Abstract
Brown-rot fungi are types of fungi that selectively degrade cellulose and hemicellulose from wood and are perhaps the most important agents involved in the degradation of wood products and dead wood in forest ecosystem. Two new brown-rot species, collected from southern China, are nested within the clades of Fomitopsis sensu stricto and Oligoporus sensu stricto, respectively. Their positions are strongly supported in the Maximum Likelihood phylogenetic tree of the concatenated the internal transcribed spacer (ITS) regions, the large subunit of nuclear ribosomal RNA gene (nLSU), the small subunit of nuclear ribosomal RNA gene (nuSSU), the small subunit of mitochondrial rRNA gene (mtSSU), the largest subunit of RNA polymerase II (RPB1), the second largest subunit of RNA polymerase II (RPB2) and the translation elongation factor 1-α gene (TEF1) sequences. Fomitopsisbambusae, only found on bamboo, is characterised by its resupinate to effused-reflexed or pileate basidiocarps, small pores (6–9 per mm), the absence of cystidia, short cylindrical to oblong-ellipsoid basidiospores measuring 4.2–6.1 × 2–2.3 μm. Oligoporuspodocarpi is characterised by white to pale cream pore surface, round or sometimes angular pores (5–6 per mm), broadly ellipsoid to reniform basidiospores measuring 3.8–4.2 × 2–2.3 μm and growing on Podocarpus. Illustrated descriptions of these two novel species, Fomitopsisbambusae and Oligoporuspodocarpi, are provided.
Keywords: Brown-rot fungi, multi-gene phylogeny, phylogeny, taxonomy
Introduction
Wood-inhabiting basidiomycota can be grouped into two categories, white-rot and brown-rot fungi, according to their ability for decaying or decomposing wood. Brown-rot fungi selectively degrade cellulose and hemicellulose from wood and decayed material becomes reddish-brown or tan, crisp, causing massive cracks in the middle of a longitudinal crisscross. However, white-rot fungi can degrade all the components of wood and decayed material, become white or pale-yellow or light reddish-brown and expose the fibrous structure. The number of brown rot fungi is remarkably smaller compared to white rot fungi (Zhang 2003; Wu et al. 2020). Gilbertson (1981) has estimated that approximately 6% of the wood-rotting basidiomycetes in North America give a brown rot. On the other hand, Dai (2012) demonstrated that 14% of Chinese polypores in northern China can cause a brown rot (Cui et al. 2019). Brown-rot fungi are perhaps the most important agents involved in the degradation of wood products and in the degradation of dead wood in forest ecosystems. It is worth emphasising that the diversity of brown rot fungi is higher in high-latitude areas than in low-latitude areas and the number of brown rot fungi decreases from north to south in China (Zhou and Dai 2012; Dai et al. 2015), so that brown-rot fungi are infrequent in tropical areas.
As a cosmopolitan brown-rot genus of polypores, Fomitopsis P. Karst., was established by Karsten, based on F.pinicola (Sw.) P. Karst. (Karsten 1881). The genus was classified in the Fomitopsidaceae morphologically (Jülich 1981) and belonged to the Antrodia clade phylogenetically (Binder et al. 2005; Ortiz-Santana et al. 2013; Han et al. 2016). Han et al. (2016) confirmed that species, previously belonging to Fomitopsis sensu lato, were embedded in seven lineages and eleven species form the core group of Fomitopsis. In addition, four species Fomitopsiscaribensis B.K. Cui & Shun Liu, F.eucalypticola B.K. Cui & Shun Liu, F.ginkgonis B.K. Cui & Shun Liu and F.roseoalba A.M.S. Soares, Ryvarden & Gibertoni were introduced as new species and F.bondartsevae (Spirin) A.M.S. Soares & Gibertoni was proposed as a new combination (Soares et al. 2017; Tibpromma et al. 2017; Liu et al. 2019). In the latest study, ten species have been recognised in the Fomitopsispinicola complex (Haight et al. 2019; Liu et al. 2021). So far, 25 species have been accepted in Fomitopsis sensu stricto (s. str.).
Oligoporus Bref. (Polyporales, Basidiomycetes) was typified with O.farinosus Bref., 1888 (Syn. O.rennyi (Berk. & Broome) Kotl.) (Brefeld 1888). Recent phylogenetic analyses have demonstrated that Oligoporus and Tyromyces belong to different clades and that they were grouped within families Dacryobolaceae Jülich and Incrustoporiaceae Jülich (Binder et al. 2013; Floudas and Hibbett 2015; Justo et al. 2017). Shen et al. (2019) have proved Oligoporus s. str. is different from Postia s. str. in morphology and molecular phylogenetic analysis. Meanwhile, species in Postia s. str. have a broad host range growing both on angiosperm and gymnosperm wood, but Oligoporus s. str. grows only on gymnosperm wood (Donk 1971; Ryvarden and Melo 2014; Shen et al. 2019). So far, only two species have been accepted in Oligoporus s. str. (Shen et al. 2019).
During our investigations of brown-rot fungi in China, eight specimens were collected from Hainan Province in tropical China. Morphological examination shows these collections to represent two brown-rot polypores, corresponding to Fomitopsis s.s. and Oligoporuss.s. After phylogenetic analyses of the internal transcribed spacer (ITS) regions, the large subunit of nuclear ribosomal RNA gene (nLSU), the small subunit of nuclear ribosomal RNA gene (nuSSU), the small subunit of mitochondrial rRNA gene (mtSSU), the largest subunit of RNA polymerase II (RPB1), the second largest subunit of RNA polymerase II (RPB2) and the translation elongation factor 1-α gene (TEF1) sequences, two new species were confirmed as belonging to Fomitopsis s.s. and Oligoporus s.s.. In this paper, we describe and illustrate these two new species.
Materials and methods
Morphological studies
The examined specimens were deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC) in Beijing, China. Macro-morphological descriptions were based on the field notes and measurements of herbarium specimens. Colour terms followed Petersen (1996). Micro-morphological data were obtained from the dried specimens and observed under a light microscope following Chen et al. (2017) and Shen et al. (2019). Sections were studied at a magnification up to 1000× using a Nikon Eclipse 80i microscope with phase contrast illumination (Nikon, Tokyo, Japan). Drawings were made with the aid of a drawing tube. Microscopic features, measurements and drawings were made from slide preparations stained with Cotton Blue and Melzer’s Reagent. Spores were measured from sections cut from the tubes. To present the variation of spore size, 5% of measurements were excluded from each end of the range and are given in parentheses. The following abbreviations are used: IKI = Melzer’s Reagent, IKI– = neither amyloid nor dextrinoid, KOH = 5% potassium hydroxide, CB = Cotton Blue, CB– = acyanophilous, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores), Q = variation in the L/W ratios between the specimens studied, n (a/b) = number of basidiospores (a) measured from given number (b) of specimens.
