Abstract
Hepatic fibrosis is characterized by the pathological accumulation of extracellular matrix (ECM) in the liver resulting from the persistent liver injury and wound-healing reaction induced by various insults. Although hepatic fibrosis is considered reversible after eliminating the cause of injury, chronic injury left unchecked can progress to cirrhosis and liver cancer. A better understanding of the cellular and molecular mechanisms controlling the fibrotic response is needed to develop novel clinical strategies. It is well documented that activated hepatic stellate cells (HSCs) is the most principal cellular players promoting synthesis and deposition of ECM components. In the current review, we discuss pathways of HSC activation, emphasizing emerging extra- and intra-cellular signals that drive this important cellular response to hepatic fibrosis. A number of cell types and external stimuli converge upon HSCs to promote their activation, including hepatocytes, liver sinusoidal endothelial cells, macrophages, cytokines, altered ECM, hepatitis viral infection, enteric dysbiosis, lipid metabolism disorder, exosomes, microRNAs, alcohol, drugs and parasites. We also discuss the emerging signaling pathways and intracellular events that individually or synergistically drive HSC activation, including TGFβ/Smad, Notch, Wnt/β-catenin, Hedgehog and Hippo signaling pathways. These findings will provide novel potential therapeutic targets to arrest or reverse fibrosis and cirrhosis.
Keywords: hepatic fibrosis, hepatic stellate cell, myofibroblast, signal pathway
1. Introduction
Liver fibrosis is the pathologic sequela of chronic repetitive injury and is a reversible healing response in response to acute or chronic cell injury. Further development of liver fibrosis leads to cirrhosis and even liver cancer. Various factors can cause liver fibrosis, the main risk factors identified at present include viral infection, alcoholism, obesity-related steatohepatitis and so on [1,2,3]. Cirrhosis is a major cause of morbidity and mortality globally, imposing a heavy health burden on many countries. Globally, cirrhosis currently causes 1.16 million deaths and is the 11th most common cause of death each year [4]. Cirrhosis imposes a huge economic burden in the United States, with estimated annual direct costs of more than USD 2 billion and indirect costs of more than USD 10 billion [5].
Hepatic stellate cell (HSC) activation represents a critical event in fibrosis [6,7]. In normal liver, HSCs exist in a quiescent non-proliferative state, having a star-like shape with intracellular lipid droplet storage containing vitamin A as retinyl palmitate [8]. HSCs are a type of resident non-mesenchymal cells that have features of both resident fibroblasts (embedded in normal stroma) and pericytes (endothelial cells attached to capillaries). Locating in the space of Disse, HSCs are a major producer of extracellular matrix (ECM) [8,9,10], which accounts for approximately 15% of total resident cells and one third of the total nonparenchymal cells in the normal human liver [11]. Pathological, toxic, metabolic or viral diseases lead to liver cell damage and immune cell infiltration, activating the transdifferentiation of HSCs to myofibroblasts, which is known as “activation”. It is generally believed that HSCs are the main source of myofibroblasts during hepatic fibrosis and are independent of the source of damage [12,13]. In chronic liver disease, the imbalance between the pro-fibrogenic and anti-fibrogenic mechanisms leads to continuous activation of proliferating, contractile, and migrating myofibroblasts, resulting in excessive production of ECM. Large amounts of ECM deposited in the liver lead to liver fibrosis [14]. Here, we review extra- and intra-cellular mechanisms of HSC activation, emphasizing recent emerging cellular and molecular signals that trigger this important cellular response to liver injury.
2. Extracellular Factors of HSC Activation
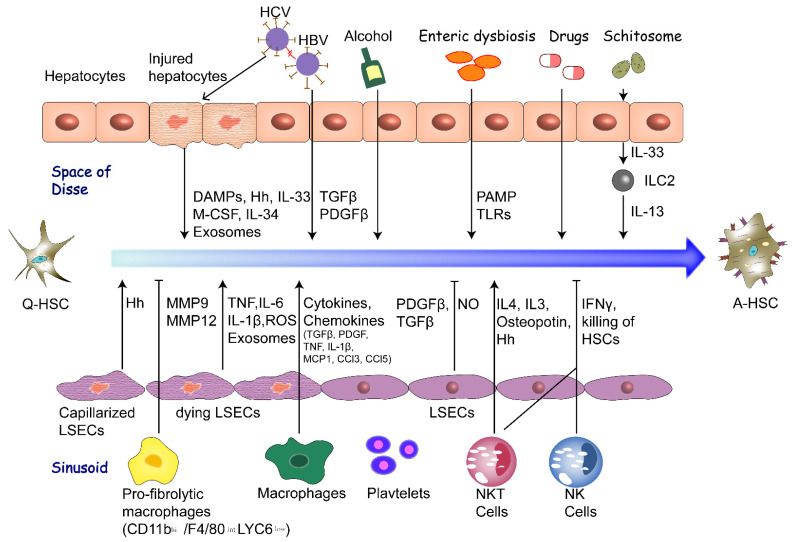
The extracellular factors that promote HSC activation have been identified as stimulation of various cell types, altered extracellular matrix, enteric dysbiosis, chronic infection of hepatitis virus, lipid metabolism disorder, exosomes, microRNA and other factors including alcohol, drugs and parasites (Figure 1).
Figure 1.
