Abstract
Fusobacterium nucleatum (Fn), a bacterium associated with a wide spectrum of infections, has emerged as a key microbe in colorectal carcinogenesis. However, the underlying mechanisms and clinical relevance of Fn in colorectal cancer (CRC) remain incompletely understood. We examined associations between Fn abundance and clinicopathologic characteristics among n=105 treatment-naïve CRC patients enrolled in the international, prospective ColoCare Study. Electronic medical charts, including pathological reports, were reviewed to document clinicopathologic features. Quantitative real-time polymerase chain reaction (PCR) was used to amplify/detect Fn DNA in pre-operative fecal samples. Multinomial logistic regression was used to analyze associations between Fn abundance and patient sex, age, tumor stage, grade, site, microsatellite instability, body mass index (BMI), alcohol consumption and smoking history. Cox proportional hazards models were used to investigate associations of Fn abundance with overall survival in adjusted models. Compared to patients with undetectable or low Fn abundance, patients with high Fn abundance (n=22) were three-fold more likely to be diagnosed with rectal vs colon cancer (Odds Ratio, OR=3.01; 95% CI=1.06–8.57; P<0.05) after adjustment for patient sex, age, BMI, and study site. Patients with Fn-high abundance also had a five-fold increased risk of being diagnosed with rectal cancer vs. right-sided colon cancer (OR=5.32, 95% CI=1.23–22.98, P=0.03). There was no statistically significant association between Fn abundance with overall survival. Our findings suggest that Fn abundance in fecal samples collected prior to surgery varies by tumor site among treatment-naïve CRC patients. Overall, fecal Fn abundance may have diagnostic and prognostic significance in the clinical management of CRC.
Keywords: colorectal cancer, tumor site, gut microbiome, Fusobacterium, feces
MICROABSTRACT
The involvement of Fusobacterium nucleatum (Fn) in colorectal cancer (CRC) patients remains poorly studied. Therefore, we analyzed pre-operatively collected fecal samples for Fn DNA among n=105 treatment-naïve CRC patients. Multinomial regression showed that patients with high Fn abundance compared to patients with low/undetectable abundance had a 3-fold increase in risk of being diagnosed with rectal cancer compared to colon cancer.
INTRODUCTION
Colorectal cancer (CRC), the third most commonly diagnosed malignancy and second leading cause of cancer deaths among men and women globally, is a multifactorial disease with complex etiology (1, 2). CRC development is influenced by lifestyle, environmental, and/or dietary factors. Additionally, the functional genetic profile of gut microbial communities has been shown to influence CRC formation and progression, as the gut microbiome exerts inflammatory and immune-mediated effects which can impact therapeutic and prognostic outcomes in patients. In particular, evidence is accumulating to suggest an association between CRC and gram-negative, non-sporulating, anaerobic Fusobacterium nucleatum (Fn) (3). Although considered a common member of the oral microbiome due to its prevalence within the subgingival biofilm in deep periodontal pockets, Fn has been involved in a wide spectrum of diseases, including oropharyngeal infections (e.g. periodontitis) and adverse pregnancy outcomes (3, 4). In 2012, Fn DNA and RNA was discovered to be enriched in CRC tissues when compared with adjacent non-tumor tissues (3, 5, 6). Subsequently, it was shown that patients diagnosed with CRC harbor identical Fn strains in their oral cavities and in the colorectal tumor (7, 8). Similarly, Fn abundance in feces was found to correlate with Fn abundance in CRC tissues (3, 9). Utilizing fecal samples, previous studies have reported that CRC patients harbor higher levels of Fn when compared with non-CRC controls (2). However, prior studies have revealed a potential for modifications of gut microbial composition with antibiotics, chemotherapeutic agents as well as radiotherapy (10–12). As such, it is critical to understand the significance of Fn in a treatment-naïve setting among CRC patients. The purpose of our study was to evaluate associations between Fn abundance and clinicopathologic characteristics utilizing fecal biospecimen collected pre-operatively from treatment-naïve CRC patients in a prospective cohort.
