Abstract
Mitochondria are essential organelles in physiology and kidney diseases, because they produce cellular energy required to perform their function. During mitochondrial metabolism, reactive oxygen species (ROS) are produced. ROS function as secondary messengers, inducing redox-sensitive post-translational modifications (PTM) in proteins and activating or deactivating different cell signaling pathways. However, in kidney diseases, ROS overproduction causes oxidative stress (OS), inducing mitochondrial dysfunction and altering its metabolism and dynamics. The latter processes are closely related to changes in the cell redox-sensitive signaling pathways, causing inflammation and apoptosis cell death. Although mitochondrial metabolism, ROS production, and OS have been studied in kidney diseases, the role of redox signaling pathways in mitochondria has not been addressed. This review focuses on altering the metabolism and dynamics of mitochondria through the dysregulation of redox-sensitive signaling pathways in kidney diseases.
Keywords: acute kidney injury (AKI), chronic kidney disease (CKD), tricarboxylic acid (TCA) cycle, mitochondrial metabolism, mitochondrial redox signaling, mitochondrial proteins, oxidative phosphorylation (OXPHOS), fatty acid (FA) β-oxidation, mitochondrial dynamics, biogenesis, mitophagy
1. Introduction
Kidney diseases are a severe health problem that causes high economic costs worldwide in medical attention, emergency, therapies, among others [1,2]. These are divided into acute kidney injury (AKI) and chronic kidney diseases (CKD). AKI encompasses a set of pathologies characterized by the rapid loss of renal function in a short period [3]. AKI is often caused by the use of chemotherapeutics agents such as cisplatin, episodes of renal ischemia/reperfusion (I/R), and exposure to contaminants [4]. AKI is associated with high morbidity and mortality, contributing to CKD development and affecting approximately between 7% and 12% of the world [5]. CKD cause renal fibrosis development [6,7,8]. The latter comprises an unsatisfactory repair process and is the consequence of severe and persistent damage that does not restore organ function [9]. Renal fibrosis, in turn, is one of the principal mechanisms involved in AKI to CKD transition [5].
Mitochondria are responsible for several cell functions such as cell growth, cell survival, and apoptosis induction, playing a significant role in kidney physiology and the development of kidney diseases. Mitochondria also coordinate the biosynthesis of lipids, amino acids, and nucleotides and bioenergetics processes such as tricarboxylic acid (TCA) cycles, electron transport systems (ETSs), and fatty acids (FA) β-oxidation [10]. During these processes, reactive oxygen species (ROS) are produced. Low levels of ROS are needed to regulate cellular signaling, but an excess of ROS induces oxidative stress (OS). OS causes oxidative damage in organelles including mitochondria and biomolecules, such as proteins, lipids, and deoxyribonucleic acid (DNA), which may be conducive to cell death. Indeed, OS is associated with AKI development and its transition to CKD, where mitochondria dysfunction is the principal characteristic of both [11,12]. Although mitochondrial metabolism, ROS production, and OS have been studied in kidney diseases, the role of redox signaling pathways in renal mitochondria impairment is not well understood. This review focuses on altering the metabolism and dynamics of mitochondria through the dysregulation of redox-sensitive signaling pathways in kidney diseases.
2. Redox-Sensitive Signaling in Kidney Diseases
ROS at low levels act as secondary messengers, activating signaling pathways and cellular enzymes and regulating several cellular processes such as cell proliferation, survival, and growth [13]. Among the plethora of ROS, hydrogen peroxide (H2O2) and nitric oxide (•NO) are the central redox signaling agents [13,14,15,16]. These ROS induce oxidative post-translational modifications (Ox-PTMs) in proteins that contain redox-sensitive amino acid residues, regulating their structure, localization, and function [17,18]. These redox-sensitive amino acids are arginine (Arg), cysteine (Cys), histidine (His), lysine (Lys), methionine (Met), proline (Pro), threonine (Thr), and tyrosine (Tyr) [19,20]. However, Cys and Met are most prone to be attacked by ROS. These amino acids contain oxidizable sulfur groups on the side chains, of which the oxidation states depend on the redox microenvironment changes [13]. Thus, the protein function is regulated by the oxidate states of these amino acids [21,22].
Cys residues perform structural functions, such as the assembly of iron-sulfur (Fe-S) groups, heme prosthetic groups, and zinc finger motifs and are essential in the active sites of enzymes. The sulfur in Cys is a large, polarizable, electron-rich atom. These characteristics give it high reactivity and the ability to adopt multiple oxidation states. In addition, pKa influences the formation of the nucleophilic thiolate anion (S−). Because the surrounding milieu influences pKa, the thiol (SH) protonation form of Cys depends on the cellular microenvironment. For example, at physiological pH (pH 7.3), the pKa of Cys is 8.3, which means that Cys is in a protonated and less reactive state [23]. However, if the cellular microenvironment becomes alkaline (pH < 7.8), Cys can adopt a deprotonated state S−. Note that positively charged amino acids adjacent to Cys can substantially decrease the pKa of SH. Furthermore, the presence of other contiguous positive charges, such as the positive partial charge of a dipole, can also reduce the pKa of Cys, making them more reactive [24]. On the other hand, since Cys oxidation reactions are predominantly bimolecular nucleophilic substitution (SN2) reactions in the protonated form, steric hindrance can prevent Cys oxidation [25]. In this way, in the steric hindrance, the surrounding amino acids and redox microenvironment will determine whether a Cys will be reactive to undergo redox modification, dictating selectivity for SH modification. Therefore, there is a wide range of Ox-PTMs that also depend on the present electrophiles. For example, H2O2 oxidizes SH, with an oxidate state of −2, in an oxidative microenvironment, producing sulfenic acid (R–SOH) with an oxidate state of 0 [26]. R-SOH can react with proximal SH groups to form disulfide bonds (S–S), with a −1 oxidate state or s-glutathionylation (R-SSG) by reacting with glutathione (GSH) (Figure 1) [27,28]. This reaction is reversed by glutaredoxin (Grx). However, under OS, R-SOH forms sulfinic acid (R–SO2H) with a +2 oxidate state or sulfonic acid (R–SO3H) with a +4 oxidate state. The latter is not enzymatically reversible [29]. R–SOH can also be condensed with another R–SOH to form thiosulfinate [R–S(O)–S–R’]. R–S(O)–S–R’, in turn, reacts with amine or amide groups to form sulfenamide (R–SN–R’) [30]. Additionally, Cys can suffer from other modifications such as S-nitrosylation and persulfonation. Regarding S-nitrosylation, it comprises the •NO covalent link to the thyil radical (R–S•) to form S-nitrosothiol (R–SNO), [31] and in persulfonation, the hydrogen sulfide (H2S) reacts with R–S• to form persulfide (R–S–SH) [20].
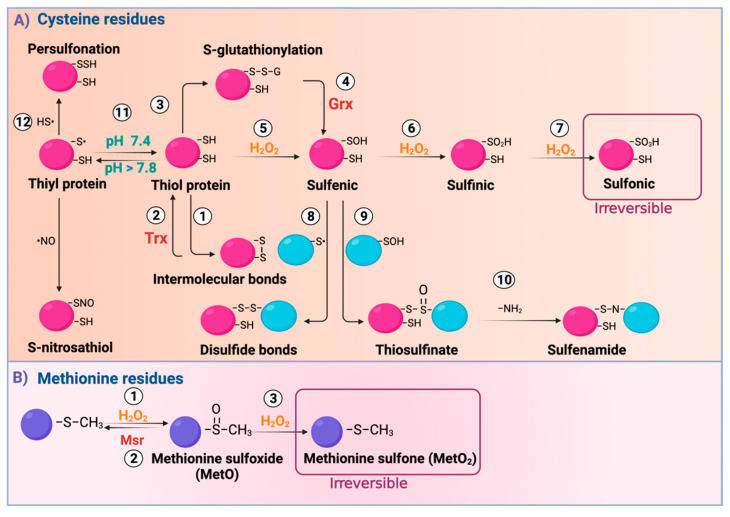
Figure 1.
Reactive oxygen species (ROS) induce modifications in cysteine (Cys) and methionine (Met) residues. (A) ROS oxidize Cys residues in its thiol form, named thiol (SH) protein, forming (1) intermolecular bonds, (2) reversed by thioredoxin (Trx), or (3) S-glutathionylation (R–SSG). R–SSG can be reversed by (4) the glutaredoxin (Grx) enzyme, acquiring a sulfenic (R–SOH) form. Moreover, (5) R–SOH is formed through oxidation by H2O2 from SH. If oxidation continues, R–SOH forms (6) sulfinic (R–SO2H) or (7) sulfonic (R–SO3H); the last is irreversible. Furthermore, R–SOH can condense with another R-SOH to form (8) disulfide bonds (R–S–S–R’) or (9) thiosulfinate [R–S(O)–S-R]. The latter can react with amide groups (–NH2), forming (10) sulfenamide (R–SN–R′). At the alkaline cellular microenvironment (pH > 7.8), R-SH proteins are deprotonated, forming (11) thiyl proteins (–S•). The latter can react with hydrogen sulfide (H2S) to form (12) persulfide (RSSH) by persulfination (R–S–S–H). –S• can also react with nitrosothiol (•NO), forming S-nitrosothiol (RSNO). (B) Met residues are oxidized to form (1) methionine sulfoxide [MetO (R–SOCH3)], reversed by (2) methionine sulfoxide reductase (Msr). However, if oxidation persists, MetO is further oxidized by H2O2 to (3) methionine sulfone [MetO2 (RSO2CH3)], which is irreversible. H2O2: hydrogen peroxide; HS•: hydrosulfide radical. Created with BioRender.com.
On the other hand, Met residues are oxidated by H2O2, generating methionine sulfoxide [MetO (R–SOCH3)] [32]. This oxidation is reversed by methionine sulfoxide reductase (Msr). However, if OS persists, MetO is transformed into methionine sulfone [MetO2 (RSO2CH3)], an enzymatically irreversible product [33].
The alkalinization of the mitochondrial matrix is due to pumping protons from the matrix into the intermembrane space (IMS). It has a tremendous impact on the potential reactivity of Cys, because alkalinization induces Cys protein residues to exist in an ionized state, which gives them a higher reactivity for ROS oxidation. Thus, mitochondria harbor a unique environment that promotes Cys modification.
As mentioned, there are Ox-PTMs classified as enzymatically reversible and irreversible. Ox-PTMs are reversible by thioredoxin (Trx), peroxiredoxin (Prx), Grx, glutathione peroxidase (GPx), Msr isoform A (MsrA), or the GSH [34,35]. Since reversible Ox-PTMs have enzymatic systems that remove oxidations, they are considered part of cellular signaling processes [34]. In contrast, irreversible ROS modifications are not considered part of cell signaling, because they do not have enzymatic reduction mechanisms [18].
Regarding cysteine persulfidation, it is a reversible Ox-PTM modification of SH to RSSH, which can be formed by reacting with H2S, more precisely HS−, and oxidized protein thiols, reaction between inorganic polysulfides and protein thiolates, and radical reaction by other reactive sulfur species [24]. Persulfidation regulates fine tune protein function, localization and interaction in cells [36]. It also modulates biological processes, including autophagy, cellular metabolism, inflammation, cell cycle, and cell death under physiological and pathological contexts [37]. It has been shown that the Cys’ architecture and spatial arrangement determine if the residue can be persulfonated and the effect on protein function. Allosteric impediments protect Cys residues from being oxidized under SOH, preventing permanent damage and preserving protein function. Likewise, in a reduced environment, this Ox-PTM is reversed [38]. Additionally, persulfonation depends on ROS levels in the cells. The latter is supported, because the transient ROS increase might augment oxidized Cys forms (SOH and S–S), highly reactive to H2S [39]. Thus, the reversibility of this Ox-PTM relies on Cys residues and the cellular microenvironment.
Ox-PTMs inactivate numerous proteins that contain Cys groups, such as protein tyrosine phosphatases (PTPs). The inactivation of PTPs prevents the deactivation of protein tyrosine kinases (PTKs), maintaining the activation of cell signaling pathways, such as mitogen-activated protein kinases (MAPK), promoting cell proliferation [40]. In addition, ROS such as H2O2 have also been shown to promote Cys oxidation and the formation of S–S in proteins such as growth factor receptors (GFRs), inducing their activation and cell growth. Since oO-PTMs regulate cellular functions involving cell proliferation, growth, migration, differentiation, and death, the concentration of ROS must be balanced to maintain cell homeostasis and prevent kidney diseases [13].
In kidney physiology, 25% of mitochondrial Cys in proteins can suffer nitrosylation, and around 70% of these proteins depend on the activity of endothelial nitric oxide synthase (eNOS) [41]. These nitrosylations are protective in the kidneys. For instance, Zhou et al. [42] reported that in I/R, the denitrosylase enzyme aldo-keto reductase family 1 member A1 (AKR1A1) is associate with kidney damage. In contrast, its deletion is protective in this organ. The authors also found that S-nitroso-coenzyme A (CoA) reductase induces pyruvate kinase isoform M2 (PKM2) nitrosylation, inhibiting its activity. This nitrosylation favors the pentose phosphatase pathway instead of glycolysis. The latter reduces equivalents increase, attributed to ROS detoxification, which has a protector effect during I/R [42]. S-glutathionylation is also a protective Ox-PTM in renal mitochondria [43]. In the kidneys, the reduction of mitochondrial protein S-glutathionylation induced by folic acid is associated with acute kidney damage [44]. The latter has been demonstrated, because of glutathionylated proteins decrease and the levels of GSH and the activity of the enzymes involved in S-glutathionylation in the mitochondria 24 h (h) after treatment with folic acid [44]. In the cisplatin-induced AKI model, the reduction of Met is a protective Ox-PTM, since MsrA deficiency exacerbates cisplatin-induced damage by increasing mitochondrial susceptibility [45]. Furthermore, mice MsrA−/− are more sensible to kidney injury induced by I/R [46]. The deficiency of MsrA, cystathionine-β-synthase (CBS), and cystathionine-γ-lyase (CSE), which are enzymes involved in the transsulfuration pathway, decreases homocysteine and H2S [46]. Low levels of H2S have also been reported after unilateral ureteral obstruction (UUO) due to CBS and CSE decrease, inducing superoxide anion radical (O2•−) and H2O2 production. The latter promotes the augment of oxidative damage markers such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) [47]. The MsrA deficiency in this model contributes to fibrosis development by increasing collagen deposition and augmenting fibrosis markers [48]. Therefore, MsrA regulates the Met metabolism and the production of H2S in the kidney [49].
Indeed, H2S regulates several kidney biological processes. CSE and CBS are expressed in the kidney, producing H2S that controls sodium reabsorption and glomerular filtration [50]. In addition, CSE is commonly expressed in endothelial and mesangial cells and podocytes [51]. In kidney diseases, H2S ameliorates renal damage [52]. It has been shown that H2S attenuates kidney injury during I/R and diabetic kidney disease (DKD). In animal models of I/R, H2S donors administration before or after I/R ameliorates renal damage by decreasing OS, inflammation, and apoptosis [53]. Furthermore, in patients, it has been found that CSE messenger RNA (mRNA) expression positively correlates with glomerular filtration rate recovery after two weeks of kidney transplantation [54], suggesting a protective role of H2S. Supporting the latter, CBS and CSE levels and H2S production are low in animal models suffering I/R [53]. Regarding DKD, patients with type 2 diabetes show lowed H2S plasma levels than non-diabetic people [55]. In UUO, the low production of H2S is also due to the fact that CBS levels are decreased [56]. In this model, H2S decrease is related to fibrosis and inflammation development. Following this, in vitro, sodium hydrosulfide (NaHS) treatment reduces fibrosis and inflammation by inhibiting transforming growth factor-beta 1 (TGFβ1) [56]. Together, these data support the relevance of H2S signaling in kidney injury.
