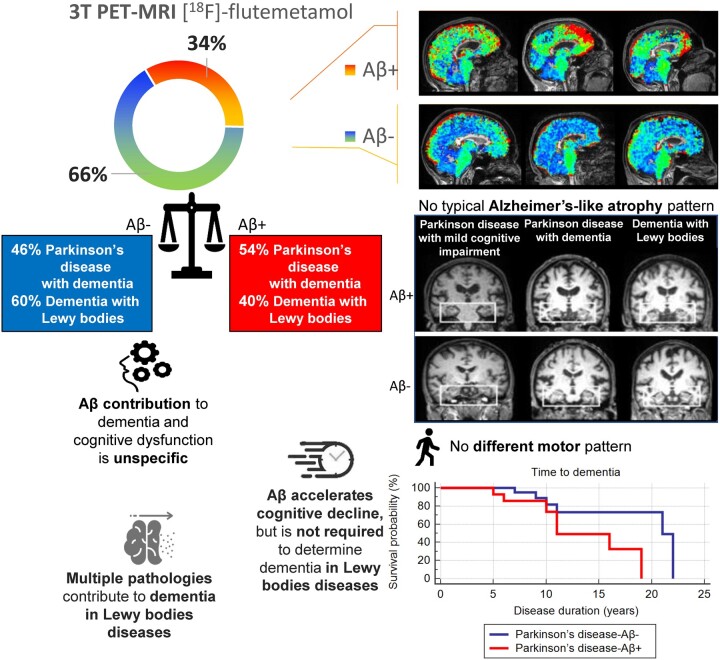
Abstract
Dementia in Lewy Body Diseases (Parkinson’s disease and dementia with Lewy Bodies) affects progression of disabilities, quality of life and well-being. Understanding its pathogenetic mechanisms is critical to properly implement disease-modifying strategies. It has been hypothesized that synuclein- and amyloid-pathology act synergistically aggravating cognitive decline in elderly patients but their precise contribution to dementia is debated. In this study, we aimed at exploring if presence of amyloid deposits influences clinical, cognitive and neuroanatomical correlates of mental decline in a cohort of 40 Parkinson’s disease patients with normal cognition (n = 5), mild cognitive impairment (n = 22), and dementia (n = 13) as well as in Dementia with Lewy Bodies (n = 10). Patients underwent simultaneous 3 T PET/MRI with [18F]-flutemetamol and were assessed with an extensive baseline motor and neuropsychological examination, which allowed level II diagnosis of mild cognitive impairment and dementia. The role of amyloid positivity on each cognitive domain, and on the rate of conversion to dementia at 1-year follow-up was explored. A Kaplan Meier and the Log Rank (Mantel–Cox) test were used to assess the pairwise differences in time-to-develop dementia in Parkinson’s disease patients with and without significant amyloidosis. Furthermore, the presence of an Alzheimer’s dementia-like morphological pattern was evaluated using visual and automated assessment of T1-weighted and T2-weighted MRI images. We observed similar percentage of amyloid deposits in Parkinson’s disease dementia and dementia with Lewy Bodies cohorts (50% in each group) with an overall prevalence of 34% of significant amyloid depositions in Lewy Body Diseases. PET amyloid positivity was associated with worse global cognition (Montreal Cognitive Assessment and Mini Mental State Examination), executive and language difficulties. At 12-month follow-up, amyloid positive Parkinson’s disease patients were more likely to have become demented than those without amyloidosis. Moreover, there was no difference in the presence of an Alzheimer’s disease-like atrophy pattern and in vascular load (at Fazekas scale) between Lewy Body Diseases with and without significant amyloid deposits. Our findings suggest that in Lewy Body Diseases, amyloid deposition enhances cognitive deficits, particularly attention-executive and language dysfunctions. However, the large number of patients without significant amyloid deposits among our cognitively impaired patients indicates that synuclein pathology itself plays a critical role in the development of dementia in Lewy Body Diseases.
Keywords: Parkinson’s disease, dementia with Lewy bodies, amyloid, PET/MRI, cognitive dysfunction
Biundo et al. used PET/3TMRI to study amyloidosis influence on cognition and conversion to dementia in Lewy Body disorders. A similar percentage of patients were amyloid-β negative, but general cognition was more severe and conversion to dementia was faster in amyloid-β positive. Amyloid-β contributes to cognitive decline in Lewy Body disorders.
Graphical Abstract
Graphical Abstract.
Introduction
Lewy Body Diseases, such as Parkinson’s disease and Dementia with Lewy bodies, manifest with different phenotypes and a combination of motor and non-motor symptoms. As research is increasingly focussed on disease modifying strategies, identifying symptoms that most contribute to disease progression has become a key objective in translational research in both conditions.1–3
Cognitive decline and dementia are established disease milestones in Parkinson’s disease as they negatively impact on progression of disability,2,4 quality of life4–6 and life expectancy.7
Parkinson’s disease with mild cognitive impairment (PD-MCI) and particularly deficits in semantic and visuospatial skills have been associated with faster cognitive decline and conversion to dementia,8–11 but the significant variability in the PD-MCI profile makes prediction very challenging in individual patients.12
The underlying pathology is also heterogeneous with amyloid plaques, tau neurofibrillary tangles (Alzheimer’s hallmarks), cortical Lewy bodies13,14 reported with variable frequency in demented patients.
Neuropathology studies suggest that in Parkinson’s disease amyloidosis is associated with worse cognitive status and shorter motor to dementia interval, similarly to dementia with Lewy Bodies.15 According to the National Institute of Aging and Alzheimer’s Association research framework, brain amyloid deposition only determines whether or not an individual is in the Alzheimer’s continuum, and by itself is not sufficient for the diagnosis of Alzheimer’s disease as well as the presence of neurodegenerative biomarkers and cognitive symptoms.16–19 Moreover, 30–40% of cognitively unimpaired elderly persons have Alzheimer’s disease neuropathologic changes at autopsy,20 and a similar proportion has abnormal amyloid biomarkers.21 Based on these findings, a synergism between synuclein and Alzheimer’s disease-type pathologies [primarily amyloid deposits (Aβ) pathology] to dementia-risk in Lewy Body Diseases is conceivable.22,23 Furthermore, amyloidosis increases with ageing, highlighting the importance to investigate patients in a specific age range.24
However, most published studies on Lewy Body Diseases include relatively small cohorts with frequently incomplete cognitive characterization, and heterogeneous patients’ age, making difficult to draw firm conclusions about the role of amyloid in Lewy Body Diseases.25–29
In this study, we assessed amyloid deposition by [18F]flutemetamol PET and investigated if this distinguishes clinical/cognitive phenotypes in patients with the whole spectrum of Parkinson’s disease and dementia with Lewy Bodies (DLB) [PD cognitively intact (PD-NC), PD-MCI, Parkinson’s with dementia (PDD) and DLB] and within a similar age range. Since imaging was performed using a simultaneous 3T PET/MRI scanner, we evaluated in the same patient, structural and molecular changes and if this is associated with prevalence of dementia at baseline and prospectively at 12-month follow-up.
Material and methods
Study population
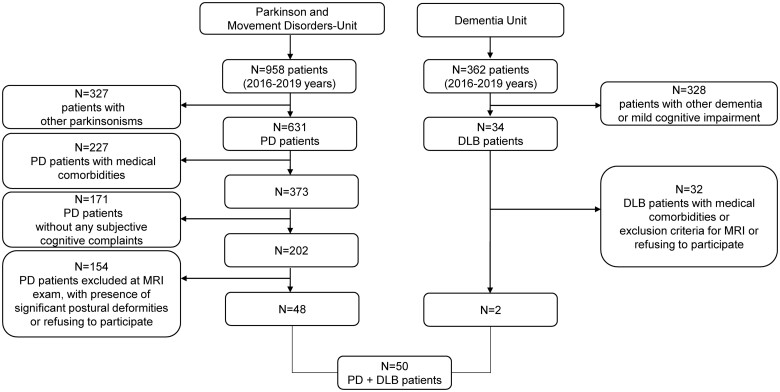
A total of 50 patients [10 DLB, 13 PDD, and 27 non-demented Parkinson’s disease (22 PD-MCI and 5 PD-NC)] were included in the study from 2016 to 2019. Patients were recruited consecutively at the Parkinson’s Disease and Movement Disorders Unit and Dementia Unit of Neurology Clinic in Padua and underwent a comprehensive clinical and neuropsychological evaluation. We considered only PD and DLB patients within an age range between 55 and 85 years, encompassing the overall spectrum of cognitive status in Lewy Body Diseases, with subjective complains and/or early dementia (based on altered activities of daily living). From a total of 1000 patients evaluated at first visit, 50 subjects were included in the study (please see Fig. 1 with the flowchart detailing the recruitment phase).
