Abstract
Background
Major depressive disorder (MDD) is a pervasive and debilitating syndrome characterized by mood disturbances, anhedonia, and alterations in cognition. While the prevalence of MDD is twice as high for women compared to men, little is known about the molecular mechanisms that drive sex differences in depression susceptibility.
Methods
We discovered that Slit Guidance Ligand 1 (SLIT1), a secreted protein essential for axonal navigation and molecular guidance during development, is downregulated in the adult ventromedial prefrontal cortex (vmPFC) of depressed women compared to healthy controls, but not depressed men. This sex-specific downregulation of Slit1 was also observed in vmPFC of mice exposed to chronic variable stress. To identify a causal, sex-specific role for SLIT1 in depression-related behavioral abnormalities, we performed knockdown (KD) of Slit1 expression in vmPFC of male and female mice.
Results
When combined with stress exposure, vmPFC Slit1 KD reflected the human condition by inducing a sex-specific increase in anxiety- and depression-related behaviors. Further, we found that vmPFC Slit1 KD decreased the dendritic arborization of vmPFC pyramidal neurons, and decreased the excitability of the neurons, in female mice, effects not observed in males. RNA-sequencing analysis of vmPFC after Slit1 KD in female mice revealed an augmented transcriptional stress signature.
Conclusions
Together, our findings establish a crucial role for SLIT1 in regulating neurophysiological and transcriptional responses to stress within the female vmPFC, and provide mechanistic insight into novel signaling pathways and molecular factors influencing sex differences in depression susceptibility.
Keywords: Major depressive disorder, SLIT1, chronic variable stress, prefrontal cortex, RNA sequencing
Introduction
Major depressive disorder (MDD) is a highly prevalent and debilitating syndrome characterized by mood changes, an inability to feel pleasure, and alterations in physiological functions, cognition, and psychomotor activity (1). Depression is caused by a complex interplay between genetic, epigenetic, and environmental factors, and its multifaceted nature has made the identification of genes contributing to depression susceptibility difficult to identify. Further confounding this complexity is the fact that susceptibility to MDD is associated with a striking sex difference: women are affected at twice the rate of men (2-4). Furthermore, men and women tend to display a distinct presentation of depression (5), with women on average having an earlier age of onset, longer depressive episodes, heightened symptom severity, and tendency to respond to different pharmacological treatments (6). In addition, females display higher rates of comorbid anxiety disorders, while males exhibit more comorbid substance use disorders (7). While the predominance of women suffering from MDD is well-established, substantially less is known about the molecular mechanisms that underlie the sex differences observed in depression risk and presentation (8).
One predominant type of neuroadaptation in response to stress is structural changes within the cerebral cortex, including dendritic remodeling, synaptic turnover, and neuronal replacement. These adaptations have been implicated as part of structural manifestations of the depressed brain (9). Postmortem studies report a decrease in synapse numbers in medial prefrontal cortex (mPFC) of subjects suffering from depression (9). Preclinical studies extend these findings, demonstrating reductions in dendritic complexity and spine density within mPFC and hippocampus in response to chronic stress (10, 11). Atrophy of mPFC pyramidal neurons is observed after even short periods of stress (12-14), indicating that these cells are especially sensitive to environmental insult. The majority of these studies have been conducted in male rodents. However, stress has been shown to promote dynamic changes in the neuronal dendritic tree in a sex-specific manner (15). Specifically in mPFC pyramidal neurons, stress induces opposing effects on apical dendritic length in male vs. female rats (16). Yet, the molecular mechanisms by which stress causes these sex-specific structural alterations in mPFC remain poorly understood.
Guidance cues, including the Slit, Netrin, and Semaphorin families, determine the fine organization of neuronal circuits during embryonic and postnatal development (17), and are being increasingly recognized as potential targets for neurological and psychiatric disorders, including depression (18-20). One molecular pathway that has been associated with neuronal plasticity in the developing brain is the SLIT/ROBO pathway (21-24). Slit Guidance Ligands (SLIT1, SLIT2, SLIT3) are evolutionary conserved secreted glycoproteins that are implicated in axonal navigation and molecular guidance in cellular migration during neural development (25-27). SLIT1, the family member most highly expressed in the central nervous system, has been shown to positively regulate dendritic growth in cortical cells in vitro (28). Although SLIT/ROBO signaling has been well-characterized in neuronal development, the role for SLIT1 in regulating plastic changes during adulthood or in response to stress have not been explored.
Here, we examined how SLIT1 function within mPFC contributes to stress susceptibility and MDD in a sex-specific manner. We first identify downregulation of Slit1 mRNA expression in the ventral mPFC (vmPFC), specifically in females, induced by chronic stress in mice and depression in humans. We then demonstrate that Slit1 KD in mouse vmPFC promotes behavioral susceptibility to stress, leads to sex-specific alterations in dendritic morphology and excitability of vmPFC pyramidal neurons, and is linked to genome-wide changes in gene expression by RNA-sequencing (RNA-seq). Overall, these investigations highlight a novel contribution of SLIT1 to stress responses and provide further insight into understanding the signaling pathways and molecular factors that contribute to sex differences in stress susceptibility and MDD.
Methods
See supplemental information for detailed Methods.
Human brain postmortem tissue
Human postmortem brain tissue and RNA-seq data from MDD and control subjects were collected, analyzed, and reported as part of a published study (29).
Animals
C57BL/6J female or male mice were used for all experiments.
Electrophysiology
Recordings were performed on vmPFC GFP-positive cells the day after miR-LacZ or miR-Slit1 infection. mEPSC peak amplitudes and interevent intervals were collected.
Dendritic morphology
Brains were collected four days after miR-LacZ or miR-Slit1 infection. GFP-positive pyramidal cells within layer II-III of the vmPFC were stained and manually traced.
RNA sequencing and data analysis
Using 7 individual female mice per group, library preparation and RNA-seq were performed according to previously described methods (30).
Statistical analysis
GraphPad Prism 8.0 software package was used for analyzing the data.