Cys persulfidation is an essential process in kidney physiology and disease, protecting against kidney injury [24]. Persulfidation in Cys 105 of mitochondrial glyceraldehyde 3-phosphate dehydrogenase (GAPDH) elevates its enzymatic activity [57]. In contrast, the persulfidation in Cys 156 or Cys 152 induces enzymatic deactivation [58]. Moreover, the persulfidation in Keap 1 Cys 150 promotes nuclear factor erythroid 2–related factor 2 (Nrf2) release and translocation to the nucleus, activating its genes targets [59]. This mechanism is critical for regulating redox homeostasis in the kidney. In renal pathologies, Nrf2 is deactivated, leading to OS increase [60,61]. In the kidney, persulfides regulate blood pressure and sodium reabsorption. In line with this, in tubular epithelial cells, the persulfidation of epidermal growth factor receptor (EGFR) (Cys 797 and 798) results in the loss of function of sodium/potassium-adenosine triphosphatase (Na+/K+-ATPase) [62]. Cys persulfide is produced by CSE and CSB, which are depleted in kidney diseases. However, a previous study showed that mice lacking CSE and CBS have significant levels of Cys persulfide proteins, which suggested the possibility of alternative processes [63,64,65]. Indeed, cysteinyl-transfer RNA (tRNA) synthetases (CARSs) act as cysteine persulfide synthases in vivo [66]. Moreover, CARSs are also involved in the regulation of mitochondrial bioenergetics (oxygen consumption and membrane potential) and dynamics (dynamin-related protein 1 (Drp1)) [66]. Thus, this mechanism may be active in kidney diseases, which deserves investigation. The latter might suggest therapeutic targeting OS-induced mitochondrial dysfunction in renal pathologies.
3. Ox-PTMs Regulate Manganese Superoxide Dismutase (Mn-SOD) in Kidney Injury
Mn-SOD is in the mitochondrial matrix, while copper/zinc-SOD (Cu/Zn-SOD) is in the space of the inner mitochondrial membrane (IMM) and IMS. These two enzymes catalyze the dismutation of O2•− to H2O2 in the mitochondrial matrix and IMS, respectively [67]. This dismutation is crucial to avoid the O2•−-induced ferric iron (Fe3+) to ferrous iron (Fe2+) reduction in Fe-S clusters of critical enzymes such as aconitase (Acn). The latter leads to the release of Fe2+ and the inactivation of these enzymes [68]. During Fenton/Haber–Weiss reaction, Fe2+ reacts with H2O2 to produce a highly reactive ROS, hydroxyl radical (•OH), so the regulation of O2•− is essential to maintain mitochondrial homeostasis. Moreover, O2•− overproduction, directly and indirectly, leads to the inactivation of Mn-SOD, promoting mitochondrial dysfunction. For instance, O2•− can react with •NO to produce peroxynitrite (ONOO−), and ONOO− can induce Mn-SOD deactivation via nitration of Tyr34 residue in its active site [69].
Mn-SOD can also be S-glutathionylated in Cys 196, avoiding irreversible oxidation of SH. Renal I/R injury induces O2•− and ONOO− production [70], increasing nitration levels of mitochondrial proteins such as Mn-SOD and cytochrome c (cyt c), inactivating them and inducing OS and mitochondrial dysfunction [71,72,73,74]. Likewise, in folic-acid-induced renal damage, Mn-SOD activity reduction in isolated mitochondria is related to decreased mitochondrial S-glutathionylation [44], making it more susceptible to nitration. Moreover, in human renal transplants and experimental rat models of chronic renal nephropathy, there are elevated levels of ONOO− [72].
In DKD, mitochondrial OS reduces Mn-SOD enzyme activity due to Tyr nitration of this enzyme. Interestingly, the use of resveratrol, a potent antioxidant, reduces OS and Tyr nitration of Mn-SOD, preserving Mn-SOD activity. Moreover, kidneys of mice treated with streptozotocin to induced diabetic nephropathy (DN) show nitration in Mn-SOD Tyr 34, which results in a decrease of Mn-SOD. However, antagonists of thromboxane A2 receptors reduce diabetes-induced renal injury, which is associated with Mn-SOS Tyr nitration reduction [75].
In models of hypertension-related kidney injury, where hypertension is induced by angiotensin II (Ang II), the production of O2•− is promoted through the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) [76]. In these models, Mn-SOD activity deactivation is also associated with Tyr nitration, inducing OS [77]. Moreover, spontaneously hypertensive rats treated with N(G)-nitro-L-arginine-methyl ester (L-NAME), a potent nitric oxide synthase (NOS) inhibitor, reduce nitration of Mn-SOD, preserving its activity [78].
OS closely regulates aging-related kidney dysfunction, primarily by mitochondrial ROS (mtROS). A protein closely associated with aging is Klotho. This protein induces the activation of transcription factors such as forkhead box O (FoxO) that cause the expression of antioxidant enzymes such as Mn-SOD. Interestingly, Klotho−/− mouse models show high nitrotyrosine levels in Mn-SOD [79].
4. Crosstalk between NOXs and Mitochondria in Kidney Diseases
Mitochondria and NOXs are the primary ROS sources in the kidney. The NOXs family consists of seven isoforms, being NOX1, NOX2, NOX4, and NOX5, the most expressed in renal cells [80]. The O2•− production of NOX1 and NOX2 needs to assemble membrane subunit p22-phox and the cytosolic subunits p47- and p67-phox and ras-related C3 botulinum toxin substrate 1 (Rac1), while NOX4, abundantly expressed in mitochondrial membranes of renal cells, does not require cytosolic subunits and produces H2O2 [81,82,83].
The levels of NOXs augment in several AKI and CKD models, inducing ROS overproduction [84]. In the folic acid model, NOXs-linked ROS overproduction (in the kidney cortex, proximal tubules (PT), and distal tubules (DT)) is related to mtROS enhancement, because these two sources establish a pathological circle of ROS production, favoring AKI to CKD transition [44]. In the 5/6 nephrectomy model, mtROS also induce NOXs activation, increasing inflammation and fibrosis. Moreover, this mechanism contributes to fibrosis development in UUO [85]. Following the latter, several authors have established that in kidney pathologies, mtROS and NOXs-produced ROS increase mitochondrial damage and mitochondrial membrane potential depolarization (↓ΔΨm) (Figure 2) [86,87]. Ang II with the angiotensin type 1 receptor (ATR-1) also participates in the crosstalk between NOXs (NOX2 and NOX4) and mitochondria [88]. In addition to Ang II, in DN, the interaction of advanced glycation end products (AGE), produced by high glucose levels, activates NOXs by AGE receptor (RAGE) to generate ROS production [89]. Protein kinase C (PKC) epsilon (PKC-ε) also activates NOXs by inducing the phosphorylation of p47-phox, triggering ROS production [90]. NOXs-induced ROS cause the opening of a mitochondrial adenosine triphosphate (ATP)-sensitive potassium (K) channel (mt-KATP), triggering ↓ΔΨm [91]. In AKI and CKD models, the use of the NOXs inhibitor, apocynin, decreases mtROS production [92,93], supporting the idea that crosstalk between NOXs and mitochondria is involved.
Figure 2.
Crosstalk between nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and mitochondria in the kidney. Angiotensin II (Ang II) binds to the angiotensin type 1 receptor (ATR-1), which activates NOX2 and NOX4. In addition, the binding of advanced glycation end products (AGEs) to the receptor for advanced glycation end products (RAGE) induces the activation of NOX2. Furthermore, protein kinase C (PKC) epsilon (PKC-ε), activated by mitochondrial ROS (mtROS), activates NOX2 through the p47-phox subunit, inducing ROS production. NOXs-induced ROS promote the phosphorylation and opening of an mitochondrial adenosine triphosphate (ATP)-sensitive potassium K channel (mt-KATP), decreasing mitochondrial membrane potential depolarization (↓ΔΨm). ROS and mtROS activate the redox signaling pathways: transforming growth factor-beta 1 (TGFβ1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). mtROS also affect mitochondrial function by inducing damage to mitochondrial deoxyribonucleic acid (DNA) (mtDNA) and phospholipids that, in the last instance, generates mitochondrial dysfunction. The mtROS also favor the mitochondrial permeability transition pore (MPTP) opening, inducing the release of proapoptotic factors into the cytosol. p67-phox: subunit from NOX2; p22-phox: subunit from NOX2 and NOX4; Rac1: Ras-related C3 botulinum toxin substrate 1; ETS: electron transport system; CI: complex I; CII: complex II; CIII: complex III; CIV: complex IV; CV: complex V. Created with BioRender.com.
The binding of Ang II and AGEs to their receptors activates TGFβ1 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) redox-sensitive signaling pathways (Figure 2) [94]. Furthermore, mtROS can stimulate TGFβ1 through the upregulation of Smad 2/3, inducing its nuclear translocation. The latter is supported by the fact that the mitochondrial-targeting antioxidants coenzyme Q (mitoQ) and 2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO) prevents the activation of TGFβ1 along with the transcription of TGFβ1 genes [95]. mtROS also activate NF-κB by inducing monocyte/macrophage infiltration, increasing interstitial inflammation in UUO kidneys, and treating the antioxidant curcumin to decrease them and preventing interstitial inflammation [96]. mtROS also activate NF-κB in macrophages. In this sense, Herb et al. [97] demonstrated that in macrophages, mtROS, and not ROS produced by NOX2, activate NF-κB by deactivating the regulatory subunit of inhibitor IKK complex (IKKγ) through the disulfide linkage formation. However, in kidney diseases, this mechanism has not been investigated.
TGFβ1 and NF-κB are also localized in mitochondria, regulating mitochondrial proteins [98]. Moreover, the mitochondrial localization of both proteins might indicate that inflammation and fibrosis processes are regulated by mtROS production. Following the latter, in DN, hyperglycemia triggers the Smad4 translocation into mitochondria. This translocation reduces oxidative phosphorylation (OXPHOS), inducing inflammation, fibrosis, and podocyte injury [98].
The production of mtROS and ROS produced by NOXs also damages phospholipids and mitochondrial DNA (mtDNA). mtDNA is particularly susceptible to ROS, because it does not contain histones to protect it, causing DNA integrity loss and resulting in the acquisition of mutations [99]. mtROS also favor the opening of mitochondrial permeability transition pores (MPTPs) into the cytosol [86]. On the other hand, mtROS induce phospholipids oxidation, principally cardiolipin, leading to the ↓ΔΨm, MPTP opening, and ETS activity reduction [100]. Thus, the pathological crosstalk between NOXs and mitochondrial induces ROS, affecting mitochondrial metabolism and biomolecules integrity. The latter induces mitochondrial impairment, favoring the AKI to CKD transition.
5. Mitochondrial Metabolism, ROS, and OS in Kidney Diseases
The kidneys remove waste from the blood, reabsorb nutrients, regulate electrolyte balance, maintain acid-base homeostasis and regulate blood pressure [10]. These processes require high amounts of energy, which come from OXPHOS or anaerobic glycolysis, depending on the region of the kidneys. For example, the renal cortex uses OXPHOS and low amounts of glucose, while the renal medulla uses glycolysis and lactate. Therefore, the medulla necessarily uses anaerobic glycolysis due to low oxygen levels. In contrast, the renal cortex uses OXPHOS, fed mainly by FA oxidation [10,101].
A significant number of mitochondria in renal cells is located in PT, the most metabolically active [10]. OXPHOS is the principal mechanism to produce ATP in renal proximal tubular cells (RPTCs). Ninety percentage of ATP is required to reabsorption of glucose, ions, and nutrients through the sodium-potassium ATP pump (Na+/K+ ATPase) [102]. PT uses FA, such as palmitate, through FA β-oxidation to produce high ATP levels (Figure 3) [103]. Therefore, RPTCs have high levels of carnitine O-palmitoyl transferase I (CPT I) isoforms A (CPT IA) and B (CPT IB), and carnitine O-palmitoyl transferase II (CPT II) [104].
Figure 3.
Renal proximal tubule (RPTC) cells use fatty acids (FA) β-oxidation to produce adenosine triphosphate (ATP). In the kidney, the proximal tubules of the nephrons of the renal cortex use fatty acids (FA) as the primary source of energy. FA bound to the fatty acid-binding protein (FABP) enter the RPTC through the cluster of differentiation 36 (CD36). In the cytosol, acyl-coenzyme A (CoA) synthetase (ACS) (attached to the outer mitochondrial membrane (OMM)) activates FA by the addition of acetyl-CoA (CoA-SH). The latter allows FA to enter the OMM through carnitine O-palmitoyl transferase I (CPT I). CPTI exchanges acetyl-CoA for L-carnitine. In turn, FA goes to the inner mitochondrial membrane (IMM). In the IMM, carnitine O-palmitoyl transferase II (CPT II) removes the carnitine group and adds acetyl-CoA (CoA-SH). The latter allows FA to enter the mitochondrial matrix. Fatty acyl-CoA undergoes β-oxidation, generating nicotinamide adenine dinucleotide phosphate (NADH) and flavin adenine dinucleotide (FADH2). Created with Biorender.com.
5.1. FA β-Oxidation Dysfunction in Kidney Diseases
The impairment of β-oxidation has been reported in AKI and CKD. In patients and animal models, mRNAs along with β-oxidation proteins levels are decreased [105,106,107]. In the folic-acid-induced AKI model, ATP production is reduced, and mitochondria are uncoupling due to β-oxidation dysfunction [108]. Moreover, in maleic-acid (MA)-induced AKI, the FA β-oxidation-linked oxygen consumption rate (OCR) is diminished [109]. On the other hand, the transcriptomic analysis showed that the acyl-CoA dehydrogenase family member 10 (ACAD10) is downregulated in DN [110]. Moreover, in the 5/6 nephrectomy model, medium-chain acetyl dehydrogenase (MCAD) decreases at twenty-eight days [107]. Thus, the decrease in these enzymes is related to FA β-oxidation deregulation in AKI and CKD. Following the latter, FA β-oxidation has been evaluated in a course temporal in 5/6 nephrectomy, which decreases from early times [111]. Consequently, the reduction of FA β-oxidation causes intrarenal lipids accumulation, inducing lipotoxicity and impairing renal function [112]. In line with this, Nishi et al. [113] demonstrated that lipid accumulation is evident in tubular epithelial cells (TECs). Lipid deposition increases according to kidney lesion, suggesting a metabolic reprogramming that shifts β-oxidation to lipid synthesis [114,115]. According to the latter, UUO increases triglycerides synthesis from one day after obstruction [116]. Triglycerides increase is partly due to the overexpression of the transporter of the long-chain FA cluster of differentiation 36 (CD36) in PT [7,117]. CD36 also promotes signaling pathway activation such as epithelial–mesenchymal transition (EMT), inflammation, and others, leading to fibrosis. In this context, CD36−/− mice subjected to UUO show less fibrosis than sham groups [118]. However, Kang et al. [106] showed that mice that overexpress CD36 show the accumulation of lipids but low expression levels of fibrotic markers. Therefore, the authors hypothesized that impaired FA β-oxidation is sufficient to induce the development of fibrosis and lipid accumulation is the only consequence of this dysfunction [106]. Thus, it has been suggested that defective FA β-oxidation is one of the principal mechanisms associated with fibrosis development [103,106].
