Abstract
Diagnosis is one of the crucial tasks performed by primary care physicians; however, primary care is at high risk of diagnostic errors due to the characteristics and uncertainties associated with the field. Prevention of diagnostic errors in primary care requires urgent action, and one of the possible methods is the use of health information technology. Its modes such as clinical decision support systems (CDSS) have been demonstrated to improve the quality of care in a variety of medical settings, including hospitals and primary care centers, though its usefulness in the diagnostic domain is still unknown. We conducted a scoping review to confirm the usefulness of the CDSS in the diagnostic domain in primary care and to identify areas that need to be explored. Search terms were chosen to cover the three dimensions of interest: decision support systems, diagnosis, and primary care. A total of 26 studies were included in the review. As a result, we found that the CDSS and reminder tools have significant effects on screening for common chronic diseases; however, the CDSS has not yet been fully validated for the diagnosis of acute and uncommon chronic diseases. Moreover, there were few studies involving non-physicians.
Keywords: clinical decision support systems, diagnostic accuracy, health information technology, primary care
1. Introduction
Diagnosis by primary care physicians (PCPs) is an important task; however, there is always a risk of diagnostic error in the task of diagnosis. Diagnostic errors are the greatest threat to patient safety in primary care [1]. A recent study estimated that approximately 5% of adult patients in the United States experience diagnostic errors in outpatient settings every year [2]. Estimates from diagnostic error rates in selected research studies indicate that 12 million Americans suffer from diagnostic errors in primary care alone each year [2,3]. The same study found that 33% of these diagnostic errors led to “serious permanent injury” or “immediate or inevitable death”. This translates into at least 4 million people seriously harmed, including at least 1.7 million people who died, due to diagnostic errors [3]. Thus, the prevention of diagnostic errors in primary care is an urgent issue. The World Health Organization (WHO) recently noted the importance of safety and diagnostic accuracy in primary care [4], and diagnostic issues that harm patients through errors or delays in testing and treatment have emerged as a matter of grave concern in global safety [5].
Primary care commonly involves a large number of patients and decision-making in the face of uncertainty [6]. In addition, the characteristics of primary care (first contact, accessible, continuous, comprehensive, and coordinated care) make it an area at high risk of diagnostic errors [7]. More than half of medical malpractice cases against general practitioners are due to diagnostic errors [8], and their prevention requires prompt action. In primary care, where the risk of diagnostic errors is high, there are high expectations for improving the diagnostic process through CDSS and health IT technologies [5].
One of the health IT products is the clinical decision support system (CDSS). CDSS is not intended to replace a clinician’s assessment, but rather to facilitate the clinician’s correct assessment and reasoning through suggestions and alerts [1,9]. CDSS performs various functions, including giving reminders, alerting users of prescription interactions and test results, interpreting tests, predicting mortality based on epidemiological data, assisting in diagnosis, and calculating drug doses [10].
CDSS has been demonstrated to enhance the quality of care in a variety of healthcare settings, including hospitals and primary care centers [1,11]. In 2007, the US government published “A Roadmap for National Action on Clinical Decision Support” to encourage the introduction of CDSS in electronic health records (EHRs) [12]. In 2013, 41% of US hospitals with EHRs were estimated to have CDSS, and in 2017, 40.2% had advanced CDS capabilities [13]. In the UK, the government introduced Isabel software as a national healthcare system [14], and an artificial intelligence (AI)-powered triage and diagnosis system called Babylon is in operation, although some barriers remain [15]. The CDSS market is currently dominated by North America and is estimated to reach USD 1.33 billion by 2021 and USD 2.24 billion by 2026 [16]. Conversely, language remains a barrier for introduction in non-English speaking countries [17]. In developing countries, barriers have been pointed out for EHR implementation before CDSS [18].
While CDSS is useful for improving medical care in the primary care setting, few studies have been conducted in the diagnostic setting [1], and there are few reports showing measurable clinical effects [19]. Additionally, CDSS use has not been promoted due to physicians’ negative perceptions and prejudices toward CDSS as a diagnostic aid, complex data entry, and gaps in data use [13]. Moreover, there are barriers to widespread CDSS use and shortcomings of CDSS: CDSS disrupts the workflow of healthcare professionals, increases the time required to complete tasks, increases cognitive load, and decreases time spent with patients (especially in standalone systems). CDSS has several disadvantages, including overriding by physicians, alert fatigue, and risk of deskilling of healthcare providers due to long-term use [13]. In primary care, concerns about reliability, impact on workflow, and incompatibility between the gatekeeper role and CDSS recommendations are considered barriers to adoption [20]. One study found that solo practices had significantly lower rates of CDSS use, regardless of EHR use [21]. Another problem is that cost-effectiveness is unknown [13]. To eliminate barriers to CDSS adoption, various factors such as internal and external environments, individuals, and interventions have been pointed out [22]. Particularly, to successfully eliminate barriers, user-centered design and analysis of impact on performance improvement are needed [23,24].
We conducted a scoping review to verify the usefulness of CDSS in the diagnostic domain in primary care and to identify research gaps and areas of uncertainty that require further exploration.
2. Materials and Methods
This scoping review was based on the methodology of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) statement [25]. The primary review questions were: (1) what is the current status of the usefulness of the CDSS in the diagnostic area of primry care? and (2) can we identify research gaps and areas of uncertainty from existing knowledge?
2.1. Search Strategy
We searched PubMed/MEDLINE for articles written in English and published until March 2020. Suitable search terms were chosen to cover the three dimensions of interest: decision support systems, diagnosis, and primary care. The query used the Medical Subject Headings (MeSH) terms, “Decision Support Systems, Clinical” [MeSH] and “Primary Health Care” [MeSH].
Two of the authors (TM and TH) independently screened the titles and abstracts of the retrieved articles to assess their relevance based on the eligibility criteria. A third author (KK) made the final decision on papers regarding which there was no consensus between TM and TH. Backward citation tracking was also performed to identify additional relevant articles. Finally, full-text versions of the articles that were found relevant by the two reviewers were reviewed.
2.2. Eligibility Criteria
The following inclusion criteria were used for the selection of relevant studies: (1) the study should have evaluated the implementation of a CDSS in primary care and (2) CDSS included everything from simple reminders to complex computer clinical decision support systems, all of which are relevant to the diagnostic process. Additionally, in this study, reminder tools were also included in the CDSS. Studies were excluded if they were (1) related to treatment or management; (2) targeted the accessibility of CDSS in primary care settings; (3) not real clinical studies using simulations or scenario cases; (4) studies on CDSS for caregivers; (5) studies in the pediatric population; and (6) review articles and protocol studies.
