Abstract
Purpose
It is known that as the T stage of a carcinoma progresses, the prognosis becomes poorer. However, there are few studies about factors that affect the prognosis of T4 advanced colon cancer. This study aimed to identify the prognostic factors associated with disease-free survival (DFS) and overall survival (OS) in T4 colon cancer.
Methods
Patients diagnosed with stage T4 on histopathology after undergoing curative surgery for colon cancer between March 2009 and March 2018 were retrospectively analyzed for factors related to postoperative survival. Primary outcomes were DFS and OS.
Results
Eighty-two patients were included in the study. DFS and OS of the pathologic (p) T4b group were not inferior to that of the pT4a group. Multivariate analysis showed that differentiation (hazard ratio [HR], 4.994; P = 0.005), and laparoscopic surgery (HR, 0.323; P = 0.008) were significant prognostic factors for DFS, while differentiation (HR, 7.904; P ≤ 0.001) and chemotherapy (HR, 0.344; P = 0.038) were significant prognostic factors for OS.
Conclusion
Tumor differentiation, laparoscopic surgery, and adjuvant chemotherapy were found to be significant prognostic factors in patients with T4 colon cancer. Adjuvant chemotherapy and curative resections by laparoscopy might improve the prognosis in these patients.
Keywords: Colorectal neoplasms, Prognosis, Laparoscopy, Adjuvant chemotherapy
INTRODUCTION
Colorectal cancer is the fourth most common cancer worldwide [1], and the third most common cancer in Korea. In Korea, 25,330 people were newly diagnosed with colorectal cancer in 2019. The number of people who died from colorectal cancer in 2019 accounted for 10.5% of all deaths from cancer [2].
According to the TNM classification of cancers in the 8th edition of the American Joint Committee on Cancer (AJCC) cancer manual, the T4 stage of colorectal cancer indicates that the tumor has invaded the visceral peritoneum (T4a) or adjacent organs (T4b) [3]. In patients with colorectal cancer, the higher T stages are associated with poorer prognoses, such as increased rates of recurrence and decreased rates of survival [4-6]. A study found that the 5-year survival rate for node-negative colorectal cancer was 92.5% for stage I (T1N0M0 or T2N0M0), 83.6% for stage IIA (T3N0M0), 76.3% for stage IIB (T4aN0M0), and 58.8% for stage IIC (T4bN0M0) [4]. This decline in survival rates highlights the need to determine the prognostic factors affecting disease-free survival (DFS) and overall survival (OS) in patients with T4 colorectal cancer. This study aimed to identify the prognostic factors associated with DFS and OS in patients with T4 colon cancer.
METHODS
Patient selection
Between March 2009 and March 2018, Inje University Sanggye Paik Hospital admitted 161 patients who underwent radical surgery for primary colorectal cancer, adherent to the adjacent organs or structures. Data collected from the hospital database, medical records, and the National Cancer Center database were analyzed retrospectively. This study was approved by the Institutional Review Board (IRB) of Inje University Sanggye Paik Hospital (No. 2020-08-004). The IRB waived the need for informed consent for this retrospective chart review.
The inclusion criterion for this study was a pathological confirmation of T4 colon cancer for which curative resection surgery was performed (the TNM staging was based on the 8th edition AJCC cancer staging manual). The exclusion criteria were patients with rectal cancer, inadequate electronic records, recurrent colorectal cancer, positive surgical margins, distant metastasis at the time of diagnosis, any other cancers, neoadjuvant chemoradiotherapy, follow-up duration of 1 year or less after resection, and those with 30-day mortality after resection. After applying the inclusion and exclusion criteria, 82 patients with T4 colon cancer were included in the study.
