Abstract
Background
Coronavirus disease 2019 (COVID-19) has been associated with neuropsychiatric complications ranging from new-onset psychosis to delirium, dysexecutive syndromes, catatonia, and akinetic mutism (AM). AM can be conceptualized as a disorder of motivation wherein patients exhibit a loss of speech and spontaneous movement, owing to disruption of underlying frontal-subcortical circuits.
Objectives
The objectives of this study were to review the concept and differential diagnosis of AM, as well as the clinical literature on AM in COVID-19 and discuss potential implications for underlying functional neuroanatomy and mechanistic pathways, as well as clinical management.
Methods
A narrative literature review was performed using PubMed querying published articles for topics associated with AM and its occurrence in COVID-19.
Results
AM has been described in case reports and a prospective cohort study of patients with COVID with neurological complaints. Three COVID-19 AM subgroups can be distinguished, including individuals with severe respiratory illness, those with meningoencephalitis, and those with delirium and pre-existing neuropsychiatric illness. Electrophysiology and functional imaging suggest COVID-19 AM may result from underlying frontal lobe dysfunction and disruption of associated distributed circuits subserving goal-directed behavior. Distinctive combinations of pathophysiological mechanisms may be at play in the different subgroups of COVID-19 AM cases.
Conclusion
AM has been described in association with COVID-19 and may manifest in clinically heterogenous subgroups with distinct underlying mechanisms. The diagnosis of AM and evaluation of potential etiologies can be complex. The occurrence of AM contributes evidence to the hypothesis of frontal lobe dysfunction in COVID-19.
Key words: coronavirus disease 2019, neuropsychiatry/behavioral neurology, akinetic mutism
Introduction
Coronavirus disease 2019 (COVID-19) is increasingly recognized as a potent cause of encephalopathy, which is the presenting symptom in a substantial proportion of patients.1, 2, 3, 4, 5 COVID-associated encephalopathy is not a unitary phenomenon; however, the literature on COVID-19 and delirium has identified unusual characteristics such as pyramidal and extrapyramidal signs, myoclonus, and both severe agitation and hypoactivity.6
Among presentations of extreme hypoactivity are cases of akinetic mutism (AM), where patients are awake but mute with minimal spontaneous goal-directed movement due to a profound motivational deficit. AM patients are vulnerable to complications of immobility and often require total care, straining acute care and post-acute rehabilitation settings, and can trigger psychiatric consultation for diagnostic and management assistance.
COVID-associated AM is significant as it may represent the extreme end of a spectrum of motivational deficits in a larger number of patients. Furthermore, AM can be more precisely localized with regards to underlying circuitry than delirium. Its occurrence may signal specific network dysfunction in a substantial subset of COVID-associated encephalopathy cases.
Thus far, the literature on AM in COVID-19 is derived from case reports and a single cohort study.4 , 6, 7, 8, 9, 10, 11, 12 In the following review, we will discuss the concept of AM, review the literature on AM in COVID and offer hypotheses regarding the underlying functional neuroanatomy and potential etiological mechanisms. Implications for management are also discussed. The aim is to raise awareness of severe abulic states in COVID-19 encephalopathy and gauge their potential significance for our understanding of the disease.
Terminology
Cairns et al13 introduced the term akinetic mutism in 1941 to describe a state of wakeful unresponsiveness in a patient with a third ventricle cyst. His definition of AM depicted the striking co-occurrence of alertness as evinced by visual tracking with physical inertness characterized by lack of spontaneous movement, speech, and emotional expression. Importantly, akinesis and mutism were not total; command-following and short, appropriate answers could be elicited with sufficient prompting some of the time.
The initial definition by Cairns et al14 was essentially preserved in subsequent diagnostic criteria for AM published by the Aspen Neurobehavioral Workgroup, a multidisciplinary collaboration that has set terminology for disorders of consciousness since the mid-1990s. Therein, AM was considered a subtype of the minimally responsive state, later recharacterized as the minimally conscious state (MCS).15 The four criteria of AM included (1) akinesis and mutism, (2) preserved visual tracking, (3) brief interaction with vigorous and persistent prompting, and (4) lack of elementary sensorimotor dysfunction (such as quadriparesis).14 The most recent Aspen guidelines in 2018 did not make mention of AM,16 though leading contributors have continued to describe AM as a subset of MCS in other published work.17
The intact but dormant capacity for complex behavior in AM has led a number of authors to implicate frontal-subcortical circuits subserving goal-directed behavior. As per this view, AM is conceptualized as an especially severe form of abulia or apathy. Fisher18 wrote that while AM itself was uncommon, partial presentations, or “abulia minor,” were present in a wide range of neurological conditions, anticipating the current interest in apathy as a pervasive clinical issue in neuropsychiatry. However, while patients with AM would appear to meet recent consensus criteria for apathy,19 other authors have questioned the relationship of AM to apathy/abulia, in part owing to issues of diagnostic reliability which have not been systemically assessed.20 , 21 We will proceed from the conceptualization of AM as a disorder of diminished motivation, while acknowledging these considerations.
