Abstract
PolygonatumkingianumCollett et Hemsl.var.grandifolium D.M. Liu & W.Z. Zeng (1981), which sprouts twice a year, once in spring and once in autumn, differs from Polygonatumkingianum in leaves, bracts, perianth and filaments. Morphological comparison and molecular phylogeny indicate that it is identical to the newly-published Polygonatumhunanense H.H. Liu & B.Z. Wang (2021). Hence, we propose that P.kingianumvar.grandifolium should be recognised as a new synonym of P.hunanense. In addition, phylogenetic analyses confirmed that P.hunanense is sister to Polygonatumsect.Polygonatum, rather than P.kingianum of Polygonatumsect.Verticillata.
Keywords: Polygonatum hunanense , Polygonatum kingianum var. grandifolium , phylogeny, plastome
Introduction
The genus Polygonatum Mill. (Asparagaceae, tribe Polygonateae), commonly known as ‘Solomon’s Seal’, contains more than 60 species widespread in the Northern Hemisphere, with Himalayas to southwest China and north-eastern Asia as diversification centres (Tamura et al. 1997a; Jeffrey 1980, 1982; Wang et al. 2016). Species in Polygonatum are perennial herbs with rhizome, stems erect, arching or sometimes scandent, leaves alternate, opposite or whorled, inflorescences an umbel, corymb or raceme (Chen and Tamura 2000). Rhizomes of some species, like Polygonatumsibiricum Redouté, Polygonatumcyrtonema Hua and Polygonatumkingianum Collett et Hemsl., are widely used in traditional Chinese medicine. Tamura et al. separated Heteropolygonatum M.N. Tamura et Ogisu from Polygonatum (Tamura et al. 1997a) and found the topological difference of Heteropolygonatum and its relative taxa: (1) (Heteropolygonatum + Disporopsis) + Polygonatum and (2) (Heteropolygonatum + Polygonatum) + Disporopsis (Tamura et al. 1997b). Later, the former case was supported by Meng et al. (2014) and Wang et al. (2016), whereas the latter one was supported by Floden and Schilling (2018) and Zhao et al. (2019). The genus Polygonatum was divided into three sections, based on four chloroplast molecular markers, leaf arrangement and basic chromosome number: (1) Polygonatumsect.Polygonatum, which is characterised by alternate leaves and basic chromosome number x = 9–11; (2) Polygonatumsect.Sibirica only includes Polygonatumsibiricum with whorled leaves and x = 12; and (3) Polygonatumsect.Verticillata shows variable phyllotaxy and x = 13–15 (Meng et al. 2014). This is widely accepted and confirmed by multiple molecular phylogenetic studies (Wang et al. 2016; Floden and Schilling 2018; Zhao et al. 2019). However, due to hybridisation and polyploidisation in this genus, classification of some species with large morphological variations and wide distribution range remains controversial (Tamura 1990; Tamura 2008; Floden 2015; Floden and Schilling 2018; Zhao et al. 2019).
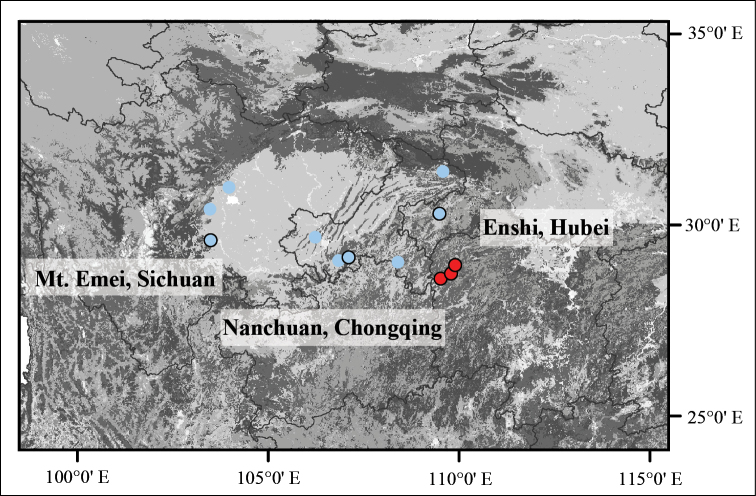
During fieldwork in the last few years, we found several populations of a unique Polygonatum species in Sichuan Province, Chongqing Municipality and Hubei Province of China (Figure 1). The plants are 1–3 m high with 3–5 whorled leaves per round, yellowish-white or greenish-white flowers and yellow or orange berries (Figure 2). It sprouts twice a year, once in spring (March to April) and once in autumn (September), whereas other species sprout only once in spring. It is likely belonging to the section Verticillata, according to the phenotype. After carefully checking the protologue and type specimens (Figure 3), we found that our collections matched the description of PolygonatumkingianumCollett et Hemsl.var.grandifolium D.M. Liu et W.Z. Zeng, which was published in Flora Sichuanica and is differing from other P.kingianum varieties by having broader leaves ((1.5–) 2.4–5 cm wide) with green leaf base (vs. 0.2–1.0 (–1.5) cm wide, leaf base red), 2-5 mm long bracts at base of pedicel (vs. 1-2 mm long, on pedicel), yellowish- or greenish-white perianth (vs. pink, red or white) (Figures 2, 4; Xu 1981). In addition, it is also similar to the newly-published Polygonatumhunanense H.H. Liu & B.Z. Wang from Hunan, China (Liu et al. 2021). In this study, molecular phylogenetic analyses were performed to reveal the phylogenetic relationships amongst P.kingianumvar.grandifolium, P.hunanense and P.kingianum.