DNA extraction and sequencing
A cetyltrimethylammonium bromide rapid plant genome extraction kit (Aidlab Biotechnologies Co., Ltd, Beijing, China) was used to extract the total genomic DNA from dried specimens according to the manufacturer’s instructions with some modifications (Song and Cui 2017; Xing et al. 2018). The ITS regions were amplified with the primer pairs ITS5 (GGA AGT AAA AGT CGT AAC AAG G) and ITS4 (TCC TCC GCT TAT TGA TAT GC) (White et al. 1990). The nLSU regions were amplified with the primer pairs LR0R (ACC CGC TGA ACT TAA GC) and LR7 (TAC TAC CAC CAA GAT CT) (http://www.biology.duke.edu/fungi/mycolab/primers.htm). The nuSSU regions were amplified with the primer pairs NS1(CCG GAG AGG GAG CCT GAG AAA C) and NS4 (CCC GTG TTG AGT CAA ATT A) (White et al. 1990). The mtSSU regions were amplified with the primer pairs MS1 (CAG CAG TCA AGA ATA TTA GTC AAT G) and MS2 (GCG GAT TAT CGA ATT AAA TAA C) (White et al. 1990). RPB1 was amplified with the primer pairs RPB1-Af (GAR TGY CCD GGD CAY TTY GG) and RPB1-Cr (CCN GCD ATN TCR TTR TCC ATR TA) (Matheny et al. 2002). RPB2 was amplified with the primer pairs fRPB2-5F (GAY GAY MGW GAT CAY TTY GG) and fRPB2-7CR (CCC ATR GCT TGY TTR CCC AT) (Matheny 2005). TEF1 was amplified with the primer pairs EF1-983F (GCY CCY GGH CAY CGT GAY TTY AT) and EF1-1567R (ACH GTR CCR ATA CCA CCR ATC TT) (Rehner and Buckley 2005). The PCR procedure followed that of Liu et al. (2019). The PCR products were purified with a Gel Extraction and PCR Purification Combo Kit (Spin-column) in Beijing Genomics Institute, Beijing, P.R. China. The purified products were then sequenced on an ABI-3730-XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA) using the same primers as in the original PCR amplifications. The sequence quality was checked following Nilsson et al. (2012). All newly-generated sequences were submitted to GenBank and were listed in Tables 1 and 2.
Table 1.
A list of species, specimens and GenBank accession numbers of sequences used in the phylogeny of Fomitopsis.
Table 2.
A list of species, specimens and GenBank accession numbers of sequences used in the phylogeny of Oligoporus.
Phylogenetic analyses
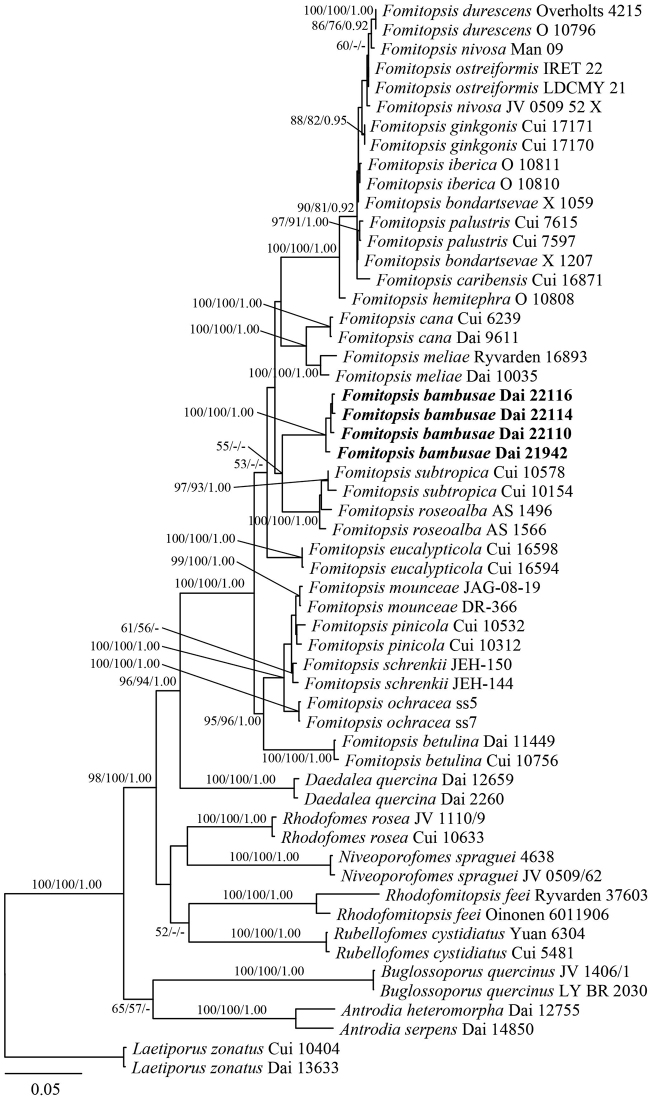
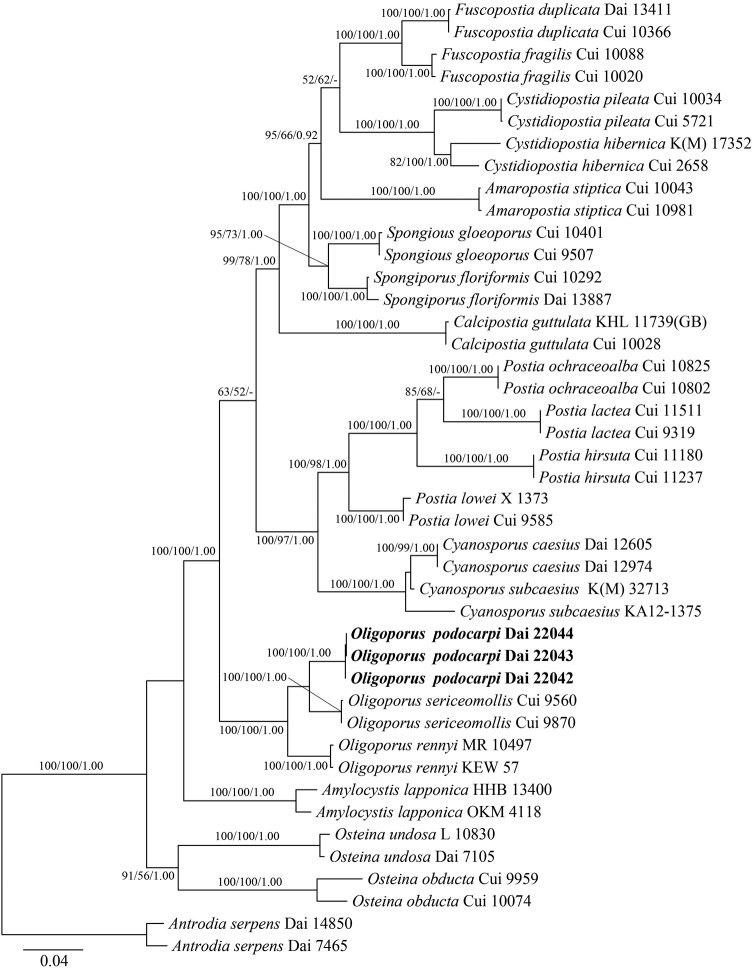
New sequences, deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) (Table 1), were aligned with additional sequences retrieved from GenBank (Table 1) using BioEdit 7.0.5.3 (Hall 1999) and ClustalX 1.83 (Thompson et al. 1997), followed by manual adjustment. Sequence alignment was deposited at TreeBase (http://purl.org/phylo/treebase/; submission ID 28131). In phylogenetic reconstruction, sequences of Laetiporuszonatus B.K. Cui & J. Song, obtained from GenBank, were used as outgroups in the phylogeny of Fomitopsis (Fig. 1) while sequences of Antrodiaserpens (Fr.) P. Karst. were used as outgroups in the phylogeny of Oligoporus (Fig. 2).
Figure 1.
Maximum Likelihood phylogenetic tree of the new Fomitopsis species, based on multi-genes sequences data. Branches are labelled with bootstrap values (MP/ML) higher than 50% and posterior probabilities (BI) more than 0.90, respectively. Bold names: New species.
Figure 2.
Maximum Likelihood phylogenetic tree of the new Oligoporus species, based on multi-genes sequences data. Branches are labelled with bootstrap values (MP/ML) higher than 50% and posterior probabilities (BI) more than 0.90, respectively. Bold names: New species.
Maximum Parsimony (MP) analysis was applied to those two phylogenies and trees construction procedure were performed in PAUP* version 4.0b10 (Swofford 2002). Settings for phylogenetic analyses in this study followed the approach of Zhu et al. (2019) and Song and Cui (2017). All characters were equally weighted and gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1000 random sequence additions. Max-trees were set to 5000, branches of zero length were collapsed and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap (BT) analysis with 1000 replicates (Felsenstein 1985). Descriptive tree statistics tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for each Maximum Parsimonious Tree (MPT) generated.