Results
Female-specific downregulation of Slit1 in vmPFC of MDD patients and chronically stressed mice
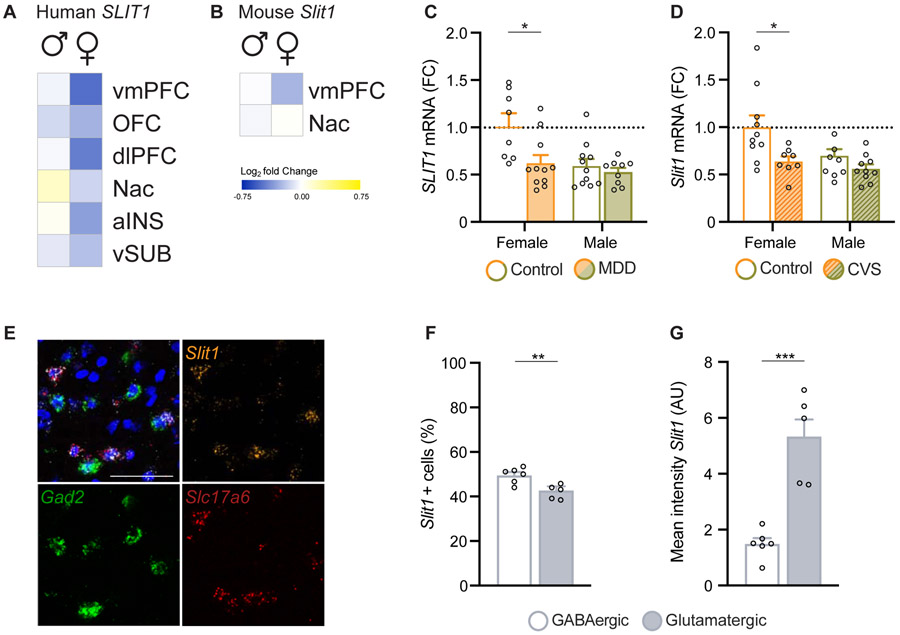
To identify sex-specific gene targets in the SLIT/ROBO pathway regulated in depression, we leveraged RNA-seq datasets previously generated by our laboratory from human depressed subjects and chronically stressed mice. The human dataset includes the transcriptomes of postmortem ventral medial prefrontal cortex (vmPFC), dorsolateral PFC (dlPFC), orbitofrontal cortex (OFC), nucleus accumbens (NAc), anterior insula (aINS), and ventral subiculum (vSUB) of male and female depressed and control human subjects (29). From this dataset, we identified a female-specific downregulation of Slit1 mRNA in vmPFC of depressed subjects compared to controls, with no change observed in any other brain region (Fig. 1A). Notably, in control subjects there was a trend for higher levels of Slit1 mRNA in vmPFC of females compared to males (Suppl. Fig. 1A). No other Slit or Robo family member displayed significantly altered expression in vmPFC of depressed females or males, and minimal regulation of these genes was apparent in the other brain regions studied (Suppl. Fig. 1B).
Figure 1. Neuronal Slit1 is downregulated in the vmPFC of depressed human females and chronically stressed female mice.
(A) Heatmap representing RNA-seq results indicating Slit1 downregulation in the vmPFC of depressed females, but not males, compared to control. vmPFC, ventromedial prefrontal cortex; dlPFC, dorsolateral PFC; OFC, orbitofrontal cortex; NAc, nucleus accumbens; aINS, anterior insula; and vSUB, ventral subiculum. (B) Heatmap representing RNA-seq results indicating Slit1 mRNA downregulation in the vmPFC of female, but not male, mice after chronic variable stress (CVS). (C) Bar graph showing qPCR validation of Slit1 mRNA downregulation in vmPFC of depressed females only (n=8-11/group). (D) Bar graph showing qPCR validation of Slit1 mRNA downregulation after CVS in female mice only (n=8-10/group). (E) Representative vmPFC images from FISH for Slit1 (orange), Gad2 (GABAergic marker, green) and Scl17a6 (glutamatergic marker, red) mRNA (scale bars, 50 μm). (F) Quantification of FISH images indicates that Slit1 is expressed in both glutamatergic and GABAergic neurons with slightly higher co-localization in Gad2 expressing cells (n=5-6 animals). (G) Quantification of Slit1 mRNA shows markedly higher expression levels per cell in glutamatergic neurons compared to GABAergic neurons (n=5-6 animals). *p<0.05, **p<0.01, ***p<0.001. Data are shown as mean ± s.e.m.
We next examined whether this sex-specific regulation of Slit1 is also observed in mice subjected to chronic stress. To that end, we used a previously generated dataset that employed a 21-day chronic variable stress (CVS) procedure, a model well-known to induce equivalent degrees of anxiety- and depression-like behaviors in both sexes of mice as well as to mimic a subset of transcriptional abnormalities associated with human depression (29). Similar to the human findings, we identified a downregulation of Slit1 mRNA in vmPFC of female mice exposed to CVS compared to controls, with no change seen in the NAc (Fig. 1B). There was no change in other Slit or Robo family members in either brain region (Suppl. Fig. 1C).
We confirmed these sex-specific gene expression findings by performing qPCR on the same human and mouse tissue samples used to generate the prior RNA-seq datasets (Fig. 1C,D). Slit1 mRNA levels were significantly lower in vmPFC of female MDD patients (Two-way ANOVA, Fgender(1,35)=9.7, p=0.0036; Fdisease(1,35) =7.6, p=0.0088) vs. female controls (post hoc Tukey test: p=0.0112). No differences were observed between male MDD and control patients. Similarly, female mice subjected to CVS displayed significantly lower levels of Slit1 mRNA in vmPFC (Two-way ANOVA, Fgender(1,34)=5.4, p=0.0256; Fdisease(1,34)=9.3, p=0.0044) compared to unstressed females (post hoc Tukey test: p=0.0227), whereas male animals exhibited no difference in Slit1 gene expression following chronic stress. These analyses confirm that stress suppresses Slit1 expression in a sex-specific manner.
To characterize the cell-type enrichment of Slit1, we explored existing datasets of cell type-specific gene expression (31,32). We identified neuronal cells as the principal cell type that exhibits high expression levels of Slit1 mRNA in the adult human (Suppl. Fig. 2A) and mouse (Suppl. Fig. 2B) brain. We next used RNAscope® fluorescent in-situ hybridization (FISH) on adult vmPFC tissue of mice to examine whether Slit1 is expressed predominantly in glutamatergic or GABAergic neurons (Fig. 1E). Slit1 mRNA was co-expressed in cells positive for either the GABAergic marker glutamic acid decarboxylase 2 (Gad2) or the glutamatergic marker vesicular glutamate transporter 2 (Slc17a6), with a slightly higher percentage of Gad2 positive neurons expressing Slit1 (t(9)=3.613, p=0.0056) (Fig. 1F). This finding is in line with reports from humans, showing equivalent expression of Slit1 in glutamatergic and GABAergic neurons in OFC (Suppl. Fig. 2C) (33). However, we found that glutamatergic neurons expressed strikingly higher levels of Slit1 compared to GABAergic neurons (t(9)=5.628, p=0.0003) (Fig. 1G). This cell-type specific difference in expression is in line with a published single cell RNA-seq study from mouse somatosensory cortex, showing strong enrichment of Slit1 in glutamatergic neurons (Suppl. Fig. 2D) (34).
