Summary
Background
Joint injury is a major risk factor for osteoarthritis and provides an opportunity to prospectively examine early processes associated with osteoarthritis. We investigated whether predefined baseline demographic and clinical factors, and protein analytes in knee synovial fluid and in plasma or serum, were associated with clinically relevant outcomes at 2 years after knee injury.
Methods
This longitudinal cohort study recruited individuals aged 16–50 years between Nov 1, 2010, and Nov 28, 2014, across six hospitals and clinics in London, UK. Participants were recruited within 8 weeks of having a clinically significant acute knee injury (effusion and structural injury on MRI), which was typically treated surgically. We measured several predefined clinical variables at baseline (eg, time from injury to sampling, extent and type of joint injury, synovial fluid blood staining, presence of effusion, self-reported sex, age, and BMI), and measured 12 synovial fluid and four plasma or serum biomarkers by immunoassay at baseline and 3 months. The primary outcome was Knee Injury and Osteoarthritis Outcome Score (KOOS4) at 2 years, adjusted for baseline score, assessed in all patients. Linear and logistic regression models adjusting for predefined covariates were used to assess associations between baseline variables and 2-year KOOS4. This study is registered with ClinicalTrials.gov, number NCT02667756.
Findings
We enrolled 150 patients at a median of 17 days (range 1–59, IQR 9–26) after knee injury. 123 (82%) were male, with a median age of 25 years (range 16–50, IQR 21–30). 98 (65%) of 150 participants completed a KOOS4 at 2 (or 3) years after enrolment (50 participants were lost to follow-up and two were withdrawn due to adverse events unrelated to study participation); 77 (51%) participants had all necessary variables available and were included in the core variable adjusted analysis. In the 2-year dataset mean KOOS4 improved from 38 (SD 18) at baseline to 79 (18) at 2 years. Baseline KOOS4, medium-to-large knee effusion, and moderate-to-severe synovial blood staining and their interaction significantly predicted 2-year KOOS4 (n=77; coefficient −20·5, 95% CI −34·8 to −6·18; p=0·0060). The only predefined biomarkers that showed independent associations with 2-year KOOS4 were synovial fluid MCP-1 (n=77; −0·015, 0·027 to −0·004 per change in 1 pg/mL units; p=0·011) and IL-6 (n=77; −0·0005, −0·0009 to −0·0001 per change in 1 pg/mL units; p=0·017). These biomarkers, combined with the interaction of effusion and blood staining, accounted for 39% of outcome variability. Two adverse events occurred that were linked to study participation, both at the time of blood sampling (one presyncopal episode, one tenderness and pain at the site of venepuncture).
Interpretation
The combination of effusion and haemarthrosis was significantly associated with symptomatic outcomes after acute knee injury. The synovial fluid molecular protein response to acute knee injury (best represented by MCP-1 and IL-6) was independently associated with symptomatic outcomes but not with structural outcomes, with the biomarkers overall playing a minor role relative to clinical predictors. The relationship between symptoms and structure after acute knee injury and their apparent dissociation early in this process need to be better understood to make clinical progress.
Funding
Versus Arthritis, Kennedy Trust for Rheumatology Research, and NIHR Oxford Biomedical Research Centre.
Introduction
Knee osteoarthritis is one of the most prevalent forms of osteoarthritis and is the primary reason for knee joint replacement; post-traumatic osteoarthritis accounts for approximately 12% of all of prevalent knee osteoarthritis.1, 2 It is thought that post-traumatic osteoarthritis occurs in approximately 50% of people after clinically significant knee injuries such as anterior cruciate ligament rupture.3 However, it is not currently possible to predict who will develop osteoarthritis after a knee injury, and there are a number of reasons why it might be important to be able to do so. First, patients report that they want better information on personalised risk so that they can plan lifestyle choices and decisions, such as returning to professional or high-impact sports. Second, identifying patients at high risk might maximise the effect, and direct the targeting, of existing treatments, such as reconstructive surgery or intense rehabilitation. Lastly, stratification of this high-risk population might facilitate trials of new therapeutics aiming to prevent osteoarthritis in high-risk groups, potentially leading to personalised medicine for the first time in this area. Previous studies have identified clinical factors that are associated with osteoarthritis that is evident on the basis of x-ray and MRI imaging, including intra-articular fracture and concurrent meniscal tear.4, 5 There is conflicting evidence about whether measures of inflammation such as effusion might be adverse prognostic factors after anterior cruciate ligament rupture;5, 6 there has been insufficient information to develop a prognostic model to date.5, 6, 7, 8
Research in context.
Evidence before this study
Acute joint injury is known to be associated with an inflammatory response detectable in synovial fluid, which varies between individuals. Many, but not all, knee injuries lead to haemarthrosis (blood in the joint persisting after injury). Those exposed to an acute knee joint injury have an increased risk of osteoarthritis, but there is currently no reliable way of predicting who will develop disease, either symptomatic or structural. Prior to the planning of this study, we searched Pubmed for cohort studies or other interventional or non-interventional studies using terms including [knee or “knee joint” OR Tibiofemoral OR ‘Tibio-femoral” OR Patellofemoral OR “Patello-femoral”] AND [Injury OR Trauma OR “ligament rupture” OR “derangement” OR “Anterior Cruciate Ligament” OR “meniscus”] from inception to March 2010 for articles published relating to biomarkers, other predictors, and outcomes after knee injury. The search was limited to studies in English language. To date there are no meta-analyses relating to either biomarker or clinical prediction of outcomes after knee injury available in this area.
Added value of this study
The presence of knee effusion and haemarthrosis at the time of knee injury were the most important predictors of clinical outcomes at 2 years in this cohort. The measurable acute joint injury inflammatory response (interleukin [IL]-6, monocyte chemoattractant protein [MCP]-1) was associated with worse patient-reported outcomes, but to a more modest degree when considering these important clinical factors. This study shows the importance of considering clinical factors in combined models during biomarker research; the study also emphasises the real utility, at least in research terms, of assessment of synovial fluid as opposed to blood concentrations of such markers. The findings highlight that prediction of persistence of symptoms and the development of structural disease might need to be considered separately when studying this type of population. The study suggests that 39% of the variability in individual outcome might be possible to predict using this information.
Implications of all the available evidence
Understanding how symptomatic outcomes relate to risk of later structural disease is crucial for progress in this area, not just in post-traumatic osteoarthritis but also osteoarthritis in general. The study suggests that clinically meaningful prediction of outcomes after knee injury and in early osteoarthritis should be possible and could inform the design of future translational or clinical studies. Methods for detecting and quantifying haemarthrosis at the time of injury are needed and could provide a clinically relevant and accessible biomarker. Understanding whether elements of the inflammatory response to joint injury associated with symptom persistence are truly prognostic biomarkers or even whether they might represent therapeutic targets for prevention of either pain or disease will need granular and longitudinal assessment of individuals in other cohorts associated with biological samples. This understanding, in conjunction with reverse translation of findings to preclinical models and first experimental medicine studies in this area might allow us to aim for prevention of osteoarthritis for the first time.