Extracellular factors promoting HSC activation. Extracellular factors including stimulation of various cell types, cyto-kines, altered ECM, hepatitis viral infection, enteric dysbiosis, lipid metabolism disorder, exosomes, alcohol, drugs (MTX, APAP) and schistosome promote or inhibit the activation of HSCs through production of various cytokines and other signaling molecules. The characteristics of A-HSCs include proliferation, contractility, fibrogenesis, inflammatory sig-naling, loss of retinoid and enhanced ECM production. HSC, hepatic stellate cell; DAMPs, damage-associated molecular patterns; Hh, Hedgehog signaling; IL-33, interleukin-33; M-CSF, macrophage colony-stimulating factor; TGFβ, Transforming growth factor β; PDGFβ, Platelet derived growth factor β; PAMP, pathogen-associated molecular patterns; TLRs, Toll-like receptors; MMP, matrix metalloproteinases; TNF, tumor necrosis factor; ROS, reactive oxygen species; MCP1, monocyte chemo-tactic protein-1; NO, nitric oxide; IFNγ, interferon γ; NK, natural killer; NKT, natural killer T; MTX, methotrexate; APAP, acetaminophen; hi, high expression; low, low expression; int, intermediate expression.
2.1. Hepatocytes
In response to injury, hepatocytes change their gene expression and secretion profile, and thus affect HSC activation. Damage-associated molecular patterns (DAMPs) released by injured hepatocytes might directly or indirectly promote HSC activation. Nucleotide binding oligomerization domain-like receptors 3 (NLRP3) is one of the main components of inflammasomes and the downstream targets of DAMPs. Mice with the constitutively active mutant NLRP3 develop severe liver inflammation with pyroptotic hepatocyte death and HSC activation [15]. When the liver is damaged, hepatocytes release IL-33, which activates the innate lymphoid cells (ILCs). In the three known cell subsets of ILCs (ILC1, ILC2 and ILC3), ILC2 drives HSC activation and promotes the occurrence of liver fibrosis, demonstrating that hepatocytes promote HSC activation [16]. In addition, damaged hepatocytes, rather than normal hepatocytes, secrete exosomes which contain microRNAs that activate HSCs [17].
2.2. Liver Sinusoidal Endothelial Cells
In the normal liver, liver sinusoidal endothelial cells (LSECs) maintain the quiescence of HSCs through heparin-binding EGF-like growth factor and paracrine factors such as nitric oxide (NO) [18,19]. Normal LSECs are fully differentiated and highly endocytic, which contain fenestrae. Prior to fibrosis, LSECs lose their fenestration and undergo capillarization due to incomplete differentiation of bone marrow-derived LSECs that are recruited to the injured liver, and are permissive for HSC activation [19,20,21]. In rats with thioacetamide-induced cirrhosis, a soluble guanylate cyclase activator, BAY 60-2770, leads to reversal of the capillarization, which further leads to quiescence of HSC and regression of fibrosis [21]. Depending on the injury environment, LSECs may promote either liver regeneration or fibrosis. In particular, the CXCR7–ID1 pathway in LSECs in response to injury promotes liver regeneration, but the FGFR1–CXCR4 pathway promotes HSC activation and fibrosis [22].
2.3. Macrophages
Accumulating evidence suggests that progressive fibrotic diseases, including hepatic fibrosis, are tightly regulated by macrophages [23]. Macrophages play dual roles in liver fibrosis progression and its resolution. Polarized and plastic activation of macrophages is traditionally classified into classic M1 and alternative M2 activation. The M1 phenotype is characterized by high expression of pro-inflammatory cytokines, high production of reactive nitrogen and oxygen intermediates, promotion of Th1 response, while the M2 phenotype characterized by highly efficient phagocytic activity, high expression of scavenging, mannose and galactose receptors and production of ornithine and polyamines via the arginase pathway [24]. During the progression of fibrosis, injury-induced inflammation triggers the recruitment of macrophages to the liver, where they produce cytokines and chemokines including transforming growth factor β (TGFβ), Platelet derived growth factor (PDGF), tumor necrosis factor (TNF), IL-1β, monocyte chemotactic protein-1 (MCP1), CCL3 and CCL5 to induce HSC activation. The recruitment of immature monocyte-derived LY6Chi macrophages are facilitated by CCL2 secreted by Kupffer cells and HSCs. However, during the regression of liver fibrosis, macrophages have a CD11bhi/F4/80intLY6Clow phenotype which is arisen from a phenotypic switch of profibrogenic LY6Chi macrophages. The LY6Clow phenotype stops the production of fibrogenic and inflammatory factors, and instead secrete matrix metalloproteinases (MMPs) such as MMP9 that promotes HSC apoptosis and MMP12 [18,25].
2.4. Fibrogenic Cytokines
TGFβ is generally considered to be the most impotent fibrogenic cytokine, as described in more detail in the following article. The downstream connective tissue growth factor (CTGF) of TGFβ is a key fibrogenic cytokine that accelerates the activation of HSCs. It has been reported that TGFβ induces the expression of CTGF through Smad and Stat3 signaling pathways in HSCs. In A-HSCs, the pro-fibrotic CTGF is also upregulated and promotes the pathogenetic processes of hepatic fibrosis, including cell proliferation, contractility, migration and ECM production [26]. A-HSCs release CTGF and other pro-fibrogenic factors which drive the deposition of ECM [27].
