Abstract
Autosomal recessive Chanarin-Dorfman syndrome (CDS, MIM #275630) is defined as a neutral lipid storage disease with ichthyosis (NLSDI) due to an accumulation of lipid droplets in a variety of different tissues including liver and muscle cells, leucocytes, fibroblasts and nerve cells It is caused by biallelic mutations in the abhydrolase domain containing 5 gene (ABHD5, MIM *604780) which is localized on the short arm of chromosome 3. Here we report an 18 month-old girl in whom we have identified the homozygous ABHD5 mutation c.700C > T, p.(Arg234*). Since none of the parents carried this point mutation, parentage was confirmed by microsatellite marker analysis. Suspected uniparental disomy (UPD) was confirmed by microsatellite genotyping over the entire chromosome 3 and indicated a maternal origin. UPD is an extremely rare event that is not necessarily pathogenic, but may cause disease if the affected chromosome contains genes that are imprinted. Here we report the first case of Chanarin-Dorfman syndrome due to a de novo ABHD5 mutation in the maternal germ cell, combined with a maternal uniparental isodisomy of chromosome 3. This case demonstrates that genetic analysis of the patient and both parents is crucial to provide correct genetic counseling.
Keywords: Chanarin-Dorfman syndrome (CDS), uniparental disomy (UPD), ABHD5, de novo mutation
1. Introduction
Chanarin-Dorfman syndrome (CDS) is a rare autosomal recessive disorder characterized by ichthyosis. Due to the accumulation of triglycerides in different tissues, other symptoms of the disease can occur: hepatosplenomegaly, mild myopathy, cataract, neurosensory deafness, liver cirrhosis and possible psychomotor development and mental impairment [1]. The presence of lipid droplets in granulocytes, also called Jordan’s anomaly, is the major diagnostic criterion for CDS, and can be found in all patients [1]. Dorfman first described the syndrome in 1974 [2]. The disease is caused by biallelic mutations in the ABHD5 gene (abhydrolase domain-containing protein 5, lysophosphatidic acid acyltransferase, MIM *604780) as first described in 2002 by Lefèvre et al. [3]. To date over 40 different mutations have been published in patients with CDS.
Uniparental disomy (UPD) describes a condition in which an individual carries a pair of chromosomes from the same parent. Several mechanisms are known to lead to (UPD). The presence of two copies of one chromosome in the ovum or sperm cell, which results in a trisomic zygote, is suspected to be the main cause of UPDs. Due to correction of the trisomic state (trisomic rescue), a disomic embryo with two different constitutions can emerge: the embryo either has one chromosome from each parent, or the embryo has two maternal or two paternal chromosomes. A second mechanism for UPD is monosomic rescue, where the monosomic chromosome is replicated. Furthermore, gametic complementation (fertilization of a disomic egg with a nullsomic sperm or vice versa) is possible [4]. In UPD, two constellations are possible: heterodisomy, where both chromosomes of one homologous chromosome pair of one parent are present or isodisomy, where two copies of the same parental chromosome are present.
Here we report a patient with a homozygous mutation in the gene ABHD5 that emerged from a de novo mutation in the maternal germ cells in combination with maternal uniparental isodisomy (UPD) of chromosome 3.
2. Materials and Methods
2.1. DNA Extraction and Sequencing
Informed consent was obtained and genomic DNA was isolated from peripheral blood lymphocytes of the patient and the parents using standard methods. We performed a next-generation sequencing (NGS) gene panel analysis with a SureSelect XTHS Custom Kit (Agilent Technologies, Inc. Santa Clara, CA, USA) and an Illumina MiSeq system in the patient. All coding exons and flanking intronic sequences of the genes ABCA12, ALOX12B, ALOXE3, CERS3, CYP4F22, NIPAL4, PNPLA1, SDR9C7, SULT2B1, TGM1 and ABHD5 were analyzed. Sanger sequencing of the DNA of the patient has validated the ABHD5 mutation.