The alterations in CPT I levels also contribute to FA β-oxidation impairment. For example, modifications in transporters of plasma acylcarnitine have been reported in CKD [119]. In this context, Prieto-Carrasco et al. [120] showed CPT I levels decreased in a temporal course from 2 to 28 days after nephrectomy, associated with progressive impairment in mitochondrial β-oxidation. The authors concluded that the decrease in CPT I favors mitochondrial β-oxidation impairment and the subsequence fibrosis development. The latter is supported by the fact that patients with CKD show a correlation between low CPT IA levels and fibrosis [121]. In this sense, the transgenic mouse models overexpressing CPT IA are able to restore oxidative metabolism, avoiding fibrosis development in UUO, folic acid nephropathy, and adenine-induced nephrotoxicity [121]. In addition, CPT IA overexpression also reduces fibrosis by decreasing TGFβ1 levels [121].
In summary, defective FA β-oxidation is observed in kidney diseases from early times, promoted through decreased mRNA expression and downregulation in the activity and levels of the proteins involved in this process and ETS activity reduction (discussed below). Later, the overexpression of CD36 contributes to lipid accumulation and the activation of mechanisms that lead to the fibrotic process. However, other factors contribute to the impairment of β-oxidation.
Oxidation and OS Production and in Kidney Diseases
Renal pathologies cause a disturbance in mitochondria homeostasis, affecting mitochondrial metabolism, which leads to AKI to CKD transition. OS might cause these alterations in mitochondrial metabolism produced during ETS, β-oxidation, and Krebs cycle activity [103]. For instance, Kowaltowski’s group [122,123] demonstrated that H2O2 is produced during the first step of FA β-oxidation, catalyzed by a very long-chain acyl-CoA dehydrogenase (VLCAD) enzyme in liver mitochondria. If H2O2 produced is not degraded, it could induce mitochondria-decoupling electron leakage during OXPHOS. Even β-oxidation may be damaged. The latter has been supported by Aparicio-Trejo et al. [44], showing that folic acid causes damage to mitochondria and decreasing the OXPHOS associated with FA β-oxidation is due to ROS overproduction. The use of the antioxidant N-acetylcysteine (NAC) prevents the reduction in OXPHOS capacity associated with FA β-oxidation impairment from 2 to 28 days of the administration, avoiding CKD transition [108]. In accordance, Briones-Herrera et al. [109] showed that MA, another inductor of OS, decreases β-oxidation and the use of antioxidant sulforaphane (SF) prevents this decrease [109].
On the other hand, Tan et al. [110] showed by transcriptomic analysis in diabetic mice that mitochondrial FA β-oxidation is downregulated. This downregulation is attributed to the overexpression of the C5 substrate of the complement system receptor 1 (C5aR1). Thus, C5aR1 is implicated in lipids metabolism in diabetes [124]. C5aR1 is also upregulated in kidney diseases, producing FA β-oxidation impairment in DN [110]. In addition, C5aR1 upregulation disrupts mitochondrial respiration, generating high levels of ROS. These results showed that C5aR1-induced ROS overproduction alters FA metabolism in DN.
During mitochondria decoupling, electron leakage from ETS occurs, which induces the reduction of oxygen (O2) to the radical O2•−, a type of ROS that triggers the production of other ROS such as H2O2 [125]. High levels of •OH and ONOO− induce OS and significantly oxidative damage of proteins, lipids, and DNA. The oxidation of lipids produces highly reactive molecules such as MDA and 4-HNE as products of chain lipid peroxidation that also induce mtDNA damage (Figure 4) [68]. Forty-eight hours after cisplatin treatment, induced AKI, 4-HNE, and MDA levels increase along with GPx4 levels decrease in the renal cortex, indicating lipid membrane peroxidation [126]. Furthermore, in MA-induced Fanconi syndrome, 24 h after injection with MA, the mitochondrial levels of 4-HNE are elevated [109]. Additionally, GPx activity decreases, favoring H2O2 accumulation and mitochondrial lipidic peroxidation [109]. In folic-acid-induced AKI, 24 h after folic acid administration, mitochondrial MDA and 4-HNE levels increase [44]. Both OS markers also increase in nephrectomy models [127,128]. Together, these results show that mitochondria suffer lipid peroxidation in AKI and AKI to CKD transition.
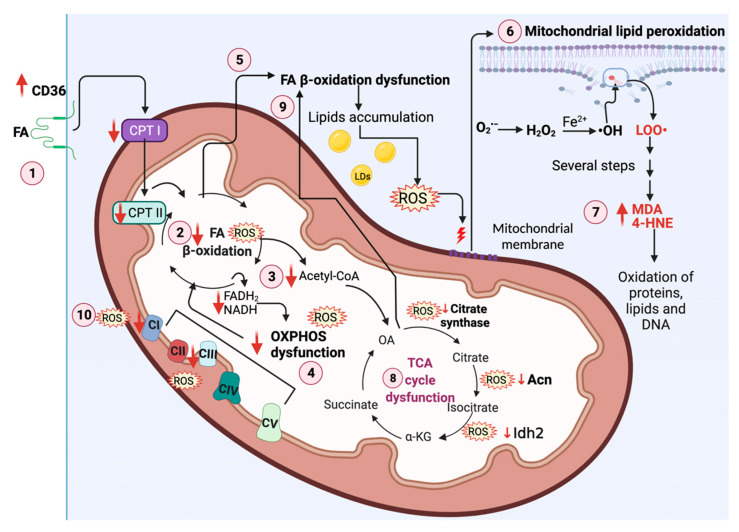
Figure 4.
ROS deregulate mitochondrial metabolism in kidney diseases. (1) In renal damage, the cluster of differentiation 36 (CD36) overexpression causes a high fatty acids (FA) uptake. In addition, carnitine O-palmitoyl transferase I (CPT I) and carnitine O-palmitoyl transferase II (CPT II) are decreased. (2) ROS cause FA β-oxidation decrease, inducing (3) a tricarboxylic acid (TCA) cycle and (4) oxidative phosphorylation (OXPHOS) capacity reduction. The decrease in β-oxidation also (5) induces the accumulation of lipids and a further ROS overproduction. The latter (6) damages mitochondrial membranes by inducing mitochondrial lipid peroxidation, (7) forming the products malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). These products are highly reactive and damage other lipids, proteins, and mitochondrial DNA (mtDNA). On the other hand, (8) ROS downregulate aconitase (Acn), citrate synthase, and isocitrate dehydrogenase isoform 2 (Idh2), inducing TCA cycle dysfunction. Moreover, (9) TCA cycle impairment induces FA β-oxidation dysfunction. (10) ROS decrease CI and CIII activities, inducing β-oxidation dysfunction [111]. O2•−, superoxide anion radical; H2O2, hydrogen peroxide; •OH, hydroxyl radical; LOO•, lipid peroxyl radical; LDs, lipid droplets; OA, oxalacetate; α-KG, alpha-ketoglutarate; DNA, deoxyribonucleic acid; NADH, nicotinamide adenine dinucleotide phosphate; FADH2, flavin adenine dinucleotide; CoA, coenzyme A; CII, complex II; CIV, complex IV; CV, complex V. Created with BioRender.com.
In kidney diseases, the uptake of lipids by CD36, along with the dysfunction of FA β-oxidation, causes lipids accumulation in lipid droplets (LDs), inducing ROS overproduction (Figure 4) [129,130]. Since ROS and their products induce severe cell damage, a cellular balance of ROS is needed. This balance is performed by different antioxidants that include enzymatic and non-enzymatic antioxidants [131]. The reduction of the antioxidant system has been widely reported in kidney diseases [11,132]. In renal ischemia and nephrotoxicity, catalase (CAT), SOD, and glutathione S-transferase (GST) levels are depleted [70,133]. Moreover, in cisplatin-induced AKI, mitochondrial GSH and NADPH levels are decreased [134]. In the 5/6 nephrectomy, the activities of CAT, SOD, GPx, GR, and GST fall at 20 h in glomeruli, PT, and DT [128]. Moreover, early after UUO, these enzymes’ mRNA and protein levels decrease, while oxidative markers increase [135]. Indeed, the decrease in antioxidant-system-induced OS has been suggested as a factor to induce the AKI to CKD transition.
The decreases in acetyl-CoA induced by FA β-oxidation impairment reduce the TCA cycle capacity. Interestingly, in AKI and CKD models, the reduction in TCA cycle enzymes is observed, before FA β-oxidation dysfunction occurs, suggesting this point as the start of the vicious cycle, which further increases the mitochondrial damage (Figure 4). In the next section, we will address the impact that ROS have on TCA cycle dysfunction.
5.2. TCA Cycle Redox-Sensitive Signaling Pathway in Kidney Diseases
The urinary excretion of the non-diabetic CKD patients shows low levels of TCA cycle metabolites (e.g., citrate, cis-aconitate, isocitrate, alpha-ketoglutarate (α-KG), and succinate) [136]. In addition, kidney biopsies have reduced aconitate, isocitrate, alpha-ketoglutarate dehydrogenase (α-KGDH), and succinate gene expression. These results show TCA cycle dysfunction [136]. In contrast, in a mouse model of DN, pyruvate, citrate, α-KGDH, and fumarate are upregulated [137]. Moreover, in UUO, succinate levels increase, attributed to TCA cycle dysfunction [138]. Note that the amount of the metabolites is tissue- and disease-dependent, so the identification of these metabolites could give advantages in the early detection of mitochondrial damage in these diseases.
TCA cycle dysfunction might be attributed to ROS alterations. In vitro studies have postulated that high glucose oxidation rates lead to the excessive production of electron donors from the TCA cycle. As a consequence, ETS becomes overloaded, promoting O2•− overproduction [139]. In line with this, podocytes treated with high glucose levels have high ROS levels, and the treatment with mitoTEMPO decreases them [140], suggesting that ROS are specifically delivered from mitochondria. Controversially, the determination of mtROS in the diabetic mouse model shows that it is reduced [141]. Further studies in vivo are needed to elucidate the mtROS overproduction-induced TCA cycle dysfunction in DN.
In the kidney, TCA cycle enzymes can be sulfenylated or S-glutathionylated. For example, Acn can be reversibly inactivated by the oxidation of the sulfhydryl group by O2•− and H2O2. However, if OS persists, Acn can be irreversibly deactivated by the disruption of the 4Fe-4S group [142]. In AKI induced by folic acid, mitochondrial Acn activity decreases, and the pre-treatment with NAC prevents it [44], suggesting that ROS promote the deactivation of Acn. In addition, the relation between Acn and citrate synthase diminishes, supporting the idea that decreasing in Acn activity is related to OS [44]. Moreover, Mapuskar et al. [143] reported that the persistent increase of O2•− decreases Acn and citrate synthase activity in cisplatin-induced kidney injury, of which the effects are ameliorated by SOD mimetic avasopasem manganese (GC4419) treatment. The authors reported that in the AKI phase, Acn and citrate synthase activities do not show changes, suggesting that high levels of ROS are required for their inactivation in this model [143]. The latter is demonstrated due to the fact that high levels of ROS are more evident in cisplatin-induced CKD [143].
In kidney pathologies, the levels of mitochondrial isocitrate dehydrogenase isoform 2 (Idh2) are decreased [144,145]. In the cisplatin model, Idh2 function is affected by decreased mitochondrial NADPH and GSH and increased H2O2 production [145]. Furthermore, Han et al. [144] showed that OS generated during I/R reduces Idh2 levels in kidney tubule cells from mice. Since S-glutathionylation deactivates Idh2, this Ox-PTM may be produced during OS under I/R conditions [146]. The deletion of the Idh2 (Idh2−/−) gene in these mice exacerbates kidney tubule injury by increasing plasma creatinine and blood urea nitrogen (BUN) levels. In addition, OS increases the reduction of mitochondrial NADP+ along with GST and GPx activities. In contrast, mitochondrial GSSG/GSH ratio augments. Idh2−/− mice show mitochondrial dysfunction and fragmentation, which induces apoptosis in kidney tubule cells [144]. After UUO, Idh2 decreases, and its deletion increases OS markers such as 4-HNE and H2O2 in mitochondrial fractions [147]. Additionally, inflammatory cell filtration was more evident in Idh2−/− than wild-type (WT) groups. Together, these results highlighted the importance of Idh2 in managing OS, and its deactivation exacerbates mitochondrial damage.
Note that the fact that ROS-induced TCA cycle dysfunction affects FA β-oxidation has been demonstrated, because TCA cycle impairment is observed early before FA β-oxidation damage in time course studies of the AKI to CKD transition, (Figure 4). In this regard, OXPHOS capacity is also decreased by ROS in early times, suggesting that both events are required to enhancement FA β-oxidation dysfunction [44,108,120].
5.3. OXPHOS Redox-Sensitive Signaling Pathway in Kidney Diseases
As mentioned above, kidney energy demand depends on OXPHOS, which in turn is regulated by Ox-PTMs. However, in the context of kidney diseases, it is poorly studied. OXPHOS capacity is downregulated in renal diseases, inducing ROS overproduction. In this regard, the production of mitochondrial H2O2 has been reported in kidney injury models, associated with ↓ΔΨm, decreasing OXPHOS capacity [8,148]. In remnant kidney from 5/6 nephrectomy, OXPHOS linked to complex I (CI) feeding decrease in a temporal course of nephrectomy from 2 to 28 days [111]. Moreover, male Sprague Dawley rats subjected to nephrectomy showed ATPβ, NDUSF8, and cytochrome c oxidase subunit 1 (Cox I) reduction [8]. Consequently, CI, complex III (CIII) activities, and cyt c diminished, impairing mitochondrial function [8]. Avila-Rojas et al. [148] reported that potassium dichromate (K2Cr2O7) decreases the CI + CII-linked S3 respiratory state. In addition, ΔΨm in CI + complex II (CII)-linked respiration and respiration associated with OXPHOS are reduced, suggesting that K2Cr2O7 principally affects the synthesis of mitochondrial ATP [148]. Although redox signaling has not been investigated in previous studies, components of OXPHOS might be regulated by Ox-PTMs.
6. ROS Induce Uncoupling Proteins (UCPs) Dysregulation in Kidney Diseases
UCPs are proton transporters (H+), which move H+ from the IMM into the mitochondrial matrix. These transporters are localized in the IMM and dissipate the proton gradient from the mitochondrial matrix into the IMS [149]. mtROS induce UCP2 activation, decreasing the proton gradient and preventing mtROS overproduction [149].
It has been shown that UCP2 deletion aggravates tubular injury in the I/R model by inducing ROS overproduction, supporting the importance of these transporters in ROS dissipation [150]. Moreover, the UCP2 inhibition worsens the damage caused by lipopolysaccharide (LPS), increasing apoptosis in TECs [151]. In CKD, Jian et al. [152] showed that in renal tubular cells (RTCs), the expression of UCP2 is induced three days after obstruction and continues after seven days, avoiding UUO-induced fibrosis. It suggests that UCP2 is crucial to avert fibrosis development induced by ROS in UUO.
Although UCP1 is commonly found in mitochondria from brown adipose tissue, it is expressed in the kidney. For instance, in AKI models induced by cisplatin or I/R, Jia et al. [153] found that UCP1 is upregulated in renal TECs and its presence is related to OS suppression. Chouchani et al. [154] showed that mtROS alter the redox status of UCP1 by inducing its sulfenylation in Cys 253, promoting UCP1 activity.
