Abstract
Human myeloid leukemia cells respond to 12-O-tetradecanoylphorbol-13-acetate (TPA) and other activators of protein kinase C (PKC) with induction of monocytic differentiation. The present studies demonstrated that treatment of U-937 and HL-60 myeloid leukemia cells with TPA, phorbol-12,13-dibutyrate, or bryostatin 1 was associated with the induction of stress-activated protein kinase (SAPK). In contrast, TPA-resistant TUR and HL-525 cell variants deficient in PKCβ failed to respond to activators of PKC with the induction of SAPK. A direct role for PKCβ in TPA-induced SAPK activity in TUR and HL-525 cells that stably express PKCβ was confirmed. We showed that TPA induced the association of PKCβ with MEK kinase 1 (MEKK-1), an upstream effector of the SAPK/ERK kinase 1 (SEK1)→SAPK cascade. The results also demonstrated that PKCβ phosphorylated and activated MEKK-1 in vitro. The functional role of MEKK-1 in TPA-induced SAPK activity was further supported by the demonstration that the expression of a dominant negative MEKK-1 mutant abrogated this response. These findings indicate that PKCβ activation is necessary for activation of the MEKK-1→SEK1→SAPK cascade in the TPA response of myeloid leukemia cells.
The human U-937 and HL-60 myeloid leukemia cell lines proliferate autonomously in the absence of exogenous hematopoietic growth factors (6, 52). These cells, however, have retained the capacity to respond to inducers of differentiation with growth arrest and the appearance of a mature phenotype. In this context, treatment of U-937 and HL-60 cells with agents that activate protein kinase C (PKC), including 12-O-tetradecanoylphorbol-13-acetate (TPA) and phorbol-12,13-dibutyrate (PDBu), induces differentiation along the monocytic lineage. Bryostatin 1, a macrocyclic lactone, also activates PKC and induces monocytic differentiation of myeloid leukemia cells (51). While these findings have indicated that factor-independent growth of myeloid leukemia cells is reversible by activation of PKC-mediated signaling, little is known about the downstream effectors responsible for induction of the differentiated monocytic phenotype.
PKC is a family of at least 12 serine/threonine protein kinase isoforms which are involved in diverse cellular responses (24, 43). The α, β, γ, δ, ɛ, μ, η, θ, and ζ forms of PKC are responsive to phorbol esters. The available evidence suggests that PKCβ is involved in TPA-induced differentiation of myeloid leukemia cells. Accordingly, TPA-resistant HL-60 cell variants are deficient in PKCβ expression (37, 42, 56, 57). Down-regulation of PKCβ expression (19) and functional defects in PKCβ (31) have also been found for TPA-resistant U-937 cell variants. In addition, defective translocation of PKCβ from the cytosol to the cell membrane has been shown for TPA-resistant variants of both U-937 and HL-60 cells (19, 64). Importantly, increased expression of PKCβ resulting from treatment with retinoic acid (64) or from transfection of the PKCβ gene (56) restores TPA inducibility of growth arrest and a differentiated monocytic phenotype. PKCβ is expressed as two isoforms, βI and βII, as a result of an alternative splicing mechanism that produces a PKCβI protein which is truncated by 50 amino acids at the carboxy terminus (32); the longer PKCβII isoform is expressed in U-937 and HL-60 cells (22, 56).
Treatment of myeloid leukemia cells with TPA is associated with changes in the expression of certain early- and late-response genes. TPA down-regulates c-myc transcripts in HL-60 cells (47) and induces expression of the c-jun gene (49, 54, 61). Similar findings have been obtained with other inducers of monocytic differentiation (49), including okadaic acid, an inhibitor of phosphoserine/threonine protein phosphatases 1 and 2A (1, 25). Activation of Jun/AP-1 contributes to induction of c-jun transcription (2) and monocytic differentiation (54). The early growth response 1 (EGR-1) gene is also activated during TPA- and okadaic acid-induced monocytic differentiation (27, 29) and is necessary for the appearance of the monocytic phenotype (41). Thus, the induction of early response genes and thereby upstream signals involved in their transcriptional activation may be directly linked to the reversal of the leukemia phenotype.
Members of the mitogen-activated protein kinase (MAPK) superfamily are involved in diverse cellular processes, including the induction of differentiation. Among the three related MAPK families identified to date, the extracellular signal-regulated protein kinases (ERK) have been identified as playing a role in differentiation. Activation of the MAPK kinase (MEK1) is necessary and sufficient for neuronal differentiation of PC12 rat pheochromocytoma cells (7) and for megakaryocyte differentiation of human K562 erythroleukemia cells (59). In contrast, overexpression of constitutively active MEK1 in U-937 cells results in growth inhibition but no phenotypic differentiation (15). In addition, activation of ERK by TPA in the TPA-resistant UT16 variant of U-937 cells suggests that ERK activation is not sufficient for induction of human myeloid leukemia cell differentiation (48).
The stress-activated protein kinases (SAPK; also known as Jun kinases or JNK) are serine/threonine protein kinases related to the MAPK family. SAPK is activated by tumor necrosis factor, diverse DNA-damaging agents, UV light, and anisomycin (12, 28, 33). SAPK phosphorylates Ser-63 and Ser-73 of the c-Jun amino terminus and thereby activates c-Jun transcription function (12, 33). The ATF2 and Elk1 transcription factors are also phosphorylated by SAPK (18, 45, 60). Whereas TPA-induced monocytic differentiation is associated with induction of c-jun (49, 54, 61) and EGR-1 (27, 29) gene expression, SAPK-mediated activation of c-Jun, ATF2, and Elk1 and thereby early response genes is associated with the appearance of the differentiated phenotype. MEK kinase 1 (MEKK-1) (34) preferentially activates SAPK/ERK kinase 1 (SEK1) (13, 36, 38) and, consequently, SAPK (46). Of interest, Bck 1p, a MEK1 kinase homolog in yeast, functions downstream of the PKC homolog PKC 1p (35). The finding that murine MEKK-1 can also function as a downstream effector of PKC 1p and can replace Bck 1p has provided support for potential interactions between PKC and MEKK-1 (3). However, the link between events activated by TPA and the MEKK-1→SEK1→SAPK pathway is unclear.
The present studies demonstrated that PKCβII is an upstream effector of TPA-induced SAPK activation. Similar findings have been obtained with other activators of PKC that induce monocytic differentiation of myeloid leukemia cells. We also showed that TPA induces the binding of PKCβII to MEKK-1 and that MEKK-1 is necessary for TPA-induced activation of SAPK.
MATERIALS AND METHODS
Cell culture.
Human U-937 myeloid leukemia cells (American Type Culture Collection [ATCC], Rockville, Md.) and the TPA-resistant clone TUR (19) were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine. Human HL-60 myeloid leukemia cells (ATCC) and the TPA-resistant clone HL-525 (23) were grown in RPMI 1640 medium supplemented with 15% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 2 mM l-glutamine. HeLa cells (ATCC) were grown in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine. U-937 and HL-60 cells were suspended at a density of 2.5 × 105/ml and treated with 16 nM TPA (Sigma Chemical Co.), 160 nM PDBu (Sigma), 10 nM bryostatin 1, 40 ng of okadaic acid (Calbiochem) per ml, or 1 μM all-trans-retinoic acid (ATRA; Hoffmann-La Roche, Basel, Switzerland).
SAPK/JNK kinase assays.