Figure 1.
Flowchart of the studied population.
All Parkinson’s disease patients fulfilled the Queen Square Brain Bank criteria for the diagnosis of probable Parkinson’s disease30; the clinical diagnosis of probable DLB was based on published consensus criteria.31 All DLB patients had parkinsonism and presented dementia either before or within 1 year from the onset of motor manifestations. The clinical diagnoses for both PD and DLB were confirmed by presence of reduced DaTscan SPECT binding in the striatum. Exclusion criteria were the treatment with deep brain stimulation, atypical Parkinsonian disorders, severe psychiatric or neurological comorbidity, and clinically relevant cerebrovascular disease on MRI. The study was approved by the Ethic Committee of the University of Padua (4340/AO/17). All patients gave written informed consent according to the Declaration of Helsinki.
Clinical and neuropsychological examination
Demographic, clinical characteristics and presence of comorbid diseases were collected by neurologists with experience in movement disorders.
Disease severity was assessed using the motor section of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III)32 and Hoehn–Yahr scale. Motor phenotypes were determined as either tremor dominant phenotype or postural instability and gait disturbance phenotype following the classification algorithm proposed by Jankovic et al.33 Following the original classification methods, the ratio of the mean MDS-UPDRS-III tremor scores (items: 15–18) to the mean MDS-UPDRS gait disturbance scores (items: 9–12) was used to define tremor phenotype (ratio ≥ 1.5), gait disturbance phenotype (ratio ≤ 1) or indeterminate phenotype (ratios >1.0 and <1.5). Finally, the sum of items 3.3a–3.3e, and sum of items 3.7a–3.8b and 3.14 were considered for rigidity and for bradykinesia. The score for each motor symptom was calculated by summing up the scores of corresponding items in MDS-UPDRS-III (see Supplementary S1).
Levodopa and dopamine agonist equivalent daily doses were calculated.34 Seven out of 23 demented patients (5/13 PDD and 2/10 DLB) were on acetylcholinesterase inhibitors and one on memantine.
Patients underwent an extensive neuropsychological examination to diagnose mild cognitive impairment and dementia according to the Movement Disorder Society Level II criteria35,36 (for further details on cognitive tests adopted, see Fiorenzato et al.37) In respect to the extensive neuropsychological assessment, the Trail making Test-B was considered ‘not feasible’ as more than the 20% of patients in both groups were not able to either initiate or complete the task and for this reason was excluded from the analyses. Age at disease onset was defined as the age at which the patient noticed the first motor symptom (or cognitive symptoms in DLB subjects) indicative of Parkinson’s disease. The expert neuropsychologist (who assessed the II level mild cognitive impairment patient at baseline), blinded to motor score and PET results, made a diagnosis of cognitive stable versus converted to dementia profile at 1-year follow-up interval. Dementia diagnosis was made whether caregivers reported significant changes in patient’s activities of daily living (altered ADLs).38
The ADLs scale, developed by Katz,39 is the most frequently used measure of older adults’ basic functional status. This scale can be self-administered or assessed by caregiver or a healthcare worker. The inability to perform ADLs results in the dependence of other individuals and/or mechanical devices. The basic ADLs are those skills required to manage one’s basic physical needs, including personal hygiene or grooming, dressing, toileting, transferring or ambulating, and eating. The total score for ADLs is 0–6. When this screen is used over time, it serves to document a person’s functional improvement or deterioration.40
Well-being and quality of life were assessed using the Parkinson’s Disease Questionnaire (PDQ-8).41 The presence of depression, anxiety and apathy was assessed using the Beck Depression Inventory-II (BDI-II), State-Trait Anxiety Inventory forms (STAI Y-1 to assess state anxiety, STAI Y-2 to assess trait anxiety) and Starkstein Apathy Scale (AS), respectively.42
Patients were evaluated in ‘on’ medication state. The cognitive tests were administered by trained neuropsychologists, in the morning, on two separate occasions within 3–5 days. All clinical assessments were performed blind to PET results.
PET/MRI acquisition
Lewy Body Diseases participants in accordance with the amyloid imaging procedure guideline43 received an intravenous injection of about 185 MBq [18F]flutemetamol (performed manually over 10 s and flushed with 30 ml of saline over about 15 ± 5 s) directly in an integrated 3T PET/MRI system (Biograph mMR; Siemens, Erlangen, Germany). Images were acquired between 0–10 and 90–110 min after injection according to Cecchin et al.44 PET data were reconstructed into a 256 × 256 matrix (voxel 2.32 × 2.32 × 2.03) using the built-in 3D Ordinary Poisson-Ordered Subset Expectation Maximization algorithm with eight iterations, 21 subsets and a 3-mm Gaussian post-filtering. Standard correction of decay, scatter, dead time and attenuation was performed. An ultrashort echo time sequence was used for attenuation correction. Anatomical volumetric data via T1-weighted-3D magnetization-prepared rapid acquisition gradient echo sequence (TR 1.900 ms, TE 2.53, slice thickness 1 mm, matrix 256 × 256, FOV 250 mm) were simultaneously acquired. Additionally, a 1 mm-isotropic T2-weighted-3D, and two-dimensional susceptibility-weighted imaging were acquired for clinical evaluation, excluding secondary parkinsonisms, the presence of vascular brain damage and allowing visual rating scales assessment.
Classification of PET amyloid images
Binary visual classification of [18F]flutemetamol scans as positive or negative is accurate and reliable for detection of cases with histology defined plaques.45 An expert nuclear medicine physician (DC, with both in-person and e-training), blinded to cognitive status and diagnosis, rated each scan as amyloid-positive (Aβ+) or negative (Aβ−). This judgement was based on the assessment of [18F]flutemetamol uptake in grey versus white matter in frontal lobes, anterior cingulate, posterior cingulate, precuneus, temporo-parietal, including insula, lateral temporal lobes and striatal region (in accordance with [18F]-Flutemetamol product information sheet: https://www.ema.europa.eu/en/medicines/human/EPAR/vizamyl).
