Abstract
Hematopoietic stem and progenitor cell (HSPC)-based gene therapy (GT) requires the collection of a large number of cells. While bone marrow (BM) is the most common source of HSPCs in pediatric donors, the collection of autologous peripheral blood stem cells (PBSCs) is an attractive alternative for GT. We present safety and efficacy data of a 10-year cohort of 45 pediatric patients who underwent PBSC collection for backup and/or purification of CD34+ cells for ex vivo gene transfer. Median age was 3.7 years and median weight 15.8 kg. After mobilization with lenograstim/plerixafor (n = 41) or lenograstim alone (n = 4) and 1−3 cycles of leukapheresis, median collection was 37 × 106 CD34+ cells/kg. The procedures were well tolerated. Patients who collected ≥7 and ≥13 × 106 CD34+ cells/kg in the first cycle had pre-apheresis circulating counts of at ≥42 and ≥86 CD34+ cells/μL, respectively. Weight-adjusted CD34+ cell yield was positively correlated with peripheral CD34+ cell counts and influenced by female gender, disease, and drug dosage. All patients received a GT product above the minimum target, ranging from 4 to 30.9 × 106 CD34+ cells/kg. Pediatric PBSC collection compares well to BM harvest in terms of CD34+ cell yields for the purpose of GT, with a favorable safety profile.
Keywords: plerixafor, lenograstim, apheresis, hematopoietic stem and progenitor cells, rare disease, mobilization, harvest, congenital
Graphical abstract
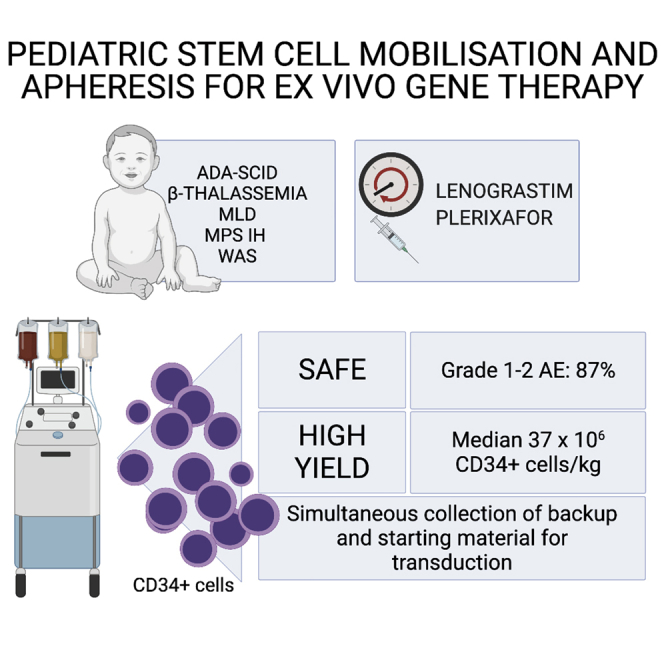
Ex vivo gene therapy requires the timely collection of large amounts of stem cells. We report that mobilization and apheresis of stem cells are safe and effective even in pediatric patients, favorably comparing with conventional bone marrow harvest. Prospectively, this may allow implementation of selection strategies of genetically modified cells.
Introduction
Autologous haemopoietic stem and progenitor cells (HSPCs) are the source material for ex vivo gene therapies in pediatric monogenic diseases.1, 2, 3, 4, 5, 6, 7 Although unmanipulated autologous haemopoietic stem cell transplantation (HSCT) requires the collection of ≥2 × 106 CD34+ cells/kg, gene therapy (GT) collection targets are usually higher, due to purification, ex vivo manipulation, extensive quality testing, freezing, and thawing.8 Furthermore, for safety purposes, an unmanipulated backup is usually stored separately before infusion of the drug product (DP).
Bone marrow (BM) harvest is the standard of care to collect HSPCs from pediatric donors.9 We have previously reported the outcome of BM harvests in a comparable cohort of patients undergoing GT,10 collecting a sufficient amount of cells without any major adverse event (AE). Mobilization and apheresis of HSPCs are standard procedures for adult donors and have been adapted to pediatric patients with a favorable safety profile.4,9,11, 12, 13 However, the pediatric experience in peripheral blood stem cell (PBSC) leukapheresis remains limited and mainly reported for patients weighing >20 kg and not systematically addressed for GT so far. In our center, we progressively transitioned to use PBSCs in GT patients with the aim of increasing the amount of HSPCs collected and reducing the invasiveness associated with the BM harvest.
Here, we report a 10-year experience of PBSC collection in pediatric patients enrolled in GT protocols and provide safety and collection efficacy data. We also evaluate the process yields from harvest to infusion and compare these results with our historical cohort of disease-matched BM harvests.10
Results
Patient population
Between April 1, 2010, and March 31, 2020, 45 consecutive patients affected by adenosine deaminase (ADA)-severe combined immunodeficiency (SCID; n = 4); β-thalassemia (n = 7); metachromatic leukodystrophy (MLD; early juvenile = 8, late infantile = 2); late infantile or early juvenile, mucopolysaccharidosis 1 Hurler (MPSIH; n = 8); or Wiskott–Aldrich syndrome (WAS; n = 16) enrolled in GT protocols were included in the study. Patients’ characteristics are summarized in Table 1. β-thalassemic patients were older than MLD and MPSIH ones, as expected by the design of the trial.14 Forty out of 45 patients performed leukapheresis upfront to collect cells for both DP manufacturing and backup, and 38/40 met this goal, whereas 2 required an additional BM harvest (Supplemental materials and methods).
Table 1.