The association between AM and structural brain injury has obscured cases reported in relation to toxic-metabolic as well as immunologic/infectious etiologies. While AM can be a chronic state, particularly in the setting of bilateral lesions, the prognosis differs based on etiology. Unsurprisingly, resolution of the underlying cause such as infection or toxic exposure typically leads to clinical improvement.22 , 23 Moreover, certain important AM etiologies, including delayed posthypoxic leukoencephalopathy, tend to exhibit substantial improvement as a feature of their natural history.24
Diagnostic Considerations
Like delirium or catatonia, AM is a syndrome rather than a disease and must be fully characterized to guide differential diagnosis. AM has been conceptualized as a profound deficit in motivation owing to damage to the mesocorticolimbic dopamine tracts or global dopamine depletion.25 Consequently, patients with AM exhibit minimal goal-directed behavior despite wakefulness and intact sensorimotor capacity. Isolating the motivational deficit in AM requires careful behavioral observation and examination to rule out dysfunction at other levels of the nervous system (Table 1 ).
Table 1.
Differential diagnosis for akinetic mutism, features, and distinguishing characteristics
| Condition | Features | Distinguishing characteristics from AM |
|---|---|---|
| MCS/PVS | Reduced arousal—typically due to brainstem injury—results in absence of goal-directed behavior | Eye tracking spared in AM and MCS but not in PVS Complex speech or action in response to environmental triggers in AM but not in MCS/PVS |
| Locked-In syndrome | Immobility due to paralysis sparing vertical eye movements and blinking, typically caused by ventral pons/midbrain infarction | Absence of motor paralysis in AM Often some preserved communication via patterned eye movements |
| Polyneuropathy/myopathy | Hypokinesis due to pain and reduced muscular function from muscle and nerve injury in critical illness | Motivation to move remains intact Speech output is not typically limited as in AM |
| Ischemic or hemorrhagic infarct | Hypokinesis due to upper motor neuron injury, typically unilateral NB: A strategic infarct may produce an AM phenotype. |
Motivation to move may be intact Speech output may be aphasic, but mutism is rare |
| Hypoactive delirium | Marked by disorientation, inattention, fluctuating consciousness in the absence of agitation | Affective state is typically distressed or fearful in delirium compared to AM Goal-directed movement—particularly in response to perceptual disturbances—are preserved in hypoactive delirium |
| Nonconvulsive status epilepticus | Presentations are diverse but mutism is common and stuporous non-responsiveness can develop | Often fluctuating presentation with motor automatisms, which are less typical for AM EEG findings may be demonstrative |
| Catatonia | Marked by immobility, mutism, rigidity; may also demonstrate hyperkinetic features such as agitation, stereotypy, mannerisms NB: Catatonia and AM share similar features and may be related pathophysiologic states. |
Affective state is typically fearful AM does not have hyperkinetic features AM does not typically respond to benzodiazepines |
AM = akinetic mutism; MCS = minimally conscious state; PVS = persistent vegetative state.
First, the examiner must consider the patient's level of arousal. In AM, patients have intact arousal but diminished motivation with lack of goal-directed behavior. Owing to diminished arousal, persistent vegetative states also exhibit an absence of goal-directed behavior. Clinically, AM can be distinguished from persistent vegetative state by the presence of eye tracking.26
The relationship between AM and MCS is more complex as AM has been categorized as a subtype of MCS.1 The most recent international guidelines defined the category of MCS plus, which refers to preservation of language function that is a positive prognostic indicator.27 Similarly, patients with AM may demonstrate transient restoration of speech or action in response to environmental stimuli, classically when speaking on the phone, known as the “telephone sign.”28 These awakenings reflect the intact but dormant capacity for integrated behavior AM, which is otherwise devastated in many cases of MCS. The absence of goal-directed behavior is typically more extreme in AM, MCS, and persistent vegetative state than typical delirium. As such, the absence of goal-directed behavior outside of MCS/persistent vegetative state should raise concern for conative disorders including AM and catatonia. Nonconvulsive status epilepticus has been reported in COVID-19 and can also precipitate mutism and stupor.29
The next distinction is whether lack of spontaneous movement is owing to encephalopathy at all. In critically ill patients with COVID-19, polyneuropathy/myopathy needs consideration as this condition can occur after prolonged sedation and paralysis. In addition, ischemic and hemorrhagic stroke can damage upper motor neuron tracts and curtail movement, with the locked-in syndrome being the most dramatic manifestation.30 The presence of these insults can obscure co-occurring motivational deficits, such as in the patient that not only “won't” but “can't.”31 The same may be true to a lesser extent in Parkinsonian states, where there is marked slowness of motor initiation and performance (noting that Parkinsonism may also result in motivational deficits related to mesocorticolimbic hypodopaminergia).32
The final consideration is that of akinetic catatonia, wherein patients can present as immobile and mute with an apparent lack of voluntary movement.28 Indeed, AM may represent a pure “motor catatonia” with overlapping pathophysiology, but lacking the subjective experience of intense fear or anxiety, which is common in patients with catatonia and may represent a human equivalent of the mammalian fear response (i.e., “playing possum”).28 , 33 , 34 Patients with catatonia are more likely to exhibit hyperkinetic features such as automatisms (stereotypies, mannerisms) and echophenomena. Interestingly, both AM and patients with catatonia may exhibit abnormal tone in the form of gegenhalten, though waxy flexibility and catalepsy are typically absent in AM.28 Medications such as amantadine or zolpidem may have therapeutic benefit in catatonia and AM,25 but AM does not typically respond to benzodiazepines.35 As such, the lorazepam challenge may also have a role for distinguishing catatonia and AM.