3. Results
3.1. General Overview
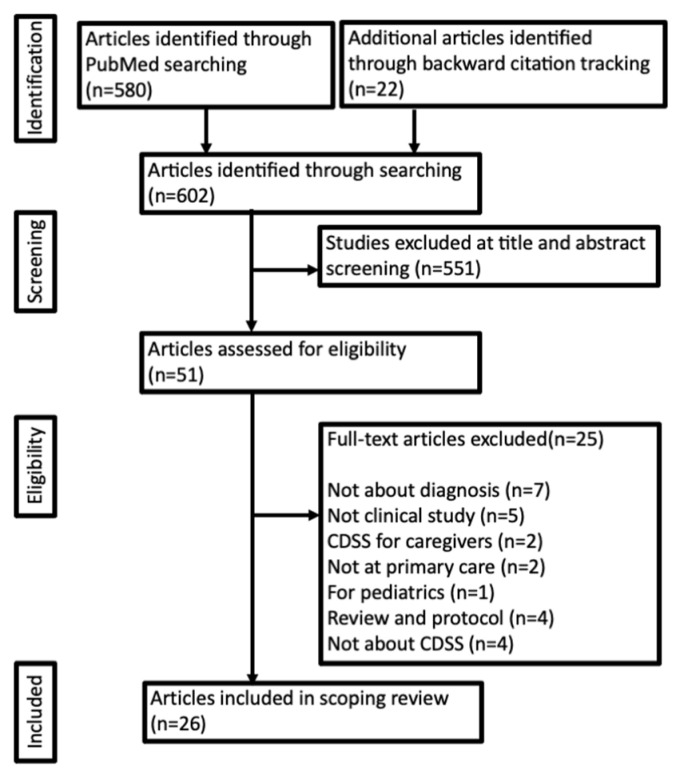
The search query returned 580 articles and backward citation tracking included 22 articles. After analyzing the titles and abstracts, 551 articles that were not relevant to the research question were discarded. Finally, 51 full-text articles were reviewed, among which 25 articles that did not satisfy the eligibility criteria were excluded. The corresponding PRISMA flow diagram is shown in Figure 1.
Figure 1.
PRISMA flow diagram.
Twenty-six clinical studies (17 randomized control trials [RCTs] and nine non-RCTs) were included (Table 1). They comprised 10 studies on combined outcomes including malignancy prevention, vaccine uptake, and lifestyle prevention [26,27,28,29,30,31,32,33,34,35]; three studies on malignancy (one each on breast cancer, cervical cancer, and colorectal cancer) [36,37,38]; five studies on cardiovascular risk factors (one each on diabetes mellitus [DM], dyslipidemia [DLP], obesity, abdominal aortic aneurysm [AAA], and chronic kidney disease [CKD]) [39,40,41,42,43]; two studies on musculoskeletal diseases (one each on osteoporosis and function) [44,45]; three studies on infectious diseases (two on hepatitis B virus [HBV] and one on human immunodeficiency virus [HIV]) [46,47,48]; and others included one study on depression, one study on dementia, and one study on domestic violence [49,50,51]. Common chronic diseases were the main target, and none of the studies included common acute diseases or uncommon chronic diseases. In almost all studies, CDSS had significant results in the screening and diagnosis of chronic diseases.
Table 1.
Characteristics of the 26 clinical studies included in this scoping review.
| Reference | Participants | Age | Study Design | Target Disease | Process Outcome | Main Outcome | Main Observation | Significant Difference | Evaluator | Support Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Rosser et al. 1991, Canada [26] | 8502 patients | Over 15 years old | Randomized controlled trials | combined outcomes | Screening rate | Rates of completion of the preventive procedures | Five screening procedures (Flu vaccine, measure blood pressure, Assess smoking status, Papanicolaou smear, Tetanus vaccine) | ○ | Physician | Reminder |
| Ornstein et al. 1991, USA [27] | 7397 patients | 40.0 ± 17.3 | Randomized controlled trials | combined outcomes | Screening rate | Adherence to preventive serivices | Five recommended preventive services (cholesterol measurements, fecal occult blood testing, mammography, Papanicolaou smears, and tetanus vaccine) | ○ | Physician | Reminder |
| McPhee et al. 1991, USA [28] | 2331 encount | No details available | Randomized controlled trials | combined outcomes | Screening rate | Rates of completion of the preventive procedures | Nine cancer prevention services and screenings (stool occult-blood test, rectal examination, pelvic examination, Papanicolaou’s smear, breast examination, smoking assessment, smoking counseling, dietary assessment, dietary counseling, sigmoidoscopy, mammography) | ○ | Physician | Reminder |
| McPhee et al. 1989, USA [29] | 1936 records | Varies by criteria | Randomized controlled trials | combined outcomes | Screening rate | Rates of completion of the cancer screening procedures | Seven cancer prevention screenings (stool occult blood test, rectal examination, sigmoidoscopy, pap smear, pelvic examination, breast examination, mammogram) | ○ | Physician | Reminder |
| McDonald et al. 1984, USA [30] | 12467 patients | No details available | Randomized controlled trials | combined outcomes | Screening rate | Response rate for reminder | Twelve actions including 5 preventive procedures (occult blood testing, mammographic screening, weight reduction diets, influenza, and pneumococcal vaccines) | ○ | Physician | Reminder |
| Hamilton et al. 2013, UK [31] | 2593 records | No details available | Nested case-control study | Lung and colorectal cancer | Screening rate and diagnosis | Diagnosis of cancer and diagnostic test | 2-week referrals for lung and colorectal cancer, requested CXR, coloscopies | ○ | Physician | Decision support system |
| Murphy et al. 2015, USA [32] | 733 records | 60.4 ± 7.4 (intervention group) | Randomized controlled trials | Lung, colorectal and prostate cancers | Time to diagnostic evaluation | Proportion and time to diagnostic evaluation of cancer | Electronic health record-based trigger (red flag criteria of colorectal, lung and prostate cancer), Proportion and time to diagnostic evaluation of cancer | ○ | Physician | E-trigger |
| Price et al. 2019, UK [33] | No details available | No details available | Cross-sectional study | Lung and colorectal cancer | Referral rate for suspected malignancy | 2-week wait referral rate | Availability and use cancer decision-support tools | × | Physician | Decision support system |
| Vetter, 2015, USA [34] | 39 records | No details available | Non-randomized controlled trials | General diagnosis | Accuracy of diagnosis | Diagnostic accuracy and clinical documentation | Chart audit tool | ○ | Nurse practitioner | Decision support system |
| Shimizu et al. 2018, Japan [35] | 100 patients | 70 ± 20.9 | Retrospective observational study | General diagnosis | Accuracy of diagnosis | Diagnostic error rate | Exposure to computor clinical decision support system | ○ | Physician | Decision support system |
| Burack et al. 1997, USA [36] | 1225 patients | Women over 40 years old | Randomized controlled trials | Breast cancer | Screening rate | Mammography rates | Mammography rates | ○ | Physician | Reminder |
| Burack et al. 1998, USA [37] | 5801 patients | Women 18–40 years old | Randomized controlled trials | Cervical cancer | Screening rate | Visitation, Pap smear | Visitation, Pap smear | × | Physician | Reminder |
| Sequist et al. 2009, USA [38] | 21860 patients | 60.3 ± 8.3 (Intervention group) | Randomized controlled trials | Colorectal cancer | Screening rate | Fecal occult blood testing, flexible sigmoidoscopy and colonoscopy | Fecal occult blood testing, flexible sigmoidoscopy, colonoscopy and detection of colorectal adenomas | ○ | Physician | Reminder |
| Litvin et al. 2016, USA [39] | No details available | Over 18 years old | Non-randomized controlled trials | Chronic kidney disease | Screening rate | CKD identification and management | Performance on chronic kidney disease clinical quality measures | ○ | Physician | Decision support system |
| Lee et al. 2007, USA [40] | 1874 encounters | 47.8 ± 17.88 (Intervention group) | Randomized controlled trials | Obesity | Diagnostic rate | Diagnostic accuracy of obesity-related diagnoses | Screening rate and diagnsis of obesity-related diagnoses | ○ | Nurse practitioner | Decision support system |
