Abstract
The mammalian prion protein (PrPC) is composed of a large intrinsically disordered N-terminal and a structured C-terminal domain, containing three alpha-helical regions and a short, two-stranded beta-sheet. Traditionally, the activity of a protein was linked to the ability of the polypeptide chain to adopt a stable secondary/tertiary structure. This concept has been extended when it became evident that intrinsically disordered domains (IDDs) can participate in a broad range of defined physiological activities and play a major functional role in several protein classes including transcription factors, scaffold proteins, and signaling molecules. This ability of IDDs to engage in a variety of supramolecular complexes may explain the large number of PrPC-interacting proteins described. Here, we summarize diverse physiological and pathophysiological activities that have been described for the unstructured N-terminal domain of PrPC. In particular, we focus on subdomains that have been conserved in evolution.
Keywords: prion, intrinsically disordered, stress protection, liquid–liquid phase separation, neurodegeneration
1. The Prion Protein
The mammalian prion protein (PrP) was first identified as a protease-resistant protein in brain extracts, which co-purified with the infectious scrapie agent [1]. The identification of the corresponding gene revealed that PrP is a constitutively expressed host protein, mainly found in neuronal and immune cells [2,3]. From these and subsequent studies, the concept emerged that the disease-causing mechanism in mammalian prion diseases is a conformational transition of the cellular isoform of PrP (PrPC) into PrPSc, an aberrantly folded conformer with neurotoxic and infectious properties [4]. The central role of PrPC in the formation of PrPSc and infectious prions is highlighted in PrPC-deficient mice and goats, which are resistant to prion infection [5,6]. Biogenesis of PrPC is characterized by a series of co- and posttranslational modifications (see [7]). After import into the endoplasmic reticulum (ER), PrP is modified in the secretory pathway with two N-linked glycans of complex structure [8,9,10,11] and a C-terminal glycosylphosphatidylinositol (GPI) anchor [12], which targets mature PrPC to the outer leaflet of the plasma membrane. Conversion into PrPSc is thought to occur after mature PrPC has reached the plasma membrane or is re-internalized for degradation [13]. However, neither the two N-linked glycans [14,15] nor the GPI anchor of PrPC [16,17] are essential for the formation of infectious prions.
2. The Intrinsically Disordered N-Terminal Domain of PrP
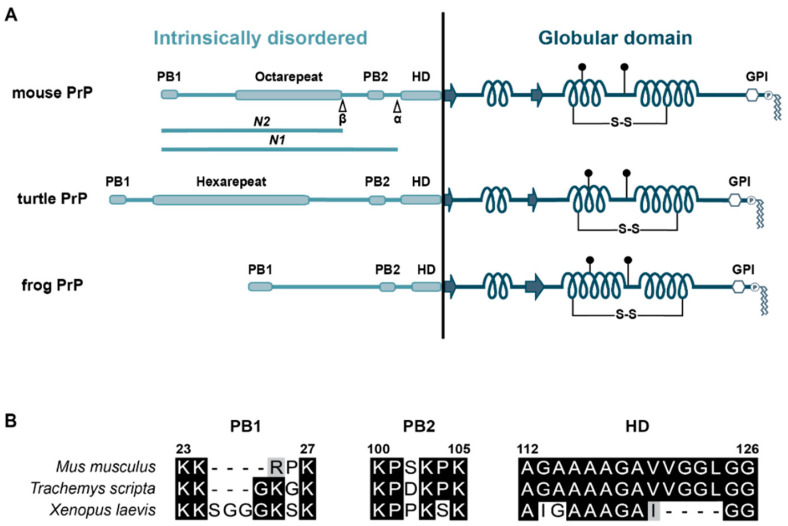
The first published structure of mouse PrPC revealed the presence of a highly structured C-terminal domain of some 100 amino acids with three alpha-helical domains and two short beta-strands [18]. Interestingly, the remaining N-terminal domain of similar length proved to be completely intrinsically disordered (Figure 1A) [19,20]. This modular structure of PrPC is conserved through evolution; moreover, the C-terminal domain of human (amino acid (aa) 121–230), chicken (aa 121–225), turtle (aa 121–225), and xenopus (aa 90–222) PrPC show extensive structural similarities [21] (Figure 1A). While the N-terminal unstructured domain shows considerable sequence diversity between tetrapod classes (Figure 1), it contains three highly conserved regions: (1) an internal hydrophobic domain (HD) first described as a putative transmembrane domain [22], (2) a stretch of positively charged amino acids distal to the ER signal peptide (PB1), and (3) a stretch of positively charged amino acids at the C-terminal end of the unstructured domain (PB2) (Figure 1B). In this review, we discuss in detail that these regions are associated with some physiological and pathophysiological activities described for PrPC. However, the N-terminal domain is not only important in the context of full-length PrPC. A considerable fraction of mature PrPC is proteolytically processed in vivo, resulting in the release of two distinct soluble, unstructured N-terminal fragments. One fragment, designated N1, is formed by α-cleavage of PrPC under physiological conditions approximately at amino acid position 110. A second cleavage around amino acid position 90 (β-cleavage) is mainly observed under pathological conditions and liberates the fragment N2 [23,24,25,26,27,28] (Figure 1A). These findings raise the intriguing question of whether PrPC processing plays a role in generating soluble N-terminal fragments with distinct biological activities (see [26,29,30]).
Figure 1.
(A) Schematic representation of PrPC from mouse, turtle, and frog. PB: polybasic motif; β: cleavage site that generates N2; α: cleavage site that generates N1; HD: hydrophobic domain; arrows: β-strand; coils: α-helices; S-S: disulfide bridge; filled circles: N-linked glycans; GPI: glycosylphosphatidylinositol anchor. (B) Sequence alignments of PB1, PB2, and the HD. Identical residues are marked in black, similar residues in gray (GenBank accession numbers: M18070.1, XP_034617687.1, AAH94089.1). The numbering of the residues refers to mouse PrPC.