Review of Literature on AM and COVID-19
AM has been described in case reports of COVID-associated encephalopathy as well as a recent cohort study.7, 8, 9, 10, 11, 12 , 36 , 37 While there is no single clinical profile associated with AM in COVID-19, there do appear to be 3 main subgroups, including patients with (1) severe respiratory illness, (2) meningoencephalitis, or (3) pre-existing neuropsychiatric vulnerability.
Most reported cases have occurred in patients with severe respiratory illness requiring intubation in the absence of meningoencephalitis. In a recent prospective cohort study, Nersesjan et al11 reported the development of AM in 8 of 61 (13%) patients who were COVID-positive enrolled on admission. In 7 of 8 cases, AM also emerged after extubation and persisted for a median of four days before spontaneous recovery. Clinical data were otherwise reported at group rather than case level. In another case report, AM also emerged after extubation in severe COVID-19, lasting for a duration of 5–7 days.7 Imaging and CSF were normal, and the patient's mental status ultimately improved after the administration of high-dose steroids and IVIg.
Another subset of cases involved patients with meningoencephalitis and mild respiratory disease, where AM occurred within a week of symptom onset. For example, 2 cases of AM were reported after several days of progressive behavioral change with mild respiratory symptoms. Both patients met criteria for meningoencephalitis based on clinical examination and elevated CSF protein and pleocytosis, though severe acute respiratory syndrome coronavirus 2 polymerase chain reaction was negative. In another case, a healthy 16-year-old girl developed fever, tachycardia, and acute-onset paranoia which progressed to AM over 5 days with normal CSF studies, serum CRP, and imaging.38 She was also treated with IVIg for presumed parainfectious autoimmune encephalitis, with equivocal response.
In our own COVID-associated delirium case series, 3 patients aged 68 to 76 years with pre-existing neuropsychiatric vulnerability and signs of systemic hyperinflammation presented with acute-onset behavioral changes and progressed from hyperactive delirium to abulia and mutism within the course of a week.5
Importantly, AM may be underreported in COVID-19, obscured under the label of encephalopathy or dysexecutive syndromes.39 Moreover, AM may represent the “tip of the iceberg” of more common but subtle abulic presentations in COVID-19. Indeed, a recent survey of “long-haulers” after severe COVID-19 demonstrated significant elevations in apathy on standardized psychometrics.40 As the literature on AM in COVID-19 develops, we may be better able to characterize prevalence, epidemiology, and clinical course, as well as potential interventions.
Etiological Implications
The functional neuroanatomy of AM and related states has been clarified by clinical correlation and neuroimaging. Frontal-subcortical circuitry involved in AM may be vulnerable to COVID-19 by a range of putative mechanisms.
Functional Neuroanatomy of AM
Early studies on the functional neuroanatomy of AM proceeded from clinical correlation with focal brain lesions, which typically involved anteromedial frontal lobes or centromedian mesodiencephalic regions.28 Culprit insults were highly varied, ranging from cerebrovascular accident, hypoxia, or neoplasm to neurodegenerative and inflammatory or infectious disease.25 Fisher18 attempted to unify these heterogenous findings by positing common disruption to the mesodiencephalofrontal activating system subserving reward and motivation-based goal-directed behavior, identifying mesocorticolimbic dopamine projections as a particular site of vulnerability.
Advances in neuroimaging and functional neuroanatomy have confirmed the general thrust of Fisher's early observations.25 Investigators have identified the anterior cingulate cortex (ACC) as the overlapping hub of functional networks affected by diverse lesions in patients with AM.25 Indeed, AM often results from bilateral anterior circulation infarcts that damage the ACC.41 Accordingly, the ACC is reciprocally connected to the cingulate motor areas and participates in cortico-striato-thalamo-cortical loops, explaining its involvement in motor and salience networks subserving motivational decision-making.42 Thus, lesions in the basal ganglia, thalamus, or midbrain can generate AM phenotypes similar to those precipitated by the frontal lesions described by Fisher.43
Evidence of Frontal Lobe Dysfunction in COVID-Associated Encephalopathy
AM may be conceptualized as a frontal lobe syndrome owing to disruption of cortico-striato-thalamo-cortical circuits originating in the ACC. Interestingly, neuroimaging and neurophysiological studies in patients with COVID-19 have yielded evidence of frontal lobe dysfunction in COVID-associated encephalopathy, including but not limited to patients with AM.