Figure 1.
Distribution map of Polygonatumhunanense (red dots with black circle, based on Liu et al. 2021) and Polygonatumkingianumvar.grandifolium (blue dots with black circle: three populations investigated in this study; blue dots: previous specimen records).
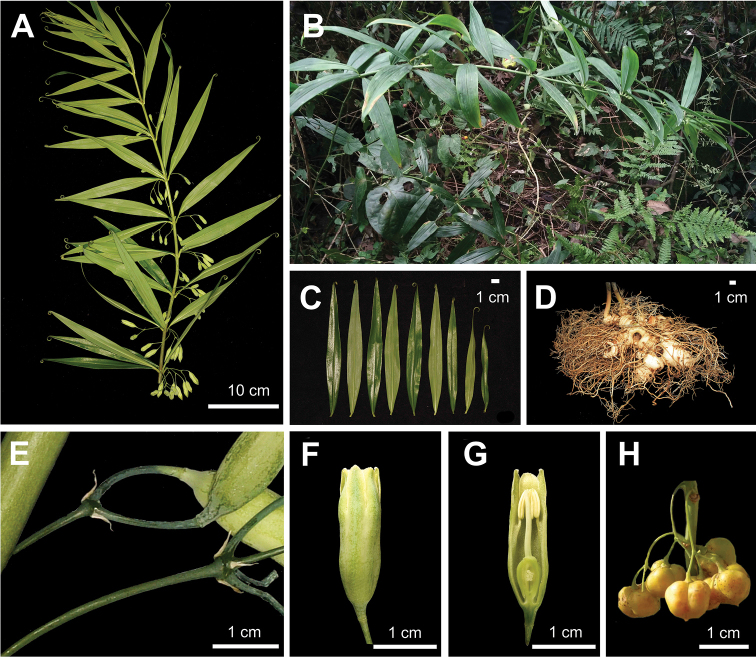
Figure 2.
Polygonatumkingianumvar.grandifoliumA stem B plant habit C leaves D rhizome E bracts F flower G longitudinal section of flower H fruit.
Figure 3.
Lectotype of Polygonatumkingianumvar.grandifolium, Hao Zhang 1231 (CDCM 00044013).
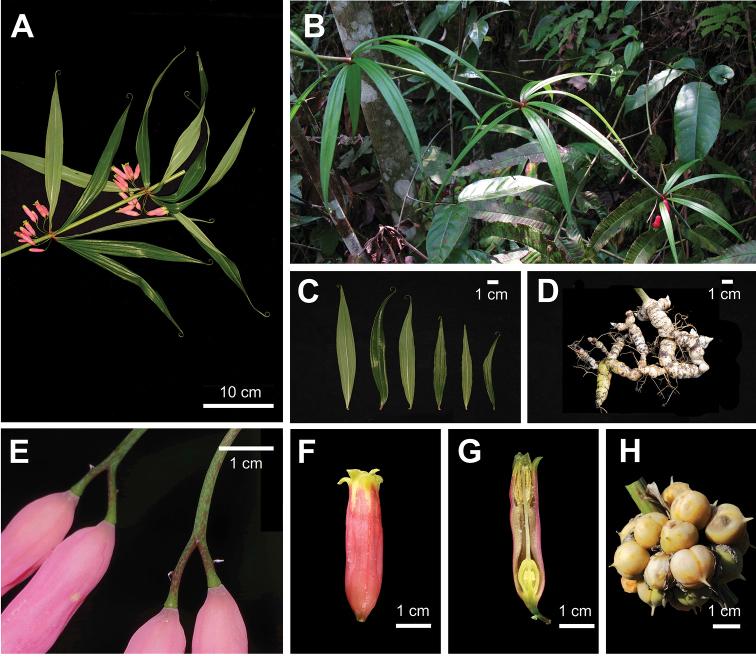
Figure 4.