Maximum Likelihood (ML) analysis was conducted with RAxML-HPC252 on Abe through the CIPRES Science Gateway (www.phylo.org) and involved 100 ML searches. All model parameters were estimated by the programme. Only the best Maximum Likelihood tree from all searches was kept. The Maximum Likelihood bootstrap values (ML-BS) were performed using a rapid bootstrapping with 1000 replicates. The phylogenetic tree was visualised using Treeview (Page 1996).
MrModeltest 2.3 (Posada and Crandall 1998; Nylander 2004) was used to determine the best-fit evolution model for two combined matrices to reconstruct phylogenetic analyses as a 6-gene dataset (ITS+nLSU+nuSSU+mtSSU+RPB2+TEF1) and a 7-gene dataset (ITS+nLSU+nuSSU+mtSSU+RPB1+RPB2+TEF1) for Bayesian Inference (BI). Bayesian Inference was calculated with MrBayes 3.2.6 (Ronquist et al. 2012), with a general time reversible (GTR) model of DNA substitution and a gamma distribution rate variation across sites. Four Markov chains were run for two runs from random starting trees for one million generations and trees were sampled every 100 generations. The burn-in was set to discard 25% of the trees. A majority rule consensus tree of all remaining trees was calculated. Branches that received bootstrap support for Maximum Parsimony (MP), Maximum Likelihood (ML) and Bayesian Posterior Probabilities (BPP) greater than or equal to 75% (MP and ML) and 0.95 (BPP) were considered as significantly supported.
Results
Molecular phylogeny
The phylogeny of Fomitopsis, based on a combined 6-gene (ITS, nLSU, nuSSU, mtSSU, RPB2, TEF1) dataset, included sequences from 64 fungal samples representing 29 taxa. They were downloaded from GenBank and generated in the present study (Table 1). The dataset had an aligned length of 4718 characters, including gaps (680 characters for ITS, 1343 characters for nLSU, 1013 characters for nuSSU, 547 characters for mtSSU, 648 characters for RPB2, 487 characters for TEF1), of which 3346 characters were constant, 1860 were variable and parsimony-uninformative, and 1212 were parsimony-informative. Maximum parsimony analysis yielded one equally-parsimonious tree (TL = 3802, CI = 0.544, RI = 0.787, RC = 0.428, HI = 0.456) and the MP tree is shown in Fig. 1. The best model for the combined ITS+nLSU+nuSSU+mtSSU+RPB2+TEF1 sequence dataset was estimated and applied in the Bayesian analysis was GTR+I+G with equal frequency of nucleotides, lset nst = 6 rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). Bayesian analysis resulted in a concordant topology with an average standard deviation of split frequencies = 0.008975.
The phylogeny of Oligoporus, combined 7-gene (ITS, nLSU, nuSSU, mtSSU, RPB1, RPB2, TEF1) dataset, included sequences from 43 fungal samples representing 21 taxa. They were downloaded from GenBank and generated in the present study (Table 2). The dataset had an aligned length of 5772 characters, including gaps (612 characters for ITS, 1302 characters for nLSU, 1009 characters for nuSSU, 491 characters for mtSSU, 1231 characters for RPB1, 648 characters for RPB2, 479 characters for TEF1), of which 4127 characters were constant, 129 were variable and parsimony-uninformative and 1516 were parsimony informative. Maximum parsimony analysis yielded four equally-parsimonious trees (TL = 3925, CI = 0.600, RI = 0.784, RC = 0.471, HI = 0.400) and a strict consensus tree of these trees is shown in Fig. 2. The best model for the combined ITS+nLSU+nuSSU+mtSSU+RPB1+RPB2+TEF1 sequence dataset was estimated and applied in the Bayesian analysis was GTR+I+G with equal frequency of nucleotides, lset nst = 6 rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). Bayesian analysis resulted in a concordant topology with an average standard deviation of split frequencies = 0.008567.
In our phylogenies (Figs 1 and 2), five samples on bamboo formed an independent lineage in the Fomitopsis s.s. clade with strong support (100% ML, 100% MP, 1.00 BPPs) and are distant from other taxa in the genus. Both morphology and rDNA sequence data confirmed that the five samples represent a new species in Fomitopsis. Meanwhile, three samples on Podocarpus were nested in the Oligoporus s.s. clade and formed an independent lineage with a robust support (100% ML, 100% MP, 1.00 BPPs). Both morphology and rDNA sequence data confirmed that the three samples represent a new species in Oligoporus.
Table 3.
A comparison of species in the Fomitopsis.
| Species | Holotype | Basidiocarps | Pileal surface | Pore surface | Pore (per mm.) | Hyphal system | Cystidia/cystidioles | Basidiospores | References |
|---|---|---|---|---|---|---|---|---|---|
| F. abieticola | China | Annual to perennial; pileate | Cream to pinkish buff | Cream to pinkish buff when fresh, becoming buff to curry-yellow when dry | Round to angular, 2–4 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 17.5–50.2 × 4.3–9.5 μm | Oblong-ellipsoid to ellipsoid, 7–9 × 4–5 µm. | Liu et al. (2021) |
| F. bambusae | China | Annual, resupinate to effused-reflexed or pileate | Pluish grey when fresh, pale mouse-grey to greyish-sepia when dry | Bluish-grey to pale mouse-grey when fresh, becoming mouse-grey to dark grey when dry | Round to angular, 6–9 | Dimitic | Cystidia absent; fusoid cystidioles present, 11–18 × 2.5–4 μm | Cylindrical to oblong ellipsoid, 4.2–6.1 × 2–2.3 µm | Present study |
| F. betulina | Norway | Annual; pileate | Whitish to mouse-coloured or brownish | White to pale brownish | Round to angular, 3–5 | Di-trimitic | Absent | Cylindrical, slightly allantoid, 5–6 × 1.5–1.7 µm. | Ryvarden and Melo (2014) |
| F. bondartsevae | Russia | Annual; effused-reflexed to pileate | Round to angular, 2–3 | Trimitic | Cystidia absent; fusoid cystidioles present, 18–26 × 4.5–6 μm | Cylindrical, 6–7.2 × 2.2–2.5 µm. | Spirin (2002) | ||
| F. cana | China | Annual; resupinate to effused-reflexed or pileate | Pale mouse-grey to dark grey, azonate | Cream to straw coloured turning mouse-grey to dark grey | Angular, 5–8 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 9–16 × 3–5 μm | Cylindrical to oblong ellipsoid,5–6.2× 2.1–3 μm. | Li et al. (2013) |
| F. caribensis | Puerto Rico. | Annual; pileate, sessile | White to cream buff when fresh, cream buff to curry-yellow at base | White to cream when fresh, becoming cream to pinkish-buff when dry | Round to angular, 6–9 | Dimitic | Cystidia absent; fusoid cystidioles occasional, hyaline, thin-walled, 12.5–23.5 × 2.5–4 μm | Cylindrical to oblong-ellipsoid, 6–7.5 × 2.3–3.1 μm. | Liu et al. (2019) |
| F. durescens | USA | Annual; sessile | Cream coloured to pale buff, drying tan | White to cream coloured, ochraceous on drying | Round to angular, 4–5 | Trimitic | Cystidia absent; fusoid cystidioles present, 14–16 × 5–6 μm | Narrowly cylindrical, 6–8 × 1.5–2.5 µm | Gilbertson and Ryvarden (1986) |