| Chaudhry et al. 2012, USA [41] | 1763 patients | Men aged 65-75 | Retrospective observational study | Abdominal aortic aneurysm | Screening rate | Screening rate of abdominal aortic aneurysm | Screening rate of abdominal aortic aneurysm | ○ | Physician | Decision support system |
| Kanealy et al. 2005, New Zealand [42] | 5628 patients | Over 50 years old | Randomized controlled trials | Diabetes | Screening rate | Screening rate of diabetes | Screening rate of diabetes | ○ | Physician | Reminder |
| Wyk et al. 2008, Netherlands [43] | 87886 patients | 43.8 ± 14.8 (intervention group) | Randomized controlled trials | Dyslipidemia | Screening rate | Screening and treated rate of dyslipidemia | Screening and treated rate of dyslipidemia | ○ | Physician | E-alert |
| Rubenstein et al. 1995, USA [44] | 557 patients | 51.4 ± 18.2 (intervention group) | Randomized controlled trials | Physical function | Functional decline | Functional Status Questionnaire | Functional Status Questionnaire and completion rate of interventions | ○ | Physician | Feedback report |
| DeJesus et al. 2012, USA [45] | 14674 patients | Women over 65 years old | Retrospective observational study | Osteoporosis | Screening rate | Completion rate of osteoporosis screening | Completion rate of osteoporosis screening, pratice rate of osteoporosis screening | ○ | Physician | Decision support system |
| DeSilva et al. 2020, USA [46] | 13707 patients | Over 12 years old | Randomized controlled trials | Hepatitis B virus infection | Screening rate | Diagnosis of chronic HBV infection | Rate of alerts opened, test order, obtain of result, positive HBV screening test | ○ | Physician | E-alert |
| Chak et al. 2018, USA [47] | 2987 patients | 38.5 ± 14.7 (intervention group) | Randomized controlled trials | Hepatitis B virus infection | Screening rate | Completion rate of hepatitis B infection screening | Completion rate of hepatitis B virus infection screening, positive rate of test | ○ | Physician | E-alert |
| Sundaram et al. 2009, USA [48] | 26042 patients | No details available | Randomized controlled trials | Human immunodeficiency virus infection | Screening rate | HIV screening rates | HIV screening rates, degree to guideline concordant, adherence to reminders, and provider attitude and knowledge. | × | Physician | Reminder |
| Miller et al. 2017, USA [49] | 19869 patients | No details available | Cross-sectional study | Depression | Screening rate | Depression screening rates | Depression screening rates, contraindications to medication, level of alert, mental health risk | ○ | Physician | Decision support system |
| Downs et al. 2006, UK [50] | 450 records | 84.9 ± 6.6 (Intervention group) | Non-randomized controlled trials | Dementia | Rates of detection of dementia | Detection rates of dementia | Detection rates of dementia, concordance with guidelines | ○ | Physician | Decision support system |
| Ahmad et al. 2009, Canada [51] | 293 patients | 43.5 ± 14.8 | Randomized controlled trials | Intimate partner violence and control | Screening rate | Initiation of discussion about risk for Intimate partner violence and control and detection of women at risk | Initiation of discussion about risk for Intimate partner violence and control and detection of women at risk | ○ | Physician | Decision support system |
3.2. Effect of CDSS on the Screening and Diagnosis of Composite Outcomes
The effect of the recommended multiple screening reminder as a composite outcome was assessed in five RCTs [26,27,28,29,30], all of which showed significantly positive results.
Two studies evaluated the impact of CDSS on clinical diagnosis without focusing on specific diseases or multiple outcomes [34,35]. Vetter’s study on nurse practitioners (NPs) in a home-based primary care setting showed that the introduction of the CDSS improved the diagnostic accuracy and appropriate documentation [34]. A retrospective study by Shimizu et al. [35] in an outpatient department of a community-based hospital examined the effects of CDSS and found that it significantly reduced diagnostic errors (odd’s ratio [OR] 15.21).
3.3. Effect of CDSS on the Screening and Diagnosis of Cancer
Hamilton et al. [31] investigated whether adding risk assessment tools would improve testing and diagnosis of lung and colorectal cancer in a before-and-after cohort study involving 165 clinics and 614 primary care physicians, using a nested qualitative study. They found 2593 uses of risk assessment tools with increased correct testing and diagnosis of lung and colorectal cancers after the addition.
Murphy, in an RCT, investigated whether E-triggers, based on electronic medical records, could reduce the time to diagnosis of colorectal, lung, and prostate cancers in two primary care practices, with 72 primary care physicians divided equally into intervention and target groups. Of the 10,673 patients, E-trigger was triggered in 1256 patients, resulting in a significant decrease in the time to diagnostic evaluation for colorectal and prostate cancers; however, no significant difference was observed for lung cancer [32].
Price et al. [33] conducted a cross-sectional study in primary care clinics to determine the relationship between the use of cancer decision support tools and the number of referrals for suspected malignancy (2-week wait). The results showed that there was no significant difference between the use of cancer decision support tools and the number of referrals, suggesting the possibility of the underuse of CDSS in primary care in the United Kingdom.
Three studies were applicable to CDSS interventions for a single malignancy. Burack and Gimotty [36] conducted an RCT in three primary care practices to evaluate the sustained effect of computerized reminders in the second year of the intervention. The results showed that over the 2 years, the reminder group had significantly higher mammography uptake than in the pre-intervention period. Burack et al. [37], in an RCT of 5801 women, evaluated the influence of reminders to patients and/or physicians on PAP smear practices and found no significant effect. Sequist et al. [38] conducted an RCT of reminders to 21,860 patients and 110 primary care physicians in 11 ambulatory care centers to determine the impact of reminders on colorectal cancer screening. The screening rate was similar and did not significantly increase between patients of physicians who received email reminders and the control group (41.9% vs. 40.2%, p = 0.47).