3. Physiological and Pathophysiological Activities of the N-Terminal Domain
The major phenotype of PrPC-deficient mice and goats is their resistance to prion diseases [5,6]. Apart from that, a number of biological activities have been attributed to PrPC, such as modulation of synaptic transmission and neuronal excitability; protection against oxidative stress; neuroprotective and neurotoxic signaling; and a role in cell differentiation, neuronal adhesion, and neuro-immune crosstalk. In the context of this review, we focus specifically on biological activities of PrP that are dependent on, or are modulated by, the N-terminal domain or conserved regions therein. For an overview of activities that have been ascribed to PrP thus far, we would like to refer the reader to previous comprehensive reviews [31,32,33,34,35,36,37,38,39,40,41,42,43].
3.1. The N-Terminal Domain and Prion Diseases
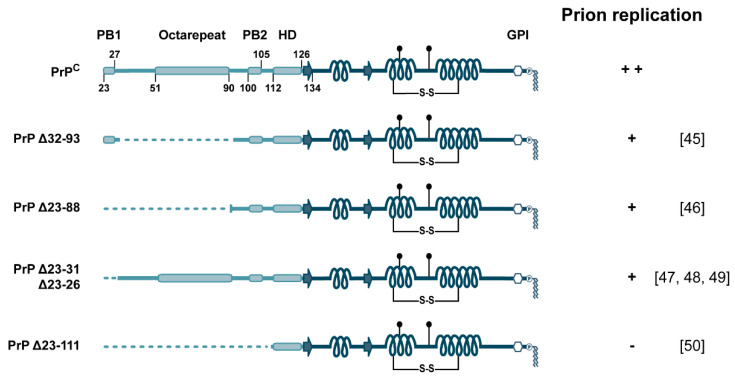
The prion protein identified in proteinase K-treated brain extracts of scrapie-infected hamsters was PrP27–30, the protease K-resistant core of PrPSc that lacks aa 23–90. PrP27–30 transmits prion disease, indicating that the infectious properties of prions are not dependent on the N-terminal part of PrP comprising N2 [1,44]. This part is also dispensable for the conversion of PrPC into PrPSc, since propagation of infectious prions is supported in transgenic mice expressing only a truncated variant of PrPC devoid of aa 32–93 [45] or aa 23–88 [46]. However, incubation time was significantly increased in these mice, revealing that the unstructured domain of PrPC modulates propagation of prions. Notably, mice expressing a variant of PrPC deleted for only aa 23–31 already display a dramatically reduced susceptibility to prion infection and accumulate significantly reduced levels of PrPSc. This study highlights an important role of the PB1 domain of PrPC in the conversion into PrPSc [47], a finding corroborated later [48,49]. On the other hand, the N2 domain (aa 23–90) or regions therein only modulate prion propagation; deleting the N1 domain prevents prion disease. Transgenic mice expressing PrPC(Δ23–111) remain healthy after inoculation with scrapie prions and do not accumulate protease-resistant PrPSc [50] (Figure 2).
Figure 2.
The role of the N-terminal domain in prion propagation. Schematic representation of PrPC variants and their ability to support replication of infectious prions in mice after inoculation with scrapie prions. The numbering of the residues refers to mouse PrPC. Respective publications are in brackets.
Inherited prion diseases in humans are caused by mutations in the PrP gene (PRNP). Excluding the octapeptide repeat insertions, all pathogenic mutations found in the N-terminal unstructured domain of PrPC are distal to amino acid 90 [51]. Interestingly, a recent high-resolution structure obtained by cryo-EM showed that the section containing residues 95–112, which is unstructured in PrPC, contains two beta-strands upon conversion into PrPSc [52].
3.2. The N-Terminal Domain and Neurotoxicity
In this section, we would like to illustrate by means of two examples how the N-terminal domain is involved in neurotoxic signaling of PrPC independently of the formation of infectious prions: first, by deleting the internal highly conserved hydrophobic domain, and second, by interacting with neurotoxic protein assemblies.
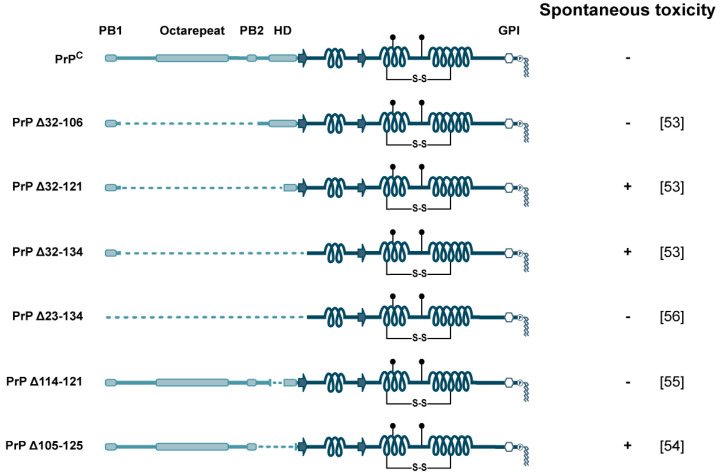
From a study designed to identify regions of PrPC involved in the formation of infectious prions, it emerged that deletions in the internal HD can convert PrPC into a neurotoxic protein that is not infectious. Transgenic mice expressing PrPC with a deletion of residues 32–80, 32–90, or 32–106 showed no overt phenotype. However, severe ataxia and neuronal cell death was observed in transgenic mice expressing PrPC (Δ32–121) or PrPC (Δ32–134) [53]. Notably, the deletion of 20 amino acids of the HD (Δ105–125) is sufficient to create a neurotoxic PrP variant [54], while a shorter deletion (Δ114–121) is not [55]. Although the relevance of the mechanisms underlying neurotoxic activity to the pathogenesis of prion diseases remains unclear, there are some noteworthy features of PrPΔHD. First, while expression of PrPΔHD causes neurodegeneration, it does not form infectious prions. Second, co-expression of wild-type PrPC suppresses the neurotoxic activity of PrPΔHD [53,54,55]. Third, transgenic mice expressing PrPC (Δ23–134) instead of PrPC (Δ32–134) display no clinical symptoms or neuropathology, indicating that PB1 is required for the toxic phenotype of PrPC (Δ32–134) [56] (Figure 3).
Figure 3.
Neurotoxic activity of PrPC variants with N-terminal deletions. Schematic representation of PrPC variants and their spontaneous activity to induce neuronal dysfunction in transgenic mice. Respective publications are in brackets.
