Abstract
Purpose
Coronavirus disease 2019 (COVID-19) has affected many parts of daily life and healthcare, including cancer screening and diagnosis. The purpose of this study was to determine whether there was an upshift in the colorectal cancer stage at diagnosis due to delays related to the COVID-19 outbreak.
Methods
From January to June of each year from 2017 to 2020, a total of 3,229 patients who were first diagnosed with colorectal cancer were retrospectively reviewed. Those enrolled from 2017 to 2019 were classified as the ‘pre-COVID’ group, and those enrolled in 2020 were classified as the ‘COVID’ group. The primary outcome was the rate of stage IV disease at the time of diagnosis.
Results
There was no statistically significant difference in the proportion of stage IV patients between the pre-COVID and COVID groups (P=0.19). The median preoperative carcinoembryonic antigen level in the COVID group was higher than in the pre-COVID group in all stages (all P<0.05). In stage I, II patients who underwent radical surgery, the lymphatic invasion was more presented in COVID patients (P=0.009).
Conclusion
We did not find significant stage upshifting in colorectal cancer during the COVID-19 outbreak. However, there were more initially unresectable stage IV colorectal cancer patients with a low conversion rate to resectable status, and more patients had factors related to poor prognosis. These results may become more apparent over time, so it is vital not to neglect cancer screening to not delay the diagnosis during the COVID-19 epidemic.
Keywords: COVID-19, Colorectal neoplasms, Early detection of cancer, Neoplasm staging
INTRODUCTION
Since the first outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China, in December 2019, it has spread worldwide. In many countries, the number of cases of COVID-19 infections has increased rapidly, and the World Health Organization (WHO) declared COVID-19 to be a pandemic on March 11, 2020. Cumulative infections total over 36 million, with over 1 million deaths recorded as of October 2020 [1]. The COVID-19 outbreak has had significant impacts on daily life. Many countries have implemented ‘lock-down’ policies to prevent the spread of COVID-19 and restrict people’s movement in different ways. In addition to this, many hospitals were shut down. It has reduced people’s access to healthcare and lowered cancer screening rates, leading to lower cancer diagnosis rates, including colorectal cancer [2-4].
Since January 2020, when the first COVID-19 occurred in Korea, approximately 25,000 confirmed cases of COVID-19 and 430 deaths have been recorded (as of October 2020) [5]. South Korea has relatively low mobility restrictions and is a country with a reasonably good quarantine system, so there was no remarkable decrease in direct access to hospitals. However, according to the data of Health Insurance Review and Assessment Service, the number of colonoscopies performed significantly decreased from January to March 2020 [6]. It is assumed that it decreased as in other countries not because of the difficulty of accessing healthcare but because of the fear of contacting other people.
The purpose of this study was to determine whether the number of patients diagnosed with colorectal cancer after the COVID-19 outbreak decreased and whether there was an upshift in stage at diagnosis.
METHODS
This study retrospectively reviewed data collected at a single tertiary colorectal cancer center. We enrolled a total of 3,229 patients diagnosed with colorectal cancer for the first time from January to June of each year from 2017 to 2020. In 2017, 2018, 2019, and 2020, 761, 808, 945, and 715 patients visited Samsung Medical Center (Seoul, Korea), respectively. 2017 to 2019 was classified as the pre-COVID group, and 2020 was classified as the COVID group.
Initial work-up included carcinoembryonic antigen (CEA) level, colonoscopy, esophagogastroscopy, abdomen and pelvis computed tomography (CT), chest CT, and rectum magnetic resonance imaging (MRI) if the tumor location was in the rectum. Further evaluation such as liver MRI or positron emission tomography/CT was done selectively if necessary. According to the results of these tests, a multidisciplinary team was formed to determine the treatment plan for patients with metastasis or other organ invasions, including whether to perform surgery immediately or administer chemotherapy. The FOLFOX/FOLFIRI plus bevacizumab or cetuximab was the usual first-line of chemotherapy for metastatic colorectal cancer. Although there is no exact protocol for the evaluation of operability after chemotherapy, generally, after 6 cycles of chemotherapy, stage IV patients rediscussed when to have surgery and decided whether to continue chemotherapy or conduct surgery and other local therapy such as radiofrequency ablation or radiotherapy and then reevaluated after 12 cycles of chemotherapy. However, this duration of chemotherapy until the operability was rediscussed differed according to the opinions of individual oncologists.