7. Redox-Sensitive Signaling Controls Mitochondrial Dynamics, Biogenesis, and Mitophagy
Mitochondrial dynamic is the balance between mitochondrial fusion and fission, and it is involved in regulating mitochondrial metabolism and cell death. Likewise, the shape and morphology of mitochondria are regulated by the metabolite and ROS concentration concentrations [155]. The integral membrane guanosine triphosphatases (GTPases) perform mitochondrial fusion: mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optical atrophy 1 (Opa1) [136]. Meanwhile, mitochondrial fission is mediated by Drp1, the mitochondrial fission factor (Mff), the adaptor protein fission protein 1 (Fis1), and the mitochondrial elongation factor 1 (Mief1) and 2 (Mief2). Chronic OS has been shown to induce mitochondrial fission [155]. However, H2O2 sublethal amounts, and acute OS cause mitochondrial hyperfusion [156]. In HeLa cells, mitochondrial hyperfusion has been associated with the S-glutathionylation of Mfn2 and the formation of Opa1 oligomers. Moreover, mitochondrial hyperfusion increases the resistance to cellular stress and cell death, since it promotes antioxidant defense enzymes activation [156]. In the next section, we analyze the current trends and paradigms of ROS-mediated signaling that form a link between redox-sensing elements and mitochondrial dynamics and their possible role in kidney diseases.
7.1. Redox-Sensitive Proteins Participating in Fission and Fusion
Redox signaling conveys external and internal signals between redox-sensitive receptors and the downstream effectors of fission machinery. Mitochondrial dynamics require the recruitment of proteins to mitochondria. Indeed, the importation of several proteins to mitochondria depends on proton electrochemical gradient H+ created by ETS at the IMM, which is called the proton motive force (PMF) [157]. In addition, several redox-sensitive proteins are activated to induce proteins translocation from the cytosol. For instance, previous studies in HeLa cells have shown that MAPKs are involved in regulating the fission process in response to OS through Ras [158,159]. In line with this, the redox-sensitive extracellular regulated kinase 2 (ERK2) protein mediates the phosphorylation of Drp1 in Ser 616 to induce mitochondrial fragmentation [158]. In addition to ERK 2, PKC-delta (PKC-δ) promotes Drp1 phosphorylation in Ser 579 under OS [160]. Drp1 is also regulated by ROS. In this context, Kim et al. [161] showed that in vascular diseases related to diabetes and aging models, protein disulfide isomerase A1 (PDIA1) is depleted, inducing the sulfenylation of Drp1 in Cys 644. The latter leads to mitochondrial fragmentation, mtROS increase, and senescence induction. Furthermore, the authors demonstrated that the restoration of the PDIA1/Drp1 axis could be used as a therapeutic strategy to improve vascular diseases [161], suggesting that PDIA1 has a thiol reductase function for Drp1. Drp1 persulfidation was previously reported, inducing its inactivation. Persulfidation is carried out by CARS2, altering mitochondrial dynamics and favoring fusion [66].
In renal pathologies, the upregulation of Drp1 is related to OS conditions [44,147,148]. Likewise, the phosphorylation of ERK 1/2 increases along with Drp1 levels in response to OS in the I/R rat model [162]. Thus, ROS might induce the recruitment of Drp1 through ERK1/2 (Figure 5).
Figure 5.
Redox-sensitive signaling regulates mitochondrial dynamics in kidney diseases. (1) ROS activate the redox-sensitive extracellular regulated kinase 2 (ERK2) protein and protein kinase C (PKC) isoform δ (PKC-δ). These proteins phosphorylate and activate dynamin-related protein 1 (Drp1), inducing its translocation to the OMM to triggering fission. Likewise, ROS overproduction upregulates Drp1, fission 1 (Fis1), and mitochondrial fission factor (Mff), inducing fission increase. (2) ROS also induce fusion decrease by downregulating optical atrophy 1 (Opa1) and mitofusin 1 (Mfn1) and 2 (Mfn2) proteins. (3) The augment of fission promotes mitophagy activation by inducing the translocation of phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (Pink1) that in turn recruit to parkin in the OMM to induce mitophagosome formation. Mitophagosome fuses with lysosome to form mitophagolysome formation. However, mitophagy flux is impaired, inducing the accumulation of damaged mitochondria. (4) Low ROS levels induce mitochondrial biogenesis by activating the redox-sensitive adenosine monophosphate (AMP)-activated protein kinase (AMPK) and the peroxisome proliferator-activated receptor (PPAR) γ coactivator-1 alpha (PGC-1α). However, high levels of ROS producing oxidative stress (OS) induce mitochondrial biogenesis decrease, triggering mitochondrial mass decrease. Created with BioRender.com.
In kidney diseases, ROS overproduction induces mitochondrial proteins fusion decrease and fission increase. In line with this, in the K2Cr2O7 rat model, Drp1 increase and curcumin treatment decrease it [148], suggesting that ROS induce Drp1 upregulation (Table 1). The latter is supported by the fact that ROS promote Drp-1 translocation to mitochondria. Drp1 is also recruited in RPTC treated with cisplatin, inducing mitochondrial fragmentation and ATP depletion [163]. Moreover, Drp1 is upregulated in Idh2−/− mice due to NADPH and GSH decrease, which increase mtROS. mtROS also downregulate Opa1 (Table 1) [44,144,145]. Thus, mtROS trigger fission increase and fusion decrease. Following the latter, Opa1 levels are diminished under H2O2 treatment, and the diminishment is even higher in Idh2 small interfering RNA (siRNA)-transfected mProx24 cells [144]. Mfn1 is another fusion protein decreased in AKI models (Table 1) [44,164].
Table 1.
ROS regulate mitochondrial dynamics, biogenesis, and mitophagy in acute kidney injury (AKI) models.
| AKI Model | In Vivo Model | Mitochondrial Dynamic Protein | Mechanism | References |
|---|---|---|---|---|
| Cisplatin-induced nephrotoxicity and I/R | C57BL/6 mice | ↑Drp1 | Drp1 translocates to the mitochondria in response to ROS overproduction. | Brooks et al. [163] |
| Maleate-induced nephrotoxicity | Male Wistar rats | ↑Drp1, Fis1 | Maleate-induced OS promotes mitochondrial fission by increasing Drp1 and Fis1. | Molina-Jijón et al. [84] |
| Cisplatin | C57BL/6 mice | ↓Opa1, Mfn1 ↑Fis1 ↑Pink1, parkin |
ROS and mtROS promote fission and decrease the mitochondrial fusion process. | Ortega-Domínguez et al. [164] |
| I/R | C57BL/6 mice | Drp1−/− | The deletion of Drp1 improves mitochondrial function by decreasing mtROS. | Perry et al. [165] |
| Cisplatin | Female C57BL/6 Idh2−/− mice | ↓Opa1 ↑Drp1 |
Idh2−/− decreases NADPH and GSH levels, inducing OS and triggering fission increase and fusion decrease. | Kong et al. [145] |
| I/R | Female C57BL/6 Idh2−/− mice | ↑Drp1, Fis1 ↓Opa1 |
Idh2−/−-induced mtROS, decreasing the levels of fusion proteins and augmenting fission proteins. | Han et al. [144] |
| Folic acid | Male Wistar rats | ↑Fis1, Drp1 ↓Opa1, Mfn1 ↑Pink1, ↓LC3 ↓PGC-1α, ↓NRF1, NRF2 |
ROS overproduction increases fission and reduces the fusion process. | Aparicio-Trejo et al. [44] |
| Nephrotoxicity by K2Cr2O7 | Male Wistar rats | ↑Drp1 ↓PGC-1α |
ROS overproduction increases fission and reduces biogenesis. | Ávila-Rojas et al. [148] |
| MA: induced Fanconi syndrome | Male Wistar rats | ↓TFAM, ↑Fis1, Drp1 ↑Parkin, p62, LC3-II |
SF prevents mitochondrial fission increase and TFAM decrease and regulates mitophagy. | Briones-Herrera et al. [109] |
Abbreviations: ↑: increase; ↓: decrease; I/R, ischemia/reperfusion; Drp1, dynamin-related protein 1; Fis1, fission 1; OS, oxidative stress; Opa1, optical atrophy 1; Mf1, mitofusin 1; Pink1, phosphatase and tensin homolog (PTEN)-induced putative kinase 1; Idh2, Isocitrate dehydrogenase isoform 2; K2Cr2O7, potassium dichromate; PGC-1α, peroxisome proliferator-activated receptor (PPAR) γ coactivator-1 alpha; TFAM, transcription factor A; NRF1, nuclear respiratory factor 1; NRF2, nuclear respiratory factor 2; p62, sequestosome; LC3, microtubule-associated protein 1A/1B-light chain 3 phosphatidylethanolamine conjugate; NADPH, nicotinamide adenine dinucleotide phosphate; GSH, glutathione; mtROS, mitochondrial reactive oxygen species; MA, maleic acid; SF, sulforaphane.
The blockage of mitochondrial fission has been suggested as a strategy to ameliorates mitochondrial damage [166]. For instance, the loss of DRP1, six hours after suffering bilateral I/R, re-establish mitochondrial function due to the reduction of mtROS production associated with fission decrease (Table 1) [165]. In addition, mice Drp1−/− does not have tubulointerstitial fibrosis [165], suggesting that the Drp1 blocking might avoid the AKI to CKD transition. Furthermore, the specific inhibitor of Drp1, the mitochondrial division inhibitor 1 (mdivi-1), ameliorates I/R-mediated AKI (Table 1) [163]. However, in the case of UUO, the usage of mdivi-1 augments fibrosis [167], which correlates with midivi-1-treated human proximal tubular cells (HK2) under hypoxic conditions, showing fibrosis markers increase [167]. Therefore, Drp1 blocking might be employed in the case of AKI, but not in CKD.
The management of redox recovery homeostasis might be utilized as a strategy to improve mitochondrial dynamics. Consistent with this, the use of antioxidants that target mitochondria has been shown to enhance the homeostasis of mitochondrial dynamics [44,148]. Therefore, ROS regulate proteins involved in mitochondrial fission and fusion. According to the latter, it has been hypothesized that the treatment with sublethal amounts of H2O2 induces acute stress, promoting a hyperfused mitochondrial state [24,168]. In folic-acid-induced kidney injury, Drp1 and Fis1 increase, and the treatment with NAC decreases them [44]. In response to cisplatin or bilateral I/R, Drp1 is recruited to mitochondria, triggering apoptosis induction by delivering cyt c and decreasing the antiapoptotic protein B cell lymphoma 2 (Bcl-2) [163]. Moreover, the activation of peroxisome proliferator-activated receptor γ (PPARγ) stabilizes mitochondrial potential, reducing ROS. The latter results in decreasing Drp1, augmenting Mfn2, Opa1 and restoring mitochondrial dynamics [8], suggesting that the rescue of mitochondrial biogenesis might recover mitochondrial dynamics due to mtROS decrease. In CKD, the levels of fusion proteins (e.g., Mfn1, Mfn2, and Opa1) are also decreased, while fission proteins are increased (e.g., Drp1 and Mff) (Table 2) [8,167,169,170], attributed to mitochondrial OS increase.
Table 2.
ROS regulate mitochondrial dynamics, biogenesis, and mitophagy in chronic kidney disease (CKD) models.
| CKD Model | In Vivo Model | Mitochondrial Dynamic Proteins Alteration | Effects | References |
|---|---|---|---|---|
| DN | Male C57BL/6J mice |
↓PGC-1α, AMPK | Reduced ROS levels decrease mitochondrial biogenesis. | Dugan et al. [141] |
| 5/6 nephrectomy | Male Sprague-Dawley rats | ↓Mfn2, Opa1 ↑Drp1 ↓PPARγ |
The use of pioglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) activator, decreases mtROS, improving mitochondrial dynamics. | Sun et al. [8] |
| 5/6 nephrectomy | Male Wistar rats | ↑Mfn1, Opa1 ↓Fis1, Drp1 |
ROS overproduction favors mitochondrial fusion. | Aparicio-Trejo et al. [128] |
| UUO | Male C57BL/6J mice | ↑LC3, Pink1, parkin | ROS-induced senescence impairs mitophagy. | Liu et al. [169] |
| UUO | Male C57BL/6J mice | ↓Drp1 ↑LC3, Pink1, parkin |
mtROS recruit Drp1 to the OMM, regulating mitophagy parkin-dependent. | Li et al. [167] |
| 5/6 Nephrectomy | Male Wistar rats | ↓NRF1, NRF2, TFAM PGC-1α, PPARα ↓Mfn2, Opa1 ↑LC3, p62 |
Mitochondrial biogenesis and dynamics are altered temporal courses. | Prieto-Carrasco et al. [111] |
Abbreviations: ↑: increase; ↓: decrease; DN, diabetic nephropathy; PPARα, peroxisome proliferator-activated receptor γ coactivator-1α; AMPK, adenosine monophosphate (AMP)-activated protein kinase; PPARγ, peroxisome proliferator-activated receptor γ; OMM, outer mitochondrial membrane; Drp1, dynamin-related protein 1; Fis1, fission 1; OS, oxidative stress; Opa1, optical atrophy 1; Mf1, mitofusin 1; Pink1, phosphatase and tensin homolog (PTEN)-induced putative kinase 1; PGC-1α, peroxisome proliferator-activated receptor (PPAR) γ coactivator-1 alpha; TFAM, transcription factor A; NRF1, nuclear respiratory factor 1; NRF2, nuclear respiratory factor 2; p62, sequestosome; LC3, microtubule-associated protein 1A/1B-light chain 3 phosphatidylethanolamine conjugate.
Ox-PTMs might regulate mitochondrial dynamics proteins; however, they are poorly studied in kidney disease. In line with this, S-nitrosylation in Drp1 Ser 644 induces Drp1 dimerization and augments its GTPase activity. Thus, NO promotes Drp1-induced mitochondrial fission [171]. Furthermore, OS can trigger mitochondrial hyperfusion [156]. Further studies are needed to clarify the role of ROS to induce Ox-PTMs regulation in mitochondrial dynamics and biogenesis in renal disease.
7.2. Redox-Sensitive Proteins Participating in Mitochondrial Biogenesis
Mitochondria biogenesis is triggered to increase the number and size of mitochondria [172]. PPARγ coactivator-1 alpha (PGC-1α) controls the biogenesis process through the transcription of nuclear respiratory factors 1 (NRF-1) and 2 (NRF-2), PPARs, transcription factor A (TFAM), estrogen, and estrogen-related receptors (ERRs), among others [173]. These proteins are redox-sensitive. For instance, the exposure to H2O2 in skeletal muscle cells for 24 h increases the activity of the PGC-1α promoter as well as mRNA expression. These effects are blocked with NAC treatment [174], showing that ROS mediate the activation of PGC-1α. However, the impact of ROS over PGC-1α depends on the concentration and cellular type. For example, low ROS levels lead to the reduced expression of PGC-1α, while high levels induce its transcription through redox-sensitive adenosine monophosphate (AMP)-activated protein kinase (AMPK) [174], which function as a cellular energy sensor by regulating mitochondrial biogenesis and maintaining redox homeostasis [175]. AMPK reduces ROS through PGC1-α, which induces the overexpression of CAT, Mn-SOD, UCP2, and nicotinamide adenine dinucleotide (NAD)-dependent deacetylase sirtuin-3 (SIRT3) [176]. Therefore, the activation of AMPK induces PGC-1α promoter activity and mRNA levels increase. PGC-1α is commonly downregulated in kidney diseases, leading to mitochondrial mass and metabolism decrease [10]. In the folic-acid-induced AKI model, PGC-1α, TFAM, NRF1, and NRF2 levels decrease 24 h after the treatment, and NAC treatment prevents this effect [44]. Moreover, male Wistar rats subject to nephrotoxicity by K2Cr2O7 show a decrease in PGC-1α levels [148]. Both studies showed that the treatment with antioxidants (NAC and curcumin) upregulates biogenesis [47,152,169]. Importantly, NAC and curcumin antioxidants have shown mitochondria protection by promoting bioenergetics preservation and maintaining redox homeostasis to avoid mtROS [16,94]. In 5/6 nephrectomy-induced CKD, the levels of PGC-1α decrease in a temporal course from two days after the nephrectomy, causing reductions in NRF1 and NRF2 [120]. The treatment with pioglitazone, an antidiabetic drug, reduces mtROS, restoring mitochondrial biogenesis. Interestingly, the biogenesis decrease in DN is attributed to the low production of O2•− [141]. AMPK activity decreases in this model, and AMPK activation induces O2•− production, activating PGC-1α [141]. Therefore, in the DN mice model, low ROS levels are essential factors to trigger mitochondrial biogenesis.