3.4. Effect of CDSS on the Screening and Diagnosis of Cardiovascular Risk Factors
Five CDSS studies were applicable for screening and diagnosing cardiovascular risk factors. They included three RCTs, one non-RCT, and one retrospective observational study. The participants were screened for CKD, AAA, obesity, DM, and DLP. Litvin et al. [39] evaluated the effect of implementing the CDSS for the identification and management of CKD in 11 primary care clinics. The results showed significant improvement in the screening and monitoring of albuminuria over 2 years. Lee et al. [40] measured the effect of CDSS in 1874 clinical sites over an 8-month period among NPs of two specialties (acute care and family) and showed that the CDSS group had 11.3% more diagnoses and 37% fewer false negatives than the control group. The introduction of CDSS has improved the diagnosis of obesity. In a before-after retrospective observational study, Chaudhry et al. [41] investigated the screening construction rate of AAA before and after the introduction of CDSS in male patients aged 65–75 years who visited the clinic in 2007 and 2008. The overall screening rate increased significantly (13%), and the percentage of patients in the completed-screening group improved by approximately fivefold, from 3.2% to 18.2%.
Kenealy et al. [42] conducted an RCT of an intervention that included computer-based reminders to improve screening for DM. The duration of the study was 2 months, and 107 family physicians participated. The results showed that computer-based reminders significantly improved the screening rate for DM (OR, 1.49). Wyk et al. [43] conducted an RCT of 38 clinics, 77 physicians, and 87886 patients in the Netherlands to determine the effect of alerts on improving dyslipidemia screening. Each clinic was assigned to one of three groups: receive alerts, on-demand support, or no intervention. After 12 months of follow-up, screening occurred 65% of the time in the alert group, compared to 35% in the on-demand group, and 25% in the target group, with a significantly higher screening rate in the alert group. The frequency of treatment for patients needing treatment was also significantly higher in the alert group (66% vs. 40% vs. 26%).
3.5. Effect of CDSS on the Screening and Diagnosis of Musculoskeletal Conditions
Rubenstein et al. [44] conducted an RCT to determine the impact of implementing the CDSS, which provides screening and feedback for improving physical functioning in older adults. Seventy-three internists and 557 patients in a primary care clinic participated; patients in the CDSS group had significantly less frequent functional decline and significantly improved emotional well-being scores compared with the control group.
DeJesus et al. [45] conducted a before-and-after retrospective observational study to measure the effect of CDSS on osteoporosis screening. Eligible patients were women aged ≥ 65 years who had never undergone a bone mineral density test and were seen in a primary care clinic. They found that the overall screening rate improved significantly from 80.1% to 84.1%, and completion of screening after the visit increased from 5.87% to 9.79%, an improvement of 66.7%.
3.6. Effect of CDSS on the Screening and Diagnosis of Infectious Diseases
DeSilva et al. [46] conducted a pilot study at nine clinics to screen for HBV infection in people born outside the United States. Eligible patients were aged ≥ 12 years and from countries with HBV infection rates of 2% or higher. E-alerts were triggered for more than 4500 patients between July 2012 and March 2013, and in 14.0% of patients, healthcare providers responded to the trigger; six previously unrecognized HBV-infected patients were identified. Although the usefulness of the triggers was demonstrated, there was no significant difference between passive and active interventions, and the response rate to the triggers decreased yearly.
Chak et al. [47] conducted a study in the United States to measure the effect of E-alerts in screening foreign-born HBV high-risk populations. Over a period of 1 year, 2987 patients were included in the study and the intervention group had significantly more screening tests performed than the control group (OR 2.64), demonstrating the usefulness of E-alerts in HBV screening.
An RCT was conducted by Sundaram et al. [48] to determine the efficacy of a computerized reminder and feedback intervention in improving HIV screening among 32 physicians in five primary care clinics. The results showed that the intervention did not significantly improve HIV screening, possibly due to the overall low rate of HIV screening and barriers to reminders.
3.7. Effect of CDSS on the Screening and Diagnosis of Other Diseases
Miller et al. [49] investigated whether drug-related E-triggers improve depression screening in a 3-year cross-sectional descriptive study. Primary care physicians screened for depression in 2.1% of patients, with a significant increase in screening rates, especially when moderate or high warnings were given.
Downs et al. [50] conducted an unblinded, clustered, randomized, controlled study to determine whether CDSS improves the detection of dementia in primary care. Thirty-six clinics were randomly assigned to the control, workshops, tutorials on CD-ROM, and CDSS embedded in the electronic health record groups. The CDSS significantly improved the detection of dementia compared to the control group in 450 records (30% vs. 11%).
An RCT of 11 family physicians and 282 patients was conducted by Ahmad et al. [51] to evaluate whether computer-assisted screening by family physicians could improve the detection of women at risk for intimate partner violence and control (IPVC); they found that CDSS significantly improved the detection of IPVC compared to the control group (18% vs. 9%).
4. Discussion
The results of this scoping review show that the CDSS and reminder tools have significant results in screening for common chronic diseases; however, the CDSS has not yet been fully validated for the diagnosis of acute disease and uncommon chronic diseases.
The clinical usefulness of CDSS in acute illness and uncommon chronic diseases seems promising. A previous study showed a 17% improvement in the diagnosis of acute abdominal pain when CDSS was introduced in an emergency room [52]. Moreover, though simulation-based, the usefulness of CDSS for primary care physicians in various diseases such as orthopedic diseases [9], ophthalmic diseases [53], and skin malignancies [54] has been suggested. Farmer developed a knowledge-based CDSS to aid in the diagnosis of shoulder disorders in the primary care setting based on computer science literature and orthopedic opinion. Although accuracy varied with each shoulder disease, the CDSS diagnostic results for 93 case studies had a sensitivity of 91%, specificity of 98%, positive likelihood ratio of 53.12, and a negative likelihood ratio of 0.08 [9]. López et al. conducted a study on the functionality and reliability of OphthalDSS, a mobile application for the diagnosis of anterior segment ocular disease diagnosis. Fifty primary care physicians in Spain used OphthalDSS and evaluated the results; 70% of physicians were satisfied with the functionality and 95% of physicians rated it as reliable [53]. Gerbert et al. investigated whether the CDSS could support primary care physicians in triaging lesions suggestive of cutaneous malignancies (basal cell carcinoma and squamous cell carcinoma). In a study of 20 primary care physicians presenting 15 skin lesions and comparing triage options, the percentage of incorrect triage choices decreased from 36.7% to 13.3% when CDSS was used [54]. Although the above study was not conducted in a clinical setting, the results show that the CDSS can be expected to improve diagnosis for a variety of diseases in primary care.
