Abstract
Context
Rheumatic conditions have a large impact on both patients and society. Many patients experience adjustment problems, such as symptoms of anxiety and depression and sleep problems, contributing to high healthcare costs. Internet-based cognitive-behavioral therapy (iCBT) has shown to support patients with somatic conditions in coping with their disease, with therapist-guided iCBT usually showing larger effects than unguided iCBT. However, the specific relevance of guided iCBT for rheumatic conditions has not been reviewed yet, which could have important implications for implementation.
Objectives
The objective of our review was to give an overview of evaluations of guided iCBT for rheumatic conditions, including physical, psychological, and impact on daily life outcomes.
Methods
This review is registered with PROSPERO with registration number CRD42020154911. The review followed PRISMA guidelines and included an assessment of risk of bias. PubMed, PsycINFO, Embase, Cochrane Library, Web of Science, and Emcare were searched until 5 October 2020. Inclusion criteria were: patients ≥18 years old with a rheumatic condition, randomized controlled trial, accessible full-text English article, original data, inclusion of psychological, and/or physical and/or impact outcomes, and therapist-guided iCBT. Study and sample characteristics, as well as clinical variables were extracted.
Results
A systematic search identified 6089 studies, of which 8 trials were included, comprising of 1707 participants in total. Significant medium to large between-group effects were found for psychological outcomes (depression, anxiety, catastrophizing, self-efficacy) and impact on daily life outcomes (impact on daily life, quality of life), whilst results for physical outcomes (pain intensity, fatigue) were mixed.
Conclusion
Whilst more research is warranted, for instance regarding physical outcomes, cost-effectiveness, safety of the intervention, and moderators of iCBT success, our results show that guided iCBT could be an important addition to medical treatment for rheumatic conditions. Guided iCBT can improve psychological and impact on daily life outcomes in patients with rheumatic conditions, which is promising for iCBT implementation in clinical practice.
Keywords: Rheumatic conditions, Chronic pain, Online cognitive-behavioral therapy, Internet interventions
Highlights
-
•
This systematic review summarized the efficacy of guided iCBT for rheumatic diseases.
-
•
Positive results were found for psychological and disease impact outcomes.
-
•
Results for physical outcomes were mixed.
-
•
ICBT can help patients develop self-management skills to cope with their disease.
-
•
ICBT can be an important addition to medical treatment for rheumatic diseases.
1. Introduction
Since rheumatic conditions are chronic and generally characterized with pain, stiffness, and fatigue, many individuals suffering from these conditions experience adjustment problems (Berger et al., 2007; Evers et al., 2011; Sturgeon et al., 2016). These adjustment problems interfere with a patient's daily life and encompass, among others, symptoms of anxiety and depression, and sleep problems (Berger et al., 2007; Evers et al., 2011; Sturgeon et al., 2016). For example, a meta-analytic review showed that pooled point prevalence rates of depression ranged from 15% to 39% in patients with rheumatoid arthritis from different countries worldwide, depending on the questionnaire and threshold used (Matcham et al., 2013). Moreover, a cross-sectional study showed a prevalence of depression and anxiety of about 50% in a Croatian sample of patients with inflammatory rheumatic disease (Petric et al., 2015). These adjustment problems have a large impact on both patients and society: patients' quality of life and their treatment adherence are often negatively affected, resulting in high healthcare use and high medical costs (Berger et al., 2007; De Achaval and Suarez-Almazor, 2010; Hresko et al., 2018; Lacasse et al., 2016; Yelin et al., 2007). Improving patients' self-management skills in coping with the consequences of a chronic disease in daily life is, therefore, increasingly recognized as an essential addition to medical treatment (Bodenheimer et al., 2002a; Bodenheimer et al., 2002b; Borenstein et al., 2017). Self-management refers specifically to the ability to monitor the condition and to use cognitive, behavioral, and emotional strategies to maintain a satisfactory quality of life (Barlow et al., 2002).
Cognitive-behavioral therapy (CBT) has shown to be an effective self-management intervention for various somatic conditions and symptoms (e.g., fibromyalgia, rheumatoid arthritis, and chronic pain [Bernardy et al., 2018; Butler et al., 2006; Cunningham and Kashikar-Zuck, 2013; Dures and Hewlett, 2012; Evers et al., 2002; Lami et al., 2013]). In CBT, disease-specific dysfunctional beliefs and behaviors are being challenged and modified using cognitive and/or behavioral exercises in a structured manner (O'Donohue and Fisher, 2012). However, broad implementation of such interventions is hampered by various barriers, including high monetary costs, time constraints in the medical setting, as well as a lack of available and trained healthcare providers (Ferwerda et al., 2018; Taylor and Chang, 2008). Moreover, patients experience barriers themselves, such as physical constraints to travel and stigma associated with psychological treatment (Evers et al., 2002; Lami et al., 2013). Internet-based cognitive-behavioral therapy (iCBT) has the potential to largely overcome these barriers, as the online treatment program can be attended at home with minimal remote professional guidance (Andersson et al., 2014; Bendig et al., 2018). Guided iCBT generally consists of several modules in a secured online environment, which include psychoeducation, assignments, relaxation exercises, and relapse prevention (Andersson et al., 2014; Van Beugen et al., 2014). The treatment is usually guided by a therapist, who mostly uses e-mail messages to provide feedback and to support motivation (Van Beugen et al., 2014).
Previous studies have indicated that guided iCBT can be as effective as face-to-face CBT for a broad range of somatic and mental conditions (Andersson et al., 2014; Carlbring et al., 2018; Bendig et al., 2018) and that (guided) iCBT has an effect on a broad range of outcomes (Buhrman et al., 2016; Ljótsson et al., 2014; Macea et al., 2010; Mehta et al., 2019; Van Beugen et al., 2014; White et al., 2020). It was shown to attain significant improvements in psychological outcomes (e.g., depressive mood, anxious symptoms [Buhrman et al., 2016; Mehta et al., 2019; Van Beugen et al., 2014]), disease-related physical outcomes (e.g., pain, fatigue, and/or headache [Buhrman et al., 2016; Van Beugen et al., 2014]), and impact on daily life outcomes (e.g., quality of life [Van Beugen et al., 2014]) among patients with chronic somatic (pain) conditions when compared to various control groups. Guided internet-based interventions appear to have larger effects than unguided internet-based interventions in mental health conditions (Baumeister et al., 2014; Karyotaki et al., 2021; Richards and Richardson, 2012), with limited evidence that intensive guidance may be equally effective as less intensive guidance (Baumeister et al., 2014). The efficacy of therapist guidance in somatic conditions and chronic pain appears to be less consistent, with some studies showing superiority of guided interventions and others finding similar effects of guided and unguided interventions (e.g., Buhrman et al., 2016; Mehta et al., 2019; Van Gils et al., 2016; Vugts et al., 2018; White et al., 2020). There are differing accounts on the importance of the qualification of coaches. In a study by Baumeister et al. (2014) the qualification of coaches providing guidance appeared of minor importance, whereas in a study by Vugts et al. (2018) functional interference effects were larger when guidance was provided at clinical level compared to master's level. The quality of the therapeutic relationship has shown to be directly related to clinical outcomes in guided iCBT (Ferwerda et al., 2016; Pihlaja et al., 2017). Besides, this type of treatment is positively reviewed by patients (Ferwerda et al., 2013). The ease of the iCBT treatment and the time saved were especially appealing to a Dutch sample of patients with rheumatoid arthritis and psoriasis (Ferwerda et al., 2013). However, an overview of the efficacy of this intervention method concerning the self-management of patients with rheumatic conditions specifically is lacking. Having such an overview will clarify what the specific benefit of guided iCBT is for this group of patients with an especially high disease burden (Berger et al., 2007; De Achaval and Suarez-Almazor, 2010; Evers et al., 2011; Hresko et al., 2018; Lacasse et al., 2016; Sturgeon et al., 2016; Yelin et al., 2007). A review can thereby set out directions for implementation of this type of care into clinical practice, as an addition to regular medical care. From previous reviews it is already clear that technology can have a large positive impact on rheumatology care, by providing a way to deliver care through online triage consultations and video-teleconferencing visits, and by monitoring disease activity remotely (e.g., Piga et al., 2017). However, the specific role of guided self-management support via iCBT to advance rheumatic patients' disease coping and quality of life is still unclear.
