Abstract
Impaired clearance of perivascular waste in the brain may play a critical role in morbidity after mild traumatic brain injury (mTBI). We aimed to determine the effect of mTBI on the burden of magnetic resonance imaging (MRI)-visible perivascular spaces (PVSs) in a cohort of U.S. military veterans and whether sleep modulates this effect. We also investigated the correlation between PVS burden and severity of persistent post-concussive symptoms. Fifty-six Iraq/Afghanistan veterans received 3 Tesla MRI as part of a prospective cohort study on military blast mTBI. White matter PVS burden (i.e., number and volume) was calculated using an established automated segmentation algorithm. Multi-variate regression was used to establish the association between mTBIs sustained in the military and PVS burden. Covariates included age, blood pressure, number of impact mTBIs outside the military, and blast exposures. Correlation coefficients were calculated between PVS burden and severity of persistent post-concussive symptoms. There was a significant positive relationship between the number of mTBIs sustained in the military and both PVS number and volume (p = 0.04). A significant interaction was found between mTBI and poor sleep on PVS volume (p = 0.04). A correlation was found between PVS number and volume, as well as severity of postconcussive symptoms (p = 0.03). Further analysis revealed a moderate correlation between PVS number and volume, as well as balance problems (p < 0.001). In Iraq/Afghanistan veterans, mTBI is associated with an increase in PVS burden. Further, an interaction exists between mTBI and poor sleep on PVS burden. Increased PVS burden, which may indicate waste clearance dysfunction, is associated with persistent post-concussive symptom severity.
Keywords: all sleep disorders, brain trauma, glymphatic, MRI, perivascular spaces
Introduction
Mild traumatic brain injury (mTBI) caused by a blast is the “signature injury” of the wars in Iraq and Afghanistan. It is estimated that 8–20% of military service members who have been deployed to Iraq or Afghanistan have sustained at least one blast mTBI.1 Moreover, a large proportion of them has been exposed to multiple blasts (as well as impact).2 The association between multiple mTBIs and severity of post-traumatic symptoms is well documented in the literature.3 However, the mechanisms underlying this relationship remain poorly understood.
Sleep disruption is a common consequence of mTBI.4 Approximately half of persons with mTBI suffer from some form of sleep disturbance, including insomnia, hypersomnia, and apnea.4–7 Recent studies have shown that post-traumatic sleep disturbances can impair the recovery process.8 Persons with post-traumatic sleep disruption report worse fatigue, anxiety, depression, headaches, cognitive deficits, and functional impairment.9,10 Therefore, sleep disturbances represent a unique modifiable treatment target that can potentially improve outcomes in subjects with mTBI. However, despite the available studies addressing the link between poor sleep and post-mTBI morbidity,11,12 the mechanisms underlying this association are yet to be defined.
The glymphatic pathway, a recently described brain-wide network of perivascular spaces (PVSs) that supports the clearance of interstitial solutes and wastes from the brain, is most active during sleep.13,14 In humans, high PVS burden is observed in conditions where glymphatic dysfunction is also observed, such as Alzheimer's disease, cerebral small vessel disease, cerebral amyloid angiopathy, and multiple sclerosis.15–18 In rodents, mTBI causes impairment of glymphatic function, evidenced by a decrease in perivascular cerebrospinal fluid (CSF) flow.19,20 Interactions between mTBI and poor sleep on glymphatic function in humans are still unknown. Elucidating such interactions could provide essential insights into the link between repeated mTBI, poor sleep, and post-mTBI impairment.
Increased PVS burden has been observed in subjects with TBI, particularly in those who reported poor sleep.21,22 However, the link between the number of mTBI, poor sleep, PVS burden, and persistent post-concussive symptoms in veterans has not been established.
The objective of this study was 2-fold: 1) determine the relationship between the number of mTBI, poor sleep, and PVS burden in a cohort of Iraq/Afghanistan military veterans after adjusting for clinically relevant covariates and 2) establish the relationship between PVS burden and persistent post-concussive symptoms in this cohort. To achieve our objectives, we utilized a validated and automated imaging segmentation tool, which allowed us to objectively quantify whole-brain PVS number, volume, morphology, and location.23,24
Methods
Study design
We performed an analysis of magnetic resonance imaging (MRI) data obtained as part of a study of outcomes in Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. The parent study was reviewed by the Institutional Review Board at the VA Puget Sound. All participants provided written informed consent before study enrollment and participation in study procedures. The analysis of deidentified data was approved by the Oregon Health & Science University Institutional Review Board.
Participants
Parent study
Participants were recruited from VA Puget Sound, as well as through outreach to veterans' organizations. All patients were male and fluent in English. Enrollment and follow-up took place between March 2011 and September 2019. Exclusion criteria for the parent study included: a neurological disorder (including moderate or severe traumatic brain injury), lifetime Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnoses of schizophrenia, other psychotic disorder, or bipolar disorder and a diagnosis of substance abuse or dependence within the past 3 months. MRI was obtained strictly for research purposes and not based on clinical need.
Current study
For inclusion criteria, OEF/OIF/OND veterans were included in the current study if they 1) had MRI data available of sufficient quality for PVS segmentation and 2) reported a history of mTBI (attributable to blast or any other cause). mTBI was defined as per the American Congress of Rehabilitation Medicine diagnostic criteria for mTBI (i.e., one or more of the following was present): 1) loss of consciousness (LOC) up to 30 min, 2) loss of memory for events surrounding the event for up to 24 h, or 3) any alteration in mental state.25
Variables
Clinical and demographic variables
These included age, race, ethnicity, and blood pressure.
Assessment of mild traumatic brain injury
Information regarding the lifetime history of both blast and impact mTBI was obtained as previously described.26,27 Briefly, a team of two physicians or physician assistants simultaneously completed semistructured interviews that yielded information regarding the number and characteristics of lifetime mTBIs and blast exposures. For each episode, participants were asked to report any loss/alteration of consciousness, post-traumatic amnesia, medical care received, and any change in duty status after the injury. For this analysis, mTBI exposure was stratified into three categories: 1) the number of mTBI with LOC sustained while in the military; 2) the number of mTBI with LOC sustained outside the military; and 3) number of blast exposures while in the military that met the definition of mTBI but were not associated with LOC.
