Abstract
Head and neck squamous cell carcinoma (HNSCC) is characterized by a high mortality rate owing to very few available oncological treatments. For many years, a combination of platinum-based chemotherapy and anti-EGFR antibody cetuximab has represented the only available option for first-line therapy. Recently, immunotherapy has been presented an alternative for positive PD-L1 HNSCC. However, the oncologists’ community foresees that a new therapeutic era is approaching. In fact, no-chemo options and some molecular targets are on the horizon. This narrative review addresses past, present, and future therapeutic options for HNSCC from a translational point of view.
Keywords: head and neck squamous cell carcinoma, immunotherapy, DNA damage response, epithelial growth factor receptor
1. Introduction
As most cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed in locally advanced or metastatic settings, this set of oncological disease is characterized by a high mortality rate. Unfortunately, in the recurrent/metastatic setting, the only available treatments are represented by systemic treatments and palliative radiotherapy and/or surgery [1]. Various topical drugs have been proposed as palliative treatments in the management of HNSCC as an adjuvant or neoadjuvant therapy, with controversial results [2,3].
Many efforts have been pursued in identifying new targets and innovative therapies to increase therapeutic options over chemotherapy, as well as to improve response rates, survival, and quality of life. This paper is aimed at dissecting the therapeutic pathway, which led to the discovery of targeted therapy. In particular, the role of immunotherapy that currently represents “the present” is addressed [4]. Finally, the crucial importance of translational research to prepare new therapies tailored on patient and tumor characteristics is underlined (see Supplementary File for the methodology applied to search literature).
2. The Past and Present: Targeting EGFR
2.1. EGFR mAb
EGFR (epithelial growth factor receptor) is a member of the ErbB/HER family, overexpressed in about 90% of HNSCCs. Its expression is related to poor survival because of resistance to radiotherapy and local treatments; nevertheless, its prognostic role is still quite controversial [5]. In 2006, cetuximab, an anti-EGFR monoclonal antibody (mAb), was approved in HNSCC treatment. Cetuximab is an IgG1 monoclonal antibody that blocks EGFR activation by specifically binding to the extracellular domain of EGFR, thus inducing EGFR internalization and downregulation. Inhibition of EGFR-downstream pathways is able to interfere with cancer growth. Moreover, cetuximab has anti-body-dependent cell-mediated cytotoxicity, owing to its IgG1 isotype, as it directs cytotoxic immune-cells against EGFR-expressing tumor cells [6,7]. In recurrent/metastatic HNSCC (R/M HNSCC), cetuximab was approved in combination with platinum-based chemotherapy, showing fairly good results in terms of overall survival, response rate, and progression-free survival [8].
Previously, other EGFR mAbs were tested without reaching clinical approval. In the SPECTRUM trial [9], panitumumab, a fully human mAb, in combination with polychemotherapy (based on platinum + fluorouracil), did not show any benefit in the metastatic setting. The difference in clinical activity between cetuximab and panitumumab was probably due to their different isotype conformation with a consequent heterogeneous induction of antibody-dependent cellular cytotoxicity (ADCC) (IgG1 for cetuximab and IgG2 for panitumumab). Similarly, zalutumumab is an IgG1 mAb that can block EGFR and induce ADCC, but it did not increase the clinical outcome in the same setting. In an exploratory, open-label, randomized, multicenter study in operable HNSCC patients, imgatuzumab (GA201), which is another primatized glycol-engineered IgG1 mAb with ADCC-related immune effects, showed promising results in terms of tumor immune infiltration. Sym004, a new generation anti-EGFR mAb, displayed only modest anti-tumor activity in a proof of concept trial, without further clinical exploration [10].
Losatuxizumab vedotin (ABBV-221) is a second-generation antibody–drug conjugate (ADC) anti-EGFR that obtained some responses in a multicenter phase 1 study also enrolling patients with HNSCC, but it was poorly tolerated for the high frequency of infusion reactions [11].
2.2. EGFR TKIs
In addition to EGFRmAb, small-molecule tyrosine kinase inhibitors (TKIs) that bind to the intracellular domain of EGFR were studied.
Two first-generation reversible EGFR-TKIs, erlotinib and gefitinib, were tested in multiple clinical trials without obtaining any benefit over the EXTREME regimen [12,13]. However, a phase II study evaluating the combination of erlotinib, carboplatin, paclitaxel, and cetuximab in patients with metastatic or recurrent HNSCC is ongoing. Nevertheless, a preliminary analysis of the first 24 patients has shown that overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) were similar to historical data obtained with the EXTREME regimen [14]. In trials enrolling patients with both metastatic and locoregional disease, dacomitinib, a second-generation irreversible EGFR TKI, led to inconclusive results [15].
Different data were obtained with the other irreversible second-generation TKI inhibitor, afatinib, which significantly improved PFS, in a phase III LUX-head and neck 3 trial, as second-line treatment when compared with the standard methotrexate [16].
EGFR mutations often confer greater responsiveness to small anti-EGFR molecules. Nevertheless, the EGFR gene is very rarely mutated in HNSCC, as is the case for lung cancers. This paramount gap can explain the unconvincing results obtained with EGFR inhibitors in various clinical studies. Despite the conflicting results obtained with the use of different anti EGFR therapies, EGFR still remains a key therapeutic target. Therefore, even if most of these drugs are not approved in clinical practice, we believe that these positive data confirm the biological importance of EGFR signaling in HNC development. The study of the EGFR pathway in HNSCC deserves further scientific efforts.
3. The Present and Future: Immunotherapy
3.1. Check-Point Inhibitors (ICIs)
Recently, immunotherapy has greatly modified the therapeutic standard of HNSCC in the R/M setting. The immune system plays a fundamental role in regulating tumor growth, as several solid and hematological tumors develop more easily in immunocompromised individuals, thus underlining the importance of “immunological surveillance” against the growth of tumor cells. On this rationale, many efforts have been focused on the development of immunotherapy drugs to restore the ability of the immune system for detecting and destroying cancer cells [17].
Specifically, progress in the understanding of mechanisms regulating the immune system activity has shed light on the crucial role of many proteins and lymphocytes. In particular, antigens associated with cytotoxic T lymphocytes-4 (CTLA-4), programmed cell death ligand 1 (PDL-1), and indoleamine-2,3-dioxygenase (IDO), as well as lymphocytes with regulatory functions (T-regulatory cells–Tregs) and myeloid-derived suppressive cells (MDSC), can profoundly modulate the immune response and belong to the so-called “immune checkpoints”, offering new therapeutic strategies.
Recently, published data have clearly indicated that immunotherapy (anti-CTLA-4 and anti-PD-1/PD-L1 antibodies) represents an important therapeutic option for HNSCC patients. However, despite promising treatment results, a significant number of patients still fail to achieve clinically meaningful benefits. For this reason, in the era of precision medicine, identifying reliable predictive factors to select patients who are most likely to benefit from treatment with immune agents is a crucial and open challenge in oncology [18].
