Abstract
Despite an early understanding of amyotrophic lateral sclerosis (ALS) as a disease affecting the motor system, including motoneurons in the motor cortex, brainstem, and spinal cord, today, many cases involving dementia and behavioral disorders are reported. Therefore, we currently divide ALS not only based on genetic predisposition into the most common sporadic variant (90% of cases) and the familial variant (10%), but also based on cognitive and/or behavioral symptoms, with five specific subgroups of clinical manifestation—ALS with cognitive impairment, ALS with behavioral impairment, ALS with combined cognitive and behavioral impairment, the fully developed behavioral variant of frontotemporal dementia in combination with ALS, and comorbid ALS and Alzheimer’s disease (AD). Generally, these cases are referred to as amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD). Clinical behaviors and the presence of the same pathognomonic deposits suggest that FTLD and ALS could be a continuum of one entity. This review was designed primarily to compare neuropathological findings in different types of ALS relative to their characteristic locations as well as the immunoreactivity of the inclusions, and thus, foster a better understanding of the immunoreactivity, distribution, and morphology of the pathological deposits in relation to genetic mutations, which can be useful in specifying the final diagnosis.
Keywords: amyotrophic lateral sclerosis, frontotemporal lobar degeneration, sporadic ALS, familial ALS, amyotrophic lateral sclerosis-frontotemporal spectrum disorder, ALS-FTSD, motor neuron disease
1. Introduction
Amyotrophic lateral sclerosis (ALS) has classically been considered a disease exclusively affecting the motor system and, thus, it is a part of a group of disorders known as motor neuron diseases (MND), which includes a whole spectrum of disorders affecting upper motor neurons (corticospinal tract), lower motor neurons (in anterior horn or motor cranial nerve nuclei in the brain stem), or both [1]. The MNDs also includes “restricted” phenotypes, including primary lateral sclerosis (PLS), progressive muscular atrophy (PMA), and progressive bulbar palsy (PBP) [2]. PLS is characterized by isolated progressive upper motor neuron dysfunction without lower motor neuron symptomatology that begins in the fifth to sixth decade with progressively developing spasticity, hyperreflexia, and mild weakness [3]. Unlike ALS, PLS is most often slowly progressive (survival times of 7.2–14.5 years) [3]. PMA includes lower motor neuron dysfunction; however, upper motor neuron signs may sometimes be present [4]. Even in PMA, disease survival times are longer than in ALS [5]. PBP can be divided into childhood- and adult-onset forms [6]. The childhood-onset form, also called Fazio-Londe disease, is an autosomal recessive disease associated with progressive impairment of the cranial nerves with two clinical subtypes—an early course, typically starting before the age of 6 years with mainly respiratory symptoms, and a late course starting between 6 and 20 years with mainly motor symptoms in the upper limbs [7]. The adult-onset form attacks the bulbar region and clinically presents with swallowing, speaking, and chewing difficulties [8].
Another MND disease is spinal muscular atrophy (SMA), an inherited condition with an autosomal-recessive pattern affecting lower motor neurons that degenerate due to a lack of survival motor neuron (SMN) protein encoded by the SMN1 gene [9]. Historically, five types of SMA, based on the age of symptoms onset and the highest physical ability achieved, are distinguished [10]. Disease severity can be modified by the SMN2 gene, which led to the creation of the first treatment [11].
Kennedy’s disease, also known as spinal and bulbar muscular atrophy, is a recessive X-linked disease affecting men (women can be carriers). It is caused by an expansion of a CAG repeat in the gene for androgen receptors [12]. Clinically, patients present with muscle weakness, proximal atrophy in the limbs, and bulbar symptomatology. Gynecomastia due to the insensitivity to androgen, heart rhythm, and urinary problems may also be present [13].
The last condition that falls under MND is post-polio syndrome (PPS), characterized by muscle weakness and/or muscle fatigability, dysphagia, dysphonia, and subsequent respiratory failure that can begin dozens of years after recovery from a poliomyelitis infection [14].
The first to describe and diagnose ALS was Jean-Martin Charcot, so ALS is sometimes referred to as Charcot’s disease [15]. Charcot found and reported damage within the lateral columns of the spinal cord that leads to chronic progressive paralysis along with contractures but without muscle atrophy. On the other hand, lesions affecting the anterior horns cause paralysis and muscle atrophy but lack contractures [16]. Another common name for ALS is Lou Gehrig’s disease in memory of the famous New York Yankees baseball player who died (1941) of ALS at the age of 37 [17].
In ALS, the word “amyotrophy” refers to muscle fiber atrophy, caused by selective neuronal degeneration in the anterior horns of the spinal cord, leading to weakness and fasciculations of affected muscles. The phrase “lateral sclerosis” refers to the hardening of the anterior and lateral corticospinal tracts as axons and myelin are replaced by glial cells [18]. Therefore, ALS is still often defined as a fatal neurodegenerative disease characterized by progressive muscle paralysis determined by the degeneration of motoneurons in the motor cortex, brainstem, and spinal cord [19] with the characteristic limb onset form accompanied by awkwardness, weakness, and tripping or stumbling. The bulbar onset form first appears as speech or swallowing difficulties [20]. However, today, we know that not all forms are characterized solely by motor neuron lesions—some patients also have a cognitive and behavioral impairment that is remarkably similar to the behavioral variant of frontotemporal dementia (bvFTD). Recently, these cases have become referred to as amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD) [21]. ALS is the most common MND in adults, with the vast majority of ALS cases being sporadic; however, about 10% have a familial history with a typical Mendelian autosomal dominant pattern of inheritance [22], although cases with autosomal recessive or X-linked inheritance are also known [23]. Regarding genetic variants, the most common cause is a hexa-nucleotide repeat expansion of GGGGCC in the first intron of chromosome 9 open reading frame 72 (C9orf72), which causes 30–50% of familial ALS (FALS) cases and 5% of sporadic ALS (SALS) [24,25]. These repeat expansions are also frequently found in frontotemporal dementia (FTD), which shows the molecular overlap between these two pathological units, i.e., ALS and FTD [26]. Clinically, the sporadic and familial forms are indistinguishable [27], although some studies suggest that the familial variant has an earlier onset [28].
The aim of the review is to compare the neuropathological findings in individual types of ALS in detail, including characteristic inclusions, their immunoprofiles and characteristic localizations, and thus, foster a better understanding of their relationship to genetic mutations, which can be useful in specifying the final diagnosis.
2. Incidence and Prevalence of ALS
The incidence of ALS in Europe is reported to be around 1–2.6 cases per 100,000 inhabitants per year, and the prevalence is 6 per 100,000 inhabitants per 100,000 population [22], although foci of higher frequencies occur in the Western Pacific [29]. The disease typically occurs at 58–60 years [22], rarely occurs before the age of 40, and generally, is slightly more common in men (the reported M:F ratio is about 1.5:1) [29]; however, most studies suggest that the bulbar onset form is more common in women [30,31]. The median survival time is 20–48 months, though 10–20% of patients survive more than ten years [20].
3. Etiology
The cause of ALS is not yet fully understood; however, the various genes involved in the pathogenesis of ALS share similar biological functions, which allowed the identification of some major molecular pathways that trigger selective damage in specific neurons [27]. The effect of head injury [32], viruses like herpes simplex [33], enterovirus [34], or retrovirus [35], exotoxins including excitotoxicity mediated by glutamate [36], the lysosomal-endosomal system [37], or disorders of the immune system leading to chronic inflammatory processes including autoimmune processes [38,39] have all been considered to be causal candidates, but none have been demonstrated so far. The role of epigenetics, especially defects in histone homeostasis (acetylation and deacetylation) suggested by transcriptional dysregulation indicating changes in chromatin structure, which is seen in both murine ALS models and patients, has also been assumed [40]. In addition, some ALS patients have higher physical exertion and lower body mass indexes (BMI) compared to the unaffected population, which could be related to the onset [38,41].
In the case of FALS, an autosomal dominant pattern of inheritance with the most common mutation being in C9orf72, superoxide dismutase 1 gene (SOD1), and fused of the sarcoma (FUS) gene are usually seen [42]. Moreover, ALS has been shown to be associated with mutations in genes with DNA/RNA regulating functions, such as TAR DNA binding protein (TARDBP) [43,44].
Recently published studies reported selective hypothalamic atrophy in both SALS and FALS cases, including the asymptomatic stage of FALS [45]. In FALS, atrophy correlates with body mass index (BMI), but no correlation with the severity of motor problems has been found [45].
Early motor manifestations of SALS, with the presence of TDP-43 insoluble and ubiquitinated inclusions, reflect the failure of complex adaptive motor skills leading to split hand presentation, gait disorders, split leg syndrome, and bulbar symptomatology associated with vocalization [46].
A characteristic histopathological feature of TDP-43 pathology is its limitation to the cortical region and subcortical nuclei with direct cortical projections [47]. The pathological TDP-43 protein is found in the cerebral cortex, corticofugal fibers, subcortical nuclei, and the motor neurons of the brainstem and anterior horns of the spinal cord [39]. The prion-like mechanism at the synaptic terminals of corticofugal axons is currently accepted, and theoretically explains the spread to neocortical regions and the relationship between ALS and FTD [39].