| F. eucalypticola | Australia | Annual to biennial; effused-reflexed to pileate | Cream to salmon-coloured when young, straw yellow to clay-pink | Cream to yellow when fresh, buff to clay-buff when dry | Round to angular, 3–5 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 15–36 × 2–5.3 μm | Cylindrical to oblong-ellipsoid, 5.8–9.1 × 2.7–5 μm. | Liu et al. (2019) |
| F. ginkgonis | China | Annual; pileate, imbricate | Dirty greyish-brown to mouse-grey | Pinkish-buff to cinnamon-buff | Round to angular, 3–6 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 12.5–27.6 × 2.8–4.1 μm | Cylindrical, 7.2–9 × 2.2–3 μm. | Liu et al. (2019) |
| F. hemitephra | New Zealand | Perennial; solitary, attached by a broad lateral base | Tobacco brown or fuscous. | White or straw to isabelline | Round or slightly angular, 6–7 | Trimitic | Cystidia absent; cystidioles, 6–8 × 3.5–4 µm | Elliptic-oblong, 4–6 × 2–2.5 μm. | Cunningham (1965) |
| F. hengduanensis | China | Annual to perennial; pileate | Pale dark grey to reddish-brown at base and cream to flesh-pink towards the margin | white to cream when fresh, becoming buff to straw-yellow | Round to angular, 6–8 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 13.2–36.5 × 2.5–5.4 μm | Oblong-ellipsoid to ellipsoid, 5.2–6 × 3.2–3.6 µm. | Liu et al. (2021) |
| F. iberica | Portugal | Annual; sessile, dimidiate, single or imbricate | White to cream when young. drying honey-coloured to brown | Pale, white, cream to straw-coloured | Round to ellipsoid, 3–4 per mm | Trimitic | Cystidia absent; pointed cystidioles present, 20–27 × 4–5–5 µm | Cylindrical to distinctly fusoid, 6–8 × 2.8–3.7 µm. | Melo and Ryvarden (1989) |
| F. kesiyae | Vietnam | Annual; pileate | Buff yellow to orange-yellow buff | White to cream when fresh, olivaceous buff to cinnamon-buff when dry | Round to angular, 6–8 | Dimitic | Cystidia absent; fusoid cystidioles occasionally present, 11.5–30.4 × 2.6–6 μm | Oblong-ellipsoid to ellipsoid, 4.8–5.3 × 3–3.5 µm. | Liu et al. (2021) |
| F. massoniana | China | Annual; effused-reflexed to pileate | Buff-yellow to apricot-orange | White to cream when fresh, cream to buff | Round, 5–7 | Dimitic | Cystidia absent; fusoid cystidioles occasionally present, 14.8–36 × 3.8–6 μm | Oblong-ellipsoid, 6.2–7.3 × 3.3–4 µm. | Liu et al. (2021) |
| F. meliae | USA | Annual or biennial; sessile, pilei single to imbricate, dimidiate | Ivory to tan or cinereous | Ochraceous | Round to angular, 5–7 | Trimitic | Cystidia absent; fusoid cystidioles present, 15–23 × 4–5 µm | Cylindrical, slightly fusiform, tapering to the apex, 6–8 × 2.5–3 µm. | Gilbertson (1981) |
| F. mounceae | Canada | Perennial; pileate | Brownish-orange to black at base and pale orange to greyish-orange towards the margin | Yellowish-white, greyish-yellow, pale orange to light ochraceous buff, bright reddish-brown when dry | Round, 3–5 | Dimitic | Cystidia obclavate to subfusiform with subacute or rounded apices, 16–35 × 3–6.5 µm | Ellipsoid to cylindrical, 5.8–6.6 × 3.4–4 µm. | Haight et al. (2019) |
| F. nivosa | Brazil | Annual to biennial; sessile, dimidiate, single to imbricate | Cream to pale sordid brown or tan | Cream to pale sordid brown or tan | Round to angular, 6–8 | Trimitic | Cystidia absent; cystidioles broadly rounded, subapically contracted, 12–15 × 4–5 µm | Cylindrical, 6–9 – 2–3 µm | Gilbertson and Ryvarden (1986) |
| F. ochracea | Canada | Perennial; pileate | Brownish-grey to greyish-brown at base and orange white to pale orange towards the margin | Pale yellow, pale orange, light ochraceous buff, reddish-brown when dry | Round, 4–5 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 20–40 × 4–6.5 μm | Broadly ellipsoid, 5.1–5.9 × 3.6–4 µm. | Stokland and Ryvarden (2008); Haight et al. (2019) |
| F. ostreiformis | Singapore | Annual; sessile or effuse-reflexed | Greyish pileal surface | White or greyish-white | Round to angular, 3–4 | Trimitic | Cystidia absent; cystidioles present, 10–17 × 2.8–4 μm | Cylindrical, 4.2–5.6 × 1.4–2.6 pm | De (1981); Hattori (2003) |
| F. palustris | USA | Perennial; sessile, horizontal, applanate | Dingy ochraceous to ochraceous buff, suffused dingy brownish-vinaceous | Vinaceous drab to brownish-vinaceous but pallid ochraceous near the margin | Angular, 7–9 | Dimitic | absent | Cylindrical, 3.7–4.7 × 2–2.5 µm. | Corner (1989); Hattori (2003) |
| F. pinicola | Europe | Perennial; pileate | Brownish-orange to black at base and buff-yellow to cinnamon towards the margin | Cream coloured becoming citric yellow when bruised | Round, 4–6 | Trimitic | Cystidia present, 18–90 × 3–9 μm | Cylindrical-ellipsoid, 6–9 × 3–4.5 µm. | Ryvarden and Melo (2014); Haight et al. (2019) |
| F. roseoalba | Brazil | Annual; pileate, resupinate to effused-reflexed | White to pink when fresh, cream to greyish when dry | White to cream when fresh and ochraceous when dried | Round to angular, 4–6 | Trimitic | absent | Ellipsoid to sub-cylindrical, 3–4.9 × 1.8–2 µm. | Tibpromma et al. (2017) |
| F. schrenkii | USA | Perennial; effused-reflexed to pileate | Greyish-orange to olive brown at base and greyish-orange to greyish-yellow towards the margin | Pale yellow, pale orange, cream buff, reddish-brown when dry | Round, 3–4 | Dimitic | Cystidia cylindrical, subulate, or subfusiform with subacute, 16–30 × 3–8 µm | Ellipsoid to broadly cylindrical, 5.7–6.7 × 3.7–4.2 µm. | Haight et al. (2019) |
| F. subpinicola | China | Annual; pileate | Apricot-orange, scarlet to fuscous | White to cream when fresh, turning buff yellow to buff when dry | Round, 6–8 | Dimitic | Cystidia absent; fusoid cystidioles occasionally present, 14.5–34.6 × 3.2–7.2 μm | Oblong-ellipsoid to ellipsoid, 4.3–5.5 × 2.7–3.3 µm. | Liu et al. (2021) |
| F. subtropica | China | Annual; resupinate to effused-reflexed or pileate | Straw-yellow when young, becoming pale mouse-grey to flesh-pink with age. | Cream to straw coloured or pale pinkish | Angular, 6–9 | Trimitic | Cystidia absent; fusoid cystidioles occasionally present, 9–15 × 3–4 μm | Cylindrical to oblong-ellipsoid, 3.2–4 × 1.8–2.1 µm. | Li and Cui (2013) |
| F. tianshanensis | China | Annual to perennial; effused-reflexed to pileate | Dark bluish-grey to yellowish-brown | Cream to pinkish-buff when fresh, becoming faint yellow to light pink when dry | Round to angular, 1–3 | Dimitic | Cystidia absent; fusoid cystidioles occasionally present, 15.5–44 × 3.3–6.5 μm | Oblong-ellipsoid, 6.3–7 × 3.2–3.8 µm. | Liu et al. (2021) |
Taxonomy
. Fomitopsis bambusae
Y.C. Dai, Meng Zhou & Yuan Yuan sp. nov.