Data collection and outcome measures
The data collected included the age, sex, body mass index, Charlson comorbidity index, location of the tumor, pathologic and histological features, tumor size, TNM stage, levels of carcinoembryonic antigen (CEA), surgical technique, adjuvant chemotherapy regimens, recurrence, length of follow-up after resection, and mortality. Patients in whom the curative resection surgery was performed using the laparoscopic technique, were defined as the ‘laparoscopic’ group. Patients who were converted from the laparoscopic technique to the open technique were assigned to the ‘open’ group. ‘Right colon’ group was defined as cases in which the tumors were located from the cecum to the splenic flexure, and the ‘left colon’ group comprised patients in whom the tumors were located from the descending colon to the sigmoid colon. Patients who received more than 50% of the planned cycles of adjuvant chemotherapy were defined as the ‘chemotherapy’ group. Patients who received chemotherapy for 6 or more cycles per the modified FOLFOX (folinic acid, fluorouracil, and oxaliplatin) regimen, or received 4 or more cycles of capecitabine only or Xelox (capecitabine and oxaliplatin) regimen were in the chemotherapy group. The outcome measures were DFS and OS.
Statistical analysis
The categorical variables are reported as counts (percentages), while age, tumor size, and CEA level are reported as means ± standard deviations. The statistical significance of any differences between the groups was evaluated using the chi-square test. Time‐to‐event analyses were performed for DFS and OS, and Kaplan-Meier estimates were plotted. Univariate Cox regression analyses were used to assess the variables that were the candidate predictors for DFS and OS. Factors with P < 0.1 on univariate analyses were entered into multivariate analyses using the Cox model. Hazard ratios (HRs) and their respective 95% confidence intervals (CIs) were calculated. A P-value of < 0.05 was considered statistically significant. The software used for the analysis was IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
The mean age of the study group was 67.8 ± 13.1 years. Forty-seven subjects (57.3%) were men. There were no statistical differences in age, Charlson comorbidity index, and nodal status between the T4a and T4b groups. The number of patients who underwent laparoscopic surgery was 52. Four patients who were taken up for laparoscopic surgery initially were converted to open surgery. After the curative-intent surgery, adjuvant chemotherapy was administered to 68 patients (82.9%). Other patients’ characteristics according to the T stage are presented in Table 1.
Table 1.
Demographic features of T4 colon cancer
| Variable | Total | Stage |
P-value | |
|---|---|---|---|---|
| T4a | T4b | |||
| No. of patients | 82 | 69 | 13 | |
| Age (yr) | 67.8 ± 13.1 | 68.0 ± 12.8 | 66.9 ± 14.8 | 0.778 |
| Sex | 0.008 | |||
| Male | 47 (57.3) | 44 (63.8) | 3 (23.1) | |
| Female | 35 (42.7) | 25 (36.2) | 10 (76.9) | |
| Body mass index (kg/m2) | 23.0 ± 3.2 | 23.1 ± 3.3 | 22.2 ± 2.6 | 0.329 |
| CCI | 0.492 | |||
| ≥4 | 35 (42.7) | 30 (43.5) | 5 (38.5) | |
| <4 | 47 (57.3) | 39 (56.5) | 8 (61.5) | |
| Location of tumor | 0.062 | |||
| Right | 51 (62.2) | 40 (58.0) | 11 (84.6) | |
| Left | 31 (37.8) | 29 (42.0) | 2 (15.4) | |
| Differentiation | 0.059 | |||
| Well | 3 (3.7) | 1 (1.4) | 2 (15.4) | |
| Moderate | 72 (87.8) | 62 (89.9) | 10 (76.9) | |
| Poorly | 3 (3.7) | 2 (2.9) | 1 (7.7) | |
| Othersa | 4 (4.9) | 4 (5.8) | 0 (0) | |
| Tumor size (cm) | 6.0 ± 1.9 | 6.0 ± 1.9 | 6.0 ± 2.1 | 0.974 |
| N stage | 0.193 | |||
| 0 | 32 (39.0) | 24 (34.8) | 8 (61.5) | |
| 1 | 30 (36.6) | 27 (39.1) | 3 (23.1) | |
| 2 | 20 (24.4) | 18 (26.1) | 2 (15.4) | |
| Invasion | ||||
| Lymphatic | 72 (87.8) | 59 (85.5) | 13 (100) | 0.159 |
| Vascular | 41 (50.0) | 35 (50.7) | 6 (46.2) | 0.500 |
| Perineural | 24 (29.3) | 19 (27.5) | 5 (38.5) | 0.314 |
| CEA (ng/mL) | 21.3 ± 58.8 | 11.7 ± 28.8 | 60.2 ± 117.5 | 0.003 |
| Operative technique | 0.044 | |||
| Open | 30 (36.6) | 22 (31.9) | 8 (61.5) | |
| Laparoscopic | 52 (63.4) | 47 (68.1) | 5 (38.5) | |
| Adjuvant chemotherapy | 68 (82.9) | 56 (81.2) | 12 (92.3) | 0.299 |
| Recurrence | 23 (28.0) | 20 (29.0) | 3 (23.1) | 0.475 |
| Local | 4 (4.9) | 4 (5.8) | 0 (0) | 0.494 |
| Distant | 21 (25.6) | 18 (26.1) | 3 (23.1) | 0.562 |
| Expire | 27 (32.9) | 23 (33.3) | 4 (30.8) | 0.566 |
Values are presented as number only, mean±standard deviation, or number (%).