Slit1 knockdown (KD) in vmPFC promotes sex-specific susceptibility to stress
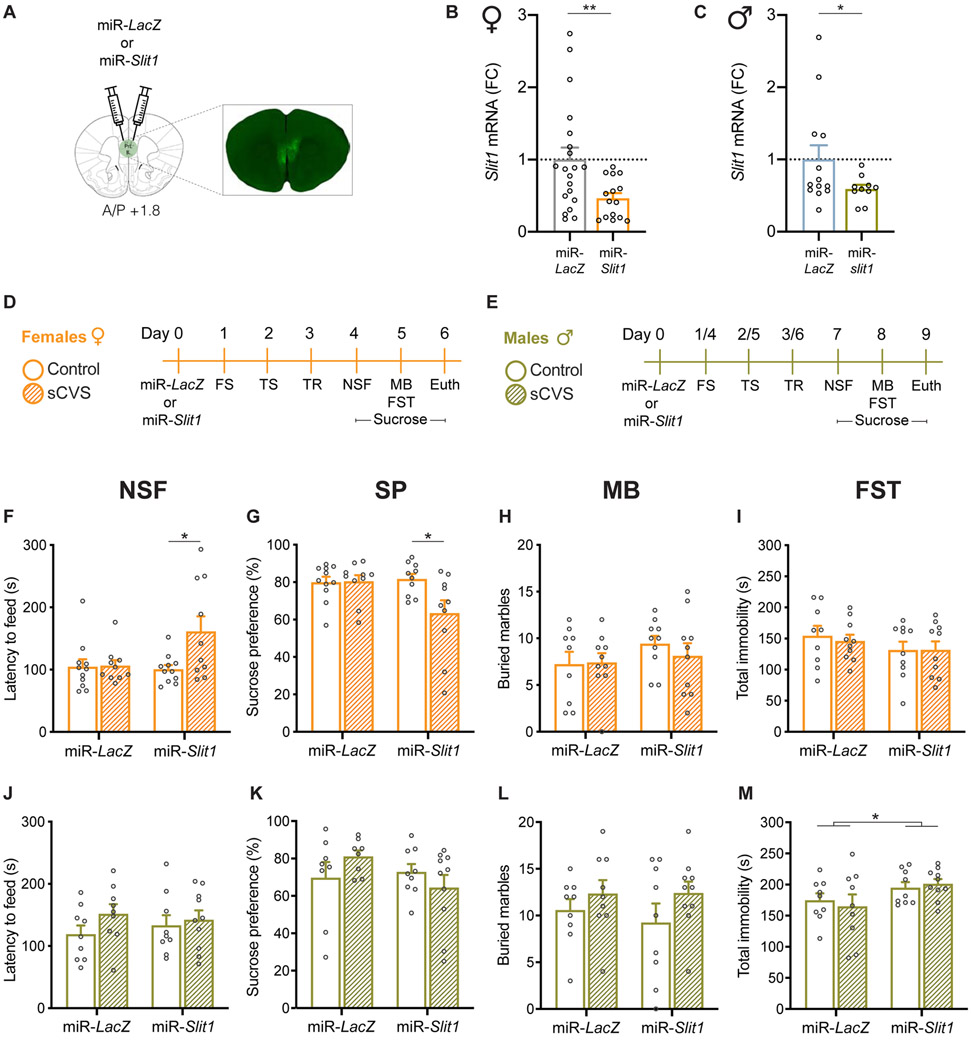
To test the causal, sex-specific role of Slit1 in depression- and anxiety-related behavioral abnormalities, we generated HSV vectors, which infect neurons selectively, expressing an artificial miRNA designed to target Slit1 (miR-Slit1) or LacZ as a control (miR-LacZ). Infusion of miR-Slit1 into vmPFC (Fig. 2A) leads to effective KD of Slit1 in this region of female (t(33)=2.651, p=0.0061) (Fig. 2B) and male (t(21)=1.782, p=0.0446) (Fig. 2C) mice. We then combined Slit1 KD with a subthreshold (sCVS) procedure to test its ability to induce sex-specific anxiety- and depression-like behaviors. Whereas sCVS by itself is insufficient to induce stress-related behavioral abnormalities in normal animals, it can reveal altered stress susceptibility when combined with viral-mediated manipulation of specific genes (35). Due to divergent stress susceptibility at baseline, female mice were subjected to 3 days of sCVS (Fig. 2D) and male mice to 6 days of sCVS (Fig. 2E), followed by behavioral testing (35). When followed by sCVS, Slit1 KD in female vmPFC increased latency to feed in the novelty suppressed feeding (NSF) test (Fig. 2F) (Two-way ANOVA, Finteraction(1,38)=4.110, p=0.0497; Fstress(1,38)=4.640, p=0.0376) compared to miR-LacZ controls (post hoc Tukey test: p=0.0415), miR-Slit1 non-stressed mice (post hoc Tukey test: p=0.0262), and miR-LacZ sCVS mice (trend, post hoc Tukey test: p=0.0589). We found no differences in latency to feed in the home cage as part of the post-test (Suppl. Fig. 3A), suggesting that the effects observed in NSF are not due to alternation in appetite between the groups. Consistent with these results, miR-Slit1 sCVS females exhibited decreased sucrose preference (SP) compared to all of the other groups (Two-way ANOVA, Finteraction(1,37)=4.781, p=0.0352; Fstress(1,37)=4.324, p=0.0446; post hoc Tukey test: miR-Slit1 sCVS vs. miR-LacZ control, p=0.0429; miR-Slit1 sCVS vs. miR-Slit1 control, p=0.0418; miR-Slit1 sCVS vs. miR-Slit1 control, p=0.0249) (Fig. 2G). We observed no differences between the groups in marble burying (MB) or the forced swim test (FST) (Fig. 2H,I). By contrast, the same manipulation in combination with 6 days of sCVS did not promote susceptibility to stress in male mice in any of these tests (figure 2J-L). Male mice in the different groups had similar latencies to feed during the NSF post-test as well (Suppl. Fig. 3B). Notably, we observed increased immobility time in the FST in male mice treated with miR-Slit1 regardless of stress exposure (Fvirus(1,33)=5.1, p=0.0297) (Fig. 2M). Together, these findings suggest that Slit1 KD in vmPFC promotes abnormal stress responses in a sex-specific manner that mimics the human phenomena.
Figure 2. Slit1 KD in vmPFC neurons controls a sex-specific anxiety- and depression-like phenotype in mice.
(A) Schematic representation of viral delivery to KD Slit1 in mouse vmPFC. (B, C) Quantification of HSV-mediated knockdown of Slit1 in the vmPFC validates significant downregulation in female (B) and male (C) mice (n=10-20). (D, E) Schematic representation of the experimental design in female (D) or male mice (E) including surgery, exposure to subthreshold chronic variable stress (sCVS), and behavioral tests used to assess the impact of Slit1 KD compared to controls. (F, J) Slit1 KD in the female vmPFC followed by sCVS increased latency to feed in the novelty suppressed feeding (NSF) test compared to their unstressed counterparts as well as unstressed controls (F), but had no effect in males (J). (G, K) miR-Slit1 sCVS females consumed less sucrose as measured in the sucrose preference (SP) test compared to all the other groups (G), with no effects observed in males (K). (H, L) No differences in the marble burying (MB) test in both females (H) and males (L). (I, M) No differences in the forced swim test (FST) in females (I), but increased immobility time in miR-Slit1 males compared to controls. (M). *p<0.05. Data are shown as means ± s.e.m.