The first hints that wild-type GPI-anchored PrPC could serve as a toxic receptor of pathogenic protein conformers emerged from an elegant study by Brandner and colleagues who grafted neural tissues overexpressing PrPC into the brains of PrP0/0 mice. After infection with scrapie prions, the PrPC-expressing graft propagated PrPSc and developed histopathological alterations characteristic of scrapie disease. However, the surrounding PrPC-deficient tissue remained healthy, despite the accumulation of PrPSc [57]. Using a cell culture model, we provided further evidence that PrPSc can induce neurotoxic signaling via an interaction with PrPC at the plasma membrane. Importantly, the unstructured N-terminal domain of PrPSc was required for this activity [58]. A series of subsequent studies then revealed that the ability of PrPC to relay toxic signals after binding to misfolded protein assemblies is of broad pathological significance. In a landmark study, the Strittmatter group showed that toxicity of oligomeric Aβ can be mediated by PrPC [59]. Moreover, PB1 and PB2 in the unstructured N-terminal domain of PrPC were mapped as the Aβ-binding sites [59,60]. While a possible pathological role of PrPC as a receptor of Aβ was initially discussed critically, numerous studies in cultured cells and transgenic mice have convincingly supported this concept (see [39,61]). Consistent with the ability of intrinsically disordered domains to interact with different substrates independently of their primary sequence, we then observed that completely unrelated beta-sheet rich oligomeric assemblies; for example, those formed by the yeast prion protein Sup35 or an artificial beta-sheet peptide can bind to the N-terminal domain of PrPC and induce cell death [62]. Finally, neurotoxic signaling of soluble α-synuclein and Tau assemblies via binding to the N-terminal domain of PrPC was demonstrated [63,64,65]. It will now be interesting to explore the intracellular signaling pathways activated by the PrPC/oligomer complex in more detail and harness them in developing therapeutic strategies for neurodegenerative diseases.
3.3. The N-Terminal Domain and Neuroprotection: A Role of Soluble Fragments
From the analysis of PrPC variants devoid of the N-terminal domain or subdomains thereof, it became apparent that some of the biological activities of full-length GPI-anchored PrPC are dependent on its N-terminal regions. We now turn to intriguing findings showing that soluble N-terminal fragments of PrPC have biological activities independently of the globular C-terminal domain. Notably, analyzing the brain interactome of soluble N1 revealed that the intrinsically disordered N-terminal domain is a major mediator of PrP interactions [66].
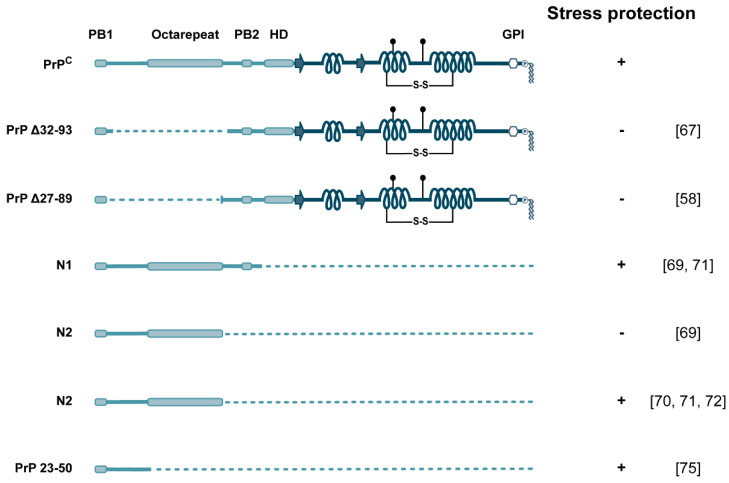
It was previously shown that the stress protective activity of full-length PrP requires the N-terminal domain [58,67,68]. Interestingly, this activity seems to be at least partially independent of the C-terminal domain and/or GPI-anchoring. By employing recombinant proteins added to cultured cells, different groups presented evidence that soluble N1 and N2 can protect against various stress paradigms [69,70,71,72]. Notably, in one study [69] N1, but not N2, displayed neuroprotective activity in vivo and in vitro by modulating the p53 signaling pathway. This finding may indicate that PB2, which is missing in N2, might be important for a biological activity specific for N1.
Another interesting example for soluble N-terminal fragments showing an activity initially ascribed to GPI-anchored full-length PrP emerged from the observation that the loss of PrPC in mice leads to a chronic demyelinating polyneuropathy affecting Schwann cells [73], a phenotype that was corroborated later in PrPC-deficient goats [74]. In a follow-up study in mice, it was then demonstrated that a soluble N-terminal fragment has the same activity as full-length PrPC in activating the G protein-coupled receptor Adgrg6. Intriguingly, this activity was dependent on PB1: substitution of the cationic residues in PB1 by alanines abolished the activity of the N-terminal fragment to activate Adgrg6 [75].
The first observation that a soluble PrP fragment can protect against neurotoxicity induced by pathogenic protein assemblies was made in transgenic mice expressing a secreted full-length PrP-immunoglobulin Fc (PrP-Fc2) fusion protein. After inoculation with prions, PrP-Fc2 was not converted into PrPSc, and the onset of prion disease was delayed [76]. A plausible mode of action would be that secreted PrP-Fc2 interacts with PrPSc and thereby interferes with its toxic signaling via GPI-anchored PrPC.
On the basis of the role of the N-terminal domain in mediating the interaction of PrPC with Aβ, we were wondering whether a soluble N1 fragment would protect against Aβ-induced toxicity. Notably, the isolated N-terminal domain of PrP cannot be expressed as a secreted protein in mammalian cells or neurons of transgenic mice, since ER import of such a C-terminally truncated PrP construct is significantly impaired [77,78,79]. We therefore employed a fusion protein composed of N1 and the Fc portion of human IgG1. Indeed, the secreted N1 fragment efficiently bound to Aβ and significantly reduced its toxic signaling via PrPC [62]. Further experimental evidence for a protective activity of the soluble N1 fragment against toxic effects of oligomeric Aβ was provided subsequently in different model systems, including primary neurons, Caenorhabditis elegans, and mouse models of Aβ-induced memory dysfunction [80,81,82,83] (Figure 4).
Figure 4.
Neuroprotective activity of PrP. Schematic representation of PrPC variants and their stress protective activity in cell culture and transgenic mouse models. Respective publications are in brackets.