Very rarely, paraneoplastic syndrome, positive for anti-Hu antibodies, and presenting as an MND, is found. MND is considered an atypical paraneoplastic syndrome that has yet to meet the criteria set by Graus et al., [48] i.e., at least one of three conditions have to be fulfilled: (1) Post-therapeutic reduction of neurological manifestations in the absence of immune modulation; (2) the presence of onconeural antibodies; and/or (3) partially characterized onconeural antibodies and a malignancy presenting within five years of the onset of neurological abnormalities [48].
4. Clinical Manifestation
Amyotrophic lateral sclerosis is an MND [49], and the typical form is characterized by upper and lower motoneuron involvement, which is present in 65–70% of cases. Another form is progressive bulbar paralysis with bulbar muscle involvement, which occurs in 25% of patients [50]. A rarer manifestation of the disease is progressive muscle atrophy with only lower motoneuron lesions; this occurs in 5–8% of ALS patients [39]. An extremely rare form of the disease is primary lateral sclerosis (PLS), characterized by a lesion limited to upper motoneurons and affecting 1–4% of patients [51]. In PLS, the clinical diagnosis is based on per exclusionem after excluding all other possible causes (structural, infectious, and demyelinating diseases leading to upper motor neuron syndrome or hereditary spastic paraplegias) [51]. Equally rare is the monomelic spinal amyotrophy variant with focal atrophy that usually presents as weakness in one limb [52,53].
Surprisingly often, i.e., in 55% of cases with clinically obvious dementia, 15% have been described [54] with characteristics of both ALS and cognitive impairment. In the dementia form (i.e., ALS-FTSD), five forms have been distinguished based on clinical presentation:
-
(1)
ALS with cognitive impairment (ALSci);
-
(2)
ALS with behavioral impairment (ALSbi);
-
(3)
ALS with combined cognitive and behavioral impairment (ALS-cbi);
-
(4)
Fully developed behavioral variant of frontotemporal dementia (bvFTD) in combination with ALS (ALS-FTD);
-
(5)
Comorbid ALS and Alzheimer’s disease (AD) [55].
In the fully developed disease, the clinical picture is relatively characteristic, but diagnostic difficulties may occur at the beginning of the clinical manifestation. Objective findings in the developed form are a mixed picture of central and peripheral quadriparesis, bulbar symptoms, hyperreflexia, spasticity, positive pyramidal signs, atrophy of the muscles of the upper and lower limbs and tongue, and massive fasciculations, especially of the limbs and tongue [29].
The disease most often (in two-thirds of cases) [29] begins with an asymmetric weakness and wasting of limited muscle groups, with the subsequent development of spasticity [29] typically manifesting in the upper limbs [26]. Clinical findings may imitate mononeuropathy or radiculopathy; however, weight loss, fasciculations, emotional lability, and frontal-lobe cognitive impairment [56] may also be present. Bulbar onset with articulation disorders and swallowing difficulties [26] are seen in 30% of cases; additionally, striking atrophy and fasciculations of the tongue [57] may be present. Limb weakness may begin to develop simultaneously with bulbar symptomatology or with a delay of 1–2 years [29]. As paralysis progresses, it leads to death due to respiratory failure within 2–3 years in bulbar onset and 3–5 years in the more typical limb onset variant [29].
Less often, and in cases with a focal onset, muscle weakness affects the neck muscles [26], and muscle fatigue is a common symptom [58]. Fasciculations or cramps appear in the plexus muscles of the upper and lower limbs (e.g., deltoid muscle and quadriceps femoris muscle) [39]. Atrophies of small muscles of the hand and foot (especially the interosseous muscles) leading to split-hand or split-leg signs may be clinically present [59,60]. An ALS diagnosis requires the absence of sensory signs, visual disturbances, and sphincter problems [52].
In ALS-FTSD, the degree of cognitive impairment and behavioral manifestation varies from case to case and ranges from mild cognitive deterioration bordering on a normal finding to marked changes in behavior and personality with a severe frontal syndrome. Cognitive impairment is present mainly in the bulbar form of ALS [61].
Patients with ALS may develop some form of bvFTD during the disease; conversely, some patients with bvFTD develop symptoms of motor neuron disease (from clinically insignificant fasciculations with hyperreflexia to typical ALS). The onset of motor problems and dementia usually differ, making the clinical picture of simultaneous development of ALS and bvFTD unusual [62]. ALS-FTSD has a significantly worse prognosis and shorter survival than patients with isolated forms (i.e., ALS or bvFTD independently) [63].
5. Differential Diagnosis
To standardize the diagnosis for clinical research and reduce misdiagnoses, the El Escorial criteria have been implemented [64]. Regarding diseases belonging to an ALS differential diagnosis and brain-affecting diseases, adult polyglucosan body disease (APBD) has clinical symptomatology consistent with upper and lower motor neuron lesions. However, cognitive decline, distal sensory loss, and bladder and bowel function disturbances also occur, which can clinically help differentiate the entities [52].
Among brainstem and spinal cord diseases, ALS can be mimicked by multiple sclerosis, which can cause both upper and lower motoneuron symptoms, and in some cases, may even resemble bulbar onset ALS [52]. In the predominantly spinal form, an MRI of the cervical spinal cord is performed to exclude other possibly treatable conditions; however, cervical spondylotic myelopathy or syringomyelia may include dissociated sensory loss, which is usually present in syringomyelia but starts at a younger age than most ALS cases [65]. Another disease that should be considered in the differential diagnosis is degenerative myeloradiculopathy, which can also be consistent with the clinical presentation of ALS [52].
Spinal and bulbar muscular atrophy (SBMA), also known as Kennedy’s disease, is a hereditary X-linked lower motor neuron disease characterized by progressive muscular weakness and should also be considered in the differential diagnosis. An initial clinical manifestation usually includes muscle cramps, muscle twitching, tremor, fatigue, and slurred speech [66].
The potential for paraneoplastic encephalomyelitis, which can sometimes manifest as a motor neuron disorder, must not be overlooked. Sensory or autonomic features and ataxia may appear later in the course [67].
In dysphagia and dysarthria, which are neuromuscular transmission disorders (especially myasthenia gravis), arterial atherosclerotic disease, infiltrative tumors, and infectious or autoimmune causes should be ruled out [68]. Oculopharyngeal muscular dystrophy may also imitate ALS, although it usually involves the extraocular muscles and muscles of eyelids in contrast to ALS. In patients presenting with bulbar symptomatology lacking extraocular involvement, a muscle biopsy may be required [52].
Benign monomelic amyotrophy is a differential diagnosis for monomelic onset of ALS [52]. Generally, it is always necessary to examine the cerebrospinal fluid and perform an MRI of the brain and spinal cord. In addition to the rare variant of oculopharyngeal muscular dystrophy mentioned above, a muscle biopsy may be indicated to rule out polymyositis [69] or inclusion body myositis [70].
Furthermore, some systemic diseases can partially mimic the clinical manifestation of ALS—for example, hyperthyroidism, which can cause hyperreflexia as a corticospinal tract sign, fasciculations, weight loss, or weakness. However, many symptoms that are not typical for ALS are often present (fine tremor, tachycardia, heat intolerance, and anxiety). Weakness may also be seen in hyperparathyroidism and mimic the lower motoneuron onset form of ALS [52].
6. Types of ALS
6.1. Sporadic ALS
Sporadic ALS (SALS) is the most common form of ALS (90%)—it occurs randomly, without any known cause, with no clearly associated risk factors, and no family history of the disease, thus the etiology of most cases of SALS remains elusive.
6.1.1. Background of SALS
Only 10% of SALS cases include known disease-associated ALS mutations [71], with C9orf72 expanded alleles being the most common [24]. Mutations in the SOD1 gene (gene for the antioxidant enzyme protecting cells from reactive superoxide radicals, endoplasmic reticulum stress, mitochondrial dysfunction, and axonal transport disruption) [72] are found in 2–3% of SALS [73]. Another interesting gene to study appears to be TARDBP, an abnormal TDP-43 fragment found in neurons and astrocytes in approximately 95% of SALS cases [74]. In less than 1% of SALS cases, mutations in hnRNP A1 and hnRNP A2B1 are detected. Oligogenic or polygenic SALS cases have an earlier age of onset [75]. The remaining cases likely represent the interplay of genetic and environmental factors.
6.1.2. Gross Findings in SALS
SALS gross findings include typically shrunken ventral/anterior roots that appear grey compared to the dorsal/posterior roots, lateral corticospinal tract, and sometimes the whole spinal cord may appear atrophic [76] (see Figure 1).
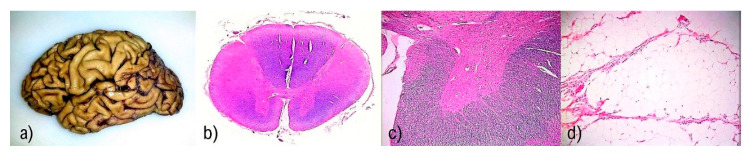
Figure 1.