Interleukin plays an important role in the activation of hepatic stellate cells. interleukin-13 (IL-13) is an immunoregulatory cytokine secreted mainly by a T-cell subset termed Th2 cells. In HSCs, IL-13 directly induces the expression of collagen I and other key fibrosis-related genes such as α-smooth muscle actin (α-SMA) [28,29]. IL-13 also induces CTGF through the Erk-mitogen-activated protein kinase (MAPK) pathway to accelerate the activation of HSCs [30]. Damaged hepatocytes secreted IL-33 which leads to accumulation and activation of innate lymphoid cells (ILC2). ILC2 Activated by IL-33 produce IL-13, inducing the activation and trans-differentiation of HSCs [16,28]. IL-17 is produced mainly by Th17 cells, but can also be produced by neutrophils and other lymphocytes. IL-17 induces the production of collagen I in HSCs by activating the STAT3 signaling pathway and pharmacological inhibition of IL-17-induced ERK1/2 or p38 significantly reduces HSCs activation and collagen expression [28].
Dead or dying endothelial cells and white blood cells release inflammatory mediators, DAMPs or danger signals, which initiate a noninfectious “sterile” inflammatory response. Among them, TNF, IL-6, IL-1β, reactive oxygen species (ROS), and Hedgehog (Hh) ligand can facilitate the initiation process of HSC activation [31,32]. TNF and IL-1β cannot directly promote HSC activation, but they prolong the survival of A-HSCs through activating NF-κB signaling pathway both in vivo and in vitro [33]. ROS provides paracrine activation signals to HSCs. When the transmembrane enzyme complex Nox1 or Nox4 regulating ROS is inactivated, liver injury, inflammation and fibrosis were significantly reduced [34].
Platelets are also important cells involved in inflammation, and PDGF and TGFβ produced by them are important cytokines that induce HSC activation [35]. PDGF is an important mitogen in the liver and one of the chemokines that promote the proliferation and migration of HSCs. Studies in humans and rodents have shown that PDGF ligands and receptors are rapidly expressed in HSCs at the onset of liver injury.
2.5. Altered ECM
A-HSCs are a major producer of ECM, and the alteration of ECM also affects HSC activation. In normal liver, laminins, type IV collagen and a mixture of proteoglycans are scattered within the hepatic ECM. HSCs express two types of collagen receptors: integrins and discoidin domain-containing receptors, and each type receives signals from ECM components to regulate cell adhesion, differentiation, proliferation and migration [25]. HSCs secrete a large amount of ECM after activation, and then progressive deposition of ECM proteins in the space of Disse gradually leads to increased density and stiffness of ECM. Furthermore, matrix composition shifts from collagen type IV, heparan sulfate proteoglycan, and laminin to fibrillar collagen type I and III. These changes act as mechanical stimuli to activate HSCs at least partially through integrin signaling pathways, forming positive feedback loops [31]. In addition, the expanded ECM promotes proliferation of HSCs by binding PDGF, hepatocyte growth factor (HGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) and other growth factors to play a storage role [36]. Furthermore, extracellular matrix protein 1 (ECM1) produced by hepatocytes interacts with αv integrins to stabilize extracellular matrix-deposited TGFβ to prevent HSC activation [37].
2.6. Enteric Dysbiosis
Gut microbes have many physiological functions, such as producing vitamin B series, digesting food particles and gaining energy from them, promoting the host immunity and antagonizing foreign invaders. Moreover, gut microbes can affect the normal physiological function of the liver through enterohepatic circulation. Normally, gut microbes play a protective role in the liver. For instance, commensal microbiota has a hepatoprotective effect, preventing liver fibrosis by reducing HSC activation [38]. However, enteric dysbiosis can cause pathological changes in the liver through HSC activation. Intestinal dysbiosis leads to release and increased exposure to pathogen-associated molecular patterns (PAMPs), activating HSCs through Toll-like receptors (TLRs) [39]. A high-fat diet increased the rate of endotoxin/lipopolysaccharide production by intestinal Gram-negative bacteria, leading to higher bacterial translocation rate, and accelerated fibrous formation in CCl4 and bile duct ligation (BDL) mice by promoting HSC activation [40].
2.7. Chronic Infection of Hepatitis Virus
Chronic infection of hepatitis virus has become one of the major risk factors for liver fibrosis worldwide [41]. Viral genes and proteins directly or indirectly promote HSC activation.
Hepatitis B virus (HBV) e antigen directly induced expression of TGFβ, and TGFβ in turn mediated the activation and proliferation of HSCs. Meanwhile, HBV e antigen promotes the release of soluble mediators that activate HSCs, resulting in the production of ECM components and related factors that lead to fibrogenesis in patients with chronic HBV infection [42]. HBV Dane particles and x and c proteins may up-regulate the mRNA levels of PDGFβ and PDGFR-β and promote the phosphorylation of PDGFR-β, leading to subsequent auto-phosphorylation. Furthermore, which induces HSC proliferation [43].
Hepatitis C virus (HCV) infects about 120–130 million people around the world. Chronic HCV infection is the cause of hepatic necroinflammatory lesions and fibrosis of variable intensity. HCV cannot directly infect human HSCs, but it has been proved that HCV viral proteins activate HSCs after direct interaction with plasma membrane in a variety of in vitro experiments [44]. Elevated expression of IL-34 and macrophage colony-stimulating factor (M-CSF) in HCV-infected hepatocytes stimulates the process of peripheral blood mononuclear cells transforming into macrophages and promotes HSC activation by enhancing TGFβ and PDGFβ signaling [45].
2.8. Lipid Metabolism Disorder
The retinoid in the human body is mainly stored in the lipid droplets of HSC cytoplasm. Under normal conditions, Q-HSCs store up to 80% of body retinols (vitamin A lipid droplets) and contribute to retinol homeostasis with visible lipid droplets in the cytoplasm. Alcohol dehydrogenases (ADHs) in HSCs are a kind of retinol metabolic enzyme that oxidizes retinol to retinaldehyde. Among the 6 different types in the ADH family, ADH3 promotes HSC activation and inhibits the activity of NK cells, which plays an important role in promoting the progression of liver fibrosis. ADH3 inhibition enhances cytotoxicity of NK cells against HSCs and reduces the expression of TGFβ1 and collagen. Ablation of ADH3 gene blocked retinol metabolism in HSCs, alleviating liver fibrosis induced by BDL and CCl4 [46].