Slit1 KD decreases dendritic arbor length and branching and alters neurophysiological properties of vmPFC neurons of female mice
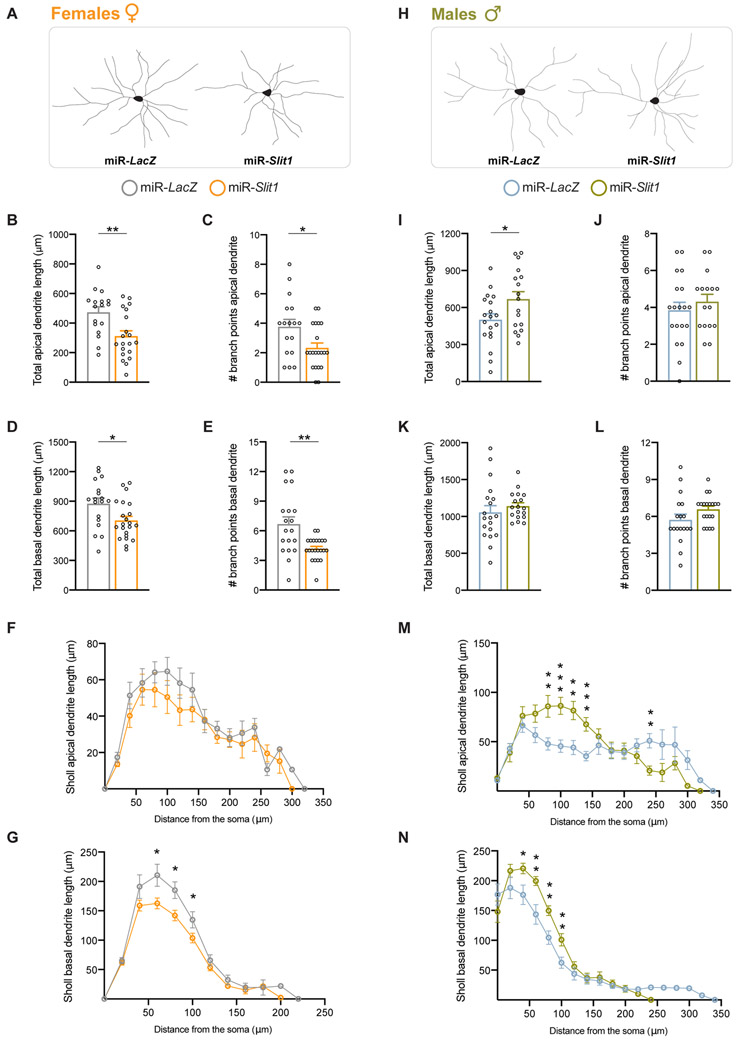
We next explored the potential mechanisms linked to the behavioral effects of Slit1 KD. SLIT1 has been reported to alter dendritic morphology in embryonic cortical mouse neurons in vitro (36), but there are no reports of its influence in the adult brain. To that end, we performed immunohistochemistry on miR-Slit1 or miR-LacZ infected vmPFC tissue of female adult mice. We focused our attention on pyramidal neurons from layer II/III of vmPFC, as past studies have identified these cells to be critical for modulating stress responses in mice (13,37). Figure 3A shows representative reconstructed pyramidal neurons for each group. Following miR-Slit1 infection, pyramidal neurons displayed a robust decrease in dendritic size and branching with no change in the soma surface area compared to miR-LacZ controls (Suppl. Fig. 4A). The total apical dendritic arbor length (t(35)=3.135, p=0.0035) (Fig. 3B) and branching (t(36)=2.490, p=0.0175) (Fig. 3C) were decreased by Slit1 KD. Similarly, Slit1 KD decreased the total basal dendritic arbor length (t(38)=2.486, p=0.0175) (Fig. 3D), branching (t(39)=3.499, p=0.0012) (Fig. 3E), and average basal dendritic arbor length (t(39)=3.896, p=0.0004) (Suppl. Fig. 4B). Scholl analysis found no difference between the two groups for apical dendritic length (Fig. 3F) or branching (Suppl. Fig. 4C), but revealed a significant reduction in the dendritic length between 60-100μm from the cell body (Fig. 3G) and the number of interactions in basal arbors (Suppl. Fig. 4D), indicating that dendritic restructuring induced by miR-Slit1 was confined to proximal basal dendritic branches.
Figure 3. Slit1 KD affects dendritic arbor length and branching in vmPFC pyramidal neurons of in a sex-specific manner.
(A) Representative tracings of pyramidal neurons in the vmPFC of female mice after miR-LacZ (left) or miR-Slit1 infection (right). (B, C) Total apical dendritic arbor length (B) and branching (C) were decreased after miR-Slit1 infection when compared to miR-LacZ controls. (D, E) Slit1 KD decreased the total basal dendritic arbor length (D) and number of branches (E) compared to controls (n=82-83 dendrites/group). (F, G) Scholl analysis revealed no change in length of apical dendrites in vmPFC pyramidal neurons infected with miR-Slit1 compared to miR-LacZ (F), but a decrease in the length (G) of basal dendrites mainly 60-100 μm from the soma. (H) Representative tracings of pyramidal neurons in the vmPFC of male mice after miR-LacZ (left) or miR-Slit1 infection (right). (I) Total apical dendritic arbor was increased after miR-Slit1 infection when compared to miR-LacZ controls. (J-L) No change was observed in apical branching (J), the total basal dendritic arbor length (K) or number of branches (L) compared to controls (n=85-91 dendrites/group). (M, N) Scholl analysis revealed an increase in the length of apical (M) and basal (N) dendrites mainly 80-140 μm from the soma. Per group: n=5 animals, 19-22 neurons. *p<0.05, **p<0.01, ***p<0.001. Data are shown as means ± s.e.m.
We repeated the same experiment in male mice. Figure 3H shows representative reconstructed pyramidal neurons for each group. Following miR-Slit1 infection, pyramidal neurons displayed no change in the soma surface area compared to miR-LacZ controls (Suppl. Fig. 4E). Slit1 KD increased the total apical dendritic arbor length in males (t(34)=2.185, p=0.0351) (Fig. 3I). No change was seen in the total apical dendritic branching (Fig. 3J), total basal dendritic arbor length (Fig. 3K) and branching (Fig. 3L), or in the average basal dendritic length (Suppl. Fig. 4F). However, Scholl analysis revealed a significant increase in the apical dendritic length of the male miR-Slit1 group compared to controls between 60-140μm from the soma (Fig. 3M) and the number of interactions (Suppl. Fig. 4G). In addition, between 40-100μm away from the cell body, we observed a significant increase in the basal dendritic length (Fig. 3N) and the number of interactions (Suppl. Fig. 4H) in the miR-Slit1 group compared to the miR-LacZ mice. Together, these results show that Slit1 KD leads to reduced dendritic length and branching in females, while increasing apical dendritic length in males, which confirms a sex-specific role for Slit1 in dendritic remodeling in the rodent adult brain.