These two examples revealed a biological function of soluble N-terminal fragments of PrPC at the outer leaflet of the plasma membrane or in the extracellular space. What is missing thus far is experimental evidence that soluble N-terminal fragments have a biological function in intracellular compartments. To date, it was shown that the N-terminal domain has the capacity to enter cells. PB1 and PB2 have similarities with cell-penetrating peptides and the fusion of PB1 or PB2 to heterologous proteins promote their cellular uptake and delivery into the cytoplasm [84,85].
4. The N-Terminal Domain Is Necessary and Sufficient for Liquid–Liquid Phase Separation of PrP
Multiple cellular processes, including receptor-mediated signaling and formation of stress granules, are coordinated by biomolecular condensates or membrane-less compartments that can form via liquid–liquid phase separation (LLPS) (see [86,87,88,89]). Furthermore, several proteins implicated in neurodegenerative diseases have been shown to undergo LLPS, leading to a concept that altered LLPS can promote the formation of protein assemblies with neurotoxic properties (see [90,91,92,93]).
Indeed, LLPS of full-length PrP has been described recently [94,95,96,97,98]; however, the molecular mechanisms underlying the formation of PrP-containing liquid droplets remain unknown.
In a recent study, we provided insight into the mechanism underlying the propensity of the mammalian prion protein to undergo LLPS [99]. Our study revealed that the intrinsically disordered N1 fragment of PrP is necessary and sufficient for the formation of biomolecular condensates, emphasizing the concept that intrinsically disordered and low-complexity regions are important drivers of phase separation [100]. Furthermore, a mutational analysis revealed that LLPS of N1 is governed at the molecular level mainly by intermolecular cation–π interactions of the positively charged residues in PB1 and PB2 with aromatic side chains [99].
Although there are only few publications to date that have examined LLPS of PrP in detail [94,95,96,97,98,99], they provided experimental evidence that LLPS could play a role in regulating (patho)physiological activities of PrP and formation of infectious prions. Consistent with the concept that biomolecular condensates can be precursors of pathogenic protein aggregates, it was shown that after LLPS of recombinant full-length PrP, a rapid liquid–solid phase transition occurred, leading to the formation of β-sheet-rich and PK-resistant amyloid [94]. Notably, the unstructured N-terminal domain of PrP was required for the initial LLPS, supporting our finding that LLPS of PrP-C1 and -C2 is impaired [99]. Interestingly, it was already described some time ago that large globular protein assemblies preceded the formation of PrP145X amyloid fibrils in vitro. While it was not shown experimentally, the images of the globular structures are indicative of biomolecular condensates formed by PrP145X via LLPS [101]. Another intriguing observation is that binding of neurotoxic Aβ oligomers to the polybasic motifs converts liquid-like droplets of full length PrP into hydrogel and induces a conformation change of PrP [97,102], suggesting that aberrant phase transition of PrPC may be associated with the activity of the PrP/Aβ complex to induce neurotoxic signaling [97]. Thus far, a possible role of LLPS in the neuroprotective activities of full length PrP or the liberated N-terminal fragments has only been indirectly demonstrated: PrP variants that lack a stress protective activity in cell culture and animal models [58,67] failed to undergo LLPS in vitro [94,99]. Vice versa, N1 formed biomolecular condensates in vitro [99] and showed stress-protective activity in cell culture models [69,71]. It will now be interesting to analyze LLPS of PrPC and its proteolytic fragments in a cellular context and, in particular, to address the role of membrane anchoring via the GPI-anchor.
Author Contributions
S.A.P. and J.K. literature research, review and editing, J.T. writing-original draft and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy–EXC 2033–390677874–RESOLV; TA 167/6-3, and TA 167/11-1 (to J.T.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bolton D.C., McKinley M.P., Prusiner S.B. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 2.Oesch B., Westaway D., Wälchli M., McKinley M.P., Kent S.B.H., Aebersold R., Barry R.A., Tempst P., Teplow D.B., Hood L.E., et al. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 3.Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J.M., et al. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985;315:331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner S.B. Scrapie prions. Annu. Rev. Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 5.Salvesen O., Espenes A., Reiten M.R., Vuong T.T., Malachin G., Tran L., Andréoletti O., Olsaker I., Benestad S.L., Tranulis M.A., et al. Goats naturally devoid of PrPC are resistant to scrapie. Veter Res. 2020;51:1. doi: 10.1186/s13567-019-0731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H.-P., DeArmond S.J., Prusiner S.B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 7.Tatzelt J., Winklhofer K.F. Folding and misfolding of the prion protein in the secretory pathway. Amyloid. 2004;11:162–172. doi: 10.1080/1350-6120400000723. [DOI] [PubMed] [Google Scholar]
- 8.Haraguchi T., Fisher S., Olofsson S., Endo T., Groth D., Tarentino A., Borchelt D.R., Teplow D., Hood L., Burlingame A., et al. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch. Biochem. Biophys. 1989;274:1–13. doi: 10.1016/0003-9861(89)90409-8. [DOI] [PubMed] [Google Scholar]
- 9.Rudd P.M., Endo T., Colominas C., Groth D., Wheeler S.F., Harvey D.J., Wormald M., Serban H., Prusiner S.B., Kobata A., et al. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc. Natl. Acad. Sci. USA. 1999;96:13044–13049. doi: 10.1073/pnas.96.23.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo T., Groth D., Prusiner S.B., Kobata A. Diversity of oligosaccharide structures linked to asparagines of the scrapie pri-on protein. Biochemistry. 1989;28:8380–8388. doi: 10.1021/bi00447a017. [DOI] [PubMed] [Google Scholar]