Visual Alzheimer's disease-like pattern assessment
Two trained observers (L.W. and A.A.) independently visually rated each participant T1-weighted and T2-weighted MRI images blinded to age, sex or diagnosis and rated the Scheltens’ scale of medial temporal atrophy (MTA),46 the Pasquier’s Global Cortical Atrophy scale and the frontal subscale (GCA and GCA-F, respectively),47 the Koedam’s scale of Posterior Atrophy (PA) and the Fazekas’s scale.48,49 In order to increase the raters’ accuracy,50 all brains MRI data were rigidly realigned to the Montreal Neurologic Institute template using FMRIB’s Linear Image Registration Tool (FLIRT-FSLv6.0, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki).51,52 This rigid transform was computed with six degrees of freedom (i.e. rotation and translation only) and was used to align each brain automatically to the anterior commissure—posterior commissure line and conform all images to the same voxel size (1 mm3 × 1 mm3 × 1 mm3) and input dimension (182 × 218 × 182). Cut-offs for the visual rating scales have previously been published.53 Normal versus abnormal cut-off points were determined for each individual in the four visual rating scales. An MTA score ≥1.5 was considered abnormal in the age-group 65–74, ≥2 for the age-group 75–84 and ≥2.5 for the age-group 85–94. For PA and GCA and GCA-F, a score ≥1 was always considered abnormal irrespective of age (age-correction did not improve diagnostic accuracy in cut-offs derivation). For Fazekas’s scale, a score ≥1 was considered indicative of at least mild vascular abnormalities.48 The combination of MTA, PA and GCA-F and Fazekas score have previously been described in relation to Alzheimer's disease.53 Namely, we identified (i) the Alzheimer's disease-like pattern if a patient presented either the limbic-predominant pattern (defined as an abnormal MTA, and a normal PA and GCA-F) or the typical Alzheimer's disease pattern (defined as an abnormal MTA in conjunction with either an abnormal PA or GCA-F or an abnormal MTA in conjunction with both an abnormal PA and GCA-F) or the hippocampal-sparing pattern (defined as a normal MTA and either an abnormal PA or GCA-F, or both an abnormal PA and GCA-F); and (ii) the Alzheimer's disease-like pattern plus Fazekas.54
Automated Alzheimer's disease-like pattern assessment
The Brain Anatomical Analysis using Diffeomorphic deformation (BAAD 4.31-http://www.shiga-med.ac.jp/~hqbioph/BAAD/Welcome_to_BAAD.html) Statistical Parametric Mapping tool (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) was used to calculate the Alzheimer's disease-score from T1-weighted-3D in order to predict the presence of an early-Alzheimer's disease pattern. This tool includes a Computational Anatomy Toolbox (CAT12)-based (http://www.neuro.uni-jena.de/cat/), T1-weighted-3D diffeomorphic segmentation after inhomogeneity correction, T1-weighted-3D quality check assessment and Total Intracranial Volume (TIV) estimation. T2-weighted-3D was included in the multimodal segmentation for correcting brain atrophy estimation from the presence of white matter lesions. BAAD’s Regions of Interest extraction integrated the Wfu_Pickatlas (http://fmri.wfubmc.edu/software/PickAtlas) and MarsBaR (http://marsbar.sourceforge.net) SPM12 toolboxes. Alzheimer's disease score is a score ranging from zero to one. With a score higher than 0.6, the patient is highly suspected as being in the Alzheimer's disease continuum.55 This score comes from multiple sampling points of brain volume of interest (VOIs) and judged by support-vector machine (SVM) model. The SVM model provided in BAAD was previously trained, including 700 patients using Alzheimer Disease Neuroimaging Initiative (ADNI, http://adni.loni.usc.edu/) data of North America and validated by Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL, https://aibl.csiro.au/) or Japanese ADNI (http://www.j-adni.org/) data. Sequential minimal optimization was used for solving the minimization SVM problem and removing outliers with the gradient difference as convergence criteria. The Alzheimer's disease-score provided by BAAD was estimated based on 10 VOIs of the Automated Anatomical Labeling atlas56: right BA28, left amygdala, left angular gyrus, left frontal inferior operculum, left superior medial frontal + right superior medial frontal, left middle occipital, left superior parietal, left Rolandic operculum and left middle temporal pole. Age, TIV and sex were included in the predictive model based on age and sex ranked healthy subject normative data. For explorative purpose, BAADs tool provide at subject level a voxel-wise non-parametric statistical map of grey matter and white matter alterations, comparing each participants brain MRI normalized and segmented image to the age- and sex-matched normative data with the SnPM12 tool (http://warwick.ac.uk/snpm).
Statistical analysis
An a priori power analysis was conducted using G*Power3.1.9.457 to test the difference between Montreal Cognitive Assessment (MoCA) score means in two independent groups using a two-tailed Wilcoxon–Mann–Whitney test. The asymptotic relative efficiency (A.R.E. method) was used for effect size estimation. A large effect size (d = 1.027) and allocation ratio N1/N2 = 0.5 were calculated based on mean (SD) MoCA scores and Aβ+ frequency reported in the Parkinson’s Progressive Markers Initiative large Parkinson’s disease cohort study.58 An alpha probability error of 0.05 was considered for sample estimation. Results showed that a total sample of 50 participants with 17 Lewy Body disorders with significant Aβ (LBDs-Aβ+ subgroup) and 33 Lewy Body disorders without significant Aβ (LBDs-Aβ− subgroup) was required to achieve a power of 0.91.
Pairwise differences in characteristics (clinical, motor and cognitive) between the two Lewy Body disorders subgroups (LBDs-Aβ+ versus LBDs-Aβ−) were assessed with Mann–Whitney U-test or Fisher’s exact test as appropriate. In order to explore the role of Aβ on each cognitive domain and cognitive test performance, we converted each cognitive raw score to Z-score using Italian normative data. Then, we calculated the Z-compound as the mean Z-score among tests of each cognitive domain and considered −1.5 SD as pathologic performance cut-off. Pearson’s Chi-square statistic was used to compare percentage of failures in LBDs-Aβ+ versus LBDs-Aβ−. Spearman’s chi-squared test was run to explore whether Aβ+ is associated with an MRI AD-like pattern in PD-MCI and Lewy Body dementia subgroups (PDD and DLB). Rate of conversion at 1-year FU was calculated and difference in rate of conversion in Parkinson’s disease subgroups (PD-Aβ+ versus PD-Aβ−) were evaluated using the McNemar test. Finally, a Kaplan–Meier and the log-rank (Mantel–Cox) test were used to assess the pairwise differences in time-to-develop dementia from disease onset in PD-Aβ+ versus PD-Aβ− subgroups. Moreover, the relative risk of hazard occurring at any given time in PD-Aβ+ to develop dementia was calculated. Statistical analyses were run using IBM-SPSS 25 (IBM SPSS Inc., Chicago, IL, USA) and python 3.8 seaborn libraries for violin plot distribution density.
Data availability
All data are available upon reasonable request.
Results
Patients’ characteristics at baseline
Thirty-three out of 50 Lewy Body Disorders patients presented with Aβ− status (5 PD-NC, 16 PD-MCI, 6 PDD and 6 DLB) and 17 with Aβ+ status (6 PD-MCI, 7 PDD and 4 DLB). Because the PDD and DLB groups did not differ in terms of cognitive and neuropsychiatric functioning or percentage of positive [18F]flutemetamol uptake, we pooled them into a single Lewy body type dementia (DEM) group (13 DEM Aβ− versus 10 DEM Aβ+) for the remaining analyses (see Supplementary Table 1). The overall Lewy Body disorders subgroups (5 PD-NC, 16 PD-MCI and 13 DEM Aβ− versus 6 PD-MCI and 10 DEM Aβ+) did not significantly differ in any general clinical characteristic except for Mini Mental State Examination (MMSE) and MoCA total score. Moreover, they were indistinguishable when detailed motor symptoms were compared (see Table 1 and Supplementary Fig. 1 for more details).
Table 1.