The Fear-Avoidance Model has been conceptualized as a theoretical model to guide pain research and management, describing developing and maintaining factors in chronic pain (Lethem et al., 1983; Vlaeyen and Linton, 2012). In this model, the role of catastrophizing, pain-related fear, and low self-efficacy is emphasized in promoting pain avoidance, resulting in an increase in physical symptoms and depression and reducing overall quality of life (Vlaeyen and Linton, 2012; Shim et al., 2018). In line with the Fear-Avoidance Model, previous research has found evidence for the efficacy of online self-management interventions for these types of outcomes (e.g., catastrophizing, self-efficacy and effects on other psychological, physical and impact on daily life outcomes) in chronic somatic (pain) conditions (e.g., Buhrman et al., 2016; Ljótsson et al., 2014; Macea et al., 2010; Mehta et al., 2019; Van Beugen et al., 2014; Vugts et al., 2018; White et al., 2020). Moreover, patients have indicated to value these outcome categories such as improvement in psychological wellbeing, reduction of symptoms, and a decrease in the impact of the disease on daily life (Carr et al., 2003; Hsiao and Fraenkel, 2017; Van der Elst et al., 2020). The current review, therefore, aims to explore the efficacy of guided iCBT in rheumatic conditions for the types of outcomes that are found to be relevant in the context of pain self-management from a theoretical, evidence-based and patient-preferred viewpoint, namely, psychological outcomes (depression, anxiety, catastrophizing, self-efficacy), physical outcomes (pain intensity, fatigue), and impact on daily life outcomes (impact on daily life, quality of life).
2. Methods
2.1. Search strategy and eligibility criteria
This review is registered with PROSPERO with registration number CRD42020154911. The review followed PRISMA guidelines and included an assessment of risk of bias. Studies were identified using databases PsycINFO, Embase, Emcare, Cochrane Library, Web of Science, and PubMed from inception until October 5th, 2020. A search string with the keywords “internet”, “cognitive-behavioral therapy”, and “chronic pain” including other related terms (see appendix) was used for a broader search on somatic conditions, in order not to exclude any specific rheumatic conditions that were labeled as “chronic pain conditions”.
As a first selection, a review team member (MV) screened titles and abstracts of studies to select those that were potentially eligible for inclusion. Next, two review team members (MV and JT) independently assessed the full text of the studies that passed the first screening for eligibility. Any disagreement between them regarding eligibility was resolved through discussion with a third team member (AE). Afterwards, a review team member (HJD) extracted data. A second review team member (JT) collected data (i.e., sample characteristics as well as clinical variables) from a random sample of the included studies in order to verify the accuracy of the included data. Finally, two review team members (MV and JT) independently checked references in relevant reviews and eligible articles for further relevant studies.
The inclusion criteria for the selection of articles were: (1) study subjects were adults (≥18 years old) with a rheumatic condition, (2) randomized controlled trial (RCT) design, (3) published in English, (4) availability of full-text article, (5) internet-based intervention (not face-to-face, onsite computerized therapy, videoconferencing, or personal digital assistants) as the main way of communication (i.e., patient spends >50% of total intervention time on an internet-based intervention), (6) intervention based on CBT principles, including a minimum of 2 CBT techniques, such as cognitive restructuring and problem-solving techniques, (7) use of therapist contact during the intervention with at least one episode of personalized patient contact (e.g., through messages), (8) use of original data, and (9) inclusion of psychological outcomes (depression, anxiety, self-efficacy, catastrophizing), and/or physical outcomes (pain intensity, fatigue), and/or impact on daily life outcomes (impact on daily life, quality of life). When more than one instrument was used for measuring the same outcome, the most validated instrument or the instrument that was most comparable to those used in other studies was included in the review. Any control group was eligible for inclusion. Studies were excluded when the intervention was primarily aimed at lifestyle change or disease monitoring, and when participants with multiple chronic somatic conditions (e.g., diabetes and rheumatic conditions) were analyzed as one group.
2.2. Data report
2.2.1. Descriptive data
The following information was collected for every article: year of publication, country of data collection, type of rheumatic disease, setting, mean age and sex of participants, presence of and type of control condition(s), number of patients included, number of completers and dropouts, reasons for dropout (if applicable), completer or intent-to-treat analyses, intervention content (intervention goals, most commonly mentioned elements in the intervention), intervention duration, type of therapist, frequency of therapist contact and mode of contact, post-treatment results, follow-up results, and adverse effects of treatment (adverse events, deterioration in outcomes). Moreover, four types of dropout rates were calculated: (1) intervention dropouts by dividing the number of patients who quit the intervention or did not fill out post-intervention questionnaires by the number of patients randomized to the intervention group, (2) measurement dropouts by dividing the number of patients from the intervention and control groups who did not return post-intervention questionnaires by the total number of patients randomized, (3) intervention follow-up dropouts by dividing the number of patients that did not return the last follow-up questionnaire by the number of patients randomized to the intervention group, and (4) measurement follow-up dropouts by dividing the number of patients from the intervention and control groups who did not return the last follow-up questionnaire by the total number of patients randomized. These dropout calculations were aimed at standardizing dropout rates across studies and were based on previous recommendations and studies (e.g., Van Beugen et al., 2014). Follow-up dropouts were added in order to report on all available follow-up data. Eysenbach (2005) suggests that dropouts at different points in time be reported, in order to point towards underlying causes for attrition.
2.2.2. Assessment of risk of bias
Two independent raters (HJD and JT) assessed the risk of bias of the eligible studies separately using the Cochrane risk of bias tool (Higgins and Green, 2011). A third rater (AE) was consulted to reach consensus when needed. The following biases were assessed: selection bias (systematic differences in baseline characteristics of different groups), performance bias (systematic differences between groups in the provision of care other than iCBT or in exposure to external factors), detection bias (systematic differences in outcome assessment), reporting bias (systematic differences between reported and unreported results), and attrition bias (systematic differences in withdrawals between conditions).
2.2.3. Measurement strategy
Based on previous literature searches during study conduction, the studies eligible for inclusion were expected to be diverse in their application of guided iCBT, in the types of rheumatic conditions that they included, and in their methodologies. Therefore, it was decided a priori that a narrative synthesis of study results would be conducted rather than a quantitative meta-analysis. Cohen's κ was calculated to determine interrater reliability (McHugh, 2012) concerning the included studies in the review. Included studies were grouped and summarized according to outcome (including psychological, physical, and impact on daily life outcomes) and type of rheumatic condition. Between-group effect sizes and significance levels of guided iCBT conditions versus control conditions were reported. Significance levels of ≤0.05 were applied. If post-hoc between-group effects were not reported, interaction effects (time x group) were reported. The type of effect sizes (e.g., Cohen's d), were reported as they were reported in the articles.
3. Results
3.1. General descriptive data
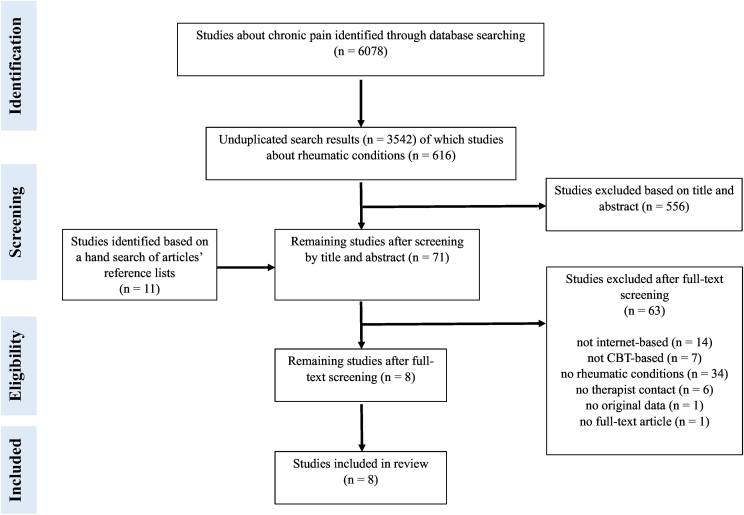
The search identified 6089 studies in total (see Fig. 1), with eight studies meeting the inclusion criteria (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008; Shigaki et al., 2013; Simister et al., 2018; Peters et al., 2017; Vallejo et al., 2015). Cohen's κ indicated near perfect agreement between the reviewers regarding the included studies in this review, κ = 0.955. The characteristics of the studies are summarized in Table 1. The included studies were carried out between 2008 and 2018. One study was performed in the Netherlands (Ferwerda et al., 2017), one in the Netherlands and Belgium (Peters et al., 2017), two in the United States of America (Lorig et al., 2008; Shigaki et al., 2013), two in Canada (Friesen et al., 2017; Simister et al., 2018), one in Spain (Vallejo et al., 2015), and one in Sweden (Hedman-Lagerlöf et al., 2018). Since the studies were mainly performed online, the setting was not always described in the articles. Two studies were run from a hospital setting (Ferwerda et al., 2017; Vallejo et al., 2015) and two studies were run from universities (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018). In the other studies the setting was unclear (Shigaki et al., 2013; Lorig et al., 2008; Peters et al., 2017; Simister et al., 2018). The study populations consisted of a combination of patients with rheumatoid arthritis, osteoarthritis, fibromyalgia, and/or another arthritic condition (Lorig et al., 2008), patients with fibromyalgia only (Friesen et al., 2017; Vallejo et al., 2015; Simister et al., 2018; Hedman-Lagerlöf et al., 2018), patients with fibromyalgia or other musculoskeletal pain (Peters et al., 2017) or patients with rheumatoid arthritis (Ferwerda et al., 2017; Shigaki et al., 2013). The mean age of the patient populations ranged from 40 to 56 years; the pooled mean age was 51.91 (SD = 11.98) years. The majority of the patients were female, with percentages ranging from 64% to 100%.