B08F1D81-E3C5-5DEE-AD3F-A6D9CEC9CCC7
MycoBank No: 839359
Figure 3.
Basidiocarps of Fomitopsisbambusae (holotype Dai 22116). Scale bar: 1.0 cm.
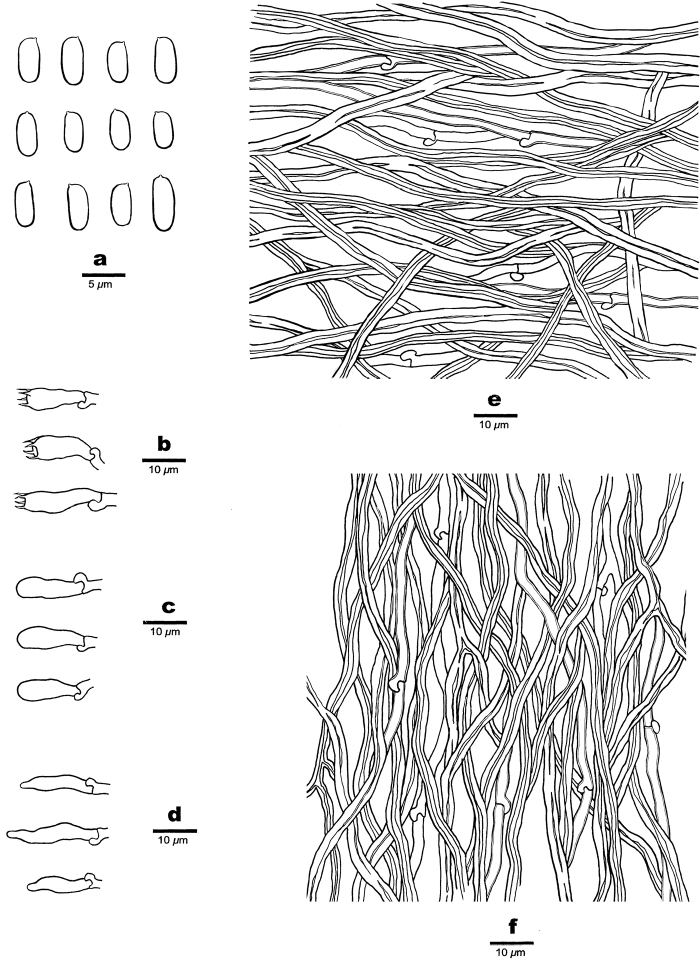
Figure 4.
Microscopic structures of Fomitopsisbambusae (drawn from the holotype) a basidiospores b basidia c basidioles d cystidioles e hyphae from context f hyphae from trama.
Diagnosis.
Fomitopsisbambusae is characterised by resupinate to effused-reflexed or pileate, soft corky basidiocarps with bluish-grey pores, small pores measuring 6–9 per mm, cylindrical to oblong ellipsoid basidiospores measuring 4.2–6.1 × 2–2.3 μm and growing on dead bamboo.
Type.
China. Hainan, Haikou, Jinniuling Park, on dead bamboo, 18.XI.2020, Yu-Cheng Dai leg., Dai 22116 (holotype BJFC036008).
Etymology.
Bambusae (Lat.): refers to the species growing on bamboo.
Fruiting body.
Basidiocarps annual, resupinate to effused-reflexed or pileate, separable from the substrate, without odour or taste and soft corky when fresh, corky and light in weight when dry. Pilei semicircular, projecting up to 1 cm, 1.5 cm wide and 5 mm thick at base; resupinate part up to 14 cm long, 6 cm wide and 2 mm thick at centre. Pileal surface bluish-grey when fresh, pale mouse-grey to greyish-sepia when dry, glabrous to slightly velutinate, rough, azonate; margin acute, incurved when dry. Pore surface bluish-grey to pale mouse-grey when fresh, becoming mouse-grey to dark grey when dry; sterile margin up to 1 mm wide; pores round to angular, 6–9 per mm; dissepiments thin, entire. Context white to cream, corky, up to 3.5 mm thick. Tubes paler than pore surface, corky, up to 1.5 mm long.
Hyphal structure.
Hyphal system dimitic; generative hyphae bearing clamp connections; skeletal hyphae IKI–, CB–; tissue unchanged in KOH.
Context.
Generative hyphae hyaline, thin- to slightly thick-walled, occasionally branched, 1.5–3 μm in diam.; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, occasionally branched, interwoven, 2–4.5 μm in diam.
Tubes.
Generative hyphae hyaline, thin- to slightly thick-walled, rarely branched, 1.5–2.5 μm in diam.; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, occasionally branched, flexuous, interwoven, 2–3 μm in diam. Cystidia absent; fusoid cystidioles present, hyaline, thin-walled, 11–18 × 2.5–4 μm. Basidia short clavate to barrel-shaped, bearing four sterigmata and a basal clamp connection, 13–19 × 4.5–5.5 μm; basidioles dominant, in shape similar to basidia, but smaller.
Spores.
Basidiospores cylindrical to oblong ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, (4–)4.2–6.1(–6.5) × (1.9–)2–2.3(–2.6) µm, L = 4.917 µm, W = 2.109 µm, Q = 2.26–2.41 (n = 90/3).
Type of rot.
Brown rot.
Additional specimens (paratypes) examined.
China. Hainan, Haikou, Jinniuling Park, on dead bamboo, 7.XI.2020, Yu-Cheng Dai leg., Dai 21942 (BJFC035841), 18.XI.2020, Dai 22104 (BJFC035996), Dai 22110 (BJFC036002) and Dai 22114 (BJFC036006).
Table 4.
A comparison of species in the Oligoporus.
| Species | Basidiocarps | Pore (per mm) | Pore surface | Cystidia | Cystidioles | Basidiospores size (μm) | Basidiospores shape | Reference |
|---|---|---|---|---|---|---|---|---|
| Oligoporus podocarpi | Resupinate | Round to angular, 5–6 | White to pale cream | Thick-walled with apically encrusted | Absent | 3.8–4.2 × 2–2.5 | Allantoid to oblong ellipsoid | Present study |
| O. rennyi | Resupinate | Angular, 2–4 | White or cream, then pale brown | Absent | Absent | 4.8–6 × 2.5–3.5 | Oblong ellipsoid | Ryvarden and Melo (2014); Shen et al. (2019) |
| O. sericeomollis | Resupinate | Round and angular, 4–6 | White or discoloured yellowish or tan | Thick-walled with apically encrusted | Present, thin-walled | 4–5 × 2–2.5 | Oblong cylindrical to ellipsoid | Ryvarden and Melo (2014); Shen et al. (2019) |
. Oligoporus podocarpi
Y.C. Dai, Chao G. Wang & Yuan Yuan sp. nov.
5209892F-1DF4-541D-98F8-F789BE9F9D3E
MycoBank No: 839360
Figure 5.
Basidiocarps of Oligoporuspodocarpi (holotype Dai 22042). Scale bar: 1.0 cm.