PolygonatumkingianumA stem B plant habit C leaves D rhizome E bracts F flower G longitudinal section of flower H fruit.
Materials and methods
Morphologic observation
Morphological characters of the living individuals from Mt. Emei, Sichuan Province, China were observed. In addition, 16 herbarium specimens of Polygonatumkingianumvar.grandifolium in IMC, CDCM and CDBI were examined. Subsequently, morphological comparisons were conducted with the living individuals, specimens and descriptions of Polygonatumhunanense and Polygonatumkingianum from flora and previous research (Jeffrey 1980; Xu 1981; Chen and Tamura 2000; Liu et al. 2021).
Sequencing, plastome assembly and annotation
In order to determine the phylogenetic status of the taxon, we sequenced three samples from Nanchuan, Chongqing Municipality, Enshi, Hubei Province and Mt. Emei, Sichuan Province, respectively (Figure 1), as well as two Polygonatumkingianum, one Polygonatumsibiricum and one Polygonatumzanlanscianense (Table 1). Representative voucher specimens are currently deposited at the Herbarium of Zhejiang University (HZU). Genomic DNAs were extracted from silica-gel dried leaves using DNA Plantzol Reagent (Invitrogen), following the manufacturer’s instructions. The libraries were prepared and sequenced using paired-end 150 bp at Beijing Genomics Institute (BGI, Shenzhen, China) on a BGISEQ-500 sequencing platform. Approximately 3G raw data were generated for each sample. Raw data were trimmed by removing adapters and low-quality reads and then a de novo approach was applied to assemble plastomes using the NOVOPlasty v.3.8.3 (Dierckxsens et al. 2017) with K-mer = 39. The plastome and rbcL gene sequences of Polygonatumstenophyllum Maxim. (KX822773) were adopted as reference and seed sequence, respectively. DOGMA (Wyman et al. 2004) was used for plastome annotation with manually checking the start/stop codons in Geneious 10.2.3 (http://www.geneious.com). In addition, plastome data of Polygonatum and outgroups (Heteropolygonatum and Disporopsis Hance) from Floden & Schilling (2018) were used for phylogenetic analyses (Table 1). To study the phylogenetic relationship between P.kingianumvar.grandifolium and P.hunanense, rbcL, trnK, psbA-trnH and trnC-petN, sequences from Liu et al. (2021) were downloaded for further phylogenetic analyses.
Table 1.
GenBank accessions of plastomes involved in this study. Samples in bold were newly sequenced in this study.
| Species name | GenBank number | Length |
|---|---|---|
| Disporopsisjinfushanensis Z.Y. Liu | MH891733 | 155,188 |
| Heteropolygonatumaltelobatum (Hayata) Y.H. Tseng, H.Y. Tzeng et C.T. Chao | MH891734 | 155,534 |
| Heteropolygonatumalternicirrhosum (Hand.-Mazz.) Floden | MH891737 | 155,510 |
| Heteropolygonatummarmoratum (H. Lév.) Floden | MH891735 | 155,447 |
| Heteropolygonatumpendulum (Z.G. Liu et X.H. Hu) M.N. Tamura et Ogisu | MH891736 | 155,436 |
| Polygonatumacuminatifolium 2 Komarov | MH891751 | 155,304 |
| Polygonatumannamense Floden | MH891738 | 155,277 |
| Polygonatumarisanense Hayata | MH891752 | 155,340 |
| Polygonatumbiflorum (Walter) Elliott | MH891756 | 155,470 |
| Polygonatumcathcartii Baker | MH891745 | 155,970 |
| Polygonatumgovanianum Royle | MH891755 | 155,089 |
| Polygonatumkingianumvar.grandifolium 1 | MW373518 | 155,609 |
| Polygonatumkingianumvar.grandifolium 2 | MW373529 | 155,632 |
