Abstract
Introduction
When stem cells are grafted into tissues, they differentiate and form specialized cells. However, the proficiency of stem cells to endure and assimilate the host cell is dependent on various growth factors and cytokines. According to various studies, these factors are available in the spent media of harvested stem cells, which can be used for treatment in regenerative medicine and cosmetic products. There are differences in cytokine secretion depending on the culture environment, which are clarified in this paper.
Methods
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) were cultured either in a bioreactor or in a flask. The conditioned medium from the hUC-MSC cultures in the flask and in the bioreactor was designated as “FM” and “BM”, respectively. We assessed the effects of FM and BM on UVB-induced oxidative stress, anti-aging, and melanogenic properties. The amount of growth factors, cell viability, hyaluronic acid (HA), pro-collagen, and pro-melanin were quantitatively evaluated in the FM and BM treated groups. The induction of HA and collagen synthesis was measured in CCD-986SK cells. For melanogenesis, the effects of FM and BM on melanin content and tyrosinase activity were measured in SK-MEL-31 cells.
Results
In the present study, the secretion of growth factors, HA, and pro-collagen was significantly higher in the BM treatment, compared to that in the FM treatment. BM protected CCD-986SK cells against death from UVB induced oxidative stress. BM increased the promoter activity of the anti-oxidant genes SOD1, CAT, and GP; and downregulated the accelerating collagen decomposition gene, MMP-1, induced by UVB irradiation. In α-melanocyte-stimulating hormone (α-MSH) stimulated SK-MEL-31 cells, BM reduced melanin production and decreased the levels of MITF, tyrosinase, TRP-1, and TRP-2. These results suggest that BM could be used as a skin protection agent, because of its anti-apoptotic, anti-aging, and anti-melanogenic properties. This could be attributed to the differences in culturing methods; it is difficult to maintain the temperature and sterility in FM culture, when compared to that in the automated culturing conditions of the BM system.
Conclusions
Collectively, our results indicate that using BM-conditioned hUC-MSC medium is very efficient process for producing raw materials for developing functional cosmetics.
Keywords: Human stem cell-conditioned medium, Growth factors, Anti-apoptosis, Anti-aging, Hyaluronic acid, Melanogenesis
1. Introduction
Stem cell-based cosmetics are an emerging science and are increasingly being used in regenerative medicine, offering a potential cure to various serious diseases, especially in unmet medical needs [1]. Longevity and aging societies are a global trend, and Korea is progressing rapidly into an aging society [2]. Stem cell replacement therapy and regenerative medicine have emerged as effective anti-aging methods. In addition, we are constantly researching and endeavoring to meet the human desire to control rapid aging and maintain youth.
Long considered both physiologic and unavoidable, skin aging is a degenerative phenomenon whereby both inherent and environmental factors intervene to produce a genuine disease. The pathobiological basis of skin aging remains poorly understood. At the cellular level, stem cell dysfunction and natural wastage appear to be key drivers, and both genetic and epigenetic factors are involved in a complex interaction that over time results in the decline of our main protective interface with the external environment. Past and current understanding of the cellular and molecular intricacies of skin aging provides a foundation for future approaches designed to revert the aging phenotype [1,3].
Collagen, elastin, and hyaluronic acid are the structural components of the skin, providing strength, elasticity, smoothness, and structure [4]. However, these structural elements decrease with age. Weakness to environmental stresses such as UV rays, smoking, toxins, pollutants, and lack of adequate nutrition cause skin damage, whereby the skin loses its elasticity, leading to the appearance of wrinkles, dark circles, and drooping. The skin is constantly exposed to the external environment; and therefore, it is susceptible to damages from physical irritation, ultraviolet rays, heat, and various pollutants. In particular, oxidative stress caused by reactive oxygen species (ROS) and UVs during routine metabolism is a major cause of skin aging [5]. The body is equipped with enzymes such as SOD, CAT, and GPx, which have the ability to control oxidation, thereby counteracting the imbalance and oxidation inhibition of oxides caused by oxidative stress induced by ROS. In addition, enzymes such as glutathione reductase (GR) and HO1 also protect the body against oxidative stress [6].
Recently, human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) were isolated and successfully cultured. The successful cultivation of functional hUC-MSCs implies that hUC-MSCs are capable of differentiating into neuronal cells and adipocytes [[7], [8], [9]], which has been demonstrated by many researchers. It has also been proven by many researchers that through the use of hUC-MSCs, viral vaccine production, treatment of skin ulcers, reduction of inflammation in the body, and recovery of damaged skin are effective [10]. This hUC-MSC conditioned medium consisting of various growth factors and cytokines is known to promote the regeneration of damaged tissue. Thus, stem cell-conditioned medium is an innovative development that can be used in skin care and hair care [1].
Growth factors and cytokines present in the conditioned medium play a role in cell division [11], growth of novel cells, and enhance the synthesis of collagen, elastin, and hyaluronic acid. Collagen synthesis helps to maintain the elasticity and reliability of the skin, making it appear younger. Cytokines are chemicals produced by stem cells that regulate cell division and promote survival. Cytokines stimulate tissue healing by attracting macrophages that reduce cell damage and activate fibroblasts that secrete collagen and elastin [1].
Growth factors and cytokines present in the conditioned medium, such as IGF, IGFBPs, VEGF, TGF-β, and PDGF are responsible for healthy skin. Aside from the skin health advantage, the conditioned medium contains essential growth factors for healthy skin growth and regeneration [12]. Growth factors present in the conditioned medium have the ability to promote skin-whitening effects and are used in skin-brightening cosmetics [13].
Therefore, in this study, hUC-MSCs were extracted separately using the same conditioned media, and the culture methods were compared to identify the optimal method. Consequently, we investigated the factors involved in the differentiation of hUC-MSCs by culturing hUC-MSCs. Lastly, we identified, measured, and compared the necessary components for wrinkle improvement, whitening, and moisturizing that depend on incubation conditions.
2. Materials and methods
2.1. Background
The hUC-MSCs used in this study were supplied by Prime Cell in the Czech Republic. Three hUCs collected from full-term births were used and evaluated separately. Ethical approval was obtained from the Institutional Review Board (IRB) of the University Hospital Brno.
2.2. Isolation of hUC-MSCs
The human umbilical cord (hUC) tissues were sanitized by treating them with phosphate-buffered saline (PBS)containing antibiotics for 10 min and then transferred to the laboratory in Hanks' Balanced Salt Solution (HBSS, Thermo Fisher Scientific, USA) within 1 h. The hUC tissues were cut into 0.3–0.5 cm pieces using sterile forceps and curved scissors. The pieces were cultured in sterile 10 mm Petri dishes containing low-glucose Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco) and 1% (v/v) penicillin/streptomycin (Gibco) at 37 °C in an incubator with a humidified atmosphere of 5% CO2, and the medium was refreshed every 24 h. A large number of fibroblast-like cells around the hUCs tissue pieces appeared one week later. The remaining hUC tissues were removed, and these primary fibroblast-like cells (passage 0) were passaged at 80% confluence using 0.25% trypsin (Gibco). The cells were resuspended in culture medium at a dilution ratio of 1:3 and expanded on a new plastic Petri dish to passage 1.
2.3. Culture of hUC-MSC
The hUC-MSCs were resuspended at 5 × 105 cells/cm2 in T-175 cm2 flasks (Thermo Fisher Scientific, USA), and hUC-MSCs were cultured in FBS-free DMEM medium (StemPro MSC SFM Xeno Free medium, Life Technologies, Gaithersburg, MD) for six days. Upon reaching 80% confluence, the monolayer was washed and harvested using Trypsin–EDTA (Thermo Fisher Scientific, USA), and the cells were split 1:4 into a new T-175 flask. The cells routinely replaced the medium when 80% of the mating was reached, which took approximately three days. The cultured medium was collected with a 0.2 μm filter replaced each time with the medium and stored in a deep freezer at −80 °C. We named it FM, which was kept in the flask.