7.3. Mitophagy, ROS, and OS in Kidney Diseases
The dysregulation of mitophagy has been previously reported in renal diseases [177,178]. At biological levels, ROS are involved in mitophagy regulation. Upon mitochondrial damage, depolarization, or mitochondrial OS, mitophagy is triggered. ROS promote the recruitment of phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (Pink1) in the outer mitochondrial membrane (OMM) [179]. The latter leads to the recruitment and phosphorylation of parkin to begin mitophagy [180]. In the AKI models, Pink1 and parkin are upregulated after damage [44,109,164]. The upregulation of these proteins is related to ROS and mtROS increase. Furthermore, in CKD models, both proteins are augmented [120,167,169]. For instance, in the 5/6 nephrectomy model, levels of Pink1, parkin, microtubule-associated protein 1A/1B-light chain 3 phosphatidylethanolamine conjugate (LC3-II), and sequestosome (p62) increase in a temporal course way [120]. However, in both cases, mitophagy has been reported impaired. Although the levels of LC3-II increase, the accumulation of p62 is evident, suggesting dysfunctional mitophagy [120]. p62 accumulation is considered a marker of mitophagy malfunction. In kidney injury, mitophagosome accumulation is evident by increasing Pink1, parkin, and p62 [181]. Together, these data suggest that ROS induce Pink1 and parkin translocation to mitochondria, but the high ROS levels exacerbate mitophagy machinery, damaging it. Mitophagy damage is supported, because antioxidants can increase mitophagy flux in AKI and CKD models [44,181].
On the other hand, excessive autophagy has been described in models of UUO, which triggers endothelial dysfunction [182]. Consistent with this, Chen et al. [183] found that treatment with NaHS, an exogenous H2S donor, in UUO mice decreases the expression levels of LC3-II/I, beclin-1, and AMPK proteins. On the contrary, the p62, CBS, and CSE levels increase compared to in the sham groups. Therefore, H2S is considered a protective mechanism, because it moderates OS that promotes the dysregulation of autophagy.
8. Concluding Remarks
ROS are second messengers that modify redox-sensitive proteins in mitochondria by inducing Ox-PTMs. Thus, low levels of ROS are necessary to render these modifications. However, in kidney diseases, ROS trigger mitochondrial dysfunction evident by alterations in FA β-oxidation, TCA cycle, OXPHOS, mitophagy, mitochondrial dynamics, and biogenesis.
Moreover, the crosstalk between NOXs and mitochondria generates ROS. The impairment in one of these elements can trigger an uncontrolled ROS production increase. Therefore, perturbations in mitochondrial redox homeostasis are common characteristics that allow the transition from AKI to CKD.
Acknowledgments
A.K.A.-R. is a Ph.D. student from Posgrado en Ciencias Biológicas at the Universidad Nacional Autónoma de México and is recipient of a scholarship from CONACyT, México (CVU 818062). We gratefully acknowledge the Postdoctoral Grant’s Program (POSTDOC) from the Dirección General de Asuntos Académicos (DGAPA), UNAM, for the postdoctoral fellow position to A.C.-G.
Abbreviations
| ↓ΔΨm | mitochondrial membrane potential depolarization |
| •NO | nitric oxide |
| •OH | hydroxyl radical |
| 4-HNE | 4-hydroxynonenal |
| α-KΓ | Alpha-ketoglutarate |
| α-KΓΔH | alpha-ketoglutarate dehydrogenase |
| ACAD10 | acyl-CoA dehydrogenase family member 10 |
| ACS | acyl-CoA synthetase |
| Acn | aconitase |
| AGE | advanced glycation end products |
| AKR1A1 | aldo-keto reductase family 1 member A1 |
| AKI | acute kidney injury |
| AMPK | adenosine monophosphate (AMP)-activated protein kinase |
| Ang II | angiotensin II |
| Arg | arginine |
| ATP | adenosine triphosphate |
| ATR-1 | angiotensin type 1 receptor |
| Bcl-2 | B cell lymphoma 2 |
| BUN | blood urea nitrogen |
| CI | complex I |
| CII | complex II |
| CIII | complex III |
| CIV | complex IV |
| CV | complex V |
| C5aR1 | C5 substrate of the complement system receptor 1 |
| CAT | catalase |
| CBS | cystathionine β-synthase |
| CD36 | cluster of differentiation-36 |
| CKD | chronic kidney diseases |
| CoA | coenzyme A |
| Cox I | cytochrome c oxidase subunit 1 |
| CPT I | carnitine O-palmitoyl transferase I |
| CPT II | carnitine O-palmitoyl transferase II |
| CPT IA | CPT I isoform A |
| CPT IB | CPT I isoform B |
| CSE | cystathionine γ-lyase |
| Cu/Zn-SOD | copper/zinc-SOD |
| Cys | cysteine |
| CARSs | cysteinyl-transfer RNA (tRNA) synthetases |
| Cyt c | cytochrome c |
| DKD | diabetic kidney disease |
| DN | diabetic nephropathy |
| DNA | deoxyribonucleic acid |
| Drp1 | dynamin-related protein 1 |
| DT | distal tubules |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial–mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| ERK2 | extracellular regulated kinase 2 |
| ERR | estrogen and estrogen-related receptors |
| ETS | electron transport system |
| FA | fatty acids |
| FABP | fatty-acid-binding protein |
| FADH2 | flavin adenine dinucleotide |
| Fe2+ | ferrous iron |
| Fe3+ | ferric iron |
| Fe-S | iron-sulfur |
| Fis1 | fission protein 1 |
| FoxO | forkhead box O |
| GC4419 | avasopasem manganese |
| GFRs | growth factor receptors |
| GPx | glutathione peroxidase |
| Grx | glutaredoxin |
| GSH | glutathione |
| GTPases | guanosine triphosphatases |
| GST | glutathione S-transferase |
| h | hours |
| HK2 | human proximal tubular cells |
| HS• | hydrosulfide radical. |
| H2O2 | hydrogen peroxide |
| H2S | gydrogen sulfide |
| His | histidine |
| I/R | ischemia/reperfusion |
| Idh2 | isocitrate dehydrogenase isoform 2 |
| IKKγ | regulatory subunit of inhibitor IKK complex |
| IMM | inner mitochondrial membrane |
| IMS | intermembrane space |
| K2Cr2O7 | potassium dichromate |
| L-NAME | N(G)-nitro-L-arginine-methyl ester |
| LC3-II | microtubule-associated protein 1A/1B-light chain 3 phosphatidylethanolamine conjugate |
| LDs | lipid droplets |
| LOO• | lipid peroxyl radical |
| LPS | lipopolysaccharides |
| Lys | lysine |
| MA | maleic acid |
| MAPK | mitogen-activated protein kinases |
| MCAD | medium-chain acetyl dehydrogenase |
| MDA | malondialdehyde |
| Met | methionine |
| MetO (R–SOCH3) | methionine sulfoxide |
| MetO2 (RSO2CH3) | methionine sulfone |
| Mff | mitochondrial fission factor |
| Mfn1 | mitofusin 1 |
| Mfn2 | mitofusin 2 |
| Mief1 | mitochondrial elongation factor 1 |
| Mief2 | mitochondrial elongation factor 2 |
| mitoTEMPO | 2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium chloride |
| mitoQ | mitochondrial-targeting antioxidants coenzyme Q |
| Mn-SOD | manganese superoxide dismutase |
| mRNA | messenger RNA |
| Msr | methionine sulfoxide reductase |
| MsrA | Msr isoform A |
| MPTP | mitochondrial permeability transition pore |
| mt-KATP | mitochondrial ATP-sensitive potassium K channel |
| mtDNA | mitochondrial DNA |
| mtROS | mitochondrial ROS |
| Na+/K+ ATPase | sodium–potassium ATP pump |
| NAC | N-acetylcysteine |
| NAD | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NaHS | sodium hydrosulfide |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOS | nitric oxide synthase |
| NOXs | NADPH oxidases |
| NRF-1 | nuclear respiratory factor 1 |
| NRF-2 | nuclear respiratory factor 2 |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| O2 | oxygen |
| O2•− | superoxide anion radical |
| OA | oxalacetate |
| OCR | oxygen consumption rate |
| OMM | outer mitochondrial membrane |
| ONOO− | peroxynitrite |
| Opa1 | optical atrophy 1 |
| OS | oxidative stress |
| Ox-PTMs | oxidative post-translational modifications |
| OXPHOS | oxidative phosphorylation |
| p62 | sequestosome |
| PDIA1 | protein disulfide isomerase A1 |
| PGC-1α | peroxisome proliferator-activated receptor (PPAR) γ coactivator-1 alpha |
| Pink1 | phosphatase and tensin homolog (PTEN)-induced putative kinase 1 |
| PKC | protein kinase C |
| PKC-ε | PKC-epsilon |
| PKC-δ | PKC-delta |
| PKM2 | pyruvate kinase isoform M2 |
| PMF | proton motive force |
| PPARs | peroxisome proliferator-activated receptor |
| Pro | proline |
| Prx | peroxiredoxin |
| PT | proximal tubules |
| PTKs | protein tyrosine kinases |
| PTM | post-translational modifications |
| PTPs | protein tyrosine phosphatases |
| R–S• | thyil radical |
| R–SNO | S-nitrosothiol |
| R–SOH | sulfenic acid |
| R–SO2H | sulfinic acid |
| R–SO3H | sulfonic acid |
| R–S(O)–S–R | thiosulfinate |
| R–SN–R’ | sulfenamide |
| R–S–S–H | persulfonation |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RAGE | AGE receptor |
| ROS | reactive oxygen species |
| RPTCs | renal proximal tubular cells |
| RTC | renal tubular cells |
| S− | thiolate anion |
| S–S | disulfide bonds |
| SF | sulforaphane |
| SH | thiol |
| siRNA | small interfering RNA |
| SIRT3 | nicotinamide adenine dinucleotide (NAD)-dependent deacetylase sirtuin-3 |
| SN2 | bimolecular nucleophilic substitution |
| SSG | glutathionylation |
| TCA | tricarboxylic acid |
| TECs | tubular epithelial cells |
| TFAM | transcription factor A |
| TGFβ1 | transforming growth factor-beta 1 |
| Thr | threonine |
| Trx | thioredoxin |
| Tyr | tyrosine |
| UCPs | uncoupling proteins |
| UCP1 | uncoupling protein 1 |
| UCP2 | uncoupling protein 2 |
| UCPs | uncoupling proteins |
| UUO | unilateral ureteral obstruction |
| VLCAD | very long-chain acyl-CoA dehydrogenase |
| WT | wild type |
Author Contributions
Conceptualization, A.K.A.-R.; writing—original draft preparation, A.K.A.-R.; writing—review and editing, A.K.A.-R., A.C.-G., O.E.A.-T., and J.P.-C.; figures preparation, A.K.A.-R.; funding acquisition, J.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT; grant number: A1-S-7495) and by Dirección General de Asuntos del Personal Académico (DGAPA; grant numbers: IN202219 and IN200922).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manns B., Hemmelgarn B., Tonelli M., Au F., So H., Weaver R., Quinn A.E., Klarenbach S., for Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease The Cost of Care for People With Chronic Kidney Disease. Can. J. Kidney Health Dis. 2019;6 doi: 10.1177/2054358119835521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collister D., Pannu N., Ye F., James M., Hemmelgarn B., Chui B., Manns B., Klarenbach S. Health Care Costs Associated with AKI. Clin. J. Am. Soc. Nephrol. 2017;12:1733–1743. doi: 10.2215/CJN.00950117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas M.E., Blaine C., Dawnay A., Devonald M.A.J., Ftouh S., Laing C., Latchem S., Lewington A., Milford D.V., Ostermann M. The Definition of Acute Kidney Injury and Its Use in Practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Fang Y., Teng J., Ding X. Acute Kidney Injury Epidemiology: From Recognition to Intervention. Contrib. Nephrol. 2016;187:1–8. doi: 10.1159/000443008. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani P., Remuzzi G., Glassock R., Levin A., Jager K.J., Tonelli M., Massy Z., Wanner C., Anders H.-J. Chronic Kidney Disease. Nat. Rev. Dis. Primers. 2017;3:17088. doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Klimova E., Aparicio-Trejo O.E., Tapia E., Pedraza-Chaverri J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules. 2019;9:141. doi: 10.3390/biom9040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susztak K., Ciccone E., McCue P., Sharma K., Böttinger E.P. Multiple Metabolic Hits Converge on CD36 as Novel Mediator of Tubular Epithelial Apoptosis in Diabetic Nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L., Yuan Q., Xu T., Yao L., Feng J., Ma J., Wang L., Lu C., Wang D. Pioglitazone Improves Mitochondrial Function in the Remnant Kidney and Protects against Renal Fibrosis in 5/6 Nephrectomized Rats. Front. Pharmacol. 2017;8:545. doi: 10.3389/fphar.2017.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewitson T.D., Holt S.G., Smith E.R. Progression of Tubulointerstitial Fibrosis and the Chronic Kidney Disease Phenotype—Role of Risk Factors and Epigenetics. Front. Pharmacol. 2017;8:520. doi: 10.3389/fphar.2017.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhargava P., Schnellmann R.G. Mitochondrial Energetics in the Kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irazabal M.V., Torres V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells. 2020;9:1342. doi: 10.3390/cells9061342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlakou P., Liakopoulos V., Eleftheriadis T., Mitsis M., Dounousi E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms—Biomarkers—Interventions, and Future Perspectives. Oxidative Med. Cell. Longev. 2017;2017:6193694. doi: 10.1155/2017/6193694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmström K.M., Finkel T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 14.Sies H., Jones D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 15.Sies H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Gregorio A., Aranda-Rivera A.K. Redox-Sensitive Signalling Pathways Regulated by Human Papillomavirus in HPV-Related Cancers. Rev. Med. Virol. 2021:e2230. doi: 10.1002/rmv.2230. [DOI] [PubMed] [Google Scholar]
