Abstract
Abstract
Over 30 years, the Gram-positive bacterium Priestia megaterium (previously known as Bacillus megaterium) was systematically developed for biotechnological applications ranging from the production of small molecules like vitamin B12, over polymers like polyhydroxybutyrate (PHB) up to the in vivo and in vitro synthesis of multiple proteins and finally whole-cell applications. Here we describe the use of the natural vitamin B12 (cobalamin) producer P. megaterium for the elucidation of the biosynthetic pathway and the subsequent systematic knowledge-based development for production purposes. The formation of PHB, a natural product of P. megaterium and potential petro-plastic substitute, is covered and discussed. Further important biotechnological characteristics of P. megaterium for recombinant protein production including high protein secretion capacity and simple cultivation on value-added carbon sources are outlined. This includes the advanced system with almost 30 commercially available expression vectors for the intracellular and extracellular production of recombinant proteins at the g/L scale. We also revealed a novel P. megaterium transcription-translation system as a complementary and versatile biotechnological tool kit. As an impressive biotechnology application, the formation of various cytochrome P450 is also critically highlighted. Finally, whole cellular applications in plant protection are completing the overall picture of P. megaterium as a versatile giant cell factory.
Key points
• The use of Priestia megaterium for the biosynthesis of small molecules and recombinant proteins through to whole-cell applications is reviewed.
• P. megaterium can act as a promising alternative host in biotechnological production processes.
Keywords: Priestia megaterium, Bacillus megaterium, Recombinant protein production, Vitamin B12, Cytochrome P450, Polyhydroxybutyrate (PHB), Plant growth-promoting bacterium, Cell-free transcription-translation
Introduction
Since its discovery in 1884 (de Bary 1884), Priestia megaterium (formerly known as Bacillus megaterium (Gupta et al. 2020)) provides a powerful cell factory for biotechnology, with numerous patents and applications in industry. The bacterium serves as a model organism for genetic studies and recombinant protein production (Vary 1992; Vary 1994). With its large size of up to 2.5 × 10 μm—“megaterium” literally means “big beast”—it has a significant larger volume compared to that of Escherichia coli (Vary et al. 2007) (Fig. 1).
Fig. 1.
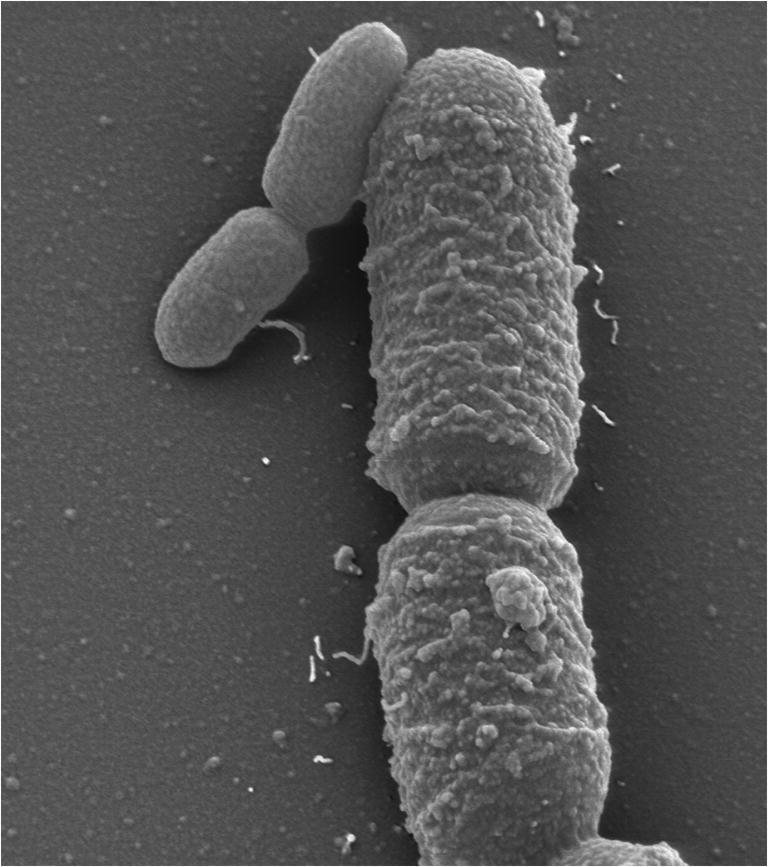
Electron microscope image of Priestia megaterium (large cells) and Escherichia coli (small cells). P. megaterium and E. coli were individually grown aerobically in rich medium at 37 °C, mixed in the middle of their exponential growth phases and examined in a field emission scanning electron microscope (FESEM) Zeiss DSM982 Gemini (magnification 6,500-fold). The picture was taken by Manfred Rohde, Helmholtz Centre for Infection Research, Braunschweig, Germany.
P. megaterium is a Gram-positive rod, has a low G+C (~38%) genome, and forms endospores. Its size alone has attracted microbiologists for many years to study its physiology and function including cell division, cell wall biosynthesis, and sporulation (Vary 1994). Now within the last decades, recent advances in modern molecular biology have unlocked its potential for biotechnology. With the expansion of synthetic biology, the many advantages of P. megaterium make it an attractive microbial cell factory to rival other model microbes such as E. coli and Bacillus subtilis (Eisenstein 2016). This review will provide an overview of the diverse potential of P. megaterium as a model Gram-positive organism for biotechnological applications including small molecules like cobalamin (vitamin B12), the polymer polyhydroxybutyrate (PHB), the production of diverse intra- and extracellular recombinant proteins, whole-cell transformations, and its function as a plant growth-promoting bacterium.
Priestia megaterium—history and genome sequencing
In October 2020, Gupta et al. showed that many Bacillus species in addition to the Subtilis and Cereus clade constitute a total of 17 new individual clades based on conserved signature indels (CSIs). They proposed that these clades should be recognized as new genera, with the name Priestia gen. nov. for the Megaterium clade containing the former Bacillus species B. megaterium, B. abyssalis, B. aryabhattai, B. endophyticus, B. filamentosus, B. flexus, and B. koreensis due to two CSIs in the oligoribonuclease NrnB which were uniquely shared by all clade members (Gupta et al. 2020).
P. megaterium can be found in diverse habits including honey (López and Alippi 2009), wine (von Cosmos et al. 2017), raw meat (Yucel et al. 2009), fish (Al Bulushi et al. 2010), sea water (Xu et al. 2014), the oral cavity of humans (Al-Thubiani et al. 2018), and most typically plants and soil (Dobrzanski et al. 2018). Consequently, its metabolism is adapted to a variety of different carbon sources including xylose (a byproduct of hemicellulose), glycerol (de Jesus et al. 2016; Korneli et al. 2013; Moreno et al. 2015), disaccharides such as cellobiose, maltose or sucrose (Youngster et al. 2017), and a range of cheap mixed saccharide sources such as sugarcane molasses (Kanjanachumpol et al. 2013).
The first genome sequences of two P. megaterium strains (DSM319 and QM B1551) were published a decade ago by Eppinger et al. (2011). Up to now, the full genome sequences including corresponding plasmids of around 20 distinct P. megaterium strains are available at the NCBI genome database. Five of these strains lack natural plasmids, while the remaining strains contain up to ten plasmids, consistent with studies already from the early 1980s which found plasmid-less strains to be an exception (Stahl and Esser 1983). The type strain DSM32 (ATCC14581) has been used to conduct basic genetic research. It is also known as the source of the cytochrome P450-BM3 (CYP102A1) (Narhi and Fulco 1986). The plasmid-less DSM319 and its variant MS941, which lacks the gene coding for the major extracellular protease NprM (Wittchen and Meinhardt 1995), are best suited candidates for plasmid-based genetic applications, including the generation of mutants and the recombinant production of proteins (Biedendieck et al. 2011). Strain QM B1551 is used in basic research, especially within the context of sporulation genetics (Manetsberger et al. 2018; Riyami et al. 2019). Strain WSH-002 has been used for co-cultivation with Ketogulonicigenium vulgare (Zhang et al. 2010) or Gluconobacter oxydans (Lü et al. 2003) to produce vitamin C (Liu et al. 2011). Strain NCT-2, which was isolated from salinization soil from greenhouses, shows high capacity in bioremediation in salinized soil (Wang et al. 2020). Similarly, strain Q3 was described as an endophytic quinclorac-degrading bacterium for bioremediation purposes (Liu et al. 2014), while strains YC4-R4 and TG1-E1 show high salt tolerance as plant growth-promoting rhizobacteria (Vílchez et al. 2018a; Vílchez et al. 2018b). Likewise, strain JX285 acts as plant growth-promoting bacterium, isolated from rhizospheric soil (Huang et al. 2019). Strain SR7 was identified in samples collected from a naturally supercritical carbon dioxide (scCO2)-rich environment. SR7 displays resistance to scCO2, which is considered to be a promising alternative to classical organic solvents and is already used in in vitro applications (Boock et al. 2019; Freedman et al. 2018).
P. megaterium in biotechnological production processes—from the biosynthesis of small molecules through to whole-cell applications
These different properties clearly highlight the diversity of P. megaterium and provide the prerequisite for its diverse applications ranging from the biosynthesis of small molecules, recombinant proteins, and biotransformations to whole-cell bioremediation. Table 1 provides a summary of recombinant proteins and other products produced using P. megaterium. Outlined data show that major applications in recombinant protein production with P. megaterium rely on a strong protein export system for secretion into the surrounding environment. Overall, proteins with biomedical applications like Clostridioides difficile toxins (Yang et al. 2008), protein vaccines (Wang et al. 2018), urokinase-like plasminogen activators (Rygus and Hillen 1991), antibody fragments (Jordan et al. 2007; Lakowitz et al. 2017), and penicillin G acylase (Mayer et al. 2019) constitute major extracellular products. Another important class of proteins is involved in the metabolism of various carbohydrates. It consists of levansucrases (Biedendieck et al. 2007a; Korneli et al. 2013; Malten et al. 2006), α-cyclodextrin glycosyltransferase (Zhou et al. 2012), dextransucrase (Malten et al. 2005b), xylanase (Zheng et al. 2012), glucose dehydrogenase (Rygus and Hillen 1991), β-galactosidase (Rygus and Hillen 1991), and mannitol dehydrogenase (Baumchen et al. 2007), to name a few. Furthermore, enzymes of vitamin B12 and heme biosynthesis (Biedendieck et al. 2010; Leech et al. 2003; Mobius et al. 2010; Moore et al. 2013a; Moore et al. 2014), reductive dehalogenases (Payne et al. 2015), and the model green fluorescent protein (GFP) (Biedendieck et al. 2007b; Biedendieck et al. 2007c; Gamer et al. 2009; Stammen et al. 2010a; Stammen et al. 2010b) complete the picture. Finally, the challenging cytochrome P450 enzymes, catalyzing for example stereospecific hydroxylation of steroids or vitamin D3, are naturally encoded by different P. megaterium genomes and were recombinantly produced using this bacterium (Abdulmughni et al. 2017a; Abdulmughni et al. 2017b; Bleif et al. 2012; Brill et al. 2014; Ehrhardt et al. 2016; Gerber et al. 2015).
Table 1.