SAPK/JNK kinase assays were performed as described previously (26) with minor modifications. Cells were lysed on ice for 30 min in lysis buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Nonidet P-40 [NP-40], 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM dithiothreitol [DTT], 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 10 mM sodium fluoride). Equal amounts of protein, as determined by a protein assay (Bio-Rad Laboratories, Richmond, Calif.), were incubated with 1 μg of anti-JNK1 antibody (sc-474; Santa Cruz Biotechnology [SBC]) for 1 h at 4°C or 1 μg of antihemagglutinin (anti-HA) antibody (clone 12CA5; Boehringer Mannheim Biochemicals) for 1 h followed by 1 h of incubation with anti-mouse immunoglobulin G (IgG) antibody (402334; Calbiochem). Protein A-Sepharose beads (Pharmacia) were added for 1 h. The immunocomplexes were washed twice with buffer A (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.1% NP-40, 1 mM sodium vanadate, 1 mM PMSF, 1 mM DTT, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 10 mM sodium fluoride), washed twice with buffer B (100 mM Tris-HCl [pH 7.6], 0.5 M LiCl, 1 mM PMSF), and then washed once with kinase buffer I (50 mM HEPES [pH 7.4], 10 mM MgCl2, 2 mM DTT, 0.1 mM sodium vanadate). The immunoprecipitates were resuspended in kinase buffer containing glutathione S-transferase (GST)-Jun (amino acids 2 to 100) and [γ-32P]ATP and incubated for 10 min at 30°C before sodium dodecyl sulfate (SDS) sample buffer was added to terminate the reaction. Samples were analyzed by SDS–10% polyacrylamide gel electrophoresis (PAGE) and autoradiography. Equal loading of the lanes was determined by Coomassie blue staining of the gel. Autoradiograms were scanned, and the intensity of the GST-Jun signals was quantitated by laser densitometry.
Cell transfections.
pEF2/PKCβII was constructed by subcloning the 2.0-kb BamHI fragment from pAcMP1/PKCβII (ATCC) into the pEF2 vector made by substituting the cytomegalovirus promoter of pcDNA3 with the elongation factor 1α promoter (9).
TUR and HL-525 cells were resuspended at 107/ml and transfected by electroporation (Gene Pulser; Bio-Rad; 0.25 V, 960 μF). TUR cells were cotransfected with pTK-Hyg (Clontech) and pEF2/PKCβII or the empty pEF2 vector (pEF2/neo). HL-525 cells were transfected with pEF2/PKCβII or pEF2/neo. Two days posttransfection, the cells were cultured in media containing 200 μg of hygromycin B (Boehringer) per ml and 800 μg of Geneticin sulfate (GIBCO-BRL) per ml. After 4 weeks of selection, cells were maintained in 100 μg of hygromycin B per ml or 400 μg of Geneticin sulfate per ml.
The 2.2-kb EcoRI fragment from a kinase-inactive mutant, MEKK-1 (K-M) (21), was subcloned into pSuperCatch (17), which contains the sequence for Flag tag (Eastman Kodak Co., Rochester, N.Y.). pEF2/Flag-MEKK-1 (K-M) was constructed by subcloning the 2.4-kb HindIII-EcoRV fragment from pSuperCatch/MEKK-1 (K-M) into the pEF2 vector.
HeLa cells were resuspended at 2.5 × 107/ml and transfected by electroporation (Gene Pulser; 0.22 V, 960 μF) with pEF2/PKCβII, pEF2/neo, full-length MEKK-1 (62), pEF2/PKCδ (9), pEF2/Flag-MEKK-1 (K-M), hemagglutinin (HA)-tagged SAPK (33), or pEBG/c-Raf-1 (K-M) (58). At 48 h posttransfection, the cells were harvested and left untreated or treated with 16 nM TPA for 15 min. Whole-cell lysates were then prepared for immunoprecipitation and immunoblot analysis.
Immunoprecipitation.
Cells were washed twice with ice-cold phosphate-buffered saline and lysed in lysis buffer. Soluble proteins were incubated with anti-PKCβII antibody (sc-210; SBC), anti-MEKK-1 antibody (antibody 11612 directed against the carboxy-terminal 15 amino acids [provided by G. Johnson]), or anti-HA antibody for 1 h followed by 1 h of incubation with anti-mouse IgG antibody. Protein A-Sepharose beads were added for 1 h. The immune complexes were washed three times with lysis buffer and subjected to immunoblot analysis.
Subcellular fractionation.
Cytosolic and membrane fractions were obtained as described previously (64). Cells were resuspended in TEM lysis buffer (20 mM Tris-HCl [pH 7.5], 0.5 mM EDTA, 0.5 mM EGTA, 10 mM DTT, 1 mM PMSF, 25 μg of aprotinin per ml, 25 μg of leupeptin per ml, 10 mM β-mercaptoethanol) and sonicated. After sedimentation of the nuclear fraction by centrifugation at 3,500 rpm (Beckman benchtop ultracentrifuge) for 10 min, the cell extracts were centrifuged at 55,000 rpm (Beckman benchtop ultracentrifuge) for 30 min. The pellets were solubilized in TEM buffer containing 1% NP-40. The supernatant (cytosolic fraction) and the solubilized membrane fraction were subjected to immunoblot analysis.
Immunoblot analysis.
Proteins were separated by SDS-PAGE with 7.5, 10, or 15% polyacrylamide gels and then transferred to nitrocellulose filters. After being blocked with 5% dried milk in PBS-Tween, the filters were incubated with the following antibody: anti-PKCα (sc-208; SBC), anti-PKCβII, anti-PKCδ (sc-937; SBC), anti-MEKK-1 (antibodies 11612 and 95-012 directed against the kinase domain [provided by G. Johnson]; antibody sc-252 directed against the carboxy-terminal 22 amino acids [SBC]), anti-HA, or anti-Flag M2 (F3165; Sigma). After being washed and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Amersham), the antigen-antibody complexes were visualized by chemiluminescence (enhanced chemiluminescence detection system; Amersham).
In vitro binding of PKCβII and MEKK-1.
Human recombinant PKCβII (2 μl, 0.271 mg/ml; Calbiochem) was incubated in buffer C (20 mM Tris-HCl [pH 7.6], 20 mM MgCl2, 2 mM CaCl2, 20 μM ATP, 500 nM TPA) with glutathione-Sepharose beads bound to GST–MEKK-1 or GST for 30 min at 30°C. The adsorbed material obtained by washing three times with lysis buffer was analyzed by immunoblotting with anti-PKCβII antibody.
In vitro phosphorylation of MEKK-1.
GST–MEKK-1 (5 μg, derived from Escherichia coli; Upstate Biotechnology catalog no. 14-176) or GST was incubated in buffer C with human recombinant PKCβII (0.5 μl) and [γ-32P]ATP for 30 min at 30°C. Phosphorylation of the reaction products was assessed by SDS-PAGE and autoradiography.
MEKK-1 activity assays.
A cDNA containing the carboxy-terminal portion of 80-kDa MEKK-1 was amplified by PCR with rat full-length MEKK-1 (62) as a template and cloned into the yeast p426GAG expression vector, which contains the GST domain under the control of the yeast GAL1 promoter (55). GST–MEKK-1 (yeast derived) or GST bound to glutathione beads was pretreated with calf intestinal alkaline phosphatase (1 μl, 27.8 U/μl; GIBCO-BRL) at 37°C for 1 h. The beads were washed three times with lysis buffer, twice with 0.5 M LiCl–100 mM Tris-HCl (pH 7.6), and once with kinase buffer II (20 mM Tris-HCl [pH 7.6], 20 mM MgCl2, 2 mM CaCl2). The beads were then incubated in buffer C with or without 0.5 μl of PKCβII for 30 min at 30°C. After the kinase reaction, the beads were washed three times with lysis buffer, twice with 0.5 M LiCl–100 mM Tris-HCl (pH 7.6) containing 1% NP-40 and 0.5% deoxycholic acid, and once with 50 mM HEPES (pH 7.4)–10 mM MgCl2. The kinase reaction was performed with 50 mM HEPES (pH 7.4)–10 mM MgCl2–20 μM ATP–[γ-32P]ATP containing 5 μg of GST-SEK1 K-R mutant [SEK1 (K-R)] for 5 min at 30°C. Chelerythrine chloride (200 μM; Sigma) was added as needed. The reaction was terminated by the addition of SDS sample buffer and boiling. The reaction products were analyzed by SDS-PAGE and autoradiography.