The involvement of cholesterol metabolism in HSC activation is not well understood, but disorders of cholesterol metabolism in other liver resident cells types may indirectly lead to HSC activation [47]. Alterations in cholesterol metabolism in nonalcoholic fatty liver disease (NAFLD) can activate Kupffer cells and induce HSC transdifferentiation [48]. Liver x receptors (LXRs) are the key regulator of cholesterol balance that govern whole body cholesterol homeostasis. Primary Lxrαβ−/− HSCs are pro-fibrotic and pro-inflammatory. These cells lose their lipid droplets more rapidly during in vitro activation and achieve the activated phenotype more quickly than cells isolated from wild-type mice [49]. Acyl-coenzyme A: cholesterol acyltransferase (ACAT1) catalyzes the conversion of free cholesterol into cholesterol ester, which avoids the excessive accumulation of free cholesterol. ACAT1 deficiency leads to elevated free cholesterol levels in HSCs, enhanced TLR4 signaling and down-regulated bone morphogenetic protein and activin membrane-bound inhibitor expression, leading to HSC sensitivity to TGFβ activation [50]. Increased production of TGFβ and other potentially unknown signaling molecules by hepatocytes induced HSC activation even in the absence of immune cells due to excessive lipid accumulation in hepatocytes (e.g., during hepatic steatosis) [47]. Treatment of hepatocytes with palmitic acid not only induced hepatocyte apoptosis, but also enhanced the ability of hepatocyte-derived exosomes to activate HSCs [17].
2.9. Exosome and MicroRNA
As important means of communication between cell populations, exosomes are nano-sized membrane vesicles that can transfer lipid, nucleic acids, proteins, and other bioactive molecules between different cell populations. Exosomes can be released by various cells, and exert numerous physiological and pathological activities, including cell growth, proliferation, differentiation, and apoptosis [51]. Recently, various cell types in the liver including hepatocytes and LSECs have been shown to interact with HSCs via exosomes, in turn modulating the biological activities of HSCs. For instance, palmitic acid stimulation enhanced the production of exosomes in hepatocytes and changed their exosomal miRNA profile. Moreover, exosomes derived from these hepatocytes stimulated the activation of HSCs [17]. LSECs secrete exosomes that express high amounts of sphingosine kinase 1, which promote HSC migration and activation [52].
A variety of microRNAs have been reported to have the potential to regulate fibrogenic signaling pathways in HSCs and participate in the activation process of HSCs, including TGF-β/Smad, Wnt/β-catenin, Hedgehog and so on [53]. For instance, miR-214 is significantly upregulated during HSC activation and leads to ECM accumulation by inhibiting the expression of suppressor-of-fused homolog, a negative regulator of hedgehog signaling pathway in LX-2 cells [54]. MiR-125b can promote HSC activation and fibrogenesis by upregulating RhoA signaling pathway and can be considered as A-HSCs specific fibrosis marker [55]. MiR-195 overexpression activates HSCs by reducing Smad7, and its inhibitors block HSC activation, reduce α-SMA expression, and enhance Smad7 expression [56]. These above observations suggest that exosomes and microRNAs may provide new clues for the therapeutic and diagnosis of hepatic fibrosis in the near future.
2.10. Other Factors
Other factors including alcohol, drugs and parasites can also influence HSC activation.
Studies have identified the impact of alcohol on the expression of epigenetic regulators during HSC activation. Alcohol directly affects HSC activation by stimulating overall changes in chromatin structure, leading to increased expression of ECM proteins. Furthermore, alcohol has the potential to promote the accumulation of elastin through directly stimulating tropoelastin gene transcription, elastin protein expression and TIMP-1 gene transcription in HSCs [31,57]. In addition, alcohol inhibits the antifibrotic process by inhibiting natural killer cell-mediated interferon-gamma-induced death of A-HSCs [58].
Some drugs affect HSC activation. Methotrexate (MTX) is commonly used for the treatment of autoimmune diseases and skin diseases, but rheumatoid arthritis and psoriasis patients who receive MTX therapy for a long time are at high risk of developing liver damage. MTX-PG, a metabolite of MTX, inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase enzyme, leading to intracellular adenosine accumulation, which in turn leads to HSC activation, ECM accumulation and liver fibrosis [59]. Acetaminophen (APAP) is one of the quantitively most consumed drugs worldwide, but overdosing often results in severe liver damage and even liver failure [60]. APAP exposure does not directly cause HSC activation, but leads to toxicity mainly in hepatocytes and mounts a hepatocyte damage dependent activation of HSCs [61,62].
It has been reported that there are complex and diverse interactions between HSCs and schistosome eggs. The number of A-HSCs increased in murine and human livers infected with schistosoma mansoni compared with healthy liver [63]. It is possible that one role for A-HSCs is to coordinate the influx of the various immune cells to mediate the granulomatous response [64]. In addition, during schistosome infection, hepatocytes overexpress IL-33, driving the activation and proliferation of a subset of hepatic innate lymphoid cells (ILC2). In turn, ILC2s produce IL-13, which drives HSC activation by regulating TGF-β1 and CTGF expression [65].