3.2. CTLA-4
CTLA-4 is the first checkpoint receptor that has been successfully studied and tested in cancer as a target [19]. It has a critical role in maintaining activation of T cells, as demonstrated by the lethal systemic immune-hyperactivation phenotype of CTLA-4 knockout mice [20]. Currently, two human anti-CTLA-4 antibodies are used and studied in clinical practice in solid tumors. Ipilimumab, approved in the treatment of advanced melanoma, and tremelimumab, under development in several solid tumors, works by binding to CTLA-4 and blocking its immunosuppressive signal. As a result, activated T cells, including those activated by tumor antigens, can continue to proliferate, producing cytokines and exerting their cytotoxic effector functions in the tumor microenvironment. The first data came from a case report. In a 46-year-old male with relapsed PDL1 positive HNSCC, Schwab et al. [21] showed that the combination ipilimumab plus nivolumab induced a partial response after 8 weeks from the start of treatment and complete response after 4 months of therapy.
3.3. Programmed Death-1 (PD-1/PDL-1)
Another immune checkpoint receptor studied is the programmed death receptor 1 (PD1). It is a member of the CD28 superfamily that delivers negative signals upon interaction with its two ligands, the programmed cell death ligand 1 (PD-L1) and the programmed cell death ligand 2 (PD-L2). Similar to CTLA-4, PD-1 plays a key role in regulating and maintaining the balance between T cell activation and in promoting self-tolerance. Unlike CTLA-4, PD-1 is widely expressed and can be found not only on the surface of T cells, but also on that of B and NK (natural killer) cells. While CTLA-4 mainly regulates the activation of T cells in lymphatic tissues, the primary role of PD-1 is to suppress the inflammatory activity of T cells in peripheral tissues during a cell-mediated or inflammatory immune response. In turn, targeting PD-1/PD-L1 can produce a wide-ranging effect. PD-L1 ligand is commonly upregulated on several human solid tumors, including HNSCC. Consequently, it represents a biomarker in clinical practice (such as, for example, in lung cancer) [22].
The expression of PD-L1 on tumor cells, as assessed by immunohistochemistry (IHC), was initially identified as a biomarker for predicting response to treatment with anti-PD-1/anti-PD-L1 therapies. This topic has been widely studied on different types of cancer with mixed results [23,24].
The factors predictive for a good response to anti-PD1 treatment are not fully understood. Indeed, PDL1 expression is only one of the potential determinants of immunotherapy efficacy. The lack of benefit in some patients with PD-L1 positive cancer implies that other molecular mechanisms are involved in resistance to checkpoint inhibition. It was also demonstrated that, in HNSCC, the combined positive score (CPS), calculated as the number of PD-L1 positive cells including tumor, lymphocytes, and macrophages, in relation to total tumor cells, appears to be more specific than the tumor proportional score (TPS). The latter measures PD-L1 expression only on tumor cells, in the selection of patients with HNSCC who may benefit from immunotherapy treatment. This feature was confirmed by the results of the first-line KEYNOTE-048 study. It demonstrated the superiority of the anti PDL-1pembrolizumab, alone or in combination with chemotherapy (cisplatin or carboplatin and 5FU), over the standard platinum-5-fluorouracil-cetuximab regimen, in patients with HNSCC PDL-1 CPS positive (CPS > 1) [25]. Currently, pembrolizumab, an anti-PD-L1 mAb, alone or in combination with platinum-based chemotherapy, represents the new standard of care in first-line therapy for HNSCC with CPS ≥ 1.
The positive response to immune checkpoints inhibitor can be also predicted by the presence of other biomarkers such as the expression of PD-L2, the other PD-1 ligand, as emerged from the results of the KEYNOTE-012 study [26]. In HNSCC, PD-L1 is overexpressed in about 50–60% of cases; therefore, PD-1/PD-L1 and PD-L2 inhibitors may represent the main class of immunotherapy drugs for this cancer type.
4. Immunotherapy Biomarkers
4.1. TMB
A large portion of HNSCC has a high tumor mutational burden (TMB). It is related to heavy cigarette smoking (typical of patients with HNSCC) and to the presence of human papillomavirus (HPV) and Epstein–Barr virus (EBV) viral infections. It is likely that tumors with a large number of somatic genome mutations develop a higher specific T cell response to tumor neoantigens, which results in greater susceptibility to immunotherapy. For this reason, TMB has been proposed as a new biomarker of response to immune agents. Several studies have explored the correlation between elevated TMB and the benefit of anti-CTLA-4 and anti-PD-1 antibodies, and mutational load has proved to be a very promising biomarker as tumors with TMB > 100 somatic mutations associated with an increase in survival [27]. Clinical findings in the HNSCC cohort of the KEYNOTE-012 study showed that patients with both elevated TMB and high PD-L1 expression responded to treatment with pembrolizumab; moreover, there was no direct association between TMB and PD-L1 expression. This confirms that TMB and PD-L1 are two independent biomarkers for the prediction of the response to immunotherapy [28]. Recently, the study published by Zhang et al. [29] found that high levels of TMB are also associated with poor prognosis, advanced stage, and large primary tumor size in HNSCC patients.
4.2. Microsatellite Instability
Microsatellite instability (MSI) refers to a specific “hypermutator phenotype” corresponding to the presence of somatic or inherited DNA mismatch repair genes mutations. In routine use, detection of MSI is done by IHC for MMR (mismatch repair) proteins or by DNA profiling. MSI-high is associated with the efficacy of PD-1 blockade in other tumor types [30]; however, the incidence of the MSI-high phenotype is very low in HNSCC and no data are available for using it in clinical practice [31].
4.3. New Immunotherapy Biomarkers and Targets
Other emerging biomarkers studied to assess the primary response to immunotherapy are tumor-infiltrating lymphocytes (TILs), HPV, IDO, inducible T-cell co-stimulator (ICOS), and NKG2A (natural killer group 2A) receptor. The role of TIL as a predictor for patient selection has been studied in various tumor types. It has been observed that, in tumor samples obtained after immunotherapy, a high density of TILs is associated with increased activity of these drugs [32]. Furthermore, it was also found that a better response rate to pembrolizumab has been observed in melanoma patients with higher CD8+ density during treatment [33]. In the cohort study published by Spector et al. [34], TILs’ levels were an independent prognostic factor in patients with HNSCC.