CCI, Charlson comorbidity index; CEA, carcinoembryonic antigen.
Mucinous carcinoma and signet ring cell.
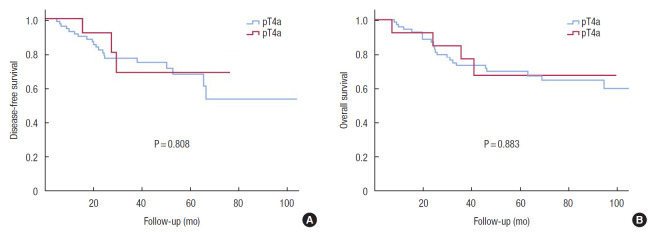
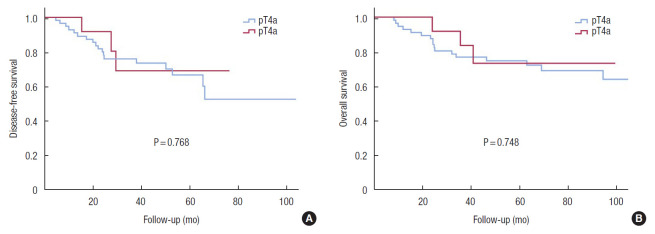
Kaplan-Meier curves demonstrated that DFS and OS of the T4b group were not inferior to those of the T4a group (Fig. 1). Comparing patients in the T4a and T4b groups who received adjuvant chemotherapy, the results were similar (Fig. 2). Differentiation (HR, 0.499; P = 0.005) and surgical technique (HR, 0.323; P = 0.008) were independent prognostic factors for DFS in the univariate and multivariate Cox regression analysis (Table 2).
Fig. 1.
Disease-free survival (A) and overall survival (B) curves comparing the groups with pathologic (p) T4a and pT4b colon cancer.
Fig. 2.
Disease-free survival (A) and overall survival (B) curves comparing patients with pathologic (p) T4a and pT4b colon cancer who received adjuvant chemotherapy. Using the Kaplan-Meier survival curve, we compared the disease-free survival and overall survival between patients in the pT4a and pT4b groups.
Table 2.
Univariate and multivariate Cox regression analyses for predictors of disease-free survival (DFS) in T4 colon cancer
| Variable | DFS |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Male sex | 1.459 | 0.618–3.444 | 0.389 | |||
| Age | 0.995 | 0.966–1.025 | 0.737 | |||
| Body mass index | 1.012 | 0.890–1.150 | 0.861 | |||
| CCI, < 4 | 0.642 | 0.278–1.482 | 0.299 | |||
| Tumor location | 0.897 | |||||
| Right | 1.000 | |||||
| Left | 1.057 | 0.460–2.429 | ||||
| Differentiation | 0.006 | 0.005 | ||||
| WD, MD | 1.000 | 1.000 | ||||
| PD, othersa | 4.730 | 1.555–14.386 | 4.994 | 1.610–15.489 | ||
| Size | 0.929 | 0.747–1.156 | 0.510 | |||
| T stage | 0.058 | |||||
| pT4a | 1.000 | |||||
| pT4b | 0.383 | 0.142–1.034 | ||||
| Positive node | 0.860 | 0.006–0.006 | 0.808 | |||
| Invasion | ||||||
| Lymphatic | 1.793 | 0.703–4.577 | 0.222 | |||
| Vascular | 3.752 | 0.503–27.969 | 0.197 | |||
| Perineural | 1.578 | 0.686–3.630 | 0.283 | |||
| CEA | 0.992 | 0.971–1.014 | 0.491 | |||
| Laparoscopic | 0.332 | 0.144–0.762 | 0.009 | 0.323 | 0.140–0.747 | 0.008 |
| Adjuvant chemotherapy | 1.425 | 0.329–6.169 | 0.635 | |||
HR, hazard ratio; CI, confidence interval; CCI, Charlson comorbidity index; WD, well differentiated; MD, moderate differentiated; PD, poorly differentiated; p, pathologic; CEA, carcinoembryonic antigen.