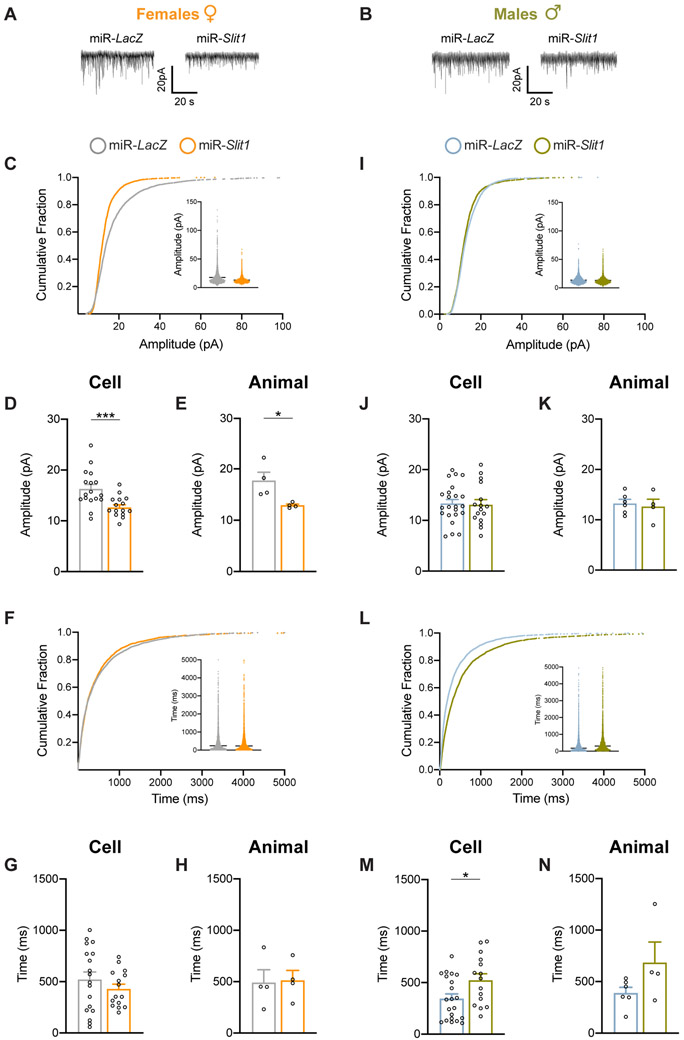
We followed up on these morphological findings by assessing the impact of Slit1 KD on the electrophysiological properties of pyramidal neurons in vmPFC of female and male mice. We show representative mEPSC traces of vmPFC neurons after miR-Slit1 or miR-LacZ infection (Fig. 4A,B). In females, miR-Slit1 reduced the amplitude of mEPSCs compared to miR-LacZ controls (Fig. 4C) per cell (Mann-Whitney U=42, n1=17, n2=15, p=0.0008) (Fig. 4D) and per animal (Mann-Whitney U=0, n1=n2=4, p=0.0286) (Fig. 4E), with no change observed in interevent interval (Fig. 4F-H). In contrast, we found no differences for male mEPSC amplitude (Fig. 4I-K), and increased interevent interval following Slit1 KD (Fig. 4L) analyzed per cell (Mann-Whitney U=86, n1=21, n2=15, p=0.0213) (Fig. 4M), although this effect did not hold up when analyzed per animal (Fig. 4N). Together, these results demonstrate that Slit1 KD alters synaptic function of adult vmPFC pyramidal neurons in a sex-specific fashion, which may contribute to aberrant behavioral responses to stress.
Figure 4. Slit1 KD modulates mEPSC amplitude in vmPFC pyramidal neurons of female mice.
(A, B) Representative membrane potential of pyramidal neurons in the vmPFC of female (A) or male (B) mice after Slit1 or LacZ KD. (C) Cumulative curves and scatter plots for mEPSC amplitude of female mice. (D, E) Bar graphs indicate that Slit1 KD decreased the amplitude of mEPSCs compared to miR-LacZ on a “per cell” (n=15-17 cells/group) (D) or “per animal” (n=4 animals/group) (E) basis. (F) Cumulative curves and scatter plots for mEPSC interevent interval of female mice. (G, H) Slit1 KD in females had no effect on mEPSC interevent interval per cell (G) or per animal (H). (I) Cumulative curves and scatter plots for mEPSC amplitude of male mice. (J, K) Slit1 KD in males had no effect on mEPSC amplitude per cell (J) or per animal (K). (L) Cumulative curves and scatter plots for mEPSC interevent interval of male mice. (M, N) Bar graphs indicate that Slit1 KD increased mEPSC interevent interval compared to miR-LacZ per cell (n=15-21 cells/group) (M), but not per animal (n=4-6 animals/group) (N). ***p<0.001.