Regarding viral infections, HPV positivity correlates with a better clinical outcome with immunotherapy, thus representing a favorable clinical prognostic biomarker in HPV-positive disease [34]. Chen et al. [35] demonstrated that p16 protein expression is highly correlated with PD-L1 expression in HNSCC samples, thus explaining why these tumors probably respond better to anti-PD-1/PD-L1 drugs. Some evidence suggests that HPV positivity is predictive of response to anti-PD1 agents. In this regard, the KEYNOTE-012, CheckMate 141, and the KEYNOTE 048 investigations showed an improvement in outcome in HPV-positive patients compared with HPV-negative patients. Despite this, HPV positivity cannot currently be considered to select patients for immunotherapy [36]. IDO plays an important role in immunity as it intervenes in the natural defense against various pathogens; it is produced in response to inflammatory stimuli and performs an immunosuppressive function by limiting the activity of T lymphocytes, on the one hand, and activating the mechanisms involved in immune tolerance, on the other hand [37]. Retrospective studies in patients with HNSCC showed that high levels of IDO expression are correlated with worse outcomes and a poorer prognosis, probably owing to the direct association of IDO with regulatory T cells (T-Reg). Although evidence from other tumors, such as melanoma, did not show improvement in outcome in the group of patients treated with IDO inhibitor epacadostat, some studies have been conducted in patients with HNSCC. In the phase I/II study ECHO-202/Keynote 037, which evaluated the combination of epacadostat plus pembrolizumab, in the two patients enrolled with HNSCC, disease stability was obtained as the best response with a disease response of 34% and a disease control rate of 39% [38]. Instead, the results of the phase III Keynote 669/Echo 304 study that evaluated the combination of epacadostat plus pembrolizumab versus pembrolizumab monotherapy versus the EXTREME regimen are awaited. A phase II study evaluating the combination of BMS-986205, an IDO1 inhibitor with nivolumab (NCT03854032) in stage II–IV patients with HNSCC, is still ongoing. Another molecule involved in immunity and being studied is ICOS, a protein stimulated by both the T cell receptor and CD28 signals. A potential therapeutic strategy to overcome resistance to anti PD1/PDL-1 could be represented by the combination of anti PD1 antibodies with the ICOS agonists. In this regard, a double-blind, randomized phase 3 study is underway evaluating the combination of an ICOS agonist, GSK3359609, with pembrolizumab versus placebo plus pembrolizumab in first-line treatment in patients with HNSCC R/M PD-L1 positive [39]. Lastly, an alternative target of the immune checkpoint could be represented by the NKG2A receptor, present on the surface of NK cells and on CD8 + T lymphocytes. Monalizumab is an IgG4 class antibody whose function is precisely to block NKG2A by promoting antitumor immunity and increasing antibody-dependent cell-mediated cytotoxicity. As the combination of monalizumab plus cetuximab showed promise in a phase II study (with a 31% response rate), a phase III study is ongoing to evaluate the combination of the two antibodies in patients with platinum-resistant R/M SHCCN previously treated with immunotherapy [40].
5. What Is New for the Future: IO Combinations
5.1. Clinically Relevant Molecular Alterations in HNSCC
The development of new technologies helped to dissect tumor genomic molecular alterations, thus identifying novel therapeutic targets. How to translate these molecular features into clinically relevant treatments options is still not clear. The level of evidence of actionable mutations in HNSCC on the basis of the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT) [41] is an instrument to help oncologists in selecting treatments. Therefore, alterations of 33 genes have been studied. Among these alterations, HRAS-activating mutations (targetable by tipifarnib, a farnesyltransferase inhibitor) and similarly for NTRK (neurotrophic tyrosine kinase receptor) fusions, seem to be very interesting. These alterations have also been proposed as new targets in combination with immunotherapy [42]. Based on positive results of palbociclib (CDK4/6 inhibitor) and afatinib in molecular subgroups from a retrospective investigation [43], CDKN2A-inactivating alterations and EGFR amplification have been ranked in a high position.
EMERGING TARGETS: MEK. ErbB family proteins like EGFR, HER2, HER3, and HER4 play important roles in many cancer types, including head and neck. Despite the few goals achieved in targeted drug development, many translational and preclinical experiences have studied the relation between ErbB proteins and drug sensitivity in HNSCC. Afatinib, an irreversible inhibitor of EGFR, HER2, and HER4, was studied in combination with the MEK inhibitor PD0325901, with the aim to inhibit cisplatin-resistant HNSCC cells lines. Afatinib was shown to inhibit the Akt/mTOR activity and to promote the phosphorylation of EGFR, HER2, and HER3, concomitantly with an up-regulation of MEK/ERK signaling. More interestingly, MEK inhibitor PD0325901 blocked ERK phosphorylation, while the combination inhibited if all these pathways synergistically [44].
Recently, MEK inhibition has also been demonstrated to overcome the limited efficacy of CDK4/6 inhibitor. In fact, Fang et al. [45] reported that treatment with trametinib (MEK inhibitor) plus palbociclib (CDK4/6 inhibitor) resulted in a G0/G1 cell cycle arrest and apoptotic cell death in HNSCC cells, along with a remarkable decrease of MAPK pathway activation. These results have been confirmed in studies conducted on xenograft mouse models [46].
DDR. DNA damage response (DDR) is a cellular process used to report the presence of DNA damage [46]. Therefore, targeting DDR is emerging as a promising therapeutic option in many cancer types, especially where platinum and/or radiotherapy (both acting on DNA damage) are milestones of treatment. In this scenario, many DDR inhibitors are also under investigation in HNSCC that could be considered a prototype of DDR-sensitivity [47].
5.2. PARP (Poly ADP-Ribose Polymerases)
In vitro studies demonstrated a high sensitivity of HNSCC (both homologous recombination (HR)-deficient and -proficient) to the radiosensitizing activity of PARP inhibitors (PARPi) [48]. Moreover, other preclinical and clinical experiences demonstrated that PARPi sensitize cancer cells (including HNSCC) to platinum-based chemotherapy, temozolamide, and topoisomerase inhibitors [49,50]. These promising synergistic effects are currently tested in different ongoing clinical trials that combine CT/RT with PARPi (e.g., NCT01758731, NCT01460888, NCT02308072). In addition, other combination strategies of PARPi plus other-than-PARPDDR inhibitors (e.g., CHK1 and WEE1 inhibitors) are also under evaluation in HNSCC [51].
5.3. DNA-PK (DNA-Dependent Protein Kinase, Catalytic Subunit)
Different DNA-PK inhibiting molecules have been developed so far. Unfortunately, most showed several pharmacokinetics issues or an unacceptable safety profile [51,52]. As with other DDR inhibitors, DNA-PK development is mainly based on combination strategies, considering that monotherapy showed only modest effects [53]. In general, cells with a defective DNA-PK activity (also artificial) are highly sensitive to radiotherapy, indicating a potential radiosensitizing activity, later confirmed in different preclinical studies [54,55]. Specifically, the radiosensitizing effect of the DNA-PK inhibitor NU7411 was confirmed in preclinical studies in different cancer types such as lung, liver, and breast cancer [56,57]. On these bases, also the combination of EGFR inhibition (involved in the DNA-PK pathway) has been studied, showing an increased radiosensitizing effect in EGFR overexpressing cells and leading to an interesting new research field of the EGFR/DNA-PK co-inhibition [58]. All these promising effects of DNA-PK inhibitors are also under investigation in the clinical setting, as multiple clinical trials in solid tumors are ongoing (not specific for HNC).