Recombinant proteins and other products produced and secreted with Priestia megaterium
| Product | Features | Product titer | Reference |
|---|---|---|---|
| Intracellular | |||
| Glucose dehydrogenase (Gdh) | Native promoter of gdh | 30 U mL−1 | (Meinhardt et al. 1989) |
| Glucose dehydrogenase (Gdh) | PxylA | 101.1 U mgProtein−1 | (Rygus and Hillen 1991) |
| Mutarotase (Mro) | 73.7 U mgProtein−1 | ||
| Urokinase-like Plas-minogen activator (Puk) | 400 U mL−1 per optical density unit | ||
| β-Galactosidase | PxylA | 4,937 mU | (Rygus and Hillen 1991) |
| PxylAopt. | 5,200 mU | (Hartz et al. 2019) | |
| Promoter of gene of putative ferrous iron transport protein | 6,300 mU | ||
| Clostridioides difficile toxin TcdA | PxylA, size: 308 kDa, his-tag | 5-10 mg L−1 | (Yang et al. 2008) |
| Clostridioides difficile toxin TcdB | PxylA, size: 270 kDa, his-tag | 10 mg L−1 | |
| Chimeric protein vaccine Tcd169 | PxylA | n.d. | (Wang et al. 2018) |
| Chimeric protein vaccine Tcd169Fl | PxylA | n.d. | |
| Chloroform reductive dehalogenase | PT7, his-tag, purification, B12-cofactor | 180 mg L−1 (calculated) | (Jugder et al. 2018) |
| Reductive dehalogenase RdhANP plus mutants | PT7, his-tag, purification, B12-cofactor | n.d. | (Payne et al. 2015) |
| Green fluorescent protein (GFP) | PxylA, Strep-tag, fed-batch, 52 gCDW L−1 | 274 mg L−1 | (Biedendieck et al. 2007c) |
| PsacB | 7.9 mg gCDW−1 | (Biedendieck et al. 2007b) | |
| PxylAopt., fed-batch, 35 gCDW L−1, ΔxylA-mutant | 1.25 g L−1 | (Stammen et al. 2010a) | |
| PT7 | 50 mg L−1 | (Gamer et al. 2009) | |
| PK1E | 61.4 mg gCDW−1 | (Stammen et al. 2010b) | |
| CbiX | PxylA, his-tag, purification | n.d. | (Leech et al. 2003) |
| CbiH60 | PxylA, his-tag | n.d. | (Moore et al. 2013a) |
| HemG | PxylA, his-tag, purification | n.d. | (Mobius et al. 2010) |
| Extracellular | |||
| Clostridioides difficile toxin TcdB | PxylA, size: 270 kDa, his-tag, SPLipA | n.d. | (Yang et al. 2008) |
| Keratinase | PxylA, native signal peptide | 186.3 U mL−1 | (Radha and Gunasekaran 2008) |
| PamyL, native signal peptide | 171.3 U mL−1 | ||
| Levansucrase SacB | PxylAopt., glycerol as C-source | 520 mg L−1 | (Korneli et al. 2013) |
| PsacB | 4252.4 U L−1 | (Biedendieck et al. 2007b) | |
| Levansucrase LevΔ773 | PxylA, SPLipA | 4 mg L−1 | (Malten et al. 2006) |
| Levansucrase LevΔ773His | PxylA, SPLipA, his-tag | 2.1 mg L−1 | |
| Levansucrase StrepLevΔ773 | PxylA, SPLipA, strep-tag | 2.7 mg L−1 | |
| Dextransucrase DsrS | PxylA, size: 188 kDa, native signal peptide | 240 U L−1 | (Malten et al. 2005b) |
| α-Cyclodextrin glycosyltransferase | PxylA, SPLipA, codon optimized | 8.9 U mL−1 | (Zhou et al. 2012) |
| Thermobifida fusca hydrolase (TFH) | PxylAopt., SPYocH | 7,200 U L−1 (7.7 mg L−1) | (Stammen et al. 2010a) |
| Endoglucanase EGI1 | PT7, 5 different signal peptides, different media | 108 mg L−1 | (Kalbarczyk et al. 2018) |
| Multimodular cellulose Cel9AT | 52 mg L−1 | ||
| Xylanase | PxylA, his+strep-tag, purification | 304.26 IU mL−1 | (Zheng et al. 2012) |
| Thermostable xylanase | PxylA, his+strep-tag, purification | 106 IU mL−1 | (Sun et al. 2015) |
| ß-glucosidase (BglZ) | PxylA | Activity measured in cell extract | (Kurniasih et al. 2014) |
| Endoglucanase (EglII) | |||
| Fusion protein EglII-BglZ | |||
| Priestia megaterium penicillin G acylase (PGA) | PxylA, SPLipA, ΔxylA-mutant | 41 mg L−1 | (Yang et al. 2006) |
| P. megaterium PGA | PxylAopt., native signal peptide, purified from growth medium, crystallization | 500 U L−1 (20.6 mgpurified enzyme L−1) | (Mayer et al. 2019) |
| Bacillus species FJAT PGA | 550 U L−1 (30.2 mgpurified enzyme L−1) | ||
| Bacillus thermotolerans PGA | 220 U L−1 (15.2 mgpurified enzyme L−1) | ||
| Hybrid PGAs | PxylAopt., native signal peptide, purified from growth medium | n.d. | (Mayer et al. 2019) |
| Single chain PGAs | n.d. | ||
| Chimeric versions of S-layer protein SslA | PxylA, cell surface display | n.d. | (Knobloch et al. 2012) |
| Antibody fragment scFV(D1.3) α-lysozyme | PxylAopt., SPLipA, his-tag, micro-bioreactor | 14 mg L−1 | (Lakowitz et al. 2017) |
| Antibody fragment D1.3 scFab α-lysozyme | PxylA, SPLipA, his-tag | 3.5 μg L−1 | (Jordan et al. 2007) |
| Whole-cell systems | |||
| Homolog Cbi-enzymes for cobalamin biosynthesis | PxylA, overexpression of 14 gene cbi-operon | Used for cobalamin production (220 μg L−1) | (Moore et al. 2014) |
| HemA | PxylA | Used for cobalamin production (2.8 μg L−1) | (Biedendieck et al. 2010) |
| HemAXCDBL | PxylA, integrated upstream of operon | Used for cobalamin production (8.5 μg L−1) | (Biedendieck et al. 2010) |
| Mannitol dehydrogenase (MDH) and formate dehydrogenase (FDH) | PxylA, 2 gene operon, codon optimized | Whole-cell transformation for D-mannitol production (22 g L−1) | (Baumchen et al. 2007) |
| Cytochrome P450 CYP106A1 | PxylAopt., coproduction with reductase Arh1 and a redox partner | Whole-cell transformation for hydroxylation of 11-keto-β-boswellic acid to 15α-hydroxy-KBA (560.7 mg L−1 day−1) | (Brill et al. 2014) |
| Cytochrome P450 CYP106A2 | PxylAopt., coproduction with redox partners AdR and Adx | Whole-cell transformation for hydroxylation of 11-keto-β-boswellic acid to 15α-hydroxy-KBA (560.7 mg L−1 day−1) | (Bleif et al. 2012) |
| Cytochrome P450 CYP109A2 | PxylAopt. | Whole-cell transformation for the conversion of vitamin D3 to 25-hydroxyvitamin D3 (54.9 mg L−1 day−1) | (Abdulmughni et al. 2017a) |
| Cytochrome P450 CYP109E1 | PxylAopt. | Whole-cell transformation for the conversion of vitamin D3 to 25-hydroxyvitamin D3 (24.5 mg L−1 day−1) | (Abdulmughni et al. 2017b) |
| Bovine cytochrome P450 CYP11A1 | PxylAopt., coproduction with redox partners AdR and Adx, codon optimized | Whole-cell transformation for the conversion of cholesterol and analogs (up to 116 mg L−1 48 h−1) | (Gerber et al. 2015) |
| Human cytochrome P450 CYP27A1 | PxylAopt., coproduction with redox partners AdR and Adx, codon optimized | Whole-cell transformation hydroxylation of cholesterol, vitamin D3 and 7-dehydrocholesterol (up to 113.14 mg L−1 48 h−1) | (Ehrhardt et al. 2016) |
Production of small molecules: cobalamin (vitamin B12) in P. megaterium
P. megaterium is a natural producer of vitamin B12 (cobalamin) and has played a prominent role in the study of cobalamin biosynthesis and its industrial production. Cobalamin is a key vitamin for higher eukaryotes, which take it from their diet and require it for B12-dependent enzymes (Banerjee and Ragsdale 2003). In nature, cobalamin is only produced by certain species of bacteria and archaea. Derived from the tetrapyrrole family, cobalamin contains a central cobalt ion octahedrally coordinated between four pyrrole nitrogens, a lower ligand (DMB, 5,6-dimethylbenzimidazole) and an interchangeable upper ligand (adenosyl or methyl group). Vitamin B12 is officially named cyanocobalamin, where the upper ligand is replaced by cyanide during downstream processing (cyanide extraction), after microbial fermentation. However, the biologically active forms for cobalamin-dependent enzymes are either adenosylcobalamin (coenzyme B12) or methylcobalamin (cofactor B12). Microbes typically use cobalamin as a prosthetic group for enzymes in primary and secondary metabolism. P. megaterium possesses a number of cofactor B12 or coenzyme B12-dependent enzymes that aid its survival in the environment. This includes ribonucleotide reductase (NrdJ), methionine synthase (MetH), methylmalonyl CoA mutase (MutAB), and ethanolamine lyase (EutBC). In particular, the coenzyme B12-dependent EutBC assimilates ethanolamine as a sole carbon and nitrogen source (Roof and Roth 1989; Wolf and Brey 1986).
P. megaterium as a model to study genetics and biosynthesis of cobalamin
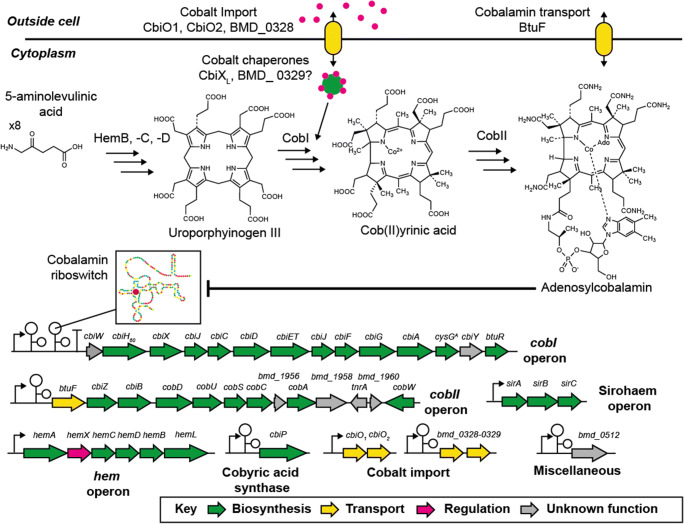
The biosynthesis of cobalamin is complex and requires about 30 enzymes. Therefore, several decades of research has been required to fully decipher the biosynthesis of cobalamin. Originally, P. megaterium was identified as a natural producer of cobalamin by studying its growth on ethanolamine, with auxotroph mutants deficient in cobalamin biosynthesis (Wolf and Brey 1986). Since then, it has provided a suitable model to study the biosynthesis of cobalamin. The biosynthesis of cobalamin in P. megaterium can be summarized in three stages: stage 1, the synthesis of uroporphyrinogen III; stage 2, assembly of the corrin ring; and stage 3, attachment of the upper and lower axial ligands to the central cobalt ion (Warren et al. 2002). For P. megaterium DSM319, the majority of its cobalamin genes are organized into the following biosynthetic operons: hem (stage 1), cobI (stage 2), and cobII (stage 3) (Eppinger et al. 2011) (Fig. 2). An exception to this rule includes the genes encoding for cobalt transport (bmd_0328, cobO1, cobO2) and a cobyric acid synthetase (cbiP), which are located separately within the genome (Fig. 2). Stage 1 uroporphyrinogen III (uro’gen III) biosynthesis is encoded by the hem operon (hemAXCDBL) in P. megaterium, whose genetic structure is similar to B. subtilis (Hansson et al. 1991). Then, cobalamin is built in two stages. For the first stage, P. megaterium operates the so-called anaerobic pathway to insert cobalt (Raux et al. 1998; Scott 2001) and build the corrin ring to achieve the first oxygen stable intermediate, cobyrinic acid (Moore et al. 2013b). Then the final steps in cobalamin biosynthesis attach the lower and upper axial cobalt ligands. This final stage is found in many prokaryotic lineages, since it also permits salvaging of vitamin B12 precursors (Maggio-Hall and Escalante-Semerena 1999). Distinctly, P. megaterium assembles the lower ligand (5,6-dimethylbenzimidazole) through an aerobic pathway for the final stages of its assembly (Collins et al. 2013). Therefore, P. megaterium has a customized cobalamin pathway to suit its requirement for molecular oxygen at different stages.
Fig. 2.
Summary of cobalamin genetics, biosynthesis, and regulation in Priestia megaterium DSM319. Upper part: cobalt and cobalamin transporters are indicated in yellow, cobalt in pink, and cobalt chaperon in green. Middle part: summary of cobalamin biosynthesis starting from 8 molecules of 5-aminolevulinic acid. The final product here is shown as adenosylcobalamin which can interact with the cobalamin riboswitches. CobI and CobII indicate all enzymes encoded by the cobI and cobII operons shown below. Lower part: all genes are represented as colored arrows. Black arrows upstream of the operons/single genes indicate promoters, black “T”s terminators and black stem-loop structures cobalamin riboswitches. All genes clustered in operons or situated on their own are annotated. Hypothetical genes are annotated as open reading frames (bmd_0000) as shown in www.megabac.tu-bs.de.
P. megaterium metabolic engineering of cobalamin production
Since cobalamin requires approximately 60 chemical steps for its total synthesis (Battersby 2000), it is essential for biotechnology to make cobalamin through microbial production. P. megaterium provides an excellent host for producing cobalamin (Biedendieck et al. 2010; Martens et al. 2002; Moore et al. 2013a; Moore et al. 2013b; Moore et al. 2014). For example, while P. megaterium wild-type strains (DSM319, DSM509, and QM B1551) make only low levels (~0.2–1.0 μg L−1) of cobalamin in the lab, unpublished industrial strains are believed to reach up to 300 mg L−1 (Martens et al. 2002). Since cobalamin biosynthesis is complex (requires 30 enzymes), there are several bottlenecks that limit its production. This includes biosynthesis of precursors, import of cobalt (Fig. 2), feedback regulation, and rate-limiting enzymes. We will discuss how these individual steps can be optimized in P. megaterium.
The supply of precursors such as uroporphyrinogen III (uro’gen III) for the main tetrapyrrole scaffold and S-adenosyl-L-methionine (SAM) for methylation represents a major bottleneck for cobalamin biosynthesis in P. megaterium. For example, glutamyl-tRNA reductase (HemA) is regulated at both the transcriptional and post-translation level (Schobert and Jahn 2002), through a negative feedback mechanism in heme biosynthesis. Overexpression of a proteolysis-resistant hemA mutant in P. megaterium increases cobalamin levels 11-fold to 2.8 μg L−1 (Biedendieck et al. 2010). Furthermore, to increase uro’gen III supply directly, chromosomal overexpression of the uro’gen biosynthesis operon (hemAXCDBL operon) increases cobalamin levels up to 8.5 μg L−1. Therefore, the supply of 5-aminolevulinic acid (5-ALA) and uroporphyrinogen III is a major limiting factor in cobalamin biosynthesis.