While diffuse EEG slowing is typically observed in delirium, frontal-predominant theta-delta slowing was reported in up to 33% of cases in a systematic review of 617 patients with COVID-encephalopathy.44 AM has also been reported to be associated with frontal EEG slowing and frontal hypometabolism on perfusion imaging in patients with COVID-19. For example, Nersesjan et al11 found frontal intermittent rhythmic delta activity in 75% cases where EEG was obtained. Cani et al12 report a case of likely parainfectious immune-mediated AM in COVID-19 associated with frontal EEG slowing and hypoperfusion on fluorodeoxyglucose positron emission tomography.
Investigators have also described specific functional networks involved in COVID-associated encephalopathy. Kas et al8 report on 7 consecutive patients with COVID-19 who received fluorodeoxyglucose positron emission tomography imaging during the acute phase and at 1 and 6 months after recovery. Frontal behavioral syndromes were the most common clinical feature in the sample, though AM was not specifically investigated. Notably, perfusion imaging showed bilateral frontal hypoperfusion most consistent in the ACC and caudate nucleus. Mild perfusion abnormalities persisted in these regions through 6-month follow-up, which may reflect a shared substrate between AM and longer-lasting executive dysfunction observed in many post-COVID patients.45
Mechanistic Hypotheses of COVID-Associated AM
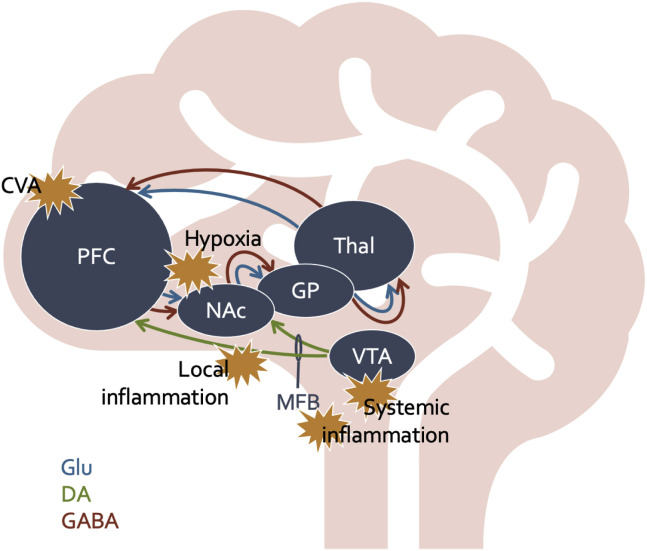
Early clinical reports suggest AM is a heterogenous phenomenon in COVID-19 occurring in the context of (1) severe respiratory illness, (2) CSF-negative encephalitis, and (3) patients with pre-existing neuropsychiatric vulnerability following hyperactive delirium. Distinct combinations of etiological mechanisms likely contribute to these respective presentations. See Table 2 for a summary of these etiological mechanisms and Figure 1 for a graphical depiction.
Table 2.
Proposed mechanisms of AM in COVID-19 and associated case reports
| Etiology | Proposed mechanisms | Hypotheses | Case references |
|---|---|---|---|
| Severe respiratory illness | Systemic inflammation Hypoxic-ischemic injury Coagulopathy |
AM may result from BBB and mesolimbic disruption from systemic inflammation; from DPHL or other direct hypoxic injury; or strategic infarcts from COVID-19 associated hypercoagulability; neuronal pathways from lung to CNS | Cani et al. 2021 Lang et al. 2020 Muccioli et al. 2020 Nersesjan et al. 2021 Wu et al. 202046 |
| Meningoencephalitis | Systemic inflammation CSF-specific antigens Local CNS invasion |
AM may result from local inflammation disrupting BBB in frontal subcortical circuitry (fenestrated endothelium and choroid plexus), producing inflammatory responses either to direct viral invasion or CSF-specific antigens, the latter of which may develop from parainfectious immune dysregulation. | Gaughan et al. 2021 Pilotto et al. 2020 Pilotto et al. 2021 Achar and Ghosh 202047 |
| Neuropsychiatric vulnerability | Systemic inflammation Underlying dopaminergic deficit/dysregulation |
AM may result from systemic inflammation disrupting dopamine synthesis, producing AM in patients with pre-existing dopaminergic dysfunction. | Ahmad and Rathore 2020 Beach et al. 2020 Brown et al. 2020 |
AM = akinetic mutism; BBB = blood-brain barrier; DPHL = posthypoxic leukoencephalopathy.