2.2. Microsatellite Analysis
We performed microsatellite analysis for markers on different chromosomes (D3S1358, THO1, D18S51, PENTA-E, D13S317, D7S820, CSF1PO, PENTA-D, D8S1179, TPOX, FGA) and for 9 markers on chromosome 3 (D3S050, D3S1263, D3S3613, D3S3527, D3S2406, D3S3045, D3S2440, D3S1311, D3S1272) by multiplex PCR and fragment analysis on an ABI Prism 3130XL Automatic DNA Sequencer (Thermo Fisher Scientific, Waltham, MA, USA).
3. Results
3.1. Clinical Manifestations
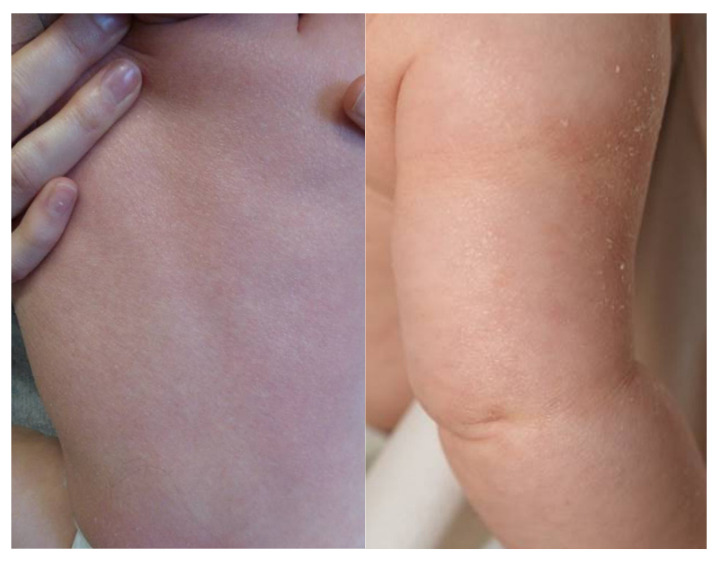
The patient is the first child of non-consanguineous parents with a Caucasian background. Pregnancy was complicated by intrauterine growth retardation in the 3rd trimester, but otherwise unremarkable. The girl was delivered spontaneously in the 40th week of gestation with a birth weight of 2350 g (<1st percentile), birth length was 45 cm (<1st percentile), and head circumference 32.5 cm (<3rd percentile). At birth, her skin was scaly without a collodion membrane. At the age of six months, she presented fine whitish scales and mild erythema (Figure 1).
Figure 1.
The affected child showed fine whitish scales and mild erythema (on the left: back; on the right: upper arm).
At the age of 12 months the girl was in good physical condition. Growth was normal with body weight and length at the 17th and 24th percentile, respectively. Apart from mild ichthyosis, slightly delayed psychomotor development and mild axial muscular hypotonia, there was no clinical evidence for other extracutaneous manifestations. The blood count was normal; however, typical lipid vacuoles were present in the leucocytes in a blood smear. Transaminase activities (AST 83 U/L, ALT 45 U/L) and creatine kinase activity (371 U/L) were slightly elevated, whereas triglyceride and total cholesterol levels were normal. Abdominal sonography showed no signs of hepatosplenomegaly or hepatic steatosis. Sonography of the muscles was unremarkable. Echocardiography showed good bilateral function and no signs of cardiomyopathy, and the electrocardiogram was normal. Ophthalmologic examination as well as audiogram and otoacoustic emissions also yielded normal results. Therapeutically, a fat-reduced and fat-modified diet was initiated to replace long-chain fatty acids by medium-chain triglycerides (MCT), and the patient was supported by additional physiotherapeutic measures. Transaminase levels and creatine kinase activity decreased under this regimen, but remained mildly elevated.
3.2. Molecular Analysis
Based on the clinical findings, an autosomal recessive congenital ichthyosis (ARCI) was initially suspected. No mutations in the known ARCI genes ABCA12, ALOX12B, ALOXE3, CERS3, CYP4F22, NIPAL4, PNPLA1, SDR9C7, SULT2B1 and TGM1 were identified. However, we found the homozygous mutation c.700C > T, p.(Arg234*) in exon 5 of ABHD5 (NM_016006.6) confirming the molecular genetic diagnosis of CDS. This mutation has previously been described in the literature [5,6] and is clearly defined as pathogenic (class 5) according to the American College of Medical Genetics and Genomics (ACMG) [7]. An overview of all reported pathogenic mutations is listed in Table S1.