PATIENTS AND METHODS
Study Population
This study population consists of patients from the international prospective ColoCare Study (Clinicaltrials.gov Identifier: NCT02328677). The ColoCare Study is a cohort of men and women aged 18 to 89 years with newly-diagnosed primary invasive CRC (stage I-IV), no prior history of cancer and undergoing surgery (13, 14). Electronic medical charts, including pathological reports, were reviewed to abstract clinical information. A total of n=75 patients recruited as part of the ColoCare Study in Heidelberg (HD), Germany (between December 2010 and December 2014) and n=30 patients recruited between October 2015 and March 2018 from the ColoCare Study site at the Huntsman Cancer Institute (HCI), Utah, met the following inclusion criteria: (i) available pre-operative fecal biospecimens collected at the patients’ homes and immediately stored in RNAlater (Sigma-Aldrich, Germany [HD] and Thermo-Fisher Scientific Inc., MA, USA [HCI]) at −80°C), (ii) a negative history of neoadjuvant therapy (chemo- and/or radiation therapy), and (iii) no antibiotic use (reported 1+ month prior to sample collection). This study was approved by the ethics committee of the medical faculty at the University of Heidelberg and the University of Utah. All study participants provided written informed consent.
Experimental Methods
DNA/RNA was extracted from a 200μl RNAlater/fecal sample using the AllPrep PowerViral DNA/RNA Kit (Qiagen Inc., USA) according to the manufacturer’s instructions, including 2 minutes of bead-beating at 4;ºC with a Mini-Beadbeater-16 (BioSpec Products, Bartlesville, OK). Quantitative real-time PCR (qRT-PCR) was performed on 0.2μl of template DNA. Reactions were performed in 20μl reactions containing primer (9) and 1× final concentration PowerUp Sybr Green MasterMix (Thermo Fisher Scientific Inc., MA, USA). All reactions were performed in duplicate. A positive control consisting of pooled samples of Fn strains and a non-template control were also included in each qRT-PCR run. DNA amplification and detection was performed with the CFX96 Real-Time System C1000 Thermal Cycler (Bio-Rad, CA, USA) using the following conditions: For Fn: 2 minutes at 50°C, 2 minutes at 95°C, and 45 cycles of 15 seconds at 95°C, 15 seconds at 57 °C and 20 seconds at 72°C. For 16S rRNA: 2 minutes at 50°C, 2 minutes at 95°C and 32 cycles of 15 seconds at 95°C, 20 seconds at 55 °C and 15 seconds at 72°C. Primer sets and concentrations used have been previously described (9, 15). Cycle thresholding (Ct) was calculated with a detection level of Ct=50 (Bio-Rad, CA, USA).
Relative abundance of Fn was calculated in proportion to total bacterial load as the ratio of average Ct values. As described in prior studies investigating Fn abundance, patients with positive (detectable) Fn DNA fecal biospecimen were classified into Fn high (n=22) and Fn low (n=22) groups based on median Fn Ct values for each study site (HD: low<0.034; high>0.034; HCI: low<0.00067; high>0.00067) (16–19). Patients with Fn negative (undetectable) abundance (n=61) were included in the Fn low group (20).
Statistical Analysis
Comparisons between Fn high vs. low groups were examined by Chi-square and t-tests for categorical and continuous variables, respectively. Tumor site was categorized as: colon and rectosigmoid junction/rectum. In addition, tumors in the colon were also categorized as right-sided colon (cecum to transverse colon) and left-sided colon tumors. Multinomial logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) to quantify associations of Fn abundance with clinicopathologic characteristics, where the reference outcome category was Fn-low/Fn-negative. Associations between clinicopathologic characteristics (e.g. tumor stage, tumor grade) as well as lifestyle characteristics, including common CRC risk factors (alcohol consumption and smoking), and Fn abundance group were assessed in independent models adjusted for age at surgery (years, continuous), patient sex, body mass index (BMI, kg/m2, continuous) and study site (HD, HCI). Cox proportional hazard models were also used to investigate overall survival after 24 months of follow-up, in crude models and in models adjusted for age, sex, and study site. Time at risk was estimated from the date of recruitment to the date of death, loss to follow-up or to the end of the follow-up period, whichever occurred first. Data were analyzed using SAS version 9.4 statistical software (SAS Institute), with a two-sided P<0.05 considered as statistically significant.