RESULTS
Activation of SAPK in myeloid leukemia cells treated with inducers of differentiation.
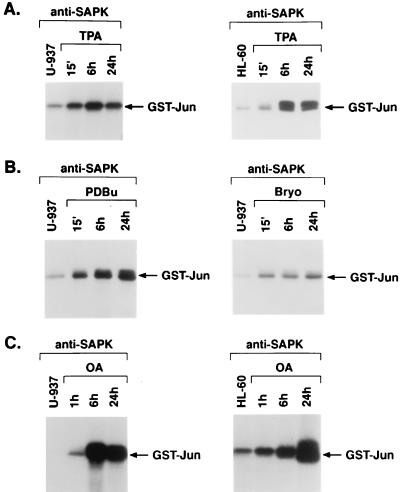
Human U-937 and HL-60 myeloid leukemia cells respond to TPA and other agents that activate PKC, such as PDBu and the non-phorbol ester bryostatin 1, with induction of monocytic differentiation. To assess the effects of these agents on SAPK activity, anti-SAPK antibody immunoprecipitates from treated cells were assayed for phosphorylation of the GST-Jun substrate. SAPK activity was induced in U-937 cells by 15 min of TPA treatment, and sustained activation of SAPK was observed through 24 h (Fig. 1A). Similar findings were obtained for TPA-treated HL-60 cells (Fig. 1A). PDBu and bryostatin 1 also induced rapid and sustained increases in SAPK activity in U-937 cells (Fig. 1B). Similar findings were obtained with these agents for HL-60 cells (data not shown). Okadaic acid, an inhibitor of protein phosphatases 1 and 2A, induces monocytic differentiation of myeloid leukemia cells (25). Treatment of U-937 and HL-60 cells with okadaic acid was associated with induction of SAPK by 1 h that was sustained at 24 h (Fig. 1C). These findings supported the induction of SAPK activity by diverse agents in association with monocytic differentiation of myeloid leukemia cells.
FIG. 1.
Activation of SAPK by TPA and other inducers of monocytic differentiation. (A) U-937 and HL-60 cells were treated with 16 nM TPA. (B) U-937 cells were treated with 160 nM PDBu or 10 nM bryostatin-1. (C) U-937 and HL-60 cells were treated with 40 ng of okadaic acid (OA) per ml. Treatment times are shown. The cells were then lysed and subjected to immunoprecipitation with anti-SAPK antibody. The immunoprecipitates were incubated with GST-Jun and [γ-32P]ATP. GST-Jun phosphorylation was assessed by SDS-PAGE and autoradiography.
Defective activation of SAPK in TPA-resistant myeloid leukemia cells.
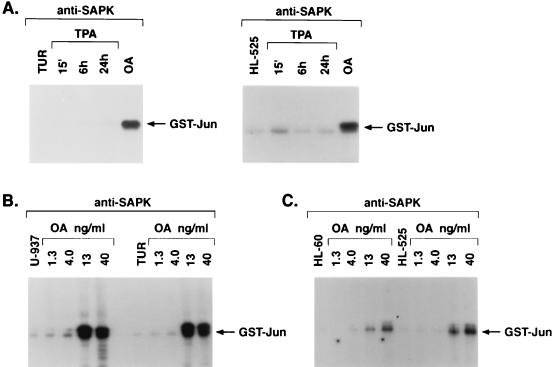
Whereas TUR and HL-525 cells fail to respond to TPA with induction of monocytic differentiation (19, 23), we examined if SAPK is induced by activators of PKC in these cells. Treatment of TUR and HL-525 cells with TPA was associated with substantial abrogation of SAPK induction compared to that in TPA-treated parental U-937 and HL-60 cells (Fig. 2A). Similar results were obtained following treatment of TUR and HL-525 cells with PDBu or bryostatin 1 (data not shown). In contrast, TUR and HL-525 cells respond to okadaic acid with induction of monocytic differentiation (19, 30) and also exhibited okadaic acid-induced increases in SAPK activity (Fig. 2A). To further assess the difference in responses to TPA and okadaic acid, dose-response relationships were studied with U-937 and TUR cells. The results demonstrated that whereas the induction of SAPK was markedly different in TPA-treated U-937 and TUR cells, the responses to okadaic acid were comparable between the two cell types (Fig. 2B). Similar results were obtained for HL-60 and HL-525 cells (Fig. 2C). These results indicated that defective activation of SAPK in TPA-treated TUR and HL-525 cells is attributable not to a loss of SAPK responsiveness but rather to defects in the activation of upstream effectors.
FIG. 2.
Defective activation of SAPK in TPA-resistant myeloid leukemia cells. (A) TUR and HL-525 cells were treated with 16 nM TPA for the indicated times or with 40 ng of okadaic acid (OA) per ml for 6 h. (B and C) U-937 and TUR cells (B) or HL-60 and HL-525 cells (C) were treated with the indicated concentrations of OA for 6 h. Anti-SAPK antibody immunoprecipitates were assayed for phosphorylation of GST-Jun.
ATRA pretreatment increases PKCβ expression and responsiveness to TPA-induced SAPK activity.
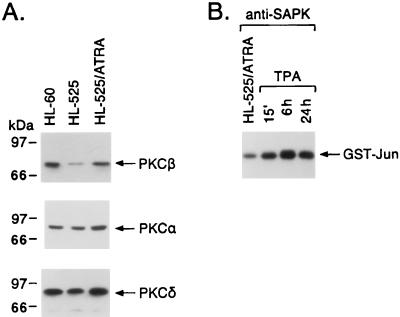
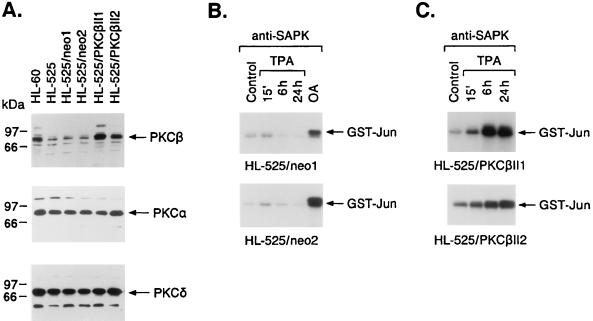
Previous studies demonstrated that TUR and HL-525 cells are deficient in PKCβ expression (19, 56). The finding that the up-regulation of PKCβ expression by ATRA treatment or transfection of the PKCβ gene restores responsiveness to TPA supports an essential role for PKCβ in TPA-induced monocytic differentiation (56, 64). To address the potential involvement of PKCβ in TPA-induced activation of SAPK, we pretreated HL-525 cells with ATRA for 3 days; as previously shown (64), this treatment increased the expression of PKCβII 4.5-fold (mean of three independent experiments) to nearly that in wild-type HL-60 cells (Fig. 3A). In contrast, PKCα expression and PKCδ expression were increased less than 1.5-fold in ATRA-pretreated HL-525 cells (Fig. 3A). ATRA pretreatment had little, if any, effect on SAPK activity (data not shown) but restored the rapid and sustained induction of SAPK activity in response to TPA exposure (Fig. 3B). These findings supported a potential role for PKCβII in TPA-induced SAPK activation.
FIG. 3.
Effects of ATRA pretreatment on PKCβ expression and responsiveness to TPA-induced SAPK activation. (A) HL-60 and HL-525 cells were cultured in the presence or absence of 1 μM ATRA for 3 days. Lysates from the indicated cells were subjected to immunoblot analysis with anti-PKCβII, anti-PKCα, and anti-PKCδ antibodies. (B) HL-525 cells were pretreated with ATRA for 3 days and then exposed to 16 nM TPA for the indicated times. Anti-SAPK antibody immunoprecipitates were assayed for GST-Jun phosphorylation.