Assessment of sleep
Sleep quality was assessed with the Pittsburg Sleep Quality Inventory (PSQI). The PSQI measures symptoms of sleep disturbances over 1 month. Responses are categorized into seven component categories. These categories are then combined to create one global score, with a range from 0–21. A global PSQI score >5 is usually used to qualify poor sleepers.28
Assessment of post-concussive symptoms
Presence of persistent post-concussive symptoms at the time of the interview was assessed with the Neurobehavioral Symptom Inventory (NSI). The NSI is a self-reported questionnaire commonly used to assess the presence and severity of post-concussive symptoms in veterans.29 It consists of 22 items, reflecting cognitive, affective, and somatic/vestibular symptoms. Items are graded on a 0–4 scale, with higher scores reflecting higher symptom severity.30
Magnetic resonance imaging acquisition and review
Three-dimensional T1-weighted magnetically prepared rapid acquisition gradient echo (repetition time [TR]/echo time [TE]/inversion time [TI] = 7.6/3.5/909 ms, imaging matrix = 256 × 256 × 176, resolution = 1 × 1 × 1 mm) and fluid-attenuated inversion recovery (FLAIR; TR/TE/TI = 4800/245/1650 ms, imaging matrix = 168 × 168 × 120, resolution 1 × 1 × 1.5 mm) MRI were collected in the sagittal plane on a 3.0 Tesla (T) Philips Achieva whole-body scanner (Philips Medical Systems, Best, The Netherlands) with a transmit-receive head coil.
Imaging pre-processing and perivascular space segmentation
T1-weighted images were segmented into tissue types (i.e., white matter [WM], cortical gray matter, subcortical gray matter, and ventricular CSF) using Freesurfer v5.1. WM masks were corrected for tissue misclassification, as described previously,31 and eroded by a single voxel to avoid potential partial volume effects. FLAIR volumes were bias field corrected using N4BiasFieldCorrection (http://picsl.upenn.edu/software/ants/). The determination of which acquisitions were too degraded to analyze was a qualitative one, though it is notable that each of these acquisitions failed WM segmentation at the Freesurfer stage.
Perivascular space segmentation
PVS segmentation was obtained using a previously described method that searches WM for hypointense objects that meet a set of morphological criteria; WM hyperintensities were avoided by consulting FLAIR.23,24 Each PVS detected by the automated algorithm was assessed by a blinded researcher (M.L.) with experience in PVS analysis; false alarms were removed from further analysis.
Statistical analysis
Statistical analysis was performed using Stata/MP software (version 15; StataCorp LP, College Station, TX). Descriptive statistics were used to summarize the cohort's characteristics. Differences in clinical characteristics among included and excluded subjects were evaluated with two-sample t-tests. Multi-variate linear regression was used to assess the relationship between PVS burden (primary outcome) and covariates based on a priori consideration of potential predictors and confounders. PVS burden was defined as two separate outcome variables: PVS number (the number of PVS per cm3 of white matter) and volume (the total volume of PVS [mm3] per cm3 of WM). The primary exposure was the number of mTBIs with LOC sustained in the military. Potential predictors and confounders were: age, systolic blood pressure, number of mTBIs with LOC sustained outside the military, the total number of blast mTBIs without LOC while in the military, and poor sleep (dichotomous yes/no based on published PSQI parameters28). We also examined poor sleep as a potential effect modifier (interaction) of the association between the number of mTBI with LOC sustained in the military and PVS burden.
Assumptions of normality, linearity, and homoscedasticity in the final model were tested with residual versus fitted plots and quantile-quantile (Q-Q) plots. Multi-collinearity was tested by calculating correlation coefficients (Supplementary Table S1) and variance inflation factors (Supplementary Table S2). Secondary outcomes included individual PVS morphological characteristics (i.e., width and length). We used the Shapiro-Wilk test to assess the normality of distribution. PVS number volume, length, and width were not normally distributed, and therefore they were log-transformed. We report p values as well as 95% confidence intervals (CIs). All reported p values are two-sided, and the threshold for statistical significance is set to an alpha of 0.05. The correlation between PVS burden and severity of post-concussive symptoms (NSI) was evaluated with Pearson's correlation coefficient. Bonferroni's correction was used to adjust for multiple comparisons.
Data availability statement
The data that support the findings of this study are available from the parent study upon request and approval through VA Puget Sound.
Results
The initial cohort consisted of 68 male OEF/OIF/OND veterans. Of those, 12 had MRI degraded by motion artifact, which prevented automated PVS quantification, and were excluded from the analysis. There were no differences in age, number of mTBI with LOC sustained in the military, number of mTBI sustained outside the military, number of blast mTBI without LOC, systolic blood pressure, sleep quality, or severity of post-concussive symptoms between subjects included and excluded from the analysis (data not shown). The characteristics of the final cohort (n = 56) are summarized in Table 1.
Table 1.
Cohort Characteristics
| Patient characteristics | (n = 56) |
|---|---|
| Age (years), median (IQR) | 32 (29–41) |
| Months between mTBI and MRI, median (IQR) | 55 (40–95) |
| Race, n (%) | |
| White | 44 (78.5) |
| Black/African American | 3 (5.3) |
| American Indian/Alaskan Native | 1 (1.7) |
| Asian | 2 (3.5) |
| Other | 6 (10.7) |
| Ethnicity, n (%) | |
| Hispanic | 7 (12.5) |
| Non-Hispanic | 49 (87.5) |
IQR, interquartile range; mTBI, mild traumatic brain injury; MRI, magnetic resoancne imaging.