Patients’ characteristics
| Designated use of HSPCs (n of patients) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Disease | n | Female/male | Age in years (range) | Weight in kg (range) | BM CD34+ cells % (range) | Previous BM harvest (n of patients) | Backup | DP manufacturing |
| ADA-SCID | 4 | 3/1 | 5.3 (3.5−10.8) | 19.8 (14.7−28.8) | 2.1 (1.8−3.7) | 1 | 4 | 2 |
| β-thalassemia | 7 | 2/5 | 6.6 (4.4−13.6) | 20.0 (15.4−54.0) | 4.1 (1.7−5.5) | 0 | 7 | 7 |
| MLD | 10 | 2/8 | 2.5 (0.6−7.7) | 13.2 (7.0−24.0) | 3.7 (0.8−6.4) | 1 | 8 | 10 |
| MPSIH | 8 | 2/6 | 1.9 (1.0−2.7) | 11.8 (11.0−14.3) | 3.9 (1.7−7.7)a | 0 | 8 | 8 |
| WAS | 16 | 0/16 | 3.7 (0.9−14.4) | 18.4 (7.5−54.1) | 4.5 (0.5−10.8) | 0 | 16 | 16 |
| Total | 45 | 9/36 | 3.7 (0.6−14.4) | 15.8 (7.0–54.1) | 3.8 (0.5−10.8) | 2 | 43 | 43 |
DP, drug product; BM, bone marrow, HSPC, hematopoietic stem and progenitor cell.
Data not available for 3 patients.
Schedule of HSPC collection
All patients underwent a single mobilization with lenograstim subcutaneously (s.c.) alone (n = 4) or in combination with plerixafor s.c. (n = 41). Four required anesthesiologic support for central venous catheter (CVC) malfunction, transient malaise, or sedation for agitation. Cells were destined to backup (n = 2), manufacturing of the DP (n = 2), or both (n = 41).
A median of 2.5 days (range 2−4.5) passed between the first dose of lenograstim and the first leukapheresis cycle. Patients who did not receive plerixafor underwent apheresis 1.1 days later, but sample size was small (n = 4). As detailed in Table 2, 13 patients underwent 1 apheresis, 27 underwent 2 aphereses, and 5 underwent 3 cycles. For each patient, all aphereses took place on consecutive days (Figure S1A).
Table 2.
Single and total apheresis yield by weight, stratified by disease
| 1st cycle |
2nd cycle |
3rd cycle |
|||||
|---|---|---|---|---|---|---|---|
| Disease | n | Yield | n | Yield | n | Yield | Total yield |
| ADA-SCID | 4 | 11.5 (1.8−30.9) | 3 | 8.2 (1.5−18.8) | 1 | 9.1 | 27.7 (3.3−34.7) |
| β-thalassemia | 7 | 31.1 (7.5−53.2) | 3 | 23.1 (15.5−30.9) | − | 45.6 (30.6−53.2) | |
| MLD | 10 | 15.5 (5.2−36.7) | 6 | 24.1 (20.4−43.6) | − | 36.5 (5.2−57.9) | |
| MPSIH | 8 | 18.2 (1.6−24.3) | 8 | 23.9 (12.3−34.2) | 2 | 13.0 (6.3−19.6) | 45.4 (31.0−55.3) |
| WAS | 16 | 20.2 (0.7−42.0) | 12 | 21.6 (9.0−35.4) | 2 | 12.7 (9.3−16.2) | 34.4 (18.7−63.8) |
| Total | 45 | 18.3 (0.7−53.2) | 32 | 32 (1.5−43.6) | 5 | 9.3 (6.3−19.6) | 37 (3.3−63.8) |
Cell counts (×106 CD34+ cells/kg) are reported as median and range (in parentheses). The number of patients is reported for each apheresis procedure.
Safety
A total of 108 AEs were recorded, as detailed in Table 3. The incidence of AEs was higher in WAS patients as compared to β-thalassemic, MLD, and ADA-SCID patients (Kruskal-Wallis test, p < 0.001, for all multiple comparisons p < 0.05). No significant difference in the incidence of AEs was observed in patients weighing <20 kg as compared to those weighing more (p = 0.25), and no correlation was found between the total number of AEs and weight or age. Infection (n = 3) was the most common grade 3 AE.
Table 3.
Summary of adverse events
| Grade |
|||||
|---|---|---|---|---|---|
| Category | 4 | 3 | 2 | 1 | Most common event (n) |
| Allergic | 3 | 4 | urticarial skin rash (3) | ||
| Blood related | 1 | 6 | 9 | anemia (6) | |
| Cardiovascular | 1 | hypertension (1) | |||
| Electrolyte disturbances | 1 | 1 | 5 | hypokalemia (3) | |
| ENT | 2 | 2 | |||
| Gastrointestinal | 1 | 8 | 7 | vomit (5) | |
| Infectious | 3 | 6 | 4 | upper airway infection (3) | |
| Kidney | 3 | ||||
| Metabolic | 1 | 2 | 6 | metabolic acidosis (9) | |
| Musculoskeletal | 2 | 6 | 7 | arthralgia (3) | |
| Neurological | 2 | 2 | headache (4) | ||
| Respiratory | 1 | 3 | bronchospasm (2) | ||
| Other | 2 | 3 | 4 | ||
| Total | 0 | 14 | 45 | 49 | |
Adverse events related to rituximab adverse events were excluded (n = 1 grade 4, n = 2 grade 3, n = 1 grade 2). ENT, ear, nose, throat.
Median hemoglobin level before mobilization was 11 g/dL; after the last leukapheresis, hemoglobin decreased to 9.9 g/dL (p = 0.015). Excluding WAS patients due to the disease-related thrombocytopenia, median platelet values before and after mobilization were 342,000/μL and 139,000/μL, respectively (p < 0.0001). 13/31 patients had counts <130,000/μL and 2 of them <50,000/μL in the absence of clinical manifestations of thrombocytopenia. Cumulatively, considering the time window between the first apheresis and the 7 days following the last apheresis, patients were exposed to a total of 89 packed red blood cells (RBCs) and 20 platelet units. During leukapheresis, 69 RBCs units were administered as priming of the circuit system and 13 platelet units as transfusion support. Four patients had no exposure to blood products.