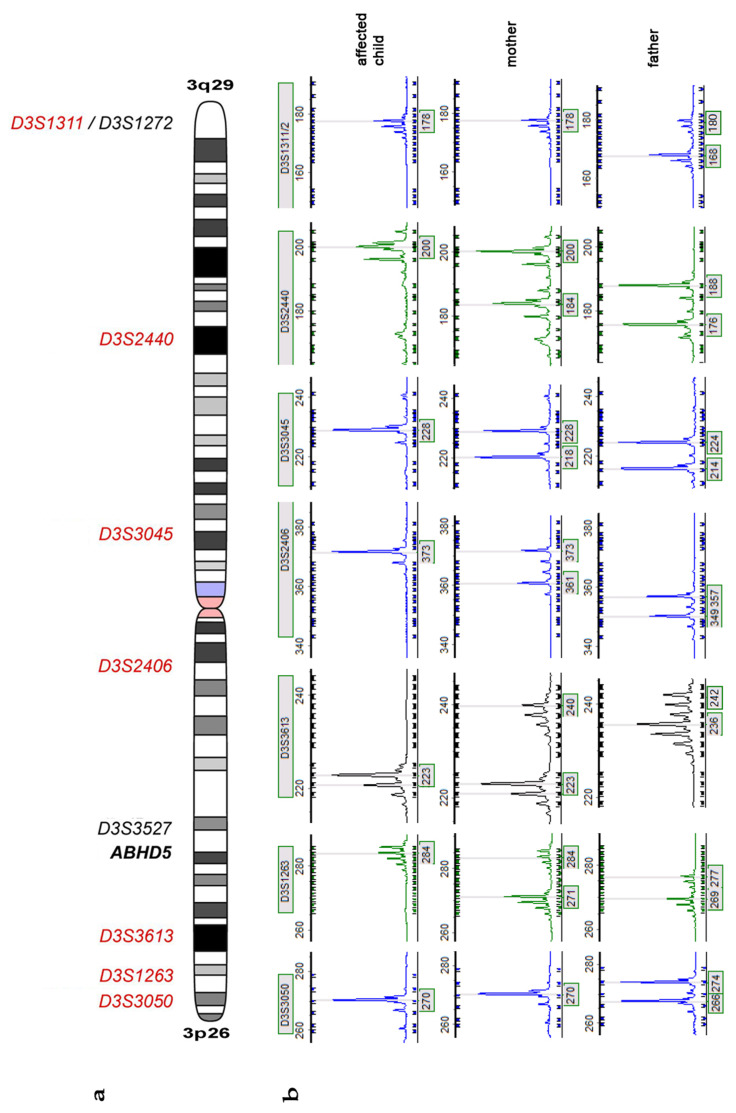
Since prenatal testing for future pregnancies was requested by the parents, they were screened for the mutation c.700C > T in the ABHD5 gene. Surprisingly, the mutation was not present in the DNA extracted from the blood of the parents. To exclude mixing up of DNA samples parenthood was confirmed by genotyping a set of microsatellites localized on different chromosomes (Supplementary Materials Table S2). Next we genotyped 9 highly polymorphic microsatellite markers on chromosome 3 (Figure 2a,b and Supp. Table S3) in the patient and her parents. Seven of the 9 markers were informative and clearly showed a maternal origin from the same chromosome in the patient, suggesting a maternal isodisomy of chromosome 3. Furthermore, since the mother did not carry the mutation in the germline, it is likely that it appeared de novo in the maternal germ cell.
Figure 2.
(a) Schematic presentation of chromosome 3 and the location of the ABHD5 gene (bold italic) with the analyzed microsatellite markers (italic); red = maternal UPD in the affected child; black = marker not informative. (b) Presentation of Microsatellite marker analysis of all informative markers. The affected child has no paternal allele and therefore did not inherit chromosome 3 of the father. The affected child shows one of the maternal alleles, suggesting a maternal isodisomy.