Perivascular space characteristics
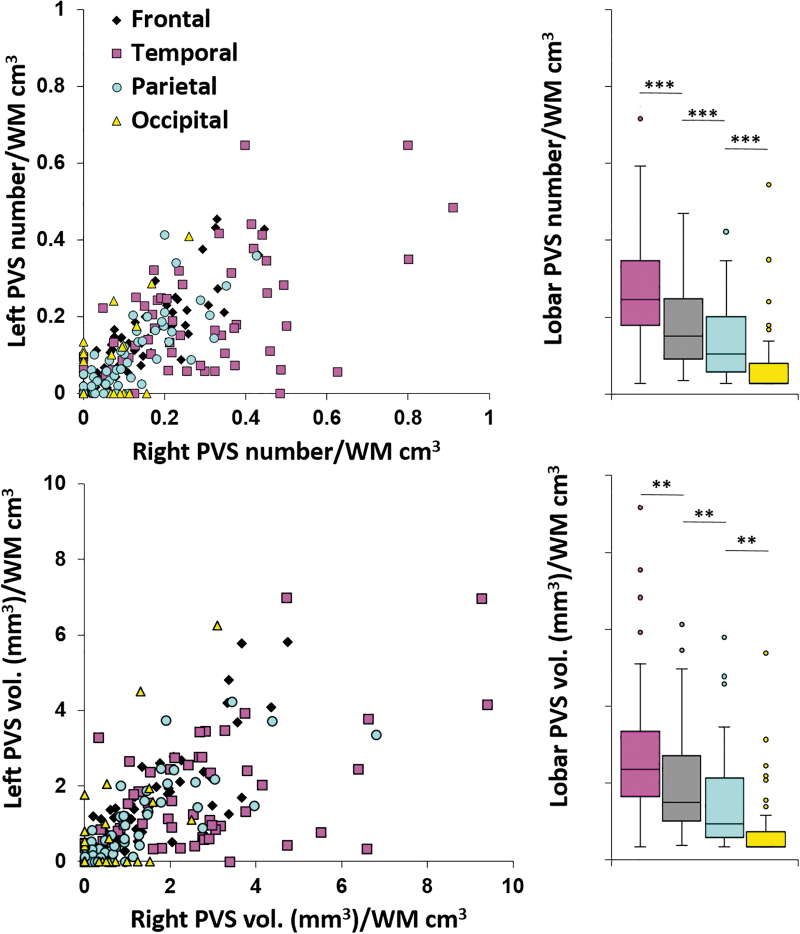
PVSs were observed in the supratentorial white matter of all 56 subjects in the cohort (Table 2). The number of PVSs observed varied widely among persons (range, 2–102). PVS number was positively correlated across hemispheres (Pearson's r = 0.87, p < 0.001), indicating a high degree of symmetry (Fig. 1). The same positive correlation was observed for PVS volume. Over both hemispheres, PVS number was the highest in the temporal lobes, followed by the frontal, parietal, and occipital lobes (all p < 0.001, top-row bar graph in Fig. 1). The same relationships were observed for PVS volume (all p < 0.01, bottom-row bar in Fig. 1).
Table 2.
Characteristics of PVS Identified with the Automated Algorithm
| Characteristic | Cohort (n = 56) | |
|---|---|---|
| PVS whole brain | Mean (SD) | Range |
| PVS total no. | 36.3 (24.4) | 2–102 |
| PVS total volume (mm3) | 351 (272.8) | 19–1202 |
| PVS no./cm3 WM | 0.13 (0.08) | 0.01–0.30 |
| PVS volume mm3/cm3 WM | 1.3 (1) | 0.08–4.40 |
| Individual PVS cluster | ||
| Width (mm) | 2.7 (0.1) | 2.3–3.0 |
| Length (mm) | 5.8 (0.4) | 5–7 |
PVS, perivascular space; WM, white matter; SD, standard deviation.
FIG. 1.
PVS burden in right and left hemispheres. PVS burden is correlated between hemispheres over subjects for both number and volume (scatter plots) in all lobes. Bar plots illustrate significantly different PVS burden by lobe. **p < 0.01; ***p < 0.001. PVS, perivascular space; WM, white matter. Color image is available online.
Mild traumatic brain injury burden and magnetic resonance–visible perivascular space characteristics
Both the number of mTBIs sustained in the military and systolic blood pressure were significantly related to PVS number and volume (Table 3; Fig. 2). Also, age was significantly related to PVS volume. After adjusting for other covariates, for each additional mTBI sustained in the military, subjects had an average 0.1 (95% CI, 0.002–0.2; p = 0.04) increase in lnPVS number/cm3 of white matter and a 0.1 (95% CI, 0.004–0.2; p = 0.04) increase in lnPVS volume (mm3)/cm3 of white matter. The number of mTBIs sustained outside the military, the number of blast mTBIs without LOC, and poor sleep did not show a significant association with PVS number or volume. To further investigate the effects of systolic blood pressure on PVS burden, subjects were stratified into two groups, based on the presence of what would be considered clinically significant systolic blood pressure (i.e., >130 mm Hg). No difference in PVS burden was found between the two groups (two-sample t-test, p = 0.19 for PVS number and p = 0.21 for PVS volume).
Table 3.
Effects of Number of mTBI with Loss of Consciousness Sustained in the Military and Age on Whole-Brain PVS Characteristics
| |
Outcome |
|||
|---|---|---|---|---|
| |
lnNo./cm3 WM |
lnVol. (mm3)/cm3 WM |
||
| β (95% CI) | p value | β (95% CI) | p value | |
| Covariates | ||||
| Age (years) | 0.02 (−0.0001 to 0.0500) | 0.05 | 0.03 (0.001–0.050) | 0.04 |
| mTBI mil. | 0.1 (0.002–0.200) | 0.04 | 0.1 (0.004–0.200) | 0.04 |
| mTBI non mil. | –0.04 (−0.20 to 0.09) | 0.49 | –0.03 (−0.2 to 0.1) | 0.7 |
| Blast exp. | 0.0003 (−0.002 to 0.003) | 0.77 | 0.0006 (−0.002 to 0.003) | 0.67 |
| SBP | 0.01 (0.003–0.030) | 0.01 | 0.02 (0.004–0.030) | 0.01 |
| Poor sleep | –0.08 (−0.76 to 0.60) | 0.81 | –0.1 (−0.8 to 0.6) | 0.78 |
| mTBI × PS | 0.4 (−0.02 to 0.80) | 0.06 | 0.48 (0.006–0.900) | 0.04 |
Bold indicates p < 0.05.