(a) Atrophy of the precentral gyrus together with atrophy of the frontal and temporal lobes may be present mainly in cases of ALS accompanied by dementia. (b) Marked atrophy and reactive astrogliosis of anterior horns, with atrophy of the anterior roots are the most striking findings in the standard staining method. Histochemical detection of myelin shows the most evident changes in the anterolateral cords in the forms of sclerosis. Stain: Luxol fast blue stain. (c) Detail of the described changes in anterior roots and horn. Stain: Luxol fast blue stain. The original magnification 200×. (d) Pronounced neurogenic atrophy and fatty pseudohypertrophy in the striated muscles of the respiratory muscles is a typical finding in ALS. Stain: Hematoxylin and eosin stain. The original magnification 200×.
The brain usually appears grossly normal, although there may be atrophy of the precentral gyrus. Atrophy is also evident on MRIs (on susceptibility-weighted images generated from gradient-echo pulse sequences (GRE/SWI)) as a bilateral hypointensity in the precentral gyrus, known as the “motor band sign [77].” In addition, atrophy of the frontal and temporal lobes may be seen, especially in cases clinically accompanied by dementia [78]. Brainstem atrophy, with a volume reduction of the medulla oblongata, may be noted as pontine atrophy. With neuroimaging methods, density reductions in the mesencephalic crura are apparent [79]. Moreover, severe atrophy of the muscles of the upper and lower limbs and tongue are also evident. All ALS variants share these gross findings; differences are described below in the relevant paragraphs.
6.1.3. Neuropathological Findings in SALS
Degenerative changes are mostly observable in the motor area and by the large number of atrophied α-motor neurons located in the anterior grey matter of the spinal cord and the motor neurons of the cranial nerve nuclei in the brainstem [80]; these are striking histopathological signs of the disease, together with affected Betz cells in the primary motor cortex.
The loss of motor neurons leads to chronic denervation with neurogenic atrophy and fatty pseudohypertrophy in the striated muscles of the limbs and respiratory muscles; type 2 muscle fibers are the most vulnerable (Figure 1) [81,82]. Differentiation of muscle fibers is easy using adenosine triphosphatase (ATPase) staining, in which type 1 fibers appear lightly stained, and type 2 fibers appear darkly stained—this pattern is seen at pH 9,4 but not at pH 4,3 or 4,6 where the staining is inverse (type 1 fibers are dark, while type 2 fibers are pale) [83]. Assemblages of muscle fibers associated with re-innervation via axonal sprouting from neighboring motor axons are also typical; nevertheless, this compensatory mechanism is insufficient for muscle regeneration [84]. The location of atrophy is important; in the bulbar form, atrophy of the tongue, diaphragm, and intercostal muscles predominate, while in the typical form of ALS, atrophy in the limb muscles prevails. However, some, especially larger muscle fibers, can be paradoxically hypertrophic. In the late stages, clusters of pyknotic nuclei may be seen.
Standard staining is dominated by changes in the anterior horns, with marked atrophy, reactive astrogliosis, and atrophy of the anterior horns. In specialized histochemical detection of myelin, the most evident changes are seen in the anterolateral cords in the form of sclerosis; however, these changes can also be found in the posterior cords in longer disease courses [39]. A key diagnostic feature is the large number of atrophied large motor neurons in the anterior horns of the spinal cord, which is apparent in cervical and lumbar intumescence. The remaining neurons show signs of regressive changes, i.e., a tendency to wrinkle, the presence of lipofuscin deposits in the cytoplasm, and central chromatolysis in the nuclei [85]. A relatively characteristic feature is also phosphorylated neurofilament aggregates found as markedly swollen axons in anterior horns, generally referred to as spheroids [86].
Different types of inclusions can be found in the cytoplasm of affected neurons. Before immunohistochemical methods were routine, Bunina bodies, small eosinophilic granular inclusions, were considered diagnostically specific for ALS [87]. Now, the availability of immunohistochemical methods has broadened neuropathological examination. Anti-ubiquitin or anti-p62 [88] antibodies have revealed other types of inclusions widely present in motor neurons of the anterior spinal horns, in the brainstem, primary motor cortex, and neuronal structures in the frontal and temporal cortical areas [89]. The inclusions may be “skein-like,” [90] having an elongated or fibrous shape, which form bizarre spherical structures in the perikaryon of neurons, or light eosinophilic round hyaline inclusions. The presence of ubiquitin indicates impaired proteasomal degradation (in addition to RNA metabolism disorders at multiple levels) [91] as a key mechanism [92]. Most of the affected neurons and glial cells contain cytoplasmic TDP-43-immunoreactive inclusions [93]. The physiological TDP-43 protein acts as a DNA/RNA binding protein that binds both mRNA and DNA and mediates mRNA splicing, transcription, and translation [94]. Skein-like inclusions in anterior horn neurons and their neurites in the spinal cord may also be optineurin (OPTN) immunoreactive in both SALS and non-SOD1 FALS [95].
Oligodendrocyte dysfunction is considered by some authors to be a primary contributing factor to ALS [96] as there is prominent degeneration of oligodendrocytes in the gray matter of the spinal cord in ALS-model mice before disease onset [97]. SOD1-dependent loss of oligodendrocytes, leading to the death of motoneurons, is discussed—firstly, it is considered to happen due to defective lactate release, and secondly, by influencing cell-to-cell contact between axons and enwrapping oligodendrocytes. When SOD1 is selectively removed from oligodendrocytes, delayed disease onset as well as a longer survival period in ALS-model mice is seen. This observation could also contribute to the theory about the relationship between the impaired function of oligodendrocytes and the vulnerability of motoneurons [97].
Considering pTDP-43, Brettschneider et al. recognized and described four stages of ALS based on the specific sequential pattern of pTDP-43. Stage I is characterized by lesions in the agranular motor cortex, brainstem motor nuclei of cranial nerves XII-X, VII, V, and spinal cord α-motoneurons. For stage 2, the involvement of the prefrontal neocortex (middle frontal gyrus), brainstem reticular formation, precerebellar nuclei, and the red nucleus is characteristic. Stage 3 shows pTDP-43 pathology in the prefrontal (gyrus rectus and orbital gyri) and postcentral neocortex plus striatum. The last stage is characterized by inclusions in the anteromedial part of the temporal lobe, including the hippocampus. At all stages, oligodendroglial aggregates are also present [98].
All forms of ALS share the neuropathological signs described above. Differences between the familial form and the form combined with dementia will be described in the following paragraphs (for summary see Table 1, Table 2 and Table 3).
Table 1.
Summary of characteristics genes and inclusions in SALS cases.
| Disease-Associated Genes | Inclusions |
|---|---|
|
|
Table 2.
Summary of characteristics genes and inclusions in FALS cases.
| Disease-Associated Genes | Inclusions |
|---|---|
|
|
Table 3.
Summary of characteristics genes and inclusions in ALS-FTSD cases.
| Disease-Associated Genes | Inclusions |
|---|---|
|
|
6.2. Familial ALS
Familial ALS (FALS) is estimated to be 10% of all ALS cases [27], with the most common being autosomal dominant inheritance; however, as mentioned above, cases with autosomal recessive or X-linked inheritance have been described [23].
6.2.1. Background of FALS
To date, 16 genes associated with FALS have been identified, and up to 80 different mutations have been detected in 10–20% of familial cases. Currently, the most important genes are associated with the metabolism of the TDP-43 protein (mostly hyperphosphorylated), which plays a crucial role in the pathogenesis of the disease. Although TDP-43 is a nuclear protein, it is transferred to the cellular cytoplasm after acute neuronal injury, where it forms stress granules [99]. In addition to the TARDBP gene itself, the fused-in-sarcoma (FUS) gene is involved in physiologically similar processes as TARDBP (i.e., a DNA/RNA binding protein involved in brain mRNA processing and transcription) and also plays a role in the pathogenesis of ALS [100]. Mutations in SOD1 (the first gene historically identified as an ALS causing gene) [27], ALS2 (ALSIN; connected to juvenile-onset ALS [101], which is ALS starting before 25 years of age) [102], and senataxin (SETX; also seen in the juvenile form of ALS) are also associated with FALS [23]. Considering the juvenile variants, spatacsin (SPG) is typically associated with autosomal recessive heritability [23]. Optineurin (OPTN) is another important gene with autosomal recessive heritability [23]. Moreover, vesicle-associated membrane protein B (VAPB), valosin-containing peptide (VCP), progranulin (PGRN), ubiquilin 2 (UBQLN2; a mutation leading to ubiquitin-mediated disruption of proteasomal degradation) [103], and sequestosome 1 (SQSTM1/p62 [104]; involved in the degradation of misfolded proteins through ubiquitin–proteasomes [105] or the autophagy-lysosome system) [106,107] are also seen in FALS. The relatively recently discovered the 72nd open reading frame on chromosome 9 (C9orf72) [108] has now been found in many patients with FALS, with the incidence estimated to be up to 20%. Patients with the SOD1 mutation rarely suffer from cognitive impairment [109].
6.2.2. Neuropathological Findings in FALS
In addition to the above-mentioned neuropathological features shared with SALS, some cases of FALS have hyaline inclusions similar to Lewy bodies in the cytoplasm of neurons. They are strongly immunoreactive with anti-superoxide dismutase (SOD1) antibodies in those cases with the SOD1 gene mutation. Inclusions immunoreactive to SOD1 are located in lower motor neurons and represent a striking pathological feature of FALS; they are due to SOD1 mutations and are also seen in SOD1 murine models [110]. Neuropathologically, FALS caused by the SOD1 mutation can be distinguished from the sporadic disease by the relative sparing of the motor cortex, slight to mild corticospinal tract involvement, which is in contrast with severe atrophy of the anterior roots, and the degeneration of lower motor neurons in the sporadic form of the disease [27].