2.4. Expansion of hUC-MSCs in the bioreactor
The bioreactor (TERUMOBCT, Lakewood, CO, USA) was fed via two circulation loops. The internal loop and the extra capillary loop, both of which have inlets for media and reagents or cells (external loop only) [14]. The medium used to cultivate hUC-MSCs in the bioreactor was the same medium used for the hUC-MSCs in the FM. The hUC-MSC medium for bioreactor cultivation was obtained from Prime Cell Therapeutics Inc., Czech Republic. The culture method was automatically set by a program embedded in the bioreactor.
The hUC-MSCs were plated at 5 × 105 cells and were cultured in FBS-free DMEM (StemPro MSC SFM Xeno Free medium, Life Technologies, Gaithersburg, MD) for eight days.
The culture supernatant was collected in a bag attached to the bioreactor, filtered through a 0.2 mm filter, and stored at −80 °C. This conditional medium was labeled as BM to indicate that it was obtained following the systematic culture of hUC-MSCs in a bioreactor.
2.5. Cell line culture
For accurate evaluation, the following cell lines were purchased: CCD-986SK cells derived from human skin fibroblasts, purchased from the Korean Cell Line Bank, KCLB, Korea (Korean Cell Line Bank; KCLB), and cultivated in Iscove's modified Dulbecco's medium (Invitrogen, Carlsbad, CA, USA) with 10% FBS and 1% penicillin/streptomycin (100 U/mL) at 37 °C in a 95% air and 5% CO2 atmosphere.SK-MEL-31 cells are a human melanoma cell line, SK-MEL-31 cells obtained from the Korean Cell Line Bank (KCL; KCLB, which is a melanoma cell line cultivated in minimum essential medium (Gibco, NY, USA) with 10% FBS and 1% penicillin/streptomycin (100 U/mL) at 37 °C in a 95% air and 5% CO2 atmosphere.
2.6. Identification of hUC-MSCs
FACS: hUC-MSCs were analyzed by fluorescence-activated cell sorting (FACSVerse, BD Bioscience-US) for cell surface marker expression. Cells were harvested using 0.25% trypsin and washed with flow cytometry buffer. For fluorescence-activated cell sorting (FACS), the sheep anti-human α-CD44 antibody conjugated with PE (R&D, Minneapolis, Minnesota, USA), rat anti-human α-CD34 antibody conjugated with PE (R&D, Minneapolis, Minnesota, USA), rat anti-human α-CD105 antibody conjugated with FITC (Abcam, Cambridge, MA, USA), and rat anti-human α-CD45 antibody conjugated with PE (R&D, Minneapolis, Minnesota, USA) were used. The antibodies were diluted in phosphate-buffered saline (PBS) and 2% bovine serum albumin (BSA) to determine their working concentrations. Staining was performed for 30 min at 4 °C.
For the immunocytochemistry assay, cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton-X 100 (Sigma–Aldrich, USA), permeabilized, and blocked with 0.5% PBST and 2% BSA in PBS. Cells were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-α-CD105, 1:200 (Abcam, Cambridge, MA, USA), mouse anti-α-CD34, 1:200 (Abcam, Cambridge, MA, USA), rat anti-α-CD45 1:200 (Abcam, Cambridge, MA, USA), and rabbit anti-α-CD44 1:200 (Abcam, Cambridge, MA, USA). After washing with PBS, the cells were incubated with secondary antibodies (FITC or rhodamine) for 1 h at room temperature. Cells were counterstained with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) for nuclear staining.
2.7. Measuring human growth factor antibody array
The human growth factor antibody array was obtained from RayBiotech (RayBio ® C-Series Human Growth Factor Antibody Array C1, #AAH-GF-1-8, RayBiotech, Inc. 3607 Parkway Lane, Suite). The human growth factor antibody array was used for the simultaneous detection of 41 human growth factors (amphiregulin, bFGF, EGF, EGF R, FGF-4, FGF-6, FGF-7, GCSF, GDNF, GM-CSF, HB-EGF, HGF, IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-6, IGF-I, IGF-I SR, IGF-II, M-CSF, M-CSF R, beta-NGF, NT-3, NT-4, PDGF Ra, PDGF Rβ, PDGF-AA, PDGF-AB, PDGF-BB, PLGF, SCF, SCF R, TGF-alpha, TGF-beta, TGF-beta2, TGF-beta3, VEGF-A, VEGF R2, VEGF R3, and VEGF-D) and was suitable for all sample types. Cytokine arrays are antibody-pair-based assays analogous to ELISA. The experimental method was to remove the membrane packaging of the plate according to the protocol provided by the manufacturer, and after the blocking solution operation, samples (FM and BM) were placed in each well and injected overnight at 4 °C. The next day, after the first and second wash, the HRP-streptavidin incubation was conducted overnight at 4 °C and the third wash. The next day, chemiluminescence detection identified the anti-body levels expressed in the membrane. After the expression was completed, the film was scanned and quantified using the ImageJ software. For comparison of expression aspects, internal positive controls were used to define the signal strength. Two duplicate spots were used for all samples. Concentrations of various human growth factors in conditioned media from the flask and bioreactor were assayed using a human growth factor antibody array kit.
The formula for the measurement is as follows:
| X (Ny) = X(y) ∗ P1/P(y) |
where: P1 = mean signal density of Positive Control spots on reference array; P(y) is the signal density of Positive Control spots on Array “y”; and X(y) = X (Ny) as the normalized signal intensity for spot “X” on Array “y”.
2.8. MTT assay on cytotoxicity
The cells (CCD-986SK: 5 × 104 cells/well, SK-MEL31: 5 × 104 cells/well) were incubated at 37 °C in a 95% air and 5% CO2 atmosphere for 24 h. Samples were treated with FM and BM at various concentrations (0%, 30%, 60%, and 100%) and incubated for a further 48 h. After washing with PBS, MTT solution (3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl-2H-tetrazolium bromide (MTT) solution was added and incubated for 4 h. The culture medium was removed, 150 μL of DMSO or H2O2 was added, shaken for 10 min at 25 °C, and absorbance was measured at 540 nm with an ELISA reader (EPOCH, BioTek).
2.9. Measurement of the content of hyaluronic acid (HA), pro-collagen, and pro-melanin
The amount of hyaluronic acid (HA), pro-collagen, and pro-melanin was contained in BM and FM. We used CCD-986SK cells for HA and pro-collagen measurements and MEL-31SK cells for Pro-melanin measurements.
The cells (CCD-986SK: 5 × 104 cells/well, SK-MEL31: 5 × 104 cells/well) were incubated at 37 °C in a 95% air and 5% CO2 atmosphere for 24 h. Samples were treated with FM and BM and incubated for an additional 72 h. Each culture medium was collected and quantified using the respective kits.
2.10. Hyaluronic acid (HA) assay
The hyaluronic acid (HA) assay was performed using the Hyaluronan Quantizing ELISA Kit (R&D Systems, USA). The experimental method followed the protocol provided by the manufacturer. CCD-986SK cells were seeded (2 × 104 cells μL 200 μl into a 96-well plate and incubated for 24 h to stabilize cell adhesion. The medium was removed and replaced with fresh PBS (three drops). Then, UV-B (20 mJ/cm2) was irradiated for 1 min and recovered. BM and FM at 30% were treated and incubated for 72 h. The cell culture medium of each condition was collected and measured using a hyaluronan quantizing ELISA kit. A TGF-β concentration of 20 ng/mL was used as the positive control.