| Polygonatumkingianumvar.grandifolium 3 | MW373520 | 155,609 |
| Polygonatumhuanum H. Lév. | MH891743 | 155,545 |
| Polygonatumkingianum 1 Collett et Hemsley | MW373516 | 155,810 |
| Polygonatumkingianum 2 | MW373517 | 155,824 |
| Polygonatummengtzense 1 F.T. Wang et Tang | MH891740 | 155,498 |
| Polygonatummengtzense 2 | MH891741 | 155,492 |
| Polygonatumoppositifolium (Wall.) Royle | MH891746 | 155,760 |
| Polygonatumorientale Desf. | MH891753 | 155,386 |
| Polygonatumpunctatum Royle ex Kunth | MH891739 | 155,333 |
| Polygonatumsibiricum 1 Redouté | MW373521 | 155,549 |
| Polygonatumstenophyllum Maxim. | KX822773 | 156,028 |
| Polygonatumstewartianum Diels | MH891749 | 155,867 |
| Polygonatumtessellatum F.T. Wang et Tang | MH891747 | 155,724 |
| Polygonatumuncinatum Diels | MH891744 | 155,694 |
| Polygonatumurceolatum (J.M.H. Shaw) Floden | MH891742 | 155,504 |
| Polygonatumverticillatum 1 (L.) Allioni | MH891748 | 155,878 |
| Polygonatumverticillatum 2 | MH891750 | 155,502 |
| Polygonatumyunnanense H. Lév. | MH891754 | 155,363 |
| Polygonatumzanlanscianense 1 Pampanini | MW373522 | 155,911 |
Phylogeny of Polygonatum
The sequence of 78 protein coding genes (CDS) shared by all plastomes were aligned using MAFFT v.7 (Katoh and Standley 2013) in Geneious 10.2.3. The rbcL, trnK, psbA-trnH and trnC-petN sequences from Liu et al. (2021) and those from the seven newly-reported plastomes were aligned using MUSCLE in Geneious 10.2.3. DNASP6 was used to do statistics of site information (Rozas et al. 2017). The phylogenetic trees were constructed using both Maximum Likelihood (ML) and Bayesian Inference (BI) methods, implemented on CIPRES Science Gateway website (https://www.phylo.org, Miller et al. 2010) with the best-fit model of DNA substitution estimated by jModelTest v.2.1.4 (Darriba et al. 2012). ML analysis was conducted using RAxML-HPC BlackBox 8.2.12 with 1000 bootstrap replicates (Stamatakis 2014). Bayesian analysis was constructed using MrBayes XSEDE 3.2.7 with two independent Markov Chain Monte Carlo chains for 10,000,000 generations and sampling every 1000 generations (Ronquist and Huelsenbeck 2003). The first 25% of calculated trees were discarded as burn-in and the remaining trees were used to construct a consensus tree to estimate the posterior probability (PP).
Results and discussion
Morphological comparisons showed that P.kingianumvar.grandifolium is almost the same as P.hunanense, except that the latter has narrower leaves (Table 2). However, they both differ from other P.kingianum varieties in leaves, bracts, perianth and filaments (Table 2). In addition, we have observed stout and no thickening filaments in P.kingianumvar.grandifolium (Figure 2G and Suppl. material 1: Figure S1) and slender filaments in P.kingianum (Figure 4G and Suppl. material 1: Figure S1). Tamura has reported that species of sect. Verticillata has slender filaments, whereas sect. Polygonatum has stout filaments and filaments of the ser. Bracteata, ser. Polygonatum and ser. Inflata are thickening in the upper part, thickening in the middle or without thickening and thickening in the lower part, respectively (Tamura 1991, 1993; Tamura et al. 1997b).
Table 2.
Comparison of morphological characters amongst P.hunanenses, P.kingianumvar.grandifolium and P.kingianum varieties. The dashed line indicates the characters are the same as the original variety.