RESULTS
Fn in fecal biospecimen of treatment-naïve CRC patients
Among 105 CRC patients, fecal samples were positive for Fn DNA in n=44 (42%) and negative in the remaining n=61 (58%) cases. The n=44 patients with detectable Fn were median split into Fn high and Fn low abundance for the respective study populations by study site to account for influence of geography on the fecal microbiome (21).
Demographic, clinical and pathological characteristics by fecal Fn abundance (high vs. low/negative) are presented in Table 1. Overall, individuals in this cohort had a mean BMI classified as overweight (27.6 kg/m2) and a mean age of 63.5 years. Eight out of every ten patients had pathologically-confirmed microsatellite stable (MSS) CRC, and two-thirds of patients (66.7%) were diagnosed with tumors located in the colon. Across Fn abundance groups, no statistically significant differences were observed for clinicopathologic or demographic characteristics. Although a higher proportion of Fn-negative/low cases were diagnosed with tumors of the colon (71.1%) compared with Fn-high cases (50.0%), these findings only reached marginal statistical significance (P=0.06).
Table 1.
Clinicopathologic characteristics of colorectal cancer patients by Fusobacterium nucleatum (Fn) abundance in iecal biospecimen.
| Characteristcs | Total |
Fn-Negative/Low1 |
Fn-High1 |
P-value2 | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 105 | 83 | 79.0 | 22 | 21.0 | ||
| ColoCare Study Site | 0.36 | ||||||
| University of Heidelberg, Germany | 75 | 71.4 | 61 | 73.5 | 14 | 63.6 | |
| Huntsman Cancer Institute, Utah | 30 | 28.6 | 22 | 26.5 | 8 | 36.4 | |
| Patient Sex | 0.56 | ||||||
| Female | 42 | 40.0 | 32 | 38.6 | 10 | 45.5 | |
| Male | 63 | 60.0 | 51 | 61.5 | 12 | 54.6 | |
| Age at Surgery | 0.24 | ||||||
| <65 years | 60 | 57.1 | 45 | 54.2 | 15 | 68.2 | |
| 65+ years | 45 | 42.9 | 38 | 45.8 | 7 | 31.8 | |
| Mean, years, (SD) | 63.5 | (11.7) | 63.8 | (11.7) | 62.5 | (11.5) | 0.64 |
| Body Mass Index 3 | 0.48 | ||||||
| Underweight/Normoweight (<25 kg/m2) | 32 | 32 | 23 | 29.1 | 9 | 42.9 | |
| Overweight (25–29.99 kg/m2) | 41 | 41 | 34 | 43.0 | 7 | 33.3 | |
| Obese (30+ kg/m2) | 27 | 27 | 22 | 27.9 | 5 | 23.8 | |
| Mean kg/m2, (SD) | 27.6 | (5.9) | 27.4 | (4.9) | 28.2 | (8.8) | 0.68 |
| Tumor Stage | 0.43 | ||||||
| Stage I | 35 | 33.3 | 29 | 34.9 | 6 | 27.3 | |
| Stage II | 32 | 30.5 | 27 | 32.5 | 5 | 22.7 | |
| Stage III | 33 | 31.4 | 24 | 28.9 | 9 | 40.9 | |
| Stage IV | 5 | 4.8 | 3 | 3.6 | 2 | 9.1 | |
| Tumor Grade 4 | 0.60 | ||||||
| Well Differentiated | 11 | 10.7 | 9 | 11.0 | 2 | 9.5 | |
| Moderately Differentiated | 75 | 72.8 | 61 | 74.4 | 14 | 66.7 | |
| Poorly Differentiated | 17 | 16.5 | 12 | 14.8 | 5 | 23.8 | |