Rapid diagnosis of rare diseases by primary care physicians remains a major challenge [55]. The UK Strategy for Rare Diseases advocates the use of ‘effective IT support’ for the diagnosis of infrequent collagen diseases in primary care. [55]. The usefulness of CDSS for rare diseases and difficult-to-diagnose cases has been previously shown on a simulation-based research [56,57]. In addition, Ronicke et al. investigated the effect of a CDSS called Adax Dx in the setting of an outpatient clinic for rare inflammatory systemic diseases [58]. A retrospective analysis of the diagnostic process in 93 confirmed diagnoses (84 cases of collagen disease) showed that 53.8% of cases had accurate diagnosis as one of the top five differential diagnoses earlier than physicians’ clinical diagnosis; in 37.6% of cases, positive diagnosis was the top differential diagnosis, suggesting the usefulness of CDSS for rare diseases. In a study by Rees et al., which developed and validated a risk prediction model to support early diagnosis of systemic lupus erythematosus (SLE), the sensitivity was 34% and the specificity was 90%; thus, early diagnosis is not possible in about two-thirds of cases. In addition, the absolute risk prediction value for SLE is usually less than 1% due to the low frequency of SLE [59]. Pearce also used primary care data to look for signs that could help in the early diagnosis of granulomatosis with polyangiitis but reported no useful signs for early detection [60]. These results suggest that there are two problems that need to be solved before introducing CDSS for the diagnosis of rare diseases in primary care settings [55]. First, it is difficult to build a model with high specificity for some diseases, and second, the lower the frequency of the disease, i.e., the lower the pre-test probability, the lower the positive predictive value. The best way to solve these problems is to improve the pre-test probability, including the exclusion of common diseases, and enhance the clinical reasoning by primary care physicians. In order to diagnose rare diseases in primary care without delay, how to use the CDSS in the augmentation of routine care will be one of the factors that primary care physicians will need to consider in the future.
Although this study demonstrated the utility of the CDSS in the screening and diagnosis of malignancies in a primary care setting, several limitations remain. The barriers to CDSS implementation and cost-effectiveness, as well as the long-term prognosis of early screening with CDSS and the benefits of CDSS diagnosis and transcription for symptomatic patients, are not yet known [61].
In our scoping review, two studies on NPs were included. In recent years, there has been a paradigm shift in the diagnostic process, with diagnoses no longer being made by physicians alone, but rather as a collaborative process involving patients and multiple professionals [62]. In a scoping review conducted by Abdellatif et al. [63] on the benefits of CDSS in nursing home settings, not only physicians but also nurses and pharmacists were found to benefit from CDSS in correcting malnutrition, pressure ulcer prevention, drug prescription, and disease management. Therefore, it is extremely important to examine the usefulness of CDSS for medical professionals other than physicians and how to utilize CDSS in multidisciplinary teams. The management of CDSS in primary care by all types of healthcare professionals and multidisciplinary teams is an important theme for the future.
One of the issues to be addressed in the future is how to adapt the CDSS to primary care settings. According to a systematic meta-review conducted by Nurek et al. [1], there are three main challenges in integrating CDSS in the field: (1) a more standardized computable approach to knowledge representation, (2) one that can be readily updated as new knowledge is gained, and (3) the need for it to trigger at appropriate points in cognitive workflow. Moreover, it also poses the barrier of failure to use dynamic vocabulary tools and to integrate with electronic health records. In fact, of the 26 articles reviewed in the present study, 20 showed an association with factors related to the use of CDSS and its barriers, including time-varying barriers, workflow, situational factors, limited clinic time, warning fatigue, and healthcare provider factors [30,31,33,34,36,37,38,39,41,42,43,44,48,49,50,51]. Patient-centered medicine is a key point in the field of primary care. When digital technologies are used in primary care, a triangle of patient-eHealth-PCPs is created, implying that the direct interaction between patients and PCPs may be affected by eHealth, and the role, importance, and meaning of human interaction in primary care must be reconsidered [64]. There is a viewpoint that digital health can enhance shared decision-making (SDM); however, others advocate that attention should be paid to maintaining “humanity” [64]. In the area of diagnosis, problems with medical information technology include that it cannot weigh in order of importance, cannot deal with comorbidities, cannot consider time series or the context of the patient, and cannot obtain input information by itself [65]. However, these disadvantages are not compatible with patient-centered medicine. It is necessary to carefully consider how to augment the advantages and disadvantages of patient-centered medicine and shared decision-making, and how to integrate them into face-to-face care without compromising quality or safety [66]. In addition, in CDSS, it is common for all input information to be entered into the system before the diagnostic process; in contrast, it is believed that in humans, the concurrent process of history taking and diagnostic thinking can easily lead to a more accurate diagnosis by calibrating the clinical information and diagnosis accordingly [67]. Moreover, there are concerns at present about the validity of the quality of medical history taking by artificial intelligence (AI) [68]; thus, the role of medical history taking in medicine practice is critical. Given that fact face-to-face communication with patients is the predominant mode of diagnosis in primary care, one of the issues to be solved is how to adapt CDSS into the current field of medicine from the user’s perspective, and how to turn its disadvantages into opportunities. This is an issue that needs to be resolved in the field of primary care medicine.
The last issue that should be added is the demand for human resources involved in the development of such diagnostic AI. In particular, the development of diagnostic AI requires the presence of clinical diagnosticians who can advise on its validation and design. Therefore, it will be important to train clinicians, including diagnosticians, who are interested in diagnostic AI and have an understanding of medical informatics.
Strengths and Limitations
In our opinion, this is the first review in the literature to focus on the category of “diagnosis” in primary care settings. In this study, we found that the CDSS is mainly used for the diagnosis and screening of common chronic diseases, and that future directions include the diagnosis of acute and rare diseases and the use of CDSS by non-physician healthcare providers.
However, this study has three limitations. First, we did not evaluate the quality of each of the included study. Second, we did not examine the barriers to the introduction of CDSS in clinical practice. Third, because it is a scoping review, there are limitations related to publication bias. In particular, some studies with negative results may not have been published.
5. Conclusions
We conducted a scoping review to determine the usefulness of the CDSS diagnosis in primary care and to identify areas that need to be explored. We found that the CDSS and reminder tools have significant results in screening for common chronic diseases; however, the CDSS has not yet been fully validated for the diagnosis of acute and uncommon chronic diseases. There have also been a few studies involving non-physicians.
Future research on the diagnosis of CDSS in primary care should focus on the usefulness of CDSS for acute and infrequent diseases, its use in situations involving patients and non-physicians, and its diagnostic accuracy. Fortunately, the issues that need to be addressed in each disease group for each clinical setting have themselves been identified. Therefore, with the progress of future research on CDSS, these issues will be solved separately.
At the same time, the value and role of the CDSS will change depending on how medical professionals in the field think about how to use the CDSS, reflecting the clinical setting and situation. In this era of AI and human augmentation, CDSS, especially in the field of diagnosis, is still in its infancy. However, the help of CDSS, a relatively new AI-based technology, will result in improved diagnoses, which is in line with the major goal of the current entire medical community. As such, the potential growth rate of this field in the future will contribute greatly to improving the quality of healthcare.
Acknowledgments
We express our appreciation to the team members from the Diagnostic Error Working Group of the Japanese Society of Hospital General Medicine for sharing their pearls of wisdom with us during this research.