2.11. Pro-collagen assay
For the pro-collagen assay, we used the protocol of Bio-Rad Gel Doc XR System, Procollagen type-1 C peptide (PIP) ELISA kit (Takara). Briefly, cells were seeded in 96-well plates under CCD-986SK, 2 × 104 cells per 200 μl, and incubated for 24 h for cell attachment and stabilization. The cells were incubated in minimal essential medium (MEM) 85%, FBS 15%, and 1% penicillin-streptomycin (10,000 U/mL) solution at 37 °C in an incubator containing 5% CO2. After 24 h, the medium was removed and replaced with fresh PBS (three drops). Then, UV-B (20 mJ/cm2) was irradiated for 1 min and recovered. BM and FM at 30% concentration were treated and incubated for 72 h. The cell culture medium of each condition was collected and measured using a procollagen type-1 C peptide (PIP) ELISA Kit. A TGF-β concentration of 20 ng/mL was used as the positive control.
2.12. Tyrosinase activity assay
For the tyrosinase activity assay, SK-MEL31 cells were injected at a dose of 1 × 105 cells per well in a 6-well plate. SK-MEL31 cells were incubated with minimum essential medium (MEM) 85%, FBS 15%, and 1% penicillin-streptomycin (10,000 U) at 37 °C in an incubator containing 5% CO2 for 24 h. The medium was removed, the cells were washed with PBS, and tyrosinase was added and incubated for 72 h. The medium should be free of phenol red if possible, so that it does not affect the absorbance measurement. The medium may be treated with α-melanocyte-stimulating hormone (α-MSH) to promote melanin production. The medium was removed, cells were carefully cleaned with PBS and mixed with 0.1 M phosphate buffer and placed on ice for 1 h, waiting for the tyrosinase to be released from the melanosomes, and centrifuged at 13,000 rpm for 20 min at 4 °C. The supernatant was mixed with 0.2% L-DOPA solution, incubated at 37 °C for 30 min, and tyrosinase activity was measured at 490 nm using an ELISA reader.
2.13. Reverse transcription (RT)-PCR
To confirm the anti-wrinkle effects, the CCD-986SK cell line was cultured at 1 × 105 cells per well in a 6-well plate and incubated for 24 h. CCD-986SK cells were incubated in a basal medium, followed by removal of the medium and then 3 drops of PBS and UV-B (20 mJ/cm2) irradiation for 1 min. The cells were washed once with PBS, and then the medium was replaced with fresh medium, followed by incubation for 48 h with complete basic growth medium (Control), FM, and BM.
SK-MEL31 cells were seeded at 1 × 105 cells per well in a 6-well plate and incubated for 24 h to observe their effect on tyrosinase, TRP-1, TRP-2, and MITF gene expression in FM and BM. Briefly, SK-MEL31 cells were incubated in a basal medium, followed by removal of the medium, followed by 3 drops of PBS and UV-B (20 mJ/cm2) irradiation (1 min). Subsequently, the cells were washed once with PBS, and then the medium was replaced with fresh one, followed by incubation for 48 h with complete basic growth medium (Control), FM, and BM, and stimulated with α-MSH treatment.
Total RNA extraction was performed using the RNeasy Mini Kit (Qiagen, MD, USA). cDNA synthesis was performed by heating 1 μg of total RNA with oligo (dT) 15 primer, dNTP (0.5 M), 1 unit RNase inhibitor, and 4-unit Omni script reverse transcriptase (Qiagen, Hilden, Germany) for 60 min at 37 °C and 5 min at 93 °C. The process was stopped by the reaction. The expression of MMP-1, MIFT, tyrosinase, TRP-1, TRP-2, SOD1, CAT, GPx and GAPDH genes was confirmed by image analysis (BIS303PC, DNR Imaging Systems Ltd, UK) by electrophoresis on a 1.5% agarose gel. Band density was measured using a densitometric program (NIH Image software, MD, USA). The sequences of MMP-1, MIFT, tyrosinase, TRP-1, TRP-2, DOS1, CAT, GPx and GAPDH oligonucleotide primers are shown in Table 1.
Table 1.
Sequences of specific primers used in Reverse Transcription (RT)- PCR analysis.
| Gene | Sequence (5’ to 3’) |
|---|---|
| MMP-1 | F 5′- CACAGCTTCCCAGCGACTC -3′ |
| R 5′- GTCCCGATGATCTCCCCTGA -3′ | |
| MIFT | F 5′-CCGTCTCTCACTG GATTGGTG-3′ |
| 5′-CGTGAATGTGTGTTCATGCCTGG-3′ | |
| Tyrosinase | F 5′- GTCCACTCACAGGGATAGCAG -3′ |
| R 5′- AGAGTCTCTGTTATGGCCGA -3′ | |
| TRP-1 | F 5′- ACTTCACTCAAGCCAACTGC -3′ |
| R 5′- AGCTTCCCATCAGATGTCGT-3′ | |
| TRP-2 | R 5′- GCTCCAAGTGGCTGTAGACC-3′ |
| R 5′- AATGCAGTGGCTTGGAAATC-3′ | |
| SOD1 | F 5′- GGGAGATGGCCCAACTACTG -3′ |
| R 5′- CCAGTTGACATCGAACCGTT -3′ | |
| CAT | F 5′- ATGGTCCATGCTCTCAAACC -3′ |
| F 5′- CAGGTCATCCAATAGGAAGG -3′ | |
| GPx | F 5′- AAGGTGCTGCTCATTGAGAATG-3′ |
| F 5′- CGTCTGGACCTACCAGGAACTT-3′ | |
| GAPDH | F 5′- TGGAATCCTGTGGCATCCATGAAAC-3′ |
| R 5′- TAAAACGCAGCTCAGTAACAGTCCG-3′ |
2.14. Statistical analysis
The data were averaged, and the sample was taken from at least three to 3–5 independent cell samples. To analyze the data, we used Student's t-test and one-way analysis of variance (ANOVA), and statistical significance was set at p < 0.05.
3. Results
3.1. Morphology and surface analysis of the human umbilical cord derived mesenchymal stem cells (hUC-MSCs)
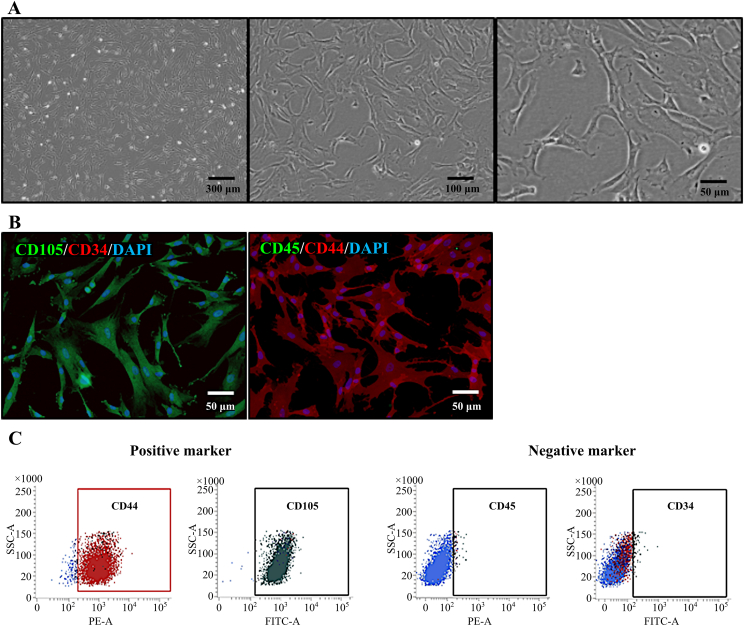
Immunostaining and fluorescence-activated cell sorting (FACS) were performed on each marker to identify the characteristics of hUC-MSCs (Passage 1) isolated from human umbilical cord tissue. The hUC-MSCs have a morphology similar to spindle- and fibroblastic-like shapes. The hUC-MSCs were successfully isolated and cultured in this experiment (Fig. 1A). Immunostaining revealed that the positive markers CD105 and CD44 were expressed, while negative markers CD34 and CD45 were not expressed (Fig. 1B). As shown in Fig. 1A and B, the hUC-MSCs were positive for specific antigen markers of CD105 and CD44, while they were negative for CD34 and CD45. The surface marker profiles of hUC-MSCs were analyzed using FACS. The percentage of expressed surface positive markers was 99.44% for CD44, 99.92% for CD105 and the percentage of negative expressed surface markers was 1.7% for CD34, and 2.3% for CD45 (Fig. 1C) [15]. The FACS results were similar to those obtained by immunostaining. Therefore, it can be confirmed that pure hUC-MSCs have unique characteristics.