| Tumor Site | 0.06 | ||||||
| Colon | 70 | 66.7 | 59 | 71.1 | 11 | 50.0 | |
| Right-sided Colon | 33 | 47.1 | 29 | 49.2 | 4 | 36.4 | 0.14 |
| Left-sided Colon | 37 | 52.9 | 30 | 50.8 | 7 | 63.6 | |
| Rectosigmoid Junction and Rectum | 35 | 33.3 | 24 | 28.9 | 11 | 50.0 | |
| H. pylori infection 5 | 0.86 | ||||||
| None | 65 | 92.9 | 54 | 93.1 | 11 | 91.7 | |
| Yes | 5 | 7.1 | 4 | 6.9 | 1 | 8.3 | |
| Microsatellite Status 6 | 0.42 | ||||||
| Microsatellite Stable (MSS) | 65 | 80.2 | 51 | 78.5 | 14 | 87.5 | |
| Microsatellite Instable (MSI) | 16 | 19.8 | 14 | 21.5 | 2 | 12.5 | |
| Alcohol Consumption 7 | 0.74 | ||||||
| None | 9 | 11.7 | 7 | 11.1 | 2 | 14.3 | |
| Yes | 68 | 88.3 | 56 | 88.9 | 12 | 85.7 | |
| Smoking History 8 | 0.11 | ||||||
| Never Smoker | 42 | 424 | 29 | 37.2 | 13 | 61.9 | |
| Current Smoker | 19 | 19.2 | 17 | 21.8 | 2 | 9.5 | |
| Former Smoker9 | 38 | 38.4 | 32 | 41.0 | 6 | 28.6 | |
| Family History of CRC 10 | 0.63 | ||||||
| None | 94 | 93.1 | 73 | 92.4 | 21 | 95.5 | |
| Yes | 7 | 6.9 | 6 | 7.6 | 1 | 4.5 | |
Among CRC patients with detectable Fn levels, individuals were median split by Fn relative abundance (n=22 low; n=22 high; HD: low<0.034; high>0.034; HCI: low<0.00067; high>0.00067).
P-values do not include unknown values.
Five patients had unknown information on body mass index.
Two patients had unknown information on tumor grade.
Thirty-five patients had unknown information on H. pylori infection status.
Twenty-four patients had unknown information on microsatellite instability.
Alcohol Consumption within last 2+ years; Twenty-eight patients had unknown information on Alcohol Consumption.
Six patients had missing information on smoking history.
Stopped smoking 2+ years ago.
Four patients had unknown information on family history of CRC.
Abbreviations: SD, standard deviation; Fn, Fusobacterium nucleatum; CRC, colorectal cancer.
Associations between fecal Fn abundance and clinicopathologic/demographic features among CRC patients
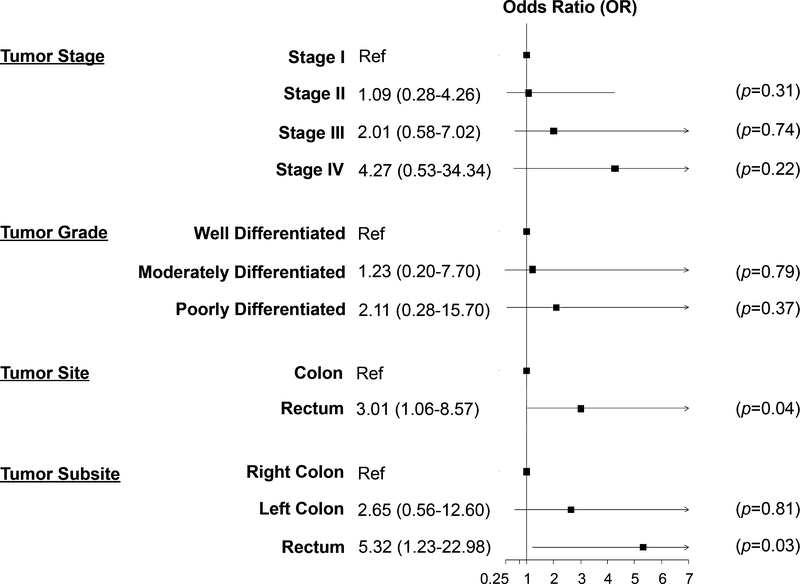
To quantify associations between Fn abundance and clinicopathologic as well as demographic characteristics, we used both unadjusted/crude and adjusted multivariable logistic regression models (Figure 1 and Supplementary Table 1). In models adjusted for patient age, sex, study site and BMI, patients with high fecal Fn abundance had a 3-fold increased likelihood of being diagnosed with rectal compared with colon tumors (OR=3.01, 95% CI=1.06–8.57, P=0.04). After further stratification by tumor site, we observed that patients with high fecal Fn abundance had a 5-fold increased risk of being diagnosed with rectal vs right-sided colon cancer (OR=5.32, 95% CI=1.23–22.98, P=0.03).