Cobalt is essential for cobalamin biosynthesis (Martens et al. 2002). Crucially, like any transition metal, regulation is required to avoid toxicity. P. megaterium has a range of unique regulatory features to control cobalt levels. In P. megaterium DSM319, the addition of cobalt (1–10 μM) alone increases cobalamin levels up to 13 μg L−1 (Moore et al. 2014). However, cobalt homeostasis and its incorporation into cobalamin biosynthesis is poorly understood. At the enzyme level, the cobaltochelatase CbiXL inserts cobalt into the tetrapyrrole macrocycle and may play a role in cobalt homeostasis (Fig. 2). Overproducing CbiXL in P. megaterium DSM509 in the presence of cobalt increases cobalamin levels by 6-fold (Biedendieck et al. 2010). Interestingly, CbiXL has an extended C-terminal domain that harbors a 4Fe-4S cluster and polyhistidine-rich motif (Leech et al. 2003). While the C-terminal extension is not essential for its chelatase activity (Leech et al. 2003), it may regulate or sense cobalt levels. For example, cobalt can substitute iron in Fe-S clusters (Ranquet et al. 2007) and polyhistidine motifs coordinate transition metals. For cobalt transport, P. megaterium has two potential cobalt transporters from the cbiO ATPase family or a single-component dual cobalt and nickel transporter (bmd_0328) (Komeda et al. 1997). Interestingly, bmd_0328 is part of two gene operons, containing an uncharacterized gene with another shorter polyhistidine motif (HXXXHH) (bmd_0329). Both genes are co-localized with an upstream cobalamin riboswitch (Fig. 2), suggesting their role in cobalamin biosynthesis. B12 riboswitches are cis-regulatory RNA elements that provide tight negative feedback control when cobalamin levels are high by sequestering either the Shine-Dalgarno site or by forming a transcription attenuator. While the role of bmd_0329 is unknown, overexpression of bmd_0328 in P. megaterium DSM319 leads to growth sensitivity in the presence of cobalt, suggesting increased cobalt import and toxicity (Moore 2011).
Unlike most prokaryotic metabolic pathways, there are no known specific transcription factors to regulate gene expression of cobalamin biosynthesis. Instead, global signals such as molecular oxygen repress cobalamin biosynthetic genes in Salmonella typhimurium (Escalante-Semerena and Roth 1987). Intriguingly, overproduction of the global anaerobic respiratory regulator FNR (fumarate and nitrate reductase regulator) in P. megaterium DSM509 increased cobalamin synthesis by 4-fold (Biedendieck et al. 2010), suggesting that cobalamin biosynthesis is globally regulated by oxygen. Instead of transcription factors, cobalamin biosynthesis is regulated by cobalamin riboswitches. The P. megaterium DSM319 genome contains eight cobalamin riboswitches. This includes genes encoding B12-independent enzymes (metE, nrdEF), complete pathways (cobI and cobII operons), cobyric acid synthetase (cbiP), and cobalt homeostasis genes (bmd_0328-bmd_0329, bmd_0512 (see above)) (Fig. 2). The cobI operon is regulated by a cobalamin riboswitch and transcription terminator and is highly sensitive (nM levels) to cobalamin (Moore et al. 2014). This is not surprising since prokaryotic cells only require trace levels of cobalamin for unrestricted growth. Instead scavenging cobalamin from the environment (Nahvi et al. 2004) is also supported by an ABC transporter btuF (located within the cobII operon) and an uncharacterized transporter (bmd_0512), both co-localized with a cobalamin riboswitch (Fig. 2).
The cobalamin riboswitches represent the major bottleneck in engineering cobalamin biosynthesis in P. megaterium (Fig. 2). To bypass this metabolic feedback, the entire cobI operon was placed under the control of a constitutive promoter on a multi-copy plasmid. Remarkably, expression in P. megaterium DSM319 in the presence of 10 μM cobalt led to major increases in cobalamin levels to 220 μg L−1, a 27.5-fold increase over the control strain (Moore et al. 2014).
Production of biopolymers using P. megaterium: polyhydroxybutyrate (PHB)
Polyhydroxyalkanoates (PHAs) are naturally occurring biopolymers synthesized by many microorganisms in response to environmental stress. They are considered to have promising potential to substitute traditional petrol-based plastics, as these so-called bioplastics show similar chemical and physical properties as conventional plastic (Chen 2009; Lu et al. 2009). PHAs were described in 1926 by the French scientist Lemoigne, who observed that P. megaterium accumulated polyhydroxybutyrate (PHB), a specific form of PHA, in the cells as distinct granules (Lemoigne 1926). Inside the cells, PHB acts as a storage device for carbon and energy and can be used again when conditions change. The hydrophobic granules are surrounded by a phospholipid monolayer in which a number of specific proteins are embedded, thereby associating with the granules (Jendrossek 2009). For the biological synthesis of PHAs, a variety of different C-sources, even crude waste material like glycerol derived from biofuel production, can be used (de Jesus et al. 2016; Naranjo et al. 2013; Solaiman et al. 2006). The key step in this process, the enzymatic polymerization of hydroxyacyl-coenzyme A (CoA) to PHA and CoA, is catalyzed by a PHA synthase. In 2001, McCool and Cannon identified the genetic organization of the five involved P. megaterium genes in two divergent orientated operons consisting of phaRBC and phaQP (McCool 2001). The phaC and phaR genes encode the two subunits of the PHA synthase (McCool 2001; Tsuge et al. 2015). Within the heterodimer, PhaC is the catalytic subunit localized with the granules, while PhaR is needed for polymerization (McCool 2001). PhaR from P. megaterium should not be confused with PhaR from other organisms like Ralstonia eutropha, where the name designates a transcriptional regulator of PHB synthesis (Lee et al. 2004). For the protein PhaB, a NADPH-dependent acetoacetyl coenzyme A reductase function was proposed. PhaB is involved in the supply of (R)-3HB-CoA monomer for the polymerization of PHB (Tsuge et al. 2015). PhaP as a phasin is localized with the granules (McCool and Cannon 1999). These non-enzymatic proteins are commonly found in PHA producers and have been shown to influence the PHA granule morphology and size (Jendrossek 2009). In P. megaterium the phaQ gene codes for a transcriptional regulator that negatively regulates the expression of phaP and phaQ. It interacts directly with PHB like PhaR in R. eutropha, although it has evolved independently (Lee et al. 2004). To ensure the abundant occurrence of phasin PhaP, but secure the required low level of the regulator PhaQ, Lee et al. speculate that the phaQ mRNA, as part of the phaQP transcript, is systematically degraded (Lee et al. 2004).
Optimizing PHB production in P. megaterium
To date, there are hardly any attempts described to develop P. megaterium toward an increased production of PHB through genetic engineering. In contrast much effort has been placed on optimizing cultivation conditions of environmental isolates of P. megaterium to increase PHB production with mainly molasses as carbon source, resulting in almost 70% PHB of cell dry weight (Gouda et al. 2001; Rodríguez-Contreras et al. 2013). For the well-known and genome sequenced strain DSM319, Godard et al. (2020) observed that the PHB content increased 5-fold to almost 30 % of cell dry weight under high salt conditions (Godard et al. 2020). Further, the production of functionalized PHB granules provides an exciting new application for P. megaterium. One recent study showed that the mammalian cytochrome P450 CYP11A1 could be immobilized and purified with PHB granules produced in P. megaterium, thereby circumventing the problem of low stability of recombinantly produced cytochromes. Here, CYP11A1 was readily localized in the phospholipid monolayer of the PHB granule in its native form verified by denaturing PAGE (Stenger et al. 2018). Another study showed that the IgG binding domain of Protein A from Staphylococcus aureus (ZZ domain) could be produced, purified, and presented on PHB granules when fused to PhaC in P. megaterium. The isolated functionalized PHB beads were capable of purifying IgG from human serum, thereby proving their functionality (Grage et al. 2017).
Production of recombinant proteins using P. megaterium: intra- und extracellular formation at g/L scale
The Gram-negative E. coli represents a well-established and heavily used host for the production and purification of recombinant proteins. However, E. coli has some major drawbacks including the presence of endotoxins (LPS) or limitations in the secretion of proteins into the growth medium, which permits easier downstream processing (Lakowitz et al. 2018; Terpe 2006). In contrast, Gram-positive bacteria lack an outer membrane, thereby omitting endotoxins and making protein secretion much more efficient. Many Gram-positives have shown promising potential for recombinant protein production, for example, multiple members of the genus Bacillus (Terpe 2006).
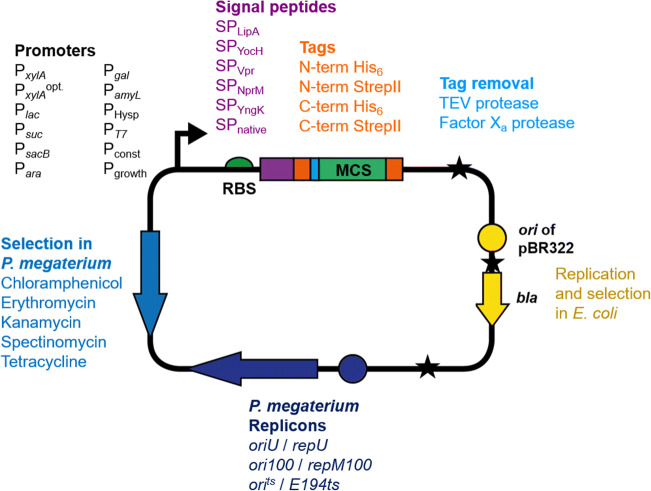
In general, P. megaterium is renowned for its high stability of recombinant plasmids, even in the absence of selective antibiotics (Radha and Gunasekaran 2008). The production of recombinant proteins using P. megaterium is typically performed by plasmid-based gene expression. The majority of these plasmids are based on the oriU/repU system derived from the pBC16 plasmid, originally found in Bacillus cereus (Bernhard et al. 1978; Rygus and Hillen 1991; Rygus et al. 1991), or on the compatible ori100/repM100 system from a plasmid found in P. megaterium QM B1551 (Eppinger et al. 2011; Gamer et al. 2009; Stevenson et al. 1998). In addition, the temperature-sensitive orits/E194ts system from plasmid pE194 is suited for genomic integration experiments (Biedendieck et al. 2010). All plasmids are designed as shuttle vectors enabling all cloning in E. coli and subsequent transfer to P. megaterium via protoplast transformation (Biedendieck et al. 2011), a technique even suited for new environmental P. megaterium isolates (Boock et al. 2019). Standard antibiotics such as tetracycline, kanamycin, chloramphenicol, erythromycin, or spectinomycin can be used as selection markers for P. megaterium (Fig. 3). Importantly, all plasmids allow stable replication and production of recombinant proteins in B. subtilis or Bacillus licheniformis (Lakowitz et al. 2017; Larsen and Bjerga 2018).
Fig. 3.
Schematic summary of Priestia megaterium plasmids used for the production, secretion, and purification of recombinant proteins. All plasmids are constructed as shuttle plasmids for cloning in E. coli (yellow elements) and replication (dark blue, different compatibility classes), selection (blue), and production of recombinant proteins in P. megaterium. Suitable promoters (black arrow) are the native (PxylA) and the optimized (PxylAopt.) xylose-inducible promoter, the lactose inducible (Plac), sucrose (Psuc, PsacB), arabinose (Para), galactosidase (Pgal), IPTG (PHysp), and starch (PamyL) promoter, the T7-RNA-polymerase-dependent promoter which is based on a two-plasmid system, and several constitutive (Pconst) and growth phase-dependent (Pgrowth) promoters. Genes encoding recombinant proteins can be fused to coding sequences of different signal peptides (purple) of the lipase A (SPLipA), the unknown secreted proteins YocH (SPYocH) and YngK (SPYngK), the natural protease NprM (SPNprM), and the serine protease VPR (SPVpr). In addition original signal peptides of the foreign recombinant protein can be used (SPnative). For purification of intra- or extracellular recombinant proteins, a fusion to N- or C-terminal His6 or StrepII tag is possible (orange). N-terminal tags can be removed of using tobacco each virus (TEV) or factor Xa protease cleavage (light blue). Black stars indicate stable places for integration of additional genetic elements as tRNAs or genes for co-expression.
Promoter systems for recombinant protein production in P. megaterium
A controlled high-level production of recombinant proteins in P. megaterium is based on the native xylose-inducible promoter/repressor system. The promoter PxylA is induced in the presence of xylose through a de-repression mechanism based on the inactivation of the repressor XylR via xylose binding. The corresponding gene xylR is encoded on the same expression plasmid (Rygus and Hillen 1991; Rygus et al. 1991). The PxylA-based expression system has undergone several systematic optimization steps resulting in intracellular recombinant protein production rates of up to 1 g L−1 (Stammen et al. 2010a) and more than 500 mg L−1 extracellularly (Korneli et al. 2013). This has resulted in the widely used P. megaterium recombinant protein production system, which comprised almost 30 plasmids and four strains and is available for commercial use from MoBiTec (Göttingen, Germany).
Moving on from the xylose-inducible system, a number of alternative promoter systems have recently been studied for protein production, expanding the P. megaterium plasmid toolbox (Fig. 3). Among these promoters are homologous sugar-inducible promoters (sucrose, arabinose, galactose, lactose) which were identified from transcriptome analyses. The employment of these systems for recombinant protein production resulted in up to 80 % yield compared to the optimized PxylA-based system (Biedendieck et al. 2007b; Hartz et al. 2019). Also a heterologous starch-inducible promoter (PamyL) from Bacillus amyloliquefaciens was found less effective compared to PxylA-based system (Radha and Gunasekaran 2008). Furthermore, the isopropyl-β-D-thiogalactopyranosid (IPTG)-inducible hyper-spank promoter (PHysp) yielded approximately 60 % of recombinant proteins compared to the PxylA-based system (Boock et al. 2019). As an alternative to these bacterial systems, phage-derived RNA polymerase (RNAP) systems have been successfully applied for protein production in multiple bacteria. For P. megaterium the genes coding for the RNAPs from the bacteriophage T7 (Gamer et al. 2009) and the E. coli phage K1E (Stammen et al. 2010b) residing on a separate plasmid were expressed under the control of PxylA. Upon xylose-based gene induction, the phage RNAPs are produced and specifically recognize the corresponding phage promoters driving the target gene expression localized on the second plasmid. The phage promoter-dependent gene expression resulted in up to 10 times more recombinant protein compared to the PxylA-based system (Gamer et al. 2009; Stammen et al. 2010b).