Fig. 1.
PRISMA flow diagram of study selection.
Table 1.
Study characteristics of included studies.
| Author, year; rheumatic condition; percentage female | Intervention and control group, n | Dropout, na (%) | Country | Intervention duration; mode, frequency, and duration of therapist contact | Type of therapist | Follow-up period |
|---|---|---|---|---|---|---|
|
Shigaki et al., 2013 Rheumatoid arthritis Percentage female: 92% |
Internet-based cognitive-behavioral therapy, 55 Waiting list, 53 |
Intervention 11 (20%) Measurement 15 (14%) Intervention follow-up 12 (22%) Measurement follow-up 20 (19%) |
USA |
Intervention duration: 10 weeks Mode/frequency/duration therapist contact: weekly telephone contact, ca. 15-30 min. Contact between patients was possible via biweekly scheduled chats, a discussion board, and a secured messaging system. |
Counselor with master's degree, trained in cognitive-behavioral therapy. | 9 months after treatment |
|
Friesen et al., 2017 Fibromyalgia Percentage female: 95% |
Internet-based cognitive-behavioral therapy, 30 Waiting list, 30 |
Intervention 5 (17%) Measurement 8 (14%) Intervention follow-up 13 (44%) Measurement follow-up 16 (27%) |
Canada |
Intervention duration: 8 weeks Mode/frequency/duration therapist contact: contact via telephone or secured messaging. Weekly 5-10 min telephone contact. |
Doctorate-level graduate student in clinical psychology. | No follow-up for the waiting list condition; 1 month follow-up after treatment for the internet-based cognitive-behavioral therapy condition. |
|
Lorig et al., 2008 Rheumatoid arthritis 28%; osteoarthritis 64%; fibromyalgia 52%; other arthritic conditions 14% Percentage female (total sample): 90% |
Internet-based cognitive-behavioral therapy, 433 Care-as-usual, 422 |
Intervention 123 (29%) Measurement 214 (25%) Intervention follow-up 126 (29%) Measurement follow-up 204 (24%) |
USA |
Intervention duration: 6 weeks Mode/frequency/duration therapist contact: online bulletin board and e-mail reminders; frequency and duration of contact unclear. |
Peer moderators. | 12 months after entry to treatment |
|
Ferwerda et al., 2017 Rheumatoid arthritis Percentage female: 64% |
Internet-based cognitive-behavioral therapy (care-as-usual + tailored internet-based cognitive-behavioral therapy), 62 Care-as-usual, 71 |
Intervention 17 (28%) Measurement 32 (24%) Intervention follow-up 33 (54%) Measurement follow-up 64 (49%) |
The Netherlands |
Intervention duration: 9–65 weeks (M = 26.07, SD = 12.22) 25% completed intervention in 17 weeks, 75% completed it in 32 weeks Mode/frequency/duration therapist contact: weekly or biweekly email contact. |
Psychologists with master's degree, under supervision of senior psychologist. | 3, 6, 9, 12 months after treatment |
|
Vallejo et al., 2015 Fibromyalgia Percentage female: 100% |
Internet-based cognitive-behavioral therapy, 20 Cognitive-behavioral therapy, 20 Waiting list, 20 |
Intervention 0 (0%) Measurement 0 (0%) Intervention follow-up 3 (15%) Measurement follow-up 7 (12%) |
Spain |
Intervention duration: 10 weeks Mode/frequency/duration therapist contact: internet-based cognitive-behavioral therapy: contact via online messaging. Frequency and duration of contact unclear. Cognitive-behavioral therapy: face-to-face group contact: 10 weekly sessions, each lasting 120 min. |
Internet-based cognitive-behavioral therapy: junior therapist under supervision of senior therapist. Cognitive-behavioral therapy: doctoral-level therapist. |
No follow-up for the waiting list condition; 3 follow-up assessments at 3, 6, and 12 months after treatment for the internet-based cognitive-behavioral therapy and cognitive-behavioral therapy conditions. |
|
Peters et al., 2017 Fibromyalgia 67%; other musculoskeletal pain 33% Percentage female (total sample): 85% |
Internet-based cognitive-behavioral therapy, 116 Positive Psychology intervention (PPI), 117 Waiting list, 51 |
Intervention 36 (31%) Measurement 78 (28%) Intervention follow-up 61 (53%) Measurement follow-up 135 (48%) |
The Netherlands/Belgium |
Intervention duration: 9 weeks Mode/frequency/duration therapist contact: both ICBT and PPI: Telephone (weeks 1, 3, 5, and 7) and e-mail (weeks 2, 4, 6, and 8) support. Average duration of telephone contact was 15-20 min. |
Both conditions: graduate or recently graduated psychologists. | No follow-up for the waiting list condition; 6 months follow-up after treatment for the Internet-based cognitive-behavioral therapy and positive psychology intervention. |
|
Simister et al., 2018 Fibromyalgia Percentage female: 95% |
Online acceptance and commitment therapy + care-as-usual, 33 Care-as-usual, 34 |
Intervention 6 (19%) Measurement 9 (14%) Intervention follow-up 8 (25%) Measurement follow-up 17 (26%) |
Canada |
Intervention duration: 2 months Mode/frequency/duration therapist contact: e-mail reminders on a weekly basis and feedback messages. |
Registered psychologist. | 3 months after treatment. |
|
Hedman-Lagerlöf et al., 2018 Fibromyalgia Percentage female: 98% |
Internet-delivered exposure therapy, 70 Waiting list, 70 |
Intervention 2 (3%) Measurement 2 (2%) Intervention follow-up 4 (6%) Measurement follow-up 4 (3%) |
Sweden |
Intervention duration: 10 weeks Mode/frequency/duration therapist contact: about 1 to 3 times/week coaching via asynchronous text messages. Reminders via text message or phone if a participant had been inactive for 4 days. In total, the average time therapists spent per participant was 2.9 h (SD = 2.1; median = 2.6; IQR = 2.6). |
Licensed psychologists or graduate psychology students. Weekly supervision by a licensed psychologist with experience in treating fibromyalgia. | No follow-up for the waiting list condition, 3 months and 6 months follow-up after treatment for the internet-delivered exposure therapy. |
See materials and methods.
In six studies, participants were allocated to either guided iCBT or a passive control condition: waiting list control (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Shigaki et al., 2013) or care-as-usual (Ferwerda et al., 2017; Lorig et al., 2008; Simister et al., 2018). Two studies used three-arm designs, in which guided iCBT was compared to both an active control condition (either face-to-face CBT or an internet-based positive psychology intervention) and a passive control condition (waiting list condition in both studies [Peters et al., 2017; Vallejo et al., 2015]). The total number of included participants was 1707; 819 participants were allocated to an active intervention condition, 137 were allocated to an active control condition (i.e., an intervention that is theorized to lead to clinically relevant changes in outcomes), and 751 participants were allocated to a passive control condition (waiting list or care-as-usual). The total number of intervention dropouts was 200 (25%), the total number of measurement dropouts was 358 (21%), the total number of intervention follow-up dropouts was 260 (32%), and the total number of measurement follow-up dropouts was 467 (28%). Regarding reasons for dropout during the guided iCBT intervention, the most common ones mentioned were physical comorbidity, reduction of symptoms (Ferwerda et al., 2017), and lack of time (Ferwerda et al., 2017; Friesen et al., 2017). In other studies, the reasons for dropout during the intervention were unclear (i.e., patients did not log in, yet unclear why; Lorig et al., 2008) or were not mentioned (Peters et al., 2017; Shigaki et al., 2013; Simister et al., 2018). Patients who dropped out at follow-up either did not return follow-up questionnaires or could not be reached to fill out the follow-up questionnaires (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008; Shigaki et al., 2013; Simister et al., 2018; Peters et al., 2017; Vallejo et al., 2015). All studies included follow-up measurements, which were applied at different time points and for different treatment conditions (see Table 1). Regarding the statistical methods used, seven studies applied intent-to-treat analyses (ITT) (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008; Simister et al., 2018; Peters et al., 2017; Vallejo et al., 2015) and one did not (Shigaki et al., 2013).