3. Intracellular Signaling Pathways of HSC Activation
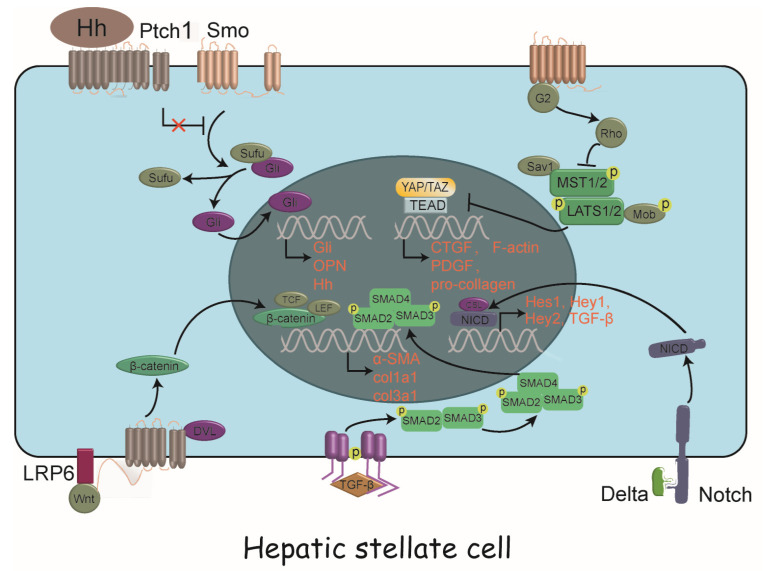
The activation of HSCs is related to a variety of cytokines and constitutes a complex regulatory network, and some of the important signal transduction pathways have gradually become clear. Herein we describe the range of intracellular signaling pathways that individually or collectively drive HSC activation (Figure 2).
Figure 2.
Intracellular signaling pathways driving HSC activation. A panoply of signals drive HSC activation, including TGF-β/SMAD pathway, Notch signaling, Wnt/β-catenin signaling, Hedgehog signaling and Hippo signaling, with complex crosstalk between them. HSC, hepatic stellate cell; Hh, hedgehog; Ptch1, patched 1; Smo, smoothened; Sufu, suppressor of fused; Gli, glioma-associated oncogene homolog; OPN, osteopontin; TCF, T lymphocyte factor; LEF, lymphocyte enhancer factor; LRP6, low-density lipoprotein receptor 6; DVL, disheveled; SMAD, small mother against decapentaplegic; α-SMA, α-smooth muscle actin; col1a1, collagen I α-1; TGF-β,transforming growth factor β; Sav1, Salvador family WW domain containing protein 1; MST1/2, mammalian STE20-like protein kinase 1 and 2; LATS1/2, large tumor suppressor kinase 1 and 2; Mob, monopolar spindle-one-binder protein; YAP, yes-associated protein; TAZ, transcriptional coactivator with PDZ-binding motif; TEAD, TEA domain transcription factor; CTGF, connective tissue growth factor; PDGF, platelet derived growth factor; NICD, Notch intracellular domain; CSL, CBF-1, Suppressor of hairless, Lag-2; Hes, hairy/enhancer of split ; Hey, hairy/enhancer of split related with YRPW motif.
3.1. TGF-β/SMAD Pathway
TGF-β signaling is considered the key fibrogenic pathway that drives HSC activation and induces ECM production. In normal liver, Q-HSCs express trace amounts of TGF-β, which is up-regulated shortly after liver injury [66]. Active HSCs produce TGF-β in response to liver injury, which forms a positive feedback loop driving fibrogenesis through SMAD2/SMAD3, while SMAD7 inhibits the activation [67]. In Q-HSCs, TLR4 activation down-regulates the TGF-β pseudo receptor Bambi to stimulate HSCs to TGF-β-induced signals [68]. Apoptotic body-engulfing macrophages secrete TGF-β and activate HSCs [69,70]. ECM1, mainly produced by hepatocytes, attenuates activation of TGF-β and its activation of HSCs to prevent liver fibrosis [37]. TGFβ-1-induced transcript 1 protein (TGFβ1i1), also named as hydrogen peroxide-inducible clone-5 (Hic-5), inhibits the activation of HSCs and liver fibrosis through reducing the TGF-β/Smad2 signaling by upregulation of Smad7 [71]. The following molecules interact with TGF-β signaling and contribute to HSC activation.
Hyaluronan (HA) is a major extracellular matrix glycosaminoglycan and a biomarker for cirrhosis. The production and deposition of HA replace functional liver tissues feature prominently in liver fibrosis. HA and HA synthase 2 (HAS2) expression was elevated in both human and murine liver fibrosis. HAS2 was transcriptionally up-regulated by TGF-β via Wilms tumor 1 to promote fibrogenic, proliferative, and mediates HSC activation through CD44, TLR4, and Notch1. Furthermore, HA expression and liver fibrosis were reduced upon HAS2 inhibition and enhanced upon HAS2 overexpression in HSCs. Depletion of HA synthesis by 4-methylumbelliferone suppresses HSC activation and liver fibrosis in mice, which may have potential to be a new therapeutic route for liver fibrosis [72].
Galectins are a family of animal beta-galactoside-binding lectins [73]. Galectin-3 has been implicated in a variety of biological processes including cell proliferation, adhesion, survival, and in the development of acute inflammation [74]. Disruption of the Gal-3 gene blocks HSC activation and collagen expression, thus reducing liver fibrosis. Specifically, in CCl4-treated, Gal-3-deficient mice, HSC activation and collagen deposition are suppressed compared with wild-type animals [75]. Additionally, treatment of LX-2 cells with recombinant Gal-1 protein can increase the phosphorylation of SMAD2, SMAD3 and ERK1/2, and bind to neuronilin-1 in a glycosylation-dependent manner to enhance HSC migration [76].