Beside inducible promoters, a number of constitutive and growth phase-dependent homologous promoters were tested for recombinant protein production. The promoters of the pyruvate dehydrogenase operon (pdhABCD) and of genes involved in glycolysis and gluconeogenesis (fba, fbp, gap, pgc, pgi, and pgk) from strain DSM319 yielded up to 75% of recombinant proteins compared to optimized PxylA-based system (Moore et al. 2018). In addition, some growth phase-dependent promoters were identified with slightly increased protein yields as the PxylA-based optimized system (Hartz et al. 2021).
Secretion of recombinant proteins with P. megaterium
As mentioned above, Bacillus excels as a good secretion host for proteins. Ninety percent of all extracellular Bacillus proteins are secreted by the secretion(SEC)-dependent pathway (Tjalsma et al. 2004; Tjalsma et al. 2000) guided by an N-terminally fused signal peptide (SP). The nascent and unfolded polypeptide is directly secreted, prior to spontaneous folding. Subsequently, the SP gets cleaved off outside of the cell, the protein is folded and released into the growth medium (Freudl 2018). For B. subtilis and others, it has been demonstrated that the combination of a specific SP with a certain recombinant protein determines the efficiency of the overall secretion process which cannot be predicted (Brockmeier et al. 2006; Freudl 2018; Hemmerich et al. 2016; Mathiesen et al. 2008). The secretion of proteins using the SEC-dependent pathway provides an excellent route if a recombinant protein is known to form insoluble inclusion bodies intracellularly (Freudl 2018). For B. subtilis 173 SEC-dependent SPs were described (Brockmeier et al. 2006). A similar number of SEC-dependent SPs was identified for P. megaterium DSM319 (www.megabac.tu-bs.de; Hiller et al. 2004). The efficiency of seven P. megaterium SPs on secretion was evaluated using the heterologous Thermobifida fusca hydrolase, resulting in highly variable levels of secreted protein (Stammen et al. 2010a) (Fig. 3). A later study found similar effects when looking at the secretion of the endoglucanase EGI1 and the multimodular cellulase Cel9AT with five (for EGI1) and four (for Cel9AT) different SPs, respectively, tested (Kalbarczyk et al. 2018). Furthermore, some studies show secretion of recombinant proteins using their original SP including the dextransucrase DsrS from Leuconostoc mesenteroides (Malten et al. 2005a), penicillin G acylases from different Bacillus species (Mayer et al. 2019; Yang et al. 2006), and a keratinase from B. licheniformis (Radha and Gunasekaran 2007; Radha and Gunasekaran 2008) (Tab. 1). For most secretion experiments, the P. megaterium strain MS941, a DSM319 variant lacking the gene coding for the extracellular neutral metalloprotease NprM, was used as this strain reveals a reduction of 98.5% of extracellular protease activity (Wittchen and Meinhardt 1995).
Further adaptations of the recombinant plasmid system in P. megaterium
Subsequently, to enable one-step protein purification, various tag-based affinity chromatography methods can be used (Terpe 2003). Thus, the P. megaterium plasmid systems were designed to create a plethora of N- and/or C-terminal fusion with His6- or StrepII-affinity tags in combination with protease cleavage sites for tag removal (Biedendieck et al. 2007c). When combined with extracellular protein production, affinity tags provide rapid tools for protein purification, especially along with continuous cultivation processes (Gädke et al. 2017a; Gädke et al. 2017b). Moreover, a codon plus system employing the co-expression of genes for tRNAs with rare codons often found in heterologous target genes showed a general positive effect on recombinant protein production by P. megaterium (Finger et al. 2015) (Fig. 3). For genomic modifications, a plasmid system based on a temperature sensitive promoter was established allowing gene integration and deletion (Biedendieck et al. 2011). Just recently, the group of Hannemann developed a CRISPR-Cas9 system for the genome editing of P. megaterium with an efficiency up to 100% (Hartz et al. 2021).
Production of biotechnological important proteins: multiple cytochrome P450 suitable for whole-cell transformations
P450 enzymes are frequently employed in metabolic reactions to catalyze challenging chemical reactions (Pochapsky 2020). They use a broad range of structurally diverse substrates and form their products with high stereo- and regio-selectivity. Humans carry 57 different P450 (CYP) enzymes involved in the metabolism of steroid hormones, other sterols, vitamin D3, eicosanoids, fatty acids, and retinoic acid (Luo and Liu 2020; Rendic and Guengerich 2021; Sarparast et al. 2020). They catalyze almost exclusively monooxygenase reactions through the activation of molecular oxygen using a single electron. The major type of reaction is the hydroxylation of difficult to activate C-H bonds. For this purpose, they contain a heme group as single-electron transfer agent. Consequently, catalyzed reactions require electron donors like NADH or NADPH. Flavin or iron-sulfur proteins transfer electrons from NADH or NADPH to the P450-bound heme (Chiliza et al. 2020; Li et al. 2020).
Since most bacterial and fungal P450s are cytosolic and soluble, these variants are better suited for biotechnology applications compared to their membrane-bound plant and mammalian homologs (Distefano et al. 2021; Finnigan et al. 2020; Iizaka et al. 2021; Toplak et al. 2021; Zhang et al. 2021b). In P. megaterium a number of different P450 enzymes were found. Seven cytosolic P450s (CYP) from different P. megaterium strains have been described. CYP106A2 from strain ATCC13368 (Berg et al. 1976; Schmitz et al. 2018) and the CYP109E1 from strain DSM319 (Jóźwik et al. 2016) rely on a FAD-dependent ferredoxin reductase and a corresponding ferredoxin as electron donor and transfer proteins. The best studied CYP102A1 from strain DSM32, also known as cytochrome P450-BM3, consists of an N-terminal P450 domain followed by a flavodoxin and a reductase domain. Therefore, this in vitro system is self-sufficient and channels electrons directly from NADPH, which accelerates monooxygenase rate in comparison to other P450s (Cook et al. 2016; Miura and Fulco 1974; Whitehouse et al. 2012). The remaining four P. megaterium P450s all require an external redox partner. These include CYP106A1 from strain DSM32 (He et al. 1989; Lee et al. 2015) and from strain DSM319 (Brill et al. 2014), CYP109A2 from strain DSM319 (Abdulmughni et al. 2017a), and CYP107DY1 from strain QM B1551. Interestingly, CYP107DY1 is the first plasmid encoded P450 found in Bacillus species. Since no CYP107 homologs can be found in the genomes of P. megaterium strains, CYP107DY1 may have been obtained by horizontal gene transfer. This observation suggests a possible role of P450s in the adaptation and even evolution of bacteria (Milhim et al. 2016).
Whole-cell systems for recombinant production of P450s in P. megaterium
To study P450s, this is typically performed using E. coli recombinant expression and studied in vitro with corresponding electron transfer proteins and cofactors. However, in vitro substrate conversion rates are often limited, which may constitute a problem for large-scale industrial applications. The use of whole-cell systems can overcome these in vitro limitations, although the import of the substrates and the export of the product might also be limited (Bernhardt and Urlacher 2014). Whole-cell systems have been biotechnologically employed for CYP106A1 from P. megaterium strain DSM319, CYP106A2 from P. megaterium strain ATCC13368 (Bleif et al. 2010; Bleif et al. 2012), and CYP109A2 (Abdulmughni et al. 2017a) and CYP109E1 (Abdulmughni et al. 2017b) from strain DSM319, all using P. megaterium as a production host (Tab. 1). In addition, E. coli-based whole-cell systems using CYP107DY1 from P. megaterium strain QM B 1551 (Milhim et al. 2016) and CYP102A1 from strain DSM32 (Chu et al. 2016) have been reported. Finally, membrane-bound mammalian P450 CYP11A1 was recombinantly produced in P. megaterium (Stenger et al. 2018).
Production of recombinant proteins: the P. megaterium cell-free transcription-translation system
For future progress with P. megaterium, a novel cell-free transcription-transcription tool was recently developed to study fundamental molecular biology and accelerate the testing of gene expression systems (Moore et al. 2018). Within synthetic biology, there has been a renewed interest in cell-free transcription-translation systems (Tinafar et al. 2019; Cole et al. 2020). Cell-free systems require a cell extract, energy solution, and plasmid DNA to synthesize recombinant proteins. These reactions can be performed in the microscale range (nL to mL range) either in test tube reactions, microtiter (96, 384, 1536 well format) plates, or microfluidics (Laohakunakorn et al. 2020). While E. coli remains the dominant cell-free system, a range of new cell-free systems has recently been developed from other major prokaryotic expression systems, including B. subtilis, Streptomyces spp., Clostridium autoethanogenum, Pseudomonas putida, and Vibrio natriegens (Gregorio et al. 2019; Cole et al. 2020). In terms of protein yield, although many systems are still in development, cell-free protein production is often monitored with GFP as a standard. For this, P. megaterium (134 ng μL−1 GFP) compares favorably to other popular Gram-positive hosts such as B. subtilis (21.6 ng μL−1) and Streptomyces lividans 66 (~100–400 ng μL−1) (Cole et al. 2020).
For P. megaterium DSM319, an optimized cell-free protocol (also active in DSM509) was recently developed (Moore et al. 2018). One key advantage of cell-free systems is the study of the biological numbers that underpin protein synthesis in combination with computational prediction models (Moore et al. 2018). A key finding of the P. megaterium cell-free system was potential rate-limiting steps in protein synthesis. For example, under the conditions studied, maximal translation rates were an order of magnitude slower than that of E. coli cell-free (Garamella et al. 2016).
In addition, the kinetics of the xylose-inducible promoter system (see above) were characterized in detail. For example, the dissociation constant for XylR and operator binding was determined at 12.9–14.2 nM with a Hill coefficient of 1.74–1.8. Finally, as an example of the power and speed of cell-free systems, using a liquid handling robot, up to 500 P. megaterium plasmids with varying promoter and RBS regions were rapidly screened for activity within 24 h. This permits the rapid characterization of DNA plasmid designs for forward engineering in P. megaterium cells (Moore et al. 2018). In summary, this recent development provides a high-yield and rapid cell-free tool for the study and engineering of P. megaterium for future metabolic engineering and synthetic biology applications.
Whole-cell applications: P. megaterium as a plant growth-promoting bacterium
Recently, within the last 20 years, scientists have discovered that the plant microbiome is essential for the survival of plants in changing environmental conditions including invasion by pathogenic microorganisms and insects (Berger and Gutjahr 2021; Genre et al. 2020; Haskett et al. 2020; Ortiz and Sansinenea 2021; Prsic and Ongena 2020; Vishwakarma et al. 2020; Zhang et al. 2021a). In conclusion, the health and growth of cultural plants can be influenced by the composition of its root and leave microbiome (Ray et al. 2020). In recent years, the beneficial effect of P. megaterium on plant growth has become a growing matter of interest. It has been described for a number of different plants including the model organism Arabidopsis thaliana (López-Bucio et al. 2007; Ortíz-Castro et al. 2008), the commercially important plants tomato (Solanum lycopersicum) (Ibort et al. 2017; Porcel et al. 2014), tea (Camellia sinensis) (Chakraborty et al. 2006), maize (Zea mays) (Al-Enazy et al. 2017; Marulanda et al. 2010), mustard (Brassica juncea L.) (Kang et al. 2014; Rajkumar and Freitas 2008), rice (Oryza sativa L.) (Feng et al. 2017), bean (Phaseolus vulgaris) (Korir et al. 2017; Ortíz-Castro et al. 2008), soybean (Glycine max) (Zhou et al. 2017), and oilseed rape (Brassica napus) (Hu et al. 2013). Three different mechanisms of plant growth promotion by P. megaterium have been described.
The first role can be summarized as biofertilizer. Phosphorus is essential for plant growth; however, its bioavailable form is often present in very low amounts (Liu 2021). The transformation of phosphorus in minerals and organic sources to their bioavailable forms occurs through secretion of organic acid in combination with acid phosphatases and phytases (Kang et al. 2014; Martínez-Viveros et al. 2010). P. megaterium secrets organic acids providing the main basis of phosphate biofertilization using secreted acid phosphatase and phytases (Hu et al. 2013). In addition, P. megaterium can provide reduced nitrogen to plants (Ding et al. 2005; Liu et al. 2006; Singh et al. 2020). Currently, a number of different P. megaterium fertilizer preparations often in combination with other bacteria are commercially available by different manufacturers for large-scale agricultural applications. These combinations including P. megaterium are also subjects of different patents. Wang et al. claim the release of potassium and phosphorus, the fixation of nitrogen, the inhibition of harmful bacteria in the soil, and the prevention of different diseases using a mixture of P. megaterium, further Bacillus strains and Gram-negative bacteria (Wang et al. 2009). Recently a fertilizer and its preparation was patented for the hydrolysis of phosphorus containing only P. megaterium but combined with organic matter and ammonium sulfate (Jianzhong 2019).
Secondly, plant growth-promoting bacterium can also be responsible for changes in environmental concentrations of phytohormones and other regulators of plant growth. Interestingly, the production of the auxin indole acetic acid (IAA) by P. megaterium resulted in a plant growth-promoting effect, as reported repeatedly for different plants (Chakraborty et al. 2006; Feng et al. 2017). Furthermore, the cultivation of A. thaliana with P. megaterium lead to higher concentrations of the isoprenoid plant hormone abscisic acids (ABA) in plant leaves, thus improving drought stress tolerance (Zhou et al. 2016). It was postulated that secretion of the polyamine spermidine by P. megaterium was responsible for the upregulation of the expression of ABA-associated genes and subsequent production of ABA in the plant. Ortíz-Castro et al. reported that cytokinin signaling plays a central role in the plant growth-promoting effect by P. megaterium on A. thaliana under defined lab conditions (Ortíz-Castro et al. 2008). In addition to the classical phytohormones, the role of so-called volatile compounds as plant growth-promoting substances is recently gaining more interest (Ryu et al. 2003; Sharifi and Ryu 2018). For P. megaterium the positive effect of the volatile compound 2-pentylfuran on the growth of A. thaliana has been observed (Zou et al. 2010). The mechanism by which this compound promotes plant growth is still unknown. Finally, acetoin produced by a P. megaterium strain promoted the growth of A. thaliana (Ryu et al. 2003).