Author Contributions
Conceptualization, T.H. and T.S.; methodology, T.H.; investigation, T.H, T.M. and K.K.; writing—original draft preparation, T.H.; writing—review and editing, T.S.; supervision, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nurek M., Kostopoulou O., Delaney B.C., Esmail A. Reducing Diagnostic Errors in Primary Care. A Systematic Meta-Review of Computerized Diagnostic Decision Support Systems by the LINNEAUS Collaboration on Patient Safety in Primary Care. Eur. J. Gen. Pract. 2015;21:8–13. doi: 10.3109/13814788.2015.1043123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh H., Meyer A.N.D., Thomas E.J. The Frequency of Diagnostic Errors in Outpatient Care: Estimations from Three Large Observational Studies Involving US Adult Populations. BMJ Qual. Saf. 2014;23:727–731. doi: 10.1136/bmjqs-2013-002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh H., Giardina T.D., Meyer A.N.D., Forjuoh S.N., Reis M.D., Thomas E.J. Types and origins of diagnostic errors in primary care settings. JAMA Int. Med. 2013;173:418–425. doi: 10.1001/jamainternmed.2013.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cresswell K.M., Panesar S.S., Salvilla S.A., Carson-Stevens A., Larizgoitia I., Donaldson L.J., Bates D., Sheikh A., World Health Organization’s (WHO) Safer Primary Care Expert Working Group Global Research Priorities to Better Understand the Burden of Iatrogenic Harm in Primary Care: An International Delphi Exercise. PLoS Med. 2013;10:e1001554. doi: 10.1371/journal.pmed.1001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panesar S.S., deSilva D., Carson-Stevens A., Cresswell K.M., Salvilla S.A., Slight S.P., Javad S., Netuveli G., Larizgoitia I., Donaldson L.J., et al. How Safe Is Primary Care? A Systematic Review. BMJ Qual. Saf. 2016;25:544–553. doi: 10.1136/bmjqs-2015-004178. [DOI] [PubMed] [Google Scholar]
- 6.Kostopoulou O., Delaney B.C., Munro C.W. Diagnostic Difficulty and Error in Primary Care--A Systematic Review. Fam. Pract. 2008;25:400–413. doi: 10.1093/fampra/cmn071. [DOI] [PubMed] [Google Scholar]
- 7.Singh H., Schiff G.D., Graber M.L., Onakpoya I., Thompson M.J. The Global Burden of Diagnostic Errors in Primary Care. BMJ Qual. Saf. 2017;26:484–494. doi: 10.1136/bmjqs-2016-005401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney B.C., Kostopoulou O. Decision Support for Diagnosis Should Become Routine in 21st Century Primary Care. Br. J. Gen. Pract. 2017;67:494–495. doi: 10.3399/bjgp17X693185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer N. An Update and Further Testing of a Knowledge-Based Diagnostic Clinical Decision Support System for Musculoskeletal Disorders of the Shoulder for Use in a Primary Care Setting. J. Eval. Clin. Pract. 2014;20:589–595. doi: 10.1111/jep.12153. [DOI] [PubMed] [Google Scholar]
- 10.Delaney B.C., Fitzmaurice D.A., Riaz A., Hobbs F.D. Can Computerised Decision Support Systems Deliver Improved Quality in Primary Care? Interview by Abi Berger. BMJ. 1999;319:1281. doi: 10.1136/bmj.319.7220.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roshanov P.S., Fernandes N., Wilczynski J.M., Hemens B.J., You J.J., Handler S.M., Nieuwlaat R., Souza N.M., Beyene J., Van Spall H.G., et al. Features of Effective Computerised Clinical Decision Support Systems: Meta-Regression of 162 Randomised Trials. BMJ. 2013;346:f657. doi: 10.1136/bmj.f657. [DOI] [PubMed] [Google Scholar]
- 12.Osheroff J.A., Teich J.M., Middleton B., STeen E.B., Wright A., Detmer D.E. A Roadmap for National Action on Clinical Decision Support. J. Am. Med. Inform. Assoc. 2007;14:141. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton R.T., Pincock T., Baumgar D.C., Sadowski D.C., Fedorak R.N., Kroeker K.I. An Overview of Clinical Decision Support Systems: Benefits, Risks, and Strategies for Success. NPJ Digit. Med. 2020;3:17. doi: 10.1038/s41746-020-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson E.J., Rubin G.P. The Utility of an Online Diagnostic Decision Support System (Isabel) in General Practice: A Process Evaluation. JRSM Short Rep. 2013;4:31. doi: 10.1177/2042533313476691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser H., Coiera E., Wong D. Safety of Patient-facing Digital Symptom Checkers. Lancet. 2018;392:2263. doi: 10.1016/S0140-6736(18)32819-8. [DOI] [PubMed] [Google Scholar]
- 16.Research and Markets Healthcare. Hospital Management. E-Healthcare. [(accessed on 25 July 2021)]; Available online: https://www.researchandmarkets.com/reports/5317115/global-clinical-decision-support-system-market.
- 17.Inokuchi R., Sato H., Nakajima S., Shinohara K., Nakamura K., Gunshin M., Hiruma T., Ishii T., Matsubara T., Kitsuta Y., et al. Development of Information Systems and Clinical Decision Support Systems for Emergency Departments: A Long Road Ahead for Japan. Emerg. Med. J. 2013;30:914. doi: 10.1136/emermed-2012-201869. [DOI] [PubMed] [Google Scholar]
- 18.Biruk S., Yilma T., Andualem M., Tilahun B. Health Professionals’ Readiness to Implement Electronic Medical Record System at Three Hospitals in Ethiopia: A Cross Sectional Study. BMC Med. Inform. Decis. Mak. 2014;14:115. doi: 10.1186/s12911-014-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ei-Kareh R., Hasan O., Schiff G.D. Use of Health Information Technology to Reduce Diagnostic Errors. BMJ Qual. Saf. 2013;22:ii40–ii51. doi: 10.1136/bmjqs-2013-001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chima S., Reece C.J., Milley K., Milton S., Mclntosh J.G., Emery J.D. Decision Support Tools to Improve Cancer Diagnostic Decision Making in Primary Care: A Systematic Review. Br. J. Gen. Pract. 2019;69:e809. doi: 10.3399/bjgp19X706745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing X., Himawan L., Law T. Availability and Usage of Clinical Decision Support Systems (CDSSs) in Office-based Primary Care Settings in the USA. BMJ Health Care Inform. 2019;26:e100015. doi: 10.1136/bmjhci-2019-100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harry M.L., Truitt A., Saman D.M., Henzler-Buckingham H.A., Allen C.T., Walton K.M., Ekstrom H.L., O’Connor P.L., Sperl-Hillen J.M., Bianco J.A., et al. Barriers and Facilitators to Implementing Cancer Prevention Clinical Decision Support in Primary Care: A Qualitative Study. BMC Health Serv. Res. 2019;19:534. doi: 10.1186/s12913-019-4326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horsky J., Schiff G.D., Johnston D., Mercincavage L., Bell D., Middleton B. Interface Design Principles for Usable Decision Support: A Targeted Review of Best Practices for Clinical Prescribing Interventions. J. Biomed. Inform. 2012;45:1202. doi: 10.1016/j.jbi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Brunner J., Chuang E., Goldzweig C., Cain C.L., Sugar C., Yano E.M. User-centered Design to Improve Clinical Decision Support in Primary Care. Int. J. Med. Inform. 2017;104:56. doi: 10.1016/j.ijmedinf.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 26.Rosser W.W., McDowell I., Newell C. Use of Reminders for Preventive Procedures in Family Medicine. Can. Med Assoc. J. 1991;145:807–814. [PMC free article] [PubMed] [Google Scholar]