5.4. ATM/ATR
ATM (ataxia-telangiectasia mutated) and ATR (ataxia-telangiectasia and Rad3 related) play a critical role in cell cycle regulation and DDR, specifically through CHK1 and CHK2 phosphorylation [59]. In HNC, 4–10% and 1–16% of the cases are characterized by ATR and ATM somatic mutations, respectively [60]. As with other DDR inhibitors, ATR/ATM targeting agents showed chemotherapy- and radiotherapy-sensitizing effects that led to preliminary clinical experience as monotherapy or in combinations [61]. M6620 (previously VX-970) is a first-in-class ATR inhibitor currently under investigation in a phase 1 trial in HPV-negative HNSCC (NCT02567422). AZD6738 is another selective ATR inhibitor that was recently demonstrated to enhance radiotherapy response in both HPV-negative and HPV-positive HNSCC in vitro [62]. A clinical trial of AZD6738 plus olaparib is currently ongoing in HNC (NCT02264678), and another biomarker-based study has recently been completed (NCT03022409).
5.5. CHK1/2
CHK1, alone or through the recruitment of RAD51, along with CHK2 (and its interaction with p53), are the main components of the DDR system [63,64]. Considering that many preclinical studies confirmed the sensitizing effect of CHK1/2 in p53-deficient cells, and that there is a high rate of Tp53 mutation in HNSCC, the CHK1/2 pathway is emerging as a promising potential new DDR inhibitor in this setting [59,65]. Prexasertib, a CHK1/2 inhibitor, was demonstrated to reduce in vitro survival fraction of HNSCC cell lines combined with cisplatin, with or without RT, mainly through the downregulation of NOTCH signaling target genes (NOTCH1, NOTCH2, and NOTCH3) and their associated ligands (JAG1, JAG2, SKP2, MAML2, and DLL1). Moreover, a significant tumor growth delay was observed in vivo in both HPV-positive and HPV-negative mouse xenografts treated with prexasertib, cisplatin, and radiotherapy without additional toxicities [66]. A phase 1 clinical trial of prexasertib combined with cisplatin and cetuximab in advanced HNSCC has completed accrual and the results are awaited (NCT02555644).
5.6. WEE1
WEE1 inhibition results in the premature entry of cells in the mitosis phase and, as CHK1 inhibitors, this effect is prevalent in p53-deficient cells [67]. Adavosertib (AZD1775) is a first-in-class WEE1 inhibitor, currently under investigation in a late-phase trial in different cancer types. Its activity in HNC was explored in combination strategies with the aim to potentiate multiple chemo- and radiotherapies [68]. The triplet combination of adavosertib, cisplatin, and docetaxel has been shown to be safe and tolerable in a phase 1 clinical trial in neoadjuvant HNC patients [69]. In addition, as stated for other DDR inhibitors, several shreds of evidence suggest the hypothesis of enhanced activity of these drugs when combined with each other [60]. Indeed, different studies proved, for instance, the synergistic effects of CHK1 and WEE1 inhibitors (e.g., adavosertib plus the CHK1 inhibitor LY2603618) [65] or triplet DDR combinations of PARPi, WEE1, and CHK1 inhibitors [51].
5.7. PI3K
Alterations of the PI3K/AKT/mTOR pathway are common in HNSCC with a prevalence of activating mutations of PI3K of 56% and 39% in HPV-positive and HPV-negative HNSCC, respectively [70,71]. Different data support the role of this pathway as an important mechanism of resistance to EGFR inhibitors and RT [72]. Despite these mechanisms, the preclinical model showed that PI3K inhibition alone led to compensatory positive feedback on the RAS/MEK/ERK or EGFR pathway inducing early resistance. On the other hand, combination therapies (e.g., targeting multiple isoforms of PI3K or combining other DDR inhibitors or DNA damaging agents) could achieve synergistic effects [73]. Moreover, as with other targeted therapies in HNSCC, effective biomarkers are still pending. Recently, NOTCH-1 loss-of-function mutations (NOTCH1mut) has shown a potential role as a predictive factor of PI3K/AKT/mTOR inhibition. Thus, in both HNSCC cell lines and xenografts models, NOTCH1mut was strongly associated with sensitivity to multiple PI3K/mTOR inhibitors and NOTCH1 inhibition or knockout in wild-type cells increased that effect. However, to overcome all these limitations, pan-PI3K inhibitors (acting on more than one isoform of PI3K) have recently emerged as potential new effective compounds [74]. Currently, buparlisib is the pan-PI3K inhibitor with the most clinical evidence. Buparlisib (BKM120) is an oral reversible PI3K inhibitor that showed anti-proliferative and pro-apoptotic effects in tumor cells, irrespective of the PIK3CA status [75]. Nevertheless, considering early safety data, its use as monotherapy has been replaced with combination strategies [76]. A phase 2 study investigating the combination of buparlisib and cetuximab has been recently completed and the results are awaited (NCT01816984). In addition, the results of a phase 2 study combining buparlisib and paclitaxel showed improved clinical efficacy with a manageable safety profile, suggesting an effective opportunity in pretreated metastatic HNSCC [77], and the phase 3 BURAN trial with this combination is still ongoing (NCT04338399).
5.8. CDK
Cyclin-dependent kinases (CDK) play a major role in cell cycle control. In the last years, different CDK4/6 inhibitors have been approved for the treatment of breast cancer and have been tested in late-phase trials in other malignancies [78,79,80]. Recently, CDK inhibition has emerged as a potential mechanism of chemo- and radiosensitization and immune stimulation, leading to preclinical and clinical research that incorporates ICIs and CDK inhibitors in different settings [81].
In HNSCC, beyond CDK4/6 inhibition, other kinases of the same family have been identified as potential biomarkers of response and poor outcome [81,82].
These pieces of evidence also led to the investigation of CDK inhibition in HNSCC in clinical settings. In a phase 1 study in R/M HNSCC, palbociclib plus cetuximab demonstrated a high disease control rate and, in a subsequent phase 2 trial in platinum- or cetuximab-resistant HPV-negative HNSCC, the combination showed efficacy comparable to PD-1 inhibitors and performed better than single-agent cetuximab [83,84]. Despite these early data, recent results from a multicenter phase 2 trial of palbociclib plus carboplatin in the R/M setting did not show improvement in survival outcome and showed that it was associated with significant myelosuppression [85]. Additional clinical trials of CDK inhibitors in HNSCC are ongoing and the results are awaited (NCT03024489, NCT04000529).
Other less frequent molecular alterations. The majority of HNSCCs show a genomic profile consistent with tobacco exposure or, alternatively, are characterized by detectable HPV DNA. Recently, different data have been published on the mutational landscape of HNSCCs showing frequent alterations in TP53, CDKN2A, PTEN, PIK3CA, and HRAS along with mutations in genes related to squamous differentiation as NOTCH1, IRF6, and TP63 [81].