Thirdly, P. megaterium can act as a biopesticide or biocontrol agent. The multiple anti-pathogenic mechanisms of P. megaterium are divers. An antifungal activity against the tea pathogen Fomes lamaoensis, the cause of brown root rot, is possibly related to the production of iron-chelating siderophores. Additionally, the enhanced secretion of the plant defense-related peroxidase, phenylalanine ammonia lyase, chitinase, and β-1,3-glucanase by P. megaterium was observed (Chakraborty et al. 2006). These enzymes are postulated to act directly against the fungal cell wall, thereby protecting the plant. The secretion of chitinase, β-1,3-glucanase, and protease by P. megaterium also mediated a protective effect against the fungus Rhizoctonia solani, the causative agent of “damping-off”, a destructive disease of plant seedlings, and against root rot in tomato (Lycopersicon esculentum Mill) (Solanki et al. 2012). The treatment of R. solani-caused diseases with P. megaterium strain ATCC55000 was patented in 1995 where its function as a biological control agent was described but lacks explanation. Moreover, the additional role of strain ATCC55000 in stimulating growth and yield in soybeans was part of the invention (Liu and Sinclair 1995). Another antifungal property of P. megaterium mediated by an unidentified volatile compound was found against the aflatoxin-producing Aspergillus flavus found on rice grains (Mannaa et al. 2017). A mixture of the three rhizobacterial bacteria P. megaterium, (Peri)Bacillus simplex, and Sinorhizobium fredii coated on soybean seeds revealed a clear protective effect against the “soybean cyst nematode” (Heterodera glycines) (Zhou et al. 2017).
Finally, coming back to the P. megaterium P450s, CYP102A1 (P450-BM3) most likely plays an important role in the regulation quorum sensing by soil bacteria through the inactivation of acyl homoserine lactones (AHLs). These molecules are known signaling molecules in the communication of Gram-negative bacteria (Chowdhary et al. 2007). Interestingly, the ability of P. megaterium to degrade AHLs suggests a link of their plant-protective, quorum-quenching activity to the quorum sensing of plant pathogenic bacteria (Dong et al. 2001). In summary, P. megaterium revealed a whole variety of molecular strategies of plant growth-promoting effects.
Conclusion and perspectives
P. megaterium is a fast-growing giant cell factory, with past and current industrial applications, and a promising alternative to standard model organisms (Eisenstein 2016). In 1994, Patricia S. Vary wrote “Prime time for Bacillus megaterium” (Vary 1994) which was followed 13 years later by the update “Bacillus megaterium - from simple soil bacterium to industrial protein production host” (Vary et al. 2007). Now, 14 years later, we have a new name for our well-known bacterium, while there are more than 20 fully sequenced genomes, a commercialized recombinant plasmid toolkit for high-yield recombinant protein production to the g per L scale, and a first cell-free transcription-translation in vitro system. In addition, our knowledge of whole-cell systems for the production of important P450 enzymes and also as a plant growth-promoting bacterium, the use in the production of bioplastics, and a prominent role in the understanding of B12 biosynthesis increased significantly.
So, what is needed next to develop P. megaterium into a competitive biotechnological production host? To support rapid and more straightforward biotechnological research, a complete genome-level single-gene knock-out library will provide an accurate picture of all non-essential genes, as is available for many model microbes. This is desirable to identify genes that may limit/benefit questions relating to recombinant protein production, metabolic capacity, or self-regulatory processes. Although the transformation of P. megaterium protoplasts is completely sufficient for the introduction of single plasmids, to test entire gene banks in this organism, a better transformation system is also needed. Since almost all genes necessary for the formation of natural competence are present in P. megaterium, an easier integration of DNA should be possible, which will serve as another important developmental feature. In addition, due to its natural size, P. megaterium represents a perfect tool for cell biological studies. Combining time-lapse microscopic studies with corresponding bioinformatic tools and modeling approaches is of interest (Münch et al. 2015).
Acknowledgements
We are very grateful to Manfred Rohde (HZI, Braunschweig, Germany) for the wonderful EM images of P. megaterium and E. coli. We acknowledge financial support by the Open Access Publication Funds of the Technische Universität Braunschweig.
Author contribution
RB and TK contributed equally to this work and were involved in the conceptualization, RB, TK, SJM, and DJ were involved in literature review and writing, RB, SJM, and DJ finalized the manuscript, RB and SJM have designed the figures, and all authors read and approved the final manuscript.
Funding
Open access funding enabled and organized by Projekt DEAL. This work was funded by the German Research Foundation (DFG), within the priority program SPP1617, “Phenotypic heterogeneity and sociobiology of bacterial populations” (TK).
Compliance with ethical standards
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rebekka Biedendieck and Tobias Knuuti contributed equally to this work.
References
- Abdulmughni A, Jóźwik IK, Brill E, Hannemann F, Thunnissen A-MWH, Bernhardt R. Biochemical and structural characterization of CYP109A2, a vitamin D3 25-hydroxylase from Bacillus megaterium. FEBS J. 2017;284:3881–3894. doi: 10.1111/febs.14276. [DOI] [PubMed] [Google Scholar]
- Abdulmughni A, Jóźwik IK, Putkaradze N, Brill E, Zapp J, Thunnissen A-MWH, Hannemann F, Bernhardt R. Characterization of cytochrome P450 CYP109E1 from Bacillus megaterium as a novel vitamin D3 hydroxylase. J Biotechnol. 2017;243:38–47. doi: 10.1016/j.jbiotec.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Al Bulushi IM, Poole SE, Barlow R, Deeth HC, Dykes GA. Speciation of Gram-positive bacteria in fresh and ambient-stored sub-tropical marine fish. Int J Food Microbiol. 2010;138:32–38. doi: 10.1016/J.IJFOODMICRO.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Al-Enazy AAR, Al-Oud SS, Al-Barakah FN, Usman ARA. Role of microbial inoculation and industrial by-product phosphogypsum in growth and nutrient uptake of maize (Zea mays L.) grown in calcareous soil. J Sci Food Agric. 2017;97:3665–3674. doi: 10.1002/jsfa.8226. [DOI] [PubMed] [Google Scholar]
- Al-Thubiani ASA, Maher YA, Fathi A, Abourehab MAS, Alarjah M, Khan MSA, Al-Ghamdi SB. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm J. 2018;26:1089–1097. doi: 10.1016/J.JSPS.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- Battersby AR. Tetrapyrroles: the pigments of life. Nat Prod Rep. 2000;17(6):507–526. doi: 10.1039/b002635m. [DOI] [PubMed] [Google Scholar]
- Baumchen C, Roth AH, Biedendieck R, Malten M, Follmann M, Sahm H, Bringer-Meyer S, Jahn D. D-mannitol production by resting state whole cell biotrans-formation of D-fructose by heterologous mannitol and formate dehydrogenase gene expression in Bacillus megaterium. Biotechnol J. 2007;2(11):1408–1416. doi: 10.1002/biot.200700055. [DOI] [PubMed] [Google Scholar]
- Berg A, Gustafsson JA, Ingelman-Sundberg M. Characterization of a cytochrome P-450-dependent steroid hydroxylase system present in Bacillus megaterium. J Biol Chem. 1976;251:2831–2838. doi: 10.1016/S0021-9258(17)33564-0. [DOI] [PubMed] [Google Scholar]
- Berger F, Gutjahr C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr Opin Plant Biol. 2021;59:101994. doi: 10.1016/j.pbi.2020.101994. [DOI] [PubMed] [Google Scholar]
- Bernhard K, Schrempf H, Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978;133:897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R, Urlacher VB. Cytochromes P450 as promising catalysts for biotechnological application: chances and limitations. Appl Microbiol Biotechnol. 2014;98:6185–6203. doi: 10.1007/s00253-014-5767-7. [DOI] [PubMed] [Google Scholar]
- Biedendieck R, Beine R, Gamer M, Jordan E, Buchholz K, Seibel J, Dijkhuizen L, Malten M, Jahn D. Export, purification, and activities of affinity tagged Lactobacillus reuteri levansucrase produced by Bacillus megaterium. Appl Microbiol Biotechnol. 2007;74(5):1062–1073. doi: 10.1007/s00253-006-0756-0. [DOI] [PubMed] [Google Scholar]
- Biedendieck R, Gamer M, Jaensch L, Meyer S, Rohde M, Deckwer WD, Jahn D. A sucrose-inducible promoter system for the intra- and extracellular protein production in Bacillus megaterium. J Biotechnol. 2007;132(4):426–430. doi: 10.1016/j.jbiotec.2007.07.494. [DOI] [PubMed] [Google Scholar]
- Biedendieck R, Yang Y, Deckwer WD, Malten M, Jahn D. Plasmid system for the intracellular production and purification of affinity-tagged proteins in Bacillus megaterium. Biotechnol Bioeng. 2007;96(3):525–537. doi: 10.1002/bit.21145. [DOI] [PubMed] [Google Scholar]
- Biedendieck R, Malten M, Barg H, Bunk B, Martens JH, Deery E, Leech H, Warren MJ, Jahn D. Metabolic engineering of cobalamin (vitamin B12) production in Bacillus megaterium. Microb Biotechnol. 2010;3(1):24–37. doi: 10.1111/j.1751-7915.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedendieck R, Borgmeier C, Bunk B, Stammen S, Scherling C, Meinhardt F, Wittmann C, Jahn D. Systems biology of recombinant protein production using Bacillus megaterium. Methods Enzymol. 2011;500:165–195. doi: 10.1016/B978-0-12-385118-5.00010-4. [DOI] [PubMed] [Google Scholar]
- Bleif S, Hannemann F, Bernhardt R, Lisurek M, Kries JPV, Jauch J, Zapp J (2010) Biokatalysator für die Hydroxylierung von Di- und Triterpenen. Germany Patent DE102009025996,
- Bleif S, Hannemann F, Zapp J, Hartmann D, Jauch J, Bernhardt R. A new Bacillus megaterium whole-cell catalyst for the hydroxylation of the pentacyclic triterpene 11-keto-beta-boswellic acid (KBA) based on a recombinant cytochrome P450 system. Appl Microbiol Biotechnol. 2012;93(3):1135–1146. doi: 10.1007/s00253-011-3467-0. [DOI] [PubMed] [Google Scholar]
- Boock JT, Freedman AJE, Tompsett GA, Muse SK, Allen AJ, Jackson LA, Castro-Dominguez B, Timko MT, Prather KLJ, Thompson JR. Engineered microbial biofuel production and recovery under supercritical carbon dioxide. Nat Commun. 2019;10:587. doi: 10.1038/s41467-019-08486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill E, Hannemann F, Zapp J, Brüning G, Jauch J, Bernhardt R. A new cytochrome P450 system from Bacillus megaterium DSM319 for the hydroxylation of 11-keto-β-boswellic acid (KBA) Appl Microbiol Biotechnol. 2014;98:1703–1717. doi: 10.1007/s00253-013-5029-0. [DOI] [PubMed] [Google Scholar]
- Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J Mol Biol. 2006;362:393–402. doi: 10.1016/j.jmb.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Chakraborty U, Chakraborty B, Basnet M. Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J Basic Microbiol. 2006;46:186–195. doi: 10.1002/jobm.200510050. [DOI] [PubMed] [Google Scholar]
- Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009;38:2434–2446. doi: 10.1039/b812677c. [DOI] [PubMed] [Google Scholar]
- Chiliza ZE, Martinez-Oyanedel J, Syed K. An overview of the factors playing a role in cytochrome P450 monooxygenase and ferredoxin interactions. Biophys Rev. 2020;12(5):1217–1222. doi: 10.1007/s12551-020-00749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary PK, Keshavan N, Nguyen HQ, Peterson JA, González JE, Haines DC. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry. 2007;46:14429–14437. doi: 10.1021/bi701945j. [DOI] [PubMed] [Google Scholar]
- Chu LL, Pandey RP, Jung N, Jung HJ, Kim EH, Sohng JK. Hydroxylation of diverse flavonoids by CYP450 BM3 variants: biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microb Cell Factories. 2016;15:135. doi: 10.1186/s12934-016-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SD, Miklos AE, Chiao AC, Sun ZZ, Lux MW. Methodologies for preparation of prokaryotic extracts for cell-free expression systems. Synth Syst Biotechnol. 2020;5(4):252–267. doi: 10.1016/j.synbio.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins HF, Biedendieck R, Leech HK, Gray M, Escalante-Semerena JC, McLean KJ, Munro AW, Rigby SEJ, Warren MJ, Lawrence AD. Bacillus megaterium has both a functional BluB protein required for DMB synthesis and a related flavoprotein that forms a stable radical species. PLoS One. 2013;8(2):e55708. doi: 10.1371/journal.pone.0055708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Finnigan JD, Cook K, Black GW, Charnock SJ. Cytochromes P450: history, classes, catalytic mechanism, and industrial application. Adv Protein Chem Struct Biol. 2016;105:105–126. doi: 10.1016/bs.apcsb.2016.07.003. [DOI] [PubMed] [Google Scholar]
- de Bary AH (1884) Vergleichende Morphologie und Biologie der Pilze, Mycetozoen und Bacterien. Von A. de Bary. Engelmann: Leipzig, 1884. Verlag von Wilhelm Engelmann, Leipzig:558. 10.5962/bhl.title.42380