Fig. 1.
Characterization of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) at Passage 2. A. The hUC-MSCs were isolated and cultured from the umbilical cord tissue. Morphology of hUC-MSCs shows successful proliferation. (Scale bar: 300 μm, 100 μm, 50 μm). B. Immunohistochemistry staining of hUC-MSCs with antibodies providing positive or negative signals. MSCs were subjected to immunolocalization of MSCs specific surface antigens using primary antibodies directed against CD 105, CD34, CD45, and CD44 and stained by secondary antibodies conjugated with FITC/Rhodamine and counterstained with DAPI. Positive markers CD105 and CD44 were expressed, and negative markers CD34 and CD45 were not expressed. (Scale bar: 50 μm). C. hUC-MSCs characterization by fluorescence-activated cell sorting (FACS). Expression level of surface markers is maintained during in vitro culture of hUC-MSCs. Positive markers CD44 and CD105 were expressed as a high percentage, and negative markers CD45 and CD34 were detected as a low percentage.
3.2. Detection of active cytokines from conditioned media of hUC-MSCs
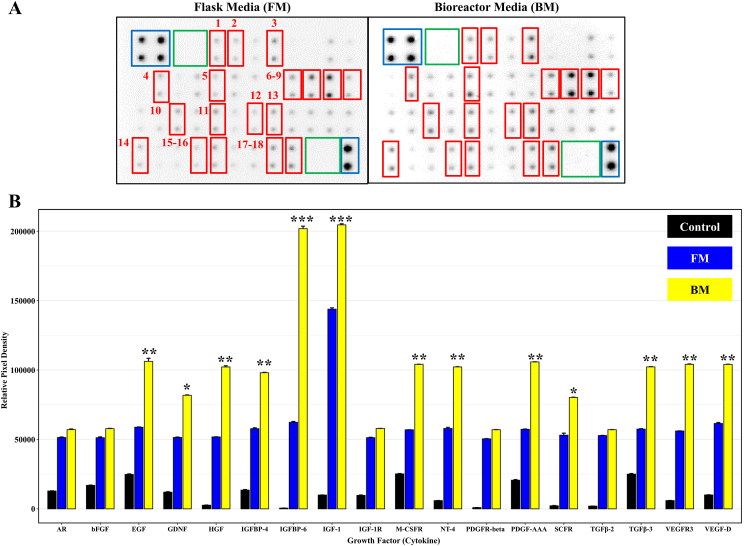
The hUC-MSCs secrete various cytokines. Recently, active cytokines secreted from stem cells have been considered to have benefits in tissue regeneration because secreted cytokines and growth factors can contribute to the host tissue microenvironment [16]. Therefore, we confirmed the paracrine effects of stem cells using a human growth factor array. This array was confirmed for semi-quantitative detection of 41 human cell culture media [17]. The culture medium of hUC-MSCs in the flask was “FM” and that of hUC-MSCs in the bioreactor was “BM.” All 41 antibodies were more strongly expressed in BM than in FM. Among these, 13 secreting growth factors were significantly detected in the FM and BM. Positive and negative controls were used in the array. The following growth factors: 1 – Epidermal Growth Factor (EGF); 2 – Fibroblast Growth Factor-6 (FGF-6); 3 – Glial cell-derived neurotropic factor (GDNF); 4 – hepatocyte growth factor (HGF); 5 – insulin-like growth factor-binding protein-4/6 (IGFBP-4/6) Insulin-like growth factor-1 (IGF-1); 6 – Macrophage Colony Stimulating Factor (M-CSFR); 7 – Neurotrophin-4 (NT-4); 8 – Platelet Derived Growth Factor-AA (PDGF-AA); 9 – Stem Cell Factor Receptor (SCFR); 10 – Vascular Endothelial Growth Factor-3 (VEGFR-3), vascular endothelial growth factor-D (VEGF-D) were expressed in FM and BM (Fig. 2A). In particular, we graphically represented 13 significant antibody results (Fig. 2B). It was confirmed that 13 cytokines, which had a significant impact on stem cell growth, were more strongly expressed in BM than in FM.
Fig. 2.
Quantification of human growth factor secretions expressed in the culture of FM and BM of hUC-MSC. A. The culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the flask is “FM” and that of hUC-MSCs in the bioreactor is “BM.” Cytokines released from hUC-MSCs's conditioned media (FM and BM) were detected using the RayBio C-Series human growth factor antibody kit. Cytokines are spotted and those released from conditioned media appear as black dots. Each antibody was spotted in duplicate. Of the total 41 cytokines, 18 cytokines with significant differences in expression between FM and BM were selected (red box). All 41 antibodies were more strongly expressed in BM than in FM. Among them: blue box – positive spot (controlled amount of biotinylated antibody printed of non-specific onto the array; used for normalization and to orientate the arrays); green box – negative spot (no antibodies used to measure the baseline responses; used for determining the level binding of the samples); red box – 1: AR, 2: bFGF, 3: EGF, 4: GDNF, 5: HGF, 6: IGFBP-4, 7: IGFBP-6, 8: IGF-1, 9: IGF-1R, 10: M-CSFR, 11: NT-4, 12: PDGFR-beta, 13: PDGF-AA, 14: SCRF, 15: TGF-beta2, 16: TGF-beta3, 17: VEGFR3, 18: VEGF-D. Cytokines presenting a detectable black spot signal are indicated. The human growth factor antibody array was used to detect paracrine effect in hUC-MSCs-conditioned media in FM (left panel) and BM (right panel). B. The result confirmed that 18 cytokines, which had a significant impact on stem cell growth, were more strongly expressed in BM than in FM. The control has nothing in it. Experiments were performed in triplicate. The experimental significance was determined by Student's t-test. Significantly differences between FM and BM were evaluated at ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001.
3.3. General effects of FM and BM on the viability of CCD-986SK cells and SK-MEL-31
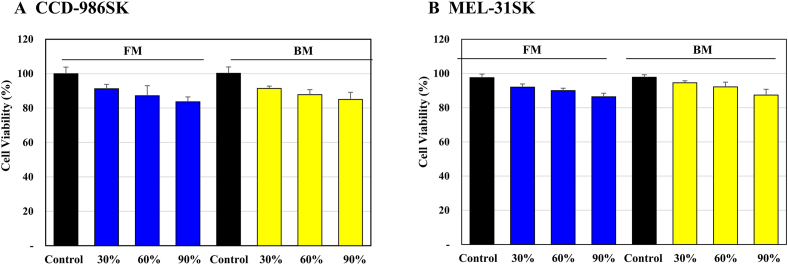
For the cytotoxicity test, concentrations were adjusted to 30%, 60%, and 100% for both BM and FM in the CCD-986SK and SK-MEL-31 cell lines to determine the effective concentration of the culture (Fig. 3A and B). It was confirmed that the cells were not cytotoxic due to an 80% survival rate at each concentration. It was confirmed that the cell viability was the best in the 30% concentration conditions of FM and BM. Therefore, the concentrations of FM and BM were set to 30%.
Fig. 3.