3.2. Intervention content and duration
The interventions all aimed at improving patients' self-management, that is, the ability to monitor the condition and use cognitive, behavioral, and emotional strategies to maintain a satisfactory quality of life (Barlow et al., 2002). Intervention goals were formulated in the studies as reducing avoidance behaviors through exposure (Hedman-Lagerlöf et al., 2018), reducing distress, improving quality of life, enhancing psychological and physical functioning, developing psychological flexibility (Ferwerda et al., 2017; Friesen et al., 2017; Lorig et al., 2008; Peters et al., 2017; Simister et al., 2018; Vallejo et al., 2015) and/or improving self-management skills (Lorig et al., 2008; Shigaki et al., 2013). The interventions consisted of several structured modules, each with different themes, such as “social functioning” and “mood”. Among the most commonly mentioned elements in these interventions were psycho-education, goal-setting, self-monitoring, relaxation, problem-solving, cognitive restructuring, attentional control, sleep hygiene, physical exercise, and relapse prevention (mentioned in 50-100% of the interventions). The guided iCBT lasted for 6 weeks in one study (Lorig et al., 2008), 8 weeks in two studies (Friesen et al., 2017; Simister et al., 2018), 9 weeks in one study (Peters et al., 2017), and 10 weeks in another three studies (Hedman-Lagerlöf et al., 2018; Shigaki et al., 2013; Vallejo et al., 2015). There was one study with a wide range in treatment duration per participant (9–65 weeks [M = 26.07, SD = 12.22]; 25% of participants completed the intervention in 17 weeks, 75% completed it in 32 weeks [Ferwerda et al., 2017]).
3.3. Therapist contact
In most studies, participating patients were guided by psychologists or psychology students (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Peters et al., 2017; Shigaki et al., 2013; Simister et al., 2018; Vallejo et al., 2015), whilst in one study patients were guided by peer moderators, who were trained online through participation in a workshop in order to co‑lead a workshop with a previously-trained moderator (Lorig et al., 2008). Regarding the mode of contact, in one study, the main mode of contact between patients and peer moderators was via a bulletin board in the secured online environment (Lorig et al., 2008). In addition, e-mail reminders were sent to nonparticipants to encourage them to participate and contact between patients was made possible via the online discussion board (Lorig et al., 2008). In another study, telephone contact was the main mode of contact, typically lasting from 15 to 30 min on a weekly basis (Shigaki et al., 2013). Besides, contact between patients was possible via biweekly scheduled chats, a discussion board, and a secured messaging system. Furthermore, in two studies, therapists sought contact via (semi)structured telephone calls combined with e-mail messages on a weekly or fortnightly basis (Peters et al., 2017; Friesen et al., 2017). The duration of these telephone calls was 5 to 10 min (Friesen et al., 2017) and 15 to 20 min (Peters et al., 2017). Finally, in four studies, the main mode of contact between patients and the therapist was via secure messaging in the online treatment program (Ferwerda et al., 2017; Hedman-Lagerlöf et al., 2018; Simister et al., 2018; Vallejo et al., 2015). In one of these studies, therapists sent feedback messages on a weekly or biweekly basis (Ferwerda et al., 2017) and in another study, therapists sent feedback messages about one to three times per week (Hedman-Lagerlöf et al., 2018). In the remaining studies, the frequency of personalized therapist contact was unclear (Simister et al., 2018; Vallejo et al., 2015).
3.4. Risk of bias
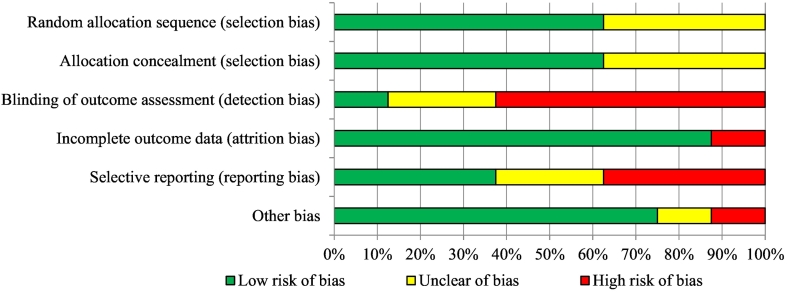
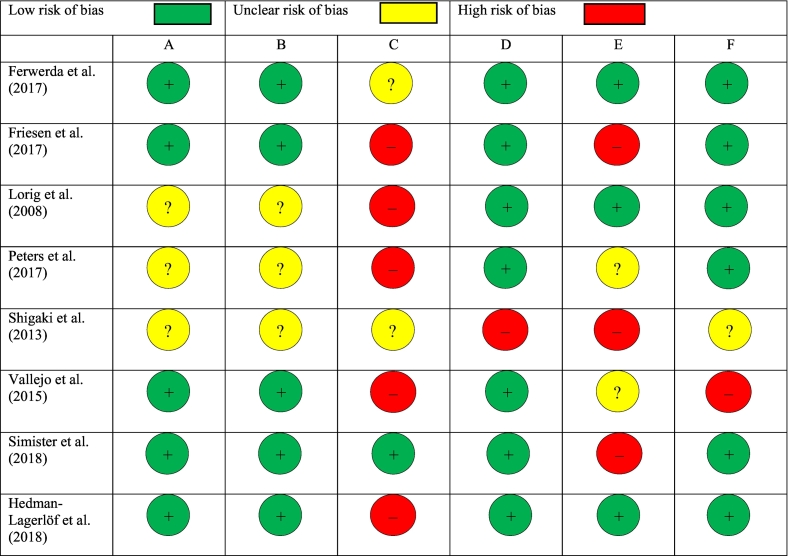
No study had a low risk of bias in all categories (see Fig. 2, Fig. 3). Concerning selection bias, more than half of the included studies (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Simister et al., 2018; Vallejo et al., 2015) reported a proper randomization method and allocation concealment (e.g., via a random number generator). In three studies this was insufficiently described, which resulted in an unclear risk of selection bias (Lorig et al., 2008; Peters et al., 2017; Shigaki et al., 2013). Regarding detection bias, one study was at a low risk of bias (Simister et al., 2018) and two other studies were at an unclear risk of bias, since blinding of outcome assessment was insufficiently described (Ferwerda et al., 2017; Shigaki et al., 2013). Five studies were at a high risk of bias concerning assessor blinding of outcome assessment, either because missing data were collected via phone (Lorig et al., 2008), or because the number of assessment points differed per group (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Peters et al., 2017; Vallejo et al., 2015). Concerning attrition bias, all studies reported intention-to-treat (ITT) analyses except for one study (Shigaki et al., 2013), which resulted in a high risk of attrition bias for this study. Regarding reporting bias, three studies pre-registered their outcomes in a trial register (Ferwerda et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008), whilst the protocols of two other studies could not be found in trial registers (Peters et al., 2017; Vallejo et al., 2015), which resulted in an unclear risk of reporting bias for the latter studies. Moreover, three studies had inconsistencies between the measurement instruments that were pre-specified in the protocol, and those that were reported in the article, which led to a high risk of reporting bias (Friesen et al., 2017; Shigaki et al., 2013; Simister et al., 2018). The risk of other bias was mostly low in the included articles, except for two studies. First, in one study, individualized guided iCBT was compared with group face-to-face CBT (Vallejo et al., 2015), which may be a complicated comparison (high risk of other bias). Second, in another study, baseline results were not reported (Shigaki et al., 2013), so the potential baseline differences between the groups could not be examined (unclear risk of other bias).
Fig. 2.
Graph with risk of bias.
Fig. 3.
Risk of bias per study. Judgment of: A = proper random sequence (selection bias); B = allocation concealment (selection bias); C = blinding of outcome assessment (detection bias); D = incomplete outcome data (attrition bias); E = selective reporting (reporting bias), F = other bias.