PVS, perivascular space; mTBI, mild traumatic brain injury; mTBI mil., mTBI with LOC sustained in the military; mTBI non mil., mTBI with LOC sustained outside the military; Blast exp., blast exposure with no LOC but meeting other criteria for mTBI; SBP, systolic blood pressure; PS, poor sleep; CI, confidence interval.
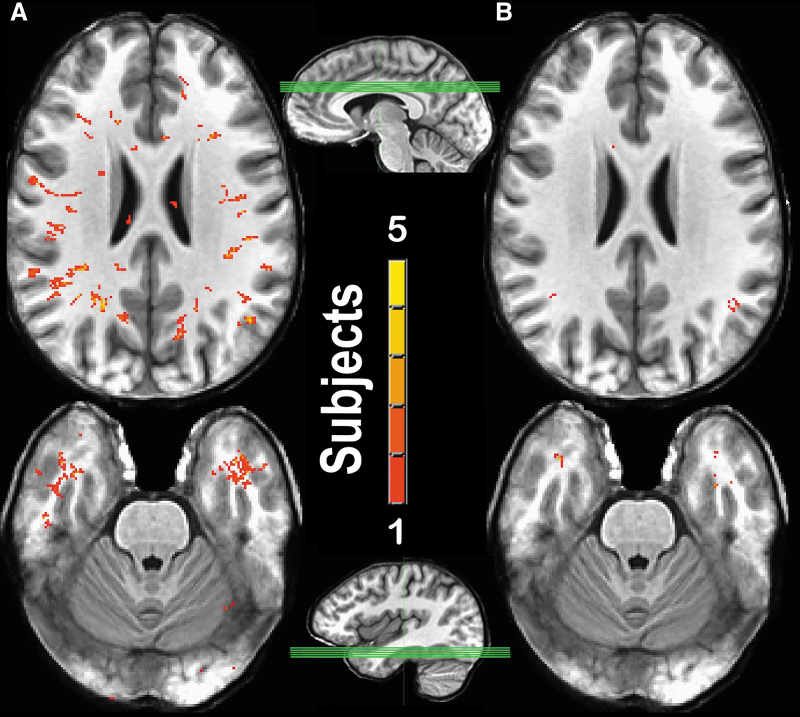
FIG. 2.
Association between mTBI number and MRI-visible PVS burden. Superimposed T1-weighted image slices through the centrum semiovale (top row) and the middle temporal lobe (bottom row) from subjects with a high prevalence (≥5) mTBI (n = 4; A) and a low prevalence (≤2) mTBI (n = 5; B). Sagittal views illustrate the slab through which the mean maximum intensity projection of slices was taken. After superimposing the slices, PVSs were color-coded. Red indicates that a particular voxel was identified as a PVS in 1 subject, whereas yellow indicates that a particular voxel was identified as a PVS in 5 subjects. MRI, magnetic resonance imaging; mTBI, mild TBI; PVS, perivascular space; TBI, traumatic brain injury. Color image is available online.
We found a significant interaction between poor sleep and the number of mTBIs sustained in the military on PVS volume. In subjects with poor sleep, the effect of mTBIs sustained in the military on PVS volume was significantly higher (β = 0.48; 95% CI, 0.006–0.9; p = 0.04) than in those reporting good sleep (Table 3). Model diagnostics indicated a good model fit and no multi-collinearity. There was no correlation between mTBI with LOC sustained in the military, number of blast mTBI without LOC, or any of the other covariates, and individual PVS morphology (i.e., width or length, data not shown). Further analysis revealed no interaction between poor sleep and systolic blood pressure on PVS number (β = −0.003; 95% CI, −0.04 to 0.04; p = 0.88) or volume (β = 0.005; 95% CI, −0.05 to 0.06; p = 0.84).
Magnetic resonance–visible perivascular space burden and post-concussive symptoms
There was a low significant positive correlation between PVS number (r = 0.28, p = 0.03) and volume (r = 0.3, p = 0.02) and overall severity of persistent post-concussive symptoms measured by the NSI (Table 4). There was no correlation between individual PVS characteristics (i.e., width, length) and severity of post-concussive symptoms. When each component of the NSI was analyzed separately, there was a significant correlation between both PVS number (r = 0.47, p = 0.0002) and volume (r = 0.48, p = 0.0002) and balance problems. These correlations remained significant after adjusting for multiple comparisons (Table 5). Other neurological complaints (i.e., dizziness, poor coordination, vision problems, noise sensitivity, and sensory problems) and mood complaints (i.e., feelings of sadness) exhibited statistically significant positive correlations with both PVS number and volume. These correlations, however, were not significant after adjusting for multiple comparisons.
Table 4.
Correlation between PVS Characteristics and Post-Concussive Symptoms Assessed by the Neurobehavioral Symptom Inventory
| |
Post-concussive symptoms |
|
|---|---|---|
| ra | p value | |
| PVS whole brain | ||
| PVS no./cm3 WM | 0.28 | 0.03 |
| PVS vol. (mm3)/cm3 WM | 0.3 | 0.02 |
| PVS cluster morphology | ||
| PVS width (mm) | 0.24 | 0.06 |
| PVS length (mm) | 0.15 | 0.26 |
Pearson's correlation coefficient.
PVS, perivascular space; WM, white matter.
Table 5.