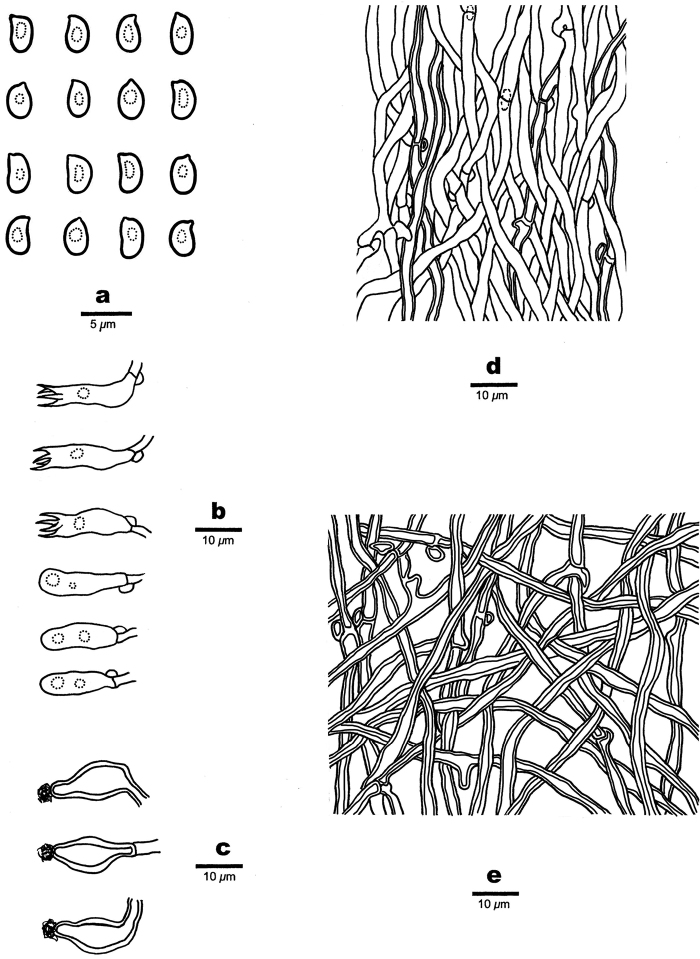
Figure 6.
Microscopic structures of Oligoporuspodocarpi (drawn from the holotype) a basidiospores b Basidia and basidioles c cystidia d hyphae from subiculum e hyphae from trama.
Diagnosis.
Oligoporuspodocarpi is characterised by soft fresh basidiocarps, becoming rigid upon drying, a monomitic hyphal system with hyaline clamped generative hyphae, the presence of apically encrusted cystidia, broadly ellipsoid to reniform, dextrinoid, cyanophilous basidiospores measuring 3.8–4.2 × 2–2.3 μm, and growing on rotten wood of Podocarpus.
Type.
China. Hainan, Changjiang, Hainan Tropical Rainforest National Park, Bawangling, rotten wood of Podocarpusimbricatus, 10.XI.2020, Yu-Cheng Dai leg., Dai 22042 (holotype BJFC035938).
Etymology.
Podocarpi (Lat.): referring to the species growing on wood of Podocarpusimbricatus.
Fruiting body.
Basidiocarps annual, resupinate, adnate, soft corky, with mushroom odour when fresh, becoming rigid when dry, mild taste, up to 3 cm long, 2 cm wide and 2.3 mm thick at the centre. Pore surface snow white when fresh, becoming cream to buff upon drying, somewhat glancing; sterile margin indistinct, thinning out, up to 0.3 mm wide; pores round to angular, 5–6 per mm; dissepiments thin, entire. Subiculum white, fibrous to soft corky when dry, up to 0.3 mm thick. Tubes concolorous with the pore surface, hard corky to brittle when dry, up to 2 mm long.
Hyphal structure.
Hyphal system monomitic; generative hyphae with clamp connections, smooth, hyaline, IKI–, CB–; tissues unchanged in KOH.
Subiculum.
Generative hyphae thick-walled with a wide lumen, occasionally branched, flexuous, interwoven, 2.5–3.8 μm in diam.
Tubes.
Generative hyphae thin- to thick-walled, occasionally branched, subparallel along the tubes to loosely interwoven, 2–3.1 μm in diam. Cystidia present, ventricose, very thick-walled, some apically encrusted. Basidia short clavate, sometimes with an intermediate constriction, with four sterigmata and a basal clamp connection, 12.5–16 × 4–5 μm; basidioles in shape similar to basidia, but smaller.
Spores.
Basidiospores broadly ellipsoid to reniform, hyaline, thin- to slightly thick-walled, smooth, often with one guttule, dextrinoid, CB+, (3.5–)3.8–4.2(–4.5) × 2–2.3(–2.5) µm, L = 3.98 μm, W = 2.14 μm, Q = 1.82–1.90 (n = 90/3).
Type of rot.
Brown rot.
Additional specimens (paratypes) examined.
China. Hainan, Changjiang, Hainan Tropical Rainforest National Park, Bawangling; rotten wood of Podocarpusimbricatus, 10.XI.2020, Yu-Cheng Dai leg., Dai 22043 (BJFC035939) and Dai 22044 (BJFC035940).
Discussion
In this study, two new species, Fomitopsisbambusae and Oligoporuspodocarpi, are described, based on morphological features and molecular data. The phylogenetic analysis of Fomitopsis (Fig. 1), inferred from ITS+nLSU+nuSSU+mtSSU+PRB2+TEF1 sequences, provides strong support (100% ML, 100% MP, 1.00 BPPs) for the placement of F.bambusae in Fomitopsis s.s. Besides, Fomitopsisbambusae formed a distinct and independent lineage, which is clearly distinguishable phylogenetically from all other known species of the genus. Fomitopsisroseoalba A.M.S. Soares and F.subtropica B.K. Cui & Hai J. Li are potentially the most closely related. Meanwhile, F.roseoalba is distinguished from F.bambusae by its larger pores (4–6 per mm vs. 6–9 per mm) and smaller basidiospores (3–4.9 × 1.8–2 µm vs. 4.2–6.1 × 2–2.3 µm, Tibpromma et al. 2017); F.subtropica is different from F.bambusae by smaller basidiospores (3.2–4 × 1.8–2.1 µm vs. 4.2–6.1 × 2–2.3 µm, Li et al. 2013).
Morphologically, Fomitopsisbambusae, F.cana (Blume & T. Nees) Imazeki, F.caribensis, F.hemitephra (Berk.) G. Cunn. and F.nivosa (Berk.) Gilb. & Ryvarden share approximately the same-sized pores (6–9 per mm). However, Fomitopsiscana differs from F.bambusae by its trimitic hyphal system, slightly larger basidiospores (5–6.2 × 2.1–3 μm, L = 5.81 μm, W = 2.6 μm vs. 4.2–6.1 × 2–2.3 µm, L = 4.917 µm, W = 2.109 µm) and grows on angiosperm wood rather than bamboo (Li et al. 2013). Fomitopsiscaribensis differs from F.bambusae by larger basidiospores (6–7.5 × 2.3–3.1 µm vs. 4.2–6.1 × 2–2.3 µm, Liu et al. 2019). Fomitopsishemitephra is distinguished from F.bambusae by its perennial habitat, woody hard basidiocarps (Cunningham 1965). Fomitopsisnivosa differs from F.bambusae by having longer basidiospores (6–9 × 2–3 µm vs. 4.2–6.1 ×2–2.3 µm, Gilbertson and Ryvarden 1986). In addition, Fomitopsisbambusae may be confused with F.ostreiformis (Berk.) T. Hatt. in having similar-sized basidiospores and also growing on bamboo, but F.ostreiformis differs from F.bambusae by the larger pores (3–4 per mm vs. 6–9 per mm) and trimitic hyphal system (De 1981).