Figure 1.
The Networks Involved in Akinetic Mutism Include the Reward Pathway and the Motivation-to-Movement Pathway, Otherwise Known as the Parallel Cortico-Striato-Thalamo-Cortical (CSTC Loops). Disruptions in the neurotransmitter flow or direct injury to key sites in these pathways will directly affect motivation and movement. Here we provide examples of locations where COVID-19 and associated systemic and local effects through various mechanisms may affect motivational circuity and produce the phenotype of AM. DA = dopamine; GABA = gamma aminobutyric acid; GP = globus pallidus; Glu = glutamate; MFB = medial forebrain bundle; NAc = nucleus accumbens; PFC = prefrontal cortex; Thal = thalamus; VTA = ventral tegmental area.
Severe Respiratory Illness
Compared to those with mild respiratory illness, mechanically ventilated patients with COVID-19 are more likely to develop systemic inflammation,48 hypoxic-ischemic injury, coagulopathy, and metabolic disturbances,49 which could all conceivably contribute to AM.
Several authors have compared COVID-associated encephalopathy to neurotoxicity associated with chimeric-antigen receptor T-cell therapy for hematologic malignancy.50, 51, 52, 53, 54 Chimeric-antigen receptor T-cell therapy triggers massive systemic cytokine release thought to disrupt blood-brain barrier function, leading to a distinctive encephalopathy characterized by mutism, loss of spontaneous movement, and aphasia.50 Cases of AM have also been reported in tacrolimus toxicity, suggesting that a systemically distributed agent can affect particular circuits, perhaps via selective vulnerability of specific regions of the blood-brain barrier.55 Regarding severe acute respiratory syndrome coronavirus 2 infection, a fenestrated endothelial region such as the ACC abutting organum vasculosum, which contains angiotensin II receptors, may be a particularly important candidate zone of AM vulnerability.56 In this way, intense peripheral inflammation in COVID-19 may produce AM among patients with severe respiratory illness.
Furthermore, hypoxemic-ischemic insult is also a possibility in mechanically ventilated patients and comprises the most common neuropathological finding in patients with severe COVID-19.2 AM has been associated with damage to the basal ganglia after acute hypoxia57 , 58 as well as posthypoxic leukoencephalopathy occurring weeks to months after the initial insult.59 Posthypoxic leukoencephalopathy after severe COVID-19 hypoxemia has been reported in the literature.60 Another study of brain magnetic resonance spectroscopy in patients with COVID-19 has revealed a molecular signature consistent with posthypoxic leukoencephalopathy, which may cause AM through damage to white matter tracts involved in motivational functions.61
Given the association of AM with cerebrovascular accidents,43 COVID-associated vascular disease is another consideration. Although large-scale cerebrovascular disease has not been reported in COVID-associated AM, the contribution of microvascular coagulopathy to neurological morbidity has yet to be fully elucidated.61 It is conceivable that strategic infarcts disrupting frontal neurocircuitry in the setting of COVID-19 may contribute to some AM cases.
CSF-Negative Meningoencephalitis
In the reported cases of AM in meningoencephalitis, symptoms correlated with elevated inflammatory cytokines in the CSF despite normal serum studies; concordantly, improvement in AM coincided with immunosuppressive therapy. These findings lend further support to an immune-mediated mechanism of AM.
Similar to AM in severe respiratory COVID-19, authors have drawn comparisons between chimeric-antigen receptor T cell neurotoxicity and CSF-negative meningoencephalitis. However, massive systemic inflammation was absent in reported cases of CSF-negative meningoencephalitis. Nonetheless, more subtle forms of immune dysregulation could conceivably disrupt blood-brain barrier integrity, precipitating the movement of inflammatory cells into the CNS.
Other proposed hypotheses implicate immune responses against nonviral antigens in the CSF. For example, evidence of intrathecal antibody synthesis has been reported in CSF studies,62 and cases of limbic encephalitis have presented in association COVID-19.9 The proinflammatory effects of COVID-19 may disrupt immune tolerance,63 leading to autoimmunity against CNS antigens. Alternatively, CSF-negative meningoencephalitis may reflect an immune response to transient, low-grade CNS involvement by severe acute respiratory syndrome coronavirus 2 difficult to detect on CSF studies. Indeed, it has been proposed that severe acute respiratory syndrome coronavirus 2 may enter the frontotemporal regions at modest levels via the olfactory bulb;64 transient viral presence in frontal lobe regions could conceivably trigger local inflammation disrupting nearby motivational networks.