We, and other researchers, have previously shown that there is an immediate biological response to acute knee joint injury, and this response is best measured in the synovial fluid.9, 10, 11 The response is typified by a variable inflammatory response,9, 10, 11, 12, 13 aspects of which are similar to the response observed in mice following surgically induced destabilisation of the medial meniscus,14 and the magnitude of inflammation is associated with early clinical outcomes in the months after knee injury.11 A number of inflammatory cytokines and growth factors, such as FGF-2, TGF-β, and IL-6, are produced in direct response to the injury of connective tissues, primarily articular cartilage; some of these responses might be associated with repair and resolution.11, 15, 16, 17 We hypothesised that an individual's immediate biological response to acute joint injury would be associated with clinical outcomes 2 years after the injury, both in terms of patient-reported outcomes and imaging-based outcomes relating to knee osteoarthritis. We focused on a panel of synovial fluid and blood markers identified in our previous studies14, 15, 16 for testing. Demographic or clinical factors that were likely to predict outcome were considered a priori in these analyses, in an attempt to only identify biomarkers of the acute biological response with clinical utility (ie, predictive after accounting for other clinical factors). The Knee Injury Cohort at the Kennedy (KICK) study was specifically designed to test this hypothesis,11 and the primary outcome analysis from the study is presented here.
Methods
Study design and participants
This longitudinal cohort study followed up individuals aged 16–50 years, recruited within 8 weeks of a clinically significant acute knee injury. Participants were recruited between Nov 1, 2010 and Nov 28, 2014, following referral for screening by the orthopaedic surgeon investigator (AW), from a population exposed to acute knee injury who were attending assessment at six hospitals and clinics in London, UK. During this time, to avoid potential sources of bias, all potentially eligible individuals were invited to participate. Inclusion criteria were: clinically significant acute knee injury within 8 weeks of recruitment; aged 16–50 years; knee effusion, evident clinically or by MRI; and evidence of one or more specified structural injuries on MRI (meniscal tear, cruciate ligament rupture, collateral ligament tear, posterolateral corner injury, traumatic chondral defects, articular or periarticular fracture, patellofemoral dislocation, or tibiofemoral dislocation) within 8 weeks of baseline visit. Exclusion criteria were: pre-existing advanced radiographic osteoarthritis (clinical Kellgren and Lawrence grade [KLG] 3 or 4) of the injured knee (ie, the index knee); inflammatory or septic arthritis of the index knee; previous or planned knee arthroplasty; active or treated systemic inflammatory disease; clinically signficant infection within 2 weeks of recruitment; pregnancy; and inability to provide blood samples.11 These criteria aligned with published considerations on the design of studies at the time of knee trauma.8 Participants were withdrawn from the study if they either withdrew their consent or developed prespecified exclusion criteria during the study (definite inflammatory arthritis, septic arthritis, arthroplasty of the index knee, or systemic inflammatory disease). Data collected to the point of a patient's withdrawal or exclusion were included in the analysis, unless otherwise requested by the participant. A CONSORT flow diagram and STROBE checklist are in the appendix (pp 22–26). Three eligible patients were involved in the design and review of this study (appendix p 1), and all participants gave written informed consent to participate before screening, according to the Declaration of Helsinki. Ethics approval was given by South East London Research Ethics Committee 5, UK (REC 10/H0706/44). The procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Procedures and outcomes
The primary outcome was the composite Knee Injury and Osteoarthritis Outcome Score (KOOS4), calculated as the mean of KOOS subscales of pain, symptoms, sports and recreation, and quality of life.18, 19 Participants completed a self-reported questionnaire that generated a KOOS4 score, and Tegner activity scores (a one-item score that grades activity based on work and sports activities on a scale of 0 to 10, where 0 is marked disability and 10 is international elite sports) were completed by the investigator (FEW) during interview with the participant by administering a standard question, as part of the investigator case report form. KOOS4 and Tegner scores were collected at baseline (within 8 weeks of knee injury and before any surgical intervention), at 3 months, and at yearly visits thereafter for up to 3 years (appendix p 2). Musculoskeletal examination including knee effusion grade was documented by a single investigator (FEW). The predefined secondary outcome was the pain subdomain of KOOS (henceforth referred to as “KOOS pain”, a patient reported outcome measure rated 0–100, where 100 is no pain) at 2 years. Predefined exploratory outcomes with sufficient sample size availability included frequent knee pain as assessed by the National Health and Nutrition Examination Survey (NHANES), which was collected as part of the investigator case report form; new radiographic tibiofemoral knee osteoarthritis (baseline KLG of 0 or 1 that had progressed to KLG ≥2 at 2 years); osteoarthritis on knee MRI as defined by Osteoarthritis Research Society International (OARSI) criteria, either new tibiofemoral osteoarthritis or new patellofemoral osteoarthritis;20 early knee osteoarthritis as defined by Luyten criteria;21 new symptomatic and radiographic osteoarthritis; and both frequent knee pain (assessed by NHANES) and new radiographic tibiofemoral knee osteoarthritis. A full list of exploratory outcomes we planned to investigate is listed in the appendix (p 3–5). Adverse events were collected at each study visit, or when they were reported by the participant. Adverse events were adjudicated as serious or not serious, and related or unrelated to study participation, by the site principal investigator. There were no meaningful deviations from protocol. One substantial protocol amendment (approved by the ethics committee on Nov 24, 2014) affected trial conduct, allowing for remote or online collection of study questionnaire data as well as in person.
Extended knee x-rays at baseline and at 2 years, acquired using a foot template in standing anteroposterior position, were scored using a KLG grading system by a single trained reader (KL), following validation against reference standard and within-set validation, which showed high intra-rater and inter-rater reliability (>85%). Features were identified using OxMorf software, which has been shown to tend towards higher sensitivity than usual clinical grading.22, 23 MRI scans of both knees were done on a 3T scanner (Siemens [Erlangen, Germany]) at a single site in London at baseline and at 2 years, using a protocol similar to the Osteoarthritis Initiative (OAI; appendix p 21). OARSI-MRI scoring20 was done by two consultant musculoskeletal radiologists, who reviewed the images at the same time and agreed scores for each proforma (KS, AL).
Biological samples were taken at baseline, 14 days, 3 months, and when attending study visits at 2 and 5 years. Samples taken included whole blood and synovial fluid; the latter was collected by needle aspiration when there was a clinical intervention such as arthrocentesis or arthroscopy, before the introduction of the arthroscope. All samples were transferred within 2 h to the laboratory and were processed and stored as previously described.11 Synovial fluid was graded for the presence of blood (appendix p 6).
12 synovial fluid and four plasma or serum biomarkers were measured by immunoassay as exposures of interest. General laboratory reagents were the best available grade from either Sigma-Aldrich (Dorset, UK) or BDH (Dorset, UK), unless otherwise stated. All Meso Scale Discovery assays and reagents were from Meso Scale Discovery, Rockville, MD, USA. All assays were done as per manufacturers' instructions and had previously undergone structured quality performance assessment for serum or plasma and synovial fluid before use (appendix p 7).11, 17
Statistical analysis
Power calculations to allow detection of a change in KOOS4 between baseline and 2 years were done at the initiation of the study, supporting a sample size of 112, with an aim to recruit 150 patients and allow approximately 30 (20%) to drop out.11
Normality of each continuous variable was assessed by quantile-quantile and kernel density plots. Fractional polynomial regression modelling was used to test whether the linear relationship between continuous variables (eg, age, body-mass index [BMI], days from injury, biomarker concentrations) and the outcome (KOOS4 and KOOS pain) prevented obtaining a reliable measure of association.24 The primary outcome measure of KOOS4 was measured at 2 years and adjusted for baseline KOOS4; analysis used a covariance (ANCOVA) approach.25 We also analysed the related secondary outcome of KOOS pain at 2 years and other predefined structural or symptomatic outcomes. We used linear regression to model the relationship between predefined clinical variables and continuous outcomes (KOOS4 and KOOS pain). We used logistic regression for the binary exploratory outcomes (KLG osteoarthritis, NHANES frequent knee pain, OARSI-MRI osteoarthritis, and Luyten early knee osteoarthritis).