3.5. Outcome measures and effect sizes
The outcome measures and between-group effects of the included two-arm studies (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008; Shigaki et al., 2013; Simister et al., 2018) are summarized in Table 2 and the results of the three-arm study (Peters et al., 2017) are summarized below (not shown in table). One study did not report between-group significance levels and effect sizes (Vallejo et al., 2015) and is therefore not discussed below. Overall, guided iCBT was associated with improvements in psychological outcome measure(s) at posttreatment (i.e., depression in five out of six studies that included this outcome [Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Simister et al., 2018; Peters et al., 2017], anxiety in three out of four studies [Ferwerda et al., 2017; Hedman-Lagerlöf et al., 2018; Peters et al., 2017], and self-efficacy in two out of three studies [Lorig et al., 2008; Shigaki et al., 2013]) when compared to a passive control condition, with effect sizes in the medium to large range (0.48 - 0.92). One out of three studies that measured pain catastrophizing, found a significant result at posttreatment (Peters et al., 2017). When guided iCBT was compared to a different guided (iCBT) treatment form (i.e., an online positive psychology intervention), no difference in efficacy was found for psychological nor physical outcomes (Peters et al., 2017).
Table 2.
Post-intervention and follow-up effects of guided iCBT for rheumatic conditions: two-armed studies with a passive control condition.
| Author, year | Outcome | Outcome measurea | Significance level of between-group effects at post-treatmentb (time x group effects were reported if post-hoc between-group effects were not reported) | Post-treatment between-group effect sizes (95% confidence intervals when reported in article)c | Significance level of between-group effects at follow-upb | Follow-up between-group effect sizes (95% confidence intervals when reported in article)c |
|---|---|---|---|---|---|---|
| Friesen et al., 2017 | General psychological | ITT | Follow-up at 1 month after treatment | |||
| Depression Anxiety Self-efficacy Pain catastrophizing |
PHQ-9 GAD-7 PSEQ PRSS |
p = 0.005 (-) p = 0.124 p = 0.060 (time x group) p = 0.066 (time x group) |
d = 0.72 (0.20 to 1.24) d = 0.40 (-0.11 to 0.91) d = 0.75 (0.23 to 1.27) d = 0.73 (0.21 to 1.25) |
There was only a follow-up for the treatment group, so no between-group effects were reported. | ||
| Physical | ||||||
| Pain Fatigue |
BPI FSI |
p = 0.001 (-) p = 0.365 (time x group) |
d = 0.87 (0.34 to 1.40) d = 0.59 (0.07 to 1.10) |
|||
|
Impact on daily life Fibromyalgia impact |
FIQ-R p = 0.008 |
d = 0.70 (0.17 to 1.22) |
||||
| Pain impact Quality of life (physical health) Quality of life (mental health) |
BPI-pain interference SF-12 Physical SF-12 Mental |
p < 0.062 (time x group) p = 0.367 p = 0.393 (time x group) |
d = 1.00 (0.46 to 1.54) d = 0.23 (20.27 to 0.74) d = 0.07 (20.44 to 0.57) |
|||
| Ferwerda et al., 2017 | General psychological | ITT | Follow-up at 3, 6, 9 and 12 months after treatment | |||
| Depression Anxiety |
BDI IRGL |
p < 0.001 (-) p < 0.001 (-) |
d = 0.54 d = 0.48 |
For all analyses, time did not have a significant effect on the outcome. As a result, all the presented results represent group differences across all post- and follow-up assessment points. | ||
| Physical | ||||||
| Fatigue Pain |
CIS IRGL |
p = 0.06 p = 0.35 |
d = 0.24 d = 0.11 |
|||
| Impact on daily life | ||||||
| Impact on daily life | Composite score of scales of the IRGL and RAND-36 |
p = 0.09 | d = 0.18 | |||
| Lorig et al., 2008d | General psychological | IT | Follow-up at 12 months after entry to treatment | |||
| Self-efficacy | Arthritis SE scale |
p = 0.018 (+ time x group) | n.r. |
p = 0.018 (+ time x group) |
n.r. | |
| Physical | ||||||
| Pain Fatigue |
VNS VNS |
p < 0.001 (- time x group) p = 0.08 (time x group) |
n.r. n.r. |
p < 0.001 (- time x group) p = 0.08 (time x group) |
n.r. n.r. |
|
| Impact on daily life | ||||||
| n.r. | ||||||
| Shigaki et al., 2013 | General psychological | Non-ITT | Follow-up at 9 months after treatment | |||
| Depression Self-efficacy |
CES-D ASES |
p = 0.14 p < 0.001 (+) |
0.44 0.92 |
p = 0.14 p < 0.001 (+) |
0.49 0.92 |
|
| Physical | ||||||
| Pain today Impact on daily life Quality of life |
RADAR QLS |
p = 0.24 p = 0.003 (+) |
0.37 0.66 |
p = 0.58 p = 0.004 (+) |
0.19 0.71 |
|
| Simister et al., 2018 | General psychological | ITT | Follow-up at 3 months after treatment | |||
| Depression Pain catastrophizing Physical Pain Impact on daily life Fibromyalgia impact |
CES-D PCS SF-MPQ FIQ-R |
p = 0.020 (- time x group) p = 0.051 (time x group) p = 0.010 (- time x group) p < 0.001 (- time x group) |
d = 0.87 (0.34 to 1.39) d = 0.36 (-0.14 to 0.87) d = 0.84 (0.32 to 1.36) d = 1.26 (0.70 to 1.80) |
p = 0.020 (- time x group) p = 0.051 (- time x group) p = 0.010 (- time x group) p < 0.001 (- time x group) |
d = 0.56 (0.04 to 1.07) d = 0.26 (-0.24 to 0.76) d = 0.11 (-0.39 to 0.62) d = 1.59 (1.00 to 2.16) |
|
| Hedman-Lagerlöf et al., 2018 | General psychological | ITT | Follow-up at 6 months and 12 months after treatment | |||
| Depression Anxiety Physical Pain Fatigue Impact on daily life |
PHQ-9 GAD-7 FIQ-pain FSS |
p < 0.001 (- time x group) p < 0.001 (- time x group) p < 0.001 (- time x group) p < 0.001 (- time x group) |
d = 0.66 (0.32 to 1.00) d = 0.67 (0.32 to 1.01) d = 0.86 (0.51 to 1.20) d = 0.88 (0.53 to 1.22) |
No between-group effects were reported at follow-up, since the waitlist condition was crossed over to treatment then. | ||
| Fibromyalgia impact Quality of Life |
FIQ BBQ |
p < 0.001 (- time x group) p < 0.001 (+ time x group) |
d = 0.90 (0.55 to 1.24) d = 0.73 (0.38 to 1.07) |
|||
ASES = Arthritis Self-Efficacy Scale; BDI = Beck Depression Inventory; BPI = Brief Pain Inventory; BBQ = Brunnsviken Brief Quality of Life Scale; CIS = Checklist Individual Strength; CES-D = Center for Epidemiologic Studies Depression Scale; FIQ-R = Fibromyalgia Impact Questionnaire – Revised; FSI = Fatigue Symptom Inventory; FSS = Fatigue Severity Scale; GAD-7 = Generalized Anxiety Disorder-7; HDS = Health Distress Scale; IRGL = Invloed van Reuma op Gezondheid en Leefwijze (Effect of Rheumatic Disease on Health and Lifestyle); ITT = intent-to-treat analysis; PCS = Pain Castastrophizing Scale; PHQ-9 = Patient Health Questionnaire-9; PSEQ = Pain Self-Efficacy Questionnaire; PRSS = Pain-Related Self-statements Scale; QLS = Quality of Life Scale; RADAR = Rapid Assessment of Disease Activity in Rheumatology; SF-12 = Short Form-12; SF-MPQ = McGill Pain Questionnaire-short form; VNS = Visual Numeric Scale.
(-) = statistically significant effect indicating a reduction in the outcome for the guided iCBT condition when compared to the control condition, (+) = statistically significant effect showing an increase in the outcome for the guided iCBT condition when compared to the control condition.
n.r. = not reported.
Since the study mentioned measurements at 6 months and at 12 months, whereas the intervention duration was 6 weeks, the 6-month measurement has been considered a post-treatment measurement in this review.
Furthermore, results regarding physical outcomes (pain intensity and fatigue) were mixed when guided iCBT was compared to a passive control condition. In four out of seven studies that measured pain severity, improvements in the guided iCBT groups were noticeable at posttreatment (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008; Simister et al., 2018). In one out of four studies that included a fatigue measure, an improvement in the guided iCBT group was found at posttreatment (Hedman-Lagerlöf et al., 2018). Large effect sizes were found for both pain severity and fatigue in the studies that showed significant results (0.84-0.88).