- 17.D’Autréaux B., Toledano M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 18.Wani R., Nagata A., Murray B.W. Protein Redox Chemistry: Post-Translational Cysteine Modifications That Regulate Signal Transduction and Drug Pharmacology. Front. Pharmacol. 2014;5:224. doi: 10.3389/fphar.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong C.M., Cheema A.K., Zhang L., Suzuki Y.J. Protein Carbonylation as a Novel Mechanism in Redox Signaling. Circ. Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 20.Anand P., Stamler J.S. Enzymatic Mechanisms Regulating Protein S-Nitrosylation: Implications in Health and Disease. J. Mol. Med. 2012;90:233–244. doi: 10.1007/s00109-012-0878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sies H. Role of Metabolic H2O2 Generation: Redox Signaling and Oxidative Stress. J. Biol. Chem. 2014;289:8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao C.C., Ma Y.S., Stadtman E.R. Modification of Protein Surface Hydrophobicity and Methionine Oxidation by Oxidative Systems. Proc. Natl. Acad. Sci. USA. 1997;94:2969–2974. doi: 10.1073/pnas.94.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winterbourn C.C., Hampton M.B. Thiol Chemistry and Specificity in Redox Signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Mailloux R.J., Jin X., Willmore W.G. Redox Regulation of Mitochondrial Function with Emphasis on Cysteine Oxidation Reactions. Redox Biol. 2014;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Conte M., Carroll K.S. The Redox Biochemistry of Protein Sulfenylation and Sulfinylation. J. Biol. Chem. 2013;288:26480–26488. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole L.B., Nelson K.J. Discovering Mechanisms of Signaling-Mediated Cysteine Oxidation. Curr. Opin. Chem. Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugo M., Turell L., Manta B., Botti H., Monteiro G., Netto L.E.S., Alvarez B., Radi R., Trujillo M. Thiol and Sulfenic Acid Oxidation of AhpE, the One-Cysteine Peroxiredoxin from Mycobacterium Tuberculosis: Kinetics, Acidity Constants, and Conformational Dynamics. Biochemistry. 2009;48:9416–9426. doi: 10.1021/bi901221s. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Ye Z.-W., Singh S., Townsend D.M., Tew K.D. An Evolving Understanding of the S-Glutathionylation Cycle in Pathways of Redox Regulation. Free Radic. Biol. Med. 2018;120:204–216. doi: 10.1016/j.freeradbiomed.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole L.B. The Basics of Thiols and Cysteines in Redox Biology and Chemistry. Free Radic. Biol. Med. 2015;80:148–157. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta V., Carroll K.S. Sulfenic Acid Chemistry, Detection and Cellular Lifetime. Biochim. Biophys. Acta Gen. Subj. 2014;1840:847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Kaya A., Lee B.C., Gladyshev V.N. Regulation of Protein Function by Reversible Methionine Oxidation and the Role of Selenoprotein MsrB1. Antioxid. Redox Signal. 2015;23:814–822. doi: 10.1089/ars.2015.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stadtman E.R., Moskovitz J., Levine R.L. Oxidation of Methionine Residues of Proteins: Biological Consequences. Antioxid. Redox Signal. 2003;5:577–582. doi: 10.1089/152308603770310239. [DOI] [PubMed] [Google Scholar]
- 34.García-Santamarina S., Boronat S., Hidalgo E. Reversible Cysteine Oxidation in Hydrogen Peroxide Sensing and Signal Transduction. Biochemistry. 2014;53:2560–2580. doi: 10.1021/bi401700f. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzawa A. Thioredoxin and Redox Signaling: Roles of the Thioredoxin System in Control of Cell Fate. Arch. Biochem. Biophys. 2017;617:101–105. doi: 10.1016/j.abb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Roorda M., Miljkovic J.L., van Goor H., Henning R.H., Bouma H.R. Spatiotemporal Regulation of Hydrogen Sulfide Signaling in the Kidney. Redox Biol. 2021;43:101961. doi: 10.1016/j.redox.2021.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawa T., Motohashi H., Ihara H., Akaike T. Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides. Biomolecules. 2020;10:1245. doi: 10.3390/biom10091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul B.D., Snyder S.H. H2S Signalling through Protein Sulfhydration and Beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 39.Nagy P., Winterbourn C.C. Rapid Reaction of Hydrogen Sulfide with the Neutrophil Oxidant Hypochlorous Acid to Generate Polysulfides. Chem. Res. Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 40.Frank G.D., Eguchi S. Activation of Tyrosine Kinases by Reactive Oxygen Species in Vascular Smooth Muscle Cells: Significance and Involvement of EGF Receptor Transactivation by Angiotensin II. Antioxid. Redox Signal. 2003;5:771–780. doi: 10.1089/152308603770380070. [DOI] [PubMed] [Google Scholar]
- 41.Doulias P.-T., Tenopoulou M., Greene J.L., Raju K., Ischiropoulos H. Nitric Oxide Regulates Mitochondrial Fatty Acid Metabolism Through Reversible Protein S-Nitrosylation. Sci. Signal. 2013;6:rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H.-L., Zhang R., Anand P., Stomberski C.T., Qian Z., Hausladen A., Wang L., Rhee E.P., Parikh S.M., Karumanchi S.A., et al. Metabolic Reprogramming by the S-Nitroso-CoA Reductase System Protects against Kidney Injury. Nature. 2019;565:96–100. doi: 10.1038/s41586-018-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang Y., Siow Y.L., Isaak C.K., O K. Downregulation of Glutathione Biosynthesis Contributes to Oxidative Stress and Liver Dysfunction in Acute Kidney Injury. [(accessed on 5 February 2021)]; doi: 10.1155/2016/9707292. Available online: https://www.hindawi.com/journals/omcl/2016/9707292/ [DOI] [PMC free article] [PubMed]
- 44.Aparicio-Trejo O.E., Reyes-Fermín L.M., Briones-Herrera A., Tapia E., León-Contreras J.C., Hernández-Pando R., Sánchez-Lozada L.G., Pedraza-Chaverri J. Protective Effects of N-Acetyl-Cysteine in Mitochondria Bioenergetics, Oxidative Stress, Dynamics and S-Glutathionylation Alterations in Acute Kidney Damage Induced by Folic Acid. Free Radic. Biol. Med. 2019;130:379–396. doi: 10.1016/j.freeradbiomed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Noh M.R., Kim K.Y., Han S.J., Kim J.I., Kim H.-Y., Park K.M. Methionine Sulfoxide Reductase A Deficiency Exacerbates Cisplatin-Induced Nephrotoxicity via Increased Mitochondrial Damage and Renal Cell Death. Antioxid. Redox Signal. 2017;27:727–741. doi: 10.1089/ars.2016.6874. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.I., Choi S.H., Jung K.-J., Lee E., Kim H.-Y., Park K.M. Protective Role of Methionine Sulfoxide Reductase A against Ischemia/Reperfusion Injury in Mouse Kidney and Its Involvement in the Regulation of Trans-Sulfuration Pathway. Antioxid. Redox Signal. 2013;18:2241–2250. doi: 10.1089/ars.2012.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung K.-J., Jang H.-S., Kim J.I., Han S.J., Park J.-W., Park K.M. Involvement of Hydrogen Sulfide and Homocysteine Transsulfuration Pathway in the Progression of Kidney Fibrosis after Ureteral Obstruction. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832:1989–1997. doi: 10.1016/j.bbadis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Kim J.I., Noh M.R., Kim K.Y., Jang H.-S., Kim H.-Y., Park K.M. Methionine Sulfoxide Reductase A Deficiency Exacerbates Progression of Kidney Fibrosis Induced by Unilateral Ureteral Obstruction. Free Radic. Biol. Med. 2015;89:201–208. doi: 10.1016/j.freeradbiomed.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 49.Lai L., Sun J., Tarafdar S., Liu C., Murphy E., Kim G., Levine R.L. Loss of Methionine Sulfoxide Reductases Increases Resistance to Oxidative Stress. Free Radic. Biol. Med. 2019;145:374–384. doi: 10.1016/j.freeradbiomed.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao X., Bian J.-S. The Role of Hydrogen Sulfide in Renal System. Front. Pharmacol. 2016;7:385. doi: 10.3389/fphar.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasinath B.S., Feliers D., Lee H.J. Hydrogen Sulfide as a Regulatory Factor in Kidney Health and Disease. Biochem. Pharmacol. 2018;149:29–41. doi: 10.1016/j.bcp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Ngowi E.E., Sarfraz M., Afzal A., Khan N.H., Khattak S., Zhang X., Li T., Duan S.-F., Ji X.-Y., Wu D.-D. Roles of Hydrogen Sulfide Donors in Common Kidney Diseases. Front. Pharmacol. 2020;11:1706. doi: 10.3389/fphar.2020.564281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han S.J., Kim J.I., Park J.-W., Park K.M. Hydrogen Sulfide Accelerates the Recovery of Kidney Tubules after Renal Ischemia/Reperfusion Injury. Nephrol. Dial. Transplant. 2015;30:1497–1506. doi: 10.1093/ndt/gfv226. [DOI] [PubMed] [Google Scholar]
- 54.Bos E.M., Wang R., Snijder P.M., Boersema M., Damman J., Fu M., Moser J., Hillebrands J.-L., Ploeg R.J., Yang G., et al. Cystathionine γ-Lyase Protects against Renal Ischemia/Reperfusion by Modulating Oxidative Stress. J. Am. Soc. Nephrol. 2013;24:759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H., Feng S.-J., Zhang G.-Z., Wang S.-X. Correlation of Lower Concentrations of Hydrogen Sulfide with Atherosclerosis in Chronic Hemodialysis Patients with Diabetic Nephropathy. Blood Purif. 2014;38:188–194. doi: 10.1159/000368883. [DOI] [PubMed] [Google Scholar]
- 56.Song K., Wang F., Li Q., Shi Y.-B., Zheng H.-F., Peng H., Shen H.-Y., Liu C.-F., Hu L.-F. Hydrogen Sulfide Inhibits the Renal Fibrosis of Obstructive Nephropathy. Kidney Int. 2014;85:1318–1329. doi: 10.1038/ki.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S Signals through Protein S-Sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarosz A.P., Wei W., Gauld J.W., Auld J., Özcan F., Aslan M., Mutus B. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) Is Inactivated by S-Sulfuration in Vitro. Free Radic. Biol. Med. 2015;89:512–521. doi: 10.1016/j.freeradbiomed.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Motohashi H., Yamamoto M. Nrf2–Keap1 Defines a Physiologically Important Stress Response Mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Bianco M., Lopes J.A., Beiral H.J.V., Filho J.D.D., Frankenfeld S.P., Fortunato R.S., Gattass C.R., Vieyra A., Takiya C.M. The Contralateral Kidney Presents with Impaired Mitochondrial Functions and Disrupted Redox Homeostasis after 14 Days of Unilateral Ureteral Obstruction in Mice. PLoS ONE. 2019;14:e0218986. doi: 10.1371/journal.pone.0218986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong W., Fu J., Liu N., Jiao C., Guo G., Luan J., Wang H., Yao L., Wang L., Yamamoto M., et al. Nrf2 Deficiency Promotes the Progression from Acute Tubular Damage to Chronic Renal Fibrosis Following Unilateral Ureteral Obstruction. Nephrol. Dial. Transplant. 2018;33:771–783. doi: 10.1093/ndt/gfx299. [DOI] [PubMed] [Google Scholar]
- 62.Ge S.-N., Zhao M.-M., Wu D.-D., Chen Y., Wang Y., Zhu J.-H., Cai W.-J., Zhu Y.-Z., Zhu Y.-C. Hydrogen Sulfide Targets EGFR Cys797/Cys798 Residues to Induce Na+/K+-ATPase Endocytosis and Inhibition in Renal Tubular Epithelial Cells and Increase Sodium Excretion in Chronic Salt-Loaded Rats. Antioxid. Redox Signal. 2014;21:2061–2082. doi: 10.1089/ars.2013.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shirozu K., Tokuda K., Marutani E., Lefer D., Wang R., Ichinose F. Cystathionine γ-Lyase Deficiency Protects Mice from Galactosamine/Lipopolysaccharide-Induced Acute Liver Failure. Antioxid. Redox Signal. 2014;20:204–216. doi: 10.1089/ars.2013.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yadav P.K., Martinov M., Vitvitsky V., Seravalli J., Wedmann R., Filipovic M.R., Banerjee R. Biosynthesis and Reactivity of Cysteine Persulfides in Signaling. J. Am. Chem. Soc. 2016;138:289–299. doi: 10.1021/jacs.5b10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano S., Ishii I., Shinmura K., Tamaki K., Hishiki T., Akahoshi N., Ida T., Nakanishi T., Kamata S., Kumagai Y., et al. Hyperhomocysteinemia Abrogates Fasting-Induced Cardioprotection against Ischemia/Reperfusion by Limiting Bioavailability of Hydrogen Sulfide Anions. J. Mol. Med. 2015;93:879–889. doi: 10.1007/s00109-015-1271-5. [DOI] [PubMed] [Google Scholar]
- 66.Akaike T., Ida T., Wei F.-Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., et al. Cysteinyl-TRNA Synthetase Governs Cysteine Polysulfidation and Mitochondrial Bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karnati S., Lüers G., Pfreimer S., Baumgart-Vogt E. Mammalian SOD2 Is Exclusively Located in Mitochondria and Not Present in Peroxisomes. Histochem. Cell Biol. 2013;140:105–117. doi: 10.1007/s00418-013-1099-4. [DOI] [PubMed] [Google Scholar]
- 68.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Yamakura F., Kawasaki H. Post-Translational Modifications of Superoxide Dismutase. Biochim. Biophys. Acta. 2010;1804:318–325. doi: 10.1016/j.bbapap.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Walker L.M., Walker P.D., Imam S.Z., Ali S.F., Mayeux P.R. Evidence for Peroxynitrite Formation in Renal Ischemia-Reperfusion Injury: Studies with the Inducible Nitric Oxide Synthase Inhibitor L-N(6)-(1-Iminoethyl)Lysine. J. Pharmacol. Exp. Ther. 2000;295:417–422. [PubMed] [Google Scholar]
- 71.MacMillan-Crow L.A., Crow J.P., Kerby J.D., Beckman J.S., Thompson J.A. Nitration and Inactivation of Manganese Superoxide Dismutase in Chronic Rejection of Human Renal Allografts. Proc. Natl. Acad. Sci. USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacMillan-Crow L.A., Cruthirds D.L., Ahki K.M., Sanders P.W., Thompson J.A. Mitochondrial Tyrosine Nitration Precedes Chronic Allograft Nephropathy. Free Radic. Biol. Med. 2001;31:1603–1608. doi: 10.1016/S0891-5849(01)00750-X. [DOI] [PubMed] [Google Scholar]
- 73.MacMillan-Crow L.A., Crow J.P., Thompson J.A. Peroxynitrite-Mediated Inactivation of Manganese Superoxide Dismutase Involves Nitration and Oxidation of Critical Tyrosine Residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 74.Cruthirds D.L., Novak L., Akhi K.M., Sanders P.W., Thompson J.A., MacMillan-Crow L.A. Mitochondrial Targets of Oxidative Stress during Renal Ischemia/Reperfusion. Arch. Biochem. Biophys. 2003;412:27–33. doi: 10.1016/S0003-9861(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 75.Xu S., Jiang B., Maitland K.A., Bayat H., Gu J., Nadler J.L., Corda S., Lavielle G., Verbeuren T.J., Zuccollo A., et al. The Thromboxane Receptor Antagonist S18886 Attenuates Renal Oxidant Stress and Proteinuria in Diabetic Apolipoprotein E-Deficient Mice. Diabetes. 2006;55:110–119. doi: 10.2337/diabetes.55.01.06.db05-0831. [DOI] [PubMed] [Google Scholar]