Pre-existing Neuropsychiatric Vulnerability
Individuals with pre-existing neuropsychiatric illness may have underlying abnormalities in brain function that predispose to abulia in the setting of COVID. Given that massive inflammatory cytokine release may disrupt dopamine synthesis and function in reward and motivation neurocircuitry,31 , 65 patients with decreased functional reserve in the dopamine system may then be especially vulnerable to AM, which is associated with dopamine depletion states, owing to selective dopamine receptor vulnerability. Indeed, AM has been described in patients with COVID-19 and schizophrenia, Parkinson's disease, and Lewy body dementia after profound delirium.4 , 66 Furthermore, prior neuropsychiatric illness may confer sensitivity not only to dopamine depletion but other insults such as hypoxic-ischemic injury, toxic-metabolic disturbance, and the neurotoxic effects of delirium itself.1 Cumulatively, multiple mechanisms may converge to produce AM features in the neurologically vulnerable.
Clinical Implications
COVID-associated AM has exhibited a transient course within the period of 1–2 weeks among the small sample of reported cases, consistent with an immunometabolic rather than structural process. Early management should aim at assessing for life-threatening or readily reversible causes of decreased responsiveness. Brain imaging, preferably MRI, should be obtained to exclude cerebrovascular accident and unusual presentations such as acute disseminated encephalomyelitis67; EEG should be performed to exclude nonconvulsive status epilepticus. Risk/benefit considerations will favor lorazepam challenge for catatonia in most cases, though caution should be used in patients with severe respiratory compromise. Lumbar puncture should be conducted when AM occurs in the absence of severe respiratory illness or fails to show steady improvement over the course of 1 week in postintubation cases to rule out CNS invasion or autoimmune meningoencephalitis as a complication of COVID-19. In addition to routine cell counts and chemistries, oligoclonal bands, as well as viral and autoimmune encephalitis panels should be obtained. Vigilant supportive care and anticoagulation should be sufficient to prevent complications of immobility given the reported time course of recovery. In persistent manifestations (i.e., cases lasting longer than 1 week without any improvement), it is reasonable to consider prodopaminergic medications such as amantadine or methylphenidate,24 which may also aid in minimizing residual apathy during rehabilitation. The role of immunotherapy is beyond the scope of this review but may offer a targeted therapeutic approach in select cases.
Conclusion
AM is marked by diminished speech and movement owing to damage to neural circuits subserving motivation. AM has been reported in patients with COVID-19 manifesting decreased responsiveness. Preliminary clinical, electrophysiological, and functional imaging data suggest disruption of frontal-subcortical circuitry in COVID encephalopathy, which may explain AM presentations. Cases of COVID-19 AM have occurred in association with (1) severe respiratory illness, (2) CSF-negative meningoencephalitis, and (3) patients with pre-existing neuropsychiatric vulnerability. The balance of putative mechanisms may differ in each context but may include immune-mediated, hypoxic-ischemic, vascular, and metabolic factors. Management of AM in COVID should include exclusion of other life-threatening or reversible causes, as well as judicious consideration of medication based on clinical trajectory.
Footnotes
Disclosure: Drs. Beach and Fricchione received speaking honoraria for a presentation on catatonia and related concepts at the American Neuropsychiatric Association 2021 Annual Meeting.
References
- 1.Kennedy M., Helfand B.K.I., Gou R.Y., et al. Delirium in older patients with COVID-19 presenting to the Emergency Department. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach S.R., Praschan N.C., Hogan C., et al. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65 doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg R.K., Paliwal V.K., Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2021;93:206–222. doi: 10.1002/jmv.26207. [DOI] [PubMed] [Google Scholar]
- 7.Muccioli L., Pensato U., Bernabè G., et al. Intravenous immunoglobulin therapy in COVID-19-related encephalopathy. J Neurol. 2020 doi: 10.1007/s00415-020-10248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kas A., Soret M., Pyatigoskaya N., et al. The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 2021;48:2543–2557. doi: 10.1007/s00259-020-05178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilotto A., Masciocchi S., Volonghi I., et al. Clinical presentation and Outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: the ENCOVID Multicenter study. J Infect Dis. 2021;223:28–37. doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilotto A., Odolini S., Masciocchi S., et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88:423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nersesjan V., Amiri M., Lebech A.-M., et al. Central and peripheral nervous system complications of COVID-19: a prospective tertiary center cohort with 3-month follow-up. J Neurol. 2021;268:3086–3104. doi: 10.1007/s00415-020-10380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cani I., Barone V., D’Angelo R., et al. Frontal encephalopathy related to hyperinflammation in COVID-19. J Neurol. 2021;268:16–19. doi: 10.1007/s00415-020-10057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairns H., Oldfield R., Pennybacker J., Whitteridge D. Akinetic mutism with an epidermoid cyst of the 3rd ventricle. Brain. 1941;64:273–290. [Google Scholar]