Predefined clinical variables and interactions (appendix p 8) were used to develop a core linear regression model (core model); these variables included time from injury to sampling, extent and type of joint injury, synovial fluid blood staining, presence of effusion, self-reported sex, age, and BMI, among others. These predefined variables were identified from four complementary sources and were documented in a statistical analysis plan before the analysis. Factors reproducibly associated with osteoarthritis progression,26 or associated with post-traumatic osteoarthritis or its progression,8 were identified by literature review, and factors associated with the immediate biological response to knee joint injury were identified from our previous studies.11 We used univariable linear regression models to assess the association of each variable with the primary outcome (adjusted for baseline KOOS4 alone). A cut-off value of p≤0·05 was used to select univariable associations for addition of variables to the core model.
We analysed the absolute change of the 12 predefined molecular markers in plasma or serum from baseline to 3 months. We used univariable linear regression models to describe the association of each predefined marker with the primary outcome (adjusted for baseline KOOS4 alone); further univariable linear regression models were then developed for each marker, adjusted for all variables in the core model.
For both clinical and molecular variables, the effects of predefined interactions were examined, when relevant. When reporting the primary analysis, R2 explained the proportion of variability in the primary outcome accounted for by the combination of the selected clinical predictors and core model and the incremental effects of the addition of selected molecular markers to the core model. This analysis was done to assess the relative contribution of these potential molecular biomarkers in a clinical context.
We used univariable logistic regression models to describe the association of each predefined variable with each (binary) secondary outcome (adjusted for baseline KOOS4 alone). There is a high chance of type I errors due to multiple testing, through fitting large numbers of regression models. As such, and in preference to Bonferroni corrections, the analysis plan specified the primary analyses clearly, with less weight given to the secondary analyses.
For primary analyses, all available data were included from all participants and all relevant visits (full available dataset). Individuals with 2-year KOOS data comprised the 2-year dataset. For regression models relating to the core model, we used data from individuals possessing all necessary variables (complete cases dataset). Data were stored on a secure online database (REDCap, Vanderbilt University, TN, USA [NIH/NCATS UL1 TR000445]). Analysis was done in STATA IC version 13 and Graphpad Prism version 8. This study is registered with ClinicalTrials.gov, number NCT02667756.
Role of the funding source
The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or in the writing of the report.
Results
184 individuals were assessed for eligibility between Nov 1, 2010, and Nov 28, 2014. 34 (18%) were excluded; one exceeded the age limit of 50 years, one had knee injury more than 8 weeks before screening, and 32 declined to take part (appendix p 22). The remaining 150 individuals were recruited at a median of 17 days (range 1–59, IQR 9–26) after knee injury. 145 individuals in our study underwent knee surgery that included washout at a median of 17 days (maximum 51 days) after injury (appendix p 2).
Before injury, 114 (76%) of 150 participants were sports professionals (ie, Tegner activity score of ≥9), and the remaining participants were active amateur sportspeople (ie, a Tegner score of 6–8; table 1). 143 (95%) participants sustained their injury during sporting activity, predominantly football or rugby. 75 (50%) of 149 participants had a medium-to-large clinical effusion detected clinically at their baseline assessment. Of the 136 participants who had synovial fluid aspirated at baseline, 50 (37%) had moderate or severe blood staining consistent with haemarthrosis (appendix p 6). 99 (66%) of 150 participants had knee x-rays during the baseline period: 64 (43%) of 150 participants had KLG 0 or 1 and 35 (23%) had KLG 2. The remaining 51 (34%) individuals had no evidence of established knee osteoarthritis on MRI at baseline.
Table 1.
Baseline characteristics of the study population
| All KICK participants (n=150) | Participants having completed KOOS4at 2 or 3 years (n=98) | Participants not having completed KOOS4at 2 or 3 years (n=52) | p value | ||
|---|---|---|---|---|---|
| Age, years | 25 (21–30) | 26 (22–32) | 24 (20–28) | 0·021 | |
| Sex | .. | .. | .. | 0·18 | |
| Male | 123 (82%) | 77 (79%) | 46 (88%) | .. | |
| Female | 27 (18%) | 21 (21%) | 6 (12%) | .. | |
| Time from injury at baseline, days (range) | 17 (9–26) | 18 (10–27) | 13 (8–24) | 0·068 | |
| Body-mass index, kg/m2 | 25 (23–28) | 25 (23–28) | 25 (23–28) | 0·61 | |
| Tegner score before injury | 10 (9–10) | 10 (7–10) | 10 (10–10) | 0·085 | |
| Tegner score at baseline | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0·74 | |
| Category of knee injury* | .. | .. | .. | 0·39 | |
| Meniscal tear | 26 (17%) | 15 (15%) | 11 (21%) | .. | |
| Single ligament rupture† only | 30 (20%) | 22 (22%) | 8 (15%) | .. | |
| ACL plus meniscal tear | 67 (45%) | 46 (47%) | 21 (40%) | .. | |
| Severe trauma | 27 (18%) | 15 (15%) | 12 (23%) | .. | |
| Clinical effusion at baseline‡ | 149 (99%) | 97 (99%) | 52 (100%) | 0·76 | |
| None | 5/149 (3%) | 3/97 (3%) | 2 (4%) | ||
| Small | 69/149 (46%) | 42/97 (43%) | 27 (52%) | .. | |
| Medium | 58/149 (39%) | 40/97 (41%) | 18 (35%) | .. | |
| Large | 17/149 (11%) | 12/97 (12%) | 5 (10%) | .. | |
| Synovial fluid, blood staining§ | 136 (91%) | 86 (88%) | 50 (96%) | 0·68 | |
| None | 42/136 (31%) | 30/86 (35%) | 12/50 (24%) | .. | |
| Mild | 37/136 (27%) | 22/86 (26%) | 15/50 (30%) | .. | |
| Moderate | 22/136 (16%) | 14/86 (16%) | 8/50 (16%) | .. | |
| Severe | 28/136 (21%) | 17/86 (20%) | 11/50 (22%) | .. | |
| Present, ungraded | 7/136 (5%) | 3/86 (3%) | 4/50 (8%) | .. | |
| KLG at baseline | 1 (0–2), n=99 | 1 (0–2), n=79 | 1 (0–2), n=20 | 0·15 | |
Data are median n (IQR; range), n (%), or n/N (%) if the denominator differs from the total number of participants presented at the top of the table. The p value compares the characteristics for the participants who completed KOOS4 at 2 or 3 years with those who did not. ACL=anterior cruciate ligament. KICK=Knee Injury Cohort at the Kennedy. KLG=Kellgren and Lawrence grade for osteoarthritis. KOOS4=Knee Injury and Osteoarthritis Outcome Score based on a composite of four subscales (pain, symptoms, sports and recreation, and quality of life).
Four types of injury were categorised by arthroscopy when done, supplemented by MRI), tending to increase with regard to the extent of trauma with increasing category: meniscal tear (evidence of at least one acute meniscal tear, without evidence of other meaningful injury), single ligament rupture (evidence of complete rupture of a single ligament, without evidence of other meaningful injury), ACL plus meniscal tear (evidence of complete rupture of ACL and at least one acute meniscal tear, without evidence of other injury), severe trauma (combined ligament [>one] rupture, patellar fracture, or dislocation [patellofemoral or tibiofemoral]).