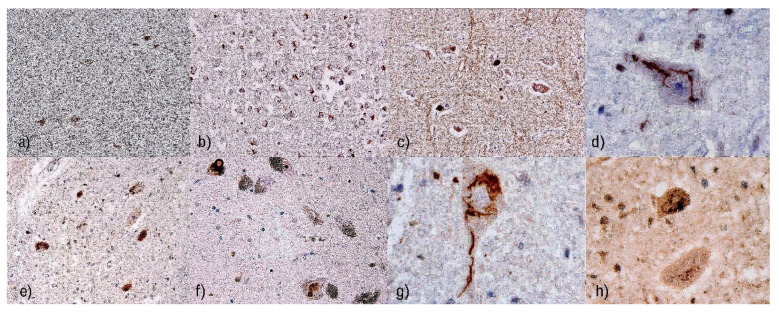
TDP-43 cytoplasmic inclusions, mainly found in the pyramidal, frontal, and temporal cortex, and hippocampal areas, are considered characteristic features of FALS with the C9orf72 mutation; the cerebellar granule cell layer, hippocampal pyramidal neurons, and neocortex all contain ubiquilin, p62, and ubiquitin inclusions that are negative relative to the TDP-43 reaction (Figure 2) [93].
Figure 2.
(a) Overview of inclusions positive in reaction with anti-phosphorylated TDP-43 (pTDP-43) antibody, original magnification 100×. (b) pTPD-43 positive cytoplasmic inclusions in the hippocampus are considered one of the characteristic features of the variant with C9orf72 mutation, magnification 100×. (c) Cytoplasmic pTDP-43-positive cytoplasmic inclusions in the hypoglossal motor neurons, original magnification 200×. (d) Detailed shape of cytoplasmatic pTDP-43-positive inclusion in the lower motor neuron. The original magnification 400×. (e) Inclusions detected with anti-p62 antibodies in the mesencephalic nuclei. The original magnification was 100×. (f) Inclusions detected by anti-p62 antibody seen in the neurons of mesencephalic nuclei. The original magnification 200×. (g) Detailed morphology of p62-positive inclusions in the cytoplasma of upper motor neuron, original magnification 400×. (h) Morphology of ubiquitin-positive “skein-like” inclusions with characteristic elongated or fibrous shape, which form bizarre spherical structures in the perikaryon of neurons. The original magnification was 400×.
Ubiquilin-positive (ubiquitin-like-positive) inclusions have been identified in spinal cord sections of patients with X-linked FALS; the inclusions co-localize with ubiquitin itself, p62, TDP-43, FUS, and OPTN but not with SOD1. UBQLN2 immunoreactivity has also been observed in spinal cord sections of sporadic ALS, ALS with dementia, and non- SOD1 FALS.
6.3. Amyotrophic Lateral Sclerosis-Frontotemporal Spectrum Disorder
In patients with amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD), inclusions positive for the anti-TDP-43 antibody reaction are usually present, so the clinical and neuropathological designation of FTLD-MND-TDP (frontotemporal lobar degeneration with motor neuron disease and TDP-43 positive inclusions) is used. However, dementia syndrome is defined as a loss of cognitive functioning and behavioral abilities to such an extent that it interferes with daily life and activities. The behavioral form (ALSbi) does not meet the condition of deterioration of self-sufficiency. Moreover, cognitive decline is often associated with a poorer prognosis [111]. Recently, the terminology was changed from “FTLD-MND” to “ALS-FTSD.” At present, the term FTLD-MND-TDP is purely neuropathological, while in clinical practice, ALS-FTSD is used [21].
6.3.1. Background of ALS-FTSD
A strong genetic burden is evident in ALS-FTSD cases [112]. The pathological expansion of the “hexa-repeat” (GGGGCC) in chromosome 9 open reading frame 72 is closely related to the behavioral variant of frontotemporal dementia (bvFTD), which occurs either in direct association with ALS or as a purely cognitive impairment lacking motor neuron dysfunction. Carriers of C9orf72 mutations have a significantly higher risk of developing cognitive impairment (40–50%) than patients lacking this mutation (8–9%) [113,114]. The spectrum of involvement in families carrying C9orf72 gene expansions (Figure 3) differs significantly from typical ALS based on purely behavioral symptomatology (bvFTD), i.e., without demonstrable motor neuron involvement up to a combination of ALS and cognitive impairment [115]. In patients with ALS with psychosis and anosognosia, the presence of C9orf72 mutation should be considered [21,116].
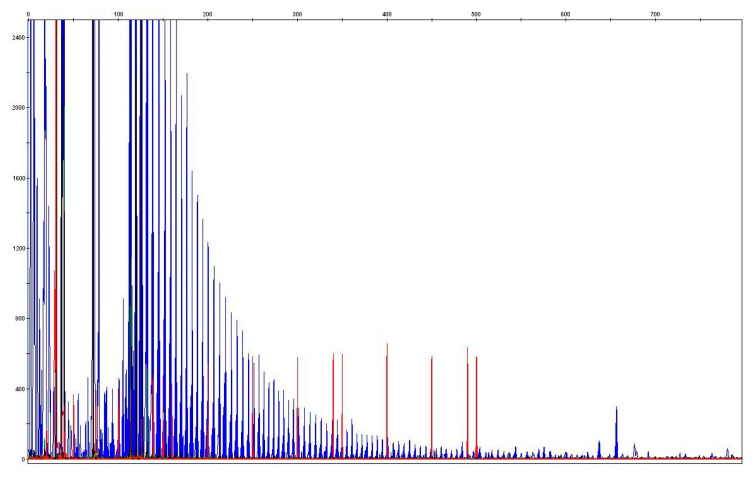
Figure 3.
Determination of copy number of hexanucleotide expansion of C9orf72. Reference values: Normal allele <20 GGGGCC repeats, permuted allele 20–100 GGGGCC repeats, complete penetrance> 100 GGGGCC repeats. The blue arrow indicates the location of 20 GGGGCC repeats and the red arrow indicates the location of 30 or more repeats.
6.3.2. Neuropathological Findings in ALS-FTSD
We can distinguish three basic types of FTLD depending on the hallmark pathological protein: (1) FTLD-tau (characterized by tau-positive inclusions), (2) FTLD-TDP (with TDP-43 inclusions), and (3) FTLD-FUS (having FUS-positive inclusions); although, a small proportion of FTD cases do not express any of the above-mentioned proteins. Those that react with ubiquitin or other markers of the ubiquitin-proteasome system are called FTLD-UPS, while completely immuno-negative cases are grouped as FTLD-NOS (not otherwise specified) [117]. Widespread ubiquilin-positive inclusions are also usually observed in the hippocampal region, including patients with the C9orf72 mutation [103].
Most ALS cases belong to the FTLD-TDP group and exhibit TDP-43 immunoreactive inclusions [118,119]; the remaining cases are in the FTLD-FUS group.
The Strong criteria [63] for the neuropathological diagnosis of ALS-FTSD remain unchanged, including the examination of the brain and spinal cord; AD must always be considered. A p62 and dipeptide repeat (DPR) pathology in the cerebellum and hippocampus is pathognomonic for C9orf72-linked ALS-FTSD [120]. The other neuropathological findings are identical to those cases lacking cognitive impairment [121]. It is probably not surprising that in genetic ALS-FTSD cases, SOD1- or FUS-immunoreactive inclusions can be found; moreover, alterations in microtubule-associated tau protein (tau) metabolism have been observed in ALS-FTSD, with the presence of tau deposits usually in the hyperphosphorylated form [92]. Since the primary function of tau is to provide stability to microtubules, site-specific tau phosphorylation therefore affects the interaction of tau and microtubules. Pathological hyperphosphorylation reduces the number of interactions between tau and microtubules, allowing tau to form less soluble oligomers and subsequently leading to the formation of fibrils [122]. In addition, research on murine models shows that having coexisting pathologies in TDP-43 and tau metabolism potentiate each other [92]. Thus, it is clear that FTD and ALS can share clinical manifestations and underlying pathophysiology, which are reflected in changes in TDP-43 and tau metabolism.
In FTLD-TDP-MND, inclusions are most common in the frontal and temporal cortex. In ALS with preserved cognition, deposits are located predominantly in the cytoplasm of motor neurons without affecting cortical structures [121].
According to the current neuropathological systemic classification, a harmonized classification system recognizes four types of FTLD-TDP pathology. Type A corresponds to Mackenzie et al. type 1 and Sampathu et al. type 3 and contains numerous short dystrophic neurites (DNs) and oval or crescent neuronal cytoplasmic inclusions (NCIs), which are located primarily in the second neocortical layer. Moderate numbers of lentiform neuronal intranuclear inclusions (NIIs) can also be present, although they are not a consistent sign of this subtype. The clinical phenotype of type A is bvFTD or progressive non-fluent aphasia and is based on mutations in the progranulin gene.
For this article, type B, equivalent to Mackenzie et al. type 3 and Sampathu et al. type 2, is the most important. Type B is characterized by moderate numbers of NCI, distributed throughout all cortical layers, with very few DNs. This condition is associated with a genetic defect in the short arm of chromosome 9, and clinically manifests as bvFTD or MND with FTD.