- de Jesus AD, Gomes GVP, da Cunha Pascoal DR, Pinho LS, Chaves LBO, Druzian JI. Simultaneous biosynthesis of polyhydroxyalkanoates and extracellular polymeric substance (EPS) from crude glycerol from biodiesel production by different bacterial strains. Appl Biochem Biotechnol. 2016;180:1110–1127. doi: 10.1007/s12010-016-2155-z. [DOI] [PubMed] [Google Scholar]
- Ding Y, Wang J, Liu Y, Chen S. Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J Appl Microbiol. 2005;99(5):1271–1281. doi: 10.1111/j.1365-2672.2005.02738.x. [DOI] [PubMed] [Google Scholar]
- Distefano AM, Setzes N, Cascallares M, Fiol DF, Zabaleta E, Pagnussat GC. Roles of cytochromes P450 in plant reproductive development. Int J Dev Biol. 2021;65:187–194. doi: 10.1387/ijdb.200100gp. [DOI] [PubMed] [Google Scholar]
- Dobrzanski T, Gravina F, Steckling B, Olchanheski LR, Sprenger RF, Espírito Santo BC, Galvão CW, Reche PM, Prestes RA, Pileggi SAV, Campos FR, Azevedo RA, Sadowsky MJ, Beltrame FL, Pileggi M. Bacillus megaterium strains derived from water and soil exhibit differential responses to the herbicide mesotrione. PLoS One. 2018;13:e0196166. doi: 10.1371/journal.pone.0196166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y-H, Wang L-H, Xu J-L, Zhang H-B, Zhang X-F, Zhang L-H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- Ehrhardt M, Gerber A, Hannemann F, Bernhardt R. Expression of human CYP27A1 in B. megaterium for the efficient hydroxylation of cholesterol, vitamin D3 and 7-dehydrocholesterol. J Biotechnol. 2016;218:34–40. doi: 10.1016/j.jbiotec.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Eisenstein M. Living factories of the future. Nature. 2016;531(7594):401–403. doi: 10.1038/531401a. [DOI] [PubMed] [Google Scholar]
- Eppinger M, Bunk B, Johns MA, Edirisinghe JN, Kutumbaka KK, Koenig SS, Creasy HH, Rosovitz MJ, Riley DR, Daugherty S, Martin M, Elbourne LD, Paulsen I, Biedendieck R, Braun C, Grayburn S, Dhingra S, Lukyanchuk V, Ball B, Ul-Qamar R, Seibel J, Bremer E, Jahn D, Ravel J, Vary PS. Genome sequences of the biotechnologically important Bacillus megaterium strains QM B1551 and DSM319. J Bacteriol. 2011;193(16):4199–4213. doi: 10.1128/JB.00449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena JC, Roth JR. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987;169(5):2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F, Ge J, Li Y, He S, Zhong J, Liu X, Yu X. Enhanced degradation of chlorpyrifos in rice (Oryza sativa L.) by five strains of endophytic bacteria and their plant growth promotional ability. Chemosphere. 2017;184:505–513. doi: 10.1016/j.chemosphere.2017.05.178. [DOI] [PubMed] [Google Scholar]
- Finger C, Gamer M, Klunkelfuß S, Bunk B, Biedendieck R. Impact of rare codons and the functional coproduction of rate-limiting tRNAs on recombinant protein production in Bacillus megaterium. Appl Microbiol Biotechnol. 2015;99:8999–9010. doi: 10.1007/s00253-015-6744-5. [DOI] [PubMed] [Google Scholar]
- Finnigan JD, Young C, Cook DJ, Charnock SJ, Black GW. Cytochromes P450 (P450s): a review of the class system with a focus on prokaryotic P450s. Adv Protein Chem Struct Biol. 2020;122:289–320. doi: 10.1016/bs.apcsb.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Freedman AJE, Peet KC, Boock JT, Penn K, Prather KLJ, Thompson JR. Isolation, development, and genomic analysis of Bacillus megaterium SR7 for growth and metabolite production under supercritical carbon dioxide. Front Microbiol. 2018;9:2152. doi: 10.3389/fmicb.2018.02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb Cell Factories. 2018;17:1–10. doi: 10.1186/s12934-018-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gädke J, Kleinfeldt L, Schubert C, Rohde M, Biedendieck R, Garnweitner G, Krull R. In situ affinity purification of his-tagged protein A from Bacillus megaterium cultivation using recyclable superparamagnetic iron oxide nanoparticles. J Biotechnol. 2017;242:55–63. doi: 10.1016/j.jbiotec.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Gädke J, Thies JW, Kleinfeldt L, Kalinin A, Starke G, Lakowitz A, Biedendieck R, Garnweitner G, Dietzel A, Krull R. Integrated in situ-purification of recombinant proteins from Bacillus megaterium cultivation using SPION in stirred tank reactors. Biochem Eng J. 2017;126:58–67. doi: 10.1016/j.bej.2017.07.001. [DOI] [Google Scholar]
- Gamer M, Frode D, Biedendieck R, Stammen S, Jahn D. A T7 RNA polymerase-dependent gene expression system for Bacillus megaterium. Appl Microbiol Biotechnol. 2009;82(6):1195–1203. doi: 10.1007/s00253-009-1952-5. [DOI] [PubMed] [Google Scholar]
- Garamella J, Marshall R, Rustad M, Noireaux V. The all E. coli TX-TL Toolbox 2.0: a platform for cell-free synthetic biology. ACS Synth Biol. 2016;5(4):344–355. doi: 10.1021/acssynbio.5b00296. [DOI] [PubMed] [Google Scholar]
- Genre A, Lanfranco L, Perotto S, Bonfante P. Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol. 2020;18(11):649–660. doi: 10.1038/s41579-020-0402-3. [DOI] [PubMed] [Google Scholar]
- Gerber A, Kleser M, Biedendieck R, Bernhardt R, Hannemann F. Functionalized PHB granules provide the basis for the efficient side-chain cleavage of cholesterol and analogs in recombinant Bacillus megaterium. Microb Cell Factories. 2015;14:1–13. doi: 10.1186/s12934-015-0300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard T, Zuhlke D, Richter G, Wall M, Rohde M, Riedel K, Poblete-Castro I, Krull R, Biedendieck R. Metabolic rearrangements causing elevated proline and polyhydroxybutyrate accumulation during the osmotic adaptation response of Bacillus megaterium. Front Bioeng Biotechnol. 2020;8:47. doi: 10.3389/fbioe.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda MK, Swellam AE, Omar SH. Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res. 2001;156:201–207. doi: 10.1078/0944-5013-00104. [DOI] [PubMed] [Google Scholar]
- Grage K, McDermott P, Rehm BHA. Engineering Bacillus megaterium for production of functional intracellular materials. Microb Cell Factories. 2017;16:211. doi: 10.1186/s12934-017-0823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio NE, Levine MZ, Oza JP. A user's guide to cell-free protein synthesis. Methods Protoc. 2019;2:24. doi: 10.3390/mps2010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Patel S, Saini N, Chen S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int J Syst Evol Microbiol. 2020;70(11):5753–5798. doi: 10.1099/ijsem.0.004475. [DOI] [PubMed] [Google Scholar]
- Hansson M, Rutberg L, Schroder I, Hederstedt L. The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol. 1991;173(8):2590–2599. doi: 10.1128/jb.173.8.2590-2599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz P, Mattes C, Schad M, Bernhardt R, Hannemann F. Expanding the promoter toolbox of Bacillus megaterium. J Biotechnol. 2019;294:38–48. doi: 10.1016/j.jbiotec.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Hartz P, Gehl M, Konig L, Bernhardt R, Hannemann F. Development and application of a highly efficient CRISPR-Cas9 system for genome engineering in Bacillus megaterium. J Biotechnol. 2021;329:170–179. doi: 10.1016/j.jbiotec.2021.02.006. [DOI] [PubMed] [Google Scholar]
- Haskett TL, Tkacz A, Poole PS. Engineering rhizobacteria for sustainable agriculture. ISME J. 2020;15:949–964. doi: 10.1038/s41396-020-00835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JS, Ruettinger RT, Liu HM, Fulco AJ. Molecular cloning, coding nucleotides and the deduced amino acid sequence of P-450BM-1 from Bacillus megaterium. Biochim Biophys Acta. 1989;1009:301–303. doi: 10.1016/0167-4781(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Hemmerich J, Rohe P, Kleine B, Jurischka S, Wiechert W, Freudl R, Oldiges M. Use of a Sec signal peptide library from Bacillus subtilis for the optimization of cutinase secretion in Corynebacterium glutamicum. Microb Cell Factories. 2016;15:1–11. doi: 10.1186/s12934-016-0604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller K, Grote A, Scheer M, Munch R, Jahn D (2004) PrediSi: prediction of signal peptides and their cleavage positions. Nucleic Acids Res 32(Web Server issue):W375–W379. 10.1093/nar/gkh378 [DOI] [PMC free article] [PubMed]
- Hu X, Roberts DP, Xie L, Maul JE, Yu C, Li Y, Zhang S, Liao X. Development of a biologically based fertilizer, incorporating Bacillus megaterium A6, for improved phosphorus nutrition of oilseed rape. Can J Microbiol. 2013;59:231–236. doi: 10.1139/cjm-2012-0579. [DOI] [PubMed] [Google Scholar]
- Huang FL, Zhang Y, LP Z, Wang S, Feng Y, NH R. Complete genome sequence of Bacillus megaterium JX285 isolated from Camellia oleifera rhizosphere. Comput Biol Chem. 2019;79:1–5. doi: 10.1016/J.COMPBIOLCHEM.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Ibort P, Molina S, Núñez R, Zamarreño ÁM, García-Mina JM, Ruiz-Lozano JM, Orozco-Mosqueda MDC, Glick BR, Aroca R. Tomato ethylene sensitivity determines interaction with plant growth-promoting bacteria. Ann Bot. 2017;120:101–122. doi: 10.1093/aob/mcx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizaka Y, Sherman DH, Anzai Y. An overview of the cytochrome P450 enzymes that catalyze the same-site multistep oxidation reactions in biotechnologically relevant selected actinomycete strains. Appl Microbiol Biotechnol. 2021;105:2647–2661. doi: 10.1007/s00253-021-11216-y. [DOI] [PubMed] [Google Scholar]
- Jendrossek D. Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes) J Bacteriol. 2009;191:3195–3202. doi: 10.1128/JB.01723-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianzhong Q (2019) A kind of bioactivation enzymatic hydrolysis phosphorus agent and preparation method thereof containing Bacillus megaterium. China Patent:CN109134083
- Jordan E, Al-Halabi L, Schirrmann T, Hust M, Dubel S. Production of single chain Fab (scFab) fragments in Bacillus megaterium. Microb Cell Factories. 2007;6:38. doi: 10.1186/1475-2859-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jóźwik IK, Kiss FM, Gricman Ł, Abdulmughni A, Brill E, Zapp J, Pleiss J, Bernhardt R, Thunnissen AMWH. Structural basis of steroid binding and oxidation by the cytochrome P450 CYP109E1 from Bacillus megaterium. FEBS J. 2016;283:4128–4148. doi: 10.1111/febs.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugder BE, Payne KAP, Fisher K, Bohl S, Lebhar H, Manefield M, Lee M, Leys D, Marquis CP. Heterologous production and purification of a functional chloroform reductive dehalogenase. ACS Chem Biol. 2018;13(3):548–552. doi: 10.1021/acschembio.7b00846. [DOI] [PubMed] [Google Scholar]
- Kalbarczyk KZ, Mazeau EJ, Rapp KM, Marchand N, Koffas MAG, Collins CH. Engineering Bacillus megaterium strains to secrete cellulases for synergistic cellulose degradation in a microbial community. ACS Synth Biol. 2018;7:2413–2422. doi: 10.1021/acssynbio.8b00186. [DOI] [PubMed] [Google Scholar]
- Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH. Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol. 2014;54:427–433. doi: 10.1007/s12088-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjanachumpol P, Kulpreecha S, Tolieng V, Thongchul N. Enhancing polyhydroxybutyrate production from high cell density fed-batch fermentation of Bacillus megaterium BA-019. Bioprocess Biosyst Eng. 2013;36:1463–1474. doi: 10.1007/s00449-013-0885-7. [DOI] [PubMed] [Google Scholar]
- Knobloch D, Ostermann K, Rodel G. Production, secretion, and cell surface display of recombinant Sporosarcina ureae S-layer fusion proteins in Bacillus megaterium. Appl Environ Microbiol. 2012;78(2):560–567. doi: 10.1128/AEM.06127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda H, Kobayashi M, Shimizu S. A novel transporter involved in cobalt uptake. Proc Natl Acad Sci U S A. 1997;94(1):36–41. doi: 10.1073/pnas.94.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korir H, Mungai NW, Thuita M, Hamba Y, Masso C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front Plant Sci. 2017;8:141. doi: 10.3389/fpls.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneli C, Biedendieck R, David F, Jahn D, Wittmann C. High yield production of extracellular recombinant levansucrase by Bacillus megaterium. Appl Microbiol Biotechnol. 2013;97(8):3343–3353. doi: 10.1007/s00253-012-4567-1. [DOI] [PubMed] [Google Scholar]
- Kurniasih SD, Alfi A, Natalia D, Radjasa OK, Nurachman Z. Construction of individual, fused, and co-expressed proteins of endoglucanase and β-glucosidase for hydrolyzing sugarcane bagasse. Microbiol Res. 2014;169:725–732. doi: 10.1016/J.MICRES.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Lakowitz A, Krull R, Biedendieck R. Recombinant production of the antibody fragment D1.3 scFv with different Bacillus strains. Microb Cell Factories. 2017;16(1):14. doi: 10.1186/s12934-017-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowitz A, Godard T, Biedendieck R, Krull R. Mini review: recombinant production of tailored bio-pharmaceuticals in different Bacillus strains and future perspectives. Eur J Pharm Biopharm. 2018;126:27–39. doi: 10.1016/j.ejpb.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Laohakunakorn N, Grasemann L, Lavickova B, Michielin G, Shahein A, Swank Z, Maerkl SJ. Bottom-up construction of complex biomolecular systems with cell-free synthetic biology. Front Bioeng Biotechnol. 2020;8:213. doi: 10.3389/fbioe.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen O, Bjerga GEK (2018) Development of versatile vectors for heterologous expression in Bacillus. Microorganisms 6(2). 10.3390/microorganisms6020051 [DOI] [PMC free article] [PubMed]