Demographical, motor and clinical features (Mean, SD) in LBDs-Aβ+ versus LBDs-Aβ−
| LBDs |
Mann–Whitney U-test | ||
|---|---|---|---|
| LBDs-Aβ− (n = 33) | LBDs-Aβ + (n = 17) | P-value | |
| Demographical | |||
| Age (years) | 70.12 (7.28) | 72.94 (4.08) | 0.1189 |
| Sex (Male) | 69% | 76% | 0.7455 |
| Education (years) | 10.46 (5.30) | 9.75 (5.25) | 0.5525 |
| Clinical characteristics | |||
| Age at symptoms’ onset (years) | 60.49 (8.89) | 64.88 (5.878) | 0.0527 |
| Disease duration < 5 years (%) | 18% | 24% | 0.7172 |
| Disease duration (years) | 10.00 (6.39) | 8.47 (5.01) | 0.4790 |
| LEDD (mg tot/die) | 752.78 (458.17) | 701.50 (389.85) | 0.7606 |
| DAED (mg tot/die) | 89.03 (105.42) | 67.29 (99.20) | 0.5372 |
| DA (%) | 56 | 53 | 0.9999 |
| RBD (%) | 56 | 56 | 1.000 |
| Hallucination (%) | 42 | 64 | 0.2321 |
| Subjective complain (%) | 61 | 77 | 0.3510 |
| Motor characteristics | |||
| H&Y > 3 (%) | 42 | 47 | 0.7689 |
| MDS-UPDRS total score | 67.17 (38.43) | 53.0 (28.62) | 0.5156 |
| MDS-UPDRS-III | 33.55 (19.44) | 38.94 (23.63) | 0.4792 |
| PIGD/TD/indeterminate phenotype (%) | 91/0/9 | 100/0/0 | 0.5133 |
| MDS-UPDRS-III-Bradykinesia (%) | 70 | 82 | 0.4992 |
| MDS-UPDRS-IV-Dyskinesias (%) | 19 | 8 | 0.6426 |
| MDS-UPDRS-IV-Fluctuation (%) | 54 | 50 | 0.9999 |
| Functional activities | |||
| ADL | 4.31 (1.75) | 4.00 (1.80) | 0.4958 |
| IADL | 3.59 (1.95) | 2.77 (1.99) | 0.1244 |
| PD-CFRS | 9.03 (7.50) | 11.68 (6.97) | 0.2263 |
| Global cognitive status | |||
| MMSE (corrected score) | 22.85 (3.78) | 20.17 (4.43) | 0.0366* |
| MoCA (corrected score) | 18.48 (4.70) | 14.97 (3.78) | 0.0183* |
DAED, dopamine agonist equivalent daily dose; DA (%), percentage of patients in dopamine agonist therapy; H&Y, Hoehn and Yahr scale; IADL, Instrumental ADL; LEDD, levodopa and dopamine agonist equivalent daily doses; PIGD phenotype (%), percentage of postural instability and gait disturbance; RBD (%), percentage of rapid eye movement sleep Behaviour Disorder; TD phenotype (%), percentage of tremor dominant disturbance; *P < 0.05.
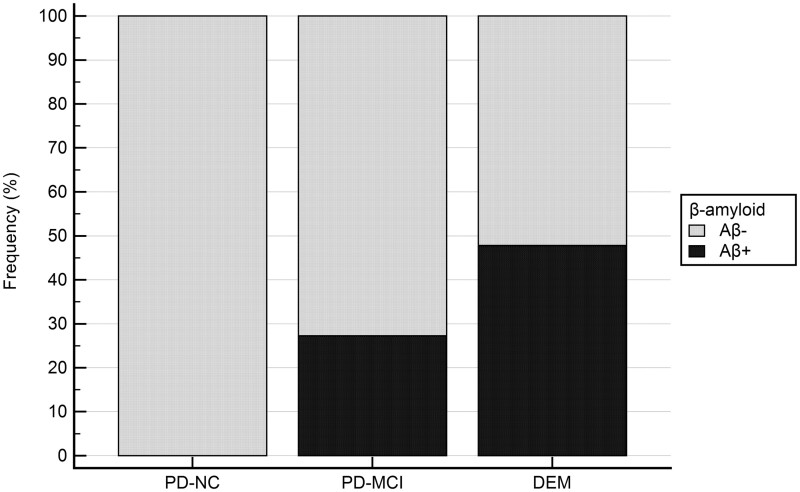
LBDs-Aβ+ patients showed worse global cognition at MoCA (P = 0.0183) and at MMSE (P = 0.0366) scales. Looking at specific test abilities, LBDs-Aβ+ versus LBDs-Aβ− showed worse performance in the executive functions as measured by the Wechsler Adult Intelligence Scale Similarities subtest (P = 0.0486) and by the Stroop Error test (P = 0.0343) and in the overall language domain (P = 0.0177), whose Z-compound score is driven by category fluency plus naming task performance (see Table 2). Finally, analyses of cognitive statuses revealed increased Aβ+ percentage according to cognitive severity (0% versus 27.3% versus 47.8% in PD-NC, PD-MCI and DEM, respectively), although Chi-squared test was not significant (see Fig. 2).
Table 2.
Comparison (percentage) of LBDs subgroups (Aβ− versus Aβ+) performing below −1.5 Z-score in each cognitive test
| LBDs |
Fisher’s exact test | ||
|---|---|---|---|
| Cognitive domains | LBDs-Aβ− (n = 33) | LBDs-Aβ+ (n = 17) | P-value |
| Attention/working memory | |||
| TMT-A | 43.7% | 64.7% | 0.2320 |
| DSST | 28.1% | 47.1% | 0.2190 |
| Z-compound | 69.7% | 88.2% | 0.1811 |
| Executive | |||
| Stroop/color task-Time | 43.7% | 41.2% | 0.9999 |
| Stroop/color task-Error | 50.0% | 82.4% | 0.0343* |
| Phonemic Fluency | 25.0% | 17.6% | 0.7250 |
| Similarities | 9.4% | 35.3% | 0.0486* |
| CDT | 25.0% | 41.2% | 0.3314 |
| Z-compound | 60.6% | 47.1% | 0.3857 |
| Memory | |||
| Prose Memory Test | 65.6% | 82.4% | 0.3228 |
| Prose Memory Test delayed | 62.5% | 76.5% | 0.3601 |
| ROCF delayed | 48.4% | 70.6% | 0.2237 |
| WPAT | 48.4% | 70.6% | 0.2237 |
| Z-compound | 36.4% | 58.8% | 0.1473 |
| Language | |||
| Naming Task | 38.7% | 52.9% | 0.3775 |
| Category Fluency | 37.5% | 41.2% | 0.9999 |
| Z-compound | 39.4% | 76.5% | 0.0177* |
| Visuospatial | |||
| VOSP | 56.2% | 68.7% | 0.5341 |
| Benton | 62.5% | 82.4% | 0.2016 |
| ROCF copy | 71.0% | 88.2% | 0.2839 |
| Z-compound | 45.5% | 41.2% | 0.9999 |
| Apraxia | |||
| Apraxia | 24.1% | 41.2% | 0.3215 |
CDT, Clock drawing test; DSST, Digit Sequencing Symbol test; ROCF copy, Rey–Osterrieth complex figure test (immediate copy); ROCF delayed, Rey–Osterrieth complex figure test (delayed copy); TMT-A, Trail making test part A; VOSP, Visual Object and Space Perception Battery; WPAT, Word paired associated test. *P < 0.05.
Figure 2.
Percentage of Aβ in LBDs patients across cognitive states. Histogram plot of percentage of Aβ+ (black) in LBDs patients. Namely, 0% of Aβ+ in PD-NC, 27.3% Aβ+ in PD MCI and 47.8% Aβ+ in DEM. Chi-square analyses comparing presence/absence of AB is not significant.
From a behavioural point of view, we did not find any significant differences between the two subgroups in depressive symptoms [BDI-II mean (SD): 8.46 (6.50) for Aβ− versus 9.67 (5.45) for Aβ+], anxiety [STAI Y-1 mean (SD): 36.96 (8.46) for Aβ− versus 38.08 (6.16) for Aβ+; STAI Y-2 mean (SD): 42.50 (10.21) for Aβ− versus 40.17 (10.36) for Aβ+], apathy [AS mean (SD): 17.71 (7.16) for Aβ− versus 18.27 (5.49) for Aβ+] and well-being [PDQ-8 mean (SD): 9.16 (5.64) for Aβ− versus 10.60 (7.26) for Aβ+].
Longitudinal analyses
At 1-year follow-up [mean: 14 months (8.00)], the 22 PD-MCI conversion rates to dementia were significantly greater in PD-Aβ+ (N 6) versus PD-Aβ− (N 16) (P < 0.0036) [0% remained stable and 100% converted (N 6) versus 81.25% remained stable (N 13) versus 18.75% converted (N 3), respectively].