The clinical and pathologic stages were determined based on the 8th edition of the American Joint Committee for Cancer. Collected baseline characteristics included age, sex, body mass index (BMI), initial CEA level, and the American Society of Anesthesiologists (ASA) physical status (PS) classification. A CEA level that exceeded 5 ng/mL was defined as elevated. We obtained pathologic TNM stage, lymphovascular invasion, perineural invasion, and tumor budding for all patients who underwent surgery. We analyzed patients’ overall characteristics in the pre-COVID and COVID groups and subanalyzed stage IV patients and stage I–III patients who underwent surgery without neoadjuvant therapy.
The primary outcome was the rate of stage IV disease at the time of diagnosis. The secondary outcomes were pathologic stage and prognostic factors (lymphovascular invasion, perineural invasion, and tumor budding) among non-stage IV patients who underwent surgery without neoadjuvant therapy.
Categorical variables were compared using the chi-square or the Fisher exact test as needed, and continuous variables were analyzed using Student t-test or Mann-Whitney U-tests. Normality of data was checked using the Shapiro-Wilk test. All analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA), and a P-value of < 0.05 was considered statistically significant.
Approval was obtained and the informed consent requirement was waived from the Institutional Review Board of Samsung Medical Center (No. SMC 2020-07-068).
RESULTS
A total of 3,229 patients were included in this study. From January to June of each year from 2017 to 2019 (pre-COVID), 2,514 patients visited Samsung Medical Center (in 2017, 2018, and 2019 a total of 761, 808, and 945 patients, respectively) after being diagnosed with colorectal cancer for the first time, and from January to June of 2020 (COVID), 715 patients visited (Fig. 1).
Fig. 1.
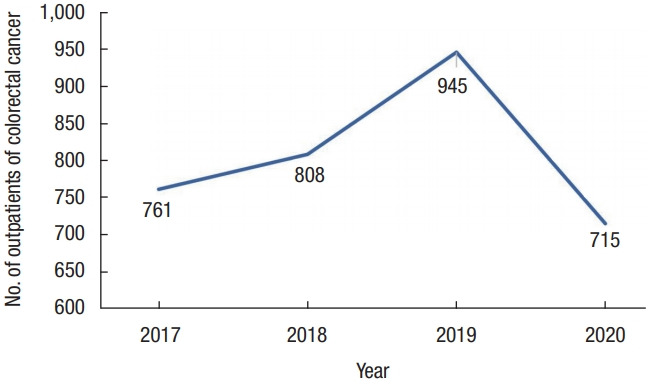
The trend of changes in the number of outpatient colorectal cancer patients by year.
The median age of pre-COVID and COVID was 61 years, and there was no statistically significant difference in cancer obstruction or perforation. The median preoperative CEA level in the pre-COVID group was 2.01 ng/mL but was 3.35 ng/mL in the COVID group (P< 0.0001). The proportion of patients with elevated CEA value (> 5 ng/mL) was significantly higher in the COVID period than in the pre-COVID period (36.2% vs. 21.6%, P < 0.0001). Moreover, the percentage of surgical candidates was lower during the COVID outbreak (73.57% vs. 82.18%, P< 0.0001). Although the ratio of stage IV patients was slightly higher (Table 1).
Table 1.
Demographics and clinical data of all stage patients (n=3,229)
| Variable | Pre-COVID (2017–2019) | COVID (2020) | P-value |
|---|---|---|---|
| No. of patients | 2,514 | 715 | |
| Age (yr)a | 61 (18–90) | 61 (17–97) | 0.80 |
| Sex | 0.64 | ||
| Male | 1,484 (59.0) | 415 (58.0) | |
| Female | 1,030 (41.0) | 300 (42.0) | |
| Body mass indexb (kg/m2) | 23.72 (14.41–40.72) | 23.62 (12.76–44.69) | 0.72 |
| Preoperative CEAa (ng/mL) | 2.01 (0.5–8,020) | 3.35 (0.3–19,110) | < 0.0001 |
| Elevated preoperative CEA, > 5 ng/mL | 525 (21.6) | 242 (36.2) | < 0.0001 |
| Cancer obstruction | 387 (15.4) | 104 (14.5) | 0.577 |
| Stent insertion | 178 (46.0) | 58 (55.8) | 0.107 |
| Stoma formation | 26 (6.7) | 2 (1.9) | |
| Bypass | 1 (0.3) | 0 (0) | |
| Cancer perforation | 31 (1.2) | 11 (1.5) | 0.524 |
| Surgery | 2,066 (82.2) | 526 (73.6) | < 0.0001 |
| Stage IV | 419 (16.7) | 134 (18.7) | 0.19 |
Values are presented as number only, amedian (range), number (%), or bmean (range).