- Lee T-R, Lin J-S, Wang S-S, Shaw G-C (2004) PhaQ, a new class of poly-beta-hydroxybutyrate (phb)-responsive repressor, regulates phaQ and phaP (phasin) expression in Bacillus megaterium through interaction with PHB. J Bacteriol 186:3015–3021. 10.1128/JB.186.10.3015-3021.2004 [DOI] [PMC free article] [PubMed]
- Lee GY, Kim DH, Kim D, Ahn T, Yun CH. Functional characterization of steroid hydroxylase CYP106A1 derived from Bacillus megaterium. Arch Pharm Res. 2015;38:98–107. doi: 10.1007/s12272-014-0366-9. [DOI] [PubMed] [Google Scholar]
- Leech HK, Raux E, McLean KJ, Munro AW, Robinson NJ, Borrelly GP, Malten M, Jahn D, Rigby SE, Heathcote P, Warren MJ. Characterization of the cobaltochelatase CbiXL: evidence for a 4Fe-4S center housed within an MXCXXC motif. J Biol Chem. 2003;278(43):41900–41907. doi: 10.1074/jbc.M306112200. [DOI] [PubMed] [Google Scholar]
- Lemoigne M. Produit de deshydratation et de polymerisation de l'acide B-oxybutyrique. Bull Soc Chim Biol. 1926;8:770–782. [Google Scholar]
- Li S, Du L, Bernhardt R. Redox partners: function modulators of bacterial P450 enzymes. Trends Microbiol. 2020;28(6):445–454. doi: 10.1016/j.tim.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Liu D (2021) Root developmental responses to phosphorus nutrition. J Integr Plant Biol 63(6):1065–1090 10.1111/jipb.13090 [DOI] [PubMed]
- Liu ZL, Sinclair JB (1995) Bacillus megetarium ATCC 55000 and method of use thereof to control R. solani US Patent 5403583
- Liu X, Zhao H, Chen S. Colonization of maize and rice plants by strain Bacillus megaterium C4. Curr Microbiol. 2006;52:186–190. doi: 10.1007/s00284-005-0162-3. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y, Zhang J, Zou W, Zhou Z, Liu J, Li X, Wang L, Chen J. Complete genome sequence of the industrial strain Bacillus megaterium WSH-002. J Bacteriol. 2011;193:6389–6390. doi: 10.1128/JB.06066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Luo K, Wang Y, Zeng A, Zhou X, Luo F, Bai L. Isolation, identification and characteristics of an endophytic quinclorac degrading bacterium Bacillus megaterium Q3. PLoS One. 2014;9:e108012. doi: 10.1371/journal.pone.0108012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López AC, Alippi AM. Diversity of Bacillus megaterium isolates cultured from honeys. LWT Food Sci Technol. 2009;42:212–219. doi: 10.1016/j.lwt.2008.05.001. [DOI] [Google Scholar]
- López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact. 2007;20:207–217. doi: 10.1094/MPMI-20-2-0207. [DOI] [PubMed] [Google Scholar]
- Lü S, Wang J, Yao J, Yu Z, Shujuan L, Jun W, Jianming Y, Zengliang Y. Study on the effect of mutated Bacillus megaterium in two-stage fermentation of vitamin C. Plasma Sci Technol. 2003;5:2011–2016. doi: 10.1088/1009-0630/5/5/014. [DOI] [Google Scholar]
- Lu J, Tappel RC, Nomura CT. Mini-review: biosynthesis of poly(hydroxyalkanoates) Polym Rev. 2009;49:226–248. doi: 10.1080/15583720903048243. [DOI] [Google Scholar]
- Luo Y, Liu JY. Pleiotropic functions of cytochrome P450 monooxygenase-derived eicosanoids in cancer. Front Pharmacol. 2020;11:580897. doi: 10.3389/fphar.2020.580897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio-Hall LA, Escalante-Semerena JC (1999) In vitro synthesis of the nucleotide loop of cobalamin by Salmonella typhimurium enzymes. Proc Natl Acad Sci U S A 96(21):11798–11803. 10.1073/pnas.96.21.11798 [DOI] [PMC free article] [PubMed]
- Malten M, Hollmann R, Deckwer WD, Jahn D. Production and secretion of recombinant Leuconostoc mesenteroides dextransucrase DsrS in Bacillus megaterium. Biotechnol Bioeng. 2005;89:206–218. doi: 10.1002/bit.20341. [DOI] [PubMed] [Google Scholar]
- Malten M, Nahrstedt H, Meinhardt F, Jahn D. Coexpression of the type I signal peptidase gene sipM increases recombinant protein production and export in Bacillus megaterium MS941. Biotechnol Bioeng. 2005;91:616–621. doi: 10.1002/bit.20523. [DOI] [PubMed] [Google Scholar]
- Malten M, Biedendieck R, Gamer M, Drews AC, Stammen S, Buchholz K, Dijkhuizen L, Jahn D (2006) A Bacillus megaterium plasmid system for the production, export, and one-step purification of affinity-tagged heterologous levansucrase from growth medium. Appl Environ Microbiol 72(2):1677–1679. 10.1128/AEM.72.2.1677-1679.2006 [DOI] [PMC free article] [PubMed]
- Manetsberger J, Ghosh A, Hall EAH, Christie G. Orthologues of Bacillus subtilis spore crust proteins have a structural role in the Bacillus megaterium QM B1551 spore exosporium. Appl Environ Microbiol. 2018;84:e01734–e01718. doi: 10.1128/AEM.01734-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaa M, Oh JY, Kim KD. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and Aflatoxin production on stored rice grains. Mycobiology. 2017;45:213–219. doi: 10.5941/MYCO.2017.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Barg H, Warren MJ, Jahn D. Microbial production of vitamin B12. Appl Microbiol Biotechnol. 2002;58(3):275–285. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- Martínez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML. Mechanisms and practical considerations involved in plant growth promotion by Rhizobacteria. J Soil Sci Plant Nutr. 2010;10:293–319. doi: 10.4067/S0718-95162010000100006. [DOI] [Google Scholar]
- Marulanda A, Azcón R, Chaumont F, Ruiz-Lozano JM, Aroca R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta. 2010;232:533–543. doi: 10.1007/s00425-010-1196-8. [DOI] [PubMed] [Google Scholar]
- Mathiesen G, Sveen A, Piard JC, Axelsson L, Eijsink VGH. Heterologous protein secretion by Lactobacillus plantarum using homologous signal peptides. J Appl Microbiol. 2008;105:215–226. doi: 10.1111/j.1365-2672.2008.03734.x. [DOI] [PubMed] [Google Scholar]
- Mayer J, Pippel J, Gunther G, Muller C, Lauermann A, Knuuti T, Blankenfeldt W, Jahn D, Biedendieck R. Crystal structures and protein engineering of three different penicillin G acylases from Gram-positive bacteria with different thermostability. Appl Microbiol Biotechnol. 2019;103(18):7537–7552. doi: 10.1007/s00253-019-09977-8. [DOI] [PubMed] [Google Scholar]
- McCool GJ, Cannon MC. PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillus megaterium. J Bacteriol. 2001;183:4235–4243. doi: 10.1128/JB.183.14.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool GJ, Cannon MC. Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol. 1999;181:585–592. doi: 10.1128/JB.181.2.585-592.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt F, Stahl U, Ebeling W. Highly efficient expression of homologous and heterologous genes in Bacillus megaterium. Appl Microbiol Biotechnol. 1989;30:343–350. doi: 10.1007/BF00296622. [DOI] [Google Scholar]
- Milhim M, Putkaradze N, Abdulmughni A, Kern F, Hartz P, Bernhardt R. Identification of a new plasmid-encoded cytochrome P450 CYP107DY1 from Bacillus megaterium with a catalytic activity towards mevastatin. J Biotechnol. 2016;240:68–75. doi: 10.1016/j.jbiotec.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Miura Y, Fulco AJ. (Omega -2) hydroxylation of fatty acids by a soluble system from Bacillus megaterium. J Biol Chem. 1974;249:1880–1888. doi: 10.1016/S0021-9258(19)42868-8. [DOI] [PubMed] [Google Scholar]
- Mobius K, Arias-Cartin R, Breckau D, Hannig AL, Riedmann K, Biedendieck R, Schroder S, Becher D, Magalon A, Moser J, Jahn M, Jahn D. Heme biosynthesis is coupled to electron transport chains for energy generation. Proc Natl Acad Sci U S A. 2010;107(23):10436–10441. doi: 10.1073/pnas.1000956107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ (2011) Elucidation of the anaerobic biosynthesis of vitamin B12 in Bacillus megaterium. University of Kent, Dissertation
- Moore SJ, Biedendieck R, Lawrence AD, Deery E, Howard MJ, Rigby SEJ, Warren MJ. Characterization of the enzyme CbiH60 involved in anaerobic ring contraction of the cobalamin (vitamin B12) biosynthetic pathway. J Biol Chem. 2013;288:297–305. doi: 10.1074/jbc.M112.422535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, Howard MJ, Rigby SEJ, Warren MJ. Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12) Proc Natl Acad Sci U S A. 2013;110:14906–14911. doi: 10.1073/pnas.1308098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Mayer MJ, Biedendieck R, Deery E, Warren MJ. Towards a cell factory for vitamin B12 production in Bacillus megaterium: bypassing of the cobalamin riboswitch control elements. New Biotechnol. 2014;31(6):553–561. doi: 10.1016/j.nbt.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Moore SJ, MacDonald JT, Wienecke S, Ishwarbhai A, Tsipa A, Aw R, Kylilis N, Bell DJ, McClymont DW, Jensen K, Polizzi KM, Biedendieck R, Freemont PS. Rapid acquisition and model-based analysis of cell-free transcription-translation reactions from nonmodel bacteria. Proc Natl Acad Sci U S A. 2018;115(19):E4340–E4349. doi: 10.1073/pnas.1715806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno P, Yañez C, Cardozo NSM, Escalante H, Combariza MY, Guzman C. Influence of nutritional and physicochemical variables on PHB production from raw glycerol obtained from a Colombian biodiesel plant by a wild-type Bacillus megaterium strain. New Biotechnol. 2015;32:682–689. doi: 10.1016/J.NBT.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Münch KM, Müller J, Wienecke S, Bergmann S, Heyber S, Biedendieck R, Münch R, Jahn D. Polar fixation of plasmids during recombinant protein production in Bacillus megaterium results in population heterogeneity. Appl Environ Microbiol. 2015;81(17):5976–5986. doi: 10.1128/AEM.00807-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi A, Barrick JE, Breaker RR. Coenzyme B12 riboswitches are widespread genetic control elements in prokaryotes. Nucleic Acids Res. 2004;32(1):143–150. doi: 10.1093/nar/gkh167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo JM, Posada JA, Higuita JC, Cardona CA. Valorization of glycerol through the production of biopolymers: the PHB case using Bacillus megaterium. Bioresour Technol. 2013;133:38–44. doi: 10.1016/j.biortech.2013.01.129. [DOI] [PubMed] [Google Scholar]
- Narhi LO, Fulco AJ. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986;261:7160–7169. doi: 10.1016/S0021-9258(17)38369-2. [DOI] [PubMed] [Google Scholar]
- Ortiz A, Sansinenea E. Recent advancements for microorganisms and their natural compounds useful in agriculture. Appl Microbiol Biotechnol. 2021;105(3):891–897. doi: 10.1007/s00253-020-11030-y. [DOI] [PubMed] [Google Scholar]
- Ortíz-Castro R, Valencia-Cantero E, López-Bucio J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav. 2008;3:263–265. doi: 10.4161/psb.3.4.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne KA, Quezada CP, Fisher K, Dunstan MS, Collins FA, Sjuts H, Levy C, Hay S, Rigby SE, Leys D. Reductive dehalogenase structure suggests a mechanism for B12-dependent dehalogenation. Nature. 2015;517(7535):513–516. doi: 10.1038/nature13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochapsky TC. A dynamic understanding of cytochrome P450 structure and function through solution NMR. Curr Opin Biotechnol. 2020;69:35–42. doi: 10.1016/j.copbio.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcel R, Zamarreño ÁM, García-Mina JM, Aroca R. Involvement of plant endogenous ABA in Bacillus megaterium PGPR activity in tomato plants. BMC Plant Biol. 2014;14:1–12. doi: 10.1186/1471-2229-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prsic J, Ongena M. Elicitors of plant immunity triggered by beneficial bacteria. Front Plant Sci. 2020;11:594530. doi: 10.3389/fpls.2020.594530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radha S, Gunasekaran P. Cloning and expression of keratinase gene in Bacillus megaterium and optimization of fermentation conditions for the production of keratinase by recombinant strain. J Appl Microbiol. 2007;103:1301–1310. doi: 10.1111/j.1365-2672.2007.03372.x. [DOI] [PubMed] [Google Scholar]
- Radha S, Gunasekaran P. Sustained expression of keratinase gene under PxylA and PamyL promoters in the recombinant Bacillus megaterium MS941. Bioresour Technol. 2008;99:5528–5537. doi: 10.1016/J.BIORTECH.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Rajkumar M, Freitas H. Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour Technol. 2008;99:3491–3498. doi: 10.1016/j.biortech.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J Biol Chem. 2007;282(42):30442–30451. doi: 10.1074/jbc.M702519200. [DOI] [PubMed] [Google Scholar]