HAb18G/CD147, a tumor-related glycoprotein expressed on the cellular membrane of HSCs, is highly expressed on activated HSCs. TGF-β upregulated HAb18G/CD147 expression in LX-2 cells, and HAb18G/CD147 transfection enhanced the profibrogenic genes expression. In mouse liver fibrosis model, HAb18G/CD147 expression increased upon the development of fibrogenesis and decreased during the liver fibrosis recovery. These data implicate that HAb18G/CD147 plays a role in HSC activation and is an effective therapeutic target in fibrosis [77].
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and have effects on liver fibrosis development and progression, which play essential roles during embryonic development [78]. In the process of CCl4-induced liver fibrosis in mice, the expression of BMP7 increased first and then decreased as well as in human patients with CLD [79]. The results of in vitro experiments show that high doses of exogenous BMP7 can inhibit the activation, migration and proliferation of TGF-β1 induced HSCs. This effect is related to the up-regulation of pSMAD1/5/8 and down-regulation of the phosphorylation of SMAD3 and p38 by BMP7. Thus, exogenous BMP7 may be used as an anti-liver fibrosis drug [80]. Intriguingly, BMP6 is upregulated in NAFLD but not in other mouse liver injury models or diseased human livers (ALD and chronic HBV or HCV infection). Recombinant BMP6 suppresses the activation of HSCs and reduces proinflammatory and profibrogenic gene expression in activated HSCs [81].
3.2. Notch Signaling
The Notch signaling pathway enables cells to communicate with their direct neighbors by ligand–receptor interaction to convey the signal into a transcriptional response to regulate tissue and organ development [82]. In mammals, there are four known receptors (Notch 1–4) and five ligands belonging to the Jagged (Jagged1, 2) and Delta-like (Delta-like, Dll1, 3, and 4) family. Signaling upon ligand–receptor binding leads to sequential proteolytic cleavage processes in the Notch receptor extracellular and transmembrane domain to release the Notch intracellular domain (NICD). In the nucleus, NICD binds to the DNA-binding recombination signal binding protein (RBP)-Jκ and activates the transcription of target genes Hes1, Hey1 and Hey2 [83].
Rat HSCs express Notch receptors in vitro and up-regulate JAG1 upon activation and differentiation to myofibroblasts [84]. The role of JAG1 in HSC biology is elusive, and more recent studies show that exposure of HSCs to JAG1 promotes α-SMA and collagen expression [84]. In TGF-β-induced human HSCs, fibrosis-related genes (col I and α-SMA), and Notch3, JAG1 and Hes1 were overexpressed compared to non-activated cells [85]. While Notch signaling can directly activates HSCs, Notch activation in neighboring cells (e.g., LSECs) also leads to HSC activation and the subsequent hepatic fibrosis. Notch activation down—regulates eNOS—sGC signaling, resulting in increased LSEC dedifferentiation, HSC activation and fibrosis [86]. Moreover, hepatocyte-specific Notch depletion in NASH mice leads to reduced fibrotic deposition and HSC activation [87], and hepatocyte Notch activation is sufficient to induce β-catenin-inactive HCC in mice with NASH [88]. Recently, a nanoparticle-mediated delivery system to target γ-secretase inhibitor to liver (GSI NPs) reduced liver fibrosis and inflammation in mice fed a NASH-provoking diet, without apparent gastrointestinal toxicity [89].
3.3. Wnt/β-Catenin Signaling
The Wnt pathway is commonly divided into β-catenin dependent (canonical) and independent (non-canonical) signaling [90]. In canonical Wnt signaling, (i) the extracellular Wnt protein is connected to the frizzled protein (Frz) on the target cell membrane and the co-receptor low-density lipoprotein receptor-related protein 5/6 (LRP5/6), thus transmitting extracellular signals to the cytoplasm by phosphorylation of loose protein (Dsh) [91]. (ii) Intracytoplasmic signaling: Dsh prevents β-catenin from phosphorylation or degradation by suppressing GSK-3β activation, thus accumulating free β-catenin [92]. (iii) Intranuclear signal transduction: When the free β-catenin in the cytoplasm reaches a certain level, it can enter the nucleus and combine with the nuclear lymphocyte enhancer factor/T lymphocyte factor (LEF/TCF) to form a β-catenin-LEF/TCF complex, leading to transcription of downstream target genes in the canonical Wnt signaling pathway.
The Wnt/β-catenin system is an evolutionary conserved signaling pathway that is vital for morphogenesis and cell organization during embryogenesis [93]. The expression of Wnt pathway components were up-regulated in hepatic fibrosis using genomic analysis from primary biliary cirrhosis livers [94]. Highly up-regulated expression of Wnt5a and its receptor frizzled 2 (Fz2) implicates this pathway in differentiation of Q-HSCs into myofibroblasts, suggesting an important role of Wnt signaling in development of liver fibrosis [95]. It is not clear whether the role of Wnt5a in promoting fibrosis is caused by inhibition, activation, or independent of β-catenin. A growing number of studies in the literature support the activation of β-catenin by Wnt signaling during HSC activation and fibrosis. Wnt–β-catenin signaling might activate HSCs through negative regulation of adipogenesis, and inhibition of this signaling pathway could contribute to the adipogenic gene profile of Q-HSCs [96]. Inhibiting Wnt signaling to β-catenin therefore might inhibit liver fibrosis. Determining the identity and cell sources of the factors that activate β-catenin in HSCs need further studies. In a single-center, open-label, phase 1 trial, administration of PRI-724, a small-molecule modulator of Wnt signaling, was tolerated by patients with HCV cirrhosis; however, liver injury as a possible related serious adverse event was observed [97].