Type C is equivalent to Mackenzie et al. type 2 and Sampathu et al. type 1; it has a predominance of elongated DNs in the upper cortical layers and few NCIs; it leads to bvFTD or semantic dementia.
Type D is associated with the inclusion of body myopathy of Paget’s disease of the bone and frontotemporal dementia caused by VCP mutations; there are large numbers of short DNs and numerous lentiform NIIs.
However, there are other examples of the wide-range neuropathological background of ALS/MND clinical symptomatology. The most important neurodegenerations in this field are tauopathies. Recently, we described tau protein deposits in corticospinal tract structures in patients with progressive supranuclear palsy (PSP) clinically mimicking MND. [123] Moreover, case reports of globular glial tauopathy (GGT) with the presence of tau-positive globular oligodendroglial inclusions (GOIs) with partial overlapping neuropathological features of progressive supranuclear palsy (PSP), clinically manifesting as MND and/or FTD, exist [124]. Neuronal, astrocytic, and especially oligodendroglial 4-repeat (4R) tau positive, mostly Gallyas negative, inclusions are present, whereas the GOIs in white matter correlate with the severity of neuropathologically confirmed degeneration [124]. Massive oligodendroglial involvement leading to changes in white matter (pallor, axonal loss, and gliosis) implies that these cases may represent primary oligodendrogliopathy [124]. Some authors also speculate that this entity could be an equivalent of “tau” multiple-system atrophy [125].
Considering comorbid cases and dementia in ALS, some comorbid ALS/AD cases have appeared in the literature; for example, one study reported that about 50% of ALS cases had Aβ deposits, and at least half of the cases had neuritic plaques in the neocortex [126]. Those patients with comorbid ALS/AD usually have progressive amnestic dementia, which is typical for AD and is accompanied by early distinctive impairment of episodic memory; MRIs of the hippocampus show significant atrophy [55].
Comorbid ALS/dementia with Lewy bodies (DLB) also exists. The prevalence of the DLB pathology in ALS is higher than in the general population, and it has been reported that Parkinsonian features develop in about 30% of ALS patients [127]. Levodopa-responsive parkinsonism accompanied by ALS, with symptoms that appear later in the course of the disease, is known as Brait–Fahn–Schwarz disease [128]. The incidence of this syndrome is high on the Japanese Kii Peninsula and the Micronesian island of Guam but is rare elsewhere in the world [129], although, Brait–Fahn–Schwarz syndrome was diagnosed in the Czech Republic (personal experience, not published yet).
7. Molecular Biomarkers of ALS
It seems that CFS-PGRN levels can be used to predict the type of FTD as it mirrors the FTD-subtype—the level is significantly lower in cases with TDP-43 pathology lacking a GRN mutation [130]. CSF-TDP-43 is also considered to be a promising CSF biomarker for ALS-FTD cases [131]. Interestingly, some researchers report higher CSF-TDP-43 levels in ALS cases than in FTLD, which might indicate more faster progression of TDP-43 pathology in ALS [132].
To predict disease development, some of the above-mentioned molecules can be used as biomarkers. Mentioned markers from the group of RNA-binding proteins in ALS are TDP-43, FUS, or hnRNPs, although some others can be used—TATA-box binding protein associated factor 15 (TAF15), which is mutated in both SALS and FALS; Ewing Sarcoma breakpoint region 1/EWS RNA binding protein 1 (EWSR1) with similar characteristics as FUS and TAF15 that they can easily aggregate leading to the toxicityn [133]; or ataxin-2 (ATXN2), which acts as a dose-sensitive modifier of TDP-43 toxic effect [134]. From the group of ALS-related genes, we could highlight SOD1, C9orf72, or spastacsin (SPG) [133], as well as non-coding RNA such as microRNA, circular RNA [133], or other molecules. It is worth mentioning serum uric acid, whose serum levels negatively correlate with the risk of death in patients with ALS [135]; the detection of higher levels of chitotriosidase in CSF correlates with microglial activation in the white matter of the spinal cord and may be helpful in patients with a short history of symptoms that are difficult to identify [136]. The identification of the blood neurofilament light chain (NFL) positively correlates with the progression of the disease, and a shorter survival period is indicated by increased NFL [137]. Some studies also indicate that patients with ALS have significantly higher levels of CSF total tau protein and a lower phosphorylated tau/total tau ratio than the control population [138].
8. Conclusions
Although much about the pathogenesis of ALS remains elusive, some major molecular pathways that can trigger selective damage in specific neurons are already known. Moreover, the disruption of individual ALS-causing genes can lead to the formation of pathological inclusions. Recognition of these features can help determine final diagnoses and also indicate potential links with other neurodegenerative diseases that need to be simultaneously considered.
Acknowledgments
The authors would like to thank Tom Secrest, for the revision of the English version of this article.
Author Contributions
The authors contributed to the paper as follows: N.J. conception, design, and draft and final manuscript preparation; R.M. supervision of the project. Both authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the MH CZ–DRO: Conceptual Development of Research Organization, the General University Hospital, Prague (VFN, 00064165); the Thomayer University Hospital, Prague (TUH, 00064190); the Grants Agency of the Ministry of Health (NV19-04-00090); and by Charles University (Project Progress Q27/LF1 and GAUK 142120).
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jackson C.E., Rosenfeld J. Motor neuron disease. Phys. Med. Rehabil. Clin. N. Am. 2001;12:335–352. doi: 10.1016/S1047-9651(18)30073-1. [DOI] [PubMed] [Google Scholar]
- 2.Ludolph A., Drory V., Hardiman O., Nakano I., Ravits J., Robberecht W., Shefner J. WFN Research Group On ALS/MND. A revision of the El Escorial criteria—2015. Amyotroph. Lateral Scler. Front. Degener. 2015;16:291–292. doi: 10.3109/21678421.2015.1049183. [DOI] [PubMed] [Google Scholar]
- 3.Singer M.A., Statland J.M., Wolfe G.I., Barohn R.J. Primary lateral sclerosis. Muscle Nerve. 2007;35:291–302. doi: 10.1002/mus.20728. [DOI] [PubMed] [Google Scholar]
- 4.Liewluck T., Saperstein D.S. Progressive Muscular Atrophy. Neurol. Clin. 2015;33:761–773. doi: 10.1016/j.ncl.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Kim W.K., Liu X., Sandner J., Pasmantier M., Andrews J., Rowland L.P., Mitsumoto H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology. 2009;73:1686–1692. doi: 10.1212/WNL.0b013e3181c1dea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudnik-Schöneborn S., Zerres K. Spinal muscular atrophies. In: Rimoin D.L., Pyeritz R.E., Korf B.R., editors. Emery and Rimoin’s Principles and Practice of Medical Genetics. 6th ed. Elsevier; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 7.Batista B.H., Almeida A.G., Nunes M.L., Pitrez P.M., Ehlers J.A. Paralisia bulbar progressiva juvenil doença de Fazio-Londe: Relato de caso Progressive bulbar palsy (Fazio-Londe disease): Case report. Arq. Neuropsiquiatr. 2002;60:830–834. doi: 10.1590/S0004-282X2002000500026. [DOI] [PubMed] [Google Scholar]
- 8.National Intitute of Neurological Disorders and Stroke Motor Neuron Diseases Fact Sheet. [(accessed on 14 June 2021)]; Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Motor-Neuron-Diseases-Fact-Sheet.