Peripheral blood cell counts and leukapheresis
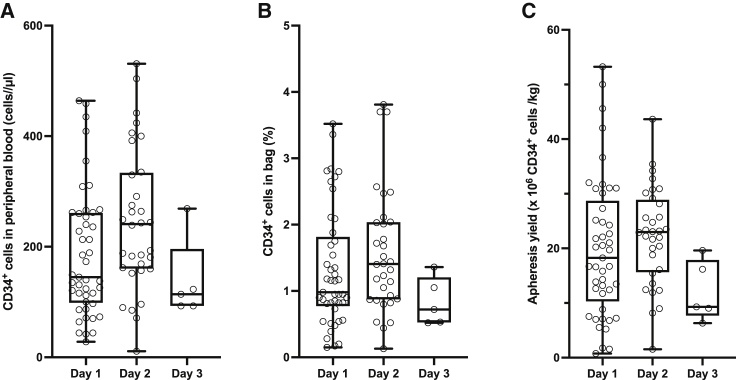
The median PBSC cell count before the first apheresis was 145 CD34+ cells/μL (range 28−464 CD34+ cells/μL). As shown in Figures 1A and S2, patients who continued the mobilization after the first apheresis had a significantly higher CD34+ cell count at day 2 (median increase of 88 CD34+ cells/μL, p < 0.0001) compared to day 1. We observed a median increase of 114 CD34+ cells/μL on day 3 compared to day 2 (p = 0.13) and of 53 CD34+ cells/μL compared to day 1 (p = 0.13).
Figure 1.
CD34+ cell counts in peripheral blood, apheresis bags, and collection yield
Box and whiskers plots illustrating the absolute number of CD34+ cells in peripheral blood before the apheresis (A) and the corresponding relative CD34+ content in the apheresis bag (B) and absolute CD34+ cell yield averaged by weight (C). Whiskers range from minimum to maximum.
Apheresis yields are summarized in Table 2. Overall, the median collection yield of the 82 apheresis was 37.0 × 106 CD34+ cells/kg, with a range of 3.3−63.8 × 106, corresponding to a median volume of 228.7 mL (range 70−891 mL).
For the first procedure, volumes ranged from 48 to 318 mL, containing 50−429 × 106 white blood cells (WBCs)/mL and 0.2%–3.5% of CD34+ cells, and weight-averaged yields ranged from 0.7 to 53.2 × 106 CD34+ cells/kg, with a median of 18.3 × 106 CD34+ cells/kg.
Volumes (median 132.5 mL, range 44−301 mL) and WBC counts (median 205 × 106 WBC/mL, range 53−374 × 106 WBC/mL) of the second apheresis did not differ significantly from the previous one. However, as illustrated in Figure 1B, the median percentage of CD34+ cells increased by 0.55% (p < 0.0001); similarly, the median yield was 23.0 × 106 CD34+ cells/kg (range 1.5−43.6 CD34+ cells/kg) corresponding to a median increase of 3.75 × 106 CD34+ cells/kg (p < 0.0001) as compared to the first apheresis in the same patients, as shown in Figure 1C.
As for the third apheresis, the collection parameters did not appear to differ significantly from the previous one, albeit the sample size was small (n = 5).
Volumes of the first and second apheresis correlated with age (both p < 0.0001) and weight (both p < 0.0001). Peripheral CD34+ cell counts before the apheresis correlated with relative CD34+ content of the apheresis bag (Spearman r 0.77, p < 0.0001 and 0.53, p = 0.0016, respectively; Figure S3A) and weight-adjusted CD34+ cell yield for both the first and second day (Spearman r 0.92, p < 0.0001 and 0.65, p < 0.0001; Figure S3B). Finally, the percentage of CD34+ cells in the bag correlated with weight-adjusted yield (Spearman r 0.81, p < 0.0001 for day 1; 0.46, p = 0.0081 for day 2; and 1, p = 0.0167 for day 3; Figure S3C). In summary, a higher peripheral CD34+ cell count corresponded to a higher percentage of CD34+ cells in the apheresis bag and both to an increased weight-adjusted yield, ultimately pointing to peripheral CD34+ cell number as a predictor of yield.
Of note, one ADA-SCID patient was a poor mobilizer, collecting 1.76 and 1.53 × 106 CD34+ cells/kg, corresponding to pre-apheresis of 28 CD34+ cells/μL on day 1 and of 11 on day 2, respectively.
Collection targets are reported in Table 4. Only one patient did not meet the target by PBSCs alone. In absolute terms and by conventional cutoffs for autologous PBSC collection,15 44 patients had an optimal collection, exceeding 5 × 106 CD34+ cells/kg, one patient fell into the “low” 2−5 × 106 CD34+ cells/kg interval, and none had a poor yield, i.e., <2 × 106 CD34+ cells/kg.
Table 4.
Minimum collection targets by protocol
| Protocol | DP manufacturing | Backup | Total |
|---|---|---|---|
| ADA-SCID | 0−NA | 1 | 1−NA |
| β-thalassemia | 5 | 2 | 7 |
| MLD | 8−10 | 0−3 | 8−13 |
| MPSIH | 8 | 3 | 11 |
| WAS | 5−10 | 3 | 8−13 |
Numbers are reported as ×106 CD34+ cells/kg. DP, drug product.
Predictors of apheresis yield
Beyond circulating peripheral CD34+ cell counts, other variables were found to be predictive of apheresis yield. Considering the entire cohort, the duration of mobilization correlated both with the yield of the first apheresis (Spearman r −0.33, p = 0.026) and the total yield (Spearman r −0.33, p = 0.029), whereas the cumulative dose of lenograstim correlated negatively with the yield of the first apheresis (Spearman r −0.65, p < 0.0001) but not with total yield (p = 0.40).
As lenograstim dosing is adjusted mostly based on peripheral WBC counts, the total lenograstim dose reflects both the WBC increase as well as the length of the mobilization (Figure S1A). Vice versa, the interval between the beginning of mobilization and the first leukapheresis reflects the increase in peripheral CD34+ cell counts and thus the individual response to the drug regimen.