| Characters | P. hunanense | P. kingianum var. grandifolium | P. kingianum | |||
|---|---|---|---|---|---|---|
| var. kingianum | var. cavaleriei | var. ericoideum | var. uncinatum | |||
| Rhizomes | moniliform or ginger-like, 1–4 cm thick | moniliform or ginger-like, 1–3.5 cm thick | subterete or submoniliform, 1–3 cm thick | – | – | – |
| Stem | 1–3.5 m, apex subscandent | 1–3 m, apex subscandent | 1–3 m, apex subscandent | – | – | ca. 60 cm |
| Phyllotaxy | whorled, 3–6 (–10) per round | whorled, 3–5 per round | whorled, 3–10 per round | – | – | whorled, 4–5 per round |
| Leaf | 5–20 (–27) cm × 0.5–2.5 (–3.2) cm, linear to lanceolate, apex strongly cirrose or curved | 13–27 cm × (1.5–) 2.4–5 cm, linear to lanceolate, apex strongly cirrose or curved | 6–16 (–20) cm × 0.2–1.0 (–1.5) cm, linear to lanceolate, membranous, apex cirrose | linear to lanceolate, somewhat coriaceous | narrow linear | short lanceolate, somewhat coriaceous, 5–6 cm × 1–1.4 cm |
| Inflorescence | (1–) 2–5 | (1–) 2–5 | (1–) 2–4 (–6) | 1–2 | 2–4 | 1–2 |
| Peduncle | 1.7–3.5 cm | 1–4 cm | 1–2 cm | stout, strongly deflexed | 2–3 cm | decurved |
| Pedicel | 0.7–1.8 cm | 0.4–1.7 cm | 0.5–1.5 cm | – | ||
| Bract | subulate to lanceolate, 3–4 mm, at base of pedicel | linear, 2–5 mm, at base of pedicel | linear, 1–2 mm, on proximal part of pedicel | |||
| white or pale yellowish-green | yellow or greenish-white | pink, red | white, tinged purple | white | white | |
| Perianth | cylindrical campanulate | cylindrical | cylindrical–campanulate | – | – | – |
| 17–22 mm | 15–27 mm | 18–25 mm | – | – | 10–13 mm | |
| Lobes | 5–6.5 mm | 4–6.5 mm | 3–5 mm | – | – | – |
| Filament | 2–3 mm, flat | 2.5–3.5 mm, stout and no thickening | 1.7–5 mm, slender | – | – | – |
| Anthers | ca. 5 mm | 2.5–5.5 mm | 4–6 mm | – | – | – |
| Ovary | 5–7 mm | ca. 4 mm | 4–6 mm | – | – | – |
| Style | ca. 9–12 mm | ca. 8–14 mm | (8–) 10–14 mm | – | – | – |
| Fruits | berries pale yellowish-green or orange, 1–1.8 cm | berries yellow or orange with black spots, 1.4–1.8 cm | berries yellow, orange or red, 1–1.5 cm | – | – | – |
| Seeds | 3–12 | 5–15 | 7–12 | – | – | – |
| Distribution | Hunan, China | Southwest China | Southwest China, Myanmar, Thailand, Vietnam | China: Sichuan, Yunnan | China: Yunnan | China: Yunnan |
| Altitude | 200–700 m | 600–1200 m | 700–3600 m | – | – | – |
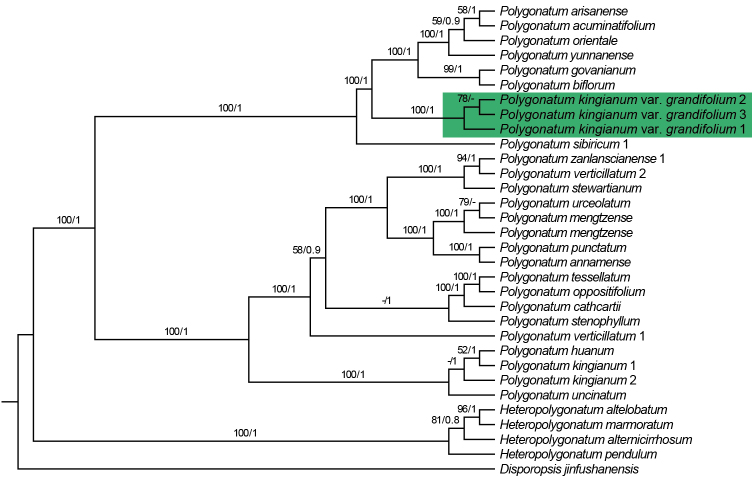
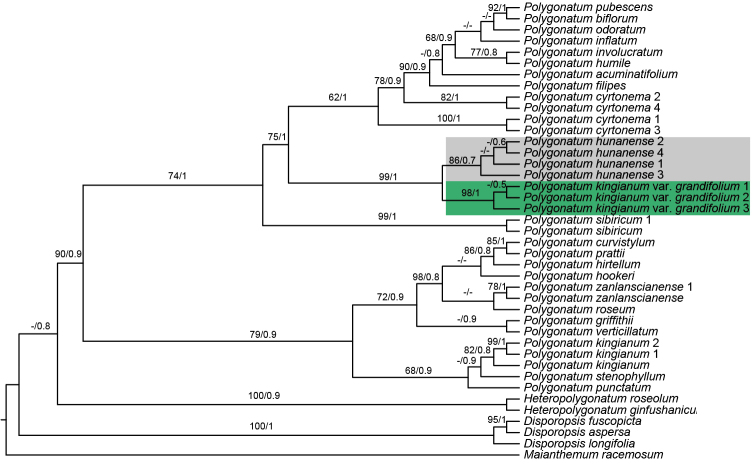
The length of seven plastomes ranged from 155,549 bp to 155,911 bp, the accession numbers being MW373516−MW373522 (Table 1). They displayed the typical quadripartite structure with 132 genes in the same order, of which 112 were unique genes including 78 protein-coding genes, 30 tRNA genes and 4 rRNA genes. The alignment CDS matrix has 66,589 characters in length, in which 1827 are variable (polymorphic) sites and 512 are parsimony-informative sites. In addition, the alignment matrix of four plastid fragments has 4,652 characters in length, of which 85 are variable (polymorphic) sites and 58 are parsimony-informative sites. The phylogenetic tree of 78 CDS supports a robust monophyletic clade of three samples of P.kingianumvar.grandifolium (BS = 100, PP = 1; Figure 5). However, it was not closely related to P.kingianum of section Verticillata, but was sister to the section Polygonatum (BS = 100, PP = 1; Figure 5). Additionally, the phylogeny, based on four plastid fragments, supported the monophyly of P.hunanense and P.kingianumvar.grandifolium (BS = 99, PP = 1; Figure 6), which suggested they should be conspecific.