- 9.Kolb S.J., Kissel J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015;33:831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burr P., Reddivari A.K.R. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2021. Spinal Muscle Atrophy. [PubMed] [Google Scholar]
- 11.Waldrop M.A., Elsheikh B.H. Spinal Muscular Atrophy in the Treatment Era. Neurol. Clin. 2020;38:505–518. doi: 10.1016/j.ncl.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Breza M., Koutsis G. Kennedy’s disease (spinal and bulbar muscular atrophy): A clinically oriented review of a rare disease. J. Neurol. 2019;266:565–573. doi: 10.1007/s00415-018-8968-7. [DOI] [PubMed] [Google Scholar]
- 13.Querin G., Sorarù G., Pradat P.-F. Kennedy disease (X-linked recessive bulbospinal neuronopathy): A comprehensive review from pathophysiology to therapy. Rev. Neurol. 2017;173:326–337. doi: 10.1016/j.neurol.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Jubelt B. Post-polio syndrome. Curr. Treat. Options Neurol. 2004;6:87–93. doi: 10.1007/s11940-004-0018-3. [DOI] [PubMed] [Google Scholar]
- 15.Jay V. The legacy of Jean-Martin Charcot. Arch. Pathol. Lab. Med. 2000;124:10–11. doi: 10.5858/2000-124-0010-TLOJMC. [DOI] [PubMed] [Google Scholar]
- 16.Goetz C.G. Amyotrophic lateral sclerosis: Early contributions of Jean-Martin Charcot. Muscle Nerve. 2000;23:336–343. doi: 10.1002/(SICI)1097-4598(200003)23:3<336::AID-MUS4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Editorial Dementia and motor neuron disease. Lancet. 1990;335:1250–1251. doi: 10.1016/0140-6736(90)91308-W. [DOI] [Google Scholar]
- 18.Rowland L.P., Shneider N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 19.Bonafede R., Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front. Cell. Neurosci. 2017;11:80. doi: 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiò A., Logroscino G., Hardiman O., Swingler R., Mitchell D., Beghi E., Traynor B.G., Eurals Consortium Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009;10:310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusina R., Mmatěj R., Cséfalvay Z., Keller J., Franková V., Vyhnálek M. Frontotemporální demence. Cesk Slov. Neurol. N. 2021;84/117:9–29. doi: 10.48095/cccsnn20219. [DOI] [Google Scholar]
- 22.Talbott E.O., Malek A.M., Lacomis D. The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 2016;138:225–238. doi: 10.1016/B978-0-12-802973-2.00013-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Sayana P., Zhang X., Le W. Genetics of amyotrophic lateral sclerosis: An update. Mol. Neurodegener. 2013;8:28. doi: 10.1186/1750-1326-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myl-lykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masrori P., van Damme P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020;27:1918–1929. doi: 10.1111/ene.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddique T., Ajroud-Driss S. Familial amyotrophic lateral sclerosis, a historical perspective. Acta Myol. 2011;30:117–120. [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta P.R., Jones A.R., Opie-Martin S., Shatunov A., Iacoangeli A., Al Khleifat A., Smith B.N., Topp S., Morrison K.E., Shaw P.J., et al. Younger age of onset in familial amyotrophic lateral sclerosis is a result of pathogenic gene variants, rather than ascertainment bias. J. Neurol. Neurosurg. Psychiatr. 2019;90:268–271. doi: 10.1136/jnnp-2018-319089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wijesekera L.L., Leigh P.N. Amyotrophic lateral sclerosis. Orphanet. J. Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCombe P.A., Henderson R.D. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Burrell J.R., Vucic S., Kiernan M.C. Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2011;12:283–289. doi: 10.3109/17482968.2011.551940. [DOI] [PubMed] [Google Scholar]
- 32.Chen H., Richard M., Sandler D.P., Umbach D.M., Kamel F. Head injury and amyotrophic lateral sclerosis. Am. J. Epidemiol. 2007;166:810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabrera J.R., Rodríguez-Izquierdo I., Jiménez J.L., Muñoz-Fernández M.A. Analysis of ALS-related proteins during herpes simplex virus-2 latent infection. J. Neuroinflamm. 2020;17:371. doi: 10.1186/s12974-020-02044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger M.M., Kopp N., Vital C., Redl B., Aymard M., Lina B. Detection and cellular localization of enterovirus RNA sequences in spinal cord of patients with ALS. Neurology. 2000;54:20–25. doi: 10.1212/WNL.54.1.20. [DOI] [PubMed] [Google Scholar]
- 35.Oluwole S.O., Yao Y., Conradi S., Kristensson K., Karlsson H. Elevated levels of transcripts encoding a human retroviral envelope protein (syncytin) in muscles from patients with motor neuron disease. Amyotroph. Lateral Scler. 2007;8:67–72. doi: 10.1080/17482960600864207. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram R.S., Gowtham L., Nayak B.S. The role of excitatory neurotransmitter glutamate in brain physiology and pathology. Asian J Pharm. Clin. Res. 2012;5:1–7. [Google Scholar]
- 37.Matej R., Botond G., Laszlo L., Kopitar-Jerala N., Rusina R., Budka H., Kovacs G.G. Increased neuronal Rab5 immunoreactive endosomes do not colocalize with TDP-43 in motor neuron disease. Exp. Neurol. 2010;225:133–139. doi: 10.1016/j.expneurol.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Turner M.R., Goldacre R., Ramagopalan S., Talbot K., Goldacre M.J. Autoimmune disease preceding amyotrophic lateral sclerosis: An epidemiologic study. Neurology. 2013;81:1222–1225. doi: 10.1212/WNL.0b013e3182a6cc13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Štětkářová I., Matěj R., Ehler E. Nové poznatky v dia gnostice a léčbě amyotrofické laterální sklerózy. Cesk Slov Neurol. N. 2018;81:546–554. doi: 10.14735/amcsnn2018546. [DOI] [Google Scholar]
- 40.Janssen C., Schmalbach S., Boeselt S., Sarlette A., Dengler R., Petri S. Differential Histone Deacetylase mRNA Expression Patterns in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2010;69:573–581. doi: 10.1097/NEN.0b013e3181ddd404. [DOI] [PubMed] [Google Scholar]
- 41.Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyo-trophic lateral sclerosis. Neuroepidemiology. 2003;22:217–228. doi: 10.1159/000070562. [DOI] [PubMed] [Google Scholar]
- 42.Geser F., Brandmeir N.J., Kwong L.K. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyo-trophic lateral sclerosis. Arch. Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 43.Vucic S., Rothstein J.D., Kiernan M.C. Advances in treating amyotrophic lateral sclerosis: Insights from pathophysiological studies. Trends Neurosci. 2014;37:433–442. doi: 10.1016/j.tins.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Wu C.H., Fal-lini C., Ticozzi N., Keagle P.J., Sapp P.C., Piotrowska K., Lowe P., Koppers M., McKenna-Yasek D., Baron D.M., et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature. 2012;488:499–503. doi: 10.1038/nature11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorges M., Vercruysse P., Müller H.P., Huppertz H.J., Rosenbohm A., Nagel G., Weydt P., Petersén Å., Ludolph A.C., Kassubek J., et al. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2017;88:1033–1041. doi: 10.1136/jnnp-2017-315795. [DOI] [PubMed] [Google Scholar]
- 46.Eisen A., Braak H., Del Tredici K., Lemon R., Ludolph A.C., Kiernan M.C. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2017;88:917–924. doi: 10.1136/jnnp-2017-315573. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami I., Arai T., Hasegawa M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol. 2019;138:751–770. doi: 10.1007/s00401-019-02077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graus F., Delattre J.Y., Antoine J.C., Dalmau J., Giometto B., Grisold W., Honnorat J., Smitt P.S., Vedeler C., Verschuuren J.J., et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatr. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown R.H., Al-Chalabi A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2013;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 50.Preston D.C., Shapiro B.E. Amyotrophic Lateral Sclerosis and its Variants. In: Preston D.C., Shapiro B.E., editors. Electromyography and Neuromuscular Disorders. 3rd ed. W.B. Saunders; London, UK: 2013. pp. 417–431. [Google Scholar]
- 51.Statland J.M., Barohn R.J., Dimachkie M.M., Floeter M.K., Mitsumoto H. Primary Lateral Sclerosis. Neurol. Clin. 2015;33:749–760. doi: 10.1016/j.ncl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghasemi M. Amyotrophic lateral sclerosis mimic syndromes. Iran J. Neurol. 2016;15:85–91. [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Ghawi E., Al-Harbi T., Al-Sarawi A., Binfalah M. Monomelic amyotrophy with proximal upper limb involvement: A case report. J. Med. Case Rep. 2016;10:54. doi: 10.1186/s13256-016-0843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein L.H., Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–380. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- 55.Rusina R., Vandenberghe R., Bruffaerts R. Cognitive and Behavioral Manifestations in ALS: Beyond Motor System Involvement. Diagnostics. 2021;11:624. doi: 10.3390/diagnostics11040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., Burrell J.R., Zoing M.C. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 57.McGee S. Examination of the Motor System: Approach to Weakness. In: McGee S., editor. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; Amsterdam, The Netherlands: 2018. pp. 551–568. [DOI] [Google Scholar]
- 58.Gibbons C.J., Thornton E.W., Young C.A. The patient experience of fatigue in motor neurone disease. Front. Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benny R., Shetty K. The split hand sign. Ann. Indian Acad. Neurol. 2012;15:175–176. doi: 10.4103/0972-2327.99700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z.-L., Cui L., Liu M., Zhang K., Liu S., Ding Q., Hu Y. Reassessment of Split-Leg Signs in Amyotrophic Lateral Sclerosis: Differential Involvement of the Extensor Digitorum Brevis and Abductor Hallucis Muscles. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreiber H., Gaigalat T., Wiedemuth-Catrinescu U., Graf M., Uttner I., Muche R., Ludolph A.C. Cognitive function in bulbar– and spinal–onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J. Neurol. 2005;252:772–781. doi: 10.1007/s00415-005-0739-6. [DOI] [PubMed] [Google Scholar]
- 62.Beeldman E., Raaphorst J., Klein Twennaar M., Govaarts R., Pijnenburg Y.A.L., de Haan R.J., de Visser M., Schmand B.A. The cognitive profile of behavioural variant FTD and its similarities with ALS: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatr. 2018;89:995–1002. doi: 10.1136/jnnp-2017-317459. [DOI] [PubMed] [Google Scholar]
- 63.Strong M.J., Abrahams S., Goldstein L.H., Woolley S., Mclaughlin P., Snowden J., Mioshi E., Roberts-South A., Benatar M., Hortobágyi T., et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooks B.R., Miller R.G., Swash M., Munsat T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the di-agnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 65.Rafalowska J., Wasowicz B. Syringomyelia simulating amyotrophic lateral sclerosis. Pol. Med. J. 1968;7:1214–1218. [PubMed] [Google Scholar]
- 66.Kennedy Disease. [(accessed on 15 June 2021)]; Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=481.