- 14.American Congress of Rehabilitation Medicine Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil. 1995;76:205–209. doi: 10.1016/s0003-9993(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 15.Giacino J.T., Ashwal S., Childs N., et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 16.Giacino J.T., Katz D.I., Schiff N.D., et al. Comprehensive systematic review update summary: disorders of consciousness: report of the guideline development, dissemination, and Implementation Subcommittee of the American Academy of neurology; the American Congress of rehabilitation medicine; and the National Institute on Disability, Independent Living, and rehabilitation research. Arch Phys Med Rehabil. 2018;99:1710–1719. doi: 10.1016/j.apmr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Giacino J.T., Fins J.J., Laureys S., Schiff N.D. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- 18.Fisher C.M. Honored guest presentation: abulia minor vs. agitated behavior. Clin Neurosurg. 1983;31:9–31. doi: 10.1093/neurosurgery/31.cn_suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 19.Miller D.S., Robert P., Ereshefsky L., et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 2021 doi: 10.1002/alz.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernat J.L. Chronic disorders of consciousness. Lancet. 2006;367:1181–1192. doi: 10.1016/S0140-6736(06)68508-5. [DOI] [PubMed] [Google Scholar]
- 21.Ghoshal S., Gokhale S., Rebovich G., Caplan L.R. The neurology of decreased activity: abulia. Rev Neurol Dis. 2011;8:e55–67. [PubMed] [Google Scholar]
- 22.Najera J.E., Alousi A., De Lima M., Ciurea S.O. Akinetic mutism - a serious complication to tacrolimus-based GVHD prophylaxis. Bone Marrow Transpl. 2013;48:157–158. doi: 10.1038/bmt.2012.110. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigo-Armenteros P., Kapetanovic-García S., Antón-Méndez L., et al. Akinetic mutism and status epilepticus due to Epstein Barr virus encephalitis. Clin Neurol Neurosurg. 2019;185 doi: 10.1016/j.clineuro.2019.105492. 105492. [DOI] [PubMed] [Google Scholar]
- 24.Shprecher D., Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26:65–72. [PMC free article] [PubMed] [Google Scholar]
- 25.Arnts H., van Erp W.S., Lavrijsen J.C.M., van Gaal S., Groenewegen H.J., van den Munckhof P. On the pathophysiology and treatment of akinetic mutism. Neurosci Biobehav Rev. 2020;112:270–278. doi: 10.1016/j.neubiorev.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Schnakers C. Update on diagnosis in disorders of consciousness. Expert Rev Neurother. 2020;20:997–1004. doi: 10.1080/14737175.2020.1796641. [DOI] [PubMed] [Google Scholar]
- 27.Thibaut A., Bodien Y.G., Laureys S., Giacino J.T. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol. 2019;267:1245–1254. doi: 10.1007/s00415-019-09628-y. [DOI] [PubMed] [Google Scholar]
- 28.Fisher C.M. ‘Catatonia’ due to disulfiram toxicity. Arch Neurol. 1989;46:798–804. doi: 10.1001/archneur.1989.00520430094024. [DOI] [PubMed] [Google Scholar]
- 29.Lin L., Al-Faraj A., Ayub N., et al. Electroencephalographic abnormalities are common in COVID-19 and are associated with Outcomes. Ann Neurol. 2021;89:872–883. doi: 10.1002/ana.26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittaker A., Anson M., Harky A. Neurological Manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treadway M.T., Cooper J.A., Miller A.H. Can’t or won’t? Immunometabolic Constraints on dopaminergic drive. Trends Cogn Sci. 2019;23:435–448. doi: 10.1016/j.tics.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makhoul K., Jankovic J. Parkinson’s disease after COVID-19. J Neurol Sci. 2021;422 doi: 10.1016/j.jns.2021.117331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Northoff G., Krill W., Wenke J., Travers H., Pflug B. The subjective experience in catatonia: systematic study of 24 catatonic patients [Internet] Psychiatrische Praxis. 1996;23:69–73. [PubMed] [Google Scholar]
- 34.Shorter E., Fink M. Oxford University Press; New York, NY: 1998. The Madness of Fear: A History of Catatonia. [Google Scholar]
- 35.Beach S.R., Gomez-Bernal F., Huffman J.C., Fricchione G.L. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1–19. doi: 10.1016/j.genhosppsych.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. COVID-19–Associated encephalopathy and cytokine-mediated Neuroinflammation. Ann Neurol. 2020;88:860–861. doi: 10.1002/ana.25855. [DOI] [PubMed] [Google Scholar]
- 37.Muccioli L., Pensato U., Cani I., et al. COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J Neuroimmunol. 2020;349 doi: 10.1016/j.jneuroim.2020.577400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaughan M., Connolly S., O’riordan S., Tubridy N., Mcguigan C., Kinsella J.A. Pediatric parainfectious encephalitis associated with COVID-19. 2021;96:541–544. doi: 10.1212/WNL.0000000000011476. [DOI] [PubMed] [Google Scholar]