- 11.Stimson E., Hope J., Chong A., Burlingame A.L. Site-specific characterization of the N-linked glycans of murine prion pro-tein by high-performance liquid chromatography/electrospray mass spectrometry and exoglycosidase digestions. Biochemistry. 1999;38:4885–4895. doi: 10.1021/bi982330q. [DOI] [PubMed] [Google Scholar]
- 12.Stahl N., Borchelt D.R., Hsiao K., Prusiner S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 13.Caughey B., Raymond G.J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 1991;266:18217–18223. doi: 10.1016/S0021-9258(18)55257-1. [DOI] [PubMed] [Google Scholar]
- 14.Sevillano A.M., Aguilar-Calvo P., Kurt T.D., Lawrence J.A., Soldau K., Nam T., Schumann T., Pizzo D.P., Nyström S., Choudhury B., et al. Prion protein glycans reduce intracerebral fibril formation and spongiosis in prion disease. J. Clin. Investig. 2020;130:1350–1362. doi: 10.1172/JCI131564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuzi N.L., Cancellotti E., Baybutt H., Blackford L., Bradford B., Plinston C., Coghill A., Hart P., Piccardo P., Barron R., et al. Host PrP Glycosylation: A Major Factor Determining the Outcome of Prion Infection. PLoS Biol. 2008;6:e100. doi: 10.1371/journal.pbio.0060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chesebro B., Trifilo M., Race R., Meade-White K., Teng C., Lacasse R., Raymond L., Favara C., Baron G., Priola S., et al. Anchorless Prion Protein Results in Infectious Amyloid Disease without Clinical Scrapie. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 17.Stohr J., Watts J.C., Legname G., Oehler A., Lemus A., Nguyen H.-O.B., Sussman J., Wille H., DeArmond S.J., Prusiner S.B., et al. Spontaneous generation of anchorless prions in transgenic mice. Proc. Natl. Acad. Sci. USA. 2011;108:21223–21228. doi: 10.1073/pnas.1117827108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riek R., Hornemann S., Wider G., Billeter M., Glockshuber R., Wüthrich K. NMR structure of the mouse prion protein domain PrP(121–231) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 19.Donne D.G., Viles J.H., Groth D., Mehlhorn I., James T.L., Cohen F.E., Prusiner S.B., Wright P.E., Dyson H.J. Structure of the recombinant full-length ham-ster prion protein PrP(29-231): The N terminus is highly flexible. Proc. Natl. Acad. Sci. USA. 1997;94:13452–13457. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riek R., Hornemann S., Wider G., Glockshuber R., Wuthrich K. NMR characterization of the full-length recombinant mu-rine prion protein, mPrP(23-231) FEBS Lett. 1997;413:282–288. doi: 10.1016/S0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 21.Calzolai L., Lysek D.A., Perez D.R., Guntert P., Wuthrich K. Prion protein NMR structures of chickens, turtles, and frogs. Proc. Natl. Acad. Sci. USA. 2005;102:651–655. doi: 10.1073/pnas.0408939102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez C.D., Yost C.S., Prusiner S.B., Myers R.M., Lingappa V.R. Unusual topogenic sequence directs prion protein bio-genesis. Science. 1990;248:226–229. doi: 10.1126/science.1970195. [DOI] [PubMed] [Google Scholar]
- 23.Chen S.G., Teplow D.B., Parchi P., Teller J.K., Gambetti P., Autilio-Gambetti L. Truncated forms of the human prion pro-tein in normal brain and in prion diseases. J. Biol. Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- 24.Mange A., Béranger F., Peoc’H K., Onodera T., Frobert Y., Lehmann S. Alpha- and beta- cleavages of the amino-terminus of the cellular prion protein. Biol. Cell. 2004;96:125–132. doi: 10.1016/j.biolcel.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Harris D.A., Huber M.T., van Dijken P., Shyng S.L., Chait B.T., Wang R. Processing of a cellular prion protein: Identifica-tion of N- and C-terminal cleavage sites. Biochemistry. 1993;32:1009–1016. doi: 10.1021/bi00055a003. [DOI] [PubMed] [Google Scholar]
- 26.Altmeppen H.C., Puig B., Dohler F., Thurm D.K., Falker C., Krasemann S., Glatzel M. Proteolytic processing of the prion pro-tein in health and disease. Am. J. Neurodegen. Dis. 2012;1:15–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Laffont-Proust I., Faucheux B.A., Hässig R., Sazdovitch V., Simon S., Grassi J., Hauw J.-J., Moya K.L., Haik S. The N-terminal cleavage of cellular prion protein in the human brain. FEBS Lett. 2005;579:6333–6337. doi: 10.1016/j.febslet.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Shyng S.L., Huber M.T., Harris D.A. A prion protein cycles between the cell surface and an endocytic compartment in cul-tured neuroblastoma cells. J. Biol. Chem. 1993;268:15922–15928. doi: 10.1016/S0021-9258(18)82340-7. [DOI] [PubMed] [Google Scholar]
- 29.Linsenmeier L., Altmeppen H., Wetzel S., Mohammadi B., Saftig P., Glatzel M. Diverse functions of the prion protein-Does proteolytic processing hold the key? Biochim. Biophys. Acta (BBA)-Bioenerg. 2017;1864:2128–2137. doi: 10.1016/j.bbamcr.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Haigh C. Copper, endoproteolytic processing of the prion protein and cell signalling. Front. Biosci. 2010;15:1086–1104. doi: 10.2741/3663. [DOI] [PubMed] [Google Scholar]
- 31.Watts J.C., Bourkas M.E.C., Arshad H. The function of the cellular prion protein in health and disease. Acta Neuropathol. 2017;135:159–178. doi: 10.1007/s00401-017-1790-y. [DOI] [PubMed] [Google Scholar]
- 32.Salvesen O., Tatzelt J., Tranulis M.A. The prion protein in neuroimmune crosstalk. Neurochem. Int. 2019;130:104335. doi: 10.1016/j.neuint.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Caughey B., Baron G.S. Prions and their partners in crime. Nature. 2006;443:803–810. doi: 10.1038/nature05294. [DOI] [PubMed] [Google Scholar]