- 67.Rowland L.P. Diagnosis of amyotrophic lateral sclerosis. J. Neurol. Sci. 1998;160(Suppl. 1):S6–S24. doi: 10.1016/S0022-510X(98)00193-2. [DOI] [PubMed] [Google Scholar]
- 68.Mélé N., Berzero G., Maisonobe T., Salachas F., Nicolas G., Weiss N., Beaudonnet G., Ducray F., Psimaras D., Lenglet T. Motor neuron disease of paraneoplastic origin: A rare but treatable condition. J. Neurol. 2018;265:1590–1599. doi: 10.1007/s00415-018-8881-0. [DOI] [PubMed] [Google Scholar]
- 69.Hudgson P. Polymyositis and Dermatomyositis in Adults. Clin. Rheum. Dis. 1984;10:85–93. doi: 10.1016/S0307-742X(21)00485-9. [DOI] [PubMed] [Google Scholar]
- 70.Dabby R., Lange D.J., Trojaborg W., Hays A.P., Lovelace R.E., Brannagan T.H., Rowland L.P. Inclusion body myositis mimicking motor neuron disease. Arch. Neurol. 2001;58:1253–1256. doi: 10.1001/archneur.58.8.1253. [DOI] [PubMed] [Google Scholar]
- 71.Wicks P., Abrahams S., Papps B., Al-Chalabi A., Shaw C.E., Leigh P.N., Goldstein L.H. SOD1 and cognitive dysfunction in familial amyotrophic lateral sclerosis. J. Neurol. 2009;256:234–241. doi: 10.1007/s00415-009-0078-0. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi Y., Homma K., Ichijo H. SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS. Adv. Biol. Regul. 2016;60:95–104. doi: 10.1016/j.jbior.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Ajroud-Driss S., Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim. Biophys. Acta. 2015;1852:679–684. doi: 10.1016/j.bbadis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Liscic R.M., Grinberg L.T., Zidar J., Gitcho M.A., Cairns N.J. ALS and FTLD: Two faces of TDP-43 proteinopathy. Eur. J. Neurol. 2008;15:772–780. doi: 10.1111/j.1468-1331.2008.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mc Cann E.P., Henden L., Fifita J.A., Zhang K.Y., Grima N., Bauer D.C., Chan Moi Fat S., Twine N.A., Pamphlett R., Kiernan M.C., et al. Evidence for polygenic and oligogenic basis of Australian sporadic amyotrophic lateral sclerosis. J. Med. Genet. 2020;14:06866. doi: 10.1136/jmedgenet-2020-106866. [DOI] [PubMed] [Google Scholar]
- 76.Ellison D., Love S., Chimelli L. Neuropathology—A Reference Text of CNS Pathology. 3rd ed. Mosby; London, UK: 2013. [Google Scholar]
- 77.Roeben B., Wilke C., Bender B., Ziemann U., Synofzik M. The motor band sign in ALS: Presentations and frequencies in a consecutive series of ALS patients. J. Neurol. Sci. 2019;406:116440. doi: 10.1016/j.jns.2019.116440. [DOI] [PubMed] [Google Scholar]
- 78.Kiernan J.A., Hudson A.J. Frontal lobe atrophy in motor neuron diseases. Brain. 1994;117:747–757. doi: 10.1093/brain/117.4.747. [DOI] [PubMed] [Google Scholar]
- 79.Bede P., Chipika R.H., Finegan E., Li Hi Shing S., Doherty M.A., Hengeveld J.C., Vajda A., Hutchinson S., Donaghy C., McLaughlin R.L., et al. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: A longitudinal neuroimaging study. NeuroImage Clin. 2019;24:102054. doi: 10.1016/j.nicl.2019.102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirano A. Neuropathology of ALS: An overview. Neurology. 1996;47(Suppl. 2):63–66. doi: 10.1212/WNL.47.4_Suppl_2.63S. [DOI] [PubMed] [Google Scholar]
- 81.Pun S., Santos A.F., Saxena S., Xu L., Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 82.Hegedus J., Putman C.T., Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyo-trophic lateral sclerosis. Neurobiol. Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Shanmukha S., Narayanappa G., Nalini A., Alladi P.A., Raju T.R. Sporadic amyotrophic lateral sclerosis (SALS)—skeletal muscle response to cerebrospinal fluid from SALS patients in a rat model. Dis. Model. Mech. 2018;11:dmm031997. doi: 10.1242/dmm.031997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Telerman-Toppet N., Coers C. Motor innervation and fiber type pattern in amyotrophic lateral sclerosis and in Charcot-Marie-Tooth disease. Muscle Nerve. 1978;1:133–139. doi: 10.1002/mus.880010205. [DOI] [PubMed] [Google Scholar]
- 85.Dickson D.W., Weller R.O. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. 2nd ed. Wiley-Blackwell; Chichester, UK: 2011. [Google Scholar]
- 86.Okamoto K., Hirai S., Shoji M., Senoh Y., Yamazaki T. Axonal swellings in the corticospinal tracts in amyotrophic lateral sclerosis. Acta Neuropathol. 1990;80:222–226. doi: 10.1007/BF00308929. [DOI] [PubMed] [Google Scholar]
- 87.Okamoto K., Mizuno Y., Fujita Y. Bunina bodies in amyotrophic lateral sclerosis. Neuropathology. 2008;28:109–115. doi: 10.1111/j.1440-1789.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 88.Cooper-Knock J., Hewitt C., Highley J.R., Brockington A., Milano A., Man S., Martindale J., Hartley J., Walsh T., Gelsthorpe C., et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain. 2012;135:751–764. doi: 10.1093/brain/awr365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rea S.L., Foster A.D., Rea S.L. The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Neural Regen. Res. 2020;15:2186–2194. doi: 10.4103/1673-5374.284977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizusawa H., Nakamura H., Wakayama I., Yen S.H., Hirano A. Skein-like inclusions in the anterior horn cells in motor neuron disease. J. Neurol. Sci. 1991;105:14–21. doi: 10.1016/0022-510X(91)90112-K. [DOI] [PubMed] [Google Scholar]
- 91.Strong M.J. The evidence for altered RNA metabolism in amyotrophic lateral sclerosis (ALS) J. Neurol. Sci. 2010;288:1–12. doi: 10.1016/j.jns.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 92.Strong M.J., Donison N.S., Volkening K. Alterations in Tau Metabolism in ALS and ALS-FTSD. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.598907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MacKenzie I.R., Frick P., Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2013;127:347–357. doi: 10.1007/s00401-013-1232-4. [DOI] [PubMed] [Google Scholar]
- 94.Cohen T.J., Lee V.M., Trojanowski J.Q. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol. Med. 2011;17:659–667. doi: 10.1016/j.molmed.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng H.X., Bigio E.H., Zhai H., Fecto F., Ajroud K., Shi Y., Yan J., Mishra M., Ajroud-Driss S., Heller S., et al. Differential Involvement of Optineurin in Amyotrophic Lateral Sclerosis with or Without SOD1 Mutations. Arch. Neurol. 2011;68:1057–1061. doi: 10.1001/archneurol.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saez-Atienzar S., Bandres-Ciga S., Langston R.G., Kim J.J., Choi S.W., Reynolds R.H., Abramzon Y., Dewan R., Ahmed S., Landers J.E., et al. Genetic analysis of amyotrophic lateral sclerosis identifies contributing pathways and cell types. Sci. Adv. 2021;7:eabd9036. doi: 10.1126/sciadv.abd9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang S.H., Li Y., Fukaya M., Lorenzini I., Cleveland D.W., Ostrow L.W., Rothstein J.D., Bergles D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brettschneider J., del Tredici K., Toledo J.B., Robinson J.L., Irwin D.J., Grossman M., Suh E., van Deerlin V.M., Wood E.M., Baek Y., et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moisse K., Mepham J., Volkening K., Welch I., Hill T., Strong M.J. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL−/− mice: Support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1296:176–186. doi: 10.1016/j.brainres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 100.Lagier-Tourenne C., Polymenidou M., Hutt K.R., Vu A.Q., Baughn M., Huelga S.C., Clutario K.M., Ling S.-C., Liang T.Y., Mazur C., et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orban P., Devon R.S., Hayden M.R., Leavitt B.R. Juvenile amyotrophic lateral sclerosis. Hum. Hypothal. Neuropsychiatr. Disord. 2007;82:301–312. doi: 10.1016/s0072-9752(07)80018-2. [DOI] [PubMed] [Google Scholar]
- 102.Ben Hamida M., Hentati F., Ben Hamida C. Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis). Conditions combining a bilateral pyramidal syndrome with limb and bulbar amyotrophy. Brain. 1990;113:347–363. doi: 10.1093/brain/113.2.347. [DOI] [PubMed] [Google Scholar]
- 103.Deng H.X., Chen W., Hong S.T., Boycott K.M., Gorrie G.H., Siddique N., Yang Y., Fecto F., Shi Y., Zhai H., et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blokhuis A.M., Groen E.J., Koppers M., van den Berg L.H., Pasterkamp R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin W.H., Park J.H., Chung K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020;53:56–63. doi: 10.5483/BMBRep.2020.53.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen-Kaplan V., Livneh I., Avni N., Fabre B., Ziv T., Kwon Y.T., Ciechanover A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA. 2016;113:E7490–E7499. doi: 10.1073/pnas.1615455113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bjørkøy G., Lamark T., Johansen T. p62/SQSTM1: A Missing Link between Protein Aggregates and the Autophagy Machinery. Autophagy. 2006;2:138–139. doi: 10.4161/auto.2.2.2405. [DOI] [PubMed] [Google Scholar]