4. Discussion
Here we report a case of Chanarin-Dorfman syndrome due to a de novo mutation in ABHD5 combined with uniparental isodisomy of maternal chromosome 3.
UPD with homozygosity of recessive alleles is being increasingly recognized as the molecular basis for several autosomal recessive disorders, however, data on the clinical prevalence and spectrum of UPD remain limited. Scuffins et al. have recently analyzed 32,067 trio exomes referred for diagnostic testing to create a profile of UPD events and their disease associations [8]. In this cohort, whole-chromosome UPD was observed in 0.31% of cases, resulting in a diagnostic finding in 0.14%. Isodisomy, as found in our patient, was more commonly observed in large chromosomes along with a higher rate of homozygous pathogenic variants [8].
Around 150 genes on specific chromosome portions are known to be imprinted [9], which means, that these genes are expressed differently during development, depending on their maternal or paternal origin. If a UPD occurs in chromosomes that contain imprinted genes, a genetic disease can result from it without the presence of a mutation.
UPD of chromosome 3 has already been reported in the literature, but no imprinting effects have been shown to date. In 2006, Xiao et al. reported a person with a paternal disomy 3 without apparent phenotypic disorders. The authors conclude that there are no paternal imprinted genes on chromosome 3 that cause rare genetic disorders [10].
Nevertheless, UPD of chromosome 3 has been reported in association with diseases. Fassihi et al. described a patient with recessive dystrophic epidermolysis bullosa and a homozygous mutation in the COL7A1 gene. The mother was heterozygous for the mutation whereas the father did not carry this mutation; microsatellite analysis of chromosome 3 subsequently confirmed a maternal isodisomy of chromosome 3. Since the patient did not show other phenotypic abnormalities, the authors suggested an absence of maternally imprinted genes on chromosome 3 [11]. Hoffman et al. similarly reported a case of Fanconi-Bickel syndrome due to a homozygous GLUT2 mutation inherited via maternal isodisomy of chromosome 3 [12]. In 2005, Schollen et al. described a patient with a congenital disorder of glycosylation and a homozygous mutation in the ALG3 gene. Neither the mother nor the father was a carrier of this mutation. Further analysis showed segmental maternal isodisomy of chromosome 3 suggesting that the disease occurred due to a combination of a de novo mutation in the ALG3 gene and segmental isodisomy of chromosome 3 [13].
Our patient showed typical clinical and biological symptoms of a classic CDS such as mild ichthyosis, Jordan abnormalities and mildly elevated transaminases. The child did not present symptoms suggestive of other diseases, which is consistent with the description that there are no maternally imprinted genes on chromosome 3.
Although UPD is a rare cause of autosomal recessive disorders, it has significant implications for both mutation screening and genetic counselling. While the risk of recurrence in subsequent pregnancies is usually 25% for couples with a child affected by CDS, in our case caused by a de novo mutation combined with maternal UPD of chromosome 3, the risk is much lower. The precise risk is difficult to estimate; however the recurrence risk of maternal UPD of chromosome 3 is likely less than 1% while the probability for a de novo ABHD5 mutation is even lower. This emphasizes that genetic testing of the patient and both parents is crucial for correct genetic counselling, not only for dominant disorders more frequently caused by de novo mutations but also for autosomal recessive disorders.
Acknowledgments
The authors are grateful to the patient and her family for supporting this study. We would like to thank Susan Cure for critical comments on the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12081164/s1, Table S1: mutations in ABHD5 (NM_016006.6), Table S2: microsatellite analysis for confirmation of parenthood, Table S3: microsatellite analysis for investigation of chromosome 3 UPD.