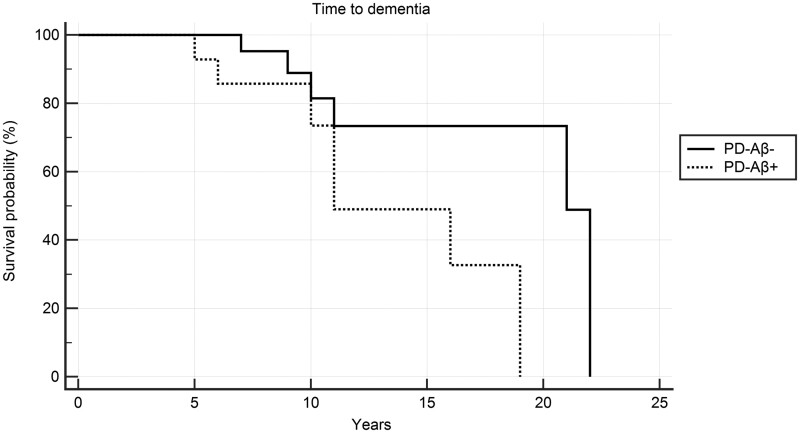
Log-rank test of the 14 PD-Aβ+ and 26 PD-Aβ− showed that the presence of Aβ anticipated the time-to-dementia (P < 0.0359). Moreover, PD-Aβ+ showed a Hazard ratio of 2.83 (CI 0.86–9.36) to develop dementia as compared to PD-Aβ− (see Kaplan–Meier curve in Fig. 3).
Figure 3.
Impact of Aβ on time to develop dementia in PD patients. Plot of Kaplan–Meier limit estimates of time to develop dementia in PD-Aβ+ versus PD-Aβ−. Logrank test P = 0.0359. Aβ+ increased Hazard ratios with 95% Confidence Interval of developing dementia = 2.83 (0.86–9.36).
MRI Alzheimer's disease-like pattern
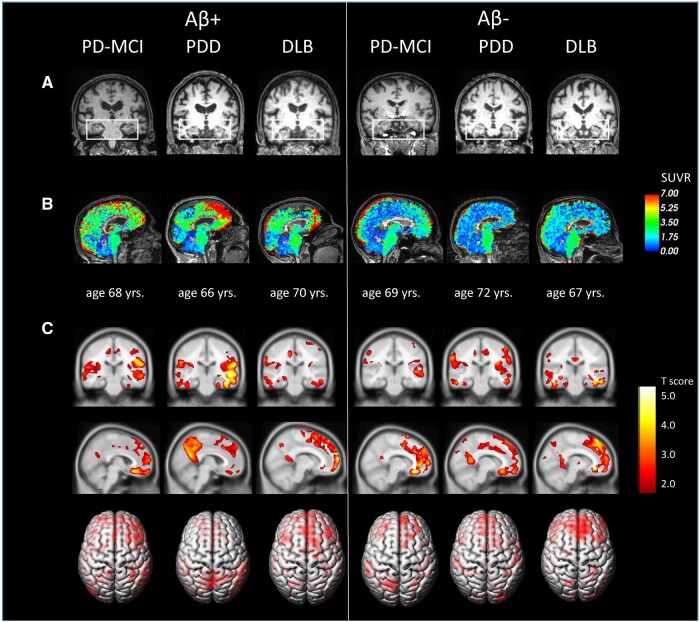
Forty-seven out of 50 Lewy Body Disorders patients {31 with Aβ− status [5 PD-NC, 15 PD-MCI, 11 DEM (5 PDD and 6 DLB)]} and 16 with Aβ+ status [6 PD-MCI, 10 DEM (7 PDD and 3 DLB)]} were included in the analysis. Three patients were excluded due to poor quality MRI resolution (presence of movement’s artefacts). We used a visual as well as automated assessment of T1-weighted and T2-weighted MRI images to identify presence of an Alzheimer's disease-like pattern. Overall, <40% of the sample showed a visually rated Alzheimer's disease-like pattern, and ∼50% of PD-MCI and DEM showed an early Alzheimer's disease-like automated score predictive of dementia. The three calculated indexes (the Alzheimer's disease-like pattern, the Alzheimer's disease-like patter plus Fazekas and the early Alzheimer's disease-like automated) were equally represented in each LBDs-Aβ subgroup (see Table 3). For a detailed summary of percentages distribution of GCA, CGA-F, MTA (left and right), PA (left and right) and Fazekas score in PD-MCI and DEM subgroups (see Supplementary Table 2). Please see Fig. 4 for representative T1-weighted 3D and PET amyloid images showing a) absence of MTA atrophy, b) FMM uptake in selected LBDs cases c) significant atrophy in fronto-striatal, anterior and posterior cingulate, precuneus and middle temporal regions compared to normative data.
Table 3.
Percentage (%) of AD-like pattern in LBDs-Aβ subgroups
| LBDs |
Fisher's exact test | ||
|---|---|---|---|
| LBDs-Aβ− (n = 31) | LBDs-Aβ+ (n = 16) | P-value | |
| AD-like VR pattern (%) | 32.3 | 37.3 | 0.7539 |
| AD-like VR pattern plus Fazekas (%) | 6.5 | 0 | 0.5411 |
| Early AD-like automated pattern (%) | 33.3 | 50.0 | 0.3470 |
AD-like VR pattern: patients with an AD-like pattern according to the presence of moderate to severe combination of MRI atrophy at visual rating scales (Global cortical atrophy-Frontal subscore, Middle temporal atrophy, posterior Atrophy-Koedam-score); AD-Like VR pattern plus Fazekas: patient with an AD pattern based on visual rating scale and a moderate to severe subcortical vascular alteration; Early AD-like automated pattern: patients with an early AD-like pattern based on an automated AD-score index using a voxel based-MRI segmentation approach.
Figure 4.
Representative images of LBDs patients according to Aβ status. (A) Visual assessments of Medial temporal lobe Index (MTA) in PD and DLB patients with/without Aβ, showing absence of mild to moderate MTA atrophy. Coronal T1w-3D views. (B) PET-MRI [18F]Flutemetamol SUVR maps obtained with cortical cerebellum as reference after three compartment Müller-Gärtner partial volume correction with PETsurfer. SUVR maps are overlaid onto each participant’s native T1-weighted 3D, showing significant Aβ deposits in frontal–striatal regions, anterior and posterior cingulate areas and precuneus. (C) Statistical non-parametric maps of significant voxel-based grey matter atrophies in frontal–striatal regions, anterior and posterior cingulate, precuneus and middle temporal areas in LBDs. Statistical maps are overlaid onto the standard MRI template after normalization to the MNI space using CAT12 tool pipeline.
Discussion
We investigated the contribution of amyloid deposition on clinical and cognitive performance and the interplay with brain atrophy in a cohort of cognitively well-characterized age-matched patients with Lewy Body Disorders using simultaneous PET/MRI imaging.
In our cohort, the overall pooled prevalence of positive cerebral PET amyloid uptake was 34% of Lewy Body Disorders patients. Interestingly, there was a similar proportion of positive and negative amyloid scans among demented participants (54% of PDD and 40% of DLB). PET amyloid positivity was associated with worse cognitive status, increased global cognitive impairment and alterations in attentive/executive and language functioning.
These findings corroborate previous evidence showing similar Aβ prevalence in Parkinson’s disease and elderly controls and a significant positive relationship between cortical beta-amyloid deposition and general cognitive impairment in Lewy Body Disorders.21,58–60 Moreover, our PD-Aβ+ patients showed faster turning to dementia than PD-Aβ−, supporting the concept that Aβ pathology is associated with more rapid cognitive decline.61–65 In addition, PET amyloid positivity was associated with a trend of worse cognitive status (0% versus 27.3% versus 47.8% in PD-NC, PD-MCI and DEM, respectively).