Figure 1. Association of Fusobacterium nucleatum with clinicopathologic characteristics among primary invasive colorectal cancer patients.
Independent models are adjusted for: patient age, sex, study site, and body mass index (continuous, kg/m2). Black boxes indicate OR point estimate and horizontal lines represent the 95% confidence interval (CI) bounds. Ref, referent.
Sensitivity analyses
To consider the possibility that colorectal tumor metastases could yield changes in fecal microbial composition, we repeated our primary analyses to exclude patients diagnosed with stage IV CRC (n=5 patients), and our results remained largely unchanged (Supplementary Table 2). High Fn abundance remained associated with a 3.35 to 5.85-fold increased risk of rectal tumors after adjusting for other covariates (colon cancer: OR=3.35, 95% CI 1.11–10.15, P=0.03; right-sided colon cancer: OR=5.85, 95% CI 1.29–26.44, P=0.03).
We also performed sensitivity analyses to exclude patients with a known family history of CRC or patients with MSI tumors. Among patients without a family history of CRC, those with a high fecal Fn abundance had a 4.7-fold increased likelihood of being diagnosed with a tumor located in the rectum (rectum vs right-sided colon tumors: OR=4.69, 95% CI 1.08–20.36, P=0.04) in adjusted models (data not shown). Among patients with MSS CRC, high abundance of fecal Fn was associated with over a 5-fold likelihood of a rectal tumor in adjusted models (rectum vs right-sided colon tumors: OR=5.83, 95% CI 1.05–32.49, P=0.04) (data not shown).
Fn abundance and overall survival
We next quantified associations between Fn abundance and overall survival in both unadjusted/crude and adjusted Cox proportional hazards models. In the unadjusted model, Fn abundance was not associated with overall survival (OR=0.84, 95% CI 0.44–1.59, P=0.59). Similar results were observed between Fn abundance and overall survival after adjustment for patient age, sex, and study site (OR=0.86, 95%CI 0.45–1.64, P=0.86) (Supplementary Table 3).
DISCUSSION
To our knowledge, this study is the first to investigate Fn abundance in neo-adjuvant therapy-naïve CRC patients who did not use antibiotics prior to surgery. The aim of our study was to explore associations between clinicopathologic features with fecal Fn abundance in a prospectively-followed cohort of CRC patients. We observed that CRC patients with a higher fecal abundance of Fn have a 3-fold to 5-fold increased risk of being diagnosed with rectal cancer compared to colon, and specifically right-sided colon, cancer. However, we did not observe an association between Fn abundance and overall survival in our cohort—in contrast to previous studies (17).
Prior studies investigating tumor location and Fusobacterium species in CRC have reported conflicting results regarding Fn abundance and disease site. Utilizing tissue samples from patients that had undergone surgical resection of the tumor, Mima et al. (2016) reported a gradually increasing proportion of Fn abundance from the rectum to the cecum (16). However, it is important to note that receipt of CRC therapy was not accounted for in this body of work.
McCoy et al. also noted that Fusobacterium species were more abundant in mucosal biopsies of the distal colon compared to right-sided colon adenomas (22). Gao et al. also reported a higher abundance of Fusobacterium (genus) in the tissues of distal colon cancer patients when compared with proximal colon cancer patients—excluding patients with known antibiotic use within 4 weeks of surgery (23). More recently, Oh et al. quantitatively measured intratumoral Fn abundance in 593 CRC tissues retrospectively collected from stage II/III CRC patients that had previously been treated with oxaliplatin-based adjuvant chemotherapy (24). While no significance differences in survival were reported between intratumoral Fn-high vs Fn-low/negative groups, the authors noted that combined status of tumor site and MSI status may be crucial characteristics that underlie the prognostic value of intratumoral Fn in tumors treated with adjuvant chemotherapy. In paired pre- and post-chemoradiation tissue samples (n=71) from locally advanced rectal cancer patients, Serna and colleagues demonstrated that Fn abundance was higher in untreated compared with treated rectal tumors (25). Altogether, given the heterogeneity in patient sample collection/types, therapies administered and CRC patient populations (e.g. by stage, tumor site), further studies are needed to understand the diagnostic, therapeutic and prognostic significance of Fn across the cancer spectrum for patients diagnosed with CRC—particularly rectal tumors.