Author Contributions
Conceptualization, J.K.; methodology, J.K.; A.H. and J.F.; validation, J.K., A.H. and J.F.; investigation, J.K., A.H. and J.F.; resources, J.F., C.H. and S.C.G.; data curation, J.K., A.H. and J.F.; writing—original draft preparation, J.K. and S.C.G.; writing—review and editing, J.K., A.H., J.F., C.H. and S.C.G.; visualization, J.K.; supervision, J.F.; project administration, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Freiburg. Ethical code number: 436/17.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cakmak E., Bagci G. Chanarin-Dorfman Syndrome: A comprehensive review. Liver Int. 2021;41:905–914. doi: 10.1111/liv.14794. [DOI] [PubMed] [Google Scholar]
- 2.Dorfman M.L., Hershko C., Eisenberg S., Sagher F. Ichthyosiform Dermatosis with Systemic Lipidosis. Arch. Dermatol. 1974;110:261–266. doi: 10.1001/archderm.1974.01630080059017. [DOI] [PubMed] [Google Scholar]
- 3.Lefèvre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J.-L., Weissenbach J., et al. Mutations in CGI-58, the Gene Encoding a New Protein of the Esterase/Lipase/Thioesterase Subfamily, in Chanarin-Dorfman Syndrome. Am. J. Hum. Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson W.P. Mechanisms leading to uniparental disomy and their clinical consequences. BioEssays. 2000;22:452–459. doi: 10.1002/(SICI)1521-1878(200005)22:5<452::AID-BIES7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Schleinitz N., Fischer J., Sanchez A., Veit V., Harle J.-R., Pelissier J.-F. Two new mutations of the ABHD5 gene in a new adult case of chanarin dorfman syndrome: An uncommon lipid storage disease. Arch. Dermatol. 2005;141:798–800. doi: 10.1001/archderm.141.6.798. [DOI] [PubMed] [Google Scholar]
- 6.Takeichi T., Sugiura K., Tso S., Simpson M., McGrath J., Akiyama M. Bi-allelic nonsense mutations inABHD5 underlie a mild phenotype of Dorfman-Chanarin syndrome. J. Dermatol. Sci. 2016;81:134–136. doi: 10.1016/j.jdermsci.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scuffins J., Keller-Ramey J., Dyer L., Douglas G., Torene R., Gainullin V., Juusola J., Meck J., Retterer K. Uniparental disomy in a population of 32,067 clinical exome trios. Genet. Med. 2021;23:1101–1107. doi: 10.1038/s41436-020-01092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prawitt D., Haaf T. Basics and disturbances of genomic imprinting. Med. Gen. 2020;32:297–304. [Google Scholar]
- 10.Xiao P., Liu P., Weber J.L., Papasian C.J., Recker R.R., Deng H.W. Paternal uniparental isodisomy of the entire chromosome 3 revealed in a person with no apparent phenotypic disorders. Hum. Mutat. 2006;27:133–137. doi: 10.1002/humu.20302. [DOI] [PubMed] [Google Scholar]
- 11.Fassihi H., Lu L., Wessagowit V., Ozoemena L.C., Jones C.A., Dopping-Hepenstal P.J.C., Foster L., Atherton D.J., Mellerio J.E., McGrath J.A. Complete maternal isodisomy of chromosome 3 in a child with recessive dystrophic epidermolysis bullosa but no other phenotypic abnormalities. J. Investig. Dermatol. 2006;126:2039–2043. doi: 10.1038/sj.jid.5700348. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman T.L., Blanco E., Lane A., Galvin-Parton P., Gadi I., Santer R., DeLeón D., Stanley C., Wilson T.A. Glucose metabolism and insulin secretion in a patient with ABCC8 mutation and Fanconi-Bickel syndrome caused by maternal isodisomy of chromosome 3. Clin. Genet. 2007;71:551–557. doi: 10.1111/j.1399-0004.2007.00802.x. [DOI] [PubMed] [Google Scholar]
- 13.Schollen E., Grunewald S., Keldermans L., Albrecht B., Korner C., Matthijs G. CDG-Id caused by homozygosity for an ALG3 mutation due to segmental maternal isodisomy UPD3(q21.3-qter) Eur. J. Med. Genet. 2005;48:153–158. doi: 10.1016/j.ejmg.2005.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.