Figure 5.
Phylogeny of Polygonatum, based on 78 protein coding genes (CDS) of plastome. Numbers above branches are Maximum Likelihood bootstrap values (BS)/Bayesian posterior probability (PP). The dash indicates support values of less than 50%. The phylogenetic position of Polygonatumkingianumvar.grandifolium is highlighted in green.
Figure 6.
Phylogeny of Polygonatum, based on rbcL, trnK, psbA-trnH and trnC-petN sequences. Numbers above branches are Maximum Likelihood bootstrap values (BS)/Bayesian posterior probability (PP). The dash indicates support values of less than 50%. The phylogenetic position of Polygonatumkingianumvar.grandifolium and P.hunanense are highlighted in green and grey, respectively.
Therefore, we propose that P.kingianumvar.grandifolium should be recognised as a new synonym of P.hunanense. In addition, both morphology and phylogeny showed that P.hunanense is different from P.kingianum and has a close relationship with sect. Polygonatum.
Taxonomic treatments
Polygonatum hunanense
H.H. Liu & B.Z. Wang
50E0C869-714C-59DE-B946-47EB803F1C55
=PolygonatumkingianumCollett & Hemsl.var.grandifolium D.M. Liu et W.Z. Zeng, Flora Sichuanica. 7: 230−231. 1981. Type: CHINA. Sichuan: Guan County, 900 m alt., 8 June 1978, Hao Zhang 1231 (lectotype, designated here: CDCM [barcode 00044013]!, Figure 3; isolectotype: CDCM [barcode 00044022]!).
Modified description of P.hunanense.
Perennials, rhizome moniliform or ginger-like, 1−4 cm in diam. Stem straight or apex subscandent, 1−3.5 m. Leaves in whorls of 3−6 (–10), sometimes alternate or opposite near base and/or apex of stem, sessile, elliptic to oblong-lanceolate, 5–20 (–27) cm long, 0.5−5 cm wide, apex strongly cirrose or curved. Inflorescences (1−) 2−5 flowered; peduncle 1−4 cm long, pendulous; bracts at base of pedicel, subulate to lanceolate, 2−5 mm. Pedicel 0.4−1.8 cm. Perianth yellowish- or greenish-white, cylindrical campanulate, slightly constricted in the middle, 1.5−2.7 cm long, perianth segments 6, arranged into 2 whorls, each 3 lobes 4−6.5 mm. Stamens 6, filaments 2−3.5 mm long, stout and no thickening, anthers 2.5−5.5 mm long. Ovary superior, globose 4−7 mm in diameter. Style 8−14 mm long. Berries pale yellowish-green or orange, 1−1.8 cm in diam., 3−15 seeds.
Phenology.
It sprouts twice a year, in spring (March to April) and autumn (September). The spring-sprouting individual flowers from April to May and fruits from December to next February. The autumn-sprouting individual flowers from November to December.
Distribution and habitat.
Polygonatumhunanense is relatively common in Chongqing Municipality, Sichuan, Hubei and Hunan Provinces, China (Figure 1). It grows in evergreen broad-leaved forests, thickets or on moist grassy slopes, at an elevation of 200 m to 1200 m. In addition, it is also widely cultivated in those areas for harvesting the rhizomes.