Despite these differences in study populations, our observation that the Fn burden in pre-operative fecal samples is associated with tumor site among treatment-naïve CRC patients aligns with the results of Gao et al. and McCoy et al. (22, 23). It is important to note, however, that our study is novel in that: we examined the abundance of Fn among CRC patients in stool samples collected from patients prior to bowel preparation, our cohort is substantially larger than these previous studies (n=105 vs 65 and 10 CRC patients, respectively), and we excluded patients with a history of antibiotic use within 4 weeks prior to surgery as well as those who underwent CRC therapy prior to stool sample collection. Given the potential disruption of neoadjuvant therapy on the gut microbiome (26), the use of fecal biospecimens from treatment-naïve patients is a critical component to our study design and corresponding findings. While we observed an association between tumor site and fecal Fn abundance, the size of our cohort limited our ability to further investigate the combined status of tumor site and MSI status, as well as other pathologic features in association with Fn abundance subgroups (high/low/negative). Future studies are warranted that are able to build upon these initial findings to explore the combined status of tumor site and MSI status associated with fecal Fn abundance at diagnosis and at subsequent timepoints.
Risk factors, clinicodemographics, and molecular features for CRC differ by tumor site. While higher BMI is associated with a greater risk of CRC, the positive relationship for BMI is weaker for rectal cancer vs proximal/distant colon cancer among men (27). Age-related and sex-specific patterns also persist by tumor site (28, 29). Thus, given the role of age, sex and BMI in colorectal carcinogenesis, we accounted for each of these characteristics in our adjusted analyses. We observed that in models adjusted for these characteristics, high fecal Fn abundance was consistently associated with an increased risk of rectal cancer diagnosis compared with (right-sided) colon tumors. Although genetic predisposition (e.g. HNPCC) and mutational burden (e.g. BRAF mutations) also contribute to CRC-site specific patterns, including prognostic outcomes (30), we were unable to investigate these characteristics in our present cohort. Together, these findings support additional investigation into potential gene-microbiome interactions may be contributing to differences in Fn abundance. Characterizing the gut microbial landscape by tumor site among CRC patients in larger studies may unravel distinct microbial patterns of potential diagnostic, therapeutic and prognostic significance within this population.
Numerous methodological choices can impact the quantification of individual microbial abundances in the microbiome. Although the use of feces to investigate microbiota remains controversial, recent studies have demonstrated that microbial signatures are largely representative of the intestinal microbiome in these non-invasively obtained biospecimens (2, 31). In particular, high levels of fecal Fn have been shown to correspond with Fn abundance detected in colorectal tumor tissues (9). Another advantage to the use of fecal samples for Fn detection is that DNA/RNA quality is preserved by storing biospecimen in applied chemical stabilizer with bacteriostatic activity at −80°C. Moreover, in our study we calculated Fn relative abundance as the ratio of Fn per 16S total abundance—based upon the results of Guo and colleagues that demonstrated that feces-based qPCR assays considering the ratio of Fn to other bacterial strains are of better prognostic value compared to Fn alone (32). Moreover, pH plays a key role in microbial environments and it is known to be significantly elevated in feces of CRC patients compared to healthy individuals. Therefore, Fn might encounter more agreeable growth conditions in the rectum—which harbors a higher pH level relative to the colon (33). Of relevance, alkaline-induced Fn biofilms exhibited altered phenotypes, including alterations in glucose and glutamate metabolism, that may reflect changes in cellular functions in diseased environments (34). Consequently, a multi-omics interrogation of metabolic profiles by tumor site (under different pH) may shed light on metabolite signatures that may be associated with Fn abundance in order to understand the potential mechanisms of Fn-associated CRC.