Cancer Genome Atlas profiling on 279 HNSCC cases provided comprehensive genomic sequencing. In HPV-related tumors, PI3KCA, TRAF3, and E2F1 amplifications have been reported as the most frequent alterations, while smoking-related HNSCCs were characterized mostly by TP53, CCND1, and CDKN2A mutations [86]. In the same analysis, beyond these two subgroups that represent the majority of HNSCCs, other types of genomic profiles have been described, related to less prevalent SC that contained inactivating alterations of NSD1, AJUBA, and FAT1 genes (involved in WNT signaling). Distinct profiles were described for tumors arising from the oral cavity. Indeed, FAT1, CASP8, CDKN2A, and NOTCH1 mutations were found more frequently in these tumors compared with other HNCs malignancies and other squamous non-HNCs cancers. Another subgroup of tumors of the oral cavity, characterized by a more favorable prognosis, showed infrequent copy number alterations along with activating mutations of or PIK3 and HRAS and, less frequently, mutations of CASP8, NOTCH1, and TP53 [86,87].
6. Conclusions
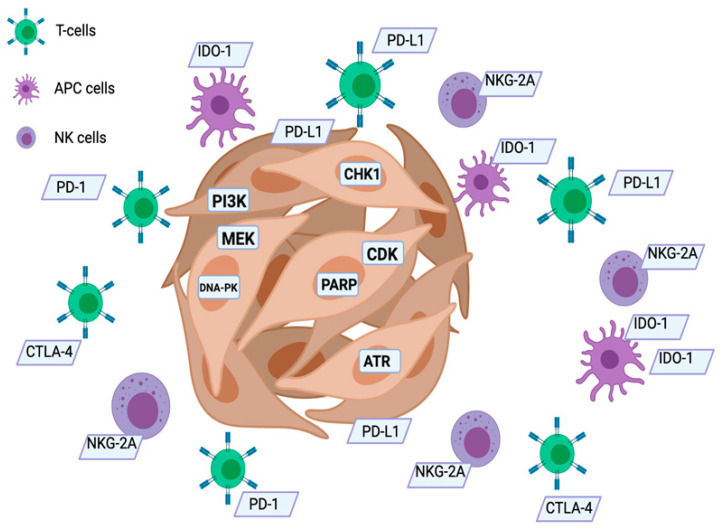
In conclusion, although new biomarkers-driven approaches and new clinical investigations are needed, possible changes in therapeutic scenario of HNSCC can be expected. A modern approach to cancer treatment should include molecular profiling of tumors that can lead to a more personalized approach (in Figure 1, you can see the possible targets that can be “hit” by the various drugs we have available). The therapeutic strategies employed, whether chemotherapy, targeted therapy, or immunotherapy, although effective, are, however, burdened by a still too high percentage of failures, and this is often not easily explained. The study of biomarkers predicting response to immunotherapy, as well as the study of the mutational status of HNSCC, or even the study of some predictive gene polymorphisms of poor or good response to some chemotherapeutic drugs (cisplatin, fluorouracil), can completely subvert the therapeutic scenario. In fact, the early identification of poor-responders as well as good-responders to the various treatments should be the achievable goal in the near future. New clinical investigations are needed to better predict the clinical relevance of tumor molecular alterations and the benefit of targeted therapy/immunotherapy. Table 1 shows the main drugs employed in HNSCC.
Figure 1.
New possible targets in HNSCC. APC: antigen presenting cells; NK: natural killer cells; PARP: poly (ADP-ribose) polymerase; IDO-1: indoleamine 2,3-dioxygenase; ATR: ataxia telangiectasia and Rad3-related protein.
Table 1.
Drugs employed in HNSCC.
| Drug | Category | Status |
|---|---|---|
| Cetuximab | Targeted Therapy (anti-EGFR) |
APPROVED |
| Panitumumab | Targeted Therapy (anti-EGFR) |
experimental |
| Losatuxizumab vedotin | Targeted Therapy (anti-EGFR) |
experimental |
| Erlotinib | Targeted Therapy (anti-EGFR) |
experimental |
| Gefitinib | Targeted Therapy (anti-EGFR) |
experimental |
| Afatinib | Targeted Therapy (anti-EGFR) |
experimental |
| Nivolumab | Immunotherapy (anti PD-1) |
APPROVED |
| Ipilimumab | Immunotherapy (anti CTLA-4) |
experimental |
| Pembrolizumab | Immunotherapy (anti PD-1) |
APPROVED |
| Atezolizumab | Immunotherapy (anti PD-L1) |
experimental |
| Monalizumab | Immunotherapy (anti-NKG2) |
experimental |
| Palbociclib | Targeted Therapy (anti-CDK 4/6) |
experimental |
| Trametinib | Targeted Therapy (anti-Mek) |
experimental |
| PARP-inhibitors | Targeted Therapy (anti-PARP) |
experimental |
| AZD6738 | Targeted Therapy (anti-ATR) |
experimental |
| Prexasertib | Targeted Therapy (anti-CHK1/2) |
experimental |
| Adavosertib | Targeted Therapy (anti-WEE1) |
experimental |
| Buparlisib | Targeted Therapy (anti-PI3K) |
experimental |
| Epacadostat | Immunotherapy (anti-IDO1) |
experimental |
| GSK3359609 | Immunotherapy (ICOS agonist) |
experimental |
Supplementary Materials
The Supplementary File are available online at https://www.mdpi.com/article/10.3390/biomedicines9081045/s1.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NccN Guidelines Head and Neck Carcinoma. Version 3.2021. [(accessed on 8 July 2021)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 2.Pentangelo G., Nisticò S., Provenzano E., Cisale G., Bennardo L. Topical 5% Imiquimod Sequential to Surgery for HPV-Related Squamous Cell Carcinoma of the Lip. Medicina. 2021;57:563. doi: 10.3390/medicina57060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennardo L., Bennardo F., Giudice A., Passante M., Dastoli S., Morrone P., Provenzano E., Patruno C., Nisticò S. Local Chemotherapy as an Adjuvant Treatment in Unresectable Squamous Cell Carcinoma: What Do We Know So Far? Curr. Oncol. 2021;28:2317–2325. doi: 10.3390/curroncol28040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ionna F., Bossi P., Guida A., Alberti A., Muto P., Salzano G., Ottaiano A., Maglitto F., Leopardo D., De Felice M., et al. Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: A Big and Intriguing Challenge Which May Be Resolved by Integrated Treatments Combining Locoregional and Systemic Therapies. Cancers. 2021;13:2371. doi: 10.3390/cancers13102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasano M., Della Corte C.M., Viscardi G., Di Liello R., Paragliola F., Sparano F., Iacovino M.L., Castrichino A., Doria F., Sica A., et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med Oncol. 2021;13 doi: 10.1177/1758835920949418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano M., Della Corte C.M., Di Liello R., Barra G., Sparano F., Viscardi G., Iacovino M.L., Paragliola F., Famiglietti V., Ciaramella V., et al. Induction of natural killer antibody-dependent cell cytotoxicity and of clinical activity of cetuximab plus avelumab in non-small cell lung cancer. ESMO Open. 2020;5:e000753. doi: 10.1136/esmoopen-2020-000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pockley A.G., Vaupel P., Multhoff G. NK cell-based therapeutics for lung cancer. Expert Opin. Biol. Ther. 2019;20:23–33. doi: 10.1080/14712598.2020.1688298. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., Erfan J., Zabolotnyy D., Kienzer H.-R., Cupissol D., et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 9.Vermorken J.B., Stöhlmacher-Williams J., Davidenko I., Licitra L., Winquist E., Villanueva C., Foa P., Rottey S., Skladowski K., Tahara M., et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 10.Machiels J.-P., Specenier P., Krauß J., Dietz A., Kaminsky M.-C., Lalami Y., Henke M., Keilholz U., Knecht R., Skartved N.J., et al. A proof of concept trial of the anti-EGFR antibody mixture Sym004 in patients with squamous cell carcinoma of the head and neck. Cancer Chemother. Pharmacol. 2015;76:13–20. doi: 10.1007/s00280-015-2761-4. [DOI] [PubMed] [Google Scholar]