- 27.Ornstein S.M., Garr D.R., Jenkins R.G., Rust P.F., Arnon A. Computer-Generated Physician and Patient Reminders. Tools to Improve Population Adherence to Selected Preventive Services. J. Fam. Pract. 1991;32:82–90. [PubMed] [Google Scholar]
- 28.McPhee S.J., Bird J.A., Fordham D., Rodnick J.E., Osborn E.H. Promoting Cancer Prevention Activities by Primary Care Physicians. Results of a Randomized, Controlled Trial. JAMA. 1991;266:538–544. doi: 10.1001/jama.1991.03470040102030. [DOI] [PubMed] [Google Scholar]
- 29.McPhee S.J., Bird J.A., Jenkins C.N., Fordham D. Promoting Cancer Screening. A Randomized, Controlled Trial of Three Interventions. Arch. Intern. Med. 1989;149:1866–1872. doi: 10.1001/archinte.1989.00390080116025. [DOI] [PubMed] [Google Scholar]
- 30.McDonald C.J., Hui S.L., Smith D.M., Tierney W.M., Cohen S.J., Weinberger M., McCabe G.P. Reminders to Physicians from an Introspective Computer Medical Record. A Two-Year Randomized Trial. Ann. Intern. Med. 1984;100:130–138. doi: 10.7326/0003-4819-100-1-130. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton W., Green T., Martins T., Elliott K., Rubin G., Macleod U. Evaluation of Risk Assessment Tools for Suspected Cancer in General Practice: A Cohort Study. Br. J. Gen. Pract. 2013;63:e30–e36. doi: 10.3399/bjgp13X660751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy D.R., Wu L., Thomas E.J., Forjuoh S.N., Meyer A.N., Singh H. Electronic Trigger-Based Intervention to Reduce Delays in Diagnostic Evaluation for Cancer: A Cluster Randomized Controlled Trial. J. Clin. Oncol. 2015;33:3560–3567. doi: 10.1200/JCO.2015.61.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price S., Spencer A., Medina-Lara A., Hamilton W. Availability and Use of Cancer Decision-Support Tools: A Cross-Sectional Survey of UK Primary Care. Br. J. Gen. Pract. 2019;69:e437–e443. doi: 10.3399/bjgp19X703745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetter M.J. The Influence of Clinical Decision Support on Diagnostic Accuracy in Nurse Practitioners. Worldviews Evid. Based Nurs. 2015;12:355–363. doi: 10.1111/wvn.12121. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T., Nemoto T., Tokuda Y. Effectiveness of a Clinical Knowledge Support System for Reducing Diagnostic Errors in Outpatient Care in Japan: A Retrospective Study. Int. J. Med. Inform. 2018;109:1–4. doi: 10.1016/j.ijmedinf.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Burack R.C., Gimotty P.A. Promoting Screening Mammography in Inner-City Settings. The Sustained Effectiveness of Com-puterized Reminders in a Randomized Controlled Trial. Med. Care. 1997;35:921–931. doi: 10.1097/00005650-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Burack R.C., Gimotty P.A., George J., McBride S., Moncrease A., Simon M.S., Dews P., Coombs J. How Reminders Given to Patients and Physicians Affected Pap Smear Use in a Health Maintenance Organization: Results of a Randomized Controlled Trial. Cancer. 1998;82:2391–2400. doi: 10.1002/(SICI)1097-0142(19980615)82:12<2391::AID-CNCR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Sequist T.D., Zaslavsky A.M., Marshall R., Fletcher R.H., Ayanian J.Z. Patient and Physician Reminders to Promote Colo-rectal Cancer Screening: A Randomized Controlled Trial. Arch. Intern. Med. 2009;169:364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litvin C.B., Hyer J.M., Ornstein S.M. Use of Clinical Decision Support to Improve Primary Care Identification and Man-agement of Chronic Kidney Disease (CKD) J. Am. Board Fam. Med. 2016;29:604–612. doi: 10.3122/jabfm.2016.05.160020. [DOI] [PubMed] [Google Scholar]
- 40.Lee N.J., Chen E.S., Currie L.M., Donovan M., Hall E.K., Jia H., John R.M., Bakken S. The Effect of a Mobile Clinical Deci-sion Support System on the Diagnosis of Obesity and Overweight in Acute and Primary Care Encounters. Adv. Nurs. Sci. 2009;32:211–221. doi: 10.1097/ANS.0b013e3181b0d6bf. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhry R., Tulledge-Scheitel S.M., Parks D.A., Angstman K.B., Decker L.K., Stroebel R.J. Use of a Web-Based Clinical Decision Support System to Improve Abdominal Aortic Aneurysm Screening in a Primary Care Practice. J. Eval. Clin. Pract. 2012;18:666–670. doi: 10.1111/j.1365-2753.2011.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenealy T., Arroll B., Petrie K.J. Patients and Computers as Reminders to Screen for Diabetes in Family Practice. Random-ized-Controlled Trial. J. Gen. Intern. Med. 2005;20:916–921. doi: 10.1111/j.1525-1497.2005.0197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Wyk J.T., van Wijk M.A., Sturkenboom M.C., Mosseveld M., Moorman P.W., van der Lei J. Electronic Alerts Versus On-Demand Decision Support to Improve Dyslipidemia Treatment: A Cluster Randomized Controlled Trial. Circulation. 2008;117:371–378. doi: 10.1161/CIRCULATIONAHA.107.697201. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein L.V., McCoy J.M., Cope D.W., Barrett P.A., Hirsch S.H., Messer K.S., Young R.T. Improving Patient Quality of Life with Feedback to Physicians About Functional Status. J. Gen. Intern. Med. 1995;10:607–614. doi: 10.1007/BF02602744. [DOI] [PubMed] [Google Scholar]