As for what concerns patients’ characteristics, female gender was associated with lower total yield (p = 0.015), with a trend for lower yield of the first apheresis (p = 0.058). Furthermore, patients who were enrolled upfront to HSPC mobilization and apheresis collected more cells than the others (p = 0.0066). Instead, no correlation was found between first apheresis or overall yield and age, weight, disease, or relative percentage of CD34+ cells in the BM.
Stepwise linear regression of first apheresis yield considering age, disease, gender, weight percentile, total lenograstim dose, and undergoing mobilization upfront identified total lenograstim dose, disease, and gender as independent predictors of first apheresis yield (R2 0.531). Gender-specific differences may at least be partly due to the effect of lenograstim, which has indeed been shown to be more effective than filgrastim in males but not in females.16 When we included the peripheral CD34+ cell counts variable in the linear regression model, disease and peripheral CD34+ cell counts were the strongest predictive variables (R2 0.843).
Plerixafor was a major contributor to harvest yield. In fact, the four patients who did not receive plerixafor collected fewer cells both during the first apheresis and overall, with a median difference of 13 × 106 CD34+ cells/kg (p = 0.0082) and 23 × 106 CD34+ cells/kg (p = 0.0005), respectively.
Regarding the subgroup of patients who received plerixafor, all received 0.24 mg/kg/day before the first apheresis. A lower yield at the first apheresis corresponded to a higher subsequent plerixafor dose (Spearman r −0.64, confidence interval [CI] −0.82 to −0.35), and all four patients who underwent a third apheresis received a high dose (0.4−0.48 mg/kg; Figure S1B). The total plerixafor dose was not correlated with the overall yield (p = 0.69) nor was there a dose-response relation between the second plerixafor dose and the yield of the second apheresis (p = 0.4).
All patients who received plerixafor except one collected ≥20 × 106 CD34+ cells/kg. Female gender was associated with lower overall yield (p = 0.012) but no significant differences in 1st apheresis yield (p = 0.073). By univariate analyses, first apheresis and total yield were not influenced by age, weight, BM CD34+ counts, nor disease (p = 0.13 and p = 0.09, respectively). Stepwise linear regression of first apheresis yield considering age, disease, gender, weight percentile, undergoing mobilization upfront, total lenograstim dose, and total plerixafor dose identified total plerixafor dose and gender as negative predictors of first apheresis yield and β-thalassemia as a positive predictor of yield (R2 0.631). Inclusion of peripheral CD34+ cell counts in the model instead replaced gender as a predictor of yield (R2 0.853).
Manipulation and engraftment
A backup was stored for 43 patients; all but one were above the minimum 2 × 106 CD34+ cell/kg threshold for a rescue autologous HSCT,17 as shown in Table S1.
Table 5 shows the median CD34+ cell count at each step in the production process, not accounting for cells that were withdrawn for research or quality control. CD34+ selection yield was in line with our historical BM cohort and previous studies.10,18 In some cases, the number of cells that was manufactured exceeded the upper infusion limits defined for each protocol. Table 5 also illustrates the infused DP dose and the predetermined dose range. One patient received a fresh formulation of the DP; 14 patients received a DP that was cryopreserved before transduction, and 28 received a DP that had been frozen after manipulation. Of note, three patients also received transduced BM cells (3.8, 6.7, and 3.66 × 106 CD34+ cell/kg; data not shown). 41/42 patients received a DP dose within the reference infusion range.
Table 5.
CD34+ cell counts across the manufacturing process
| Protocol |
n |
Starting material (×106 CD34+ cells/kg) |
Recovery from selection (×106 CD34+ cells/kg) |
Destined to transduction (×106 CD34+ cells/kg) |
Recovery from transduction (×106 CD34+ cells/kg) |
Predefined DP infusion range (×106 CD34+ cells/kg) |
DP dose (×106 CD34+ cells/kg) |
|
|---|---|---|---|---|---|---|---|---|
| MIN | MAX | |||||||
| β-thalassemia | 7 | 44.7 (18.3−50.1) | 29.6 (15.1−33) | 17.5 (14.2−18.6) | 25.2 (20.1−36.9) | 2 | 20 | 19.6 (16.3−20) |
| MLD | 10 | 33.9 (4−53.9) | 23.3 (3.5−33.8) | 23.1 (2.6−33.3) | 30.3 (4−48.3) | 2−3 | 20−30 | 26.7 (4−30) |
| MPSIH | 8 | 38 (27.3−45) | 21.9 (18.7−27.1) | 21.5 (18.3−26.7) | 19.8 (12.8−30.6) | 4 | 35 | 19.8 (12.8−30.6) |
| ADA-SCID | 2 | 40.6 (36.2−44.9) | 18.1 (15.8−20.4) | 11.1 (8.0−14.1)a | 17.9 (10.1−25.7) | 2 | 30 | 17.9 (10.1−25.7) |
| WAS | 16 | 38.1 (11.9−67.5) | 22.0 (8.8−38.5) | 21.1 (8.8−37.2) | 18.0 (5.25−61.8) | 2−3 | 20−30 | 18.0 (5.3−30.9b) |
Variables are reported as median and range (in parentheses). DP, drug product. The interval between the last apheresis and DP infusion ranged from 4 to 163 days.
The interval between the last apheresis and DP infusion ranged from 4 to 163 days
Total nucleated cells.
One patient received slightly more than 30 × 106 CD34+ cells/kg due to high busulfan exposure.
The DP dose ranged from 4 to 30.9 × 106 CD34+ cell/kg, and all patients engrafted. For patients who received a DP uniquely sourced from PBSCs, the median day of neutrophil engraftment was 24.5 (range 15−77), whereas the median day of platelet engraftment was 21 (range 10−76). No patient required reinfusion of the unmanipulated backup. No correlation was observed between the DP dose and the number of days to neutrophil or platelet engraftment.
Discussion
Autologous HSPC-based GT is becoming a new paradigm for the treatment of inborn errors of immunity,6,7,19 metabolism,2,3,20 and haemopoiesis.1,4,14 Three medicinal products based on HSPC have been authorized in the European Union (EU), and others are in advanced clinical development.1 HSPCs may be collected by BM harvest or mobilization and leukapheresis for DP manufacture; however, no standards or guidelines are available for their collection in pediatric patients for the purpose of GT.