Correlation between PVS Number and Volume (mm3)/cm3 of White Matter and Post-Concussive Symptoms
| |
PVS no./cm3 WM |
PVS volume (mm3)/cm3 WM |
||
|---|---|---|---|---|
| Symptom | ra | p value | ra | p value |
| Dizziness | 0.32 | 0.01 | 0.35 | 0.006 |
| Poor balance | 0.47 | 0.0002b | 0.48 | 0.0002b |
| Poor coordination | 0.36 | 0.005 | 0.38 | 0.003 |
| Headaches | 0.22 | 0.1 | 0.26 | 0.05 |
| Nausea | 0.26 | 0.04 | 0.25 | 0.05 |
| Vision problems | 0.35 | 0.007 | 0.35 | 0.007 |
| Light sensitivity | 0.22 | 0.09 | 0.18 | 0.16 |
| Hearing difficulties | 0.11 | 0.41 | 0.09 | 0.48 |
| Noise sensitivity | 0.27 | 0.04 | 0.27 | 0.03 |
| Numbness | 0.29 | 0.02 | 0.3 | 0.02 |
| Change in taste or smell | 0.1 | 0.44 | 0.06 | 0.64 |
| Loss of appetite | 0.05 | 0.67 | 0.06 | 0.63 |
| Poor concentration | 0.16 | 0.21 | 0.17 | 0.2 |
| Forgetfulness | –0.08 | 0.53 | –0.09 | 0.5 |
| Difficulty making decisions | 0.03 | 0.77 | 0.06 | 0.62 |
| Slowed thinking | 0.11 | 0.41 | 0.11 | 0.39 |
| Fatigue | 0.13 | 0.32 | 0.15 | 0.24 |
| Difficulty falling asleep | 0.22 | 0.09 | 0.21 | 0.1 |
| Feeling anxious | 0.24 | 0.06 | 0.25 | 0.05 |
| Feeling depressed | 0.27 | 0.04 | 0.31 | 0.01 |
| Irritability | –0.1 | 0.43 | –0.09 | 0.48 |
| Poor frustration | 0.12 | 0.36 | 0.13 | 0.31 |
Pearson's correlation coefficient.
Significant after Bonferroni's correction (α = 0.002).
PVS, perivascular space; WM, white matter.
Discussion
In this cohort of Iraq/Afghanistan veterans, the number of mTBIs with LOC sustained in the military is correlated with PVS burden, after adjusting for other clinically important covariates. Also, quality of sleep modulates the relationship between the number of mTBIs sustained in the military and PVS volume. In persons with poor sleep, the increase in PVS volume observed with each subsequent mTBI is higher than in those who sleep well. Last, a significant positive correlation exists between PVS burden and the severity of post-concussive symptoms in veterans, particularly balance problems. Although these findings do not address the directionality or causality of the relationship between mTBI, poor sleep, PVS burden, and persistent post-concussive symptoms, these data suggest a potential mechanistic link between them.
Previous studies have documented a larger number of PVS in subjects with mTBI.16,22,32 These studies used visual rating scales that provide no information concerning PVS volume, morphology, or location. Also, such scales often rely on the subjective selection of a single axial slice, which limits their sensitivity. Besides, previous reports did not consider important covariates, which may affect PVS burden.33,34 Our study builds on this pre-existing evidence by using a validated and automated method to segment PVS in 3T MRI data, which allows us to characterize PVS morphology (i.e., width, length, volume, and linearity) and location of each PVS throughout supratentorial WM.23,24
Disruption of glymphatic exchange offers a potential mechanism for the association between mTBI, increased PVS burden, and persistent post-concussive symptoms. In rodents, PVSs play an essential role in glymphatic exchange. According to this model, interstitial fluid flows into the PVS before exiting the brain.35 Under normal conditions, this flow is facilitated by the astroglial water channel, aquaporin-4, and allows for the removal of metabolic waste such as amyloid-β and p-tau.13 In animal models of blast TBI, the glymphatic pathway is partially responsible for the clearance of p-tau that accumulates in the PVS immediately after an injury.36 Importantly, in rodents, mTBI causes loss of perivascular aquaporin-4 polarization in astrocytic endfeet, with subsequent glymphatic dysfunction and impaired waste clearance.19 If a similar loss of perivascular aquaporin-4 polarization occurs in humans, CSF exit from the PVS into the interstitial space could be impaired, leading to chronic dilatation of the PVS, disruption of glymphatic flow, and waste accumulation.37 Accumulation of waste, including amyloid-β, p-tau, and lactate, has been implicated in the pathophysiology of post-traumatic neurodegeneration.13,19,35,38–40
Increased PVS burden after mTBI may be a reflection of a dysfunctional perivascular draining pathway. It is plausible that impaired clearance of waste by-products generated in excess by mTBI could lead to the propagation or persistence of post-concussive symptoms. Alternatively, increased PVS burden could reflect other post-traumatic pathological processes. Giza and colleagues described a “neurometabolic cascade” after mTBI, which involves neuroinflammation and breakdown of the blood–brain barrier.41 After mTBI, neuroinflammatory mediators, along with inflammatory cells, accumulate around the cerebral blood vessels.42 The precise relation between post-traumatic neuroinflammation and PVS dilatation remains to be determined. However, it has been hypothesized that post-traumatic accumulation of neuroinflammatory mediators and inflammatory cells and breakdown of blood–brain barrier permeability could lead to PVS enlargement and alterations of fluid clearance.43 Whether attributable to disrupted glymphatic function or other pathological processes, our findings indicate a mechanistic link between mTBI and PVS burden.
We found a significant interaction between mTBI and poor sleep for predicting PVS burden. Of the two PVS burden surrogates (i.e., number and volume), only PVS volume was affected by this interaction. The resolution of a 3T MRI scan may not be sufficient to detect small PVS, making total PVS volume a more sensitive measurement of PVS burden. These findings highlight the importance of reporting measurements other than the total PVS number when analyzing the effects of different exposures on PVS burden. Using polysomnography and visual PVS rating scales, previous studies have found a direct correlation between PVS burden and total sleep time.21 In addition to the limitations inherent to the use of visual scales, this previous work did not address the interaction between the number of mTBIs and poor sleep on PVS characteristics. Our data agree with the existing literature and show that the relation between the number of mTBIs and PVS burden is different in veterans who report poor sleep versus those who do not.
Poor sleep is being increasingly recognized as a mediator that can potentially affect the rate of recovery after mTBI.8 Evidence from animal models demonstrates that the glymphatic pathway is most active during sleep. This notion is evidenced by the sleep-induced expansion of the interstitial space and increase in convective exchange of cerebrospinal and interstitial fluid14,44 Convective flow through the brain parenchyma increases the rate of waste clearance during sleep. Based on these pre-clinical studies and the data presented here, we hypothesize a two-hit mechanism: The first hit is post-traumatic impairment of glymphatic function. The second hit is the development of poor sleep, which prevents an already dysfunctional glymphatic pathway from clearing waste efficiently.