Mucinous carcinoma and signet ring cell.
The univariate Cox regression for OS was significant for the following variables: location of cancer, differentiation, surgical technique, and adjuvant chemotherapy. After the multivariate Cox analysis, differentiation (HR, 7.904; P< 0.001) and chemotherapy (HR, 0.344; P= 0.038) remained as significant prognostic factors (Table 3).
Table 3.
Univariate and multivariate Cox regression analyses for predictors of overall survival (OS) in T4 colon cancer
| Variable | OS |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Male sex | 1.175 | 0.544–2.538 | 0.682 | |||
| Age | 1.004 | 0.974–1.036 | 0.778 | |||
| Body mass index | 1.106 | 0.977–1.251 | 0.112 | |||
| CCI, < 4 | 1.109 | 0.514–2.393 | 0.792 | |||
| Tumor location | 0.024 | 0.289 | ||||
| Right | 1.000 | 1.000 | ||||
| Left | 0.346 | 0.138–0.869 | 0.583 | 0.215–1.582 | ||
| Differentiation | < 0.001 | < 0.001 | ||||
| WD, MD | 1.000 | 1.000 | ||||
| PD, othersa | 6.924 | 2.704–17.726 | 7.904 | 2.827–22.101 | ||
| Size | 0.859 | 0.703–1.048 | 0.134 | |||
| T stage | 0.883 | |||||
| pT4a | 1.000 | |||||
| pT4b | 0.923 | 0.319–2.675 | ||||
| Positive nodes | 1.203 | 0.550–2.632 | 0.643 | |||
| Invasion | ||||||
| Lymphatic | 1.248 | 0.374–4.156 | 0.719 | |||
| Vascular | 0.787 | 0.367–1.688 | 0.539 | |||
| Perineural | 1.566 | 0.714–3.436 | 0.263 | |||
| CEA | 0.999 | 0.984–1.013 | 0.841 | |||
| Laparoscopic | 0.421 | 0.197–0.900 | 0.026 | 0.550 | 0.253–1.196 | 0.132 |
| Adjuvant chemotherapy | 0.354 | 0.146–0.857 | 0.021 | 0.344 | 0.125–0.944 | 0.038 |
HR, hazard ratio; CI, confidence interval; CCI, Charlson comorbidity index; WD, well differentiated; MD, moderate differentiated; PD, poorly differentiated; p, pathologic; CEA, carcinoembryonic antigen.
Mucinous carcinoma and signet ring cell.
DISCUSSION
Before modification of the T4 stage in the AJCC 7th edition, the peritoneal invasion was considered to have a poor prognosis. However, later studies have also shown that peritoneal invasion had a 10% to 20% higher survival rate over that of adjacent organ invasion [7, 8]. These findings led to the modification of the T4 stage in the AJCC 7th edition. After this modification, further studies found that T4a colon cancer had a poorer prognosis compared to T4b colon cancer. Baguena et al. [9] found that the T4a stage had poorer oncologic outcomes compared to those of the T4b stage. This could be attributed to the resection of T4b tumors en bloc, without any cancer cells spreading into the peritoneal cavity, whereas with T4a tumors this might not be possible [9, 10]. Although the current study assumed that the different stages of T4 affect the long-term outcomes, no significant differences in DFS and OS with respect to this parameter were found.