- 11.Cleary J.M., Calvo E., Moreno V., Juric D., Shapiro G.I., Vanderwal C.A., Hu B., Gifford M., Barch D., Roberts-Rapp L., et al. A phase 1 study evaluating safety and pharmacokinetics of losatuxizumab vedotin (ABBV-221), an anti-EGFR antibody-drug conjugate carrying monomethyl auristatin E, in patients with solid tumors likely to overexpress EGFR. Investig. New Drugs. 2020;38:1483–1494. doi: 10.1007/s10637-020-00908-3. [DOI] [PubMed] [Google Scholar]
- 12.William W.N., Jr., Tsao A.S., Feng L., Ginsberg L.E., Lee J.J., Kies M.S., Glisson B.S., Kim E.S. Single Arm, Phase II Study of Cisplatin, Docetaxel, and Erlotinib in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinomas. Oncologist. 2018;23:526-e49. doi: 10.1634/theoncologist.2017-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan E.-H., Goh C., Lim W.T., Soo K.C., Khoo M.L., Tan T., Tan D.S.W., Ang M.K., Ng Q.S., Tan P.H., et al. Gefitinib, cisplatin, and concurrent radiotherapy for locally advanced head and neck cancer: EGFR FISH, protein expression, and mutational status are not predictive biomarkers. Ann. Oncol. 2012;23:1010–1016. doi: 10.1093/annonc/mdr327. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia A.K., Mehra R., Khan S.A., Egleston B.L., Alpaugh R.K., Lango M., Ridge J.A., Burtness B. Phase II trial of carboplatin/paclitaxel and cetuximab, followed by carboplatin/paclitaxel/cetuximab and erlotinib, in metastatic or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2016;34:6027. doi: 10.1200/JCO.2016.34.15_suppl.6027. [DOI] [Google Scholar]
- 15.Bergonzini C., Leonetti A., Tiseo M., Giovannetti E., Peters G.J. Is there a role for dacomitinib, a second-generation irreversible inhibitor of the epidermal-growth factor receptor tyrosine kinase, in advanced non-small cell lung cancer? Expert Opin. Pharmacother. 2020;21:1287–1298. doi: 10.1080/14656566.2020.1746269. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y., Ahn M.-J., Chan A., Wang C.-H., Kang J.-H., Kim S.-B., Bello M., Arora R., Zhang Q., He X., et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019;30:1831–1839. doi: 10.1093/annonc/mdz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mei Z., Huang J., Qiao B., Lam A.K.-Y. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020;12:1–9. doi: 10.1038/s41368-020-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobefaro R., Viscardi G., Di Liello R., Massa G., Iacovino M.L., Sparano F., Ferrara R., Signorelli D., Proto C., Prelaj A., et al. Efficacy and safety of immunotherapy in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2020;38:e21601. doi: 10.1200/JCO.2020.38.15_suppl.e21601. [DOI] [Google Scholar]
- 19.Zhao Y., Yang W., Huang Y., Cui R., Li X., Li B. Evolving Roles for Targeting CTLA-4 in Cancer Immunotherapy. Cell. Physiol. Biochem. 2018;47:721–734. doi: 10.1159/000490025. [DOI] [PubMed] [Google Scholar]
- 20.Shi L., Meng T., Zhao Z., Han J., Zhang W., Gao F., Cai J. CRISPR knock out CTLA-4 enhances the anti-tumor activity of cytotoxic T lymphocytes. Gene. 2017;636:36–41. doi: 10.1016/j.gene.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Schwab K.S., Kristiansen G., Schild H.H., Held S.E., Heine A., Brossart P. Successful Treatment of Refractory Squamous Cell Cancer of the Head and Neck with Nivolumab and Ipilimumab. Case Rep. Oncol. 2018;11:17–20. doi: 10.1159/000485562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Corte C.M., Barra G., Ciaramella V., Di Liello R., Vicidomini G., Zappavigna S., Luce A., Abate M., Fiorelli A., Caraglia M., et al. Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J. Exp. Clin. Cancer Res. 2019;38:1–12. doi: 10.1186/s13046-019-1257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter K.A., Socinski M.A., Villaruz L.C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol. Diagn. Ther. 2018;22:1–10. doi: 10.1007/s40291-017-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Liello R., Cimmino F., Simón S., Giunta E.F., de Falco V., Martín-Martorell P. Role of liquid biopsy for thoracic cancers immunotherapy. Explor. Target. Antitumor Ther. 2020;1:183–199. doi: 10.37349/etat.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burtness B., Harrington K., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 26.Solinas C., Aiello M., Rozali E., Lambertini M., Willard-Gallo K., Migliori E. Programmed cell death-ligand 2: A neglected but important target in the immune response to cancer? Transl. Oncol. 2020;13:100811. doi: 10.1016/j.tranon.2020.100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenizia F., Pasquale R., Roma C., Bergantino F., Iannaccone A., Normanno N. Measuring tumor mutation burden in non-small cell lung cancer: Tissue versus liquid biopsy. Transl. Lung Cancer Res. 2018;7:668–677. doi: 10.21037/tlcr.2018.09.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra R., Seiwert T.Y., Gupta S., Weiss J., Gluck I., Eder J.P., Burtness B., Tahara M., Keam B., Kang H., et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Li B., Peng Y., Wu F., Li Q., Lin Z., Xie S., Xiao L., Lin X., Ou Z., et al. The prognostic value of TMB and the relationship between TMB and immune infiltration in head and neck squamous cell carcinoma: A gene expression-based study. Oral Oncol. 2020;110:104943. doi: 10.1016/j.oraloncology.2020.104943. [DOI] [PubMed] [Google Scholar]
- 30.Ciardiello D., Vitiello P.P., Cardone C., Martini G., Troiani T., Martinelli E., Ciardiello F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019;76:22–32. doi: 10.1016/j.ctrv.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Cilona M., Locatello L.G., Novelli L., Gallo O. The Mismatch Repair System (MMR) in Head and Neck Carcinogenesis and Its Role in Modulating the Response to Immunotherapy: A Critical Review. Cancers. 2020;12:3006. doi: 10.3390/cancers12103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Ruiter E.J., Ooft M.L., Devriese L.A., Willems S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. OncoImmunology. 2017;6:e1356148. doi: 10.1080/2162402X.2017.1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang A.C., Postow M.A., Orlowski R.J., Mick R., Bengsch B., Manne S., Xu W., Harmon S., Giles J.R., Wenz B., et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spector M.E., Bellile E., Amlani L., Zarins K., Smith J., Brenner J.C., Rozek L., Nguyen A., Thomas D., McHugh J.B., et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Neck Surg. 2019;145:1012–1019. doi: 10.1001/jamaoto.2019.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S.-W., Li S.-H., Shi D.-B., Jiang W.-M., Song M., Yang A.-K., Li Y.-D., Bei J.-X., Chen W.-K., Zhang Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Markers. 2019;34:398–405. doi: 10.1177/1724600819884722. [DOI] [PubMed] [Google Scholar]