General effects of FM and BM on the viability of CCD-986SK and SK-MEL-31 cells. The culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the flask is “FM” and that of hUC-MSCs in the bioreactor is “BM.” Cell viability was assessed with the colorimetric MTT assay. BM and FM were treated by various concentration (30%, 60%, and 100% of BM and FM) on CCD-986SK (A) and SK-MEL-31(B) to confirm cytotoxicity. The survival rate was more than 80% at each concentration and no cytotoxicity was confirmed. The control has nothing in it. Experiments were performed in triplicate.
3.4. Measurement of the amount for hyaluronic acid (HA), pro-collagen, and pro-melanin
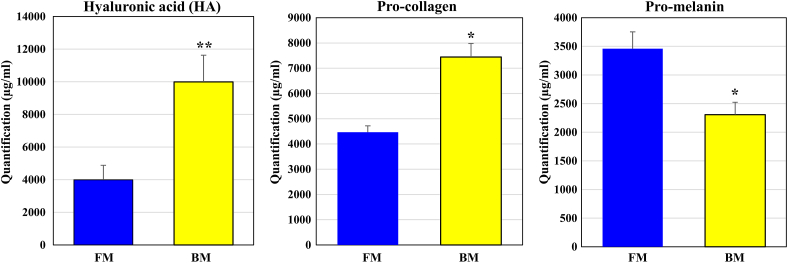
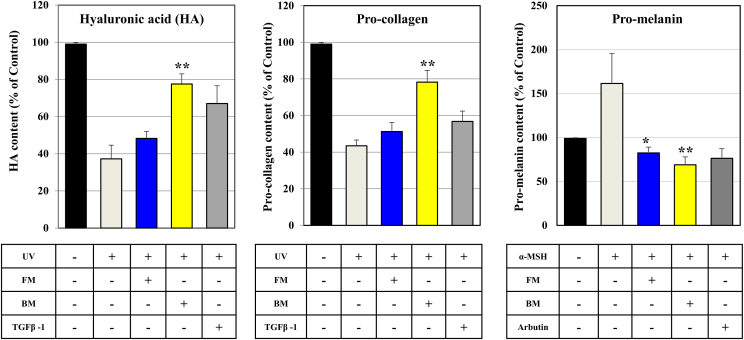
The contents of hyaluronic acid (HA), pro-collagen, and pro-melanin in BM and FM was measured using an ELISA kit (Fig. 4). As a result, HA, pro-collagen, and pro-melanin were significantly increased in both FM and BM. The quantitative results of HA and Pro-collagen confirmed that BM contained more than FM, and Pro-collagen showed that BM was lower than FM. Considering that the unit is large, the figures below the decimal point are deleted. These results suggest that the effect of BM on skin moisturization and wrinkle improvement was better than that of FM. However, in Pro-melanin, the increase was greater in FM than in BM. These results suggest that skin whitening is more effective in the BM.
Fig. 4.
The quantification of concentration for Hyaluronic acid, Pro-collagen, Pro-melanin in FM and BM. The culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the flask is “FM” and that of hUC-MSCs in the bioreactor is “BM.” The concentration Hyaluronic acid (HA), Pro-collagen, Pro-melanin was evaluated using ELISA kits. Results are expressed as respective control. Experiments were performed in triplicate. HA and Pro-collage showed high levels in BM and Pro-melanin showed high levels in FM. The experimental significance was determined by Student's t-test. Significantly differences between FM and BM were evaluated at ∗P < 0.05; and ∗∗P < 0.01.
3.5. BM increase pro-collagen, hyaluronic acid expression and represses pro-melanin
UV irradiation from the sun reduces type I pro-collagen formation by fibroblasts in the skin We used cultured CCD-986SK cells, adult human skin fibroblasts, as a representation to examine the mechanisms of this effect. We first inspected the effects of UV irradiation on the underlying and TGF-β1-stimulated type I pro-collagen depress. We confirmed that the group exposed to UV and BM-treated groups further increased the formation of pro-collagen and hyaluronic acid than the FM-treated groups and increased by the comparator TGF-β1 treatment group. In particular, it is worth noting that the group treated with BM in pro-collagen measurements was higher than that in the positive control group, TGF-β1 (Fig. 5).
Fig. 5.
Concentration for hyaluronic acid in FM and BM. The culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the flask is “FM” and that of hUC-MSCs in the bioreactor is “BM.” Effects on HA, Pro-collagen synthesis by FM and BM treats in CCD-986SK cells; and role of FM and BM synthesis in melanoma cells line (SK-MEL-31). Experiments were performed in triplicate. Significant differences between FM and BM and the non-irradiated control were evaluated at ∗P < 0.05 and ∗∗P < 0.01.
To investigate the whitening effect of stem cell culture medium, the activity of tyrosinase, which plays a key role in melanin synthesis, was measured using SK-MEL-31 cell lines (human melanoma cell line). In addition, α-MSH, which is known to cause melanin, and arbutin, a whitening material known to inhibit melanin production, was treated. As a result, BM had lower intracellular melanin levels than FM and treated with α-MSH and arbutin (Fig. 5). From these results, we were able to further confirm that BM is more effective than FM in aging suppression function, skin moisturizing, and whitening effects.
3.6. Measurement of anti-wrinkle related mRNA expression using RT-PCR
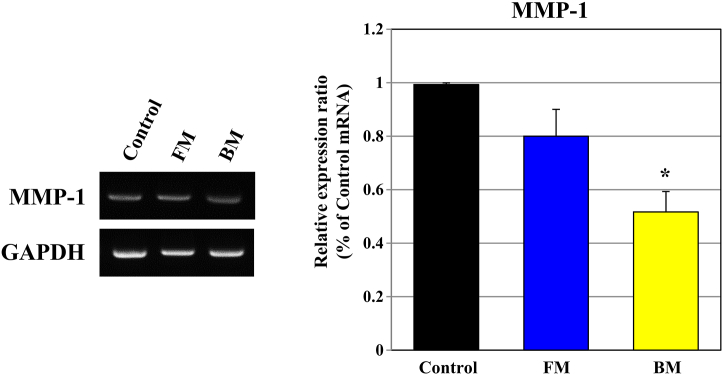
A major clinical symptom of skin aging, wrinkles, and reduced skin elasticity can result in a decrease in extracellular matrix proteins in the dermis. In UVB-stimulated CCD-986SK cells, adult human skin fibroblasts, they are broken down by MMPs such as collagenase, elastase, and gelatinase, causing damage to the length and distribution of collagen fibers, and collagen associated with skin cell wrinkles is synthesized in fibroblasts [18,19]. As the amount of pro-collagen decreases due to UV rays, the amount of collagen decreases. Thus, the amount of intracellular collagen synthesis can be determined by measuring the amount of MMP-1 in cells. The MMP-1 expression experiment showed that BM showed an approximately 2 lower growth rate compared to control and FM (Fig. 6). As a result, we confirmed that BM increases the pro-collagen reduction due to UV-B in fibroblasts and can be a preventable raw material for wrinkle production by inhibiting the increased MMP-1 expression.
Fig. 6.
Effect of BM on UVB-induced production of inhibition of MMP-1 protein in CCD-986SK. The culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the flask is “FM” and that of hUC-MSCs in the bioreactor is “BM.” Effect of FM and BM on MMP-1 gene expression. UVB (20 mJ/cm2)-exposed CCD-986SK cells were cultured for 48 h in the presence of control, BM, and FM. Control: the control group was stimulated with UVB with no basic culture medium. Results were expressed as % of GAPDH and data represent the means ± SD. Significant differences between FM and BM and the control were evaluated at ∗P < 0.01.