- Raux E, Lanois A, Warren MJ, Rambach A, Thermes C. Cobalamin (vitamin B12) biosynthesis: identification and characterization of a Bacillus megaterium cobI operon. Biochem J. 1998;335(Pt 1):159–166. doi: 10.1042/bj3350159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Lakshmanan V, Labbe JL, Craven KD. Microbe to microbiome: a paradigm shift in the application of microorganisms for sustainable agriculture. Front Microbiol. 2020;11:622926. doi: 10.3389/fmicb.2020.622926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic SP, Guengerich FP. Human family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update. Arch Toxicol. 2021;95(2):395–472. doi: 10.1007/s00204-020-02971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyami BA, Ghosh A, Rees EJ, Christie G (2019) Novel cortex lytic enzymes in Bacillus megaterium QM B1551 spores. FEMS Microbiol Lett 366(12). 10.1093/femsle/fnz146 [DOI] [PubMed]
- Rodríguez-Contreras A, Koller M, Miranda-de Sousa Dias M, Calafell-Monfort M, Braunegg G, Marqués-Calvo MS. High production of poly(3-hydroxybutyrate) from a wild Bacillus megaterium Bolivian strain. J Appl Microbiol. 2013;114:1378–1387. doi: 10.1111/jam.12151. [DOI] [PubMed] [Google Scholar]
- Roof DM, Roth JR. Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium. J Bacteriol. 1989;171(6):3316–3323. doi: 10.1128/jb.171.6.3316-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygus T, Hillen W. Inducible high-level expression of heterologous genes in Bacillus megaterium using the regulatory elements of the xylose-utilization operon. Appl Microbiol Biotechnol. 1991;35(5):594–599. doi: 10.1007/BF00169622. [DOI] [PubMed] [Google Scholar]
- Rygus T, Scheler A, Allmansberger R, Hillen W. Molecular cloning, structure, promoters and regulatory elements for transcription of the Bacillus megaterium encoded regulon for xylose utilization. Arch Microbiol. 1991;155:535–542. doi: 10.1007/BF00245346. [DOI] [PubMed] [Google Scholar]
- Ryu C-M, Farag MA, Hu C-h, Reddy MS, Wei H-X, Pare PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarparast M, Dattmore D, Alan J, Lee KSS. Cytochrome P450 metabolism of polyunsaturated fatty acids and neurodegeneration. Nutrients. 2020;12:3523. doi: 10.3390/nu12113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Janocha S, Kiss FM, Bernhardt R. CYP106A2 - a versatile biocatalyst with high potential for biotechnological production of selectively hydroxylated steroid and terpenoid compounds. Biochim Biophys Acta, Proteins Proteomics. 2018;1866:11–22. doi: 10.1016/j.bbapap.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Schobert M, Jahn D. Regulation of heme biosynthesis in non-phototrophic bacteria. J Mol Microbiol Biotechnol. 2002;4(3):287–294. [PubMed] [Google Scholar]
- Scott AI. Reflections on the discovery of nature's pathways to vitamin B12. Chem Rec. 2001;1(3):212–227. doi: 10.1002/tcr.1009. [DOI] [PubMed] [Google Scholar]
- Sharifi R, Ryu CM. Sniffing bacterial volatile compounds for healthier plants. Curr Opin Plant Biol. 2018;44:88–97. doi: 10.1016/j.pbi.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Singh RK, Singh P, Li HB, Song QQ, Guo DJ, Solanki MK, Verma KK, Malviya MK, Song XP, Lakshmanan P, Yang LT, Li YR. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: a comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020;20(1):220. doi: 10.1186/s12870-020-02400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman DKY, Ashby RD, Foglia TA, Marmer WN. Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates) Appl Microbiol Biotechnol. 2006;71:783–789. doi: 10.1007/s00253-006-0451-1. [DOI] [PubMed] [Google Scholar]
- Solanki MK, Robert AS, Singh RK, Kumar S, Pandey AK, Srivastava AK, Arora DK. Characterization of mycolytic enzymes of Bacillus strains and their bio-protection role against Rhizoctonia solani in tomato. Curr Microbiol. 2012;65:330–336. doi: 10.1007/s00284-012-0160-1. [DOI] [PubMed] [Google Scholar]
- Stahl U, Esser K. Plasmid heterogeneity in various strains of Bacillus megaterium. Eur J Appl Microbiol Biotechnol. 1983;17:248–251. doi: 10.1007/BF00510424. [DOI] [Google Scholar]
- Stammen S, Muller BK, Korneli C, Biedendieck R, Gamer M, Franco-Lara E, Jahn D. High-yield intra- and extracellular protein production using Bacillus megaterium. Appl Environ Microbiol. 2010;76(12):4037–4046. doi: 10.1128/AEM.00431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammen S, Schuller F, Dietrich S, Gamer M, Biedendieck R, Jahn D (2010b) Application of Escherichia coli phage K1E DNA-dependent RNA polymerase for in vitro RNA synthesis and in vivo protein production in Bacillus megaterium. Appl Microbiol Biotechnol 88:529–539. 10.1007/s00253-010-2732-y [DOI] [PubMed]
- Stenger B, Gerber A, Bernhardt R, Hannemann F. Functionalized poly(3-hydroxybutyric acid) bodies as new in vitro biocatalysts. Biochim Biophys Acta, Proteins Proteomics. 2018;1866:52–59. doi: 10.1016/j.bbapap.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Stevenson DM, Kunnimalaiyaan M, Müller K, Vary PS. Characterization of a theta plasmid replicon with homology to all four large plasmids of Bacillus megaterium QM B1551. Plasmid. 1998;40:175–189. doi: 10.1006/PLAS.1998.1359. [DOI] [PubMed] [Google Scholar]
- Sun MZ, Zheng HC, Meng LC, Sun JS, Song H, Bao YJ, Pei HS, Yan Z, Zhang XQ, Zhang JS, Liu YH, Lu FP. Direct cloning, expression of a thermostable xylanase gene from the metagenomic DNA of cow dung compost and enzymatic production of xylooligosaccharides from corncob. Biotechnol Lett. 2015;37(9):1877–1886. doi: 10.1007/s10529-015-1857-6. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2003;60(5):523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- Tinafar A, Jaenes K, Pardee K. Synthetic Biology Goes Cell-Free BMC Biol. 2019;17(1):64. doi: 10.1186/s12915-019-0685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalsma H, Bolhuis A, Jongbloed JDH, Bron S, van Dijl JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev. 2000;64:515–547. doi: 10.1128/MMBR.64.3.515-547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalsma H, Antelmann H, Jongbloed JDH, Braun PG, Darmon E, Dorenbos R, Dubois F, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, Dubois J-yF, Dijl JMV. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol Mol Biol Rev. 2004;68:207–233. doi: 10.1128/MMBR.68.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak M, Matthews A, Teufel R. The devil is in the details: the chemical basis and mechanistic versatility of flavoprotein monooxygenases. Arch Biochem Biophys. 2021;698:108732. doi: 10.1016/j.abb.2020.108732. [DOI] [PubMed] [Google Scholar]
- Tsuge T, Hyakutake M, Mizuno K. Class IV polyhydroxyalkanoate (PHA) synthases and PHA-producing Bacillus. Appl Microbiol Biotechnol. 2015;99:6231–6240. doi: 10.1007/s00253-015-6777-9. [DOI] [PubMed] [Google Scholar]
- Vary PS. Development of genetic engineering in Bacillus megaterium. Biotechnology. 1992;22:251–310. [PubMed] [Google Scholar]
- Vary PS. Prime time for Bacillus megaterium. Microbiology. 1994;140:1001–1013. doi: 10.1099/13500872-140-5-1001. [DOI] [PubMed] [Google Scholar]
- Vary PS, Biedendieck R, Fuerch T, Meinhardt F, Rohde M, Deckwer WD, Jahn D. Bacillus megaterium-from simple soil bacterium to industrial protein production host. Appl Microbiol Biotechnol. 2007;76:957–967. doi: 10.1007/s00253-007-1089-3. [DOI] [PubMed] [Google Scholar]
- Vílchez JI, Tang Q, Kaushal R, Wang W, Lv S, He D, Chu Z, Zhang H, Liu R, Zhang H (2018a) Complete genome sequence of Bacillusmegaterium strain TG1-E1, a Plant drought tolerance-enhancing bacterium. Microbiol Resour Announc 7:e00842–e00818. 10.1128/MRA.00842-18 [DOI] [PMC free article] [PubMed]
- Vílchez JI, Tang Q, Kaushal R, Wang W, Lv S, He D, Chu Z, Zhang H, Liu R, Zhang H. Genome sequence of Bacillus megaterium strain YC4-R4, a plant growth-promoting rhizobacterium isolated from a high-salinity environment. Genome Announc. 2018;6:4–5. doi: 10.1128/genomeA.00527-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma K, Kumar N, Shandilya C, Mohapatra S, Bhayana S, Varma A. Revisiting plant-microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Front Microbiol. 2020;11:560406. doi: 10.3389/fmicb.2020.560406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Cosmos NH, Watson BA, Fellman JK, Mattinson DS, Edwards CG. Characterization of Bacillus megaterium, Bacillus pumilus, and Paenibacillus polymyxa isolated from a Pinot noir wine from Western Washington State. Food Microbiol. 2017;67:11–16. doi: 10.1016/J.FM.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Lan B, Yuan J, Qu Y (2009) Microbial fertilizer and preparation thereof China Patent CN-101372425-A
- Wang S, Wang Y, Cai Y, Kelly CP, Sun X. Novel chimeric protein vaccines against Clostridium difficile infection. Front Immunol. 2018;9:2440. doi: 10.3389/fimmu.2018.02440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang D, Chu S, Zhi Y, Liu X, Zhou P. Genomic analysis of Bacillus megaterium NCT-2 reveals its genetic basis for the bioremediation of secondary salinization soil. Int J Genomics. 2020;2020:4109186. doi: 10.1155/2020/4109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. The biosynthesis of adenosylcobalamin (vitamin B12) Nat Prod Rep. 2002;19(4):390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- Whitehouse CJC, Bell SG, L-l W. P450 BM3 (CYP102A1): connecting the dots. Chem Soc Rev. 2012;41:1218–1260. doi: 10.1039/C1CS15192D. [DOI] [PubMed] [Google Scholar]
- Wittchen KD, Meinhardt F. Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl Microbiol Biotechnol. 1995;42:871–877. doi: 10.1007/BF00191184. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Brey RN. Isolation and genetic characterizations of Bacillus megaterium cobalamin biosynthesis-deficient mutants. J Bacteriol. 1986;166(1):51–58. doi: 10.1128/jb.166.1.51-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin Y, Huang Z, Liu Z. Characterization and site-directed mutagenesis of an α-galactosidase from the deep-sea bacterium Bacillus megaterium. Enzym Microb Technol. 2014;56:46–52. doi: 10.1016/J.ENZMICTEC.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Biedendieck R, Wang W, Gamer M, Malten M, Jahn D, Deckwer W-D. High yield recombinant penicillin G amidase production and export into the growth medium using Bacillus megaterium. Microb Cell Factories. 2006;5:36. doi: 10.1186/1475-2859-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Zhou B, Wang J, He X, Sun X, Nie W, Tzipori S, Feng H (2008) Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol 8:192. 10.1186/1471-2180-8-192 [DOI] [PMC free article] [PubMed]
- Youngster T, Wushensky JA, Aristilde L. Profiling glucose-induced selective inhibition of disaccharide catabolism in Bacillus megaterium QM B1551 by stable isotope labelling. Microbiology. 2017;163:1509–1514. doi: 10.1099/mic.0.000540. [DOI] [PubMed] [Google Scholar]
- Yucel N, Aslim B, Özdoğan H. In vitro antimicrobial effect of Satureja wiedemanniana against Bacillus species isolated from raw meat samples. J Med Food. 2009;12:919–923. doi: 10.1089/jmf.2008.0144. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu J, Shi Z, Liu L, Chen J. Manipulation of B. megaterium growth for efficient 2-KLG production by K. vulgare. Process Biochem. 2010;45:602–606. doi: 10.1016/j.procbio.2009.11.016. [DOI] [Google Scholar]
- Zhang J, Cook J, Nearing JT, Zhang J, Raudonis R, Glick BR, Langille MGI, Cheng Z. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol Res. 2021;245:126690. doi: 10.1016/j.micres.2020.126690. [DOI] [PubMed] [Google Scholar]
- Zhang X, Guo J, Cheng F, Li S (2021b) Cytochrome P450 enzymes in fungal natural product biosynthesis. Nat Prod Rep 38(6):1072–1099 10.1039/d1np00004g [DOI] [PubMed]
- Zheng H, Liu Y, Liu X, Han Y, Wang J, Lu F. Overexpression of a Paenibacillus campinasensis xylanase in Bacillus megaterium and its applications to biobleaching of cotton stalk pulp and saccharification of recycled paper sludge. Bioresour Technol. 2012;125:182–187. doi: 10.1016/j.biortech.2012.08.101. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu H, Du G, Li J, Chen J. Production of alpha-cyclodextrin glycosyltransferase in Bacillus megaterium MS941 by systematic codon usage optimization. J Agric Food Chem. 2012;60(41):10285–10292. doi: 10.1021/jf302819h. [DOI] [PubMed] [Google Scholar]
- Zhou C, Ma Z, Zhu L, Xiao X, Xie Y, Zhu J, Wang J. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int J Mol Sci. 2016;17(6):976. doi: 10.3390/ijms17060976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Zhu X, Liu R, Xiang P, Chen J, Liu X, Duan Y, Chen L. Management of the soybean cyst nematode Heterodera glycines with combinations of different rhizobacterial strains on soybean. PLoS One. 2017;12:e0182654. doi: 10.1371/journal.pone.0182654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Li Z, Yu D. Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J Microbiol. 2010;48:460–466. doi: 10.1007/s12275-010-0068-z. [DOI] [PubMed] [Google Scholar]