Previous experience with mobilization was reported in healthy pediatric donors for allogeneic transplantation or autologous HSPC transplantation for malignancies.9,12,21, 22, 23 Our analysis focuses on a large cohort of pediatric subjects with nonmalignant diseases with various comorbidities related to the underlying disorder and include also infants <1 year of age and weighing less than 10 kg.
We show that mobilization and leukapheresis in the context of autologous GT for pediatric subjects have a favorable short-term safety profile and result in adequate cell collection for backup and DP manufacturing. All patients received a DP that respected the specifications in terms of minimum CD34+ cells/kg content, and all eventually engrafted.
The vast majority of patients was fully compliant, and about one-half of them experienced no or minimal adverse effects during the mobilization and collection procedure. A number of AEs have already been reported to be related to the mobilization or apheresis procedure.9,21,24 We found some AEs to be confined to specific diseases, e.g., metabolic acidosis in MLD,25 despite the fact that leukapheresis and plerixafor rather carry a risk of alkalosis.26,27 The frailty of WAS patients, who showed the highest rate of AEs, is not surprising, as thrombocytopenia, immunodeficiency, immune dysregulation, and auto-inflammatory manifestations can understandingly be exacerbated by mobilizing drugs and other procedures. Although it is not possible to exclude that procedure-related AEs also occurred at later time points, extending the time frame would have reduced specificity of the analysis and suffered from the impact of major confounding factors, i.e., chemotherapy and GT.
The number of circulating CD34+ cells is known to be a reliable indicator of the expected apheresis yield.28 Indeed, we found a clear linear relation between the number of circulating CD34+ cells and relative number of CD34+ cells in the bag. As volumes instead correlated with age and weight, the weight-averaged CD34+ cell yield resulted directly proportional to the percentage of CD34+ cells in the bag. In our cohort, apheresis yield was influenced by gender, possibly due to suboptimal efficacy of lenograstim in females, and underlying disease; drug dosages were increased in patients with initial lower responses.
There is no consensus on the definition of “poor pediatric mobilizer.”29,30 Our GT protocols require the collection of ≥7−13 × 106 CD34+ cells/kg, significantly higher than conventional cutoffs.28 This is due to the fact that autologous HSPC GT requires higher numbers for cell manipulation for drug manufacturing and unmanipulated backup. Therefore, the traditional definition of poor mobilizer may be too loose; in our series, patients who collected ≥7 × 106 CD34+ cells/kg in the first cycle had pre-apheresis circulating counts of ≥42 CD34+ cells/μL, and those who collected ≥13 × 106 CD34+ cells/kg had ≥86 CD34+ cells/μL.
As compared to the historical BM cohort,10 patients who collected HSPCs weighed more (median 15.8 kg versus 10.6 kg, p = 0.0003) and were older (median 3.7 versus 1.5 years, p = 0.0013), whereas gender distribution was similar (p = 0.63 excluding WAS, p = 0.12 including WAS).
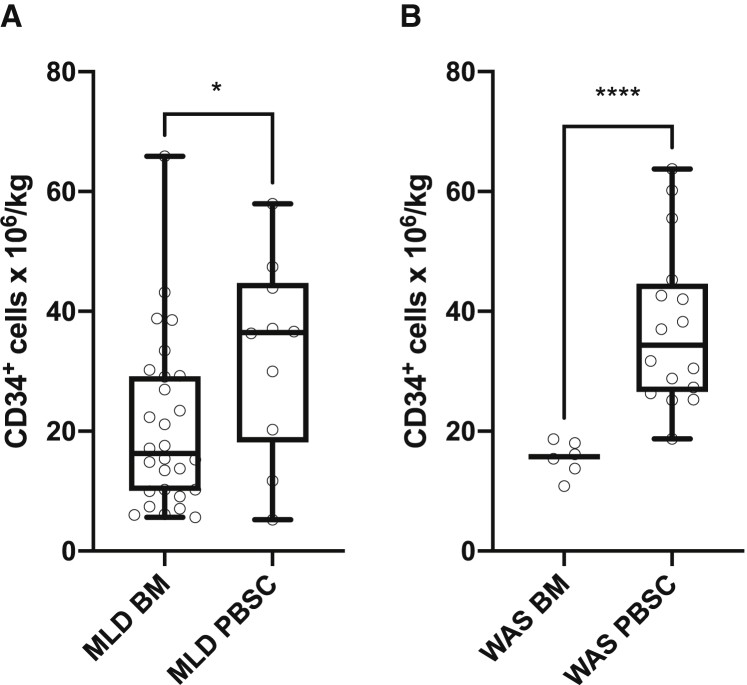
As illustrated in Figure 2, the leukaphereses of MLD and WAS patients yielded more cells as compared with BM collections (p = 0.047 and p < 0.0001, respectively). A similar difference was found between the pooled BM harvests and the pooled leukaphereses (median of 17 versus 37 × 106 CD34+ cells/kg, p < 0.0001). Indeed, linear regression analysis on the whole patient population revealed that neither age nor weight influenced the HSPC yield and that the difference between BM and PBSC yield was explained entirely by the different procedure.
Figure 2.
Comparison of CD34+ cell yield of mobilization and leukapheresis versus bone marrow harvest
(A) MLD patients. (B) WAS patients. Whiskers range from minimum to maximum. ∗ p<0.05 ∗∗∗∗ p <0.0001.
Among the 40 patients who underwent mobilization upfront, 37 did not require a second HSPC collection from either PBSC or BM. Instead, 57/57 patients of the BM harvest cohort required a separate backup collection, either by BM harvest (n = 54) or mobilization and leukapheresis (n = 3).