3.4. Hedgehog Signaling
The canonical Hedgehog (Hh) pathway is a conserved, highly complex signaling cascade, with many players and intricate regulation [98]. Patient and mouse data have shown that hepatic fibrosis is associated with Hedgehog activation [99]. It can be simplified into four fundamental components: (i) the ligand Hedgehog, (ii) the receptor Patched (Patch), (iii) the signal transducer Smoothened (Smo), and (iv) the effector transcription factor, Gli. Canonical Hh signaling occurs along a highly specialized organelle, the primary cilium [98]. In the absence of Hedgehog ligand, Patch prevents Smo from entering the primary cilium, repressing Smo activity. This allows the sequential phosphorylation of Gli by several kinases. Phosphorylated Gli is susceptible for ubiquitination by Skip-Cullin-F-box (SCF) protein/β-Transducing repeat Containing Protein (TrCP), which primes Gli to limited degradation in the proteasome. When hedgehog binds to Patch, it removes Patch from the PC, allowing Smo to enter the PC. The entry of Smo into the PC allows Smo activation. Active Smo abrogates phosphorylation and subsequent degradation of Gli. Full length Gli translocates to the nucleus where it acts as a transcription factor for several target genes [100,101].
In healthy adult liver, the Hh pathway expression is relatively dormant with low production of ligands by liver-resident cells and robust expression of Hh inhibitors, such as Hh-interacting protein (Hhip), by Q-HSCs [102]. During fibrogenic liver repair, emerging evidence has demonstrated a critical role of canonical Hh signaling, which supports that conditional deletion of Smo in α-SMA+ myofibroblasts inhibited liver fibrosis [103]. Furthermore, Hedgehog ligands can activate HSCs and induce their transdifferentiation from a quiescent phenotype into a myofibroblastic phenotype responsible for matrix deposition [100]. Moreover, the activation of Hh pathway inhibits apoptotic signals, enhances the viability and proliferative capacity of myofibroblasts and stimulates additional production of endogenous Hh ligands in an autocrine or paracrine manner, which drives a positive feedback loop to amplify Hh signaling [104]. Deregulation of the Hh signaling network may contribute to the pathogenesis and sequelae of liver damage [105]. Hhip expression falls by 90%, followed by Shh expression in HSCs and Hh pathway activation [103,106]. During the NIDDK-sponsored PIVENS trial (NCT00063622), treatment response paralleled to loss of Shh+ hepatocytes and improvement in Hh-regulated processes that promote NASH progression, indicating that VitE treatment and improvement in NASH were associated with changes in Hh signaling activity [107].
3.5. Hippo Signaling
The Hippo signaling pathway is a kinase chain composed of a series of conserved protein kinases and transcription factors, which mainly control organ size by regulating cell proliferation and apoptosis [108]. Hippo signaling is activated by the binding of upstream membrane protein receptors and ligands to generate extracellular growth inhibition signals and activate a group of highly conserved serine/threonine kinases MST [109]. YAP and TAZ are regarded as mechanoactivated coordinators of the matrix-driven feedback loop that intensify and sustains fibrosis [110]. Studies have shown that TGF, PDGF, Ankrd1, procollagen, PAI 1, F-actin, Fibronectin, K19 and other fibrosis-related genes are also regulated by the YAP-TEAD complex [110,111]. Liver injury in mice and humans promotes levels of YAP/TAZ/CYR61 in hepatocytes, hence attracting macrophages to the liver to induce inflammation and fibrosis [112].
YAP accumulates in the nucleus during the early activation of hepatic stellate cells. Inhibiting YAP can prevent HSC activation and fibrogenesis, and reduce the expression of α-SMA and type I collagen [111]. YAP expression was up-regulated in fibrotic liver tissue of model mice induced by CCl4 and returned to normal levels after stopping CCl4. In HSC-T6 cells treated with TGF-β1, YAP expression increased. In addition, the overexpression of YAP inhibited the apoptosis of activated HSC-T6 cells [111,113]. YAP in the cytoplasm of HSCs enters the nucleus after activation, combines with the transcription factor TEAD1-4, promotes the transcription of genes such as CTGF and PDGF-BB, and promotes HSC transdifferentiation and proliferation [114]. The I148M variant of the PNPLA3 gene represents a higher risk of severe liver fibrosis, and PNPLA3 I148M up-regulates Hedgehog and YAP Signaling in human HSCs [115]. These indicated that YAP can be used as an effective target to inhibit HSC activation. Conversely, others have argued YAP activation in HSCs is beneficial to liver regeneration. Preventing HSC and YAP activation by manipulating Hedgehog signaling also suppressed liver regeneration and hepatocyte proliferation [116]. Hence, it seems that YAP activation in HSCs represented beneficial non-cell-autonomous effects in the short term but detrimental effects in the long term [117].
During the repair of liver ischemia-reperfusion injury, HSCs were found to be significantly activated and proliferated. LATS1 and its adaptor protein MOB1 (Mps one binder, Mps) are inactivated, and YAP and TAZ in HSCs are selectively activated. At the same time, the expression of CTGF and survivin are up-regulated, and HSC proliferation and concomitant activation of YAP and TAZ occurred not only in injured liver, but not observed in non-ischemic liver. In the process of liver recovery after IR injury, HSC proliferation is obvious [116].