We found a positive association between systolic blood pressure and PVS burden. Previous studies have shown increased PVS burden in subjects with hypertension, particularly in the basal ganglia.45 Several mechanisms have been proposed to explain this association. These mechanisms include direct mechanical trauma secondary to CSF pulsatility,33 alteration of vessel permeability,46 and ischemic injury leading to ex vacuo dilatation.47 Despite the anatomical differences between the basal ganglia and centrum semiovale vasculature,17 our data agree with the existing literature and suggest that the relation between systolic blood pressure and PVS burden is also observed in the WM.
In our cohort, we observed a low, but significant, positive correlation between PVS burden and aggregate persistent post-concussive symptom severity. The low correlation coefficients may be explained, at least partly, because aggregate post-concussive symptom measurements assess a wide variety of symptoms. These symptoms involve different areas of the brain and independent neuroanatomical pathways. By analyzing each symptom separately and adjusting for multiple comparisons, we identified a correlation between PVS burden and balance problems. Balance is a complex neurological task that requires the integrity of several anatomical structures. Vestibular, visual, cerebellar, proprioceptive, and motor pathways are all involved in maintaining balance. This complexity makes balance particularly susceptible to the effects of mTBI.48 Whether the relationship between balance problems and PVS burden is an indicator of a direct effect of glymphatic dysfunction on balance remains to be determined.
Our findings are of particular interest to the veteran population given the exposure to repeated mTBI as part of their training and deployment, often returning to duty while still symptomatic.2,27,49 Active duty service members who sustain an mTBI and show increased PVS burden on MRI may be at risk of persistent post-concussive symptoms, particularly if they also have poor sleep. Further research is needed to determine whether increased PVS burden represents a reliable biomarker of glymphatic dysfunction, which can be used for risk stratification and return to duty in this population.
This study has several limitations. First, although the automated PVS detection was assessed by a blind reviewer and “false positives” were removed, we did not account for the potential “false negative” PVS (i.e., those PVS missed by the algorithm). As with many segmentation algorithms, it is difficult to definitively identify misses, especially when investigating objects that in non-dilated cases are too small to be detected at clinical-strength MRI. We made a decision to preserve the objectivity of an automated algorithm at the expense of an increase in the likelihood of missed PVS and so chose not to manually draw the comparatively small number of PVSs that were visible, but not identified by the segmentation algorithm. Second, specific MRI sequences, such as T2*-weighted acquisition, were not available. Therefore, the lineage of the vessels inside the PVS (arterial vs. venular) was not delineated. Future studies, including ultra-high-field MRI, are needed to gain some clarity on this question. Third, poor sleep was assessed subjectively. Although the PSQI is a self-report sleep questionnaire, it has been validated against objective measurements of sleep in patients with TBI.28,50
Implementing objective sleep measures, such as actigraphy or polysomnography, will allow future studies to more accurately establish the relationship between mTBI burden, different aspects of sleep, and PVS characteristics. Fourth, just like with any convenience sample, it is possible that selection bias was introduced while creating the cohort. Fifth, although the interaction between mTBI burden and poor sleep on PVS burden is a necessary condition to establish a causal relationship, it is not sufficient. More extensive longitudinal studies are necessary to properly establish a causal relationship between poor post-traumatic sleep and PVS burden. Sixth, in our study, PVS burden was only measured in the WM. Previous work has shown that PVSs occur in other areas of the brain, including the basal ganglia, midbrain, and cerebellum.51,52 It is possible that by restricting our analysis to the WM, we limited our sensitivity to detect the full effect of mTBI on PVS burden. Moreover, different mTBI etiologies (e.g., military vs. non-military) may be associated with PVS changes in specific structures. Future studies evaluating the relation between mTBI and PVS burden in structures other than the WM are warranted.
Our study shows that in Iraq/Afghanistan veterans, the number of mTBIs sustained in the military is associated with PVS burden, and this relationship is modulated by poor sleep. Also, we show that in this cohort, increased PVS burden is associated with worse postconcussive symptoms, particularly poor balance.
Supplementary Material
Authors' Contributions
Juan Piantino: designed and conceptualized study; analyzed the data; drafted the manuscript for intellectual content. Daniel Schwartz: designed and conceptualized study; analyzed the data; drafted the manuscript for intellectual content. Madison Luther: data collection and analysis, drafting and revision of manuscript. Craig Newgard: data analysis, drafting and revision of manuscript. Lisa Silbert: design and conceptualized study, drafting and revision of manuscript. Murray Raskind: design and conceptualized study, drafting and revision of manuscript. Kathleen Pagulayan: design and conceptualized study, drafting and revision of manuscript. Natalia Kleinhans: design and conceptualized study, drafting and revision of manuscript. Jeffrey Iliff: design and conceptualized study, drafting and revision of manuscript. Elaine Peskind: design and conceptualized study, drafting and revision of manuscript.
Funding Information
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI; K23HL150217-01), Department of Veterans Affairs Rehabilitation Research and Development Service Merit Review grant B77421, and National Institute of Aging (NIA; AG066518).
Author Disclosure Statement
Juan Piantino has receive fresearch support from National Heart, Lung, and Blood Institute (NHLBI) K23HL150217. Lisa Silbert has received research support from the National Institute of Aging (NIA) Layton Oregon Aging and Alzheimer's Disease Center (P30 AG 066518). Jeffrey Iliff receives funding from the NIA (P30 AG066509, R01AG054456), and NINDS (R01 NS089709). Elaine Peskind has receive fresearch support from the Department of Veterans Affairs Rehabilitation Research and Development Service Merit Review grant B77421.