- 36.Bauml J.M., Aggarwal C., Cohen R.B. Immunotherapy for head and neck cancer: Where are we now and where are we going? Ann. Transl. Med. 2019;7:S75. doi: 10.21037/atm.2019.03.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yentz S., Smith D. Indoleamine 2,3-Dioxygenase (IDO) Inhibition as a Strategy to Augment Cancer Immunotherapy. BioDrugs. 2018;32:311–317. doi: 10.1007/s40259-018-0291-4. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell T.C., Hamid O., Smith D.C., Bauer T.M., Wasser J.S., Olszanski A., Luke J.J., Balmanoukian A.S., Schmidt E.V., Zhao Y., et al. Epacadostat Plus Pembrolizumab in Patients with Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037) J. Clin. Oncol. 2018;36:3223–3230. doi: 10.1200/JCO.2018.78.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplon H., Muralidharan M., Schneider Z., Reichert J.M. Antibodies to watch in 2020. mAbs. 2020;12:1703531. doi: 10.1080/19420862.2019.1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borel C., Jung A.C., Burgy M. Immunotherapy Breakthroughs in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Cancers. 2020;12:2691. doi: 10.3390/cancers12092691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mateo J., Chakravarty D., Dienstmann R., Jezdic S., Gonzalez-Perez A., Lopez-Bigas N., Ng C.K.Y., Bedard P., Tortora G., Douillard J.-Y., et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) Ann. Oncol. 2018;29:1895–1902. doi: 10.1093/annonc/mdy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilardi M., Wang Z., Proietto M., Chillà A., Calleja-Valera J.L., Goto Y., Vanoni M., Janes M.R., Mikulski Z., Gualberto A., et al. Tipifarnib as a Precision Therapy for HRAS-Mutant Head and Neck Squamous Cell Carcinomas. Mol. Cancer Ther. 2020;19:1784–1796. doi: 10.1158/1535-7163.MCT-19-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Deiry W.S., Goldberg R.M., Lenz H., Shields A.F., Gibney G.T., Tan A.R., Brown J., Eisenberg B., Heath E.I., Phuphanich S., et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA: A Cancer J. Clin. 2019;69:305–343. doi: 10.3322/caac.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin X., Liao J., Yang Z., Fan X., Cullen K.J., Chen L., Dan H. Inhibition of cisplatin-resistant head and neck squamous cell carcinoma by combination of Afatinib with PD0325901, a MEK inhibitor. Am J Cancer Res. 2019;9:1282–1292. [PMC free article] [PubMed] [Google Scholar]
- 45.Fang Z., Jung K.H., Lee J.E., Cho J., Lim J.H., Hong S.-S. MEK blockade overcomes the limited activity of palbociclib in head and neck cancer. Transl. Oncol. 2020;13:100833. doi: 10.1016/j.tranon.2020.100833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glorieux M., Dok R., Nuyts S. Novel DNA targeted therapies for head and neck cancers: Clinical potential and biomarkers. Oncotarget. 2017;8:81662–81678. doi: 10.18632/oncotarget.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verhagen C.V., de Haan R., Hageman F., Oostendorp T.P., Carli A.L., O’Connor M.J., Jonkers J., Verheij M., Brekel M.W.V.D., Vens C. Extent of radiosensitization by the PARP inhibitor olaparib depends on its dose, the radiation dose and the integrity of the homologous recombination pathway of tumor cells. Radiother. Oncol. 2015;116:358–365. doi: 10.1016/j.radonc.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H., Zhang Z., Borczuk A., Powell C., Balajee A.S., Lieberman H.B., Halmos B. PARP inhibition selectively increases sensitivity to cisplatin in ERCC1-low non-small cell lung cancer cells. Carcinog. 2012;34:739–749. doi: 10.1093/carcin/bgs393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacheco J.M., Byers L.A. Temozolomide plus PARP Inhibition in Small-Cell Lung Cancer: Could Patient-Derived Xenografts Accelerate Discovery of Biomarker Candidates? Cancer Discov. 2019;9:1340–1342. doi: 10.1158/2159-8290.CD-19-0850. [DOI] [PubMed] [Google Scholar]
- 51.Morgan M.A., Lawrence T.S. Molecular Pathways: Overcoming Radiation Resistance by Targeting DNA Damage Response Pathways. Clin. Cancer Res. 2015;21:2898–2904. doi: 10.1158/1078-0432.CCR-13-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown J.S., O’Carrigan B., Jackson S.P., Yap T.A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2016;7:20–37. doi: 10.1158/2159-8290.CD-16-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson D., Amrein L., Panasci L., Aloyz R. Small Molecules, Inhibitors of DNA-PK, Targeting DNA Repair, and Beyond. Front. Pharmacol. 2013;4:5. doi: 10.3389/fphar.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helleday T., Petermann E., Lundin C., Hodgson B., Sharma R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 55.Weaver A.N., Cooper T.S., Rodriguez M., Trummell H.Q., Bonner J.A., Rosenthal E.L., Yang E.S. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget. 2015;6:26995–27007. doi: 10.18632/oncotarget.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu L., Shang Z.-F., Hsu F.-M., Zhang Z., Tumati V., Lin Y.-F., Chen B.P.C., Saha D. NSCLC cells demonstrate differential mode of cell death in response to the combined treatment of radiation and a DNA-PKcs inhibitor. Oncotarget. 2015;6:3848–3860. doi: 10.18632/oncotarget.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y., Thomas H.D., Batey M.A., Cowell I., Richardson C.J., Griffin R.J., Calvert A.H., Newell D.R., Smith G.C., Curtin N. Preclinical Evaluation of a Potent Novel DNA-Dependent Protein Kinase Inhibitor NU7441. Cancer Res. 2006;66:5354–5362. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- 58.Nowsheen S., Bonner J.A., LoBuglio A.F., Trummell H., Whitley A.C., Dobelbower M.C., Yang E.S. Cetuximab Augments Cytotoxicity with Poly (ADP-Ribose) Polymerase Inhibition in Head and Neck Cancer. PLOS ONE. 2011;6:e24148. doi: 10.1371/journal.pone.0024148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menolfi D., Zha S. ATM, ATR and DNA-PKcs kinases—The lessons from the mouse models: Inhibition≠ deletion. Cell Biosci. 