Characterization of PKCβII transfectants.
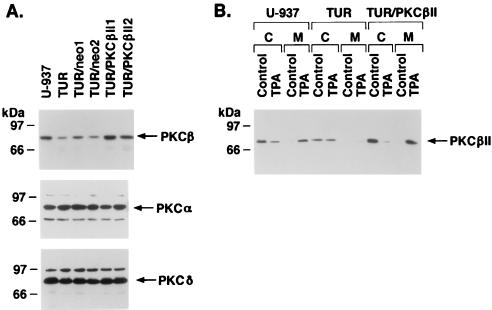
To provide more definitive evidence for the involvement of PKCβ as an upstream effector of SAPK, TUR cells that stably expressed the PKCβII gene were prepared. Separate TUR transfectants expressing the null vector (TUR/neo) demonstrated PKCβII levels comparable to those in TUR cells (Fig. 4A). In contrast, TUR transfectants expressing the PKCβII gene (TUR/PKCβII) exhibited PKCβII levels that approximated those in U-937 cells (Fig. 4A). Also, there was no apparent effect on the level of PKCα or PKCδ expression in TUR/neo or TUR/PKCβII transfectants (Fig. 4A). Treatment of U-937 cells with TPA was associated with translocation of PKCβII from the cytosolic to the membrane fraction (Fig. 4B). In contrast, translocation of PKCβII to the membrane fraction was defective in TPA-treated TUR cells (Fig. 4B). Similar defects in translocation were observed for the TUR/neo cells (data not shown), whereas PKCβII was translocated to the membrane fraction following TPA treatment of TUR/PKCβII cells (Fig. 4B). These results indicated that whereas parental TUR cells are deficient in both PKCβII expression and TPA-induced translocation, TUR transfectants expressing exogenous PKCβII display normal membrane association of PKCβII following TPA treatment.
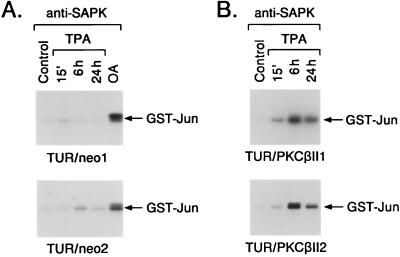
FIG. 4.
Expression of PKCβII in stable TUR transfectants. (A) TUR cells were stably transfected with pEF2/neo or pEF2/PKCβII. After selection, lysates were subjected to immunoblotting with anti-PKCβII, anti-PKCα, and anti-PKCδ antibodies. (B) The indicated cells were left untreated or were treated with 16 nM TPA for 15 min. Cytosolic (C) and membrane (M) fractions were subjected to immunoblotting with anti-PKCβII antibody.
Role for PKCβII in induction of SAPK activity.
Treatment of the TUR/neo clones with TPA demonstrated an attenuated induction of SAPK activity like that observed for nontransfected TUR cells (Fig. 5A). The TUR/PKCβII clones, however, responded to TPA with a rapid and sustained activation of SAPK (Fig. 5B). Comparable findings were obtained for the HL-525/neo and HL-525/PKCβII transfectants (Fig. 6A). Whereas the TPA-treated HL-525/neo transfectants exhibited an attenuated induction of SAPK activity, the HL-525/PKCβII transfectants responded to TPA with activation of SAPK (Fig. 6B and C). These results supported the involvement of PKCβII in TPA-induced SAPK activation. The TUR/PKCβII and HL-525/PKCβII clones also responded to TPA with cessation of growth, adherence, and increases in nonspecific esterase (NSE) staining, whereas the TUR/neo and HL-525/neo clones failed to exhibit these characteristics of monocytic differentiation (Table 1).
FIG. 5.
Activation of SAPK in TUR transfectants. TUR cells stably transfected with pEF2/neo (A) or pEF2/PKCβII (B) were exposed to 16 nM TPA for the indicated times. Anti-SAPK antibody immunoprecipitates were assayed for GST-Jun phosphorylation. Cells were also treated with 40 ng of OA per ml for 6 h.
FIG. 6.
TPA-induced activation of SAPK in HL-525 cells stably expressing PKCβII. (A) HL-525 cells were stably transfected with pEF2/neo or pEF2/PKCβII. After selection, lysates were subjected to immunoblotting with anti-PKCβII, anti-PKCα, and anti-PKCδ antibodies. (B and C) HL-525/neo (B) and HL-525/PKCβII (C) clones were treated with 16 nM TPA for the indicated times. Anti-SAPK antibody immunoprecipitates were assayed for GST-Jun phosphorylation. Cells were also treated with 40 ng of OA per ml for 6 h.
TABLE 1.
Effects of TPA on differentiation of TUR and HL-525 cell transfectantsa
| Cell type | Cell growth (% of control) | Adherent cells (% of total cells) | % of cells showing NSE staining |
|---|---|---|---|
| U-937 | 30 | 96 | >90 |
| TUR/neo-1 | 87 | 2 | <5 |
| TUR/neo-2 | 83 | 5 | <5 |
| TUR/PKCβII-1 | 49 | 93 | >75 |
| TUR/PKCβII-2 | 47 | 91 | >75 |
| HL-60 | 29 | 97 | >90 |
| HL-525/neo-1 | 101 | 3 | <5 |
| HL-525/neo-2 | 97 | 3 | <5 |
| HL-525/PKCβII-1 | 35 | 89 | >85 |
| HL-525/PKCβII-2 | 41 | 91 | >80 |
Cells were seeded onto six-well tissue culture dishes (2 × 105 cells/well) and cultured in the presence or absence of 16 nM TPA for 48 h. Viability, as determined by trypan blue exclusion, was >95% for adherent and nonadherent cells. NSE staining was performed at 72 h. Results are expressed as the mean of three determinations (standard error, <10%).
Functional interaction between PKCβII and the MEKK-1→SAPK pathway.
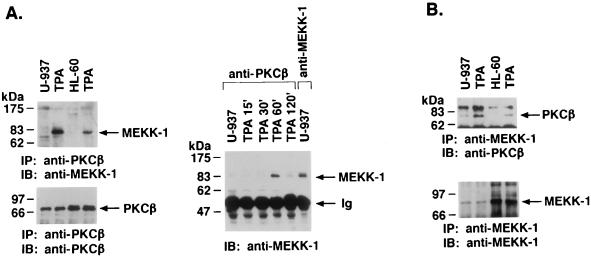
SAPK is activated by a cascade involving MEKK-1 and SEK1 (34, 36, 38, 46, 63). To determine whether PKCβII interacts with the MEKK-1→SEK1→SAPK pathway, anti-PKCβII antibody immunoprecipitates were analyzed by immunoblotting with anti-MEKK-1 antibody 11612. There was no detectable MEKK-1 in the anti-PKCβII antibody immunoprecipitates from untreated U-937 cells (Fig. 7A). In contrast, treatment with TPA resulted in the association of PKCβII and the ∼80-kDa fragment (4) of MEKK-1 (Fig. 7A, left panel). Similar findings were obtained for HL-60 cells (Fig. 7A, left panel). Kinetic studies demonstrated that the association between PKCβII and MEKK-1 was induced maximally at 1 h of TPA treatment (Fig. 7A, right panel). Compared to immunoprecipitation of control cell lysates with the anti-MEKK-1 antibody, approximately 20 to 25% of total MEKK-1 associated with PKCβII at 1 h of TPA treatment (Fig. 7A, right panel, last lane). The same findings were obtained with other anti-MEKK-1 antibodies (sc-252 and 95-012) (data not shown). In the reciprocal experiment, anti-MEKK-1 antibody immunoprecipitates were analyzed with an anti-PKCβII antibody. The results confirmed a TPA-dependent association of PKCβII and MEKK-1 (Fig. 7B). These findings suggested that activated PKCβII interacts with MEKK-1.