We also found negative cerebral amyloid uptake in both demented and PD-MCI patients who after 1 year turned to dementia. Indeed, a systematic review of more than 2000 autopsy brains in PDD, AD and DLB showed presence of limbic and neocortical Lewy body pathology in all PDD cases, while moderate or severe Aβ pathology was observed in only half of the cases and tau in only one-third.65 Moreover, high concentration of cortical Lewy bodies pathology best predicts dementia in DLB, and this correlates with clinical DLB symptoms.13 These findings support previous clinicopathological studies suggesting that multiple underlying pathologies contribute to dementia in PD, including Lewy Body pathology.66–69 Unfortunately, given the lack of dedicated imaging tracers we cannot document cerebral distribution of synuclein pathology and assess if it is responsible of the variability in dementia rates, we observed in our patients with Lewy Body Disorders.
Neuropsychological data showed that amyloidosis in Lewy Body Disorders patients worsens general cognition (measured by means of MoCA and MMSE scales), attentive–executive abilities (in particular, verbal reasoning and abstraction, cognitive flexibility, processing speed and response inhibition behaviour) as measured by Similarities and Stroop Error tasks; and language functioning (specifically verbal recall and picture naming) as assessed by category fluency and naming tasks.
These findings are also in line with our previous studies showing that both MoCA and MMSE are feasible scales to assess cognitive alterations in presence of frank dementia.12,70 However, in Lewy Body Disorders, MoCA is more sensitive and clinically useful cognitive screening instrument9,71 and correlates well with CSF Aβ 1–42 levels.58,72 Moreover, attentive/executive dysfunctions have been already observed in Aβ-positive elderlies without dementia,73 and in Aβ+ early-stage Parkinson’s disease patients with increased cortical and subcortical amyloid depositions.58
In our study, we also found significantly altered language abilities in LBDs-Aβ+ specifically by using tasks requiring access to semantic world knowledge. Evidence identified in the semantic recall dysfunctions a strong risk factor of the development of Parkinson’s disease related dementia, with these impairments reflecting a posterior temporal spread of Parkinson’s disease pathology.9,74 Moreover, it has been recently observed that markers of Alzheimer's disease-type co-pathology are implicated in impaired language performance in Lewy Body Disorders75 and that alterations in semantic fluency and naming tasks in these patients are associated with both Lewy body and Alzheimer's disease neuropathology in limbic/temporal areas.29,76–78
Interestingly, there was no difference in proportion of patients presenting Alzheimer's disease-like atrophy pattern at MRI in LBDs-Aβ+ versus LBDs-Aβ− patients with and without dementia. Cognitive performance including long-term memory was also similar in line with the concept that amyloid plaques are not the primary driver of dementia in LBDs, but they contribute in conjunction with alpha-synuclein and possibly hyper-phosphorylated tau deposition. Moreover, the lack of significant hippocampal atrophy and memory dysfunctions in our LBDs-Aβ+ patients, well supports data from a recent neuropathological study showing that tau pathology but not Aβ42 levels correlates with hippocampal volume and general cognitive status in non-demented Parkinson’s disease.79
Our study also provides some ground in the debate of PDD/DLB single versus different neuropathological aetiology. The Diagnostic Manual of Mental Disorders-fifth edition lists these two neurocognitive disorders as two separate entities. However, they share similar morphological hallmarks (cortical–subcortical alpha-synuclein, Lewy-body plus beta-amyloid and, to a less extent, plus tau pathologies),80 but different clinical onset and cognitive severity profile70 indicating some dissimilarities. By adopting the common convention of the 1-year rule, the major conclusion is that DLB shows a more severe neuropsychiatric phenotype than PDD.70,81 Neuropathological findings do not always support the arbitrary temporal distinction, with some studies observing substantial overlap of PDD and DLB phenotypes in many clinical and neuropathological investigations,82–85 including higher degrees of regional Aβ plaques in DLB than PDD.84,86–88 Overall, we can conclude that it is impossible to distinguish these two phenotypes on an individual examination without considering the temporal sequence of the event.62,89,90 In this context, our findings of similar Aβ percentage in PDD/DLB subgroups, and previous PET imaging studies of similar uptake in PDD and DLB using different tracers (PIB/F18-fluorbetapir/FBB),91–93 support only a marginal contribution from Aβ in differentiating LBDs cognitive progression trajectories.
We think that this study provides relevant evidence in establishing the role of amyloid in Lewy Body Disorders. For the first time, thanks to the use of concomitant PET/MRI scanning we extracted and compared valuable structural and molecular data.
Second, we covered the whole spectrum of Lewy Body Disorders and included a prospective evaluation at 1 year to assess cognitive worsening.
Finally, our LBDs-Aβ+ group did not differ in age, gender, clinical and behavioural features from LBDs-Aβ− patients. Specifically, our LBDs-Aβ+ group did not show differences in motor severity or motor onset, prevalence of postural instability and gait disturbance phenotype, age at symptoms onset, dopamine dose and kind as well as in neuropsychiatric functioning.
We acknowledge our study has limitations. First, only patients with early or moderate dementia were included, with no neuropathological confirmation and therefore, prospective findings should be taken with caution. Moreover, the results of similar rate of Aβ+ in PDD and DLB may be potentially biased due to the small sample size. In addition, recruitment source can also be a confounding factor as our patients were identified mainly at the movement disorder clinic rather than by the dementia centre (only 2 DLB), where patients may be more likely to have comorbid Alzheimer's disease.
Given the small sample size and the short follow time, subgroup differences should be confirmed in larger study. Increased statistical power is needed to definitively exclude amyloid deposition as a feature that distinguishes DLB from PDD. Finally, our Aβ+ patients were diagnosed based on Alzheimer's disease-range cortical Aβ deposition. Evidence suggests that lower Alzheimer's disease-range threshold may have clinical relevance in Lewy body diseases.
In conclusion, our study shows that the presence of amyloidosis plays an integral role in the neurodegenerative progression in LBDs and aggravates global cognition (MoCA, MMSE), attentive/executive and semantic recall abilities. Nevertheless, amyloidosis is not mandatory for dementia in Lewy Body Disorders as half of our demented patients were Aβ−. Moreover, the cognitive and neuroimaging profile in our LBDs-Aβ+ cohort does not present the typical morphological AD cognitive pattern since we did not observe both long-term memory decline, neither MRI AD-like atrophy pattern. Finally, the presence of Aβ does not differentiate demographic and clinical profile in Parkinson’s disease subgroups and DLB. Our findings provide grounds for an unspecific contribution of Aβ to dementia and cognitive dysfunction in Lewy body disorders.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors are grateful to GE-Healthcare for the liberal contribution of the doses of [18]F-Flutemetamol used in the present work. The authors thank Dr Giovanni Gentile for graphic editing.
Conflict of interests
A.A. has received compensation for consultancy and speaker related activities from UCB, Boehringer Ingelheim, General Electric, Britannia, AbbVie, Kyowa Kirin, Ever Pharma, Zambon, Bial, Neuroderm, Theravance Biopharma, Roche, Medscape; he receives research support from Bial, Lundbeck, Roche, Angelini Pharmaceuticals, Horizon 2020—Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer–Ingelheim for legal cases on pathological gambling.