Conservation status.
To our knowledge, this species is widely distributed in low elevations of southwest China. Therefore, we propose to treat it as Least Concern (LC) according to the IUCN Red List Categories and Criteria version 14 (August 2019). However, due to the medicinal value of the genus, many of its populations are destroyed by unmanaged exploitation.
Other specimens examined.
China. Chongqing Municipality: Nanchuan District, Sanquan, 24 January 1984, Zhengyu Liu 4958 (fl., IMC!); ibidem, 10 July 1991, Zhengyu Liu 917801 (fr. IMC!); Nanchuan District, Jinfo Mountain, 13 September 1985, Zhengyu Liu 851732 (fr. IMC!); ibidem, 28 June 1999, Zhengyu Liu 974488 (fl., IMC!); ibidem, 28 June 1999, Zhengyu Liu 974498 (fl., IMC!); ibidem, 28 June 1999, Zhengyu Liu 990498 (fl., IMC!); ibidem, 28 August 1999, Zhengyu Liu 975059 (fr. IMC!); Jiangjin District, Simian Mountain, 16 July 2000, Zhengyu Liu 2004036 (fl., IMC!); Pengshui County, Longmenxia, 24 June 1988, Fading Fu & Yaling Cao 0264 (CDBI!); Qijiang District, Zhongfeng Town, Lianghekou, 22 October 2012, The Qijiang team 13-500222-LY-411-01 (IMC!); ibidem, 22 October 2012, The Qijiang team 13-500222-LY-411-02 (fr., IMC!); Qijiang District, Shihao & Wanlong, 12 October 2014, The Qijiang team Qianjiang-0310 (IMC!); Wuxi County, Shuangyang, 16 July 1996, Zhengyu Liu 760044 (fl., IMC!). Hubei Province: Enshi City, Hegongwei Village, 06 November 2016, Jinping Si & Jingjing Liu 33-4 (HZU!). Sichuan Province: Pengzhou City, 22 June 1978, Tianfu Yang & Yunjin Chen 1231 (CDCM!); Qionglai City, 11 June 1979, Chengdu University of TCM 0668 (fl., CDCM!).
Supplementary Material
Acknowledgements
The authors are grateful to Lili Ying, Yan Lian (CDCM) and Jinbo Tan (SZ). This work was supported by the State Key Laboratory of Subtropical Silviculture (No. KF2019-6), the Major Science and Technology Project for Selection and Breeding of New Varieties of Agriculture (Chinese medicinal materials) in Zhejiang Province (No. 2016C02058-4) and the Science and Technology Basic Resources Investigation Program of China “Survey and Germplasm Conservation of Plant Species with Extremely Small Populations in South-west China” (No. 2017FY100100).
Citation
Xia M, Liu Y, Liu J, Chen D, Shi Y, Bai Z, Xiao Y, Peng C, Si J, Li P, Qiu Y (2021) A new synonym of Polygonatum in China, based on morphological and molecular evidence. PhytoKeys 175: 137–149. https://doi.org/10.3897/phytokeys.175.63383
Contributor Information
Jinping Si, Email: lssjp@163.com.
Pan Li, Email: panli_zju@126.com.
Supplementary materials
Figure S1. Longitudinal section of flower of P.hunanense and P.kingianum
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Maoqin Xia, Ying Liu, Jingjing Liu, Donghong Chen, Yan Shi, Zhicong Bai, Yu Xiao, Chen Peng, Jinping Si, Pan Li, Yingxiong Qiu
Data type
species data
References
- Chen XQ, Tamura MN. (2000) Polygonatum Mill. In: Wu ZY, Raven PH. (Eds) Flora of China (Vol.24). Science Press, Beijing & Missouri Botanical Garden Press, Beijing/St. Louis, 225–235.