Slit1 KD enhances stress-induced transcriptional activation
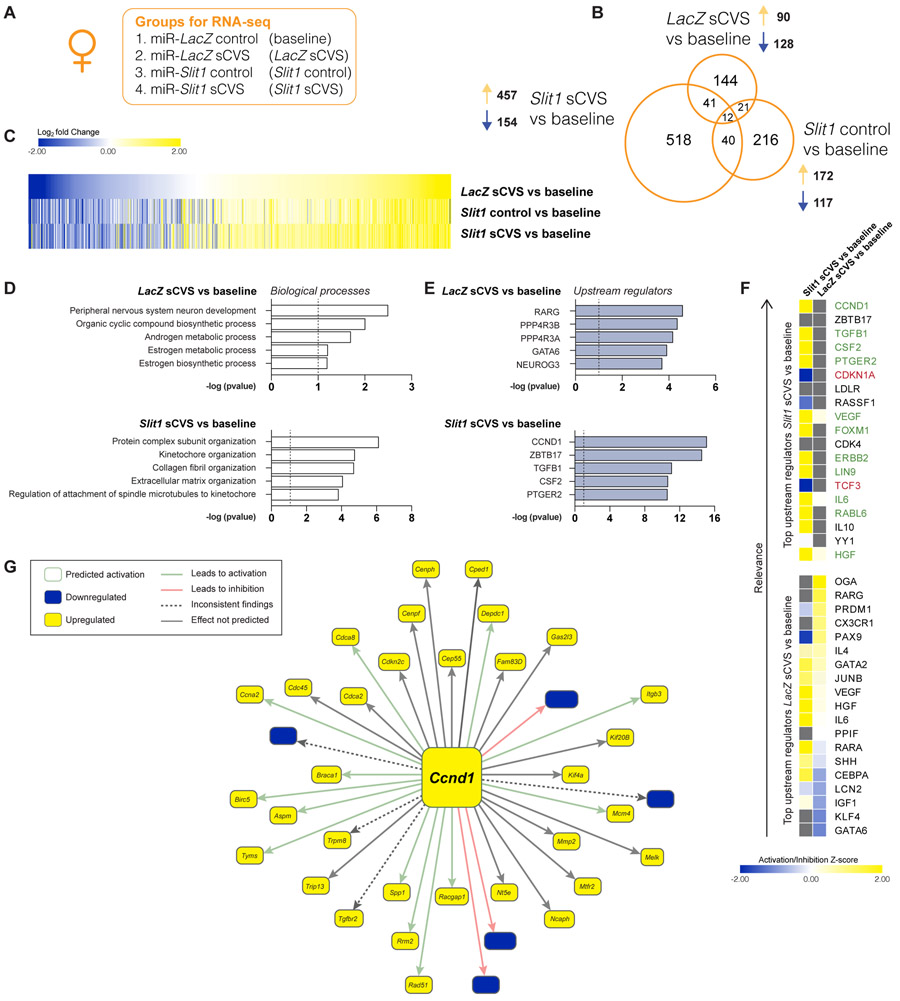
Next, we used RNA-seq to explore the molecular mechanisms by which Slit1 controls neuronal morphology, physiology, and stress-related behavior. We surveyed genome-wide transcriptional changes in female mouse vmPFC tissue after miR-Slit1 or miR-LacZ infection at baseline or after sCVS (Fig. 5A). Analysis of differentially expressed genes (DEGs), using cutoff: p<0.05 and fold-change>30%, showed that Slit1 KD in vmPFC neurons alone increased the expression of 172 genes and reduced expression levels of 117 genes (Fig. 5B). In combination with sCVS, Slit1 KD increased the expression of several-fold more genes with only modest increases in the number of downregulated transcripts. The union heatmaps presented in Figure 5C confirms an enhanced transcriptional upregulation in response to sCVS in the Slit1 KD condition compared to LacZ controls. Gene ontology (GO) analysis revealed that the DEGs in the miR-LacZ sCVS group were enriched for biological processes related to hormonal responses, such as estrogen biosynthesis and androgen metabolism, while the miR-Slit1 sCVS group terms were directly relating to known functions of SLIT1, including extracellular matrix organization (38,39) and collagen fibril organization (40) (Fig. 5D). Notably, miR-Slit1 followed by subthreshold stress alters a large subset of genes related to different aspects of synaptic functioning (Suppl. Table 3).
Figure 5. RNA-seq reveals transcriptional alterations in the vmPFC of female mice after Slit1 KD that predict Ccnd1 as an upstream regulator.
(A) List of experimental groups (n=7/group). (B) Venn diagrams showing the overlap in the list of differentially expressed genes (DEGs) between the groups (DEG cutoff: fold change >30% and p<0.05). (C) Union heatmap comparing DEG patterns between the LacZ sCVS vs. baseline (top lane), Slit1 control vs. baseline (middle lane), or Slit1 sCVS vs. baseline (bottom lane) groups. Slit KD augmented the effects of sCVS predominantly on upregulated genes. (D) Top enriched GO ontology terms from analysis of the DEGs following LacZ or Slit1 KD and sCVS in female vmPFC compared to baseline. (E) Top predicted upstream regulators of DEGs regulated by sCVS after LacZ or Slit1 KD compared to baseline. (F) Top 20 upstream regulators and their activation or inhibition Z-score for Slit1 sCVS (left panel) or LacZ sCVS (right panel) vs. baseline (green letters = activation; red= inhibition; grey letters = non). (G) Illustration of the gene network identified within the DEGs of the Slit1 sCVS vs. baseline comparison, which is predicted to have Ccnd1 as the top upstream regulator.
We performed Ingenuity Pathway Analysis (IPA) to identify predicted transcriptional upstream drivers of the genes regulated by Slit1 manipulation (Suppl. Table 4). We identified Ccnd1, encoding the cell cycle regulatory protein cyclin D1 (41), as the highest-ranked upstream regulator for the miR-Slit1 sCVS vs. baseline DEG list (Fig. 5E). In contrast, Ccnd1 was not a predicted regulator of the DEGs for the miR-LacZ sCVS vs. baseline comparison. Our IPA analysis also predicted that Ccnd1 is upregulated in the miR-Slit1 sCVS condition (Fig. 5F). Indeed, we found that Ccnd1 mRNA levels are significantly upregulated in our miR-Slit1 sCVS vs. baseline RNA-seq data. Additionally, of the genes predicted to be regulated by Ccnd1, 38 are differentially expressed within our miR-Slit1 sCVS vs. baseline comparison (Fig. 5G). Interestingly, however, Ccnd1 expression is not altered in vmPFC in human depression or mouse CVS (data not shown). Together, these results suggest that Slit1 KD alters behavioral and neurophysiological responses to stress potentially through modulation of CCND1 signaling pathways.
Discussion
Our current understanding of the molecular mechanisms leading to an increased depression risk in women is limited. Previously, our laboratory reported that the molecular abnormalities associated with MDD are strikingly divergent between males and females, with limited overlap of transcriptome changes in the two sexes (29), a finding replicated by another group in an independent cohort (42). The present study identified Slit1 as a sex-specific target that is linked to female MDD risk. We show that this neuronal-enriched gene is downregulated in vmPFC of female, but not male, MDD patients and, likewise, is lowered in vmPFC of female and not male mice after CVS. This pattern can be interpreted as masculinization of Slit1 expression by stress and depression given the higher basal levels of Slit1 seen in normal females. We go on to demonstrate that viral-mediated Slit1 KD in vmPFC neurons promotes behavioral sensitivity to stress in a sex-specific manner: females display increased depressive- and anxiety-like behaviors in the NSF and SP tests. In contrast, male mice did not show behavioral differences in these tests upon Slit1 KD, but exhibited increased immobility in the FST. This result might relate to sex differences in depression symptoms (5), in which women report higher levels of comorbid anxiety compared to males (7). We demonstrate further in female mice that Slit1 KD alters synaptic physiology and leads to shrinking of dendritic trees of vmPFC pyramidal neurons, as well as augments the effects of stress on gene expression on a genome-wide scale. Together, this work identifies Slit1 as a novel target in MDD and provides insight into signaling pathways and molecular factors contributing to sex differences in depression and stress susceptibility. As well, this study is another example that, despite the highly heterogeneous and complex nature of MDD, manipulating a single target gene in mouse mPFC can alter behavioral responses to stress in a sex-specific fashion (30,35).