26 of 30 patients had an isolated ACL rupture, and four had complete rupture of a single collateral ligament or posterior cruciate ligament.
Five participants had effusion at time of initial clinical assessment or MRI that had resolved by baseline assessment; effusion size was missing for one participant.
Grading system for synovial fluid blood staining is presented in appendix (p 6).
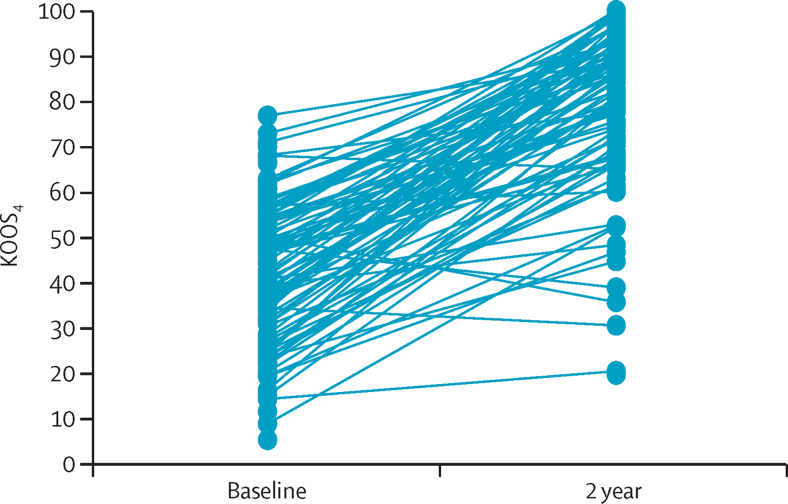
50 participants were lost to follow-up and two were withdrawn from the study due to protocol violation before the 2-year visit, therefore 98 (65%) of 150 participants completed a KOOS4 at 2 or 3 years after enrolment (3-year data were used in nine patients for whom 2-year data were not available; table 1). Participants who did not complete a 2-year or 3-year assessment were more likely to be younger and to have a higher Tegner activity score, but were otherwise similar at baseline to those who completed these assessments (table 1). In the 2-year dataset mean KOOS4 improved from 38 (SD 18) at baseline to 79 (18) at 2 years (figure 1). Baseline KOOS4 accounted for 11% of the variation in KOOS4 over time (coefficient 0·4, 95% CI 0·2–0·6; p=0·0010), and thus we adjusted for baseline KOOS4 when assessing associations of other predefined variables. Among other clinical factors, only medium-to-large knee effusion at baseline and moderate-to-severe synovial fluid blood staining were significantly associated with 2-year KOOS4 (table 2). The interaction of effusion and blood staining was adopted into the core model (coefficient −20·5, 95% CI −34·8 to −6·18; p=0·0060; R2=0·30; appendix p 10).
Figure 1.
The primary outcome KOOS4, for all individuals in the 2-year dataset at baseline and at 2 years
The KOOS4 is a composite score calculated from a patient-reported outcome measure, from 0 (extreme knee symptoms) to 100 (no knee symptoms). KOOS4=Knee Injury and Osteoarthritis Outcome Score based on a composite of four subscales (pain, symptoms, sports and recreation, and quality of life).
Table 2.
Univariable associations of the predefined clinical variables with the outcome KOOS4 at 2 years
| n | Coefficient (95% CI) | p value | R2 | ||
|---|---|---|---|---|---|
| Established risk factors for osteoarthritis | |||||
| Sex, female (ref: male) | 92 | −0·4 (−8·9 to 8·2) | 0·93 | 0·11 | |
| Age, years | 92 | 0·1 (−0·2 to 0·4) | 0·59 | 0·12 | |
| Body-mass index, kg/m2 | 91 | 0·4 (−0·2 to 1·0) | 0·17 | 0·13 | |
| Index knee osteoarthritis by KLG at baseline (ref: grade 0) | 75 | .. | .. | .. | |
| KLG 1 | .. | 4·1 (−4·9 to 13·1) | 0·36 | 0·12 | |
| KLG 2 | .. | −2·4 (−12·1 to 7·3) | 0·62 | 0·12 | |
| KLG 3 | .. | −2·2 (−11·9 to 7·5) | 0·65 | 0·12 | |
| Previous knee surgery on index knee (ref: no) | 92 | .. | .. | .. | |
| Meniscal surgery | .. | −3·1 (−13·4 to 7·1) | 0·55 | 0·12 | |
| Ligament surgery | .. | −1·1 (−11·1 to 8·9) | 0·82 | 0·12 | |
| Other surgery | .. | −6·8 (−17·2 to 3·7) | 0·20 | 0·12 | |
| Any clinical malalignment* (ref: no) | 92 | −2·1 (−9·6 to 5·4) | 0·58 | 0·11 | |
| Previous history of index knee injury (ref: no) | 92 | −3·0 (−10·2 to 4·2) | 0·41 | 0·12 | |
| Family history of knee osteoarthritis (ref: no) | 92 | 1·6 (−5·0 to 8·2) | 0·63 | 0·12 | |
| Factors associated with knee outcomes in our previous studies | |||||
| Time from joint injury, days | 92 | −0·1 (−0·3 to 0·0) | 0·070 | 0·13 | |
| Extent or nature of joint injury | 92 | .. | .. | .. | |
| Single complete ligament rupture (ref: meniscal tear) | .. | 5·1 (−3·7 to 14·0) | 0·25 | 0·14 | |
| Single complete ligament rupture and meniscal tear (ref: meniscal tear only) | .. | 0·9 (−9·9 to 8·0) | 0·84 | 0·14 | |
| Extended injury (ref: meniscal tear only) | .. | −4·9 (−18·2 to 8·4) | 0·47 | 0·14 | |
| Non-professional physical activity preinjury (ref: professional) | 92 | 0·2 (−7·2 to 7·6) | 0·95 | 0·11 | |
| Moderate-to-severe synovial fluid blood staining (ref: none or mild)† | 79 | −10·1 (−18·6 to −1·6) | 0·020 | 0·19 | |
| Other exploratory factors at baseline‡ | |||||
| Medium or large clinical effusion (ref: none or small) | 91 | −7·2 (−13·5 to −0·9) | 0·030 | 0·14 | |
| Interaction (medium or large effusion) × (moderate or severe synovial fluid blood staining)§ | 78 | −22·2 (−36·8 to −7·5) | 0·0036 | 0·25 | |
The coefficients or effect sizes, 95% CIs, p values, and R2 are shown for the univariable associations by linear regression of key predefined clinical variables with KOOS4 at 2 years when adjusted for KOOS4 at baseline, in the number of patients (n) with available data for that variable in the 2-year dataset, out of the 98 individuals for whom KOOS4 data were available at 2 years (and using KOOS4 at 3 years when missing). KLG=Kellgren and Lawrence grade for osteoarthritis. KOOS4=Knee Injury and Osteoarthritis Outcome Score based on a composite of four subscales (pain, symptoms, sports and recreation, and quality of life). Ref=reference category.
At baseline or 3 months.
Grading system for synovial fluid blood staining is given in appendix p 6.
All predefined exploratory factors that we tested are shown in appendix p 8; only associations from this category with a p value of ≤0·05 are shown here.
Seven interactions were predefined: blood staining and injury categories, blood staining and any chondral defects, blood staining and fracture or dislocation, clinical effusion and concurrent non-steroidal anti-inflammatory drug use, clinical effusion and KLG, clinical effusion and blood staining (shown here), and IL-6 and other protein synovial fluid concentrations. Only interactions of variables found to be associated with the outcome were tested; significant associations (p≤0·05) are shown here and were selected for the core model (appendix p 10).