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9(8): 772–772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckxsens N, Mardulyn P, Smits G. (2017) NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Research 45. 10.1093/nar/gkw955 [DOI] [PMC free article] [PubMed]
- Floden A. (2015) Three new Solomon’s Seals (Polygonatum: Asparagaceae) from the Eastern Himalaya. Phytotaxa 236(3): 273–278. [2): 125–131.] 10.11646/phytotaxa.236.3.8 [DOI] [Google Scholar]
- Floden A, Schilling EE. (2018) Using phylogenomics to reconstruct phylogenetic relationships within tribe Polygonateae (Asparagaceae), with a special focus on Polygonatum. Molecular Phylogenetics and Evolution 129: 202–213. 10.1016/j.ympev.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Jeffrey C. (1980) The genus Polygonatum (Liliaceae) in eastern Asia. Kew Bulletin 34(3): 435–471. 10.2307/4109822 [DOI] [Google Scholar]
- Jeffrey C. (1982) Further note on eastern Asian Polygonatum (Liliaceae). Kew Bulletin 37(2): 335–339. 10.2307/4109978 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4): 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HH, Ma YS, Wang BZ, Jie HD, Xiang SJ, Yang JN, Jie YC. (2021) Morphological and molecular characters on a new species of Polygonatumhunanense from Hunan, China. Crop Research 35(1): 88–94. [Google Scholar]
- Meng Y, Nie ZL, Deng T, Wen J, Yang YP. (2014) Phylogenetics and evolution of phyllotaxy in the Solomon’s seal genus Polygonatum (Asparagaceae: Polygonateae). Botanical Journal of the Linnean Society 176(4): 435–451. 10.1111/boj.12218 [DOI] [Google Scholar]
- Miller AM, Pfeiffer P, Schwartz T. (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop 14: 1–8. 10.1109/GCE.2010.5676129 [DOI] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics (Oxford, England) 19(12): 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. (2017) DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Molecular Biology and Evolution 34(12): 3299–3302. 10.1093/molbev/msx248 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 30(9): 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura MN. (1990) Biosystematic studies on the genus Polygonatum (Liliaceae) I. Karyotype analysis of species indigenous to Japan and its adjacent regions. Cytologia 55(3): 443–466. 10.1508/cytologia.55.443 [DOI] [Google Scholar]
- Tamura MN. (1991) Biosystematic studies on the genus Polygonatum (Liliaceae) II. Morphology of staminal filaments of species indigenous to Japan and its adjacent regions. Acta Phytotaxonomica et Geobotanica 42: 1–18. [Google Scholar]
- Tamura MN. (1993) Biosystematic studies on the genus Polygonatum (Liliaceae) III. Morphology of staminal filaments and karyology of eleven Eurasian species. Bot. Jahrb. Syst. 115: 1–26. [Google Scholar]
- Tamura MN. (2008) Biosystematic studies on the genus Polygonatum (Asparagaceae) V. Taxonomic revision of species in Japan. Acta Phytotaxonomica et Geobotanica 59: 15–29. [Google Scholar]
- Tamura MN, Ogisu M, Xu JM. (1997a) Heteropolygonatum, a new genus of the tribe Polygonateae (Convallariaceae) from West China. Kew Bulletin 52(4): 949–956. 10.2307/4117821 [DOI] [Google Scholar]
- Tamura MN, Schwarzbach AE, Kruse S, Reski R. (1997b) Biosystematic studies on the genus Polygonatum (Convallariaceae) IV. Molecular phylogenetic analysis based on restriction site mapping of the chloroplast gene trnK. Feddes Repertorium 108(3–4): 159–168. 10.1002/fedr.4921080306 [DOI] [Google Scholar]
- Wang JJ, Yang YP, Sun H, Wen J, Deng T, Nie ZL, Meng Y. (2016) The biogeographic South-North divide of Polygonatum (Asparagaceae tribe Polygonateae) within Eastern Asia and its recent dispersals in the Northern Hemisphere. PLoS ONE 11: e0166134. 10.1371/journal.pone.0166134 [DOI] [PMC free article] [PubMed]
- Wyman SK, Jansen RK, Boore JL. (2004) Automatic annotation of organellar genomes with DOGMA. Bioinformatics (Oxford, England) 20(17): 3252–3255. 10.1093/bioinformatics/bth352 [DOI] [PubMed] [Google Scholar]
- Xu JM. (1981) Polygonatum Mill. In: Sichuan Flora Editorial Board (Eds) Flora Sichuanica. Sichuan People’s Publishing House, Chengdu, 7, 230−232.
- Zhao LH, Zhou SD, He XJ. (2019) A phylogenetic study of Chinese Polygonatum (Polygonateae, Asparagaceae). Nordic Journal of Botany 37(2): njb.02019. 10.1111/njb.02019 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Longitudinal section of flower of P.hunanense and P.kingianum
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Maoqin Xia, Ying Liu, Jingjing Liu, Donghong Chen, Yan Shi, Zhicong Bai, Yu Xiao, Chen Peng, Jinping Si, Pan Li, Yingxiong Qiu
Data type
species data