SLIT1 has been found to increase the growth and branching of mouse cortical dendrites in vitro (36). As dendritic branching and synaptic plasticity are critical for learned responses to stress and other environmental factors (43), we investigated whether Slit1 manipulation affects the dendritic structure of vmPFC pyramidal neurons. Our morphological data show that KD of Slit1 decreases arbor length and branching of the apical and basal dendrites of these neurons in female mice. In contrast, we found increased apical arbor length in male mice. These results thereby confirmed a sex-specific role for Slit1 in regulating dendritic morphology in the adult mouse brain. The apical and basal dendrites of pyramidal neurons in PFC occupy distinct cortical layers, and receive information from axons originating from distinct cortical brain regions (44). Whereas the apical tuft is thought to integrate feedback information, the basal dendrites receive feedforward sensory input (45), which allows the pyramidal neurons to integrate separate sets of inputs independently. Maintaining the right balance is essential for the proper relay of cortical information processing. The loss of branching and elongation at the level of dendrites may lead to inadequate responses to environmental stimuli, which could affect neuronal plasticity and leave female mice more susceptible to stress (10).
Previous studies, predominantly conducted in male rodents, have shown that chronic stressors were capable of inducing atrophy of apical and basal dendrites in layer II-III mPFC pyramidal neurons (12,46,47). However, a recent study demonstrated that repeated stress decreased branching of layer II/III apical dendrites in vmPFC of both male and female rats (48). Other studies have shown sex-specific results following stress. For example, Garrett et al. showed that chronic stress decreased apical dendritic branch number and length of male rat pyramidal neurons in PFC, whereas the same stressors increased apical dendritic length in female rats (16). Similarly, social instability stress during adolescence reduced apical length in female rats, while reducing basilar length in males (49). These findings along with ours highlight the need for future studies systematically testing the effects of different types of stressors during different developmental windows on dendritic morphology, as well as spine volume and number, in both sexes.
Dysregulation of synaptic plasticity in mPFC has been implicated as a sex-specific process in MDD (50). Our data show that glutamatergic neurons expressed many-fold higher levels of Slit1 compared to GABAergic neurons, and that Slit1 KD decreases the branching of pyramidal cell dendrites in vmPFC of female mice. We therefore tested whether KD of Slit1 also alters the excitability of these cells. Our analysis of postsynaptic currents revealed that Slit1 KD significantly lowered the amplitude of mEPSCs, which suggests postsynaptic changes. Similarly, inhibition of miR-214-3p in mPFC reversed depressive-like behavior in male mice that underwent chronic social defeat, a finding that was accompanied by an increase in mEPSC amplitude and number of dendritic spines (51). The synaptic changes caused by Slit1 KD are consistent with the changes we observed in dendrite morphology. The synaptic changes might be related to the wide range of synaptic proteins whose mRNA expression levels are altered upon Slit1 KD in vmPFC in our RNA-seq dataset. Additionally, given that Slit1 is expressed in GABAergic neurons as well, future studies should explore the effects of Slit1 KD on inhibitory postsynaptic currents and excitatory/inhibitory balance.
To examine the underlying molecular contributions to the observed changes in vmPFC morphology and function, we performed RNA-seq after Slit1 KD. This analysis revealed that Slit1 KD augments expression of genes that are induced by sCVS. Further, reduction of Slit1 regulates DEGs that relate directly to the functioning of the SLIT/ROBO pathway, such as extracellular matrix organization (38,39) and collagen fibril organization (40). Our findings implicate Ccnd1, encoding cyclin D1 (41), as a major driver of the transcriptional changes following Slit1 KD in female vmPFC. Cyclin D1, which is expressed in the cortex of the adult brain (52), belongs to the conserved cyclin-dependent kinase (CDK) family which drives transitions through cell cycles and cell proliferation (53). In line with our findings, CCND1 has been shown to regulate neuronal morphology and the organization of dendritic trees during development (54). Additionally, CCND1 is upregulated in the amygdala following stress (55). SLIT/ROBO are suggested to influence members of the RHO GTPase family, such as RHOA, which are key regulators of the actin cytoskeletal and neuronal morphology (56). Interestingly, there is evidence that CCND1 acts as an effector of RHOA (57,58). In our miR-Slit1 sCVS vs. baseline comparison, RHOA was also identified as a predicted upstream regulator of Ccnd1. While Ccnd1 was not altered in our human MDD or mouse CVS data sets (29), it was upregulated following a subthreshold stress paradigm in combination with Slit1 KD. This suggests that Ccnd1 may play a molecular role in the initial acute stress phase in females. Together, we hypothesize that the CCND1 pathway activated by RHOA as a result of Slit1 downregulation in the adult female vmPFC contributes to reduced dendritic branching as part of the molecular, morphological, and physiological changes leading to increased susceptibility to stress and depression. In this vein, it is interesting to note that, while SLIT1 is expressed predominantly in neurons, CCND1 is expressed predominantly in non-neuronal cells, which highlights bidirectional, inter-cellular communication as a key pathophysiological mechanism for stress-related disorders.