Thus, mobilization and leukapheresis allowed the collection of more HSPCs for the purpose of GT, in a shorter period of time, irrespectively of weight; additional harvests may be required for a minority of patients. With the consideration of the complexity of the GT treatment path, the urgency of treating diseases such as MLD, MPSIH, and WAS; the simultaneous collection of backup; and cells for GT manufacturing represents an important advantage over BM harvest. Shorter duration of anesthesia, lower intravascular fluctuation, and reduced pain represent additional advantages over BM collections. Indeed, the most recent GT trials have exploited the collection of PBSCs to allow for large DP infusions;2,13,14,31 however, methods and goals of collection in children have not been addressed systematically. We believe our data may be useful for the optimization of the collection procedures and for the drafting of guidelines on PBSC collection in pediatric patients for the purpose of GT.
Furthermore, we show that the collection of very large amounts of CD34+ cells is feasible and may prospectively allow the implementation of strategies to enrich specific subpopulations of genetically modified HSPC,32, 33, 34, 35, 36 whereas compensating for the cell loss expected from the purification procedures. Our results are also relevant for collection strategies in the context of allogeneic HSCT. Harvesting large amounts of PBSCs with lenograstim and plerixafor may in fact allow us to overcome significant weight discrepancies between pediatric family donors and HSCT recipients. In any event, the expected benefits of collecting and manipulating large cell doses must be weighed against the procedural risks and the increase in marginal cost.
The first limitation of this work is its retrospective nature, which is partly mitigated by prospective data collection. The second limitation is the potential variability due to the different disease background, coupled with the small size of patient subgroups. One cannot exclude that increased data accrual will lead to new or slightly different conclusions.
In summary, our work provides the necessary basis for an informed decision on the benefits and drawbacks of PBSC collection in pediatric patients.
Materials and methods
Patient population
We included all consecutive patients <18 years who underwent mobilization between April 1, 2010, and March 31, 2020, at Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale San Raffaele (OSR). Legal guardians provided written, informed consent according to Italian law. Patients were affected by ADA deficiency (ADA-SCID), β-thalassemia, MLD, MPSIH, or WAS. Patients were enrolled in GT clinical trials (n = 32; ClinicalTrials.gov: NCT02453477,14 NCT03392987, NCT01560182,3 NCT03488394,20 NCT01515462,5 and NCT0383748319) and treated under compassionate use (n = 7), hospital exemption (n = 4), or Strimvelis (n = 2, included in this study only for backup collection). All studies were approved by the OSR Ethical Committee and Italian competent authorities.
The baseline BM aspirate CD34+ cell count was considered for predictive analysis. HSPCs were mobilized with lenograstim s.c. alone or with plerixafor s.c. Dosing was adjusted according to peripheral WBC and CD34+ counts. Leukapheresis was performed with Spectra COBE or the Optia Apheresis System (Terumo Blood and Cell Technologies [BCT]) and the WBC set or Spectra Optia IDL Set, respectively, through a percutaneous CVC; ≲3 blood vol was processed. During the collection, venous blood gas analyses were checked serially, and calcium gluconate and sodium bicarbonate were administered accordingly. For patients weighing <25 kg or with low hematocrit, the extra-corporeal circuit was primed with irradiated allogenic-packed RBCs.
Collection targets are reported as stated in the corresponding trial protocol5,14,19,20 (and ClinicalTrials.gov: NCT03392987), Strimvelis summary of product characteristics,37 or in the individualized patient’s treatment plans. The number of CD34+ cells was determined by flow cytometry (International Society of Hematotherapy and Graft Engineering [ISHAGE]); CD34+ cell content of apheresis bags was normalized by patients’ weight. Leukaphereses were enriched for CD34+ cells by Miltenyi CliniMACS and used as starting material for manufacturing of DPs by transduction with viral vectors at Molmed (currently AGC Biologics). The leukapheresis fractions dedicated to backup were cryopreserved.
AEs were recorded in the case report forms or clinical charts and graded according to the Common Terminology Criteria for Adverse Events. AEs occurring between the beginning of the mobilization and the 14 days following the last apheresis or, if occurring earlier, the first dose of conditioning chemotherapy were considered for analysis.
Statistical analysis
Statistical analysis was done with Prism version 8 (GraphPad Software, San Diego, CA, USA) or SPSS Statistics version 24 (IBM, Armonk, NY, USA). Continuous variables are summarized with median and range; correlation was assessed with Spearman r coefficient and linear regression, whereas differences were assessed by two-tailed Mann-Whitney test or Wilcoxon matched-pairs signed-rank test. Comparison among three or more groups was performed by Kruskal-Wallis and Dunn’s multiple comparison tests. Relations between categorical variables were assessed with Fisher’s exact test. Relationship with multiple independent variables was assessed with stepwise multiple linear regression, provided no significant outliers were present.
Groups with at least 5 data points were considered for statistical analysis; however, the four ADA-SCID patients were not a priori excluded from multiple comparison analyses. Significant p values are summarized on figures as follows: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
Acknowledgments
The authors would like to thank Fondazione Telethon for support. We thank the medical and nursing team of the Pediatric Immunohematology Unit and Stem Cell Transplant Program of the IRCCS San Raffaele Scientific Institute for their professional care of patients during hospitalization; Laura Castagnaro and the OSR quality team; Stefano Zancan, data managers, study coordinators, research nurses, and administrative personnel of the San Raffaele Telethon Institute for Gene Therapy (SR-TIGET) Clinical Trial Office (TCTO); and Alessandro Nonis for statistical support. Orchard Therapeutics is the current sponsor of GT studies for ADA-SCID, WAS, β-thalassemia, and MLD. The graphical abstract was created with BioRender.com. Several authors are members of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases (ERN-RITA); Inborn Error Working Party of EBMT and Italian Primary Immunodeficiencies Network (IPINET); and Associazione Italiana Ematologia e Oncologia Pediatrica (AIEOP). A.A. is the recipient of the Else Kröner Fresenius Prize for Medical Research 2020. This work was supported by Fondazione Telethon.