Our phylogeny of Oligoporus (Fig. 2), based on ITS+nLSU+nuSSU+mtSSU+PRB1+PRB2+TEF1 sequence, demonstrated Oligoporus s.s. formed a monophyletic lineage with a robust rating (100% ML, 100% MP, 1.00 BPPs), which is distant from Postia s.s. Though Oligoporus and Postia are similar to each other in morphological characteristics, some significant differences remain. For instance, Postia s.s. has effuse-reflexed to pileate basidiocarps, thin-walled and acyanophilous basidiospores (Donk 1971; Ryvarden and Melo 2014; Shen et al. 2019), while Oligoporus s.s. has resupinate basidiocarps, slightly thick-walled and cyanophilous basidiospores (Shen et al. 2019). Phylogenetically, Oligoporuspodocarpi is nested in the Oligoporus s.s. clade with a strong support (100% ML, 100% MP, 1.00 BPPs) and related to O.rennyi (Berk. & Broome) Donk and O.sericeomollis (Romell) Bondartseva (Fig. 2). These three species, representing Oligoporus s.s., have resupinate basidiocarps, white to cream pore surface and thick-walled, dextrinoid, cyanophilous basidiospores. However, Oligoporusrennyi differs from O.podocarpi in the very fragile dry basidiocarps, the lack of cystidia and the presence of chlamydospores (Donk 1971; Ryvarden and Melo 2014). Oligoporussericeomollis is different from O.podocarpi by fragile dry basidiocarps, longer basidiospores (4–5 × 2–2.5 μm vs. 3.8–4.2 × 2–2.3 µm) and the extremely bitter taste (Núñez and Ryvarden 2001; Ryvarden and Melo 2014). Mophologically, Oligoporuspodocarpi is similar to Postiasimanii (Pilát) Jülich, Cystidiopostiahibernica (Berk. & Broome) B.K. Cui, L.L. Shen & Y.C. Dai and Rhodoniarancida (Bres.) B.K. Cui, L.L. Shen & Y.C. Dai by resupinate basidiocarps, white to cream pore surface (Jülich 1982; Núñez and Ryvarden 2001; Ryvarden and Melo 2014; Shen et al. 2019). However, Postiasimanii has smaller pores (6–8 per mm) and allantoid, thin-walled basidiospores measuring 4–5.3 × 0.8–1.2 µm (Jülich 1982; Ryvarden and Melo 2014). Cystidiopostiahibernica and Rhodoniarancida are different from Oligoporuspodocarpi by larger pores (2–3 per mm in C.hibernica, 2–4 per mm in R.rancida) and allantoid, thin-walled basidiospores (4.3–6 × 1.4–1.9 µm in C.hibernica, 5–7 × 2–2.5 µm in R.rancida) (Ryvarden and Melo 2014; Shen et al. 2019).
Supplementary Material
Acknowledgements
The research is supported by the National Natural Science Foundation of China (Project No. 32000010).
Funding Statement
the National Natural Science Foundation of China (Project No. 32000010)
References
- Binder M, Hibbett DS, Larsson KH, Larsson E, Langer E, Langer G. (2005) The phylogenetic distribution of resupinate forms across the major clades of mushroom forming fungi (Homobasidiomycetes). Systematics Biodiversity 3: 113–157. 10.1017/S1477200005001623 [DOI] [Google Scholar]
- Binder M, Justo A, Riley R, Salamov A, López-Giráldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev IV, Hibbett DS. (2013) Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105: 1350–1373. 10.3852/13-003 [DOI] [PubMed] [Google Scholar]
- Brefeld O. (1888) Basidiomyceten 3. Autobasidiomyceten. Untersuchungen aus dem Gesammtgebiete der Mykologie 8: 1–184. [Google Scholar]
- Chen YY, Wu F, Wang M, Cui BK. (2017) Species diversity and molecular systematics of Fibroporia (Polyporales, Basidiomycota) and its related genera. Mycological Progress 16: 521–533. 10.1007/s11557-017-1285-1 [DOI] [Google Scholar]
- Corner EJH. (1989) Ad Polyporaceas V. Beihefte zur Nova Hedwigia 96: 1–218. [Google Scholar]
- Cui BK, Li HJ, Ji X, Zhou JL, Song J, Si J, Dai YC. (2019) Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Diversity 97: 137–302. 10.1007/s13225-019-00427-4 [DOI] [Google Scholar]
- Cui BK, Vlasák J, Dai YC. (2014) The phylogenetic position of Osteinaobducta (Polyporales, Basidiomycota) based on samples from Northern Hemisphere. Chiang Mai Journal of Science 41: 838–845. [Google Scholar]
- Cunningham GH. (1965) Polyporaceae of New Zealand. Bulletin of the New Zealand Department of Scientific and Industrial Research 164: 1–304. 10.1017/S0267190500002877 [DOI] [Google Scholar]
- Dai YC. (2012) Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53: 49–80. 10.1007/s10267-011-0134-3 [DOI] [Google Scholar]
- Dai YC, Wei YL, Zhou LW. (2015) Polypore richness along an elevational gradient: A case study in Changbaishan Nature Reserve, Northeastern China. Fungal Ecology 13: 226–228. 10.1016/j.funeco.2014.07.002 [DOI] [Google Scholar]
- De AB. (1981) Taxonomy of Polyporusostreiformis in relation to its morphological and cultural characters. Canadian Journal of Botany-revue Canadienne de Botanique 59: 1297–1300. 10.1139/b81-174 [DOI] [Google Scholar]
- Donk MA. (1960) The generic names proposed for Polyporaceae. Persoonia 1: 173–302. [Google Scholar]
- Donk MA. (1971) Notes on European polypores 8. Persoonia 6: 201–218. [Google Scholar]
- Felsenstein J. (1985) Confidence intervals on phylogenetics: An approach using the bootstrap. Evolution 39: 783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- Floudas D, Hibbett DS. (2015) Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biology 119: 679–719. 10.1016/j.funbio.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Gilbertson RL. (1981) North American wood-rotting fungi that cause brown rots. Mycotaxon 12: 372–416. 10.1007/BF00575090 [DOI] [Google Scholar]
- Gilbertson RL, Ryvarden L. (1986) North American polypores 1. Abortiporus – Lindtneria. Fungiflora, Oslo.
- Gilbertson RL, Ryvarden L. (1987) North American Polypores 2. Megasporoporia – Wrightoporia. Fungiflora, Oslo.
- Haight JE, Laursen GA, Glaeser JA, Taylor DL. (2016) Phylogeny of Fomitopsispinicola: A species complex. Mycologia 108: 925–938. 10.3852/14-225R1 [DOI] [PubMed] [Google Scholar]
- Haight JE, Nakasone KK, Laursen GA, Redhead SA, Taylor DL, Glaesera JA. (2019) Fomitopsismounceae and F.schrenkii – two new species from north America in the F.pinicola complex. Mycologia 111: 1–19. 10.1080/00275514.2018.1564449 [DOI] [PubMed] [Google Scholar]
- Hall TA. (1999) Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. 10.1021/bk-1999-0734.ch008 [DOI] [Google Scholar]
- Han ML, Chen YY, Shen LL, Song J, Vlasák J, Dai YC, Cui BK. (2016) Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Diversity 80: 343–373. 10.1007/s13225-016-0364-y [DOI] [Google Scholar]
- Hattori T. (2003) Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. V. Species described in Tyromyces (2). Mycoscience 44: 265–276. 10.1007/S10267-003-0114-3 [DOI] [Google Scholar]
- Jülich W. (1981) Higher taxa of Basidiomycetes. Bibliography of Systematic Mycology 85: 1–485. [Google Scholar]
- Jülich W. (1982) Notes on some Basidiomycetes (Aphyllophorales and Heterobasidiomycetes). Persoonia 11: 421–428. [Google Scholar]
- Justo A, Miettinen O, Floudas D, Ortiz-Santana B, Sjökvist E, Lindner D, Nakasone K, Niemelä T, Larsson KH, Ryvarden L, Hibbett DS. (2017) A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121: 798–824. 10.1016/j.funbio.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Karsten PA. (1881) Symbolae ad mycologiam Fennicam. 8. Meddelanden af Societas pro Fauna et Flora Fennica 6: 7–13. [Google Scholar]
- Kim CS, Jo JW, Kwag YN, Lee S-g, Kim S-Y, Shin C-H, Han S-K. (2015) Mushroom flora of Ulleung-gun and a newly recorded Bovista species in the Republic of Korea. Mycobiology 43: 239–257. 10.5941/MYCO.2015.43.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Han ML, Cui BK. (2013) Two new Fomitopsis species from southern China based on morphological and molecular characters. Mycological Progress 12: 709–718. 10.1007/s11557-012-0882-2 [DOI] [Google Scholar]
- Liu S, Han ML, Xu TM, Wang Y, Wu DM, Cui BK. (2021) Taxonomy and phylogeny of the Fomitopsispinicola Complex with descriptions of six new species from East Asia. Frontiers in Microbiology 12: e644979. 10.3389/fmicb.2021.644979 [DOI] [PMC free article] [PubMed]
- Liu S, Song CG, Cui BK. (2019) Morphological characters and molecular data reveal three new species of Fomitopsis (Basidiomycota). Mycological Progress 18: 1317–1327. 10.1007/s11557-019-01527-w [DOI] [Google Scholar]
- Matheny PB. (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Molecular Phylogenetics and Evolution 35: 1–20. 10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, Hall BD. (2002) Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. 10.3732/ajb.89.4.688 [DOI] [PubMed] [Google Scholar]
- Melo I, Ryvarden L. (1989) Fomitopsisiberica Melo & Ryvarden sp. nov. Boletim da Sociedade Broteriana 62: 227–230. [Google Scholar]
- Nilsson RH, Tedersoo L, Abarenkov K, Ryberg M, Kristiansson E, Hartmann M, Schoch CL, Nylander JAA, Bergsten J, Porter TM, Jumpponen A, Vaishampayan P, Ovaskainen O, Hallenberg N, Bengtsson-Palme J, Eriksson KM, Larsson KH, Larsson E, Kõljalg U. (2012) Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. MycoKeys 4: 37–63. 10.3897/mycokeys.4.3606 [DOI] [Google Scholar]
- Núñez M, Ryvarden L. (2001) East Asian Polypores, Synopsis Fungorum 14, vol 2. Fungiflora, Oslo, Norway, 229–231.