- 39.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis H.E., Assaf G.S., Mccorkell L., Wei H., Low R.J., Re’em Y., et al. Characterizing long COVID in an International Cohort: 7 Months of symptoms and their impact. 2020. Available from: [DOI] [PMC free article] [PubMed]
- 41.Németh G., Hegedüs K., Molnâr L. Akinetic mutism associated with bicingular lesions: Clinicopathological and functional anatomical correlates. Eur Arch Psychiatry Neurol Sci. 1988;237:218–222. doi: 10.1007/BF00449910. [DOI] [PubMed] [Google Scholar]
- 42.Fricchione G., Beach S. Handbook of Clinical Neurology [Internet] Elsevier B.V.; Cambridge, MA: 2019. Cingulate-basal ganglia-thalamo-cortical aspects of catatonia and implications for treatment; pp. 223–252. [DOI] [PubMed] [Google Scholar]
- 43.Nagaratnam N., Nagaratnam K., Ng K., Diu P. Akinetic mutism following stroke. J Clin Neurosci. 2004;11:25–30. doi: 10.1016/j.jocn.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Antony A.R., Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021:1–6. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y., Xu X., Chen Z., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020:1–28. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achar A., Ghosh C. COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain relevance. Cells. 2020;9:2360. doi: 10.3390/cells9112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herold T., Jurinovic V., Arnreich C., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong M., Liang X., Wei Y.D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol. 2020;189:1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gust J., Taraseviciute A., Cameron ·, Turtle J., Turtle C.J., Org C. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32:1091–1101. doi: 10.1007/s40263-018-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mack A.H., Schofield H.L. Applying (or not?) CAR-T neurotoxicity experience to COVID-19 delirium and agitation. Psychosomatics. 2020;61:859–860. doi: 10.1016/j.psym.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pensato U., Muccioli L., Cani I., et al. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol. 2021;8:968–979. doi: 10.1002/acn3.51348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilotto A., Masciocchi S., Volonghi I., et al. SARS-CoV-2 encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toniolo S., Di Lorenzo F., Scarioni M., Frederiksen K.S., Nobili F. Is the frontal lobe the Primary Target of SARS-CoV-2? J Alzheimers Dis. 2021:1–7. doi: 10.3233/JAD-210008. [DOI] [PubMed] [Google Scholar]
- 55.Sierra-Hidalgo F., Martínez-Salio A., Moreno-García S., de Pablo-Fernández E., Correas-Callero E., Ruiz-Morales J. Akinetic mutism Induced by tacrolimus. Clin Neuropharmacol. 2009;32:293–294. doi: 10.1097/WNF.0b013e3181a77fab. [DOI] [PubMed] [Google Scholar]
- 56.Kinsman B., Simmonds S., Browning K., Wenner M., Farquhar W., Stocker S. Integration of Hypernatremia and angiotensin II by the organum vasculosum of the Lamina Terminalis regulates thirst. J Neurosci. 2020;40:2069–2079. doi: 10.1523/JNEUROSCI.2208-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulick-Soper C.V., McKee J.L., Wolf R.L., et al. Pearls & Oy-sters: bilateral globus pallidus lesions in a patient with COVID-19. Neurology. 2020;95:454–457. doi: 10.1212/WNL.0000000000010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tengvar C., Johansson B., Sörensen J. Frontal lobe and cingulate cortical metabolic dysfunction in acquired akinetic mutism: a PET study of the interval form of carbon monoxide poisoning. Brain Inj. 2004;18:615–625. doi: 10.1080/02699050310001622806. [DOI] [PubMed] [Google Scholar]
- 59.Choi S., Lee S.H., Kim E.S., Eoh W. Delayed post-hypoxic leucoencephalopathy presenting akinetic mutism after traumatic cervical cord injury: a case report. Br J Neurosurg. 2013;27:529–531. doi: 10.3109/02688697.2013.771140. [DOI] [PubMed] [Google Scholar]
- 60.Lang M., Buch K., Li M.D., et al. Leukoencephalopathy associated with severe COVID-19 infection: Sequela of hypoxemia? Am J Neuroradiol. 2020;41:1641–1645. doi: 10.3174/ajnr.A6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boldrini M., Canoll P.D., Klein R.S. How COVID-19 affects the brain. JAMA Psychiatry. 2021;78:682–683. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis A., Frontera J., Placantonakis D.G., et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. 2021;421 doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Guennec L., Devianne J., Jalin L., et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020;61:e90–e94. doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pensato U., Muccioli L., Janigro D., Guarino M., Bisulli F., Cortelli P. Akinetic mutism in COVID-19-related encephalopathy: a cytokine-mediated maladaptive sickness behavioral response? Brain Behav Immun Health. 2021;15 doi: 10.1016/j.bbih.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown E.G., Chahine L.M., Goldman S.M., et al. The effect of the COVID-19 pandemic on People with Parkinson’s disease. J Park Dis. 2020;10:1365–1377. doi: 10.3233/JPD-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267:2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]