Neither time from injury to sampling nor the extent of the injury were significantly associated with 2-year KOOS4 (table 2), and re-addition of these variables to the core model using the complete cases dataset (n=77) did not suggest that they substantially contributed (appendix p 10). No other factors (eg, age, sex, or BMI) contributed further to this core model when tested on univariable analysis (appendix p 10).
Concentrations of six blood biomarkers in participants in the KICK cohort compared with healthy samples have been previously published (IL-6, MCP-1, activin A, MMP-3, TIMP-1, and TSG-6),11 and four were measured again in this study (table 3; appendix p 18). Of 12 predefined synovial fluid markers, only MCP-1 and IL-6 showed independent associations with 2-year KOOS4 when accounting for the core model (table 3). After adjustment for variables in the core model, adding the interaction between synovial fluid MCP-1 and IL-6 accounted for 39% of variability of KOOS4 at 2 years, indicating that the biomarkers played a minor role. By contrast, none of the four predefined blood biomarkers, measured either at baseline or over the subsequent 3 months (appendix p 18), was associated with 2-year KOOS4 when adjusted for core variables, blood staining, clinical effusion, and their interaction (table 2).
Table 3.
Univariable associations of baseline synovial fluid and plasma or serum biomarkers with KOOS4at 2 years
|
KOOS4at 2 years, adjusted by baseline KOOS4 |
KOOS4at 2 years, adjusted by core variables (baseline KOOS4, and effusion × blood staining) |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Coefficient (95% CI) | p value | R2 | n | Coefficient (95% CI) | p value | R2 | |
| Synovial fluid | ||||||||
| TSG-6 | 83 | −0·01 (−0·03 to 0·02) | 0·61 | 0·15 | 77 | 0·06 (−0·02 to 0·03) | 0·60 | 0·30 |
| IL-18BP | 83 | −0·002 (−0·005 to 0·002) | 0·39 | 0·17 | 77 | −0·00002 (−0·003 to 0·003) | 0·99 | 0·30 |
| IL-18 | 80 | −0·08 (−0·15 to −0·01) | 0·030 | 0·20 | 74 | −0·02 (−0·11 to 0·08) | 0·75 | 0·30 |
| Tenascin C | 78 | −0·067 (−0·137 to 0·004) | 0·065 | 0·18 | 72 | −0·03 (−0·11 to 0·06) | 0·52 | 0·29 |
| TGFβ-1 | 78 | −0·0028 (−0·0053 to −0·0003) | 0·030 | 0·30 | 72 | −0·002 (−0·004 to 0·001) | 0·14 | 0·38 |
| TIMP-1 | 83 | −0·0026 (−0·0055 to 0·0003) | 0·078 | 0·22 | 77 | −0·002 (−0·004 to 0·009) | 0·20 | 0·32 |
| MMP-3 | 83 | −0·00001 (−0·0006 to 0·0006) | 0·97 | 0·15 | 77 | 0·0004 (−0·0002 to 0·0009) | 0·19 | 0·31 |
| Activin A | 83 | 0·0012 (−0·0007 to 0·0031) | 0·21 | 0·18 | 77 | 0·0015 (−0·0003 to 0·0034) | 0·10 | 0·33 |
| IL-8 | 83 | −0·07 (−0·19 to 0·05) | 0·23 | 0·20 | 77 | −0·03 (−0·15 to 0·09) | 0·61 | 0·30 |
| FGF-2 | 81 | −0·05 (−0·09 to −0·01) | 0·023 | 0·17 | 75 | −0·02 (−0·06 to 0·02) | 0·28 | 0·29 |
| MCP-1 | 83 | −0·02 (−0·03 to −0·01) | 0·00005 | 0·30 | 77 | −0·015 (−0·027 to −0·004) | 0·011 | 0·37 |
| IL-6 | 83 | −0·0006 (−0·0010 to −0·0003) | 0·0012 | 0·22 | 77 | −0·0005 (−0·0009 to −0·0001) | 0·017 | 0·34 |
| MCP-1 × IL-6 | .. | .. | .. | .. | 77 | −0·0000005 (−0·000001 to −0·0000001) | 0·018 | 0·39 |
| Blood | ||||||||
| MMP-3 | 91 | −0·02 (−0·50 to 0·47) | 0·94 | 0·11 | 77 | 0·061 (−0·59 to 0·71) | 0·85 | 0·25 |
| NP-Y | 90 | 0·10 (−0·02 to 0·22) | 0·11 | 0·12 | 76 | 0·12 (−0·071 to 0·31) | 0·21 | 0·26 |
| IL-6 | 91 | −6·58 (−13·27 to 0·10) | 0·054 | 0·16 | 77 | −4·3 (−11·4 to 2·8) | 0·23 | 0·27 |
| FGF-2 | 91 | −0·01 (−0·13 to 0·11) | 0·85 | 0·11 | 77 | −0·010 (−0·13 to 0·11) | 0·86 | 0·25 |
Concentrations of 12 predefined analytes in synovial fluid and four predefined blood analytes and their association with KOOS4 at 2 years. For the core variable adjusted analysis, all results are shown for the complete case dataset (n=77; ie, individuals who had all necessary variables available, of the 98 individuals for whom KOOS4 was available at 2 [and 3] years). For each biomarker, the effect size is for a 1 pg/mL unit change. KOOS4=Knee Injury and Osteoarthritis Outcome Score based on a composite of four subscales (pain, symptoms, sports and recreation, and quality of life). TSG-6=tumour necrosis factor stimulated gene-6 (tumour necrosis factor-inducible gene 6 protein [TNFAIP6]). IL=interleukin. BP=binding protein. TGFβ-1=transforming growth factor β-1. TIMP-1=tissue inhibitor of metalloproteinases-1 (metalloproteinase inhibitor 1). MMP-3=metalloproteinase-3 (stromelysin-1 [SL-1]). FGF-2=fibroblast growth factor 2. MCP-1=monocyte chemoattractant protein-1 (C-C motif chemokine 2 [CCL2]). NP-Y=neuropeptide-Y.
Mean KOOS pain at 2 years was 88·7 (SD 12·1), and associations generally mirrored those for KOOS4 (data not shown). There was high correlation between KOOS4 and KOOS pain at 2 years, in support of this observation (appendix p 20).
There were no patients with MRI-evident osteoarthritis by OARSI-MRI criteria who did not also have radiographic tibiofemoral knee osteoarthritis on x-ray. Therefore, only exploratory outcomes that included x-ray criteria were analysed. 22 (15%) of 150 participants had new tibiofemoral radiographic osteoarthritis at 2 years (table 4). Irrespective of other injured structures, the presence of MRI-evident meniscal tear at baseline was associated with new radiographic osteoarthritis at 2 years (any tear vs none, odds ratio [OR] 5·70 [95% CI 1·25–25·92], p=0·024; lateral tear vs none, 4·50 [0·85–23·8], p=0·077; appendix p 12).
Table 4.