3.7. Measurement of whitening-related mRNA expression using RT-PCR
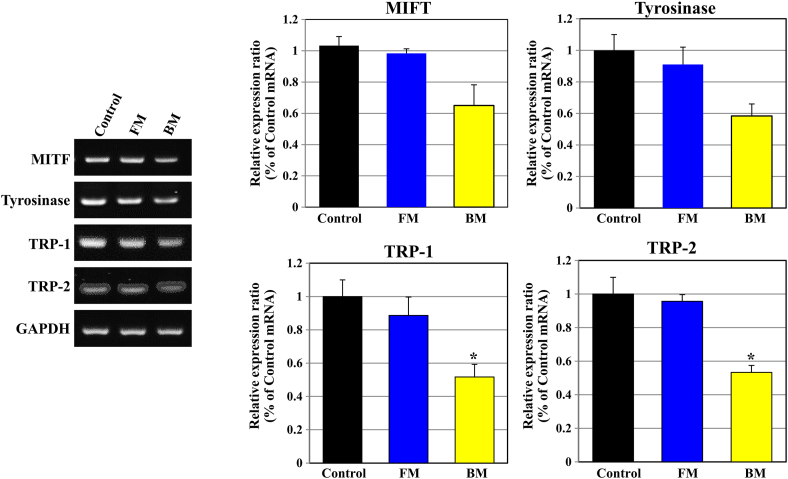
Melanin synthesis is converted to DOPA quinone via DOPA by tyrosine, TRP-1, and TRP-2, with a substrate of one amino acid [20]. TRP-1 and TRP-2 finally synthesize melanin by converting 5,6-dihydroxyindole-2-carboxylic acid (DHICA) into indole-5,6-quinone-2-carboxylic acid (IQCA), which is a dark brown color, and have been widely studied for whitening effectiveness as a pathway to suppress melanin production and the activity of enzymes involved in promoting the breakdown of melanin [21,22]. MITF promotes the transcription of tyrosinase, TRP-1, and 2, an important transcriptional modulating factor in melanin synthesis [23]. We confirmed MITF, TRP-1, TRP-2, and tyrosinase mRNA expressions after treatment with SK-MEL-31 cells to determine the effect of FM and BM on tyrosinase, enzymes associated with melanin synthesis. We confirmed that the expression MITF, TRP-1, TRP-2, and tyrosinase mRNA on SK-MEL-31, which processed BM, was suppressed over FM. In BM, mRNA levels of MIFT, tyrosinase, TRP-1, and TRP-2 showed approximately 40%–50% inhibition compared to the control group (Fig. 7). It was confirmed that the above results suggest that BM inhibit the expression of genes in the tyrosinase pathway. This suggests the possibility of using BM as a whitening functional cosmetic material.
Fig. 7.
Inhibition effects culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) extract on whitening related RNAs of melanogenesis in SK-MEL31 cells. The culture medium of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the flask is “FM” and that of hUC-MSCs in the bioreactor is “BM.” Effects of FM and BM on the expression of MITF, tyrosinase, TRP-1 and TRP-2 in SK-MEL-31 cells treated with basic culture medium (Control), FM, and BM are shown. FM and BM play an important role in the expression of MIFT and tyrosinase and production of melanin. The effect on the expression of MITF, tyrosinase, TRP-1, and TRP-2 (a transcriptional promoter), was analyzed with RT-PCR. The control group is basic culture medium instead of FM and BM in SK-MEL31. Significant differences were evaluated at ∗P < 0.01.
3.8. Expression analysis of antioxidant-related genes using RT-PCR
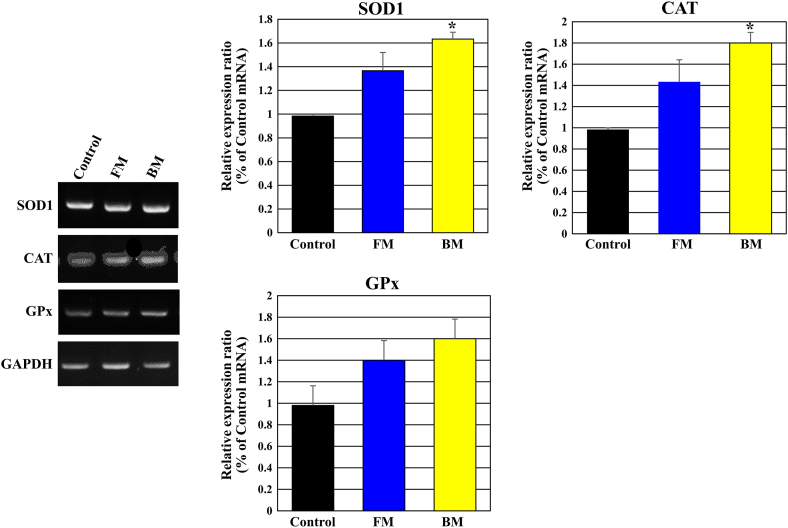
SOD, a leading primary antioxidant in the human body, converts O2-, an oxidative stress-causing ROS into H2O2 and oxygen [24]. In mammals, SOD families are classified as SOD1, SOD2, and extracellular superoxide dismutase (EC-SOD; SOD3), according to the types of metal ions present in the SOD active center; SOD1 is present in cytoplasm, SOD2 in mitochondria, and SOD3 in three different locations [25]. CAT decomposes H2O2 into water and oxygen, one of the ROSs that is inevitable in living organisms that maintain life through respiration with antioxidant enzymes. CAT is found extensively in organisms varying from higher animals, plants, to microorganisms [26]. H2O2, produced by SOD, is degraded as GPx oxidizes GSH to GSSG. GSSG is reduced to GSH by glutathione reductase (GR). There is a balance between the two forms, GSH and GSSG, in the human body. In general, more than 98% exists as GSH in cells and only 2% is converted to GSSG due to oxidative stress [27]. The expression of the antioxidant genes, SOD1, CAT, and GPx were evaluated to determine the antioxidant effect of FM and BM in the human melanoma cell line, SK-MEL- 31, treated with UVB. The expression of SOD1, CAT, and GPx was higher in the BM treatment, compared to that in the FM treatment, with a high sulfur oxidation efficacy in the BM treated cells. This maintains intracellular reduction and protects the cell from oxidative stress (Fig. 8).
Fig. 8.
Effects of FM and BM on the expression of antioxidant genes in CCD-986SK cells treated with UVB irradiation. UVB (20 mJ/cm2)-exposed CCD-986SK cells were cultured for 48 h in the presence of Control, BM, or FM. The control group is CCD-986SK cells treated with basic culture medium instead of FM and BM. The expression of SOD1, CAT, and GPx increased when CCD-986SK cells were treated with FM or BM, compared to that in the control group following UVB irradiation. ∗P < 0.01 was considered statistically significant.
4. Discussion
In this study, we confirmed the whitening and anti-wrinkle effects of functional cosmetics in the incubation medium of hUC-MSCs. Furthermore, we compared and evaluated the culture medium secreted from hUC-MSC culture in detail using quantum bioreactor cell extension systems and commonly used flask-based methods.
We investigated 41 human growth factors related to improvement of skin condition present in FM and BM and found that 18 cytokines (AR, bFGF, EGF, GDNF, HGF, IGFBP-4, IGFBP-6, IGF-1, and M−CS) exist in BM than in FM. Many studies have assessed the role of each cytokine in skin improvement and metastasis during the anti-aging process [[28], [29], [30], [31], [32], [33]].
In addition, several studies have confirmed that it is related to factors that help regenerate skin functions such as whitening and wrinkle improvement among cytokines with high expression in BM [34,35]. Therefore, we can expect that the BM we checked will be of considerable help in improving the skin.
To determine the concentration of culture medium used in this study, MTT was conducted using CCD-986SK (Human Fibroblast Cell Line) and SK-MEL-31 (Human Melanoma Cell Line) cell lines, and it was confirmed that the concentration of culture medium did not affect cell survival even if 90% of cells were considered. However, we decided for 30%, considering the economy for commercialization. If all the skin regeneration-related experiments were conducted using the undiluted solution, it is expected that all the compounds would maximize their effectiveness.