To conclude, this study implicates a role for SLIT1 in the adult brain and in MDD, and provides new insight into the growing field that recognizes the potential of axon guidance molecules as targets for adult neurological and psychiatric disorders such as depression. Further experiments are needed to extend our work to investigate the mechanisms that underlie the sex-specific effects observed in our study. Nevertheless, our findings shed light on a novel signaling pathway and its molecular constituents for the development of innovative treatments for MDD.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| BLOCK-iT Pol II miR RNAi kit | Invitrogen | K493500 | ||
| RNAscope® Multiplex Fluorescent v2 Assay | Advanced Cell Diagnostics | 323110 | ||
| mm-Slit1 | Mus musculus | Advanced Cell Diagnostics | 502491 | Channel 1 Probe |
| mm-glutamic acid decarboxylase 2 | Mus musculus | Advanced Cell Diagnostics | 415071-C2 | Channel 2 Probe |
| mm-vesicular glutamate transporter 2 | Mus musculus | Advanced Cell Diagnostics | 319171-C3 | Channel 3 Probe |
| Alexa 488 Donkey anti-Chicken | Jackson ImmunoResearch | 703546155 | Secondary antibody IHC | |
| ProLong™ Diamond Antifade Mountant | Thermo fisher scientific | P36961 | ||
| RNeasy Micro Kit (50) | Qiagen | 74004 | ||
| iScript™ cDNA Synthesis Kit | Biorad | 1708891 | ||
| TruSeq RNA CD Index Plate (96 Indexes, 96 samples) | illumina | 20019792 | ||
| TruSeq Stranded Total RNA Library Prep Gold (48 samples) | illumina | 20020598 | ||
| Agencourt AMPure XP, 60 mL | Beckman Coulter | A63881 | ||
| Agencourt RNAClean XP | Beckman Coulter | A63987 | ||
| RNase-Free DNase Set (50) | Qiagen | 79254 | ||
| qPCR primers | IDT | Custom |
Acknowledgments
This work was funded by R01MH051399 and P50MH096890 and by the Hope for Depression Research Foundation (HDRF). We wish to thank Robert Blitzer and Amanda Fakira from Mount Sinai’s electrophysiology core facility; Bridget Wicinski, Merina Varghese, and Patrick Hof for their training in dendritic morphology analysis; John Kutlu and James Simpson for their graphical training; and Hilde Schot for her never ending support.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fava M, Kendler KS. Major Depression Disorder Neuron. 2000;28(2):335–41. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC. Epidemiology of women and depression. Journal of Affective Disorders. 2003;74(1):5–13. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehner C Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. . Acta Psychiatr Scand. 2003;108:163–74. [DOI] [PubMed] [Google Scholar]
- 5.Sramek JJ, Murphy MF, Cutler NR. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci. 2016;18(4):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141–50. [DOI] [PubMed] [Google Scholar]
- 7.Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry. 2008;49(3):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Issler O, Nestler EJ. The molecular basis for sex differences in depression susceptibility. Current Opinion in Behavioral Sciences 2018. p. 1–6. [Google Scholar]
- 9.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18(9):1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare BD, Duman RS. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15(11):1714–22. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26(21):5733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Yi JH, Choi K, Hong S, Shin KS, Kang SJ. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neurosci. 2014;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chédotal A, Richards LJ. Wiring the brain: the biology of neuronal guidance. Cold Spring Harb Perspect Biol. 2010;2(6):a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Battum EY, Brignani S, Pasterkamp RJ. Axon guidance proteins in neurological disorders. Lancet Neurol. 2015;14(5):532–46. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Berrío A, Lopez JP, Bagot RC, Nouel D, Dal Bo G, Cuesta S, et al. DCC Confers Susceptibility to Depression-like Behaviors in Humans and Mice and Is Regulated by miR-218. Biol Psychiatry. 2017;81(4):306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Berrío A, Hernandez G, Nestler EJ, Flores C. The Netrin-1/DCC Guidance Cue Pathway as a Molecular Target in Depression: Translational Evidence. Biol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96(6):795–806. [DOI] [PubMed] [Google Scholar]
- 22.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96(6):785–94. [DOI] [PubMed] [Google Scholar]
- 23.Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96(6):807–18. [DOI] [PubMed] [Google Scholar]
- 24.Blockus H, Chédotal A. Slit-Robo signaling. Development. 2016;143(17):3037–44. [DOI] [PubMed] [Google Scholar]
- 25.Marin O, Plump AS, Flames N, Sanchez-Camacho C, Tessier-Lavigne M, Rubenstein JL. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130(9):1889–901. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24(6):1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H. Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell. 2011;146(1): 164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S-H, Chen W-C, Lu K-H, Chen P-J, Hsieh S-C, Pan T-M, et al. Down-regulation of Slit–Robo Pathway Mediating Neuronal Cytoskeletal Remodeling Processes Facilitates the Antidepressive-like Activity of Gastrodia elata Blume. J Agric Food Chem. 2014;62(43):10493–503. [DOI] [PubMed] [Google Scholar]
- 29.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23(9):1102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, et al. Sex-Specific Role for the Long Non-coding RNA LINC00473 in Depression. Neuron. 2020;106(6):912–26.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlenkov A, Wang M, Roussos P, Rudchenko S, Barbu M, Bibikova M, et al. Substantial DNA methylation differences between two major neuronal subtypes in human brain. Nucleic Acids Res. 2016;44(6):2593–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138–42. [DOI] [PubMed] [Google Scholar]
- 35.Hodes GE. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to sub-chronic variable stress. Journal of Neuroscience. 2015;35(50):16362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, et al. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33(1):47–61. [DOI] [PubMed] [Google Scholar]
- 37.Shrestha P, Mousa A, Heintz N. Layer 2/3 pyramidal cells in the medial prefrontal cortex moderate stress induced depressive behaviors. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4(12A):2169–87. [DOI] [PubMed] [Google Scholar]
- 39.Bhat KM. Post-guidance signaling by extracellular matrix-associated Slit/Slit-N maintains fasciculation and position of axon tracts in the nerve cord. PLoS Genet. 2017;13(11):e1007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, et al. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003;120(9):1059–70. [DOI] [PubMed] [Google Scholar]
- 41.Bartkova J, Lukas J, Müller H, Lützhøft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57(3):353–61. [DOI] [PubMed] [Google Scholar]
- 42.Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry. 2018;84(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spratling MW. Cortical region interactions and the functional role of apical dendrites. Behav Cogn Neurosci Rev. 2002;1(3):219–28. [DOI] [PubMed] [Google Scholar]
- 45.Larkum M A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2013;36(3):141–51. [DOI] [PubMed] [Google Scholar]
- 46.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. [DOI] [PubMed] [Google Scholar]
- 47.Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17(9):1998–2006. [DOI] [PubMed] [Google Scholar]
- 48.Urban KR, Geng E, Bhatnagar S, Valentino RJ. Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons. Neurobiol Stress. 2019;10:100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breach MR, Moench KM, Wellman CL. Social instability in adolescence differentially alters dendritic morphology in the medial prefrontal cortex and its response to stress in adult male and female rats. Dev Neurobiol. 2019;79(9-10):839–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22(5):535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng ZF, Zheng HL, Chen JG, Luo Y, Xu JF, Zhao G, et al. miR-214-3p Targets β-Catenin to Regulate Depressive-like Behaviors Induced by Chronic Social Defeat Stress in Mice. Cereb Cortex. 2019;29(4):1509–19. [DOI] [PubMed] [Google Scholar]
- 52.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 53.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl). 2016;94(12):1313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pogoriler J, Millen K, Utset M, Du W. Loss of cyclin D1 impairs cerebellar development and suppresses medulloblastoma formation. Development. 2006;133(19):3929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cong M, Wen L, Han F, Xu Y, Shi Y. Alterations in cyclin D1 and cyclin-dependent kinase 4 expression in the amygdalae of post-traumatic stress disorder rats. Mol Med Rep. 2017;16(6):8351–8. [DOI] [PubMed] [Google Scholar]
- 56.Blockus H, Chédotal A. The multifaceted roles of Slits and Robos in cortical circuits: from proliferation to axon guidance and neurological diseases. Curr Opin Neurobiol. 2014;27:82–8. [DOI] [PubMed] [Google Scholar]
- 57.Keely PJ. Rho GTPases as early markers for tumour progression. Lancet. 2001;358(9295):1744–5. [DOI] [PubMed] [Google Scholar]
- 58.Watts KL, Cottrell E, Hoban PR, Spiteri MA. RhoA signaling modulates cyclin D1 expression in human lung fibroblasts; implications for idiopathic pulmonary fibrosis. Respir Res. 2006;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.