COVID, coronavirus disease; CEA, carcinoembryonic antigen.
Among stage IV patients, the median CEA level of the COVID group was 14.7 ng/mL, in contrast to 8.59 ng/mL in the pre-COVID group (P= 0.021). The difference in the proportion of patients with an elevated CEA level did not show significance (pre-COVID vs. COVID, 61.8% vs. 69.1%; P= 0.163). The proportion of stage IV patients who were initially unresectable was 91.0% in the COVID group with a 12.3% conversion rate to resectable status and 82.8% in the pre-COVID group with a 20.7% conversion rate (all P< 0.05) (Table 2).
Table 2.
Characteristic of stage IV patients (n=553)
| Variable | Pre-COVID (2017–2019) | COVID (2020) | P-value |
|---|---|---|---|
| No. of patients | 419 (16.7) | 134 (18.7) | |
| Preoperative CEA (ng/mL) | 8.59 (0.5–8,020) | 14.7 (0.72–19,110) | 0.021 |
| Elevated preoperative CEA, > 5 ng/mL | 233 (61.8) | 76 (69.1) | 0.163 |
| Stage IVA | 234 (55.8) | 69 (51.5) | 0.366 |
| Stage IVB | 88 (21.0) | 36 (26.9) | |
| Stage IVC | 97 (23.2) | 29 (21.6) | |
| Initially unresectable | 347 (82.8) | 122 (91.0) | 0.021 |
| Conversion to resectable | 72 (20.7) | 15 (12.3) | 0.039 |
Values are presented as number (%) or median (range).
COVID, coronavirus disease; CEA, carcinoembryonic antigen.
Among stage I–III patients who underwent radical surgery without any neoadjuvant therapy such as chemotherapy or chemoradiotherapy, there were no differences in age, sex, BMI, ASA PS classification, or the proportion of hereditary colon cancer between the pre-COVID and COVID subgroups. The COVID subgroup had a higher median CEA value (1.69 ng/mL vs. 2.87 ng/mL, P< 0.0001) and a higher proportion of patients who showed elevated CEA level (12.9% vs. 29.1%, P< 0.0001) than the pre-COVID subgroup. However, there was no difference in stage, T classification, or N classification between the 2 groups. However, among stage I and II colorectal cancer patients, the lymphatic invasion was more common during the COVID-19 outbreak than before (pre-COVID vs. COVID, 14.9% vs. 22.1%; P = 0.009). Other factors such as vascular invasion, perineural invasion, and tumor budding were similar between groups (Table 3).
Table 3.
Characteristics of patients who had done curative surgery without neoadjuvant therapy (n=1,928)
| Variable | Pre-COVID (2017–2019) | COVID (2020) | P-value |
|---|---|---|---|
| No. of patients | 1,522 | 406 | |
| Agea (yr) | 62 (19–89) | 62 (17–92) | 0.235 |
| Sex | 0.943 | ||
| Male | 854 (56.1) | 227 (55.9) | |
| Female | 668 (43.9) | 179 (44.1) | |
| Body mass indexb (kg/m2) | 23.92 (14.41–37.27) | 23.84 (12.75–44.69) | 0.893 |
| ASA PS classification | 0.650 | ||
| I | 438 (28.8) | 114 (28.1) | |
| II | 963 (63.3) | 255 (62.8) | |
| III | 115 (7.6) | 34 (8.4) | |
| IV | 5 (0.3) | 3 (0.7) | |
| Hereditary colon cancer | 0.335 | ||
| HNPCC | 13 (0.9) | 4 (1.0) | |
| FAP | 6 (0.4) | 4 (1.0) | |
| Preoperative CEAa (ng/mL) | 1.69 (0.5–342.86) | 2.87 (0.3–268.00) | < 0.0001 |
| Elevated preoperative CEA, > 5 ng/mL | 196 (12.9) | 118 (29.1) | < 0.0001 |
| Character of surgery | > 0.999 | ||
| Elective | 1,518 (99.7) | 405 (99.8) | |
| Emergent | 4 (0.3) | 1 (0.2) | |
| Cancer obstruction | 202 (13.3) | 56 (13.8) | 0.784 |
| Cancer perforation | 18 (1.2) | 5 (1.2) | > 0.999 |
| TNM stage | 0.167 | ||
| 0+I | 409 (27.0) | 118 (29.3) | |
| II | 511 (33.8) | 116 (28.8) | |
| III | 594 (39.2) | 169 (41.9) | |
| T classification | 0.448 | ||
| Tis + T1 | 276 (18.2) | 86 (21.3) | |
| T2 | 230 (15.2) | 62 (15.3) | |
| T3 | 783 (51.7) | 193 (47.8) | |
| T4 | 226 (14.9) | 63 (15.6) | |
| N classification | 0.329 | ||
| N0 | 914 (60.5) | 235 (58.3) | |
| N1 | 430 (28.5) | 113 (28.0) | |