References
- 1.Hoge, C.W., McGurk, D., Thomas, J.L., Cox, A.L., Engel, C.C., and Castro, C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 2.Galarneau, M.R., Woodruff, S.I., Dye, J.L., Mohrle, C.R., and Wade, A.L. (2008). Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J. Neurosurg. 108, 950–957 [DOI] [PubMed] [Google Scholar]
- 3.Belanger, H.G., Spiegel, E., and Vanderploeg, R.D. (2010). Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J. Int. Neuropsychol. Soc. 16, 262–267 [DOI] [PubMed] [Google Scholar]
- 4.Mathias, J.L., and Alvaro, P.K. (2012). Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 13, 898–905 [DOI] [PubMed] [Google Scholar]
- 5.Beetar, J.T., Guilmette, T.J., and Sparadeo, F.R. (1996). Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch. Phys. Med. Rehabil. 77, 1298–1302 [DOI] [PubMed] [Google Scholar]
- 6.Ouellet, M.C., Beaulieu-Bonneau, S., and Morin, C.M. (2006). Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J. Head Trauma Rehabil. 21, 199–212 [DOI] [PubMed] [Google Scholar]
- 7.Orff, H.J., Ayalon, L., and Drummond, S.P. (2009). Traumatic brain injury and sleep disturbance: a review of current research. J. Head Trauma Rehabil. 24, 155–165 [DOI] [PubMed] [Google Scholar]
- 8.Wickwire, E.M., Williams, S.G., Roth, T., Capaldi, V.F., Jaffe, M., Moline, M., Motamedi, G.K., Morgan, G.W., Mysliwiec, V., Germain, A., Pazdan, R.M., Ferziger, R., Balkin, T.J., MacDonald, M.E., Macek, T.A., Yochelson, M.R., Scharf, S.M., and Lettieri, C.J. (2016). Sleep, sleep disorders, and mild traumatic brain injury. what we know and what we need to know: findings from a national working group. Neurotherapeutics 13, 403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castriotta, R.J., Wilde, M.C., Lai, J.M., Atanasov, S., Masel, B.E., and Kuna, S.T. (2007). Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep Med. 3, 349–356 [PMC free article] [PubMed] [Google Scholar]
- 10.Chaput, G., Giguere, J.F., Chauny, J.M., Denis, R., and Lavigne, G. (2009). Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 10, 713–716 [DOI] [PubMed] [Google Scholar]
- 11.Kostyun, R.O., Milewski, M.D., and Hafeez, I. (2015). Sleep disturbance and neurocognitive function during the recovery from a sport-related concussion in adolescents. Am. J. sports Med. 43, 633–640 [DOI] [PubMed] [Google Scholar]
- 12.Murdaugh, D.L., Ono, K.E., Reisner, A., and Burns, T.G. (2018). Assessment of sleep quantity and sleep disturbances during recovery from sports-related concussion in youth athletes. Arch. Phys. Med. Rehabil. 99, 960–966 [DOI] [PubMed] [Google Scholar]
- 13.Iliff, J.J., Wang, M., Liao, Y., Plogg, B.A., Peng, W., Gundersen, G.A., Benveniste, H., Vates, G.E., Deane, R., Goldman, S.A., Nagelhus, E.A., and Nedergaard, M. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie, L., Kang, H., Xu, Q., Chen, M.J., Liao, Y., Thiyagarajan, M., O'Donnell, J., Christensen, D.J., Nicholson, C., Iliff, J.J., Takano, T., Deane, R., and Nedergaard, M. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez, J., Berezuk, C., McNeely, A.A., Scott, C.J., Gao, F., and Black, S.E. (2015). Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J. Alzheimers Dis. 43, 415–424 [DOI] [PubMed] [Google Scholar]
- 16.Potter, G.M., Chappell, F.M., Morris, Z., and Wardlaw, J.M. (2015). Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 39, 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patankar, T.F., Mitra, D., Varma, A., Snowden, J., Neary, D., and Jackson, A. (2005). Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am. J. Neuroradiol. 26, 1512–1520 [PMC free article] [PubMed] [Google Scholar]
- 18.Charidimou, A., Jaunmuktane, Z., Baron, J.C., Burnell, M., Varlet, P., Peeters, A., Xuereb, J., Jager, R., Brandner, S., and Werring, D.J. (2014). White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology 82, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliff, J.J., Chen, M.J., Plog, B.A., Zeppenfeld, D.M., Soltero, M., Yang, L., Singh, I., Deane, R., and Nedergaard, M. (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen, J., Wright, D.K., Yamakawa, G.R., Shultz, S.R., and Mychasiuk, R. (2020). Repetitive mild traumatic brain injury alters glymphatic clearance rates in limbic structures of adolescent female rats. Sci. Rep. 10, 6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opel, R.A., Christy, A., Boespflug, E.L., Weymann, K.B., Case, B., Pollock, J.M., Silbert, L.C., and Lim, M.M. (2019). Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J. Cereb. Blood Flow Metab, 39, 2258–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inglese, M., Grossman, R.I., Diller, L., Babb, J.S., Gonen, O., Silver, J.M., and Rusinek, H. (2006). Clinical significance of dilated Virchow-Robin spaces in mild traumatic brain injury. Brain Inj. 20, 15–21 [DOI] [PubMed] [Google Scholar]
- 23.Boespflug, E.L., Schwartz, D.L., Lahna, D., Pollock, J., Iliff, J.J., Kaye, J.A., Rooney, W., and Silbert, L.C. (2018). MR imaging-based multimodal autoidentification of perivascular spaces (mMAPS): automated morphologic segmentation of enlarged perivascular spaces at clinical field strength. Radiology 286, 632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz, D.L., Boespflug, E.L., Lahna, D.L., Pollock, J., Roese, N.E., and Silbert, L.C. (2019). Autoidentification of perivascular spaces in white matter using clinical field strength T1 and FLAIR MR imaging. NeuroImage 202, 116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruff, R.M., Iverson, G.L., Barth, J.T., Bush, S.S., and Broshek, D.K. (2009). Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch. Clin. Neuropsychol. 24, 3–10 [DOI] [PubMed] [Google Scholar]