Regarding impact on daily life outcomes, two out of three studies found significant improvements in quality of life (Hedman-Lagerlöf et al., 2018; Shigaki et al., 2013) and three out of four studies found significant improvements in impact of the condition on daily life (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Simister et al., 2018) when guided iCBT was compared to a passive control condition, with medium to large effects (0.66-1.26).
All outcomes considered, internet-based exposure therapy consistently obtained positive results for psychological as well as physical and impact on daily life outcomes when compared to a waiting list control condition (Hedman-Lagerlöf et al., 2018). When comparing subgroups across all studies, no apparent differences in effects were found between studies on guided iCBT in fibromyalgia or musculoskeletal pain on the one hand and studies on guided iCBT in rheumatoid arthritis on the other hand. In the studies that included follow-up measurements, the post-treatment between-group effects were largely maintained at follow-up (see Table 2).
3.6. Adverse effects of treatment
One study described adverse events (Hedman-Lagerlöf et al., 2018), with 34% (24/70) of the internet-based exposure therapy participants reporting an adverse event at post-treatment (mainly increased pain), compared with 6% (4/70) of the participants in the waiting list control condition. Three percent (2/70) of the treatment group reported that the increased pain remained significant at the 6-month and 12-month follow-up. A regression analysis did not show a significant relationship between adverse events and treatment outcome and there were no reports of serious adverse events related to treatment. Two studies reported on deterioration in outcomes specifically (Friesen et al., 2017; Simister et al., 2018). In the study of Simister et al. (2018), participants receiving online acceptance and commitment therapy regressed on measures of valued living and cognitive fusion from post-treatment to follow-up, compared to participants receiving treatment-as-usual. In the study of Friesen et al. (2017), 13% of participants (4/30) in both the treatment and waiting list control condition showed clinical deterioration on the GAD-7 and scored within the clinical ranges at post-treatment. Three percent (1/30) of participants in the treatment condition and 10% (3/30) of participants in the waiting list control condition, demonstrated clinical deterioration on the PHQ-9 measure and scored within the clinical ranges at post-treatment. Three percent (1/30) of both the treatment and waiting list control condition participants showed clinical deterioration and scored within the clinical ranges for both the GAD-7 and PHQ-9 at post-treatment.
4. Discussion
To our knowledge, this is the first review to systematically summarize the efficacy of guided iCBT as a self-management intervention for rheumatic conditions. An overview of the efficacy of this type of intervention was provided, based on psychological, physical, and impact on of daily life measures in eight included studies. The findings showed that guided iCBT has the potential to improve rheumatic patients' psychological functioning and the impact of the disease on daily life, whilst the effects on physical outcomes were mixed. A promising result in the current review was that the improvements in the guided iCBT groups were mostly maintained at follow-up. This highlights the potential of this treatment form to improve the wellbeing of patients with rheumatic conditions in the long term. Still, these findings should be interpreted with some caution, considering the varying levels of methodological quality of the included studies.
Medium to large effects of guided iCBT were found for the psychological outcomes of depression (Ferwerda et al., 2017; Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Simister et al., 2018; Peters et al., 2017), anxiety (Ferwerda et al., 2017; Hedman-Lagerlöf et al., 2018; Peters et al., 2017), self-efficacy (Lorig et al., 2008; Shigaki et al., 2013), and pain catastrophizing (Peters et al., 2017) as well as for the impact of the disease on daily life (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Simister et al., 2018) and quality of life (Hedman-Lagerlöf et al., 2018; Shigaki et al., 2013). A similar trend, albeit more modest, was described in previous research (Buhrman et al., 2016; Mehta et al., 2019; Van Beugen et al., 2014; Vugts et al., 2018; White et al., 2020). For instance, four meta-analytic reviews on guided (iCBT) interventions in chronic pain or chronic somatic conditions in general, found improvements in psychological outcome measures with small (Van Beugen et al., 2014; White et al., 2020), small to moderate (Buhrman et al., 2016) and moderate (Mehta et al., 2019) effect sizes. Moreover, in two different meta-analyses on computer-based interventions for patients with chronic pain or functional somatic syndromes, small to medium effects were found for impact on daily life outcomes (Buhrman et al., 2016; Vugts et al., 2018). The positive effects of guided iCBT on psychological functioning and impact of the disease on daily life as described in the current review, emphasize the value of psychological treatment as an addition to medical treatment of rheumatic conditions. Better psychological functioning, such as higher self-efficacy and lower levels of depression has previously been associated with better treatment adherence, lower disease severity, lower healthcare use, and a higher quality of life (De Achaval and Suarez-Almazor, 2010). Furthermore, the current review shows the value of internet-based exposure therapy, which consistently obtained positive effects for psychological as well as physical and impact on daily life outcomes (Hedman-Lagerlöf et al., 2018). Internet-based exposure therapy focuses on exposing patients to their pain without the use of avoidance behaviors. This type of therapy may thus be an effective treatment for patients with rheumatic conditions who exhibit these pain avoidance behaviors which can ultimately exacerbate chronic pain and disability, in line with the previously described Fear-Avoidance Model (Lethem et al., 1983; Shim et al., 2018; Vlaeyen and Linton, 2012).
Regarding physical outcomes, the results were mixed with a slight majority of studies showing positive effects of guided iCBT on pain (Friesen et al., 2017; Hedman-Lagerlöf et al., 2018; Lorig et al., 2008; Simister et al., 2018) and a majority of studies showing no effects of guided iCBT on fatigue (Ferwerda et al., 2017; Friesen et al., 2017; Lorig et al., 2008). Previous meta-analyses on somatic conditions in general, however, confirmed significant effects for these outcomes in favor of iCBT (Buhrman et al., 2016; Van Beugen et al., 2014; Vugts et al., 2018). For example, previously, small to large significant effects for physical outcomes were found in a meta-analytic review on guided iCBT in chronic somatic conditions in general (Van Beugen et al., 2014). Besides, small to medium effects for these outcomes were found in two meta-analyses on computer-based interventions for patients with chronic pain or functional somatic syndromes (Buhrman et al., 2016; Vugts et al., 2018). The included interventions in the review were not primarily aimed at improving patients' physical health but rather at improving patients' skills in coping with the consequences of a chronic disease in daily life, which may have contributed to the mixed results concerning physical outcomes. The findings emphasize the value of guided iCBT as an addition to pharmacological care, where iCBT has a primary focus on improving psychological outcomes and the impact of the disease on daily life.
Regarding potential negative effects of guided iCBT, adverse events were reported in only one study and deterioration in outcomes in two studies. Whilst a minority of participants in the discussed studies experienced more symptoms in certain areas after treatment (Hedman-Lagerlöf et al., 2018; Friesen et al., 2017) or participants showed deterioration in a minority of measurement scales (Simister et al., 2018), it is unknown what factors exactly contributed to these results. Previous articles have stressed the need for further exploration of potential negative effects of iCBT and their causes (Andersson et al., 2019; Etzelmueller et al., 2020; Holmes et al., 2018; Rozental et al., 2018). For instance, further research should focus on the relationship between participant characteristics and deterioration in outcomes to optimize iCBT. Besides, future studies should consistently report on adverse events to inform on the safety of the treatment.
The findings of this review should be considered in the light of some limitations. First, the small number of included studies with their diverse application of iCBT limits the generalizability of the effects of guided iCBT in rheumatic conditions. Second, the high attrition rates in the selected studies (i.e., 25% intervention dropouts, 21% measurement dropouts, 32% intervention follow-up dropouts, and 28% measurement follow-up dropouts) and small sample size in some studies also limit broad interpretation of the summarized results. Non-adherence is a common issue in (internet-based) behavioral interventions (Van Beugen et al., 2014) and although there were measures taken to prevent this in the included articles (e.g., personalized reminders and feedback), it is unclear to what extent these methods were helpful. Future research is needed to assess the effects of persuasive technology features (e.g., online praise, reminders, rewards, modeling [Oinas-Kukkonen and Harjumaa, 2008]) on increasing adherence. Third, the selection of patients in the included articles differed in type of rheumatic condition, which complicates generalization of results. Due to a low number of included studies in this review, and heterogeneity in both measurement instruments and in study quality, we decided against separate (meta-analytic) analyses of outcomes per condition. Besides, the sample size of included studies consisted mostly of female participants, which is generally reflective of prevalence rates in rheumatic conditions (Van Vollenhoven, 2009). However, the generalization of results to male patients with rheumatic conditions may be more complicated. Fourth, all studies included an unclear risk of bias and/or a high risk of bias of some form, which may affect interpretation of results. For instance, methods were regularly insufficiently described which resulted in an unclear risk of bias in several areas (e.g., random sequence generation, allocation concealment). RCTs without reported adequate allocation concealment have been shown to overestimate treatment effects (Pildal et al., 2007). Moreover, publication bias could not be reliably evaluated due to lack of power, since the review contains less than 10 studies (Dalton et al., 2016). A publication bias could not be precluded. Fifth, mediators and moderators of treatment effects could not be examined in this review due to the limited number of included studies. Future studies should focus on how different iCBT intervention components (e.g., relaxation, psycho-education) impact psychological, physical, and impact on daily life outcomes in rheumatic conditions. Furthermore, future studies should address which patient characteristics are related to the feasibility, acceptability, and efficacy of guided iCBT for rheumatic conditions and what subgroups can benefit most from which iCBT components. For instance, it is previously suggested that adults with a lower age, female sex, higher educational levels, and higher initial interference of functioning are relatively more responsive towards online interventions (Vugts et al., 2018). Such predictive factors, and how the needs of other subgroups can be met as well, need to be further researched. Lastly, considering the high healthcare costs of rheumatic conditions (Berger et al., 2007; Hresko et al., 2018; Lacasse et al., 2016; Yelin et al., 2007), cost-effectiveness analyses are important to include in future research to advance implementation of iCBT for rheumatic conditions in clinical practice.