| N2 | 166 (11.0) | 55 (13.6) | |
| Stage I + II (n = 1,123) | 897 | 226 | |
| Lymphatic invasion | 134 (14.9) | 50 (22.1) | 0.009 |
| Perineural invasion | 265 (29.5) | 56 (24.8) | 0.157 |
| Vascular invasion | 16 (1.8) | 5 (2.2) | 0.592 |
| Tumor budding | 275 (30.7) | 63 (27.9) | 0.415 |
Values are presented as number only, amedian (range), number (%), or bmean (range).
COVID, coronavirus disease; ASA, American Society of Anesthesiologists; PS, physical status; HNPCC, hereditary nonpolyposis colorectal cancer; FAP, familial adenomatous polyposis; CEA, carcinoembryonic antigen.
DISCUSSION
Several studies have explored the impacts of COVID-19 on endoscopy volume and delayed diagnosis [2-4]. Colonoscopy is essential for the diagnosis of colon cancer, and in the COVID-19 pandemic, decreased volume of colonoscopy can delay the diagnosis of colon cancer. In Lui et al. [2], endoscopy volumes dropped more than 50%, and the number of patients diagnosed with colorectal cancer dropped 38.1% in Hong Kong after the COVID-19 outbreak. They predicted that the proportion of patients with colorectal cancer stage upshifting would be 6.4% at 6 months. In South Korea, colonoscopy volumes also dropped almost 50% in March 2020 compared to December 2019 [6]. Although our study did not investigate decreases in colon cancer screening, as supported by the studies mentioned above, we hypothesized that the rate of advanced colorectal cancer would increase as the diagnosis rate decreased.
We collected data for January to June for each year of the study to compare the number of patients diagnosed with colorectal cancer during the same period. In the pre-COVID period, the number of patients who visited Samsung Medical Center increased gradually, 761, 808, and 945 in 2017, 2018, and 2019, respectively, while in 2020, the number of patients decreased to 715. From this, we can cautiously infer that COVID-19 may have affected this decrease.
We expected that the number of stage IV patients to increase during the COVID-19 outbreak. However, only a slight, nonsignificant increase in the proportion of stage IV patients was seen in the COVID period. Although COVID-19 may not be associated with stage IV upshifting, the observation period may have been insufficient to determine the impact of COVID-19 on stage migration.
Instead, the number of initially unresectable stage IV patients was high in the COVID period compared to pre-COVID. It may indicate that the disease progressed during the COVID-19 outbreak, and delayed diagnosis of colorectal cancer could have affected the progression. Furthermore, 72 of 347 patients (20.7%) who were initially unresectable at pre-COVID changed to operable with a mean of 10.13 cycles of chemotherapy, and 15 of 122 patients (12.3%) who were unresectable during COVID-19 were converted to operable status with a mean of 7 cycles of chemotherapy. However, another consideration is that the patients diagnosed with metastatic colorectal cancer during the COVID-19 outbreak had insufficient chemotherapy to be surgical candidates during our observation period; thus, the proportion of operable metastasis may be relatively low. So, further study with a longer period should be conducted to check the differences exactly [7-9].
We also analyzed characteristics of the subgroup of patients who underwent surgery with definite curative intent without any neoadjuvant therapy. We thought there would be more advanced cases even among operable colorectal cancer during COVID-19. However, there was no difference in the pathologic stage of pre-COVID and COVID. COVID-19 may not have affected disease staging, but the results may have been underestimated due to the short observation period.