- 26.Petrie, E.C., Cross, D.J., Yarnykh, V.L., Richards, T., Martin, N.M., Pagulayan, K., Hoff, D., Hart, K., Mayer, C., Tarabochia, M., Raskind, M.A., Minoshima, S., and Peskind, E.R. (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J. Neurotrauma 31, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagulayan, K.F., Rau, H., Madathil, R., Werhane, M., Millard, S.P., Petrie, E.C., Parmenter, B., Peterson, S., Sorg, S., Hendrickson, R., Mayer, C., Meabon, J.S., Huber, B.R., Raskind, M., Cook, D.G., and Peskind, E.R. (2018). Retrospective and prospective memory among OEF/OIF/OND veterans with a self-reported history of blast-related mTBI. J. Int. Neuropsychol. Soc. 24, 324–334 [DOI] [PubMed] [Google Scholar]
- 28.Buysse, D.J., Reynolds, C.F., 3rd, Monk, T.H., Berman, S.R., and Kupfer, D.J. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 [DOI] [PubMed] [Google Scholar]
- 29.Belanger, H.G., Silva, M.A., Donnell, A.J., McKenzie-Hartman, T., Lamberty, G.J., and Vanderploeg, R.D. (2017). Utility of the neurobehavioral symptom inventory as an outcome measure: a VA TBI model systems study. J. Head Trauma Rehabil. 32, 46–54 [DOI] [PubMed] [Google Scholar]
- 30.Cicerone, K.D., and Kalmar, K. (1995). Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J. Head Trauma Rehabil. 10, 1–17 [Google Scholar]
- 31.Promjunyakul, N., Lahna, D., Kaye, J.A., Dodge, H.H., Erten-Lyons, D., Rooney, W.D., and Silbert, L.C. (2015). Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. NeuroImage. Clin. 8, 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orrison, W.W., Hanson, E.H., Alamo, T., Watson, D., Sharma, M., Perkins, T.G., and Tandy, R.D. (2009). Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J. Neurotrauma 26, 689–701 [DOI] [PubMed] [Google Scholar]
- 33.Heier, L.A., Bauer, C.J., Schwartz, L., Zimmerman, R.D., Morgello, S., and Deck, M.D. (1989). Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am. J. Neuroradiol. 10, 929–936 [PMC free article] [PubMed] [Google Scholar]
- 34.Ding, J., Sigurethsson, S., Jonsson, P.V., Eiriksdottir, G., Charidimou, A., Lopez, O.L., van Buchem, M.A., Guethnason, V., and Launer, L.J. (2017). Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol. 74, 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliff, J.J., Lee, H., Yu, M., Feng, T., Logan, J., Nedergaard, M., and Benveniste, H. (2013). Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber, B.R., Meabon, J.S., Hoffer, Z.S., Zhang, J., Hoekstra, J.G., Pagulayan, K.F., McMillan, P.J., Mayer, C.L., Banks, W.A., Kraemer, B.C., Raskind, M.A., McGavern, D.B., Peskind, E.R., and Cook, D.G. (2016). Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience 319, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen, M.K., Mestre, H., and Nedergaard, M. (2018). The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliff, J.J., Wang, M., Zeppenfeld, D.M., Venkataraman, A., Plog, B.A., Liao, Y., Deane, R., and Nedergaard, M. (2013). Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundgaard, I., Lu, M.L., Yang, E., Peng, W., Mestre, H., Hitomi, E., Deane, R., and Nedergaard, M. (2017). Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 37, 2112–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bigler, E.D. (2013). Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 7, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giza, C.C., and Hovda, D.A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75, Suppl. 4, S24–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holfelder, K., Schittenhelm, J., Trautmann, K., Haybaeck, J., Meyermann, R., and Beschorner, R. (2011). De novo expression of the hemoglobin scavenger receptor CD163 by activated microglia is not associated with hemorrhages in human brain lesions. Histol. Histopathol. 26, 1007–1017 [DOI] [PubMed] [Google Scholar]
- 43.Brown, R., Benveniste, H., Black, S.E., Charpak, S., Dichgans, M., Joutel, A., Nedergaard, M., Smith, K.J., Zlokovic, B.V., and Wardlaw, J.M. (2018). Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 114, 1462–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piantino, J., Lim, M.M., Newgard, C.D., and Iliff, J. (2019). Linking traumatic brain injury, sleep disruption and post-traumatic headache: a potential role for glymphatic pathway dysfunction. Curr. Pain Headache Rep. 23, 62. [DOI] [PubMed] [Google Scholar]
- 45.Francis, F., Ballerini, L., and Wardlaw, J.M. (2019). Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: a systematic review and meta-analysis. Int. J. Stroke 14, 359–371 [DOI] [PubMed] [Google Scholar]
- 46.Benhaïem-Sigaux, N., Gray, F., Gherardi, R., Roucayrol, A.M., and Poirier, J. (1987). Expanding cerebellar lacunes due to dilatation of the perivascular space associated with Binswanger's subcortical arteriosclerotic encephalopathy. Stroke 18, 1087–1092 [DOI] [PubMed] [Google Scholar]
- 47.Pullicino, P.M., Miller, L.L., Alexandrov, A.V., and Ostrow, P.T. (1995). Infraputaminal ‘lacunes’. Clinical and pathological correlations. Stroke 26, 1598–1602 [DOI] [PubMed] [Google Scholar]
- 48.Parrington, L., Fino, P.C., Swanson, C.W., Murchison, C.F., Chesnutt, J., and King, L.A. (2019). Longitudinal assessment of balance and gait after concussion and return to play in collegiate athletes. J. Athl. Train. 54, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lange, R.T., Brickell, T.A., Ivins, B., Vanderploeg, R.D., and French, L.M. (2013). Variable, not always persistent, postconcussion symptoms after mild TBI in U.S. military service members: a five-year cross-sectional outcome study. J. Neurotrauma 30, 958–969 [DOI] [PubMed] [Google Scholar]
- 50.Masel, B.E., Scheibel, R.S., Kimbark, T., and Kuna, S.T. (2001). Excessive daytime sleepiness in adults with brain injuries. Arch. Phys. Med. Rehabil. 82, 1526–1532 [DOI] [PubMed] [Google Scholar]
- 51.Jungreis, C.A., Kanal, E., Hirsch, W.L., Martinez, A.J., and Moossy, J. (1988). Normal perivascular spaces mimicking lacunar infarction: MR imaging. Radiology 169, 101–104 [DOI] [PubMed] [Google Scholar]
- 52.Hirabuki, N., Fujita, N., Fujii, K., Hashimoto, T., and Kozuka, T. (1994). MR appearance of Virchow-Robin spaces along lenticulostriate arteries: spin-echo and two-dimensional fast low-angle shot imaging. AJNR Am. J. Neuroradiol. 15, 277–281 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the parent study upon request and approval through VA Puget Sound.