FIG. 7.
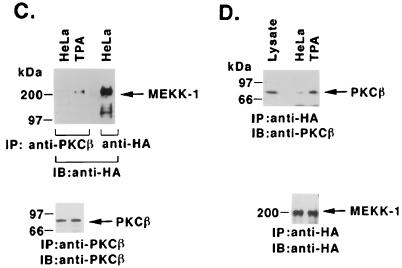
TPA-induced association of PKCβII and MEKK-1. (A) U-937 and HL-60 cells were treated with 16 nM TPA for 1 h (left panel) or for the indicated times (right panel). Cell lysates were immunoprecipitated (IP) with anti-PKCβII or anti-MEKK-1 antibody (11612; right panel, last lane). The immunoprecipitates (total applied to each lane) were subjected to immunoblot (IB) analysis with anti-PKCβII or anti-MEKK-1 antibody. Half of the anti-MEKK-1 antibody immunoprecipitate was applied to the right panel, last lane. Ig, immunoglobulin. (B) U-937 and HL-60 cells were treated with 16 nM TPA for 1 h. Anti-MEKK-1 antibody immunoprecipitates were analyzed by immunoblotting (IB) with anti-PKCβII or anti-MEKK-1 antibody. (C and D) HeLa cells were cotransfected with 10 μg of pEF2/PKCβII and 10 μg of HA-tagged full-length MEKK-1. At 48 h after transfection, the cells were treated with 16 nM TPA for 15 min. Cell lysates were immunoprecipitated (IP) with anti-PKCβII (C) or anti-HA (D) antibody and then subjected to immunoblot (IB) analysis with anti-HA (C) or anti-PKCβII (D) antibody. As a control, lysates were subjected directly to immunoblotting with anti-PKCβII antibody (left lane in panel D).
To confirm the interaction between PKCβII and MEKK-1, we performed transient expression studies with HeLa cells which, as previously shown (5), have undetectable levels of PKCβII. HeLa cells were cotransfected with pEF2/PKCβII and a vector expressing HA-tagged full-length MEKK-1. After 48 h, the transfected cells were treated with TPA, and cell lysates were subjected to immunoprecipitation with anti-PKCβII or anti-HA antibody. Analysis of the precipitates with anti-HA or anti-PKCβII antibody demonstrated that TPA induced the association of PKCβII and full-length MEKK-1 (Fig. 7C and D). Together with the results of studies with myeloid leukemia cells, these findings indicated that PKCβII binds to the truncated and full-length forms of MEKK-1 and that this association is induced by TPA-dependent activation of PKCβII.
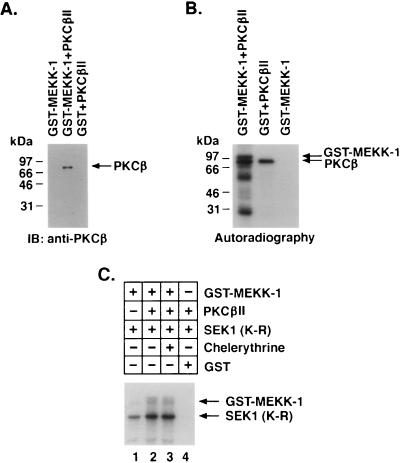
To assess whether the interaction between PKCβII and MEKK-1 is direct, we incubated purified PKCβII with GST–MEKK-1 or GST. Analysis of the material adsorbed to glutathione beads demonstrated binding of PKCβII to GST–MEKK-1 and not GST (Fig. 8A). These findings indicated that PKCβII interacts directly with MEKK-1. To determine whether PKCβII phosphorylates MEKK-1, we incubated PKCβII with GST–MEKK-1 or GST in the presence of [γ-32P]ATP. Analysis of the reaction products demonstrated phosphorylation of GST–MEKK-1 (Fig. 8B, left lane). Autophosphorylation of PKCβII was also detectable, but phosphorylation of GST was not (Fig. 8B, right lane). Because these findings indicated that PKCβII phosphorylates MEKK-1, we examined if PKCβII affects MEKK-1 activity. GST–MEKK-1 prepared from yeast and treated with alkaline phosphatase phosphorylated the kinase-inactive SEK1 (K-R) substrate (Fig. 8C, lane 1). Preincubation of GST–MEKK-1 with PKCβII and then removal of the PKCβII led to induction of MEKK-1 activity (Fig. 8C, lane 2). Similar findings were obtained in the presence of the PKC inhibitor chelerythrine chloride (Fig. 8C, lane 3). The results of three independent experiments demonstrated that preincubation of GST–MEKK-1 with PKCβII increased MEKK-1 activity 2.4-fold (mean of three independent experiments). These findings indicated that PKCβII phosphorylates and thereby activates MEKK-1 in vitro.
FIG. 8.
PKCβII phosphorylates and activates MEKK-1 in vitro. (A) Purified PKCβII was incubated with GST–MEKK-1 (lane 2) or GST (lane 3). As a control, PKCβII was omitted from the incubation with GST–MEKK-1 (lane 1). Material adsorbed to glutathione-agarose beads was analyzed by immunoblotting (IB) with anti-PKCβII antibody. (B) PKCβII was incubated with kinase-inactive GST–MEKK-1 (E. coli derived) or GST in the presence of [γ-32P]ATP. As a control, GST–MEKK-1 was incubated with [γ-32P]ATP. The reaction products were analyzed by SDS-PAGE and autoradiography. (C) Kinase-active GST–MEKK-1 (yeast derived) bound to glutathione beads was incubated with alkaline phosphatase. After being washed, the beads were incubated in the absence or presence of purified PKCβII and ATP. The GST–MEKK-1-containing beads were washed again and then incubated with SEK1 (K-R) and [γ-32P]ATP. Chelerythrine chloride (200 μM) was added to the incubation shown in lane 3. The reaction products were analyzed by SDS-PAGE and autoradiography.