Some studies have reported that patients with T4 colon cancer who underwent laparoscopic surgery had lesser blood loss compared to the amount of blood lost during open surgery of similar duration. In addition, the laparoscopic group had a faster postoperative recovery and the survival outcomes were equivalent to those of the open group [6, 11, 12]. In the current study, while the comparison of the open and the laparoscopic groups showed no statistically significant differences in age, tumor size, T4 stage, tumor location, and differentiation, those who underwent laparoscopic surgery showed a better prognosis for DFS. Some studies explain the benefit of laparoscopy as being a consequence of the surgical stress response, impaired immune responses, cytokine release, and intraoperative tumor manipulation associated with open surgery. Surgical response after colon surgery impairs immunity [13, 14], and the suppressed immune system after surgery can increase the spread of the tumor [15]. Whelan et al. [16] observed that postoperative cellular immune function is well preserved with a laparoscopic approach. It has also been reported that the non-touch technique results in a lower rate of detection of the tumor cells in the draining vein and portal venous system [17, 18]. Therefore, laparoscopic surgery has the advantages of controlling the spread of the tumor and promoting long-term survival [16-19]. For patients in the current study, the condition of the patients or the severity of the disease might have influenced the surgeon’s decision when selecting the surgical method. Thus, due to the retrospective design of the study, this selection bias might have led to a favorable prognosis for the laparoscopic group.
Klaver et al. [20] concluded while that laparoscopic surgery for T4a colon cancer might be safe, it is not appropriate for T4b colon cancer. They explained that it was difficult to perform microscopically margin-negative resections in patients with T4b colon cancer because the tumor has invaded the adjacent organs. However, research has shown that a multivisceral resection can be performed to achieve a clear resection margin. This can be beneficial for long-term survival in locally advanced primary colon cancer [21]. In this study, patients who underwent curative resection for both, T4a and T4b colon cancer, were included. This might be the reason for no statistically significant difference in the prognosis between T4a and T4b colon cancer. Therefore, the prognosis for T4b colon cancer might improve, if curative resections are performed in these patients.
While studies have shown that the location of the tumor affects the long-term outcome of cancer, there is no definitive conclusion about which location has a poor prognosis. Some studies have observed that the right-sided colon cancers have a poorer prognosis than those on the left side. They explained that this was due to genetic differences, more advanced staging, and fewer curative resections [22-24]. In contrast, a study by Warschkow et al. [25] found that the prognosis for localized right-sided colon cancer is better than that for left-sided colon cancer. In the current study, there were no significant differences between cancers in the right colon and left colon with respect to DFS and OS. Analysis of the prognosis, including margin-negative rectal cancer, showed that rectal cancer had a poorer prognosis compared to that of colon cancer. Li et al. [26] reported that the site of the tumor played an important role, for example, the rectum had a poorer prognosis than the colon. They also reported that colon cancer and rectal cancer are different diseases because of the embryological, morphological, and biological differences between the 2 sites. When comparing colon and rectal cancer, there are also differences such as concurrent chemoradiotherapy before surgery or radiotherapy after surgery. Considering these differences, our study excluded rectal cancer.
While the benefits of adjuvant chemotherapy in advanced colon cancer are well known, there are not many studies on the effectiveness of adjuvant chemotherapy on the prognosis of T4 colon cancer. Macari et al. [5] analyzed the effect of adjuvant chemotherapy on the survival of patients with T4 colon cancer; however, the results for both DFS and OS were not significant. In the current study, while there was no significant relationship between adjuvant chemotherapy and DFS, the multivariate analysis indicated that adjuvant chemotherapy had a significant benefit for OS. Furthermore, the group of patients who received adjuvant chemotherapy (81.2% in the T4a group and 92.3% in the T4b group) showed no statistical differences between the OS.
The limitations of this study are that it was a single-center, retrospective study. In addition, the cases were not randomly selected and the distribution between T4a and T4b was unequal. The number of patients in the T4b group was small; hence, no statistically significant independent prognostic factors could be identified in the T4b group. Furthermore, the cause of mortality during the research period was unknown and the OS was not verified as cancer-specific survival. Despite these limitations, due to which the results cannot be generalized, the results of this study indicate the prognostic factors affecting DFS and OS in T4 colon cancer and add to the body of evidence in this area.