- Nylander JAA. (2004) MrModeltest v.2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- Ortiz-Santana B, Lindner DL, Miettinen O, Justo A, Hibbett DS. (2013) A phylogenetic overview of the Antrodia clade (Basidiomycota, Polyporales). Mycologia 105: 1391–1411. 10.3852/13-051 [DOI] [PubMed] [Google Scholar]
- Page RMD. (1996) Treeview: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. 10.1093/bioinformatics/12.4.357 [DOI] [PubMed] [Google Scholar]
- Pegler DN, Saunders EM. (2014) British poroid species formerly placed in the genus Tyromyces (Coriolaceae). Mycologist 8: 24–31. 10.1016/S0269-915X(09)80678-2 [DOI] [Google Scholar]
- Petersen JH. (1996) Farvekort. The Danish Mycological Society’s color-chart. Foreningen til Svampekundskabens Fremme, Greve.
- Pildain MB, Rajchenberg M. (2013) The phylogenetic position of Postia s.l. (Polyporales, Basidiomycota) from Patagonia, Argentina. Mycologia 105: 357–367. 10.3852/12-088 [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. (1998) Modeltest: Testing the model of DNA substitution. Bioinformatics 14: 817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Rehner SA, Buckley E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. 10.3852/mycologia.97.1.84 [DOI] [PubMed] [Google Scholar]
- Renvall P. (1992) Basidiomycetes at the timberline in Lapland 4. Postialateritian. sp. and its rust-coloured relatives. Karsternia 32: 43–60. 10.29203/ka.1992.291 [DOI] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hőhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvarden L. (1981) Type studies in the Polyporaceae13. Species described by J.H. Léveillé. Mycotaxon 13: 175–186. [Google Scholar]
- Ryvarden L. (1991) Genera of polypores, nomenclature and taxonomy. Synopsis Fungorum 5: 1–363. [Google Scholar]
- Ryvarden L, Gilbertson RL. (1993) European polypores 1. Abortiporus-Lindtneria. Synopsis Fungorum 6: 1–387. [Google Scholar]
- Ryvarden L, Melo I. (2014) Poroid fungi of Europe. Synopsis Fungorum 31: 1–455. [Google Scholar]
- Shen LL, Wang M, Zhou JL, Xing JH, Cui BK, Dai YC. (2019) Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 42: 101–126. 10.3767/persoonia.2019.42.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares AM, Nogueira-Melo G, Plautz Jr HL, Gibertoni TB. (2017) A new species, two new combinations and notes on Fomitopsidaceae (Agaricomycetes, Polyporales). Phytotaxa 331: e75. 10.11646/phytotaxa.331.1.5 [DOI]
- Song J, Cui BK. (2017) Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evolutionary Biology 17: e102. 10.1186/s12862-017-0948-5 [DOI] [PMC free article] [PubMed]
- Spirin VA. (2002) The new species from the genus Antrodia. Mikologiya i fitopatologiya 36: 33–35. [Google Scholar]
- Swofford DL. (2002) PAUP*: Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland. 10.1111/j.0014-3820.2002.tb00191.x [DOI]
- Thangamalai MS, Alwin P, Packiaraj J, Rajaiah S. (2018) Bioprospection of Basidiomycetes and molecular phylogenetic analysis using internal transcribed spacer (ITS) and 5.8S rRNA gene sequence. Scientific Reports 8: e10720. 10.1038/s41598-018-29046-w [DOI] [PMC free article] [PubMed]
- Thompson JD, Gibson TJ, Plewniak F, Franois J, Higgins DG. (1997) The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Symposium Series 25: 4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, Santiago ALCMA, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li J, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Ottoni AM, Mešic A, Dutta AK, Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Chakraborty DXLD, Ercole E, Wu F, Simonini G, Vasquez G, Silva GA, Plautz Jr HL, Ariyawansa HA, Lee H, Kušan I, Song J, Sun J, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšík M, Samarakoon MC, Wijayawardene NN, Kim NK, Matočec N, Singh PN, Tian Q, Bhatt RP, Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Marathe SD, Khan S, Hongsanan S, Adhikari S, Mehmood T, Bandyopadhyay TK, Svetasheva TY, Nguyen TTT, Antonín V, Li WJ, Wang Y, Indoliya Y, Tkalčec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, Luangsa-ard J, Xu J, Yan J, Kang JC, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen TC, Karunarathna SC. (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83: 1–261. 10.1007/s13225-017-0378-0 [DOI] [Google Scholar]
- Walker J. (1996) An opinion on the validity of the generic name Postia Fries 1874 (Eumycota: Aphyllophorales). Australasian Mycological Society Newsletter 15: 23–26. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: InnisMA Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols: A guide to methods and applications.Academic, San Diego, 315–322.
- Wu F, Yuan HS, Zhou LW, Yuan Y, Cui BK, Dai YC. (2020) Polypore diversity in South China. Mycosystema 39: 653–681. [Google Scholar]
- Xing JH, Sun YF, Han YL, Cui BK, Dai YC. (2018) Morphological and molecular identification of two new Ganoderma species on Casuarinaequisetifolia from China. MycoKeys 34: 93–108. 10.3897/mycokeys.34.22593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YJ, Pegler DN, Chase MW. (2005) Molecular variation in the Postiacaesia complex. FEMS Microbiology Letters 242: 109–116. 10.1016/j.femsle.2004.10.046 [DOI] [PubMed] [Google Scholar]
- Zhang DZ. (2003) Three brown rot polypores new to Taiwan and their cultural studies. Taiwania 48: 1–5. 10.6165/tai.2003.48(1).1 [DOI] [Google Scholar]
- Zhou LW, Dai YC. (2012) Recognizing ecological patterns of wood-decaying polypores on gymnosperm and angiosperm trees in northeast China. Fungal Ecology 5: 230–235. 10.1016/j.funeco.2011.09.005 [DOI] [Google Scholar]
- Zhu L, Ji X, Si J, Cui BK. (2019) Morphological characters and phylogenetic analysis reveal a new species of Phellinus with hooked hymenial setae from Vietnam. Phytotaxa 1: 91–99. 10.11646/phytotaxa.356.1.8 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.