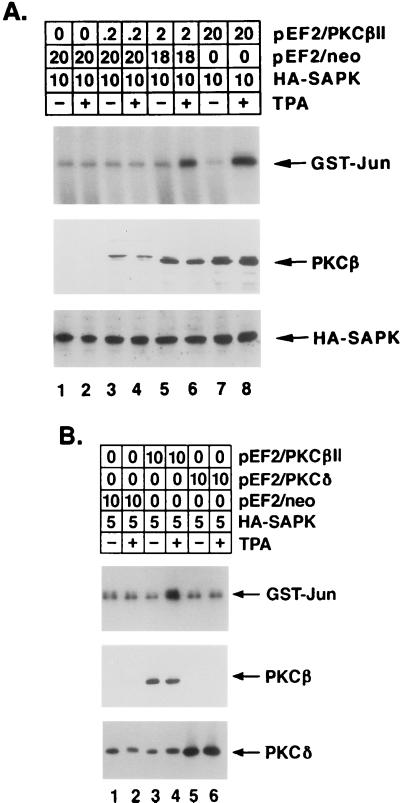
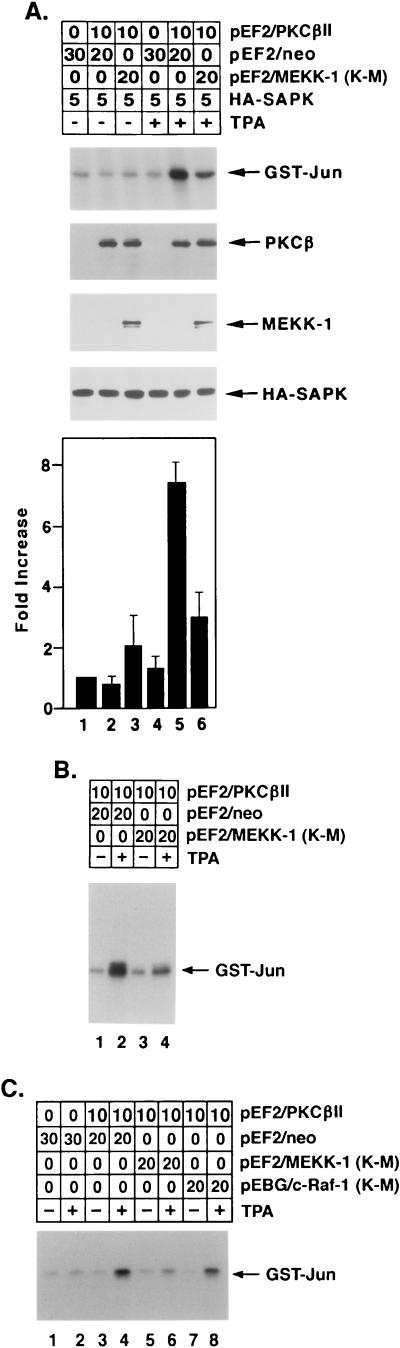
To determine whether PKCβII contributes to TPA-induced SAPK activation by a MEKK-1-dependent mechanism, cotransfection studies were performed with HeLa cells, pEF2/PKCβII, and HA-tagged SAPK. Analysis of anti-HA antibody immunoprecipitates for phosphorylation of GST-Jun demonstrated that the induction of SAPK by TPA was dependent on the level of PKCβII expression (Fig. 9A). In contrast, overexpression of the TPA-responsive PKCδ isoform had no detectable effect on TPA-induced SAPK activation (Fig. 9B). Because these findings supported the specificity of PKCβII in the induction of SAPK, the involvement of MEKK-1 in a TPA→PKCβII→SAPK cascade was assessed by cotransfection with a kinase-inactive, dominant negative mutant, MEKK-1 (K-M) (21). The results demonstrated that while TPA induced SAPK activation by a PKCβII-dependent mechanism, the expression of MEKK-1 (K-M) blocked the response (Fig. 10A). To extend these findings by assessing the activation of endogenous SAPK, similar experiments were performed with HeLa cells transfected with pEF2/PKCβII and pEF2/Flag-MEKK-1 (K-M). Analysis of anti-SAPK antibody immunoprecipitates for phosphorylation of GST-Jun confirmed that the induction of endogenous SAPK by TPA was also dependent on PKCβII expression and was blocked by the MEKK-1 (K-M) mutant (Fig. 10B). To show that MEKK-1 (K-M) specifically inhibits TPA-induced SAPK activation, we compared the effects of MEKK-1 (K-M) to those of a kinase-inactive MEK1 mutant, c-Raf-1 (K-M). In contrast to the inhibition by MEKK-1 (K-M), there was no detectable effect of the overexpression of c-Raf-1 (K-M) on TPA-induced SAPK activity (Fig. 10C). The c-Raf-1 (K-M) mutant was, however, effective in inhibiting TPA-induced ERK2 activity (data not shown). Collectively, these findings indicated that PKCβII associates with MEKK-1 by a TPA-dependent mechanism and thereby contributes to the induction of the MEKK-1→SAPK cascade.
FIG. 9.
PKCβII-dependent SAPK activation in TPA-treated HeLa cells. HeLa cells were transfected with the indicated amounts (micrograms) of pEF2/PKCβII, pEF2/PKCδ, pEF2/neo, and HA-tagged SAPK. At 48 h posttransfection, the cells were left untreated or were treated with 16 nM TPA for 15 min. Cell lysates were immunoprecipitated with anti-HA antibody, and the anti-HA antibody immunoprecipitates were assayed for phosphorylation of GST-Jun. Lysates were also subjected to immunoblot analysis with anti-PKCβII, anti-HA, and anti-PKCδ antibodies to assess the levels of expression of transfected PKCβII, HA-tagged SAPK, and PKCδ (lower panels). Panel A shows a dose dependence on PKCβII expression level, and panel B shows the specificity of PKCβII in comparison with PKCδ.
FIG. 10.
TPA-induced activation of SAPK by a PKCβII- and MEKK-1-dependent mechanism. (A) HeLa cells were transfected with the indicated amounts of pEF2/PKCβII, pEF2/neo, pEF2/Flag-MEKK-1 (K-M), and HA-tagged SAPK. At 48 h posttransfection, cells were left untreated or were treated with 16 nM TPA for 15 min. Anti-HA antibody immunoprecipitates were assayed for phosphorylation of GST-Jun. Lysates of the transfected cells were also subjected to immunoblot analysis with anti-PKCβII, anti-Flag M2, and anti-HA antibodies to assess the levels of expression of transfected PKCβII, Flag-MEKK-1 (K-M), and HA-tagged SAPK. The levels of GST-Jun phosphorylation were quantitated on the basis of the intensity of the signals, and the results are expressed as the mean ± standard error of three independent experiments (lowest panel). (B) HeLa cells were transfected with the indicated amounts of pEF2/PKCβII, pEF2/neo, and pEF2/Flag-MEKK-1 (K-M). HA-tagged SAPK was not transfected in this experiment. At 48 h posttransfection, cells were left untreated or were treated with 16 nM TPA for 15 min. Anti-SAPK antibody immunoprecipitates were assayed for phosphorylation of GST-Jun. (C) HeLa cells were transfected with the indicated amounts of pEF2/PKCβII, pEF2/neo, pEF2/Flag-MEKK-1 (K-M), and pEBG/c-Raf-1 (K-M). At 48 h posttransfection, cells were left untreated or were treated with 16 nM TPA for 15 min. Anti-SAPK antibody immunoprecipitates were analyzed for phosphorylation of GST-Jun.
DISCUSSION
Role for PKCβII in TPA-induced SAPK activity and monocytic differentiation.
Initial studies demonstrated that treatment of human myeloid leukemia cells with TPA and other activators of PKC is associated with induction of monocytic differentiation (6). These findings indicated that the growth factor-independent phenotype of myeloid leukemia cells is reversible. Although certain insights were available regarding the involvement of PKC activation in inducing leukemia cell differentiation, the precise roles, if any, of the 12 known isoforms of the PKC family in this process have been unclear. Significantly, myeloid leukemia cells resistant to TPA-induced differentiation were found to be deficient in PKCβ expression (19, 37, 42, 56, 57). Also, induction of PKCβ restored TPA-induced growth arrest and monocytic differentiation (56, 64).
The present results demonstrated that TPA-induced SAPK activation is defective in PKCβ-deficient TUR and HL-525 cells. Similar defects in SAPK activation were observed for TUR and HL-525 cells when PDBu and bryostatin 1 were used as activators of PKC. In contrast, TUR and HL-525 cells responded to okadaic acid, an inhibitor of phosphoserine/threonine phosphatases, with activation of SAPK. Other studies have demonstrated that TUR and HL-525 cells respond to okadaic acid with induction of monocytic differentiation (19, 50). These findings demonstrated that leukemia cells deficient in PKCβ retain the capacity to differentiate along the monocytic lineage through certain agents that induce signals other than the activation of PKC. The involvement of PKCβ and, particularly, PKCβII in TPA-induced monocytic differentiation was directly supported by stable transfection of a PKCβII expression vector in TUR and HL-525 cells. The PKCβII transfectants responded to TPA with the activation of SAPK, growth arrest, and the appearance of a differentiated monocytic phenotype. These findings thus support a role for PKCβII in both TPA-induced SAPK activity and monocytic differentiation.
Previous studies showed that TPA has little, if any, effect on SAPK activation in diverse cell types, including epithelial HeLa cells (12, 33, 38, 63). In contrast, TPA effectively activates SAPK in human myeloid leukemia cells (14–16, 20, 44). However, the events responsible for cell type-specific induction of SAPK activation by TPA have been unclear. PKCβ expression is undetectable in NIH 3T3 cells (39) and HeLa cells (5), which are unresponsive to TPA in terms of SAPK activation. Together with the present results, these findings indicate that PKCβ expression is necessary for TPA-induced SAPK activation.
