 https://www.childrenshospitals.org/phis. [Google Scholar]
- 20.Shafi S, Aboutanos M, Brown CV-R, et al. Measuring anatomic severity of disease in emergency general surgery. J Trauma Acute Care Surg. 2014;76:884–887. doi: 10.1097/TA.0b013e3182aafdba. [DOI] [PubMed] [Google Scholar]
- 21.Bachur RG, Levy JA, Callahan MJ, et al. Effect of reduction in the use of computed tomography on clinical outcomes of appendicitis. JAMA Pediatr. 2015;169:755–760. doi: 10.1001/jamapediatrics.2015.0479. [DOI] [PubMed] [Google Scholar]
- 22.Michelson KA, Dart AH, Bachur RG, et al. Measuring complications of serious pediatric emergencies using ICD-10. Health Serv Res. 2021;56:225–234. doi: 10.1111/1475-6773.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bureau UC. US Census Bureau website; United States: 2010. Census Regions and Divisions of the United States. [Google Scholar]
- 24.Baxter KJ, Nguyen HT, Wulkan ML, et al. Association of health care utilization with rates of perforated appendicitis in children 18 years or younger. JAMA Surg. 2018;153:544–550. doi: 10.1001/jamasurg.2017.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray DT, Mizrahi T. Trends in appendicitis and perforated appendicitis prevalence in children in the United States, 2001-2015. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016;22:557–563. doi: 10.1016/j.bbmt.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69:345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 28.Chiaravalli S, Ferrari A, Sironi G, et al. A collateral effect of the COVID-19 pandemic: delayed diagnosis in pediatric solid tumors. Pediatr Blood Cancer. 2020;67(10):e28640. doi: 10.1002/pbc.28640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czeisler MÉ, Marynak K, Clarke KE, et al. Delay or avoidance of medical care because of COVID-19–related concerns—United States, June 2020. Morb Mortal Wkly Rep. 2020;69:1250. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vagal A, Mahoney M, Allen B, et al. Rescheduling nonurgent care in radiology: implementation during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Radiol. 2020;17:882–889. doi: 10.1016/j.jacr.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alder AC, Fomby TB, Woodward WA, et al. Association of viral infection and appendicitis. Arch Surg. 2010;145:63–71. doi: 10.1001/archsurg.2009.250. [DOI] [PubMed] [Google Scholar]
- 32.Hardin Jr DM. Acute appendicitis: review and update. Am Fam Phys. 1999;60:2027. [PubMed] [Google Scholar]
- 33.Lynch DT, Lott L, Cebe K, et al. Adenovirus-associated acute appendicitis: an under-recognized relationship? Mil Med. 2017;182:e1765–e1768. doi: 10.7205/MILMED-D-16-00308. [DOI] [PubMed] [Google Scholar]
- 34.Richardsen I, Schöb D, Ulmer T, et al. Etiology of appendicitis in children: the role of bacterial and viral pathogens. J Invest Surg. 2016;29:74–79. doi: 10.3109/08941939.2015.1065300. [DOI] [PubMed] [Google Scholar]
- 35.Finkelstein P, Picado O, Muddasani K, et al. A retrospective analysis of the trends in acute appendicitis during the COVID-19 pandemic. J Laparoendosc Adv Surg Tech. 2021;31:243–246. doi: 10.1089/lap.2020.0749. [DOI] [PubMed] [Google Scholar]
- 36.Kvasnovsky CL, Shi Y, Rich BS, et al. Limiting hospital resources for acute appendicitis in children: lessons learned from the US epicenter of the COVID-19 pandemic. J Pediatr Surg. 2020;56(5):900–904. doi: 10.1016/j.jpedsurg.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington Y, Rauch DA, Leary JC, et al. How generalizable is freestanding children’s hospital data such as PHIS (pediatric health information system)? Am Acad Pediatr. 2021;(3):567–569. [Google Scholar]
- 38.Fisher JC, Tomita SS, Ginsburg HB, et al. Increase in pediatric perforated appendicitis in the New York City metropolitan region at the epicenter of the COVID-19 Outbreak. Ann Surg. 2021;273:410. doi: 10.1097/SLA.0000000000004426. [DOI] [PMC free article] [PubMed] [Google Scholar]