2020;10:1–15. doi: 10.1186/s13578-020-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aung K.L., Siu L.L. Genomically personalized therapy in head and neck cancer. Cancers Head Neck. 2016;1:1–10. doi: 10.1186/s41199-016-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto V.N., Thylur D., Bauschard M., Schmale I., Sinha U.K. Overcoming radioresistance in head and neck squamous cell carcinoma. Oral Oncol. 2016;63:44–51. doi: 10.1016/j.oraloncology.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Dok R., Glorieux M., Bamps M., Nuyts S. Effect of ATR Inhibition in RT Response of HPV-Negative and HPV-Positive Head and Neck Cancers. Int. J. Mol. Sci. 2021;22:1504. doi: 10.3390/ijms22041504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ou Y.-H., Chung P.-H., Sun T.-P., Shieh S.-Y. p53 C-Terminal Phosphorylation by CHK1 and CHK2 Participates in the Regulation of DNA-Damage-induced C-Terminal Acetylation. Mol. Biol. Cell. 2005;16:1684–1695. doi: 10.1091/mbc.e04-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai Y., Grant S. New Insights into Checkpoint Kinase 1 in the DNA Damage Response Signaling Network. Clin. Cancer Res. 2010;16:376–383. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busch C.-J., Kröger M.S., Jensen J., Kriegs M., Gatzemeier F., Petersen C., Muenscher A., Rothkamm K., Rieckmann T. G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of Chk1 and Wee1. Radiother. Oncol. 2017;122:260–266. doi: 10.1016/j.radonc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 66.Zeng L., Nikolaev A., Xing C., Della Manna D.L., Yang E.S. CHK1/2 Inhibitor Prexasertib Suppresses NOTCH Signaling and Enhances Cytotoxicity of Cisplatin and Radiation in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2020;19:1279–1288. doi: 10.1158/1535-7163.MCT-19-0946. [DOI] [PubMed] [Google Scholar]
- 67.O’Connor M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 68.Kao M., Green C., Sidorova J., Méndez E. Strategies for Targeted Therapy in Head and Neck Squamous Cell Carcinoma Using WEE1 Inhibitor AZD1775. JAMA Otolaryngol. Neck Surg. 2017;143:631–633. doi: 10.1001/jamaoto.2016.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Méndez E., Rodriguez C.P., Kao M.C., Raju S.C., Diab A., Harbison R.A., Konnick E.Q., Mugundu G.M., Santana-Davila R., Martins R., et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2018;24:2740–2748. doi: 10.1158/1078-0432.CCR-17-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai Y., Dodhia S., Su G. Dysregulations in the PI3K pathway and targeted therapies for head and neck squamous cell carcinoma. Oncotarget. 2017;8:22203–22217. doi: 10.18632/oncotarget.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bozec A., Ebran N., Radosevic-Robin N., Sudaka A., Monteverde M., Toussan N., Etienne-Grimaldi M.-C., Nigro C.L., Merlano M., Penault-Llorca F., et al. Combination of mTOR and EGFR targeting in an orthotopic xenograft model of head and neck cancer. Laryngoscope. 2015;126:E156–E163. doi: 10.1002/lary.25754. [DOI] [PubMed] [Google Scholar]
- 72.Simpson D.R., Mell L.K., Cohen E.E. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015;51:291–298. doi: 10.1016/j.oraloncology.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Josephs D.H., Sarker D. Pharmacodynamic Biomarker Development for PI3K Pathway Therapeutics. Transl. Oncogenom. 2015;7(Suppl. 1):33–49. doi: 10.4137/TOG.S30529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sambandam V., Frederick M.J., Shen L., Tong P., Rao X., Peng S., Singh R., Mazumdar T., Huang C., Li Q., et al. PDK1 Mediates NOTCH1-Mutated Head and Neck Squamous Carcinoma Vulnerability to Therapeutic PI3K/mTOR Inhibition. Clin. Cancer Res. 2019;25:3329–3340. doi: 10.1158/1078-0432.CCR-18-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Estévez L.G., García E., Hidalgo M. Inhibiting the PI3K signaling pathway: Buparlisib as a new targeted option in breast carcinoma. Clin. Transl. Oncol. 2015;18:541–549. doi: 10.1007/s12094-015-1410-z. [DOI] [PubMed] [Google Scholar]
- 76.Fruman D.A., Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soulières D., Faivre S., Mesía R., Remenár É., Li S.-H., Karpenko A., Dechaphunkul A., Ochsenreither S., Kiss L.A., Lin J.-C., et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18:323–335. doi: 10.1016/S1470-2045(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 78.Serzan M.T., Farid S., Liu S.V. Drugs in development for small cell lung cancer. J. Thorac. Dis. 2020;12:6298–6307. doi: 10.21037/jtd-2019-sclc-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spring L.M., Wander S.A., Zangardi M., Bardia A. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr. Oncol. Rep. 2019;21:1–9. doi: 10.1007/s11912-019-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Leary B., Finn R.S., Turner B.O.N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 81.Riess C., Irmscher N., Salewski I., Strüder D., Classen C.-F., Große-Thie C., Junghanss C., Maletzki C. Cyclin-dependent kinase inhibitors in head and neck cancer and glioblastoma—backbone or add-on in immune-oncology? Cancer Metastasis Rev. 2021;40:153–171. doi: 10.1007/s10555-020-09940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X., Pan Y., Yan M., Bao G., Sun X. Identification of potential crucial genes and molecular mechanisms in glioblastoma multiforme by bioinformatics analysis. Mol. Med. Rep. 2020;22:859–869. doi: 10.3892/mmr.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michel L., Ley J., Wildes T.M., Schaffer A., Robinson A., Chun S.-E., Lee W., Lewis J., Trinkaus K., Adkins D. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016;58:41–48. doi: 10.1016/j.oraloncology.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adkins D., Ley J., Neupane P., Worden F., Sacco A.G., Palka K., E Grilley-Olson J., Maggiore R., Salama N., Trinkaus K., et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: A multicentre, multigroup, phase 2 trial. Lancet Oncol. 2019;20:1295–1305. doi: 10.1016/S1470-2045(19)30405-X. [DOI] [PubMed] [Google Scholar]
- 85.Swiecicki P.L., Durm G., Bellile E., Bhangale A., Brenner J.C., Worden F.P. A multi-center phase II trial evaluating the efficacy of palbociclib in combination with carboplatin for the treatment of unresectable recurrent or metastatic head and neck squamous cell carcinoma. Investig. New Drugs. 2020;38:1550–1558. doi: 10.1007/s10637-020-00898-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chai A.W.Y., Lim K.P., Cheong S.C. Translational genomics and recent advances in oral squamous cell carcinoma. Semin. Cancer Biol. 2020;61:71–83. doi: 10.1016/j.semcancer.2019.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.