- 76.Wang H.D., Xu S., Johns D.G., Du Y., Quinn M.T., Cayatte A.J., Cohen R.A. Role of NADPH Oxidase in the Vascular Hypertrophic and Oxidative Stress Response to Angiotensin II in Mice. Circ. Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 77.Guo W., Adachi T., Matsui R., Xu S., Jiang B., Zou M.-H., Kirber M., Lieberthal W., Cohen R.A. Quantitative Assessment of Tyrosine Nitration of Manganese Superoxide Dismutase in Angiotensin II-Infused Rat Kidney. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- 78.Majzunova M., Kvandova M., Berenyiova A., Balis P., Dovinova I., Cacanyiova S. Chronic NOS Inhibition Affects Oxidative State and Antioxidant Response Differently in the Kidneys of Young Normotensive and Hypertensive Rats. Oxidative Med. Cell. Longev. 2019;2019:5349398. doi: 10.1155/2019/5349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kimura T., Shiizaki K., Akimoto T., Shinzato T., Shimizu T., Kurosawa A., Kubo T., Nanmoku K., Kuro-O M., Yagisawa T. The Impact of Preserved Klotho Gene Expression on Antioxidative Stress Activity in Healthy Kidney. Am. J. Physiol. Physiol. 2018;315:F345–F352. doi: 10.1152/ajprenal.00486.2017. [DOI] [PubMed] [Google Scholar]
- 80.Bedard K., Krause K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 81.Babelova A., Avaniadi D., Jung O., Fork C., Beckmann J., Kosowski J., Weissmann N., Anilkumar N., Shah A.M., Schaefer L., et al. Role of Nox4 in Murine Models of Kidney Disease. Free Radic. Biol. Med. 2012;53:842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 82.Serrander L., Cartier L., Bedard K., Banfi B., Lardy B., Plastre O., Sienkiewicz A., Fórró L., Schlegel W., Krause K.-H. NOX4 Activity Is Determined by MRNA Levels and Reveals a Unique Pattern of ROS Generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H., Jose P.A. Coordinated Contribution of NADPH Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Metabolic Syndrome and Its Implication in Renal Dysfunction. Front. Pharmacol. 2021;12:670076. doi: 10.3389/fphar.2021.670076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molina-Jijón E., Aparicio-Trejo O.E., Rodríguez-Muñoz R., León-Contreras J.C., Cárdenas-Aguayo M.d.C., Medina-Campos O.N., Tapia E., Sánchez-Lozada L.G., Hernández-Pando R., Reyes J.L., et al. The Nephroprotection Exerted by Curcumin in Maleate-Induced Renal Damage Is Associated with Decreased Mitochondrial Fission and Autophagy: Curcumin Decreases Maleate-Induced Renal Dysfunction. BioFactors. 2016;42:686–702. doi: 10.1002/biof.1313. [DOI] [PubMed] [Google Scholar]
- 85.Kim S., Kim S.J., Yoon H.E., Chung S., Choi B.S., Park C.W., Shin S.J. Fimasartan, a Novel Angiotensin-Receptor Blocker, Protects against Renal Inflammation and Fibrosis in Mice with Unilateral Ureteral Obstruction: The Possible Role of Nrf2. Int. J. Med. Sci. 2015;12:891–904. doi: 10.7150/ijms.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dikalov S. Cross Talk between Mitochondria and NADPH Oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wenzel P., Mollnau H., Oelze M., Schulz E., Wickramanayake J.M.D., Müller J., Schuhmacher S., Hortmann M., Baldus S., Gori T., et al. First Evidence for a Crosstalk Between Mitochondrial and NADPH Oxidase-Derived Reactive Oxygen Species in Nitroglycerin-Triggered Vascular Dysfunction. Antioxid. Redox Signal. 2008;10:1435–1448. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 88.Daiber A. Redox Signaling (Cross-Talk) from and to Mitochondria Involves Mitochondrial Pores and Reactive Oxygen Species. Biochim. Biophys. Acta Bioenerg. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 89.Hallan S., Sharma K. The Role of Mitochondria in Diabetic Kidney Disease. Curr. Diabetes Rep. 2016;16:61. doi: 10.1007/s11892-016-0748-0. [DOI] [PubMed] [Google Scholar]
- 90.Rathore R., Zheng Y.-M., Niu C.-F., Liu Q.-H., Korde A., Ho Y.-S., Wang Y.-X. Hypoxia Activates NADPH Oxidase to Increase [ROS]i and [Ca2+]i through Mitochondrial ROS–PKCε Signaling Axis in Pulmonary Artery Smooth Muscle Cells. Free Radic. Biol. Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aparicio-Trejo O.E., Tapia E., Sánchez-Lozada L.G., Pedraza-Chaverri J. Mitochondrial Bioenergetics, Redox State, Dynamics and Turnover Alterations in Renal Mass Reduction Models of Chronic Kidney Diseases and Their Possible Implications in the Progression of This Illness. Pharmacol. Res. 2018;135:1–11. doi: 10.1016/j.phrs.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 92.Cheng X., Zheng X., Song Y., Qu L., Tang J., Meng L., Wang Y. Apocynin Attenuates Renal Fibrosis via Inhibition of NOXs-ROS-ERK-Myofibroblast Accumulation in UUO Rats. Free Radic. Res. 2016;50:840–852. doi: 10.1080/10715762.2016.1181757. [DOI] [PubMed] [Google Scholar]
- 93.Asaba K., Tojo A., Onozato M.L., Goto A., Quinn M.T., Fujita T., Wilcox C.S. Effects of NADPH Oxidase Inhibitor in Diabetic Nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 94.Aranda-Rivera A.K., Cruz-Gregorio A., Aparicio-Trejo O.E., Ortega-Lozano A.J., Pedraza-Chaverri J. Redox Signaling Pathways in Unilateral Ureteral Obstruction (UUO)-Induced Renal Fibrosis. Free Radic. Biol. Med. 2021;172:65–81. doi: 10.1016/j.freeradbiomed.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 95.Jain M., Rivera S., Monclus E.A., Synenki L., Zirk A., Eisenbart J., Feghali-Bostwick C., Mutlu G.M., Budinger G.R.S., Chandel N.S. Mitochondrial Reactive Oxygen Species Regulate Transforming Growth Factor-β Signaling. J. Biol. Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuwabara N., Tamada S., Iwai T., Teramoto K., Kaneda N., Yukimura T., Nakatani T., Miura K. Attenuation of Renal Fibrosis by Curcumin in Rat Obstructive Nephropathy. Urology. 2006;67:440–446. doi: 10.1016/j.urology.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 97.Herb M., Gluschko A., Wiegmann K., Farid A., Wolf A., Utermöhlen O., Krut O., Krönke M., Schramm M. Mitochondrial Reactive Oxygen Species Enable Proinflammatory Signaling through Disulfide Linkage of NEMO. Sci. Signal. 2019;12:eaar5926. doi: 10.1126/scisignal.aar5926. [DOI] [PubMed] [Google Scholar]
- 98.Li J., Sun Y.B.Y., Chen W., Fan J., Li S., Qu X., Chen Q., Chen R., Zhu D., Zhang J., et al. Smad4 Promotes Diabetic Nephropathy by Modulating Glycolysis and OXPHOS. EMBO Rep. 2020;21:e48781. doi: 10.15252/embr.201948781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hahn A., Zuryn S. Mitochondrial Genome (MtDNA) Mutations That Generate Reactive Oxygen Species. Antioxidants. 2019;8:392. doi: 10.3390/antiox8090392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo C., Sun L., Chen X., Zhang D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regener. Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alsahli M., Gerich J.E. Renal Glucose Metabolism in Normal Physiological Conditions and in Diabetes. Diabetes Res. Clin. Pract. 2017;133:1–9. doi: 10.1016/j.diabres.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 102.Pfaller W., Rittinger M. Quantitative Morphology of the Rat Kidney. Int. J. Biochem. 1980;12:17–22. doi: 10.1016/0020-711X(80)90035-X. [DOI] [PubMed] [Google Scholar]
- 103.Simon N., Hertig A. Alteration of Fatty Acid Oxidation in Tubular Epithelial Cells: From Acute Kidney Injury to Renal Fibrogenesis. Front. Med. 2015;2:52. doi: 10.3389/fmed.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Markwell M.A., McGroarty E.J., Bieber L.L., Tolbert N.E. The Subcellular Distribution of Carnitine Acyltransferases in Mammalian Liver and Kidney. A New Peroxisomal Enzyme. J. Biol. Chem. 1973;248:3426–3432. doi: 10.1016/S0021-9258(19)43946-X. [DOI] [PubMed] [Google Scholar]
- 105.Stadler K., Goldberg I.J., Susztak K. The Evolving Understanding of the Contribution of Lipid Metabolism to Diabetic Kidney Disease. Curr. Diabetes Rep. 2015;15:40. doi: 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang H.M., Ahn S.H., Choi P., Ko Y.-A., Han S.H., Chinga F., Park A.S.D., Tao J., Sharma K., Pullman J., et al. Defective Fatty Acid Oxidation in Renal Tubular Epithelial Cells Has a Key Role in Kidney Fibrosis Development. Nat. Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fedorova L.V., Tamirisa A., Kennedy D.J., Haller S.T., Budnyy G., Shapiro J.I., Malhotra D. Mitochondrial Impairment in the Five-Sixth Nephrectomy Model of Chronic Renal Failure: Proteomic Approach. BMC Nephrol. 2013;14:209. doi: 10.1186/1471-2369-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aparicio-Trejo O.E., Avila-Rojas S.H., Tapia E., Rojas-Morales P., León-Contreras J.C., Martínez-Klimova E., Hernández-Pando R., Sánchez-Lozada L.G., Pedraza-Chaverri J. Chronic Impairment of Mitochondrial Bioenergetics and β-Oxidation Promotes Experimental AKI-to-CKD Transition Induced by Folic Acid. Free Radic. Biol. Med. 2020;154:18–32. doi: 10.1016/j.freeradbiomed.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 109.Briones-Herrera A., Ramírez-Camacho I., Zazueta C., Tapia E., Pedraza-Chaverri J. Altered Proximal Tubule Fatty Acid Utilization, Mitophagy, Fission and Supercomplexes Arrangement in Experimental Fanconi Syndrome Are Ameliorated by Sulforaphane-Induced Mitochondrial Biogenesis. Free Radic. Biol. Med. 2020;153:54–70. doi: 10.1016/j.freeradbiomed.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Tan S.M., Ziemann M., Thallas-Bonke V., Snelson M., Kumar V., Laskowski A., Nguyen T.-V., Huynh K., Clarke M.V., Libianto R., et al. Complement C5a Induces Renal Injury in Diabetic Kidney Disease by Disrupting Mitochondrial Metabolic Agility. Diabetes. 2020;69:83–98. doi: 10.2337/db19-0043. [DOI] [PubMed] [Google Scholar]
- 111.Aparicio-Trejo O.E., Rojas-Morales P., Avila-Rojas S.H., León-Contreras J.C., Hernández-Pando R., Jiménez-Uribe A.P., Prieto-Carrasco R., Sánchez-Lozada L.G., Pedraza-Chaverri J., Tapia E. Temporal Alterations in Mitochondrial β-Oxidation and Oxidative Stress Aggravate Chronic Kidney Disease Development in 5/6 Nephrectomy Induced Renal Damage. Int. J. Mol. Sci. 2020;21:6512. doi: 10.3390/ijms21186512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gai Z., Wang T., Visentin M., Kullak-Ublick G.A., Fu X., Wang Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients. 2019;11:722. doi: 10.3390/nu11040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishi H., Higashihara T., Inagi R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients. 2019;11:1664. doi: 10.3390/nu11071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herman-Edelstein M., Scherzer P., Tobar A., Levi M., Gafter U. Altered Renal Lipid Metabolism and Renal Lipid Accumulation in Human Diabetic Nephropathy. J. Lipid Res. 2014;55:561–572. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Declèves A.-E., Zolkipli Z., Satriano J., Wang L., Nakayama T., Rogac M., Le T.P., Nortier J.L., Farquhar M.G., Naviaux R.K., et al. Regulation of Lipid Accumulation by AMP-Activated Kinase [Corrected] in High Fat Diet-Induced Kidney Injury. Kidney Int. 2014;85:611–623. doi: 10.1038/ki.2013.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moosavi S.M.S., Ashtiyani S.C., Hosseinkhani S., Shirazi M. Comparison of the Effects of L: -Carnitine and Alpha-Tocopherol on Acute Ureteral Obstruction-Induced Renal Oxidative Imbalance and Altered Energy Metabolism in Rats. Urol. Res. 2010;38:187–194. doi: 10.1007/s00240-009-0238-9. [DOI] [PubMed] [Google Scholar]
- 117.Bobulescu I.A. Renal Lipid Metabolism and Lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010;19:393–402. doi: 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okamura D.M., Pennathur S., Pasichnyk K., López-Guisa J.M., Collins S., Febbraio M., Heinecke J., Eddy A.A. CD36 Regulates Oxidative Stress and Inflammation in Hypercholesterolemic CKD. J. Am. Soc. Nephrol. 2009;20:495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu J.-J., Ghosh S., Kovalik J.-P., Ching J., Choi H.W., Tavintharan S., Ong C.N., Sum C.F., Summers S.A., Tai E.S., et al. Profiling of Plasma Metabolites Suggests Altered Mitochondrial Fuel Usage and Remodeling of Sphingolipid Metabolism in Individuals With Type 2 Diabetes and Kidney Disease. Kidney Int. Rep. 2017;2:470–480. doi: 10.1016/j.ekir.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prieto-Carrasco R., García-Arroyo F.E., Aparicio-Trejo O.E., Rojas-Morales P., León-Contreras J.C., Hernández-Pando R., Sánchez-Lozada L.G., Tapia E., Pedraza-Chaverri J. Progressive Reduction in Mitochondrial Mass Is Triggered by Alterations in Mitochondrial Biogenesis and Dynamics in Chronic Kidney Disease Induced by 5/6 Nephrectomy. Biology. 2021;10:349. doi: 10.3390/biology10050349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miguel V., Tituaña J., Herrero J.I., Herrero L., Serra D., Cuevas P., Barbas C., Puyol D.R., Márquez-Expósito L., Ruiz-Ortega M., et al. Renal Tubule Cpt1a Overexpression Protects from Kidney Fibrosis by Restoring Mitochondrial Homeostasis. J. Clin. Investig. 2021;131 doi: 10.1172/JCI140695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cardoso A.R., Kakimoto P.A.H.B., Kowaltowski A.J. Diet-Sensitive Sources of Reactive Oxygen Species in Liver Mitochondria: Role of Very Long Chain Acyl-CoA Dehydrogenases. PLoS ONE. 2013;8:e77088. doi: 10.1371/journal.pone.0077088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kakimoto P.A.H.B., Tamaki F.K., Cardoso A.R., Marana S.R., Kowaltowski A.J. H2O2 Release from the Very Long Chain Acyl-CoA Dehydrogenase. Redox Biol. 2015;4:375–380. doi: 10.1016/j.redox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walport M.J. Complement. [(accessed on 18 June 2021)]; Available online: https://www.nejm.org/doi/10.1056/NEJM200104053441406.