Interaction of PKCβII with MEKK-1 in TPA-treated myeloid leukemia cells.
MEKK-1 is distinct from the MEK activator Raf and functions as an upstream effector of the SAPK pathway (38, 63). Recent studies demonstrated that MEKK-1 is cleaved by caspases during the induction of anoikis or apoptosis associated with the loss of integrin-mediated contacts (4). The cleavage of MEKK-1 is blocked by the cowpox virus CrmA protein, which inhibits certain caspases (4). In U-937 cells, which grow in suspension, MEKK-1 is constitutively expressed as an ∼80-kDa form. Overexpression of CrmA in U-937 cells (9) has no apparent effect on the expression of the ∼80-kDa form of MEKK-1 (data not shown). Similarly, U-937 cells that overexpress the p35 caspase inhibitor (9) or the antiapoptotic Bcl-xL protein (10) also express only the ∼80-kDa form of MEKK-1 (data not shown). These findings suggest that in U-937 cells, the expression of MEKK-1 as an ∼80-kDa protein is due to mechanisms other than caspase cleavage.
The present results demonstrate that treatment of U-937 cells with TPA is associated with the induction of PKCβII binding to the ∼80-kDa form of MEKK-1. Whereas cleavage can contribute, at least in part, to the activation of MEKK-1 (4), other events involving phosphorylation may be required by upstream effectors. In this context, our in vitro studies with the ∼80-kDa form of MEKK-1 provide support for activation by PKCβII. Studies with cells also provide support for a functional interaction between PKCβII and MEKK-1. TPA-induced activation of SAPK in HeLa cells was dependent on PKCβII expression, and this response was blocked by a dominant negative MEKK-1 mutant. These findings could also be explained by an indirect interaction between PKCβII and MEKK-1 that, for example, involves other proteins which are activated by PKCβII and function as upstream effectors of MEKK-1. However, the binding of PKCβII to MEKK-1 in vitro and the PKCβII-induced activation of MEKK-1 suggest that the interaction between these proteins is direct.
Role for PKCβ in induction of monocytic differentiation.
Previous work showed that monocytic differentiation of myeloid leukemia cells is associated with the induction of c-jun, junB, c-fos, and EGR-1 expression (11, 29, 47, 49). The absence of jun, fos, and EGR-1 gene induction in TPA-treated TUR cells supports a defect in upstream signals that confer the activation of these genes (19, 30). HL-525 cells also exhibit attenuated induction of c-jun and c-fos transcripts in response to TPA treatment (8). The finding that the stable introduction of PKCβII expression in TUR and HL-525 cells restores TPA induction of monocytic differentiation suggests that the defect in the induction of early-response gene expression is due to a PKCβ deficiency. Indeed, TUR and HL-525 cells that stably express the PKCβII vector respond to TPA with induction of the c-jun and EGR-1 genes (data not shown).
Induction of the c-fos gene may not be obligatory for the TPA-induced monocytic differentiation of myeloid leukemia cells (40). In contrast, other studies have demonstrated that the induction of Jun/AP-1 activity and the c-jun gene is functionally related to the induction of monocytic differentiation (54). EGR-1 expression has also been found to be essential for differentiation along the monocytic lineage (41). Thus, the induction of diverse early-response genes is probably required for the activation of signals responsible for the appearance of the monocytic phenotype. Whereas SAPK phosphorylates the c-Jun, ATF2, and Elk-1 transcription factors, which contribute to the induction of early-response gene expression, activation of the SAPK pathway by differentiating agents, such as TPA, may contribute to reversal of the phenotype that characterizes myeloid leukemia cells. This notion is consistent with the previous observation that c-jun overexpression in U-937 cells induces partial differentiation and facilitates differentiation induced by TPA (53). However, there is no direct evidence that SAPK activation is essential for the induction of monocytic differentiation. The present findings provide support for the involvement of PKCβ as an upstream effector of SAPK activation, early-response gene expression, and induction of myeloid leukemia cell differentiation.
ACKNOWLEDGMENTS
We thank M. Cobb for HA-tagged full-length MEKK-1, L. Zon and J. Kyriakis for HA-SAPK, S. Ohno for the kinase-inactive MEKK-1 (K-M) mutant, A. Yamakawa for pSuperCatch, G. Johnson for anti-MEKK-1 antibodies, G. Tzivion and J. Avruch for c-Raf-1 (K-M), and G. Petit for bryostatin 1.
This investigation was supported by Public Health Service grants CA42802 and CA68252 awarded by the National Cancer Institute.
REFERENCES
- 1.Adunyah S E, Unlap T M, Franklin C C, Kraft A S. Induction of differentiation and c-jun expression in human leukemic cells by okadaic acid, an inhibitor of protein phosphatases. J Cell Physiol. 1992;151:415–426. doi: 10.1002/jcp.1041510223. [DOI] [PubMed] [Google Scholar]
- 2.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 3.Blumer K J, Johnson G L, Lange-Carter C A. Mammalian mitogen-activated protein kinase kinase kinase (MEKK) can function in a yeast mitogen-activated protein kinase pathway downstream of protein kinase C. Proc Natl Acad Sci USA. 1994;91:4925–4929. doi: 10.1073/pnas.91.11.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S M. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 5.Chun J S, Jacobson B S. Differential translocation of protein kinase C ɛ during HeLa cell adhesion to gelatin substratum. J Biol Chem. 1996;271:13008–13012. doi: 10.1074/jbc.271.22.13008. [DOI] [PubMed] [Google Scholar]
- 6.Collins S J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 7.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Datta R, Hallahan D, Kharbanda S, Rubin E, Sherman M, Huberman E, Weichselbaum R, Kufe D. Involvement of reactive oxygen intermediates in the induction of c-jun gene transcription by ionizing radiation. Biochemistry. 1992;31:8300–8306. doi: 10.1021/bi00150a025. [DOI] [PubMed] [Google Scholar]
- 9.Datta R, Kojima H, Banach D, Bump N J, Talanian R V, Alnemri E S, Weichselbaum R R, Wong W W, Kufe D W. Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis. J Biol Chem. 1997;272:1965–1969. doi: 10.1074/jbc.272.3.1965. [DOI] [PubMed] [Google Scholar]
- 10.Datta R, Manome Y, Taneja N, Boise L H, Weichselbaum R R, Thompson C B, Slapak C A, Kufe D W. Overexpression of Bcl-xL by cytotoxic drug exposure confers resistance to ionizing radiation-induced internucleosomal DNA fragmentation. Cell Growth Differ. 1995;6:363–370. [PubMed] [Google Scholar]
- 11.Datta R, Sherman M L, Stone R M, Kufe D. Expression of the jun-B gene during induction of monocytic differentiation. Cell Growth Differ. 1991;2:43–49. [PubMed] [Google Scholar]
- 12.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 14.Franklin C C, Kraft A S. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 15.Franklin C C, Kraft A S. Constitutively active MAP kinase kinase (MEK1) stimulates SAP kinase and c-Jun transcriptional activity in U937 human leukemic cells. Oncogene. 1995;11:2365–2374. [PubMed] [Google Scholar]
- 16.Franklin C C, Unlap T, Adler V, Kraft A S. Multiple signal transduction pathways mediate c-Jun protein phosphorylation. Cell Growth Differ. 1993;4:377–385. [PubMed] [Google Scholar]
- 17.Georgiev O, Bourquin J P, Gstaiger M, Knoepfel L, Schaffner W, Hovens C. Two versatile eukaryotic vectors permitting epitope tagging, radiolabelling and nuclear localisation of expressed proteins. Gene. 1996;168:165–167. doi: 10.1016/0378-1119(95)00764-4. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Campbell D, Dérijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 19.Hass R, Hirano M, Kharbanda S, Rubin E, Kufe D. Resistance to phorbol ester-induced differentiation of a U-937 myeloid leukemia cell variant with a signaling defect upstream to Raf-1 kinase. Cell Growth Differ. 1993;4:657–663. [PubMed] [Google Scholar]