In conclusion, when en bloc resection was performed, the prognosis of T4b colon cancer was not inferior to that of T4a colon cancer. Differentiation, surgical technique, and adjuvant chemotherapy were found as prognostic factors in patients with T4 colon cancer. Adjuvant chemotherapy along with curative resections by laparoscopy might improve the prognosis in these patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2019. Cancer Res Treat. 2019;51:431–7. doi: 10.4143/crt.2019.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454–5. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 4.Gao P, Song YX, Wang ZN, Xu YY, Tong LL, Sun JX, et al. Is the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) database. BMC Cancer. 2013;13:123. doi: 10.1186/1471-2407-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macari D, Kawak S, Raofi V, Wasvary H, Jaiyesimi I. Recurrence pattern and outcomes in T4 colon cancer: a single institution analysis. J Surg Oncol. 2019;121:337–41. doi: 10.1002/jso.25766. [DOI] [PubMed] [Google Scholar]
- 6.Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, et al. Clinically suspected T4 colorectal cancer may be resected using a laparoscopic approach. BMC Cancer. 2016;16:714. doi: 10.1186/s12885-016-2753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–71. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256–63. doi: 10.1200/JCO.2009.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baguena G, Pellino G, Frasson M, Rosello S, Cervantes A, Garcia-Granero A, et al. Prognostic impact of pT stage and peritoneal invasion in locally advanced colon cancer. Dis Colon Rectum. 2019;62:684–93. doi: 10.1097/DCR.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 10.Keshava A, Chapuis PH, Chan C, Lin BP, Bokey EL, Dent OF. The significance of involvement of a free serosal surface for recurrence and survival following resection of clinicopathological stage B and C rectal cancer. Colorectal Dis. 2007;9:609–18. doi: 10.1111/j.1463-1318.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Wu Q, Gu C, Hu T, Bi L, Wang Z. Comparison of short and long-time outcomes between laparoscopic and conventional open multivisceral resection for primary T4b colorectal cancer. Asian J Surg. 2019;42:401–8. doi: 10.1016/j.asjsur.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Kim KY, Hwang DW, Park YK, Lee HS. A single surgeon’s experience with 54 consecutive cases of multivisceral resection for locally advanced primary colorectal cancer: can the laparoscopic approach be performed safely? Surg Endosc. 2012;26:493–500. doi: 10.1007/s00464-011-1907-7. [DOI] [PubMed] [Google Scholar]
- 13.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 14.Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 15.Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc. 1999;13:233–5. doi: 10.1007/s004649900952. [DOI] [PubMed] [Google Scholar]
- 16.Whelan RL, Franklin M, Holubar SD, Donahue J, Fowler R, Munger C, et al. Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc. 2003;17:972–8. doi: 10.1007/s00464-001-8263-y. [DOI] [PubMed] [Google Scholar]
- 17.Sales JP, Wind P, Douard R, Cugnenc PH, Loric S. Blood dissemination of colonic epithelial cells during no-touch surgery for rectosigmoid cancer. Lancet. 1999;354:392. doi: 10.1016/S0140-6736(99)92164-5. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi N, Egami H, Kai M, Kurusu Y, Takano S, Ogawa M. Notouch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery. 1999;125:369–74. [PubMed] [Google Scholar]
- 19.Bouvy ND, Marquet RL, Jeekel J, Bonjer HJ. Laparoscopic surgery is associated with less tumour growth stimulation than conventional surgery: an experimental study. Br J Surg. 1997;84:358–61. [PubMed] [Google Scholar]
- 20.Klaver CE, Kappen TM, Borstlap WA, Bemelman WA, Tanis PJ. Laparoscopic surgery for T4 colon cancer: a systematic review and meta-analysis. Surg Endosc. 2017;31:4902–12. doi: 10.1007/s00464-017-5544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohan HM, Evans MD, Larkin JO, Beynon J, Winter DC. Multivisceral resection in colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:2929–36. doi: 10.1245/s10434-013-2967-9. [DOI] [PubMed] [Google Scholar]
- 22.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 23.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–94. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suttie SA, Shaikh I, Mullen R, Amin AI, Daniel T, Yalamarthi S. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis. 2011;13:884–9. doi: 10.1111/j.1463-1318.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- 25.Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer. 2016;16:554. doi: 10.1186/s12885-016-2412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Li JY, Zhao AL, Gu J. Colorectal cancer or colon and rectal cancer? Clinicopathological comparison between colonic and rectal carcinomas. Oncology. 2007;73:52–7. doi: 10.1159/000120628. [DOI] [PubMed] [Google Scholar]