- 34.Biasini E., Turnbaugh J.A., Unterberger U., Harris D.A. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012;35:92–103. doi: 10.1016/j.tins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legname G. Elucidating the function of the prion protein. PLOS Pathog. 2017;13:e1006458. doi: 10.1371/journal.ppat.1006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton A., Zamponi G.W., Ferguson S.S.G. Glutamate receptors function as scaffolds for the regulation of β-amyloid and cellular prion protein signaling complexes. Mol. Brain. 2015;8:18. doi: 10.1186/s13041-015-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch T., Martin-Lannerée S., Mouillet-Richard S. Functions of the Prion Protein. Prog. Mol. Biol. Transl. Sci. 2017;150:1–34. doi: 10.1016/bs.pmbts.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Wulf M.-A., Senatore A., Aguzzi A. The biological function of the cellular prion protein: An update. BMC Biol. 2017;15:34. doi: 10.1186/s12915-017-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purro S.A., Nicoll A.J., Collinge J. Prion Protein as a Toxic Acceptor of Amyloid-β Oligomers. Biol. Psychiatry. 2018;83:358–368. doi: 10.1016/j.biopsych.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Resenberger U.K., Winklhofer K.F., Tatzelt J. Neuroprotective and Neurotoxic Signaling by the Prion Protein. Top. Curr. Chem. 2011;305:101–119. doi: 10.1007/128_2011_160. [DOI] [PubMed] [Google Scholar]
- 41.Brody A.H., Strittmatter S.M. Synaptotoxic Signaling by Amyloid Beta Oligomers in Alzheimer’s Disease through Prion Protein and mGluR5. Stud. Surf. Sci. Catal. 2018;82:293–323. doi: 10.1016/bs.apha.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakkebø M.K., Mouillet-Richard S., Espenes A., Goldmann W., Tatzelt J., Tranulis M.A. The Cellular Prion Protein: A Player in Immunological Quiescence. Front. Immunol. 2015;6:450. doi: 10.3389/fimmu.2015.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiesa R. The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease. PLoS Pathog. 2015;11:e1004745. doi: 10.1371/journal.ppat.1004745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKinley M.P., Bolton D.C., Prusiner S.B. A protease-resistant protein is a structural component of the Scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- 45.Flechsig E., Shmerling D., Hegyi I., Raeber A.J., Fischer M., Cozzio A., Mering C., Aguzzi A., Weissmann C. Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron. 2000;27:399–408. doi: 10.1016/S0896-6273(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 46.Supattapone S., Muramoto T., Legname G., Mehlhorn I., Cohen F.E., DeArmond S.J., Prusiner S.B., Scott M.R. Identification of Two Prion Protein Regions That Modify Scrapie Incubation Time. J. Virol. 2001;75:1408–1413. doi: 10.1128/JVI.75.3.1408-1413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turnbaugh J.A., Westergard L., Unterberger U., Biasini E., Harris D.A. The N-Terminal, Polybasic Region Is Critical for Prion Protein Neuroprotective Activity. PLoS ONE. 2011;6:e25675. doi: 10.1371/journal.pone.0025675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalifé M., Reine F., Paquet-Fifield S., Castille J., Herzog L., Vilotte M., Moudjou M., Moazami-Goudarzi K., Makhzami S., Passet B., et al. Mutated but Not Deleted Ovine PrP C N-Terminal Polybasic Region Strongly Interferes with Prion Propagation in Transgenic Mice. J. Virol. 2016;90:1638–1646. doi: 10.1128/JVI.02805-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das N.R., Miyata H., Hara H., Chida J., Uchiyama K., Masujin K., Watanabe H., Kondoh G., Sakaguchi S. The N-Terminal Polybasic Region of Prion Protein Is Crucial in Prion Pathogenesis Independently of the Octapeptide Repeat Region. Mol. Neurobiol. 2020;57:1203–1216. doi: 10.1007/s12035-019-01804-5. [DOI] [PubMed] [Google Scholar]
- 50.Westergard L., Turnbaugh J.A., Harris D.A. A Naturally Occurring C-terminal Fragment of the Prion Protein (PrP) Delays Disease and Acts as a Dominant-negative Inhibitor of PrPSc Formation. J. Biol. Chem. 2011;286:44234–44242. doi: 10.1074/jbc.M111.286195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mead S., Lloyd S., Collinge J. Genetic Factors in Mammalian Prion Diseases. Annu. Rev. Genet. 2019;53:117–147. doi: 10.1146/annurev-genet-120213-092352. [DOI] [PubMed] [Google Scholar]
- 52.Kraus A., Hoyt F., Schwartz C.L., Hansen B., Hughson A.G., Artikis E., Race B., Caughey B. Structure of an infectious mammalian prion. bioRxiv. 2021 doi: 10.1101/2021.02.14.431014. [DOI] [PubMed] [Google Scholar]
- 53.Shmerling D., Hegyi I., Fischer M., Blättler T., Brandner S., Götz J., Rülicke T., Flechsig E., Cozzio A., Mering C., et al. Expression of animo-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell. 1998;93:203–214. doi: 10.1016/S0092-8674(00)81572-X. [DOI] [PubMed] [Google Scholar]
- 54.Li A., Christensen H.M., Stewart L.R., Roth K.A., Chiesa R., Harris D.A. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. EMBO J. 2007;26:548–558. doi: 10.1038/sj.emboj.7601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baumann F., Tolnay M., Brabeck C., Pahnke J., Kloz U., Niemann H.H., Heikenwalder M., Rülicke T., Bürkle A., Aguzzi A. Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J. 2007;26:538–547. doi: 10.1038/sj.emboj.7601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westergard L., Turnbaugh J.A., Harris D.A. A Nine Amino Acid Domain Is Essential for Mutant Prion Protein Toxicity. J. Neurosci. 2011;31:14005–14017. doi: 10.1523/JNEUROSCI.1243-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandner S., Isenmann S., Raeber A., Fischer M., Sailer A., Kobayashi Y., Marino S., Weissmann C., Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 58.Rambold A.S., Müller V., Ron U., Ben-Tal N., Winklhofer K.F., Tatzelt J. Stress-protective activity of prion protein is cor-rupted by scrapie-prions. EMBO J. 2008;27:1974–1984. doi: 10.1038/emboj.