- 20.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 21.Hirano M, Osada S-I, Aoki T, Hirai S-I, Hosaka M, Inoue J-I, Ohno S. MEK kinase is involved in tumor necrosis factor α-induced NF-κB activation and degradation of IκB-α. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 22.Hocevar B A, Fields A P. Selective translocation of βII-protein kinase C to the nucleus of human promyelocytic (HL60) leukemia cells. J Biol Chem. 1991;266:28–33. [PubMed] [Google Scholar]
- 23.Homma Y, Henning-Chubb C B, Huberman E. Translocation of protein kinase C in human leukemia cells susceptible or resistant to differentiation induced by phorbol 12-myristate 13-acetate. Proc Natl Acad Sci USA. 1986;83:7316–7319. doi: 10.1073/pnas.83.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 25.Kharbanda S, Datta R, Rubin E, Nakamura T, Hass R, Kufe D. Regulation of c-jun expression during induction of monocytic differentiation by okadaic acid. Cell Growth Differ. 1992;3:391–399. [PubMed] [Google Scholar]
- 26.Kharbanda S, Ren R, Pandey P, Shafman T D, Feller S M, Weichselbaum R R, Kufe D W. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 27.Kharbanda S, Rubin E, Datta R, Hass R, Sukhatme V, Kufe D. Transcriptional regulation of the early growth response 1 gene in human myeloid leukemia cells by okadaic acid. Cell Growth Differ. 1993;4:17–23. [PubMed] [Google Scholar]
- 28.Kharbanda S, Saleem A, Shafman T, Emoto Y, Weichselbaum R, Woodgett J, Avruch J, Kyriakis J, Kufe D. Ionizing radiation stimulates a Grb2-mediated association of the stress activated protein kinase with phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:18871–18874. doi: 10.1074/jbc.270.32.18871. [DOI] [PubMed] [Google Scholar]
- 29.Kharbanda S M, Nakamura T, Stone R, Hass R, Bernstein S, Datta R, Sukhatme V, Kufe D. Expression of the early growth response 1 and 2 zinc finger genes during induction of monocytic differentiation. J Clin Investig. 1991;88:571–577. doi: 10.1172/JCI115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharbanda S M, Saleem A, Hirano M, Emoto Y, Sukhatme V, Blenis J, Kufe D. Activation of early growth response 1 gene transcription and pp90rsk during induction of monocytic differentiation. Cell Growth Differ. 1994;5:259–265. [PubMed] [Google Scholar]
- 31.Kiley S C, Adams P D, Parker P J. Cloning and characterization of phorbol ester differentiation-resistant U937 cell variants. Cell Growth Differ. 1997;8:221–230. [PubMed] [Google Scholar]
- 32.Kubo K, Ohno S, Suzuki K. Primary structures of human protein kinase C beta I and beta II differ only in their C-terminal sequences. FEBS Lett. 1987;223:138–142. doi: 10.1016/0014-5793(87)80524-0. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 34.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 35.Lee K S, Levin D E. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol. 1992;12:172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin A, Minden A, Martinetto H, Claret F-X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 37.MacFarlane D E, Manzel L. Activation of β-isozyme of protein kinase C (PKCβ) is necessary and sufficient for phorbol ester-induced differentiation of HL-60 promyelocytes. J Biol Chem. 1994;269:4327–4331. [PubMed] [Google Scholar]
- 38.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 39.Mischak H, Goodnight J, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase c-δ and -ɛ in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 40.Mitchell T, Sariban E, Kufe D. Effects of 1-β-d-arabinofuranosylcytosine on proto-oncogene expression in human U-937 cells. Mol Pharmacol. 1986;30:398. [PubMed] [Google Scholar]
- 41.Nguyen H Q, Liebermann H B, Liebermann D A. The zinc finger transcription factor EGR-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa M, Komada F, Uemura Y, Hidaka H, Shirakawa S. Decreased expression of type II protein kinase C in HL-60 variant cells resistant to induction of cell differentiation by phorbol diester. Cancer Res. 1990;50:621–626. [PubMed] [Google Scholar]
- 43.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 44.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Phosphorylation of c-Jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 45.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 47.Sariban E, Mitchell T, Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985;316:64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- 48.Seimiya H, Sawabe T, Toho M, Tsuruo T. Phorbol ester-resistant monoblastoid leukemia cells with a functional mitogen-activated protein kinase cascade but without responsive protein tyrosine phosphatases. Oncogene. 1995;11:2047–2054. [PubMed] [Google Scholar]
- 49.Sherman M, Stone R, Datta R, Bernstein S, Kufe D. Transcriptional and post-transcriptional regulation of c-jun expression during monocytic differentiation of human myeloid leukemia cells. J Biol Chem. 1990;265:3320–3323. [PubMed] [Google Scholar]
- 50.Slapak C, Kharbanda S, Saleem A, Kufe D W. Defective translocation of protein kinase C in multidrug resistant HL-60 cells confers a reversible loss of phorbol ester-induced monocytic differentiation. J Biol Chem. 1993;268:12267–12273. [PubMed] [Google Scholar]
- 51.Stone R M, Sariban E, Pettit G R, Kufe D W. Bryostatin 1 activates protein kinase C and induces monocytic differentiation of HL-60 cells. Blood. 1988;72:208–213. [PubMed] [Google Scholar]
- 52.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 53.Szabo E, Preis L H, Birrer M J. Constitutive cJun expression induces partial macrophage differentiation in U-937 cells. Cell Growth Differ. 1994;5:439–446. [PubMed] [Google Scholar]
- 54.Szabo E, Preis L H, Brown P H, Birrer M J. The role of jun and fos gene family members in 12-O-tetradecanoylphorbol-13-acetate induced hemopoietic differentiation. Cell Growth Differ. 1991;2:475–482. [PubMed] [Google Scholar]
- 55.Takekawa M, Posas F, Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 1997;16:4973–4982. doi: 10.1093/emboj/16.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tonetti D A, Henning-Chubb C, Yamanishi D T, Huberman E. Protein kinase C-β is required for macrophage differentiation of human HL-60 leukemia cells. J Biol Chem. 1994;269:23230–23235. [PubMed] [Google Scholar]
- 57.Tonetti D A, Horio M, Collart F R, Huberman E. Protein kinase C beta gene expression is associated with susceptibility of human promyelocytic leukemia cells to phorbol ester-induced differentiation. Cell Growth Differ. 1992;3:739–745. [PubMed] [Google Scholar]
- 58.Tzivion G, Luo Z, Avruch J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 1998;394:88–92. doi: 10.1038/27938. [DOI] [PubMed] [Google Scholar]
- 59.Whalen A M, Galasinski S C, Shapiro P S, Nahreini T S, Ahn N G. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 61.William F, Wagner F, Karin M, Kraft A S. Multiple doses of diacylglycerol and calcium ionophore are necessary to activate AP-1 enhancer activity and induce markers of macrophage differentiation. J Biol Chem. 1990;265:18166–18171. [PubMed] [Google Scholar]
- 62.Xu S, Robbins D J, Christerson L B, English J M, Vanderbilt C A, Cobb M H. Cloning of rat MEK kinase 1 cDNA reveals an endogenous membrane-associated 195-kDa protein with a large regulatory domain. Proc Natl Acad Sci USA. 1996;93:5291–5295. doi: 10.1073/pnas.93.11.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 64.Yang K D, Kharbanda S, Datta R, Huberman E, Kufe D, Stone R. All-trans retinoic acid reverses phorbol ester resistance in a human myeloid leukemia cell line. Blood. 1994;83:490–496. [PubMed] [Google Scholar]