In conclusion, the findings in this review suggest that guided iCBT may relieve psychological symptoms and the impact of the disease on daily life in patients with rheumatic conditions, such as fibromyalgia, rheumatoid arthritis, and osteoarthritis, which is promising for the implementation of iCBT for these conditions in clinical practice. A focus on guided iCBT as a self-management intervention could aid patients to develop a skill set to cope with the consequences of their chronic disease in the short and long term. Exposure as part of this skill set may be a promising development to improve psychological, as well as physical, and impact on daily life outcomes. It appears relevant to offer guided iCBT as part of a multidisciplinary stepped-care model, where patients with rheumatic conditions who experience adjustment problems can receive this treatment in addition to their pharmacological treatment. Patients could be screened for distress and those scoring high on distress could be offered an iCBT treatment tailored to their adjustment problems and risk and resilience factors in a step-up approach (e.g., Evers et al., 2014). Finally, in order to advance implementation, more, larger and higher-quality studies are needed to firmer establish the efficacy of guided iCBT for rheumatic conditions, whilst including a focus on moderator analyses, feasibility, safety of the intervention, and cost-effectiveness as well.
Declaration of competing interest
Dr. Kloppenburg reports fees for consultancy (Abbvie, Pfizer, Levicept, GlaxoSmithKline, Merck-Serono, Kiniksa, Flexion) and was a local investigator of an industry-driven trial (Abbvie). She received other funds from the Dutch Society of Rheumatology, royalties from Wolters Kluwer (UptoDate) and Springer Verlag (Reumatologie en klinische immunologie), and grants from Pfizer and IMI-APPROACH, outside the submitted work. The other authors report no financial conflicts of interest.
Acknowledgments
Acknowledgements
We thank Milon van Vliet, research assistant, for her help in conducting a literature search and selecting eligible articles for inclusion in the review.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.invent.2021.100444.
Contributor Information
Jessy A. Terpstra, Email: j.a.terpstra@fsw.leidenuniv.nl.
Rosalie van der Vaart, Email: r.van.der.vaart@fsw.leidenuniv.nl.
Margreet Kloppenburg, Email: G.Kloppenburg@lumc.nl.
Andrea W.M. Evers, Email: a.evers@fsw.leidenuniv.nl.
Appendix A. Supplementary data
Supplementary material
References
- 1.Andersson G., Cuijpers P., Carlbring P. Guided internet-based vs face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13:288–295. doi: 10.1002/wps.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson G., Titov N., Dear B.F. Internet-delivered psychological treatments: from innovation to implementation. World Psychiatry. 2019;18:20–28. doi: 10.1002/wps.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow J., Wright C., Sheasby J. Self-management approaches for people with chronic conditions: a review. Patient Educ. Couns. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 4.Baumeister H., Reichler L., Munzinger M. The impact of guidance on internet-based mental health interventions — a systematic review. Internet Interv. 2014;1:205–215. [Google Scholar]
- 5.Bendig E., Bauerei N., Ebert D.D. Internet-based interventions in chronic somatic disease. Dtsch. Arztebl. Int. 2018;115:659–665. doi: 10.3238/arztebl.2018.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger A., Dukes E., Martin S. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int. J. Clin. Pract. 2007;61:1498–1508. doi: 10.1111/j.1742-1241.2007.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardy K., Klose P., Welsch P. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome - a systematic review and meta-analysis of randomized controlled trials. Eur. J. Pain. 2018;22:242–260. doi: 10.1002/ejp.1121. [DOI] [PubMed] [Google Scholar]
- 8.Bodenheimer T., Wagner E.H., Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 9.Bodenheimer T., Wagner E.H., Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288:1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 10.Borenstein D.G., Hassett A.L., Pisetsky D.S. Pain management in rheumatology research, training, and practice. Clin. Exp. Rheumatol. 2017;35:S2–S7. [PubMed] [Google Scholar]
- 11.Buhrman M., Gordh T., Andersson G. Internet interventions for chronic pain including headache: a systematic review. Internet Interv. 2016;4:17–34. doi: 10.1016/j.invent.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler A.C., Chapman J.E., Forman E.M. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin. Psychol. Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Carlbring P., Andersson G., Cuijpers P. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn. Behav. Ther. 2018;47:1–18. doi: 10.1080/16506073.2017.1401115. [DOI] [PubMed] [Google Scholar]
- 14.Carr A., Hewlett S., Hughes R. Rheumatology outcomes: the patient's perspective. J. Rheumatol. 2003;30:880–883. [PubMed] [Google Scholar]
- 15.Cunningham N.R., Kashikar-Zuck S. Nonpharmalogical treatment of pain in rheumatic diseases and other musculoskeletal pain conditions. Curr. Rheumatol. Rep. 2013;15:306. doi: 10.1007/s11926-012-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalton J.E., Bolen S.D., Mascha E.J. Publication bias: the elephant in the review. Anesth. Analg. 2016;123:812–813. doi: 10.1213/ANE.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Achaval S., Suarez-Almazor M.E. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int. J. Clin. Rheumtol. 2010;5:313–326. doi: 10.2217/ijr.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dures E., Hewlett S. Cognitive-behavioural approaches to selfmanagement in rheumatic disease. Nat. Rev. Rheumatol. 2012;8:553–559. doi: 10.1038/nrrheum.2012.108. [DOI] [PubMed] [Google Scholar]
- 19.Etzelmueller A., Vis C., Karyotaki E. Effects of internet-based cognitive behavioral therapy in routine care for adults in treatment for depression and anxiety: systematic review and meta-analysis. J. Med. Internet Res. 2020;22 doi: 10.2196/18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evers A.W.M., Kraaimaat F.W., van Riel P.L.C.M. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002;100:141–153. doi: 10.1016/s0304-3959(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 22.Evers A.W., Zautra A., Thieme K. Stress and resilience in rheumatic diseases: a review and glimpse into the future. Nat. Rev. Rheumatol. 2011;7:409–415. doi: 10.1038/nrrheum.2011.80. [DOI] [PubMed] [Google Scholar]
- 20.Evers A.W., Gieler U., Hasenbring M.I. Incorporating biopsychosocial characteristics into personalized healthcare: a clinical approach. Psychother. Psychosom. 2014;83:148–157. doi: 10.1159/000358309. [DOI] [PubMed] [Google Scholar]
- 23.Eysenbach G. The law of attrition. J. Med. Internet Res. 2005;7 doi: 10.2196/jmir.7.1.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferwerda M., van Beugen S., van Burik A. What patients think about E-health: patients' perspective on internet-based cognitive behavioral treatment for patients with rheumatoid arthritis and psoriasis. Clin. Rheumatol. 2013;32:869–873. doi: 10.1007/s10067-013-2175-9. [DOI] [PubMed] [Google Scholar]
- 27.Ferwerda M., van Beugen S., van Riel P.C. Measuring the therapeutic relationship in internet-based interventions. Psychother. Psychosom. 2016;85:47–49. doi: 10.1159/000435958. [DOI] [PubMed] [Google Scholar]
- 25.Ferwerda M., van Beugen S., van Middendorp H. A tailored-guided internet-based cognitive-behavioral intervention for patients with rheumatoid arthritis as an adjunct to standard care: results of randomized controlled trial. Pain. 2017;158:868–878. doi: 10.1097/j.pain.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 26.Ferwerda M., van Beugen S., van Middendorp H. Tailored, therapist-guided internet-based cognitive behavioral therapy compared to care as usual for patients with rheumatoid arthritis: economic evaluation of a randomized controlled trial. J. Med. Internet Res. 2018;20 doi: 10.2196/jmir.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friesen L.N., Hadjistavropoulos H.D., Schneider L.H. Examination of an internet-delivered cognitive behavioural pain management course for adults with fibromyalgia: a randomized controlled trial. Pain. 2017;158:593–604. doi: 10.1097/j.pain.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 29.Hedman-Lagerlöf M., Hedman-Lagerlöf E., Axelsson E. Internet-delivered exposure therapy for fibromyalgia: a randomized controlled trial. Clin. J. Pain. 2018;34:532–542. doi: 10.1097/AJP.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011] The Cochrane Collaboration; 2011. www.cochrane-handbook.org [Google Scholar]