One of the noteworthy results of the present study is that there were more patients with lymphatic invasion among stage I and II colorectal cancer. Previous studies showed that lymphovascular invasion is significantly associated with poor prognosis in overall survival and disease-free survival in stage I and II colorectal cancer [10-14]. The higher rate of lymphatic invasion observed in stage I and II colorectal cancer patients during the COVID-19 outbreak may suggest a poor prognosis among these patients.
Preoperative CEA level was higher in the COVID group in every analysis, and there were more patients with an elevated CEA level (> 5 ng/mL) in the COVID group in non-stage IV patients. CEA is a proven tumor marker for monitoring and detecting tumor recurrence and evaluating treatment response in patients diagnosed with colorectal adenocarcinoma [15, 16]. Some studies also showed that an elevated preoperative CEA level over 5 ng/mL was associated with poor prognosis, and it could be an independent prognostic factor for stage I–III colorectal cancer patients [15-17]. Moreover, other studies showed elevated preoperative CEA level was associated with poor oncologic outcomes in stage IV colorectal cancer [18, 19]. In our study, although the proportion of stage IV patients with elevated CEA was not significantly different between the 2 groups, each group’s median CEA values showed significant difference. Based on these previous studies and the present study, COVID patients may have worse oncologic results than pre-COVID patients.
This study has several limitations. The most significant limitation is that the period used to determine the impact of COVID-19 was short. With a sufficient observation period, the impact of COVID-19 could be reliably assessed. However, it is noteworthy that meaningful differences between the pre- and COVID periods were shown even during the short observation period. Moreover, this is a single-center study and, although Samsung Medical Center is a large center in South Korea, it does not represent national trends. Thus, multicenter or national data collection is needed to understand national trends.
This study also has strengths. According to the data of WHO, health expenditure per capita in 2018 was $2,543 in South Korea, less than $10,624 in the United States, and $4,315 in the United Kingdom [20]. In addition, the number of visits to a doctor per capita in 2018 was 16.9, the highest among the Organization for Economic Co-operation and Development (OECD) countries [21]. As such, South Korea is one of the countries with easy access to hospitals and low medical costs, including routine health check-ups, and hospitals have not even been shut down due to the COVID-19 pandemic. In this respect, in other countries where the COVID-19 situation is more complicated than Korea and those with high medical costs or low access to hospitals, there could be more dramatic differences in colorectal cancer progression.
In conclusion, we did not find a significant stage upshifting in colorectal cancer associated with the COVID-19 outbreak. However, during this period, the number of first-visit outpatients’ decrease, more stage IV colorectal cancer patients were initially unresectable, and more patients who underwent surgery had poor prognostic factors. COVID-19 is an ongoing concern, and these results may become more apparent over time. It is vital not to neglect cancer screening to not delay the diagnosis during the COVID-19 epidemic in South Korea and worldwide. Moreover, it is necessary to establish and maintain a safe cancer screening system in the era of infectious diseases to prepare well even if other infectious diseases outbreak in the future. A longer observation period and multicenter studies are needed to confirm the results of this study.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.World Health Organization (WHO) Geneva: WHO; 2020. Coronavirus disease (COVID-19) pandemic [Internet] [cited 2020 Oct 10]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 2.Lui TK, Leung K, Guo CG, Tsui VW, Wu JT, Leung WK. Impacts of the coronavirus 2019 pandemic on gastrointestinal endoscopy volume and diagnosis of gastric and colorectal cancers: a population-based study. Gastroenterology. 2020;159:1164–6. doi: 10.1053/j.gastro.2020.05.037. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Ovidio V, Lucidi C, Bruno G, Lisi D, Miglioresi L, Bazuro ME. Impact of COVID-19 pandemic on colorectal cancer screening program. Clin Colorectal Cancer. 2021;20:e5–11. doi: 10.1016/j.clcc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maida M, Sferrazza S, Savarino E, Ricciardiello L, Repici A, Morisco F, et al. Impact of the COVID-19 pandemic on gastroenterology divisions in Italy: a national survey. Dig Liver Dis. 2020;52:808–15. doi: 10.1016/j.dld.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Central Disease Management Headquarters of Republic of Korea . Cheongju, KR: Korea Disease Control and Prevention Agency; 2020. COVID-19 [Internet] [cited 2020 Oct 10]. Available from: http://ncov.mohw.go.kr/ [Google Scholar]