While use of a prospective cohort with fecal biospecimen from treatment-naïve CRC patients without a prior history of antibiotic use in the month prior to surgery is a strength of our study, we acknowledge the limitations of our work. Although key immunological patterns have only been observed in cancer patients with Fn and were not generalizable to F. genus (35), CRC isolates have been reported to encompass five subspecies of Fn (3). However, the primer set used in our study specifically quantifies Fn and is unable to detect Fusobacteria spp. (F. genus) on a larger taxonomic level. Similar to previous studies in the field, we investigated associations by Fn abundance using two groups (high vs low/negative). However, binary investigation of Fn may have limited our ability to detect dose-dependent effects of Fn on CRC characteristics. Larger cohort studies are warranted to further dissect these associations, including the potential combined status of tumor site and MSI status previously reported (24). Lastly, dietary patterns have been reported to facilitate intestinal inflammation (36). Although initial evidence suggests that diets rich in dietary fiber and whole grains are associated with a lower risk for Fn-positive CRC, but not Fn-negative CRC (37), we were unable to incorporate dietary patterns into the present work. As such, future epidemiologic studies are needed in order to understand the role of diet in Fn-associated CRC.
In conclusion, our prospective cohort study revealed that fecal Fn abundance is associated with tumor site. In particular, high abundance of Fn in fecal samples collected from treatment-naïve patients with no prior history of antibiotic use in the month prior to surgery was significantly associated with increased likelihood of rectal cancer diagnosis compared with (right-sided) colon tumors. Given the growing significance of the gut microbiome in carcinogenesis, these findings further support the role of Fn in CRC and the potential for personalized preventive and therapeutic interventions that target this microbe in order to reduce the CRC burden.
Supplementary Material
CLINCAL PRACTICE POINTS.
Fusobacterium nucleatum (Fn) is enriched in colorectal tumor tissue compared to adjacent non-tumor tissue. In 2017, Bullman et al. described presence of Fn in both primary tumor tissue and distant metastases tissue of colorectal cancer (CRC) patients. The authors further demonstrated that antibiotic treatment of patient-derived Fn-positive xenograft mice decelerated tumor growth. Accumulating evidence suggests a distinct role of Fn in CRC development and progression, but data from prospectively followed patient cohorts are sparse. We determined Fn DNA in pre-operatively collected fecal samples of n=105 treatment-naïve CRC patients and utilized multinomial regression to investigate associations of Fn abundance with clinicopathologic patient characteristics.
In our study, patients with high Fn abundance were three-fold more likely to be diagnosed with rectal cancer than colon cancer. In an analysis further distinguishing between left- and right-sided colon cancer, patients with high Fn abundance had a five-fold increased risk of being diagnosed with rectal cancer compared to right-sided colon cancer.
Our study shows that pre-operative, treatment-naïve Fn abundance differs substantially between patients diagnosed with rectal compared to colon tumors. Thus, Fn abundance may be a relevant prognostic factor for rectal cancer. Utilizing targeted measures, such as antibiotic treatment, to decrease Fn burden in rectal cancer patients, may provide novel preventive and therapeutic avenues in the clinical management of rectal cancer.