- 108.Ji A.L., Zhang X., Chen W.W., Huang W.J. Genetics insight into the amyotrophic lateral sclerosis/frontotemporal dementia spectrum. J. Med. Genet. 2017;54:145–154. doi: 10.1136/jmedgenet-2016-104271. [DOI] [PubMed] [Google Scholar]
- 109.Lopate G., Baloh R.H., Al-Lozi M.T., Miller T.M., Fernandes Filho J.A., Ni O., Leston A., Florence J., Schierbecker J., Allred P. Familial ALS with extreme phenotypic variability due to the I113T SOD1 mutation. Amyotroph. Lateral Scler. 2010;11:232–236. doi: 10.3109/17482960902898069. [DOI] [PubMed] [Google Scholar]
- 110.Kato S., Hayashi H., Nakashima K., Nanba E., Kato M., Hirano A., Nakano I., Asayama K., Ohama E. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am. J. Pathol. 1997;151:611–620. [PMC free article] [PubMed] [Google Scholar]
- 111.Rusina R., Ridzon P., Kulišťák P., Keller O., Bartos A., Buncova M., Fialová L., Koukolík F., Matej R. Relationship between ALS and the degree of cognitive impairment, markers of neurodegeneration and predictors for poor outcome. A prospective study. Eur. J. Neurol. 2009;17:23–30. doi: 10.1111/j.1468-1331.2009.02717.x. [DOI] [PubMed] [Google Scholar]
- 112.Gregory J.M., Fagegaltier D., Phatnani H., Harms M.H. Genetics of Amyotrophic Lateral Sclerosis. Curr. Genet. Med. Rep. 2020;8:121–131. doi: 10.1007/s40142-020-00194-8. [DOI] [Google Scholar]
- 113.Chiò A., Moglia C., Canosa A., Manera U., Vasta R., Brunetti M., Barberis M., Corrado L., D’Alfonso S., Bersano E., et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology. 2019;93:e984–e994. doi: 10.1212/WNL.0000000000008063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moore K.M., Nicholas J., Grossman M., McMillan C.T., Irwin D.J., Massimo L., van Deerlin V.M., Warren J.D., Fox N.C., Rossor M.N., et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020;19:145–156. doi: 10.1016/S1474-4422(19)30394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Millecamps S., Boillée S., Le Ber I., Seilhean D., Teyssou E., Giraudeau M., Moigneu C., Vandenberghe N., Danel-Brunaud V., Corcia P., et al. Phenotype difference between ALS patients with expanded repeats inC9ORF72and patients with mutations in other ALS-related genes. J. Med. Genet. 2012;49:258–263. doi: 10.1136/jmedgenet-2011-100699. [DOI] [PubMed] [Google Scholar]
- 116.Khan B.K., Yokoyama J.S., Takada L.T., Sha S.J., Rutherford N.J., Fong J.C., Karydas A.M., Wu T., Ketelle R.S., Baker M.C., et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated withC9ORF72hexanucleotide expansion. J. Neurol. Neurosurg. Psychiatr. 2012;83:358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mackenzie I.R., Neumann M., Baborie A., Sampathu D.M., Du P.D., Jaros E., Perry R.H., Trojanowski J.Q., Mann D.M.A., Lee V.M.Y. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davidson Y., Kelley T., MacKenzie I.R.A., Pickering-Brown S., Du Plessis D., Neary D., Snowden J.S., Mann D.M.A. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 119.MacKenzie I.R., Bigio E.H., Ince P.G., Geser F., Neumann M., Cairns N.J., Kwong L.K., Forman M.S., Ravits J., Stewart H., et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis withSOD1 mutations. Ann. Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 120.Al-Sarraj S., King A., Troakes C., Smith B., Maekawa S., Bodi I., Rogelj B., Al-Chalabi A., Hortobágyi T., Shaw C. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 121.Geser F., Lee V.M.-Y., Trojanowski J.Q. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: A spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30:103–112. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fichou Y., Al-Hilaly Y.K., Devred F., Smet-Nocca C., Tsvetkov P.O., Verelst J., Winderickx J., Geukens N., Vanmechelen E., Perrotin A., et al. The elusive tau molecular structures: Can we translate the recent breakthroughs into new targets for intervention? Acta Neuropathol. Commun. 2019;7:1–17. doi: 10.1186/s40478-019-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stejskalova Z., Rohan Z., Rusina R., Tesar A., Kukal J., Kovacs G.G., Bartos A., Matej R. Pyramidal system involvement in progressive supranuclear palsy—A clinicopathological correlation. BMC Neurol. 2019;19:42. doi: 10.1186/s12883-019-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmed Z., Doherty K.M., Silveira-Moriyama L.S., Bandopadhyay R., Lashley T., Mamais A., Hondhamuni G., Wray S., Newcombe J., O’Sullivan S.S., et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: An emerging group of 4-repeat tauopathies. Acta Neuropathol. 2011;122:415–428. doi: 10.1007/s00401-011-0857-4. [DOI] [PubMed] [Google Scholar]
- 125.Lantos P.L., Quinn N. Multiple system atrophy. In: Dickson D.W., editor. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. 1st ed. ISN Neuropath Press; Basel, Switzerland: 2003. pp. 203–214. [Google Scholar]
- 126.Bowser R., Hamilton R.L. Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 2004;107:515–522. doi: 10.1007/s00401-004-0843-1. [DOI] [PubMed] [Google Scholar]
- 127.Forrest S.L., Crockford D.R., Sizemova A., McCann H., Shepherd C.E., McGeachie A.B., Affleck A.J., Carew-Jones F., Bartley L., Kwok J.B., et al. Coexisting Lewy body disease and clinical parkinsonism in frontotemporal lobar degeneration. Neurology. 2019;92:e2472–e2482. doi: 10.1212/WNL.0000000000007530. [DOI] [PubMed] [Google Scholar]
- 128.Brait K., Fahn S., Schwarz G.A. Sporadic and familial parkinsonism and motor neuron disease. Neurology. 1973;23:990. doi: 10.1212/WNL.23.9.990. [DOI] [PubMed] [Google Scholar]
- 129.Manno C., Lipari A., Bono V., Taiello A.C., La Bella V. Sporadic Parkinson disease and Amyotrophic Lateral Sclerosis complex (Brait–Fahn–Schwartz Disease) J. Neurol. Sci. 2013;326:104–106. doi: 10.1016/j.jns.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 130.Kumar-Singh S. Progranulin and TDP-43: Mechanistic Links and Future Directions. J. Mol. Neurosci. 2011;45:561–573. doi: 10.1007/s12031-011-9625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Majumder V., Gregory J.M., Barria M.A., Green A., Pal S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: A systematic review and meta-analysis. BMC Neurol. 2018;18:90. doi: 10.1186/s12883-018-1091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Junttila A., Kuvaja M., Hartikainen P., Siloaho M., Helisalmi S., Moilanen V., Kiviharju A., Jansson L., Tienari P.J., Remes A.M., et al. Cerebrospinal Fluid TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis Patients with and without the C9ORF72 Hexanucleotide Expansion. Dement. Geriatr. Cogn. Disord. Extra. 2016;6:142–149. doi: 10.1159/000444788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang X., Ji Y., Wang W., Zhang L., Chen Z., Yu M., Shen Y., Ding F., Gu X., Sun H. Amyotrophic Lateral Sclerosis: Molecular Mechanisms, Biomarkers, and Therapeutic Strategies. Antioxidants. 2021;10:1012. doi: 10.3390/antiox10071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elden A.C., Kim H.-J., Hart M.P., Chen-Plotkin A.S., Johnson B.S., Fang X., Armakola M., Geser F., Greene R., Lu M.M., et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang F., Zhang Q., Ke Y., Hao J., Lu L., Lu N., Chen X. Serum uric acid levels in patients with amyotrophic lateral sclerosis: A meta-analysis. Sci. Rep. 2018;8:1–6. doi: 10.1038/s41598-018-19609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steinacker P., Feneberg E., Halbgebauer S., Witzel S., Verde F., Oeckl P., van Damme P., Gaur N., Gray E., Grosskreutz J., et al. Chitotriosidase as biomarker for early stage amyotrophic lateral sclerosis: A multicenter study. Amyotroph. Lateral Scler. Front. Degener. 2021;22:276–286. doi: 10.1080/21678421.2020.1861023. [DOI] [PubMed] [Google Scholar]
- 137.Verde F., Steinacker P., Weishaupt J.H., Kassubek J., Oeckl P., Halbgebauer S., von Tumani H., Arnim C.A.F., Dorst J., Feneberg E., et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2019;90:157–164. doi: 10.1136/jnnp-2018-318704. [DOI] [PubMed] [Google Scholar]
- 138.Agnello L., Colletti T., Lo Sasso B., Vidali M., Spataro R., Gambino C.M., Giglio R.V., Piccoli T., Bivona G., La Bella V., et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021;28:1868–1875. doi: 10.1111/ene.14789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.