- 6.Heathcare Bigdata Hub . Health Insurance Review & Assessment Service: Wonju, KR; c2015. Statistics of public interest medical practice (Colonoscopy) [Internet] [cited 2021 Jun 13]. Available from: http://opendata.hira.or.kr/op/opc/olapMfrnIntrsDiagBhvInfo.do#none. [Google Scholar]
- 7.Cremolini C, Antoniotti C, Stein A, Bendell J, Gruenberger T, Rossini D, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020 Aug 20; doi: 10.1200/JCO.20.01225. [Epub]. [DOI] [PubMed] [Google Scholar]
- 8.Elshenawy MA, Badran A, Aljubran A, Alzahrani A, Rauf MS, Eldali A, et al. Survival benefit of surgical resection after first-line triplet chemotherapy and bevacizumab in patients with initially unresectable metastatic colorectal cancer. World J Surg Oncol. 2020;18:163. doi: 10.1186/s12957-020-01930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 2017;3:e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan H, Dong Q, Zheng B, Hu X, Xu JB, Tu S. Lymphovascular invasion is a high risk factor for stage I/II colorectal cancer: a systematic review and meta- analysis. Oncotarget. 2017;8:46565–79. doi: 10.18632/oncotarget.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang HH, Zhang ZY, Wang XY, Tang X, Liu HL, Wang AL, et al. Prognostic significance of lymphovascular invasion in colorectal cancer and its association with genomic alterations. World J Gastroenterol. 2019;25:2489–502. doi: 10.3748/wjg.v25.i20.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular invasion and perineural invasion negatively impact overall survival for stage II adenocarcinoma of the colon. Dis Colon Rectum. 2019;62:181–8. doi: 10.1097/DCR.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 13.Lim SB, Yu CS, Jang SJ, Kim TW, Kim JH, Kim JC. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum. 2010;53:377–84. doi: 10.1007/DCR.0b013e3181cf8ae5. [DOI] [PubMed] [Google Scholar]
- 14.Zhong JW, Yang SX, Chen RP, Zhou YH, Ye MS, Miao L, et al. Prognostic value of lymphovascular invasion in patients with stage III colorectal cancer: a retrospective study. Med Sci Monit. 2019;25:6043–50. doi: 10.12659/MSM.918133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SH, Tsai WS, You JF, Hung HY, Yeh CY, Hsieh PS, et al. Preoperative carcinoembryonic antigen as a poor prognostic factor in stage I-III colorectal cancer after curative-intent resection: a propensity score matching analysis. Ann Surg Oncol. 2019;26:1685–94. doi: 10.1245/s10434-019-07184-3. [DOI] [PubMed] [Google Scholar]
- 16.Hall C, Clarke L, Pal A, Buchwald P, Eglinton T, Wakeman C, et al. A review of the role of carcinoembryonic antigen in clinical practice. Ann Coloproctol. 2019;35:294–305. doi: 10.3393/ac.2019.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baqar AR, Wilkins S, Staples M, Angus Lee CH, Oliva K, McMurrick P. The role of preoperative CEA in the management of colorectal cancer: a cohort study from two cancer centres. Int J Surg. 2019;64:10–15. doi: 10.1016/j.ijsu.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Chew MH, Teo JY, Kabir T, Koh PK, Eu KW, Tang CL. Stage IV colorectal cancers: an analysis of factors predicting outcome and survival in 728 cases. J Gastrointest Surg. 2012;16:603–12. doi: 10.1007/s11605-011-1725-1. [DOI] [PubMed] [Google Scholar]
- 19.Huh JW, Lee WY, Park YA, Cho YB, Yun SH, Kim HC, et al. Prognostic factors associated with primary cancer in curatively resected stage IV colorectal cancer. J Cancer Res Clin Oncol. 2014;140:435–41. doi: 10.1007/s00432-013-1580-4. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Geneva: WHO; c2021. Current health expenditure (CHE) per capita in US$: by country, 2018 [Internet] [cited 2021 Jun 13]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/current-health-expenditure-(che)-per-capita-in-us$. [Google Scholar]
- 21.The Organisation for Economic Co-operation and Development (OECD) Paris: OECD; 2021. OECD data: Doctor’s consultations (indicator) [Internet] [cited 2021 Jun 15]. Available from: [DOI] [Google Scholar]