- 125.Wallace D.C. Mitochondria and Cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Z., Zhang H., Yi B., Yang S., Liu J., Hu J., Wang J., Cao K., Zhang W. VDR Activation Attenuate Cisplatin Induced AKI by Inhibiting Ferroptosis. Cell Death Dis. 2020;11:73. doi: 10.1038/s41419-020-2256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deng B., Yang W., Wang D., Cheng L., Bu L., Rao J., Zhang J., Xie J., Zhang B. Peptide DR8 Suppresses Epithelial-to-Mesenchymal Transition via the TGF-β/MAPK Signaling Pathway in Renal Fibrosis. Life Sci. 2020;261:118465. doi: 10.1016/j.lfs.2020.118465. [DOI] [PubMed] [Google Scholar]
- 128.Aparicio-Trejo O.E., Tapia E., Molina-Jijón E., Medina-Campos O.N., Macías-Ruvalcaba N.A., León-Contreras J.C., Hernández-Pando R., García-Arroyo F.E., Cristóbal M., Sánchez-Lozada L.G., et al. Curcumin Prevents Mitochondrial Dynamics Disturbances in Early 5/6 Nephrectomy: Relation to Oxidative Stress and Mitochondrial Bioenergetics. Biofactors. 2017;43:293–310. doi: 10.1002/biof.1338. [DOI] [PubMed] [Google Scholar]
- 129.Ge M., Fontanesi F., Merscher S., Fornoni A. The Vicious Cycle of Renal Lipotoxicity and Mitochondrial Dysfunction. Front. Physiol. 2020;11:732. doi: 10.3389/fphys.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martínez-Klimova E., Aparicio-Trejo O.E., Gómez-Sierra T., Jiménez-Uribe A.P., Bellido B., Pedraza-Chaverri J. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in the Promotion of Fibrosis in Obstructive Nephropathy Induced by Unilateral Ureteral Obstruction. BioFactors. 2020;46:716–733. doi: 10.1002/biof.1673. [DOI] [PubMed] [Google Scholar]
- 131.Cruz-Gregorio A., Manzo-Merino J., Lizano M. Cellular Redox, Cancer and Human Papillomavirus. Virus Res. 2018;246:35–45. doi: 10.1016/j.virusres.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 132.Gyurászová M., Gurecká R., Bábíčková J., Tóthová Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020;2020:5478708. doi: 10.1155/2020/5478708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma N., Wei W., Fan X., Ci X. Farrerol Attenuates Cisplatin-Induced Nephrotoxicity by Inhibiting the Reactive Oxygen Species-Mediated Oxidation, Inflammation, and Apoptotic Signaling Pathways. Front. Physiol. 2019;10:1419. doi: 10.3389/fphys.2019.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Santos N.A.G., Catão C.S., Martins N.M., Curti C., Bianchi M.L.P., Santos A.C. Cisplatin-Induced Nephrotoxicity Is Associated with Oxidative Stress, Redox State Unbalance, Impairment of Energetic Metabolism and Apoptosis in Rat Kidney Mitochondria. Arch. Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 135.Kaeidi A., Taghipour Z., Allahtavakoli M., Fatemi I., Hakimizadeh E., Hassanshahi J. Ameliorating Effect of Troxerutin in Unilateral Ureteral Obstruction Induced Renal Oxidative Stress, Inflammation, and Apoptosis in Male Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393:879–888. doi: 10.1007/s00210-019-01801-4. [DOI] [PubMed] [Google Scholar]
- 136.Hallan S., Afkarian M., Zelnick L.R., Kestenbaum B., Sharma S., Saito R., Darshi M., Barding G., Raftery D., Ju W., et al. Metabolomics and Gene Expression Analysis Reveal Down-Regulation of the Citric Acid (TCA) Cycle in Non-Diabetic CKD Patients. EBioMedicine. 2017;26:68–77. doi: 10.1016/j.ebiom.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim N.H., Hyeon J.S., Kim N.H., Cho A., Lee G., Jang S.Y., Kim M.-K., Lee E.Y., Chung C.H., Ha H., et al. Metabolic Changes in Urine and Serum during Progression of Diabetic Kidney Disease in a Mouse Model. Arch. Biochem. Biophys. 2018;646:90–97. doi: 10.1016/j.abb.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 138.Liu H., Li W., He Q., Xue J., Wang J., Xiong C., Pu X., Nie Z. Mass Spectrometry Imaging of Kidney Tissue Sections of Rat Subjected to Unilateral Ureteral Obstruction. Sci. Rep. 2017;7:41954. doi: 10.1038/srep41954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.-P., et al. Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 140.Chen J., Chen J.-K., Harris R.C. EGF Receptor Deletion in Podocytes Attenuates Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015;26:1115–1125. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dugan L.L., You Y.-H., Ali S.S., Diamond-Stanic M., Miyamoto S., DeCleves A.-E., Andreyev A., Quach T., Ly S., Shekhtman G., et al. AMPK Dysregulation Promotes Diabetes-Related Reduction of Superoxide and Mitochondrial Function. [(accessed on 17 June 2021)]; doi: 10.1172/JCI66218. Available online: https://www.jci.org/articles/view/66218/pdf. [DOI] [PMC free article] [PubMed]
- 142.Mailloux R.J., Hamel R., Appanna V.D. Aluminum Toxicity Elicits a Dysfunctional TCA Cycle and Succinate Accumulation in Hepatocytes. J. Biochem. Mol. Toxicol. 2006;20:198–208. doi: 10.1002/jbt.20137. [DOI] [PubMed] [Google Scholar]
- 143.Mapuskar K.A., Wen H., Holanda D.G., Rastogi P., Steinbach E., Han R., Coleman M.C., Attanasio M., Riley D.P., Spitz D.R., et al. Persistent Increase in Mitochondrial Superoxide Mediates Cisplatin-Induced Chronic Kidney Disease. Redox Biol. 2019;20:98–106. doi: 10.1016/j.redox.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han S.J., Jang H.-S., Noh M.R., Kim J., Kong M.J., Kim J.I., Park J.-W., Park K.M. Mitochondrial NADP+-Dependent Isocitrate Dehydrogenase Deficiency Exacerbates Mitochondrial and Cell Damage after Kidney Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017;28:1200–1215. doi: 10.1681/ASN.2016030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kong M.J., Han S.J., Kim J.I., Park J.-W., Park K.M. Mitochondrial NADP + -Dependent Isocitrate Dehydrogenase Deficiency Increases Cisplatin-Induced Oxidative Damage in the Kidney Tubule Cells. Cell Death Dis. 2018;9:716. doi: 10.1038/s41419-018-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kil I.S., Park J.-W. Regulation of Mitochondrial NADP+-Dependent Isocitrate Dehydrogenase Activity by Glutathionylation. J. Biol. Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 147.Kim J.I., Noh M.R., Yoon G.-E., Jang H.-S., Kong M.J., Park K.M. IDH2 Gene Deficiency Accelerates Unilateral Ureteral Obstruction-Induced Kidney Inflammation through Oxidative Stress and Activation of Macrophages. Korean J. Physiol. Pharmacol. 2021;25:139–146. doi: 10.4196/kjpp.2021.25.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Avila-Rojas S.H., Aparicio-Trejo O.E., Briones-Herrera A., Medina-Campos O.N., Reyes-Fermín L.M., Martínez-Klimova E., León-Contreras J.C., Hernández-Pando R., Tapia E., Pedraza-Chaverri J. Alterations in Mitochondrial Homeostasis in a Potassium Dichromate Model of Acute Kidney Injury and Their Mitigation by Curcumin. Food Chem. Toxicol. 2020;145:111774. doi: 10.1016/j.fct.2020.111774. [DOI] [PubMed] [Google Scholar]
- 149.Mailloux R.J., Harper M.-E. Uncoupling Proteins and the Control of Mitochondrial Reactive Oxygen Species Production. Free Radic. Biol. Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 150.Zhou Y., Cai T., Xu J., Jiang L., Wu J., Sun Q., Zen K., Yang J. UCP2 Attenuates Apoptosis of Tubular Epithelial Cells in Renal Ischemia-Reperfusion Injury. Am. J. Physiol. Physiol. 2017;313:F926–F937. doi: 10.1152/ajprenal.00118.2017. [DOI] [PubMed] [Google Scholar]
- 151.Zhong X., He J., Zhang X., Li C., Tian X., Xia W., Gan H., Xia Y. UCP2 Alleviates Tubular Epithelial Cell Apoptosis in Lipopolysaccharide-Induced Acute Kidney Injury by Decreasing ROS Production. Biomed. Pharmacother. 2019;115:108914. doi: 10.1016/j.biopha.2019.108914. [DOI] [PubMed] [Google Scholar]
- 152.Jiang L., Qiu W., Zhou Y., Wen P., Fang L., Cao H., Zen K., He W., Zhang C., Dai C., et al. A MicroRNA-30e/Mitochondrial Uncoupling Protein 2 Axis Mediates TGF-Β1-Induced Tubular Epithelial Cell Extracellular Matrix Production and Kidney Fibrosis. Kidney Int. 2013;84:285–296. doi: 10.1038/ki.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jia P., Wu X., Pan T., Xu S., Hu J., Ding X. Uncoupling Protein 1 Inhibits Mitochondrial Reactive Oxygen Species Generation and Alleviates Acute Kidney Injury. EBioMedicine. 2019;49:331–340. doi: 10.1016/j.ebiom.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chouchani E.T., Kazak L., Jedrychowski M.P., Lu G.Z., Erickson B.K., Szpyt J., Pierce K.A., Laznik-Bogoslavski D., Vetrivelan R., Clish C.B., et al. Mitochondrial ROS Regulate Thermogenic Energy Expenditure and Sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liesa M., Shirihai O.S. Mitochondrial Dynamics in the Regulation of Nutrient Utilization and Energy Expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shutt T., Geoffrion M., Milne R., McBride H.M. The Intracellular Redox State Is a Core Determinant of Mitochondrial Fusion. EMBO Rep. 2012;13:909–915. doi: 10.1038/embor.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hernansanz-Agustín P., Enríquez J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants. 2021;10:415. doi: 10.3390/antiox10030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kashatus J.A., Nascimento A., Myers L.J., Sher A., Byrne F.L., Hoehn K.L., Counter C.M., Kashatus D.F. Erk2 Phosphorylation of Drp1 Promotes Mitochondrial Fission and MAPK-Driven Tumor Growth. Mol. Cell. 2015;57:537–551. doi: 10.1016/j.molcel.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. Mitotic Phosphorylation of Dynamin-Related GTPase Drp1 Participates in Mitochondrial Fission. J. Biol. Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 160.Qi X., Disatnik M.-H., Shen N., Sobel R.A., Mochly-Rosen D. Aberrant Mitochondrial Fission in Neurons Induced by Protein Kinase C{delta} under Oxidative Stress Conditions in Vivo. Mol. Biol. Cell. 2011;22:256–265. doi: 10.1091/mbc.e10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim Y.-M., Youn S.-W., Sudhahar V., Das A., Chandhri R., Cuervo Grajal H., Kweon J., Leanhart S., He L., Toth P.T., et al. Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Rep. 2018;23:3565–3578. doi: 10.1016/j.celrep.2018.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rojas-Morales P., León-Contreras J.C., Granados-Pineda J., Hernández-Pando R., Gonzaga G., Sánchez-Lozada L.G., Osorio-Alonso H., Pedraza-Chaverri J., Tapia E. Protection against Renal Ischemia and Reperfusion Injury by Short-Term Time-Restricted Feeding Involves the Mitochondrial Unfolded Protein Response. Free Radic. Biol. Med. 2020;154:75–83. doi: 10.1016/j.freeradbiomed.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 163.Brooks C., Wei Q., Cho S.-G., Dong Z. Regulation of Mitochondrial Dynamics in Acute Kidney Injury in Cell Culture and Rodent Models. J. Clin. Investig. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ortega-Domínguez B., Aparicio-Trejo O.E., García-Arroyo F.E., León-Contreras J.C., Tapia E., Molina-Jijón E., Hernández-Pando R., Sánchez-Lozada L.G., Barrera-Oviedo D., Pedraza-Chaverri J. Curcumin Prevents Cisplatin-Induced Renal Alterations in Mitochondrial Bioenergetics and Dynamic. Food Chem. Toxicol. 2017;107:373–385. doi: 10.1016/j.fct.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 165.Perry H.M., Huang L., Wilson R.J., Bajwa A., Sesaki H., Yan Z., Rosin D.L., Kashatus D.F., Okusa M.D. Dynamin-Related Protein 1 Deficiency Promotes Recovery from AKI. J. Am. Soc. Nephrol. 2018;29:194–206. doi: 10.1681/ASN.2017060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bhatia D., Capili A., Choi M.E. Mitochondrial Dysfunction in Kidney Injury, Inflammation, and Disease: Potential Therapeutic Approaches. Kidney Res. Clin. Pract. 2020;39:244–258. doi: 10.23876/j.krcp.20.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li S., Lin Q., Shao X., Zhu X., Wu J., Wu B., Zhang M., Zhou W., Zhou Y., Jin H., et al. Drp1-Regulated PARK2-Dependent Mitophagy Protects against Renal Fibrosis in Unilateral Ureteral Obstruction. Free Radic. Biol. Med. 2020;152:632–649. doi: 10.1016/j.freeradbiomed.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 168.Willems P.H.G.M., Rossignol R., Dieteren C.E.J., Murphy M.P., Koopman W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015;22:207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 169.Liu T., Yang Q., Zhang X., Qin R., Shan W., Zhang H., Chen X. Quercetin Alleviates Kidney Fibrosis by Reducing Renal Tubular Epithelial Cell Senescence through the SIRT1/PINK1/Mitophagy Axis. Life Sci. 2020;257:118116. doi: 10.1016/j.lfs.2020.118116. [DOI] [PubMed] [Google Scholar]
- 170.Sang X.-Y., Xiao J.-J., Liu Q., Zhu R., Dai J.-J., Zhang C., Yu H., Yang S.-J., Zhang B.-F. Regulators of Calcineurin 1 Deficiency Attenuates Tubulointerstitial Fibrosis through Improving Mitochondrial Fitness. FASEB J. 2020;34 doi: 10.1096/fj.202000781RRR. [DOI] [PubMed] [Google Scholar]
- 171.Barsoum M.J., Yuan H., Gerencser A.A., Liot G., Kushnareva Y., Gräber S., Kovacs I., Lee W.D., Waggoner J., Cui J., et al. Nitric Oxide-Induced Mitochondrial Fission Is Regulated by Dynamin-Related GTPases in Neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Villena J.A. New Insights into PGC-1 Coactivators: Redefining Their Role in the Regulation of Mitochondrial Function and Beyond. FEBS J. 2015;282:647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 173.Hock M.B., Kralli A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- 174.Irrcher I., Ljubicic V., Hood D.A. Interactions between ROS and AMP Kinase Activity in the Regulation of PGC-1alpha Transcription in Skeletal Muscle Cells. Am. J. Physiol. Cell Physiol. 2009;296:C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 175.Yan Y., Zhou X.E., Xu H.E., Melcher K. Structure and Physiological Regulation of AMPK. Int. J. Mol. Sci. 2018;19:3534. doi: 10.3390/ijms19113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rabinovitch R.C., Samborska B., Faubert B., Ma E.H., Gravel S.-P., Andrzejewski S., Raissi T.C., Pause A., St-Pierre J., Jones R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 177.Bhatia D., Choi M.E. The Emerging Role of Mitophagy in Kidney Diseases. J. Life Sci. 2019;1:13–22. doi: 10.36069/JoLS/20191203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Y., Cai J., Tang C., Dong Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells. 2020;9:338. doi: 10.3390/cells9020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xiao B., Goh J.-Y., Xiao L., Xian H., Lim K.-L., Liou Y.-C. Reactive Oxygen Species Trigger Parkin/PINK1 Pathway-Dependent Mitophagy by Inducing Mitochondrial Recruitment of Parkin. J. Biol. Chem. 2017;292:16697–16708. doi: 10.1074/jbc.M117.787739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kondapalli C., Kazlauskaite A., Zhang N., Woodroof H.I., Campbell D.G., Gourlay R., Burchell L., Walden H., Macartney T.J., Deak M., et al. PINK1 Is Activated by Mitochondrial Membrane Potential Depolarization and Stimulates Parkin E3 Ligase Activity by Phosphorylating Serine 65. Open Biol. 2012;2:120080. doi: 10.1098/rsob.120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reyes-Fermín L.M., Avila-Rojas S.H., Aparicio-Trejo O.E., Tapia E., Rivero I., Pedraza-Chaverri J. The Protective Effect of Alpha-Mangostin against Cisplatin-Induced Cell Death in LLC-PK1 Cells Is Associated to Mitochondrial Function Preservation. Antioxidants. 2019;8:133. doi: 10.3390/antiox8050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li L., Zepeda-Orozco D., Black R., Lin F. Autophagy Is a Component of Epithelial Cell Fate in Obstructive Uropathy. Am. J. Pathol. 2010;176:1767–1778. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen Q., Yu S., Zhang K., Zhang Z., Li C., Gao B., Zhang W., Wang Y. Exogenous H2S Inhibits Autophagy in Unilateral Ureteral Obstruction Mouse Renal Tubule Cells by Regulating the ROS-AMPK Signaling Pathway. Cell. Physiol. Biochem. 2018;49:2200–2213. doi: 10.1159/000493824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.