The amount of HA and pro-collagen originally contained in BM and FM showed that HA and pro-collagen had higher BM than FM. Skin aging is the result of the inherent chronological aging process superimposed by environmental factors, principally exposed to ultraviolet (UV) radiation [3,12]. These results show that, when tested in UV-B-promoted fibroblast cells, CCD-986SK, the BM produced more HA and pro-collagen than FM and positive controls, which demonstrated to be more effective in anti-wrinkle and skin regeneration.
Alterations in collagen and elastin in the ECM are mainly responsible for the clinical signs of skin aging such as wrinkles, drooping, and unauthenticity [1,3,12,13,36]. The shrinkage of collagen and elastin fibers in skin aging is predominantly due to the increased expression of their degradative enzymes, collagenases (MMP-1), gelatinases, and elastases [1,3,12,13,36]. Collagen fibers are degraded by MMP-1 and elastin fibers by elastases [37]. Collagen, among the components constituting the connective tissue of skin cells, is an important constituent protein, accounting for about 90%. Collagen is not affected by proteases such as trypsin but is degraded by collagenase. Among the major enzymes that degrade collagen, it is commonly to occur by MMP-1 induced by the transcription factor active protein-1. MMP-1 is a degrading factor that inhibits collagen synthesis by affecting collagen type. Therefore, the expression of MMP-1 at the protein level was determined by inhibiting the activity of MMP-1, which is thought to reduce collagen degradation and thus suppress the maintenance of skin elasticity and wrinkling. BM was found to significantly reduce the expression of MMP-1 in UV-irradiated CCD-986SK cells compared to FM. Our results suggest that BM may be used as a skin aging material that inhibits ultraviolet-induced MMP-1 production in human skin cells.
Tyrosinase is the most important enzyme involved in melanin production. Tyrosine in melanosomes is oxidized to DOPA and DOPA quinone, which is metabolized to DOPA chromium, indole carboxylic acid, indole quinone, by the action and automatic oxidation reaction of enzymes [38]. To measure the inhibitory effect of melanin polymer biosynthesis in the skin, the inhibition of tyrosinase activity by BM and FM was measured, and BM showed the lowest expression and showed the best inhibition. Melanin synthesis is also converted to DOPA quinone via DOPA by tyrosinase, TRP-1, and TRP-2 with the substrate of one of the amino acids by the polymerization of amino acids or proteins through a non-enzyme and spontaneous oxidation process [20]. TRP-1 and TRP-2 convert 5,6-dihydroxyindole-2-carboxylic acid (DHICA) to indole-5,6-quinone2-carboxylic acid (IQCA), which ultimately synthesizes melanin as a skin whitening agent [21,22]. Studies have also been conducted to suppress melanin synthesis using proteins such as tyrosinase, TRP-1, TRP-2, and MITF. In intracellular signaling pathways, MITF promotes the transcription of tyrosinase, TRP-1, and TRP-2 as important transcriptional regulatory factors during melanin synthesis [39].
In our study, FM showed similar melanin inhibition compared to a positive control when treated with the melanin inhibitor arbutin after α-MSH treatment in human melanin cells, but BM showed a lower rate of inhibition than the positive control. In addition, the effects of BM and FM on the protein and mRNA expression of MITF, TRP-1, TRP-2, and tyrosinase were measured to evaluate the effects of BM and FM on genes involved in the synthesis of melanin. Melanoma cells (SK-MEL-31)-treated FM and BM confirmed that mRNA expression of MITF, TRP-1, TRP-2, and tyrosinase was inhibited as they were lower than the control, showing the largest inhibition of expression in BM. In this study, the protein and mRNA expression of MITF, TRP-1, TRP-2, and tyrosinase in BM and FM were measured to verify their activity as whitening functional cosmetic materials, and significant results were obtained.
ROS are highly reactive and play an important role in cell signaling; however, they damage cell structures through oxidative stress. Oxidative stress causes deformation and damage of DNA, lipids, proteins, and other biomolecules in the cells, necrosis, and apoptosis, resulting in aging [40]. The expression of SOD1, CAT, and GPx was reduced in CCD-986SK cells, in response to UVB treatment. The RT-PCR analysis confirmed that the levels of these enzymes were higher in the BM treatment than in the FM treatment, indicating that BM positively influenced the expression of these antioxidant enzyme genes to elicit an antioxidant effect.
It has been reported that stem cell culture has many advantages as a functional cosmetic material, but the results of this study could provide a basis for the use of BM as a skin whitening and anti-wrinkle functional cosmetic material in cell standards [1,7,41,42]. To our knowledge, this is the first study to reveal differences in cytokine elements secreted into the culture when umbilical cord-derived mesenchymal stem cells are grown using the existing flask and the new bioreactor culture methods. There is also a difference in the analysis of whitening and wrinkle improvement, which is the most important in functional cosmetics, and it will be efficient to gradually change to the bioreactor method when calculating the convenience, labor, and material costs of cell culture in detail.
All whitening and wrinkle improvement experiments showed better results in BM than in FM, and we expect this to be related to bioreactor culture conditions. Despite being raised with the same cells and cultures, the expanded hUC-MSCs in the bioreactor obtained higher levels of cytokines in the culture medium than in the flask at the time of harvest. The Quantum bioreactor is an unusual method of ingenerating raised levels of cytokines from hUC-MSCs in less time and at passages when compared to flasks.
In conclusion, our research indicated that BM has advantages over FM, considering that the risk of contaminants in Quantum was significantly reduced [29]. This could be attributed to the better cell culture conditions in BM. Bioreactor culture avoids trypsin–EDTA treatments and multiple freezing/thawing cycles, when compared to that in flask culture. This increases cell viability, proliferation rates, and cell recovery.
The enhanced anti-wrinkling and skin moisturizing effects could be attributed to boosted skin regeneration in the anti-aging treatments, because of large amounts of growth factors in the spheroid culture system [43]. The quantification of intracellular functional proteins and secreted factors would enable better understanding of the biology of hUC-MSCs in these abundant culture systems [29].
This study focused on cell efficacy; in future, animal experiments are needed to evaluate the whitening and anti-wrinkle effects of BM on the skin, to enable cosmeceutical developments.
Authors’ contributions
Y.J Choi performed the experiments and analyzed the data; Dr. Koristek, Ing. Petr Koška, and Lucie Jurečková of PrimeCell Lab. provided resources (hUC-MSCs and hUC-MSC's conditioned medium) for the experiments.
Declaration of competing interest
None.
Acknowledgements
This research was financially supported by the Ministry of Trade, Industry, and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through the International Cooperative R&D program [Grant number N0002262].