Glossary
- ADL =
activity of daily living
- Aβ =
amyloid deposits
- Aβ+ =
amyloid-positive
- Aβ− =
amyloid-negative
- AS =
Starkstein Apathy Scale
- BAAD =
The Brain Anatomical Analysis using Diffeomorphic deformation
- BDI-II =
Beck Depression Inventory-II
- DEM =
Lewy body type dementia
- DLB =
dementia with Lewy Bodies
- FMM =
[18F]flutemetamol
- GCA =
the Pasquier's Global Cortical Atrophy scale
- GCA-F =
the Pasquier's Global Cortical Atrophy scale-frontal subscale
- LBD-Aβ+ =
Lewy Body disorders with significant Aβ
- LBD-Aβ− =
Lewy Body disorders without significant Aβ
- MDS-UPDRS =
Movement Disorder Society Unified Parkinson’s Disease
- MDS-UPDRS-III =
Motor section of Movement Disorder Society Unified Parkinson's Disease
- MMSE =
Mini Mental State Examination
- MoCA =
Montreal Cognitive Assessment
- MTA =
medial temporal atrophy
- PA =
the Koedam's scale of Posterior Atrophy
- PD-Aβ+ =
Parkinson’s disease patients with significant Aβ
- PD-Aβ− =
Parkinson’s disease without significant Aβ
- PDD =
Parkinson’s disease dementia
- PD-MCI =
Parkinson’s disease with mild cognitive impairment
- PD-NC =
Parkinson’s disease with normal cognition
- PDQ-8 =
Parkinson’s Disease Questionnaire
- STAI Y-1 =
State-Trait Anxiety Inventory forms-state
- STAI Y-2 =
State-Trait Anxiety Inventory forms-trait state
- SVM =
support-vector machine
- TIV =
Total Intracranial Volume
- VOIs =
volume of interests
References
- 1.Fereshtehnejad SM, Postuma RB.. Subtypes of Parkinson's disease: What do they tell us about disease progression? Curr Neurol Neurosci Rep. 2017;17(4):34. [DOI] [PubMed] [Google Scholar]
- 2.Tsiouris KM, Konitsiotis S, Koutsouris DD, Fotiadis DI.. Prognostic factors of Rapid symptoms progression in patients with newly diagnosed parkinson's disease. Artif Intell Med. 2020;103:101807. [DOI] [PubMed] [Google Scholar]
- 3.Antonini A, Bravi D, Sandre M, Bubacco L.. Immunization therapies for Parkinson's disease: State of the art and considerations for future clinical trials. Expert Opin Investig Drugs. 2020;29(7):685–695. [DOI] [PubMed] [Google Scholar]
- 4.Duncan GW, Khoo TK, Yarnall AJ, et al. Health-related quality of life in early Parkinson's disease: The impact of nonmotor symptoms. Mov Disord. 2014;29(2):195–202. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Kurz MW.. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289(1-2):18–22. [DOI] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG.. The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. [DOI] [PubMed] [Google Scholar]
- 7.Macleod AD, Taylor KS, Counsell CE.. Mortality in Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2014;29(13):1615–1622. [DOI] [PubMed] [Google Scholar]
- 8.Hu MT, Szewczyk-Królikowski K, Tomlinson P, et al. Predictors of cognitive impairment in an early stage Parkinson's disease cohort. Mov Disord. 2014;29(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biundo R, Weis L, Facchini S, et al. Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism Relat Disord. 2014;20(4):394–399. [DOI] [PubMed] [Google Scholar]
- 10.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA.. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(7):1787–1798. [DOI] [PubMed] [Google Scholar]
- 11.Campos LS, Guimarães RP, Piovesana LG, Azevedo PC, Santos LM, D'Abreu A.. Clinical predictors of cognitive impairment and psychiatric complications in Parkinson's disease. Arq Neuropsiquiatr. 2015;73(5):390–395. [DOI] [PubMed] [Google Scholar]
- 12.Biundo R, Weis L, Antonini A.. Cognitive decline in Parkinson's disease: The complex picture. Npj Parkinsons Dis. 2016;2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng C, Gathagan RJ, Lee VM.. Distinct α-synuclein strains and implications for heterogeneity among α-synucleinopathies. Neurobiol Dis. 2018;109(Pt B):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng C, Trojanowski JQ, Lee VM.. Protein transmission in neurodegenerative disease. Nat Rev Neurol. 2020;16(4):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin DJ, Hurtig HI.. The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism. 2018;8(4):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifford J, Bennett D, Blennow K, et al. Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kepe V, Moghbel MC, Långström B, et al. Amyloid-β positron emission tomography imaging probes: A critical review. J Alzheimers Dis. 2013;36(4):613–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Høilund-Carlsen PF, Barrio JR, Gjedde A, Werner TJ, Alavi A.. Circular inference in dementia diagnostics. J Alzheimers Dis. 2018;63(1):69–73. [DOI] [PubMed] [Google Scholar]
- 19.Nelson PT, Head E, Schmitt FA, et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121(5):571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. [DOI] [PubMed] [Google Scholar]
- 21.Jansen WJ, Ossenkoppele R, Knol DL, et al. Amyloid Biomarker Study Group. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA. 2015;313(19):1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomperts SN, Locascio JJ, Rentz D, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin DJ, Lee VM, Trojanowski JQ.. Parkinson's disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifford J, Weigand S, Rocca W, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: A cross-sectional study. Lancet Neurol. 2014;13(10):997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melzer TR, Stark MR, Keenan RJ, et al. Beta amyloid deposition is not associated with cognitive impairment in Parkinson's disease. Front Neurol. 2019;10:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colom-Cadena M, Grau-Rivera O, Planellas L, et al. Regional overlap of pathologies in Lewy body disorders. J Neuropathol Exp Neurol. 2017;76(3):216–224. [DOI] [PubMed] [Google Scholar]
- 27.Sierra M, Gelpi E, Martí MJ, Compta Y.. Lewy- and Alzheimer-type pathologies in midbrain and cerebellum across the Lewy body disorders spectrum. Neuropathol Appl Neurobiol. 2016;42(5):451–462. [DOI] [PubMed] [Google Scholar]
- 28.Bougea A, Stefanis L, Emmanouilidou E, Vekrelis K, Kapaki E.. High discriminatory ability of peripheral and CFSF biomarkers in Lewy body diseases. J Neural Transm (Vienna). 2020;127(3):311–322. [DOI] [PubMed] [Google Scholar]
- 29.Coughlin D, Xie SX, Liang M, et al. Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol. 2019;85(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelb DJ, Oliver E, Gilman S.. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. [DOI] [PubMed] [Google Scholar]
- 31.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonini A, Abbruzzese G, Ferini-Strambi L, et al. MDS-UPDRS Italian Validation Study Group. Validation of the Italian version of the Movement Disorder Society–Unified Parkinson's Disease Rating Scale. Neurol Sci. 2013;34(5):683–687. [DOI] [PubMed] [Google Scholar]
- 33.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: A base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. [DOI] [PubMed] [Google Scholar]
- 34.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE.. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 35.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 37.Fiorenzato E, Strafella AP, Kim J, et al. Dynamic functional connectivity changes associated with dementia in Parkinson's disease. Brain. 2019;142(9):2860–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kats S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW.. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 39.Katz S.Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. [DOI] [PubMed] [Google Scholar]
- 40.Edemekong PF, Bomgaars DL, Sukumaran S, Levy SB. Activities of Daily Living. 2021 Jun 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Jan–. PMID: 29261878. [PubMed] [Google Scholar]
- 41.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N.. The Parkinson's Disease Questionnaire (PDQ-39): Development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26(5):353–357. [DOI] [PubMed] [Google Scholar]
- 42.Yamanishi T, Tachibana H, Oguru M, et al. Anxiety and depression in patients with Parkinson's disease. Intern Med. 2013;52(5):539–545. [DOI] [PubMed] [Google Scholar]
- 43.Minoshima S, Drzezga AE, Barthel H, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med. 2016;57(8):1316–1322. [DOI] [PubMed] [Google Scholar]
- 44.Cecchin D, Barthel H, Poggiali D, et al. A new integrated dual time-point amyloid PET/MRI data analysis method. Eur J Nucl Med Mol Imaging. 2017;44(12):2060–2072. [DOI] [PubMed] [Google Scholar]
- 45.Seibyl J, Catafau AM, Barthel H, et al. Impact of training method on the robustness of the visual assessment of 18F-Florbetaben PET scans: Results from a phase-3 study. J Nucl Med. 2016;57(6):900–906. [DOI] [PubMed] [Google Scholar]