- 45.DeJesus R.S., Angstman K.B., Kesman R., Stroebel R.J., Bernard M.E., Scheitel S.M., Hunt V.L., Rahman A.S., Chaudhry R. Use of a Clinical Decision Support System to Increase Osteoporosis Screening. J. Eval. Clin. Pract. 2012;18:89–92. doi: 10.1111/j.1365-2753.2010.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeSilva M.B., Kodet A., Walker P.F. A Best Practice Alert for Identifying Hepatitis B-Infected Patients. Am. J. Trop. Med. Hyg. 2020;103:884–886. doi: 10.4269/ajtmh.20-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chak E., Taefi A., Li C.S., Chen M.S., Harris A.M., MacDonald S., Bowlus C. Electronic Medical Alerts Increase Screening for Chronic Hepatitis B: A Randomized, Double-Blind, Controlled Trial. Cancer Epidemiol. Biomark. Prev. 2018;27:1352–1357. doi: 10.1158/1055-9965.EPI-18-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundaram V., Lazzeroni L.C., Douglass L.R., Sanders G.D., Tempio P., Owens D.K. A Randomized Trial of Computer-Based Reminders and Audit and Feedback to Improve HIV Screening in a Primary Care Setting. Int. J. STD AIDS. 2009;20:527–533. doi: 10.1258/ijsa.2008.008423. [DOI] [PubMed] [Google Scholar]
- 49.Miller M.J., Burns C.F., Kapusnik-Uner J., Carreno R., Matuszewski K.A. Depression Screening for Prescribed Medications with Mental Health Risk: Considerations for Clinical Decision Support, Workflow Redesign, and Health Information Exchange Arrangements. Res. Soc. Adm. Pharm. 2017;13:485–493. doi: 10.1016/j.sapharm.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Downs M., Turner S., Bryans M., Wilcock J., Keady J., Levin E., O’Carroll R., Howie K., Iliffe S. Effectiveness of Educational Interventions in Improving Detection and Management of Dementia in Primary Care: Cluster Randomised Controlled Study. BMJ. 2006;332:692–696. doi: 10.1136/bmj.332.7543.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad F., Hogg-Johnson S., Stewart D.E., Skinner H.A., Glazier R.H., Levinson W. Computer-Assisted Screening for In-timate Partner Violence and Control: A Randomized Trial. Ann. Intern. Med. 2009;151:93–102. doi: 10.7326/0003-4819-151-2-200907210-00124. [DOI] [PubMed] [Google Scholar]
- 52.Cooper J.G., West R.M., Clamp S.E., Hassan T.B. Does Computer-Aided Clinical Decision Support Improve the Management of Acute Abdominal Pain? A Systematic Review. Emerg. Med. J. 2011;28:553–557. doi: 10.1136/emj.2009.086801. [DOI] [PubMed] [Google Scholar]
- 53.López M.M., López M.M., de la Torre Díez I.T., Jimeno J.C.P., López-Coronado M. MHealth App for iOS to Help in Diagnostic Decision in Ophthalmology to Primary Care Physicians. J. Med. Syst. 2017;41:81. doi: 10.1007/s10916-017-0731-6. [DOI] [PubMed] [Google Scholar]
- 54.Gerbert B., Bronstone A., Maurer T., Hofmann R., Berger T. Decision Support Software to Help Primary Care Physicians Triage Skin Cancer: A Pilot Study. Arch. Dermatol. 2000;136:187–192. doi: 10.1001/archderm.136.2.187. [DOI] [PubMed] [Google Scholar]
- 55.Pearce F., Lanyon P.C., Watts R.A. Can Prediction Models in Primary Care Enable Earlier Diagnosis of Rare Rheumatic Diseases? Rheumatology. 2018;57:2065–2066. doi: 10.1093/rheumatology/kex508. [DOI] [PubMed] [Google Scholar]
- 56.Ramnarayan P., Roberts C.G., Coren M., Nanduri V., Tomlinson A., Taylor M.P., Wyatt J.C., Britto J.F. Assessment of the potential impact of a reminder system on the reduction of diagnostic errors: A quasi-experimental study. BMC Med. Inform. Decis. Mak. 2006;6:22. doi: 10.1186/1472-6947-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldman M.D., Petersen A.J., Karliner L.S., Tice J.A. Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment. J. Gen. Intern. Med. 2007;23(Suppl. S1):57–63. doi: 10.1007/s11606-007-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronicke S., Hirsch M.C., Türk E., Larionov K., Tientcheu D., Wagner A.D. Can a Decision Support System Accelerate Rare Disease Diagnosis? Evaluating the Potential Impact of Ada DX in a Retrospective Study. Orphanet J. Rare Dis. 2019;14:69. doi: 10.1186/s13023-019-1040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rees F., Doherty M., Lanyon P., Davenport G., Riley R.D., Zhang W., Grainge J.W. Early Clinical Features in Systemic Lupus Erythematosus: Can They Be Used to Achieve Earlier Diagnosis? A Risk Prediction Model. Arthritis Care Res. 2016;69:8334. doi: 10.1002/acr.23021. [DOI] [PubMed] [Google Scholar]
- 60.Pearce F.A., Habbard R.B., Grainge M.J., Watts R.A., Abhishek A., Lanyon P.C. Can granulomatosis with polyangiitis be diagnosed earlier in primary care? A case-control study. QJM Int. J. Med. 2018;111:39–45. doi: 10.1093/qjmed/hcx194. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton W., Walter F.M., Rubin G., Neal R.D. Improving Early Diagnosis of Symptomatic Cancer. Nat. Rev. Clin. Oncol. 2016;13:740–749. doi: 10.1038/nrclinonc.2016.109. [DOI] [PubMed] [Google Scholar]
- 62.Graber M.L. Progress Understanding Diagnosis and Diagnostic Errors: Thoughts at Year 10. Diagnosis. 2020;7:151–159. doi: 10.1515/dx-2020-0055. [DOI] [PubMed] [Google Scholar]
- 63.Abdellatif A., Bouaud J., Nghiem D., Lafuente-Lafuente C., Belmin J., Seroussi B. Clinical Decision Support Systems in Nursing Homes: A Scoping Review. Stud. Health Technol. Inform. 2020;270:542–546. doi: 10.3233/SHTI200219. [DOI] [PubMed] [Google Scholar]
- 64.Boers S.N., Jongsma K.R., Lucivero F., Aardoom J., Büchner F.L., de Vries M., Honkoop P., Houwink E.J.F., Kasteleyn M.J., Meijer E., et al. SERIES: EHealth in Primary Care. Part 2: Exploring the Ethical Implications of Its Application in Primary Care Practice. Eur. J. Gen. Pract. 2020;26:26–32. doi: 10.1080/13814788.2019.1678958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harada T., Shimizu T., Kaji Y., Suyama Y., Matsumoto T., Kosaka C., Shimizu H., Nei T., Watanuki S. A Perspective from a Case Conference on Comparing the Diagnostic Process: Human Diagnostic Thinking vs. Artificial Intelligence (AI) Decision Support Tools. Int. J. Environ. Res. Public Health. 2020;17:6110. doi: 10.3390/ijerph17176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Kleij R.M.J.J., Kasteleyn M.J., Meijer E., Bonten T.N., Houwink E.J.F., Teichert M., van Luenen S., Vedanthan R., Evers A., Car J., et al. SERIES: EHealth in Primary Care. Part 1: Concepts, Conditions and Challenges. Eur. J. Gen. Pract. 2019;25:179–189. doi: 10.1080/13814788.2019.1658190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu T. The 6C model for accurately capturing the patient’s medical history. Diagnosis. 2021 doi: 10.1515/dx-2020-0126. [DOI] [PubMed] [Google Scholar]
- 68.Harada Y., Katsukura S., Kawamura R., Shimizu T. Efficacy of Artificial-Intelligence-Driven Differential-Diagnosis List on the Diagnostic Accuracy of Physicians: An Open-Label Randomized Controlled Study. Int. J. Environ. Res. Public Health. 2021;18:2086. doi: 10.3390/ijerph18042086. [DOI] [PMC free article] [PubMed] [Google Scholar]