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurén J., Gimbel D.A., Nygaard H.B., Gilbert J.W., Strittmatter S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S., Yadav S.P., Surewicz W.K. Interaction between Human Prion Protein and Amyloid-β (Aβ) Oligomers. J. Biol. Chem. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith L.M., Strittmatter S.M. Binding Sites for Amyloid-β Oligomers and Synaptic Toxicity. Cold Spring Harb. Perspect. Med. 2017;7:a024075. doi: 10.1101/cshperspect.a024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Resenberger U.K., Harmeier A., Woerner A.C., Goodman J.L., Müller V., Krishnan R., Vabulas R.M., Kretzschmar H.A., Lindquist S., Hartl F.U., et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira D.G., Ferreira M.T., Miranda H.V., Batalha V.L., Coelho J., Szegö É.M., Marques-Morgado I., Vaz S.H., Rhee J.S., Schmitz M., et al. α-synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat. Neurosci. 2017;20:1569–1579. doi: 10.1038/nn.4648. [DOI] [PubMed] [Google Scholar]
- 64.Ondrejcak T., Klyubin I., Corbett G.T., Fraser G., Hong W., Mably A.J., Gardener M., Hammersley J., Perkinton M.S., Billinton A., et al. Cellular Prion Protein Mediates the Disruption of Hippocampal Synaptic Plasticity by Soluble Tau In Vivo. J. Neurosci. 2018;38:10595–10606. doi: 10.1523/JNEUROSCI.1700-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbett G.T., Wang Z., Hong W., Colom-Cadena M., Rose J., Liao M., Asfaw A., Hall T.C., Ding L., DeSousa A., et al. PrP is a central player in toxicity mediated by soluble aggregates of neurodegeneration-causing proteins. Acta Neuropathol. 2020;139:503–526. doi: 10.1007/s00401-019-02114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ulbrich S., Janning P., Seidel R., Matschke J., Gonsberg A., Jung S., Glatzel M., Engelhard M., Winklhofer K., Tatzelt J. Alterations in the brain interactome of the intrinsically disordered N-terminal domain of the cellular prion protein (PrPC) in Alzheimer’s disease. PLoS ONE. 2018;13:e0197659. doi: 10.1371/journal.pone.0197659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitteregger G., Vosko M., Krebs B., Xiang W., Kohlmannsperger V., Nolting S., Hamann G.F., Kretzschmar H.A. The Role of the Octarepeat Region in Neuroprotective Function of the Cellular Prion Protein. Brain Pathol. 2007;17:174–183. doi: 10.1111/j.1750-3639.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakthivelu V., Seidel R.P., Winklhofer K.F., Tatzelt J. Conserved Stress-protective Activity between Prion Protein and Shadoo. J. Biol. Chem. 2011;286:8901–8908. doi: 10.1074/jbc.M110.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guillot-Sestier M.-V., Sunyach C., Druon C., Scarzello S., Checler F. The α-Secretase-derived N-terminal Product of Cellular Prion, N1, Displays Neuroprotective Function in Vitro and in Vivo. J. Biol. Chem. 2009;284:35973–35986. doi: 10.1074/jbc.M109.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haigh C.L., Drew S.C., Boland M.P., Masters C.L., Barnham K.J., Lawson V.A., Collins S.J. Dominant roles of the polybasic proline motif and copper in the PrP23-89-mediated stress protection response. J. Cell Sci. 2009;122:1518–1528. doi: 10.1242/jcs.043604. [DOI] [PubMed] [Google Scholar]
- 71.Collins S.J., Tumpach C., Groveman B.R., Drew S.C., Haigh C.L. Prion protein cleavage fragments regulate adult neural stem cell quiescence through redox modulation of mitochondrial fission and SOD2 expression. Cell. Mol. Life Sci. 2018;75:3231–3249. doi: 10.1007/s00018-018-2790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haigh C.L., McGlade A.R., Collins S.J. MEK1 transduces the prion protein N2 fragment antioxidant effects. Cell. Mol. Life Sci. 2014;72:1613–1629. doi: 10.1007/s00018-014-1777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bremer J., Baumann F., Tiberi C., Wessig C., Fischer H., Schwarz P., Steele A.D., Toyka K.V., Nave K.-A., Weis J., et al. Axonal prion protein is required for peripheral myelin maintenance. Nat. Neurosci. 2010;13:310–318. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]
- 74.Skedsmo F.S., Malachin G., Vage D.I., Hammervold M.M., Salvesen O., Ersdal C., Ranheim B., Stafsnes M.H., Bartosova Z., Bruheim P., et al. Demyelinating polyneuropathy in goats lacking prion protein. FASEB J. 2019;34:2359–2375. doi: 10.1096/fj.201902588R. [DOI] [PubMed] [Google Scholar]
- 75.Küffer A., Lakkaraju A.K., Mogha A., Petersen S.C., Airich K., Doucerain C., Marpakwar R., Bakirci P., Senatore A., Monnard A., et al. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536:464–468. doi: 10.1038/nature19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meier P., Genoud N., Prinz M., Maissen M., Rulicke T., Zurbriggen A., JRaeber A., Aguzzi A. Soluble dimeric prion protein binds PrP(Sc) in vivo and antagonizes prion disease. Cell. 2003;113:49–60. doi: 10.1016/S0092-8674(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 77.Miesbauer M., Pfeiffer N.V., Rambold A.S., Müller V., Kiachopoulos S., Winklhofer K.F., Tatzelt J. α-Helical Domains Promote Translocation of Intrinsically Disordered Polypeptides into the Endoplasmic Reticulum. J. Biol. Chem. 2009;284:24384–24393. doi: 10.1074/jbc.M109.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heske J., Heller U., Winklhofer K.F., Tatzelt J. The C-terminal Globular Domain of the Prion Protein Is Necessary and Sufficient for Import into the Endoplasmic Reticulum. J. Biol. Chem. 2004;279:5435–5443. doi: 10.1074/jbc.M309570200. [DOI] [PubMed] [Google Scholar]