- 46.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: Diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55(10):967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheltens P, Pasquier F, Weerts JG, Barkhof F, Leys D.. Qualitative assessment of cerebral atrophy on MRI: Inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol. 1997;37(2):95–99. [DOI] [PubMed] [Google Scholar]
- 48.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA.. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. [DOI] [PubMed] [Google Scholar]
- 49.Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM.. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavallin L, Løken K, Engedal K, et al. Overtime reliability of medial temporal lobe atrophy rating in a clinical setting. Acta Radiol. 2012;53(3):318–323. [DOI] [PubMed] [Google Scholar]
- 51.Jenkinson M, Smith S.. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. [DOI] [PubMed] [Google Scholar]
- 52.Jenkinson M, Bannister P, Brady M, Smith S.. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira D, Cavallin L, Larsson EM, et al. ; AddNeuroMed consortium and the Alzheimer's Disease Neuroimaging Initiative. Practical cut-offs for visual rating scales of medial temporal, frontal and posterior atrophy in Alzheimer's disease and mild cognitive impairment. J Intern Med. 2015;278(3):277–290. [DOI] [PubMed] [Google Scholar]
- 54.Mortamais M, Artero S, Ritchie K.. White matter hyperintensities as early and independent predictors of Alzheimer's disease risk. J Alzheimers Dis. 2014;42 (Suppl 4):S393–S400. [DOI] [PubMed] [Google Scholar]
- 55.Vigers P, Shiino A, Tooyama I.. Diagnosis of amyloid-positive mild cognitive impairment using structural magnetic resonance imaging: The worth of multiple regions of interest. Integr Mol Med. 2015;2(3):205–213. [Google Scholar]
- 56.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 57.*ΡFaul F, Erdfelder E, Lang AG, Buchner AG.. Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 58.Fiorenzato E, Biundo R, Cecchin D, et al. Brain amyloid contribution to cognitive dysfunction in early-stage Parkinson's disease: The PPMI dataset. J Alzheimers Dis. 2018;66(1):229–237. [DOI] [PubMed] [Google Scholar]
- 59.Petrou M, Dwamena BA, Foerster BR, et al. Amyloid deposition in Parkinson's disease and cognitive impairment: A systematic review. Mov Disord. 2015;30(7):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim EW, Aarsland D, Ffytche D, et al. Amyloid-β and Parkinson's disease. J Neurol. 2019;266(11):2605–2619. [DOI] [PubMed] [Google Scholar]
- 61.Na S, Jeong H, Park JS, Chung YA, Song IU.. The impact of amyloid-beta positivity with 18F-Florbetaben PET on neuropsychological aspects in Parkinson's disease dementia. Metabolites. 2020;10(10):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017;16(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doraiswamy PM, Sperling RA, Johnson K, et al. ; AV45-A11 Study Group. Florbetapir F 18 amyloid PET and 36-month cognitive decline: A prospective multicenter study. Mol Psychiatry. 2014;19(9):1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nedelska Z, Schwarz CG, Lesnick TG, et al. Association of longitudinal β-amyloid accumulation determined by positron emission tomography with clinical and cognitive decline in adults with probable Lewy body dementia. JAMA Netw Open. 2019;2(12):e1916439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith C, Malek N, Grosset K, Cullen B, Gentleman S, Grosset DG.. Neuropathology of dementia in patients with Parkinson's disease: A systematic review of autopsy studies. J Neurol Neurosurg Psychiatry. 2019;90(11):1234–1243. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Esparcia P, López-González I, Grau-Rivera O, et al. Dementia with Lewy bodies: Molecular pathology in the frontal cortex in typical and rapidly progressive forms. Front Neurol. 2017;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akhtar RS, Xie SX, Brennan L, et al. Amyloid-beta positron emission tomography imaging of Alzheimer's pathology in Parkinson's disease dementia. Mov Disord Clin Pract. 2016;3(4):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72(4):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotzbauer PT, Cairns NJ, Campbell MC, et al. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol. 2012;69(10):1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martini A, Weis L, Schifano R, et al. Differences in cognitive profiles between Lewy body and Parkinson's disease dementia. J Neural Transm (Vienna). 2020;127(3):323–330. [DOI] [PubMed] [Google Scholar]
- 71.Fiorenzato E, Weis L, Falup-Pecurariu C, et al. Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J Neural Transm. 2016;123(12):1435–1442. [DOI] [PubMed] [Google Scholar]
- 72.Goldman JG, Andrews H, Amara A, et al. Fox Investigation of New Biomarker Discovery. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: Relationships among biomarkers and Parkinson's disease Features. Mov Disord. 2018;33(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80(15):1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258–1264. [DOI] [PubMed] [Google Scholar]
- 75.Howard E, Irwin DJ, Rascovsky K, et al. Cognitive profile and markers of Alzheimer disease-type pathology in patients with Lewy body dementias. Neurology. 2021;96(14):e1855–e1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Nazer R, Adler CH, Beach TG, et al. Regional neuropathology distribution and verbal fluency impairments in Parkinson's disease. Parkinsonism Relat Disord. 2019;65:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peavy GM, Edland SD, Toole BM, Hansen LA, Galasko DR, Mayo AM.. Phenotypic differences based on staging of Alzheimer's neuropathology in autopsy-confirmed dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;31:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64(12):2069–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Becker S, Granert O, Timmers M, et al. Association of hippocampal subfields, CSF biomarkers, and cognition in patients with Parkinson disease without dementia. Neurology. 2021;96(6):e904–e915. [DOI] [PubMed] [Google Scholar]
- 80.Jellinger KA.Dementia with Lewy bodies and Parkinson's disease-dementia: Current concepts and controversies. J Neural Transm (Vienna). 2018;125(4):615–650. [DOI] [PubMed] [Google Scholar]
- 81.Bougea A, Stefanis L, Paraskevas GP, et al. Neuropsychiatric symptoms and α-Synuclein profile of patients with Parkinson's disease dementia, dementia with Lewy bodies and Alzheimer's disease. J Neurol. 2018;265(10):2295–2301. [DOI] [PubMed] [Google Scholar]
- 82.Jellinger KA.Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci. 2009;24:114–125. [DOI] [PubMed] [Google Scholar]
- 83.Lippa CF, Duda JE, Grossman M, et al. DLB/PDD Working Group. DLB and PDD boundary issues: Diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–819. [DOI] [PubMed] [Google Scholar]
- 84.Halliday GM, Song YJ, Harding AJ.. Striatal β-amyloid in dementia with Lewy bodies but not Parkinson's disease. J Neural Transm (Vienna). 2011;118(5):713–719. [DOI] [PubMed] [Google Scholar]
- 85.Tsuboi Y, Uchikado H, Dickson DW.. Neuropathology of Parkinson's disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 2007;13 (Suppl 3):S221–S224. [DOI] [PubMed] [Google Scholar]
- 86.Jellinger KA, Attems J.. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112(3):253–260. [DOI] [PubMed] [Google Scholar]
- 87.Ruffmann C, Calboli FC, Bravi I, et al. Cortical Lewy bodies and Aβ burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol Appl Neurobiol. 2016;42(5):436–450. [DOI] [PubMed] [Google Scholar]
- 88.Hepp DH, Vergoossen DL, Huisman E, et al. Netherlands Brain Bank. Distribution and load of amyloid-β pathology in Parkinson disease and dementia with Lewy bodies. J Neuropathol Exp Neurol. 2016;75(10):936–945. [DOI] [PubMed] [Google Scholar]
- 89.Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–1934. [DOI] [PubMed] [Google Scholar]
- 90.Walker L, Stefanis L, Attems J.. Clinical and neuropathological differences between Parkinson's disease, Parkinson's disease dementia and dementia with Lewy bodies - Current issues and future directions. J Neurochem. 2019;150(5):467–474. [DOI] [PubMed] [Google Scholar]
- 91.Petrou M, Bohnen NI, Muller ML, Koeppe RA, Albin RL, Frey KA.. Abeta-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology. 2012;79(11):1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palermo G, Tommasini L, Aghakhanyan G, et al. Clinical correlates of cerebral amyloid deposition in Parkinson's disease dementia: Evidence from a PET study. J Alzheimers Dis. 2019;70(2):597–609. [DOI] [PubMed] [Google Scholar]
- 93.Yoo HS, Lee S, Chung SJ, et al. Dopaminergic depletion, β-amyloid burden, and cognition in Lewy body disease. Ann Neurol. 2020;87(5):739–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request.