Secondary binary clinical outcomes at 2 years
| Symptoms or structure | Patients with available outcome data in total cohort | Patients with positive outcome in available dataset | Patients with positive outcome in total cohort | |
|---|---|---|---|---|
| NHANES frequent knee pain at 2 years | Symptoms | 64/150 (43%) | 24/64 (38%) | 24/150 (16%) |
| New radiographic TF knee osteoarthritis*† | Structure | 56/150 (37%) | 22/56 (39%) | 22/150 (15%) |
| OARSI-MRI criteria,20 either new TF osteoarthritis or new PF osteoarthritis‡ | Structure | 52/150 (35%) | 14/52 (27%) | 14/150 (9%) |
| Luyten early knee osteoarthritis criteria21 | Symptoms and structure | 81/150 (54%) | 26/81 (32%) | 26/150 (17%) |
| New symptomatic§, and radiographic* osteoarthritis | Symptoms and structure | 61/150 (41%) | 9/61 (15%) | 9/150 (6%) |
Data are n/N (%) unless otherwise specified. NHANES=National Health and Nutrition Examination Survey. TF=tibiofemoral. OASRI=Osteoarthritis Research Society International. PF=patellofemoral. KLG=Kellgren and Lawrence grade for osteoarthritis.
Baseline KLG is 0 or 1 and KLG at 2 years is 2 or more.
X-ray available at both baseline and 2 years.
MRI available at both baseline and 2 years.
Defined by NHANES.
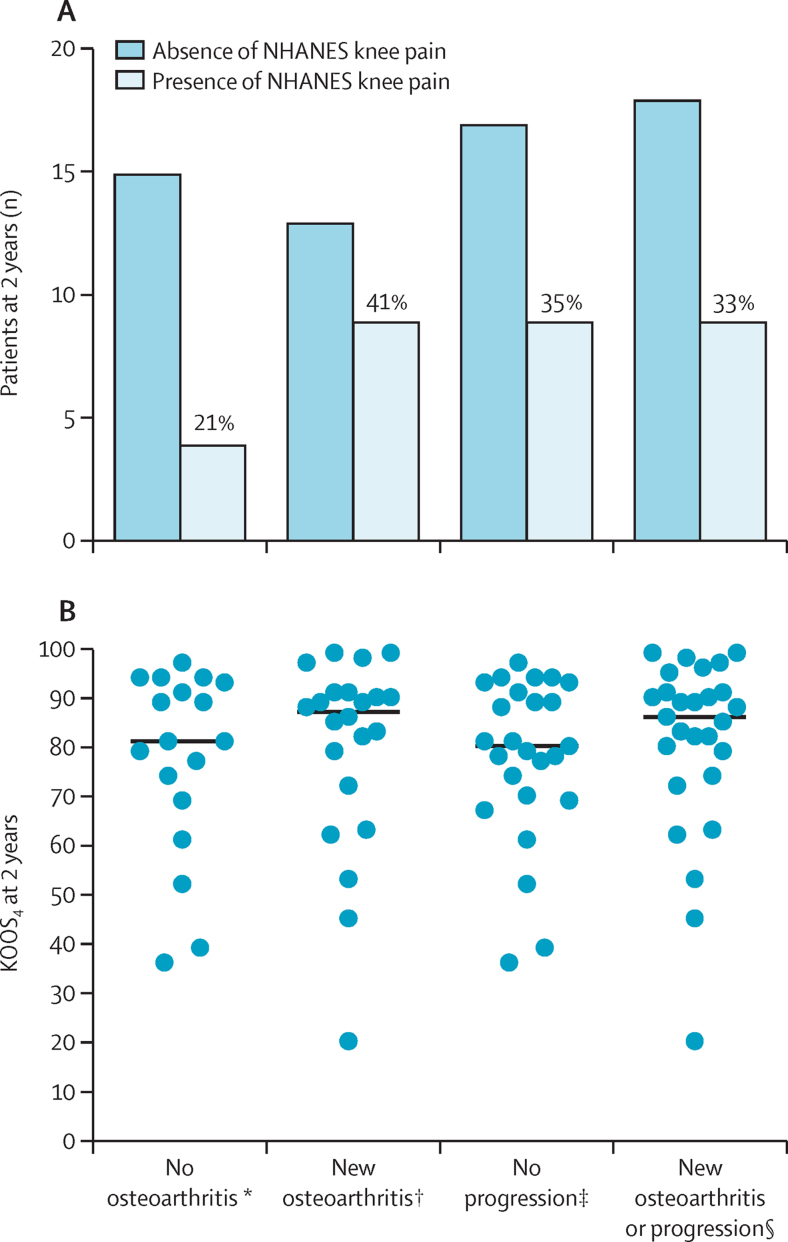
Of note, 13 (59%) of 22 participants with new tibiofemoral radiographic osteoarthritis at 2 years did not report concurrent NHANES frequent knee symptoms. There was discordance between the radiographic status of the study population and their symptomatic status at 2 years, either by NHANES question or by KOOS4 (figure 2).
Figure 2.
NHANES frequent knee pain and KOOS4 at 2 years, according to radiographic osteoarthritis status
(A) NHANES frequent knee pain at 2 years in the 53 participants in the 2-year dataset also undergoing knee x-ray at 2 years. The percentage of patients reporting NHANES frequent knee pain for each category are printed above the relevant column. Fisher's exact test to test 2 × 2 contingency for no osteoarthritis versus new osteoarthritis, in the presence or absence of NHANES symptoms, showed no significant difference (two-sided test, p=0·20). Fisher's exact test to test 2 × 2 contingency for no progression versus progression or new osteoarthritis, in presence or absence of NHANES symptoms, showed no significant difference (two-sided test, p>0·99). (B) KOOS4 at 2 years in the 52 study participants in the 2-year dataset also undergoing knee x-ray at 2 years. The Kruskal Wallis test to test for differences between groups showed no significant difference (p=0·69). KOOS4=Knee Injury and Osteoarthritis Outcome Score based on a composite of four subscales (pain, symptoms, sports and recreation, and quality of life).NHANES=National Health and Nutrition Examination Survey. *No evidence of radiographic osteoarthritis at baseline or 2 years. †New radiographic osteoarthritis (Kellgren and Lawrence grade for osteoarthritis [KLG] ≥2) at 2 years (new osteoarthritis). ‡Evidence of KLG 1 or more at baseline with no progression (no progression). §This category includes new osteoarthritis (as previously defined) or progression (progression of at least 1 grade on KLG, where KLG is 1 or more at baseline).
The presence of baseline haemarthrosis was also associated with Luyten early knee osteoarthritis criteria at 2 years in the 2-year dataset (OR 3·78 [1·27–11·19], p=0·016; appendix p 11). The only factor found to be associated with new symptomatic, radiographic osteoarthritis at 2 years in this dataset was the presence of a medium-to-large effusion at 3 months (14·0 [1·86–105·27], p=0·010; appendix p 14). Similar findings were also observed for new radiographic osteoarthritis (appendix p 12). None of the predefined baseline synovial fluid or blood markers were associated with new radiographic osteoarthritis or new symptomatic, radiographic osteoarthritis (appendix pp 16–17). The model including the interaction of blood staining, effusion, and MCP-1 and IL-6 (tables 2, 3) was not associated with either new radiographic osteoarthritis or radiographic progression (data not shown).
Two participants were withdrawn from the study (in both cases because of probable septic arthritis of the index knee joint around the time of the 3-month visit). These adverse events were not related to study participation. There were two adverse events linked to study participation, both at the time of blood sampling (one presyncopal episode and one tenderness and pain at the site of venepuncture). There were no serious adverse events related to study participation.