Author contributions
Conceptualization, D.C. and F.T.; data curation, D.C., F.T., E.A., and P.M.; formal analysis, D.C. and F.T.; funding acquisition, M.P.C., M.E.B., and A.A.; investigation, D.C., F.T., V.C., B.G., Francesca Ferrua, S.M., M.M., F.B., G.C., Francesca Fumagalli, G.V., P.S., R.M., and L.S.; methodology, S.G., M.Z., V.G., C.P., and M.P.C.; resources, A.A.; supervision, A.A., F.C., M.E.B., and M.P.C.; visualization, D.C.; writing – original draft, D.C. and F.T.; writing – review & editing, M.P.C., M.E.B., and A.A.
Declaration of interests
SR-TIGET is a joint venture between Fondazione Telethon and OSR. Gene therapies for ADA-SCID, WAS, MLD, β-thalassemia, and MPSIH developed at SR-TIGET were licensed to Orchard Therapeutics (OTL) in 2018 and 2019. A.A. is the principal investigator (PI) of the above clinical trials. M.E.B. is the current PI of the MPSIH clinical trial.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2021.05.013.
Supplemental information
References
- 1.Ferrari G., Thrasher A.J., Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 2021;22:216–234. doi: 10.1038/s41576-020-00298-5. [DOI] [PubMed] [Google Scholar]
- 2.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., Armant M., Dansereau C., Lund T.C., Miller W.P. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 4.Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M. Gene therapy in patients with transfusion-dependent β-thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 5.Ferrua F., Cicalese M.P., Galimberti S., Giannelli S., Dionisio F., Barzaghi F., Migliavacca M., Bernardo M.E., Calbi V., Assanelli A.A. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–e253. doi: 10.1016/S2352-3026(19)30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Zhang F., Adams S., Bjorkegren E., Bayford J., Brown L., Davies E.G. Hematopoietic Stem Cell Gene Therapy for Adenosine Deaminase-Deficient Severe Combined Immunodeficiency Leads to Long-Term Immunological Recovery and Metabolic Correction. Sci. Transl. Med. 2011;3:97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- 7.Cicalese M.P., Ferrua F., Castagnaro L., Pajno R., Barzaghi F., Giannelli S., Dionisio F., Brigida I., Bonopane M., Casiraghi M. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood. 2016;128:45–54. doi: 10.1182/blood-2016-01-688226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purtill D., Antonenas V., Chiappini P., Tong D., O’Flaherty E., Bajel A., Kabani K., Larsen S., Tan S., Hutchins C. Variable CD34+ recovery of cryopreserved allogeneic HPC products: transplant implications during the COVID-19 pandemic. Blood Adv. 2020;4:4147–4150. doi: 10.1182/bloodadvances.2020002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Styczynski J. Young child as a donor of cells for transplantation and lymphocyte based therapies. Transfus. Apheresis Sci. 2018;57:323–330. doi: 10.1016/j.transci.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Tucci F., Frittoli M., Barzaghi F., Calbi V., Migliavacca M., Ferrua F., Fumagalli F., Lorioli L., Castagnaro L., Facchini M. Bone marrow harvesting from paediatric patients undergoing haematopoietic stem cell gene therapy. Bone Marrow Transplant. 2019;54:1995–2003. doi: 10.1038/s41409-019-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rettig M.P., Ansstas G., DiPersio J.F. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karow A., Wilhelm A., Ammann R.A., Baerlocher G.M., Pabst T., Mansouri Taleghani B., Roessler J., Leibundgut K. Peripheral blood progenitor cell collection in pediatric patients optimized by high pre-apheresis count of circulating CD34+ cells and high blood flow. Bone Marrow Transplant. 2019;54:885–893. doi: 10.1038/s41409-018-0353-8. [DOI] [PubMed] [Google Scholar]
- 13.Tisdale J.F., Pierciey F.J., Jr., Bonner M., Thompson A.A., Krishnamurti L., Mapara M.Y., Kwiatkowski J.L., Shestopalov I., Ribeil J.A., Huang W. Safety and feasibility of hematopoietic progenitor stem cell collection by mobilization with plerixafor followed by apheresis vs bone marrow harvest in patients with sickle cell disease in the multi-center HGB-206 trial. Am. J. Hematol. 2020;95:E239–E242. doi: 10.1002/ajh.25867. [DOI] [PubMed] [Google Scholar]
- 14.Marktel S., Scaramuzza S., Cicalese M.P., Giglio F., Galimberti S., Lidonnici M.R., Calbi V., Assanelli A., Bernardo M.E., Rossi C. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019;25:234–241. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 15.Duong H.K., Savani B.N., Copelan E., Devine S., Costa L.J., Wingard J.R., Shaughnessy P., Majhail N., Perales M.-A., Cutler C.S. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2014;20:1262–1273. doi: 10.1016/j.bbmt.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Fischer J.C., Frick M., Wassmuth R., Platz A., Punzel M., Wernet P. Superior mobilisation of haematopoietic progenitor cells with glycosylated G-CSF in male but not female unrelated stem cell donors. Br. J. Haematol. 2005;130:740–746. doi: 10.1111/j.1365-2141.2005.05678.x. [DOI] [PubMed] [Google Scholar]
- 17.Panch S.R., Szymanski J., Savani B.N., Stroncek D.F. Sources of hematopoietic stem and progenitor cells and methods to optimize yields for clinical cell therapy. Biol. Blood Marrow Transplant. 2017;23:1241–1249. doi: 10.1016/j.bbmt.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Gaipa G., Dassi M., Perseghin P., Venturi N., Corti P., Bonanomi S., Balduzzi A., Longoni D., Uderzo C., Biondi A. Allogeneic bone marrow stem cell transplantation following CD34+ immunomagnetic enrichment in patients with inherited metabolic storage diseases. Bone Marrow Transplant. 2003;31:857–860. doi: 10.1038/sj.bmt.1704024. [DOI] [PubMed] [Google Scholar]