- 31.Holmes E.A., Ghaderi A., Harmer C.J. The lancet psychiatry commission on psychological treatments research in tomorrow's science. Lancet Psychiatry. 2018;5:237–286. doi: 10.1016/S2215-0366(17)30513-8. [DOI] [PubMed] [Google Scholar]
- 32.Hresko A., Lin T.C., Solomon D.H. Medical care costs associated with rheumatoid arthritis in the US: a systematic literature review and meta-analysis. Arthritis Care Res. 2018;70:1431–1438. doi: 10.1002/acr.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsiao B., Fraenkel L. Incorporating the patient's perspective in outcomes research. Curr. Opin. Rheumatol. 2017;29:144–149. doi: 10.1097/BOR.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karyotaki E., Efthimiou O., Miguel C. Internet-based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta-analysis. JAMA Psychiatry. 2021;78:361–371. doi: 10.1001/jamapsychiatry.2020.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacasse A., Bourgault P., Choinière M. Fibromyalgia-related costs and loss of productivity: a substantial societal burden. BMC Musculoskelet. Disord. 2016;17:168. doi: 10.1186/s12891-016-1027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lami M.J., Martínez M.P., Sánchez A.I. Systematic review of psychological treatment in fibromyalgia. Curr. Pain Headache Rep. 2013;17:345. doi: 10.1007/s11916-013-0345-8. [DOI] [PubMed] [Google Scholar]
- 37.Lethem J., Slade P.D., Troup J.D. Outline of a fear-avoidance model of exaggerated pain perception–I. Behav. Res. Ther. 1983;21:401–408. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 38.Ljótsson B., Atterlöf E., Lagerlöf M. Internet-delivered acceptance and values-based exposure treatment for fibromyalgia: a pilot study. Cogn. Behav. Ther. 2014;43:93–104. doi: 10.1080/16506073.2013.846401. [DOI] [PubMed] [Google Scholar]
- 39.Lorig K.R., Ritter P.L., Laurent D.D. The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum. 2008;59:1009–1017. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- 40.Macea D.D., Gajos K., Calil Y.A.D. The efficacy of web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. J. Pain. 2010;11:917–929. doi: 10.1016/j.jpain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Matcham F., Rayner L., Steer S. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2013;52:2136–2148. doi: 10.1093/rheumatology/ket169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHugh M.L. Interrater reliability: the kappa statistic. Biochem. Med. (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta S., Peynenburg V.A., Hadjistavropoulos H.D. Internet-delivered cognitive behaviour therapy for chronic health conditions: a systematic review and meta-analysis. J. Behav. Med. 2019;42:169–187. doi: 10.1007/s10865-018-9984-x. [DOI] [PubMed] [Google Scholar]
- 44.O'Donohue W.T., Fisher J.E. In: Cognitive Behavior Therapy. Core Principles for Practice. O’Donohue W.T., Fisher J.E., editors. John Wiley & Sons Inc.; Hoboken (NJ): 2012. The core principles of cognitive behavior therapy; pp. 1–12. [Google Scholar]
- 45.Oinas-Kukkonen H., Harjumaa M. In: Persuasive Technology. PERSUASIVE 2008. Lecture Notes in Computer Science. Oinas-Kukkonen H., Hasle P., Harjumaa M., Segerståhl K., Øhrstrøm P., editors. Springer-Verlag Berlin Heidelberg; Berlin: 2008. A systematic framework for designing and evaluating persuasive systems; pp. 164–176. [Google Scholar]
- 46.Peters M.L., Smeets E., Feijge M. Happy despite pain. a randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patients with chronic pain. Clin. J. Pain. 2017;33:962–975. doi: 10.1097/AJP.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petric M., Kaliterna D.M., Perkovic D. Anxiety and depression in inflammatory rheumatic diseases. Rheumatology (Sunnyvale) 2015;5:166. [Google Scholar]
- 48.Piga M., Cangemi I., Mathieu A. Telemedicine for patients with rheumatic diseases: systematic review and proposal for research agenda. Semin. Arthritis Rheum. 2017;47:121–128. doi: 10.1016/j.semarthrit.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Pihlaja S., Stenberg J.H., Joutsenniemi K. Therapeutic alliance in guided internet therapy programs for depression and anxiety disorders - a systematic review. Internet Interv. 2017;17:1–10. doi: 10.1016/j.invent.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pildal J., Hróbjartsson A., Jørgensen K.J. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int. J. Epidemiol. 2007;36:847–857. doi: 10.1093/ije/dym087. [DOI] [PubMed] [Google Scholar]
- 51.Richards D., Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32:329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Rozental A., Castonguay L., Dimidjian S. Negative effects in psychotherapy: commentary and recommendations for future research and clinical practice. BJPsych Open. 2018;4:307–312. doi: 10.1192/bjo.2018.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shigaki C., Smarr K.L., Siva C. RAHelp: an online intervention for individuals with rheumatoid arthritis. Arthritis Care Res. 2013;65:1573–1581. doi: 10.1002/acr.22042. [DOI] [PubMed] [Google Scholar]
- 54.Shim E.J., Hahm B.J., Go D.J. Modeling quality of life in patients with rheumatic diseases: the role of pain catastrophizing, fear-avoidance beliefs, physical disability, and depression. Disabil. Rehabil. 2018;40:1509–1516. doi: 10.1080/09638288.2017.1300691. [DOI] [PubMed] [Google Scholar]
- 55.Simister H.D., Tkachuk G.A., Shay B.L. Randomized controlled trial of online acceptance and commitment therapy for fibromyalgia. J. Pain. 2018;19:741–753. doi: 10.1016/j.jpain.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Sturgeon J.H., Finan P.H., Zautra A.J. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nat. Rev. Rheumatol. 2016;12:532–542. doi: 10.1038/nrrheum.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor C.B., Chang V.Y. Issues in the dissemination of cognitive–behavior therapy. Nord. J. Psychiatry. 2008;62(sup47):37–44. doi: 10.1080/08039480802315673. [DOI] [PubMed] [Google Scholar]
- 58.Vallejo M.A., Ortega J., Rivera J. Internet versus face-to-face group cognitive-behavioral therapy for fibromyalgia: randomized controlled trial. J. Psychiatr. Res. 2015;68:106–113. doi: 10.1016/j.jpsychires.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Van Beugen S., Ferwerda M., Hoeve D. Internet-based cognitive behavioral therapies for patients with chronic somatic conditions: a meta-analytic review. J. Med. Internet Res. 2014;16 doi: 10.2196/jmir.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van der Elst K., Mathijssen E.G.E., Landgren E. What do patients prefer? A multinational, longitudinal, qualitative study on patient-preferred treatment outcomes in early rheumatoid arthritis. RMD Open. 2020;6 doi: 10.1136/rmdopen-2020-001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Gils A., Schoevers R.A., Bonvanie I.J. Self-help for medically unexplained symptoms: a systematic review and meta-analysis. Psychosom. Med. 2016;78:728–739. doi: 10.1097/PSY.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 62.Van Vollenhoven R.F. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med. 2009;7:12. doi: 10.1186/1741-7015-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlaeyen J.W., Linton S.J. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Vugts M.A.P., Joosen M.C.W., van der Geer J.E. The effectiveness of various computer-based interventions for patients with chronic pain or functional somatic syndromes: a systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White V., Linardon J., Stone J.E. Online psychological interventions to reduce symptoms of depression, anxiety, and general distress in those with chronic health conditions: a systematic review and meta-analysis of randomized controlled trials. Psychol. Med. 2020;17:1–26. doi: 10.1017/S0033291720002251. [DOI] [PubMed] [Google Scholar]
- 66.Yelin E., Murphy L., Cisternas M.G. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material