Discussion
This prospective cohort study suggested that the single biggest factor associated with adverse outcomes at 2 years after knee injury was the presence of blood in the knee joint, followed by the presence of effusion, and to a more modest degree elements of the joint injury inflammatory response measured in synovial fluid. It has long been known that haemarthrosis is associated with arthritis, primarily from literature around haemophilic arthropathy and introduction of blood into joints in animal models.27, 28, 29 Haemarthrosis at the time of injury has been associated with synovial fluid-based inflammation in cross-sectional studies,30 but to our knowledge this is the first study to grade and test the association of haemarthrosis at the time of knee injury with longitudinal outcomes. These findings raise several important clinical and research considerations. Historically, patients with an acute knee injury with suspected haemarthrosis would undergo acute arthroscopic washout of the joint or needle arthrocentesis, to drain blood. In the UK and other countries, the number of knee arthroscopic surgeries has declined over the past decade, including for trauma. 145 individuals in our study underwent knee surgery that included washout at a median of 17 days (maximum 51 days) after injury (appendix p 2). This procedure would have removed blood, yet it did not affect the association of haemarthrosis with the primary outcome.
If the grade of haemarthrosis might be an important biomarker of outcome after acute knee injury, how might it be measured? Aside from invasive procedures such as needle arthrocentesis being done for clinical reasons, imaging-based approaches or blood-based markers that have strong positive predictive value for the detection and grading of blood in the joint could be used in larger scale prediction studies or clinical trials. It is important to recognise that the nature and extent of the injury and the time from injury to baseline will be associated with the presence of both haemarthrosis and effusion. However, neither the nature nor extent of the injury were independently predictive of outcome when haemarthrosis and effusion were considered in this study. It could be that haemarthrosis and effusion simply represent these effects best, although how representative they are could of course depend on how the extent of injury is captured; we chose a categorical approach that we felt was clinically meaningful.
We set out to test whether the synovial fluid protein response at the time of injury would be associated independently with patient-reported outcomes; in preclinical models, production of these proteins is thought to arise from new synthesis by connective tissues including articular cartilage.14, 31 It is worth noting that our linear regression models considered the effect of a 1 pg/mL increase in concentration of the given biomarker on the clinical outcome, whereas in practice, concentrations of biomarkers at the time of injury can differ by orders of magnitude between individuals. Therefore, although the effect sizes for these biomarkers in the regression model appear small, their contribution to the association with outcome might still be of clinical relevance.
The IL-6 response to tissue injury could be biologically important; IL-6 is synthesised by chondrocytes and synoviocytes, has the potential to initiate joint damage and sensitise nociceptors, and has been implicated previously in osteoarthritis.32, 33 However, there is evidence that genetic deletion of IL-6 can worsen murine osteoarthritis.34 We previously reported that concentration of IL-6 was associated with early knee outcomes and best represented this biological response to injury, perhaps because of substantial upregulation and interindividual variation.10, 11 However, high concentrations of IL-6 in synovial fluids at baseline have been associated with a greater clinical improvement over the first 3 months,11 whereas in our study higher concentrations were associated with worse longer-term symptomatic outcomes. These observations could be entirely plausible and compatible, particularly in view of the apparent disconnect between symptoms and structure at 2 years (and potentially earlier).
MCP-1 also contributed to the model of pain outcome at 2 years. Of note, Ccl-2 (the mouse homologue of MCP-1) has been associated with knee pain generation after destabilisation of the medial meniscus and the monocyte response to injury, and in mice, neutralising Ccl-2 improves outcomes. As such, MCP-1 remains a translational molecule of interest in the response to joint trauma and post-traumatic osteoarthritis.35, 36, 37
Our findings suggest that persisting effusion could predict adverse structural outcome, adding evidence in support of clinical beliefs held by many surgical knee specialists.5 Whether this association is driven by the effects of the effusion (muscle wasting, asymmetry, reduced activity) or by its causes (synovial inflammation, joint instability, connective tissue injury products) is not possible to infer here. That blood in the joint is associated with our primary outcome measure and is also the only identifiable predictor of positive Luyten criteria for early osteoarthritis provides some early construct validity. Although the Luyten criteria were not designed for post-traumatic osteoarthritis, they are intended for younger individuals with early disease, which seemed relevant in our study.21 Finally, a previously reported association between meniscal tear and radiological change after joint trauma was replicated, lending further validity to our findings.5
Our work has some limitations. With any predictive model, testing its validity and replication (ideally in independent datasets) and its generalisability is important. This cohort has some unusual features (young, predominantly male, sporting) in addition to incomplete follow-up (65%) that could limit the generalisability of our results. This incomplete follow-up could also lead to follow-up bias, with more symptomatic individuals more likely to remain in follow-up. Furthermore, as this cohort almost universally received surgical intervention at baseline, the findings should be considered in this context; they might not be the same for a non-surgically managed cohort. This was an exploratory study and was not designed to provide a validated prognostic model. Any findings must be replicated to support their clinical significance. However, it is unusual to collect synovial fluid and also to grade blood staining within it; to date we have not identified any existing international cohort with more than 40 individuals that included this information, and we hope to work with, or develop, independent cohorts in the future to remedy this shortage. Our findings of lack of associations of these biomarkers with selected structural outcomes might be because there is no true effect, or that the effects of blood in the joint outweigh the effects of these protein concentrations (or might interfere with their analysis), or because 2 years is too early to look for associations. Associations with secondary outcomes are exploratory and might not be powered to detect significant associations. The smaller sample size of patients attending for imaging at 2 years could have been underpowered to detect associations with our biomarkers of interest.
Further studies are needed to test the validity of these findings and whether adverse clinical outcomes at 2 years predict later radiographic disease.
Data sharing
Individual participant data that underlies the results reported in this manuscript can be made available after deidentification (text, tables, figures, and appendices); the study protocol, statistical analysis plan, and data dictionary can also be made available. These items will be available between 12 and 36 months after article publication. Access criteria are that applicants are bone fide academic researchers whose proposed use of the data is within the consent given by participants, is methodologically sound to achieve aims in the proposal, which might include individual participant data meta-analysis, and whose use has been approved by the study access committee (FEW, TLV, AW). Proposals should be directed to FEW at fiona.watt@kennedy.ox.ac.uk. Data requestors will need to sign a data transfer or access agreement.
Declaration of interests
TLV reports consultancy fees from GlaxoSmithKline, UCB, and Mundipharma and has also received research grants from Galapagos, Fidia, and Samumed. NKA reports consultancy fees from Pfizer/Lilly and received a grant in a related area of research from Merck. AJ reports consultancy fees from Freshfields Bruckhaus Deringer and from Anthera Pharmaceuticals. AW is a board member and holds stock in Fortius Clinic, has received research grants from Smith and Nephew, is a board member and shareholder in Innovate Orthopaedics, and a shareholder in DocComs. FEW has received clinical study grants from Pfizer and Astellas Pharma, reports consultancy fees from Pfizer, and is part of a consortium receiving some of its research funding from Galapagos, Fidia, and Samumed. All other authors report no competing interests.
Acknowledgments
Acknowledgments
This work was supported by project grants from the Kennedy Trust for Rheumatology Research, Versus Arthritis ‘Pain Challenge' project grant (21509), and Centre for OA Pathogenesis Versus Arthritis (grants 20205 and 21621). FEW is supported by a UK Research and Innovation (UKRI) Future Leaders Fellowship (MR/S016538/1) and was also supported during this work in part by the UK National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. FEW and NKA are members of the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis, University of Oxford (grant 21595). AJ was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. The views expressed are our own and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health. We have not been paid to write this article by a pharmaceutical company or other agency. We thank Versus Arthritis for funding this work and the Kennedy Trust for Rheumatology Research and UKRI for supporting the KICK cohort and FEW. We wish to thank Jeremy Saklatvala for his role in the conception of the study. We also thank Charlotte Kerr and Gretchen Brewer for administrative support, Joanne Milligan for technical support, and Brian Marsden, Vinod Kumar, and Andrew Freidin for database and data management support. We also thank KICK study participants and sites, particularly the Fortius Clinic for their assistance.