- 19.Ferrua F., Cicalese M.P., Galimberti S., Giannelli S., Dionisio F., Barzaghi F., Migliavacca M., Bernardo M.E., Calbi V., Tucci F. Lentiviral Hematopoietic Stem and Progenitor Cell Gene Therapy for Wiskott-Aldrich Syndrome (WAS): Up to 8 Years of Follow up in 17 Subjects Treated Since 2010. Blood. 2019;134:3346. [Google Scholar]
- 20.Gentner B., Bernardo M.E., Tucci F., Zonari E., Fumagalli F., Pontesilli S., Acquati S., Silvani P., Ciceri F., Rovelli A. Extensive Metabolic Correction of Hurler Disease By Hematopoietic Stem Cell-Based Gene Therapy: Preliminary Results from a Phase I/II Trial. Blood. 2019;134:607. [Google Scholar]
- 21.Styczynski J., Balduzzi A., Gil L., Labopin M., Hamladji R.M., Marktel S., Yesilipek M.A., Fagioli F., Ehlert K., Matulova M., European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party study. Blood. 2012;119:2935–2942. doi: 10.1182/blood-2011-04-349688. [DOI] [PubMed] [Google Scholar]
- 22.Doberschuetz N., Soerensen J., Bonig H., Willasch A., Rettinger E., Pfirrmann V., Salzmann-Manrique E., Schäfer R., Klingebiel T., Bader P., Jarisch A. Mobilized peripheral blood stem cell apheresis via Hickman catheter in pediatric patients. Transfusion. 2019;59:1061–1068. doi: 10.1111/trf.15113. [DOI] [PubMed] [Google Scholar]
- 23.Pulsipher M.A., Levine J.E., Hayashi R.J., Chan K.W., Anderson P., Duerst R., Osunkwo I., Fisher V., Horn B., Grupp S.A. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: the pediatric blood and marrow transplant consortium experience (PBMTC) 1996-2003. Bone Marrow Transplant. 2005;35:361–367. doi: 10.1038/sj.bmt.1704743. [DOI] [PubMed] [Google Scholar]
- 24.Pulsipher M.A., Chitphakdithai P., Logan B.R., Navarro W.H., Levine J.E., Miller J.P., Shaw B.E., O’Donnell P.V., Majhail N.S., Confer D.L. Lower risk for serious adverse events and no increased risk for cancer after PBSC vs BM donation. Blood. 2014;123:3655–3663. doi: 10.1182/blood-2013-12-542464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorioli L., Cicalese M.P., Silvani P., Assanelli A., Salvo I., Mandelli A., Fumagalli F., Fiori R., Ciceri F., Aiuti A. Abnormalities of acid-base balance and predisposition to metabolic acidosis in Metachromatic Leukodystrophy patients. Mol. Genet. Metab. 2015;115:48–52. doi: 10.1016/j.ymgme.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee G., Arepally G.M. Anticoagulation techniques in apheresis: from heparin to citrate and beyond. J. Clin. Apher. 2012;27:117–125. doi: 10.1002/jca.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karres D., Ali S., van Hennik P.B., Straus S., Josephson F., Thole G., Glerum P.J., Herberts C., Babae N., Herold R. EMA Recommendation for the pediatric indications of plerixafor (Mozobil) to enhance mobilization of hematopoietic stem cells for collection and subsequent autologous transplantation in children with lymphoma or malignant solid tumors. Oncologist. 2020;25:e976–e981. doi: 10.1634/theoncologist.2019-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armitage S., Hargreaves R., Samson D., Brennan M., Kanfer E., Navarrete C. CD34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 1997;20:587–591. doi: 10.1038/sj.bmt.1700938. [DOI] [PubMed] [Google Scholar]
- 29.Morland B., Kepak T., Dallorso S., Sevilla J., Murphy D., Luksch R., Yaniv I., Bader P., Rößler J., Bisogno G. Plerixafor combined with standard regimens for hematopoietic stem cell mobilization in pediatric patients with solid tumors eligible for autologous transplants: two-arm phase I/II study (MOZAIC) Bone Marrow Transplant. 2020;55:1744–1753. doi: 10.1038/s41409-020-0836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sevilla J., Guillén M., Castillo A., Prudencio M., González-Vicent M., Lassaletta Á., Cormenzana M., Ramírez M., Pérez-Martínez A., Madero L., Díaz-Pérez M.Á. Defining “poor mobilizer” in pediatric patients who need an autologous peripheral blood progenitor cell transplantation. Cytotherapy. 2013;15:132–137. doi: 10.1016/j.jcyt.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Esrick E.B., Lehmann L.E., Biffi A., Achebe M., Brendel C., Ciuculescu M.F., Daley H., MacKinnon B., Morris E., Federico A. Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. N. Engl. J. Med. 2021;384:205–215. doi: 10.1056/NEJMoa2029392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agudelo D., Duringer A., Bozoyan L., Huard C.C., Carter S., Loehr J., Synodinou D., Drouin M., Salsman J., Dellaire G. Marker-free coselection for CRISPR-driven genome editing in human cells. Nat. Methods. 2017;14:615–620. doi: 10.1038/nmeth.4265. [DOI] [PubMed] [Google Scholar]
- 33.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zonari E., Desantis G., Petrillo C., Boccalatte F.E., Lidonnici M.R., Kajaste-Rudnitski A., Aiuti A., Ferrari G., Naldini L., Gentner B. Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy. Stem Cell Reports. 2017;8:977–990. doi: 10.1016/j.stemcr.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciceri F., Bonini C., Stanghellini M.T.L., Bondanza A., Traversari C., Salomoni M., Turchetto L., Colombi S., Bernardi M., Peccatori J. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 36.Vavassori V., Mercuri E., Marcovecchio G.E., Castiello M.C., Schiroli G., Albano L., Margulies C., Buquicchio F., Fontana E., Beretta S. Modeling, optimization, and comparable efficacy of T cell and hematopoietic stem cell gene editing for treating hyper-IgM syndrome. EMBO Mol. Med. 2021;13:e13545. doi: 10.15252/emmm.202013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Medicines Agency Strimvelis. Product Information. https://www.ema.europa.eu/en/documents/product-information/strimvelis-epar-product-information_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.