Acknowledgments
FUNDING
This work was supported by grants from the National Institutes of Health/National Cancer Institute (U01 CA206110, R01 CA189184, R01 CA211705, and R01 CA207371 to C.M.U.; NIH/NHGRI T32 HG008962 to A.N.H.), the German Consortium of Translational Cancer Research (DKTK) and the German Cancer Research Center, the Matthias Lackas Foundation, Stiftung LebensBlicke, and Claussen-Simon Stiftung (Germany), the Huntsman Cancer Foundation, the Immunology, Inflammation, and Infectious Disease Initiative at the University of Utah. Y.E. was supported by a full study scholarship of the Konrad Adenauer Foundation. K.P.J. was supported by a grant from the German Research Foundation (DFG/CRC1371, P10). The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest with this work. C.M.U. has as a cancer center director oversight over research funded by several pharmaceutical companies, but has not received funding directly herself.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan CA, Garrett WS. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17(3):156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gursoy UK, Kononen E, Uitto VJ. Intracellular replication of fusobacteria requires new actin filament formation of epithelial cells. APMIS. 2008;116(12):1063–70. [DOI] [PubMed] [Google Scholar]
- 5.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komiya Y, Shimomura Y, Higurashi T, Sugi Y, Arimoto J, Umezawa S, et al. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut. 2019;68(7):1335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gethings-Behncke C, Coleman HG, Jordao HWT, Longley DB, Crawford N, Murray LJ, et al. Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2020;29(3):539–48. [DOI] [PubMed] [Google Scholar]
- 9.Tunsjo HS, Gundersen G, Rangnes F, Noone JC, Endres A, Bemanian V. Detection of Fusobacterium nucleatum in stool and colonic tissues from Norwegian colorectal cancer patients. Eur J Clin Microbiol Infect Dis. 2019;38(7):1367–76. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–85. [DOI] [PubMed] [Google Scholar]
- 11.Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65(11):1906–15. [DOI] [PubMed] [Google Scholar]
- 12.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15(3):382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich CM, Gigic B, Bohm J, Ose J, Viskochil R, Schneider M, et al. The ColoCare Study: A Paradigm of Transdisciplinary Science in Colorectal Cancer Outcomes. Cancer Epidemiol Biomarkers Prev. 2019;28(3):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himbert C, Ose J, Nattenmuller J, Warby CA, Holowatyj AN, Bohm J, et al. Body Fatness, Adipose Tissue Compartments, and Biomarkers of Inflammation and Angiogenesis in Colorectal Cancer: The ColoCare Study. Cancer Epidemiol Biomarkers Prev. 2019;28(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 2011;86(3):351–6. [DOI] [PubMed] [Google Scholar]
- 16.Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, Masugi Y, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol 2016;7(11):e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh HJ, Kim JH, Bae JM, Kim HJ, Cho NY, Kang GH. Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy. J Pathol Transl Med. 2019;53(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015;137(6):1258–68. [DOI] [PubMed] [Google Scholar]
- 20.Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74(5):1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta VK, Paul S, Dutta C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front Microbiol 2017;8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One 2013;8(1):e53653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao R, Kong C, Huang L, Li H, Qu X, Liu Z, et al. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(11):2073–83. [DOI] [PubMed] [Google Scholar]
- 24.DeSantis CE, Miller KD, Dale W, Mohile SG, Cohen HJ, Leach CR, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin 2019. [DOI] [PubMed] [Google Scholar]
- 25.Serna G, Ruiz-Pace F, Hernando J, Alonso L, Fasani R, Landolfi S, et al. Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–502. [DOI] [PubMed] [Google Scholar]
- 27.Murphy N, Ward HA, Jenab M, Rothwell JA, Boutron-Ruault MC, Carbonnel F, et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin Gastroenterol Hepatol. 2019;17(7):1323–31 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holowatyj AN, Lewis MA, Pannier ST, Kirchhoff AC, Hardikar S, Figueiredo JC, et al. Clinicopathologic and Racial/Ethnic Differences of Colorectal Cancer Among Adolescents and Young Adults. Clin Transl Gastroenterol. 2019;10(7):e00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White A, Ironmonger L, Steele RJC, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014;27(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25(4):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo S, Li L, Xu B, Li M, Zeng Q, Xiao H, et al. A Simple and Novel Fecal Biomarker for Colorectal Cancer: Ratio of Fusobacterium Nucleatum to Probiotics Populations, Based on Their Antagonistic Effect. Clin Chem. 2018;64(9):1327–37. [DOI] [PubMed] [Google Scholar]
- 33.Ohigashi S, Sudo K, Kobayashi D, Takahashi O, Takahashi T, Asahara T, et al. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci. 2013;58(6):1717–26. [DOI] [PubMed] [Google Scholar]
- 34.Chew J, Zilm PS, Fuss JM, Gully NJ. A proteomic investigation of Fusobacterium nucleatum alkaline-induced biofilms. BMC Microbiol. 2012;12:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol 2015;1(5):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Tabung FK, Zhang X, Nowak JA, Qian ZR, Hamada T, et al. Diets That Promote Colon Inflammation Associate With Risk of Colorectal Carcinomas That Contain Fusobacterium nucleatum. Clin Gastroenterol Hepatol. 2018;16(10):1622–31 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta RS, Nishihara R, Cao Y, Song M, Mima K, Qian ZR, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol 2017;3(7):921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.