Contributors
FEW, TLV, KS, and AW contributed to the study concept, and the planning, design, and conduct of the study. MG was responsible for project administration and data curation. MG, KL, NKA, EP, RH, BH, and JA contributed to the data collection, imaging, and laboratory analysis. LH, LG, AL, and KS contributed to the imaging acquisition and analysis. CG, AJ, NKA, and FEW contributed to the data analysis and reporting. CG did the statistical analysis. All authors developed drafts of the manuscript and approved the final draft. CG, MG, and FEW had access to and verified the underlying data. All authors had full access to all of the data and the final responsibility to submit for publication.
Supplementary Material
References
- 1.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of posttraumatic osteoarthritis. J Athl Train. 2017;52:491–496. doi: 10.4085/1062-6050-51.5.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 4.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20:719–725. doi: 10.1097/01.bot.0000211160.05864.14. [DOI] [PubMed] [Google Scholar]
- 5.van Meer BL, Oei EH, Meuffels DE. Degenerative changes in the knee 2 years after anterior cruciate ligament rupture and related risk factors: a prospective observational follow-up study. Am J Sports Med. 2016;44:1524–1533. doi: 10.1177/0363546516631936. [DOI] [PubMed] [Google Scholar]
- 6.Roemer FW, Englund M, Turkiewicz A. Molecular and structural biomarkers of inflammation at two years after acute anterior cruciate ligament injury do not predict structural knee osteoarthritis at five years. Arthritis Rheumatol. 2019;71:238–243. doi: 10.1002/art.40687. [DOI] [PubMed] [Google Scholar]
- 7.Spindler KP, Huston LJ, Chagin KM. Ten-year outcomes and risk factors after anterior cruciate ligament reconstruction: a MOON longitudinal prospective cohort study. Am J Sports Med. 2018;46:815–825. doi: 10.1177/0363546517749850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt FE, Corp N, Kingsbury SR. Towards prevention of post-traumatic osteoarthritis: report from an international expert working group on considerations for the design and conduct of interventional studies following acute knee injury. Osteoarthritis Cartilage. 2019;27:23–33. doi: 10.1016/j.joca.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Res Ther. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Struglics A, Larsson S, Kumahashi N, Frobell R, Lohmander LS. Changes in cytokines and aggrecan ARGS neoepitope in synovial fluid and serum and in C-terminal crosslinking telopeptide of type II collagen and N-terminal crosslinking telopeptide of type I collagen in urine over five years after anterior cruciate ligament rupture: an exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis Rheumatol. 2015;67:1816–1825. doi: 10.1002/art.39146. [DOI] [PubMed] [Google Scholar]
- 11.Watt FE, Paterson E, Freidin A. Acute molecular changes in synovial fluid following human knee injury: association with early clinical outcomes. Arthritis Rheumatol. 2016;68:2129–2140. doi: 10.1002/art.39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.Amano K, Huebner JL, Stabler TV. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am J Sports Med. 2018;46:890–899. doi: 10.1177/0363546517749834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burleigh A, Chanalaris A, Gardiner MD. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64:2278–2288. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 15.Chong KW, Chanalaris A, Burleigh A. Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo. Arthritis Rheum. 2013;65:2346–2355. doi: 10.1002/art.38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watt FE, Ismail HM, Didangelos A. Src and fibroblast growth factor 2 independently regulate signaling and gene expression induced by experimental injury to intact articular cartilage. Arthritis Rheum. 2013;65:397–407. doi: 10.1002/art.37765. [DOI] [PubMed] [Google Scholar]
- 17.Watt FE, Hamid B, Garriga C. The molecular profile of synovial fluid changes upon joint distraction and is associated with clinical response in knee osteoarthritis. Osteoarthritis Cartilage. 2020;28:324–333. doi: 10.1016/j.joca.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331–342. doi: 10.1056/NEJMoa0907797. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DJ, Arden N, Conaghan PG. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19:963–969. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luyten FP, Bierma-Zeinstra S, Dell'Accio F. Toward classification criteria for early osteoarthritis of the knee. Semin Arthritis Rheum. 2018;47:457–463. doi: 10.1016/j.semarthrit.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Goulston LM, Sanchez-Santos MT, D'Angelo S. A comparison of radiographic anatomic axis knee alignment measurements and cross-sectional associations with knee osteoarthritis. Osteoarthritis Cartilage. 2016;24:612–622. doi: 10.1016/j.joca.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leyland KM, Hunter D, Judge A. Bezier curves for measuring joint space on knee radiographs—reproducibility and validity. Osteoarthritis Cartilage. 2011;19(suppl):s180–s181. (abstr). [Google Scholar]
- 24.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 25.Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kingsbury SR, Corp N, Watt FE. Harmonising data collection from osteoarthritis studies to enable stratification: recommendations on core data collection from an Arthritis Research UK clinical studies group. Rheumatology (Oxford) 2016;55:1394–1402. doi: 10.1093/rheumatology/kew201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vulpen LF, van Meegeren ME, Roosendaal G. Biochemical markers of joint tissue damage increase shortly after a joint bleed; an explorative human and canine in vivo study. Osteoarthritis Cartilage. 2015;23:63–69. doi: 10.1016/j.joca.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Acharya SS. Exploration of the pathogenesis of haemophilic joint arthropathy: understanding implications for optimal clinical management. Br J Haematol. 2012;156:13–23. doi: 10.1111/j.1365-2141.2011.08919.x. [DOI] [PubMed] [Google Scholar]
- 29.Lafeber FP, Miossec P, Valentino LA. Physiopathology of haemophilic arthropathy. Haemophilia. 2008;14(suppl 4):3–9. doi: 10.1111/j.1365-2516.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 30.Swärd P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20:1302–1308. doi: 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Gardiner MD, Vincent TL, Driscoll C. Transcriptional analysis of micro-dissected articular cartilage in post-traumatic murine osteoarthritis. Osteoarthritis Cartilage. 2015;23:616–628. doi: 10.1016/j.joca.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–359. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 33.Latourte A, Cherifi C, Maillet J. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76:748–755. doi: 10.1136/annrheumdis-2016-209757. [DOI] [PubMed] [Google Scholar]
- 34.de Hooge AS, van de Loo FA, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage. 2005;13:66–73. doi: 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Miotla Zarebska J, Chanalaris A, Driscoll C. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthritis Cartilage. 2017;25:406–412. doi: 10.1016/j.joca.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghu H, Lepus CM, Wang Q. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76:914–922. doi: 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longobardi L, Temple JD, Tagliafierro L. Role of the C-C chemokine receptor-2 in a murine model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2017;25:914–925. doi: 10.1016/j.joca.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlies the results reported in this manuscript can be made available after deidentification (text, tables, figures, and appendices); the study protocol, statistical analysis plan, and data dictionary can also be made available. These items will be available between 12 and 36 months after article publication. Access criteria are that applicants are bone fide academic researchers whose proposed use of the data is within the consent given by participants, is methodologically sound to achieve aims in the proposal, which might include individual participant data meta-analysis, and whose use has been approved by the study access committee (FEW, TLV, AW). Proposals should be directed to FEW at fiona.watt@kennedy.ox.ac.uk. Data requestors will need to sign a data transfer or access agreement.