- 79.Mohammadi B., Linsenmeier L., Shafiq M., Puig B., Galliciotti G., Giudici C., Willem M., Eden T., Koch-Nolte F., Lin Y.-H., et al. Transgenic Overexpression of the Disordered Prion Protein N1 Fragment in Mice Does Not Protect Against Neurodegenerative Diseases Due to Impaired ER Translocation. Mol. Neurobiol. 2020;57:2812–2829. doi: 10.1007/s12035-020-01917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fluharty B.R., Biasini E., Stravalaci M., Sclip A., Diomede L., Balducci C., La Vitola P., Messa M., Colombo L., Forloni G., et al. An N-terminal Fragment of the Prion Protein Binds to Amyloid-β Oligomers and Inhibits Their Neurotoxicity in Vivo. J. Biol. Chem. 2013;288:7857–7866. doi: 10.1074/jbc.M112.423954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Béland M., Bédard M., Tremblay G., Lavigne P., Roucou X. Aβ induces its own prion protein N-terminal fragment (PrPN1)–mediated neutralization in amorphous aggregates. Neurobiol. Aging. 2014;35:1537–1548. doi: 10.1016/j.neurobiolaging.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Guillot-Sestier M.-V., Sunyach C., Ferreira S.T., Marzolo M.-P., Bauer C., Thevenet A., Checler F. α-Secretase-derived Fragment of Cellular Prion, N1, Protects against Monomeric and Oligomeric Amyloid β (Aβ)-associated Cell Death. J. Biol. Chem. 2012;287:5021–5032. doi: 10.1074/jbc.M111.323626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott-McKean J.J., Surewicz K., Choi J.-K., Ruffin V.A., Salameh A.I., Nieznanski K., Costa A., Surewicz W.K. Soluble prion protein and its N-terminal fragment prevent impairment of synaptic plasticity by Aβ oligomers: Implications for novel therapeutic strategy in Alzheimer’s disease. Neurobiol. Dis. 2016;91:124–131. doi: 10.1016/j.nbd.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lundberg P., Magzoub M., Lindberg M., Hällbrink M., Jarvet J., Eriksson L.E., Langel Ü., Gräslund A. Cell membrane translocation of the N-terminal (1–28) part of the prion protein. Biochem. Biophys. Res. Commun. 2002;299:85–90. doi: 10.1016/S0006-291X(02)02595-0. [DOI] [PubMed] [Google Scholar]
- 85.Wadia J.S., Schaller M., Williamson R.A., Dowdy S.F. Pathologic Prion Protein Infects Cells by Lipid-Raft Dependent Macropinocytosis. PLoS ONE. 2008;3:e3314. doi: 10.1371/journal.pone.0003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Banani S.F., Lee H.O., Hyman A.A., Rosen M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dignon G.L., Best R.B., Mittal J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020;71:53–75. doi: 10.1146/annurev-physchem-071819-113553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Flynn B.G., Mittag T. The role of liquid–liquid phase separation in regulating enzyme activity. Curr. Opin. Cell Biol. 2021;69:70–79. doi: 10.1016/j.ceb.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 90.Alberti S., Dormann D. Liquid–Liquid Phase Separation in Disease. Annu. Rev. Genet. 2019;53:171–194. doi: 10.1146/annurev-genet-112618-043527. [DOI] [PubMed] [Google Scholar]
- 91.Babinchak W.M., Surewicz W.K. Liquid–Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation. J. Mol. Biol. 2020;432:1910–1925. doi: 10.1016/j.jmb.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Darling A.L., Shorter J. Combating deleterious phase transitions in neurodegenerative disease. Biochim. Biophys. Acta (BBA)-Bioenerg. 2021;1868:118984. doi: 10.1016/j.bbamcr.2021.118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zbinden A., Pérez-Berlanga M., De Rossi P., Polymenidou M. Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev. Cell. 2020;55:45–68. doi: 10.1016/j.devcel.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Tange H., Ishibashi D., Nakagaki T., Taguchi Y., Kamatari Y.O., Ozawa H., Nishida N. Liquid–liquid phase separation of full-length prion protein initiates conformational conversion in vitro. J. Biol. Chem. 2021;296:100367. doi: 10.1016/j.jbc.2021.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang J.-J., Li X.-N., Liu W.-L., Yuan H.-Y., Gao Y., Wang K., Tang B., Pang D.-W., Chen J., Liang Y. Neutralizing Mutations Significantly Inhibit Amyloid Formation by Human Prion Protein and Decrease Its Cytotoxicity. J. Mol. Biol. 2020;432:828–844. doi: 10.1016/j.jmb.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 96.Matos C.O., Passos Y.M., Amaral M.J.D., Macedo B., Tempone M.H., Bezerra O.C.L., Moraes M.O., Almeida M.S., Weber G., Missailidis S., et al. Liquid-liquid phase separation and fibrillation of the prion protein modulated by a high-affinity DNA aptamer. FASEB J. 2020;34:365–385. doi: 10.1096/fj.201901897R. [DOI] [PubMed] [Google Scholar]
- 97.Kostylev M.A., Tuttle M.D., Lee S., Klein L.E., Takahashi H., Cox T.O., Gunther E.C., Zilm K.W., Strittmatter S.M. Liquid and Hydrogel Phases of PrPC Linked to Conformation Shifts and Triggered by Alzheimer’s Amyloid-β Oligomers. Mol. Cell. 2018;72:426–443.e12. doi: 10.1016/j.molcel.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Passos Y.M., Amaral M.J.D., Ferreira N.C., Macedo B., Chaves J.A.P., de Oliveira V.E., Gomes M.P.B., Silva J.L., Cordeiro Y. The interplay between a GC-rich oligonucleotide and copper ions on prion protein conformational and phase transitions. Int. J. Biol. Macromol. 2021;173:34–43. doi: 10.1016/j.ijbiomac.2021.01.097. [DOI] [PubMed] [Google Scholar]
- 99.Kamps J., Lin Y.-H., Oliva R., Bader V., Winter R., Winklhofer K.F., Tatzelt J. The N-terminal domain of the prion protein is required and sufficient for liquid-liquid phase separation; a crucial role of the Aβ-binding domain. J. Biol. Chem. 2021;297:100860. doi: 10.1016/j.jbc.2021.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Darling A.L., Liu Y., Oldfield C.J., Uversky V.N. Intrinsically Disordered Proteome of Human Membrane-Less Organelles. Proteomics. 2018;18:e1700193. doi: 10.1002/pmic.201700193. [DOI] [PubMed] [Google Scholar]
- 101.Moore R.A., Hayes S.F., Fischer E.R., Priola S.A. Amyloid Formation via Supramolecular Peptide Assemblies. Biochemistry. 2007;46:7079–7087. doi: 10.1021/bi700247y. [DOI] [PubMed] [Google Scholar]
- 102.König A.S., Rösener N.S., Gremer L., Tusche M., Flender D., Reinartz E., Hoyer W., Neudecker P., Willbold D., Heise H. Structural details of amyloid β oligomers in complex with human prion protein as revealed by solid-state MAS NMR spectroscopy. J. Biol. Chem. 2021;296:100499. doi: 10.1016/j.jbc.2021.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]