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Kim H.J., Jung M.S., Hur Y.K., Jung A.H. A study on clinical effectiveness of cosmetics containing human stem cell conditioned media. Biomed Dermatol. 2020;4:9. [Google Scholar]
- 2.Kim K.W., Kim O.S. Super aging in South Korea unstoppable but mitigatable: a sub-national scale population projection for best policy planning. Spat Demogr. 2020;8:155–173. doi: 10.1007/s40980-020-00061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell-Goldman E., Murphy G.F. The pathobiology of skin aging: new insights into an old dilemma. Am J Pathol. 2020;190:1356–1369. doi: 10.1016/j.ajpath.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papakonstantinou E., Roth M., Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lephart E. Equol's anti-aging effects protect against environmental assaults by increasing skin antioxidant defense and ECM proteins while decreasing oxidative stress and inflammation. Cosmetics. 2018;5:16. [Google Scholar]
- 6.Xu H., Zheng Y.W., Liu Q., Liu L.P., Luo F.L., Zhou H.C. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. Intech. 2017 doi: 10.5772/intechopen.72747. [DOI] [Google Scholar]
- 7.Beeravolu N., Khan I., McKee C., Dinda S., Thibodeau B., Wilson G. Isolation and comparative analysis of potential stem/progenitor cells from different regions of human umbilical cord. Stem Cell Res. 2016;16:696–711. doi: 10.1016/j.scr.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Ding D.C., Chang Y.H., Shyu W.C., Lin S.Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B., Zhang J., Shi H., Mao F., Wang J., Yan Y. A novel method to isolate mesenchymal stem cells from mouse umbilical cord. Mol Med Rep. 2018;17:861–869. doi: 10.3892/mmr.2017.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nourian Dehkordi A., Mirahmadi Babaheydari F., Chehelgerdi M., Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:111. doi: 10.1186/s13287-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noverina R., Widowati W., Ayuningtyas W., Kurniawan D., Afifah E., Laksmitawati D.R. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs) Clin Nutr Exp. 2019;24:34–44. [Google Scholar]
- 12.Amirthalingam M., Bhat S., Dighe P.A., Seetharam R.N. Human mesenchymal stromal cells-derived conditioned medium based formulation for advanced skin care: in vitro and in vivo evaluation. J Stem Cell Res Dev. 2019;5:12. [Google Scholar]
- 13.Sohn S.J., Yu J.M., Lee E.Y., Nam Y.J., Kim J., Kang S. Anti-aging properties of conditioned media of epidermal progenitor cells derived from mesenchymal stem cells. Dermatol Ther. 2018;8:229–244. doi: 10.1007/s13555-018-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley P.J., Mei Z., Durett A.G., Cabreira-Hansen Mda G., Klis M., Li W. Efficient manufacturing of therapeutic mesenchymal stromal cells with the use of the Quantum Cell Expansion System. Cytotherapy. 2014;16:1048–1058. doi: 10.1016/j.jcyt.2014.01.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang B.J., Ryu H.H., Park S.S., Koyama Y., Kikuchi M., Woo H.M. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects. J Vet Sci. 2012;13:299–310. doi: 10.4142/jvs.2012.13.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniak P.R., McDevitt T.C. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng C., Li Y., Lu L., Zhu J., Li H., Hu J. Efficient one-step induction of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) produces MSC-derived neurospheres (MSC-NS) with unique transcriptional profile and enhanced neurogenic and angiogenic secretomes. Stem Cell Int. 2019;2019:9208173. doi: 10.1155/2019/9208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas-Rodríguez S., Folgueras A.R., López-Otín C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864:2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Kim S.J., Nam Y.M., Kim Y.M., Ko S.K. Inhibition of MMP-1 expression and collagen synthesis activity of ultrasonication processed ginseng flower buds extract. Korean J Pharmacogn. 2015;46:154–159. [Google Scholar]
- 20.Seo E.J., Hong E.S., Choi M.H., Kim K.S., Lee S.J. Antioxidant and skin whitening effects of Ramnus yoshinoi extracts. Kor J Food Sci Technol. 2010;42:750–754. [Google Scholar]
- 21.Kim H.H., Park G.H., Park K.S., Lee J.Y., Kim T.H., An B.J. The effect of Aster glehni Fr. Schm. extracts on whitening and anti-wrinkle. J Life Sci. 2010;20:1034–1040. [Google Scholar]
- 22.Mallick S., Singh S.K., Sarkar C., Saha B., Bhadra R. Human placental lipid induces melanogenesis by increasing the expression of tyrosinase and its related proteins in vitro. Pigm Cell Res. 2005;18:25–33. doi: 10.1111/j.1600-0749.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 23.Buscà R., Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigm Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 24.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukai T., Ushio-Fukai M. Super oxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. [Google Scholar]
- 27.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park C.W., Kim K.S., Bae S., Son H.K., Myung P.K., Hong H.J. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells. 2009;2:59–68. doi: 10.15283/ijsc.2009.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechanteur C., Baila S., Janssens M.E., Giet O., Briquet A Baudoux E. Large-scale clinical expansion of mesenchymal stem cells in the GMP-compliant, closed automated Quantum® Cell Expansion System: comparison with expansion in traditional T-flasks. J Stem Cell Res Ther. 2014;4:8. [Google Scholar]
- 30.de Araújo R., Lôbo M., Trindade K., Silva D.F., Pereira N. Fibroblast growth factors: a controlling mechanism of skin aging. Skin Pharmacol Physiol. 2019;32:275–282. doi: 10.1159/000501145. [DOI] [PubMed] [Google Scholar]
- 31.Borg M., Brincat S., Camilleri G., Schembri-Wismayer P., Brincat M., Calleja-Agius J. The role of cytokines in skin aging. Climacteric. 2013;16:514–521. doi: 10.3109/13697137.2013.802303. [DOI] [PubMed] [Google Scholar]
- 32.Aldag C., Nogueira Teixeira D., Leventhal P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: a review of the literature. Clin Cosmet Invest Dermatol. 2016;9:411–419. doi: 10.2147/CCID.S116158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noordam R., Gunn D.A., Tomlin C.C., Maier A.B., Griffiths T., Catt S.D. Serum insulin-like growth factor 1 and facial ageing: high levels associate with reduced skin wrinkling in a cross-sectional study. Br J Dermatol. 2013;168:533–538. doi: 10.1111/bjd.12131. [DOI] [PubMed] [Google Scholar]
- 34.Bevan D., Gherardi E., Fan T.P., Edwards D., Warn R. Diverse and potent activities of HGF/SF in skin wound repair. J Pathol. 2004;203:831–838. doi: 10.1002/path.1578. [DOI] [PubMed] [Google Scholar]
- 35.Pertusi G., Tiberio R., Graziola F., Boggio P., Colombo E., Bozzo C. Selective release of cytokines, chemokines, and growth factors by minced skin in vitro supports the effectiveness of autologous minced micrografts technique for chronic ulcer repair. Wound Repair Regen. 2012;20:178–184. doi: 10.1111/j.1524-475X.2011.00762.x. [DOI] [PubMed] [Google Scholar]
- 36.Pawitan J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res Int. 2014:965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philips N., Conte J., Chen Y.J., Natrajan P., Taw M., Keller T. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch Dermatol Res. 2009;301:487–495. doi: 10.1007/s00403-009-0950-x. [DOI] [PubMed] [Google Scholar]
- 38.Lin C.B., Babiarz L., Liebel F., Roydon-Price E., Kizoulis M., Gendimenico G.J. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J Invest Dermatol. 2002;119:1330–1340. doi: 10.1046/j.1523-1747.2002.19615.x. [DOI] [PubMed] [Google Scholar]
- 39.Chang T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials. 2012;5:1661–1685. [Google Scholar]
- 40.Tan S., Sagara Y., Liu Y., Maher P., Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesarova L., Jaresova K., Simara P., Koutna I. Umbilical cord-derived mesenchymal stem cells are able to use bFGF treatment and represent a superb tool for immunosuppressive clinical applications. Int J Mol Sci. 2020;21:5366. doi: 10.3390/ijms21155366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darvishi F., Moradi M., Madzak C., Jolivalt C. Production of laccase by recombinant Yarrowia lipolytica from molasses: bioprocess development using statistical modeling and increase productivity in shake-flask and bioreactor cultures. Appl Biochem Biotechnol. 2017;181:1228–1239. doi: 10.1007/s12010-016-2280-8. [DOI] [PubMed] [Google Scholar]
- 43.Kwon S.H., Bhang S.H., Jang H.K., Rhim T., Kim B.S. Conditioned medium of adipose-derived stromal cell culture in three-dimensional bioreactors for enhanced wound healing. J Surg Res. 2015;194:8–17. doi: 10.1016/j.jss.2014.10.053. [DOI] [PubMed] [Google Scholar]