YAP plays a critical role in cell metabolism [118]. The proliferation of activated HSCs exhibits similar metabolic requirements as tumor cells. Studies show essential role of glutamine breakdown in the proliferation and phenotype development of HSCs, which is controlled by Hippo and Hh signaling [119,120].
3.6. Crosstalk of Intracellular Pathways
The activation of HSCs is complicated, involving multiple signaling molecules and multiple signaling pathways. These signaling pathways intersect and influence each other, and act together in the entire process of the activation and proliferation of HSCs.
Hippo and TGF-β/SMAD: It is demonstrated that YAP signaling works by promoting the binding of SMAD7 to activated TGF-β receptor type I, thereby eliminating downstream TGF-β signal transduction. At the same time, TAZ binds to SMAD2/3/4 heteromers in a TGF-β-dependent manner and recruits them into TGF-β response elements [121]. TAZ knockout experiments also show that TAZ plays a key role in the nuclear accumulation of SMAD2/3/4 complex in response to TGF-β and subsequent transactivation of target genes. In addition, The Hippo pathway scaffold protein RASSF1 is recruited by TGF-β to TGF-β receptor I, and is degraded by the E3 ubiquitin ligase ITCH co-recruited by the receptor, which in turn inactivates the MST/LATS kinase cascade and promotes YAP/SMAD2 interaction and subsequent nuclear translocation [121,122].
TGF-β and Notch: Excessive activation of TGF-β regulates the Notch signaling pathway in the process of liver fibrosis in rats. Inhibiting the TGF-β signaling pathway can block the Notch signaling pathway, and Notch signaling can participate in the occurrence of liver fibrosis by activating the TGF-β/SMAD pathway. TGF-β inhibitor down-regulated the expression of Notch1, Hes1 and Hes5, and inhibited Notch signal mRNA and protein expression [123]. TGF-β1 also induced the high expression of Notch1, JAG1, Hes1 in HSC. The expression of the above-mentioned markers in mouse HSC was significantly reduced after TGF-β1 knockout. After blocking the Notch pathway with specific inhibitors, the expression of Notch1 and α-SMA in HSCs was significantly reduced. These results indicate that TGF-β1 signal controls the activation of HSCs by regulating the expression of Notch signaling pathway markers [124].
Hedgehog and Hippo: The activation of the Hedgehog pathway promotes the post-transcriptional response of YAP by increasing the level of YAP protein, so Hedgehog signaling positively regulates YAP [125]. Blocking Hedgehog signaling can inhibit YAP activation in cultured HSCs, and downregulating YAP can inhibit YAP and Hedgehog-induced target gene expression, and inhibit HSC transdifferentiation into myofibroblasts, showing that the Hedgehog pathway can regulate the YAP protein of the regenerated liver in mice [126]. Previous studies have found that the Hedgehog pathway controls the HSCs activation by regulating cellular glycolysis. Conditional interruption of Hh signaling in myofibroblasts reduces the number of glycolytic myofibroblasts and the degree of liver fibrosis in mice [127]. Nevertheless, new research shows that the Hedgehog-YAP signaling pathway can promote the activation of HSCs by regulating the metabolism (i.e., the breakdown of glutamine) during the HSCs transdifferentiation into myofibroblasts [120]. Therefore, glutamine decomposition can control the accumulation of myofibroblasts in mice and may become a therapeutic target for liver fibrosis.
Hedgehog and Notch: Activating the Notch pathway in HSCs can stimulate them to become myofibroblasts through a mechanism involving epithelial-mesenchymal transition, which needs to cross the typical Hedgehog pathway. It is suggested that when HSCs are converted to myofibroblasts, it activates Hh signal, undergoes epithelial to mesenchymal transition, and increases the expression of Notch signal. However, blocking Notch signaling in myofibroblasts can inhibit Hh signaling activity and cause mesenchymal epithelial transition; inhibiting Hh pathway can inhibit Notch signaling transduction and also induce mesenchymal epithelial transition [128].
4. Conclusions
Chronic liver injury with any etiology can progress to fibrosis and the end-stage diseases cirrhosis and hepatocellular carcinoma. However, currently the development of anti-fibrotic drugs has not yet resulted in clinically approved therapeutics, underscoring the complex biology and challenges involved when targeting the intricate cellular signaling. The current review highlights key extra- and intra-cellular pathways involved in HSC activation, with potential value for the development of refined therapeutic strategies for hepatic fibrosis.
Author Contributions
Conceptualization, Y.Y., J.Z. and C.L.; writing—original draft preparation, Y.Y., J.Z. and L.X.; writing—review and editing, Y.Y., J.Z. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from Wuhan Municipal Science and Technology Bureau (2020020601012210), and Natural Science Foundation of Hubei Province (2017CFB620).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torres-Hernandez A., Wang W., Nikiforov Y., Tejada K., Torres L., Kalabin A., Wu Y., Haq M.I.U., Khan M.Y., Zhao Z., et al. Targeting SYK signaling in myeloid cells protects against liver fibrosis and hepatocarcinogenesis. Oncogene. 2019;38:4512–4526. doi: 10.1038/s41388-019-0734-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee U.E., Friedman S.L. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parola M., Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019;65:37–55. doi: 10.1016/j.mam.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Qi X., De Stafano V., Guo X. Treatment of Patients with Cirrhosis. N. Engl. J. Med. 2016;375:2103–2104. doi: 10.1056/NEJMc1612334. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi P., Wang S., Friedman S.L. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021;33:242–257. doi: 10.1016/j.cmet.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J., Zhang Z., Zhang Y., Li W., Zheng W., Yu J., Wang B., Chen L., Zhuo Q., Chen L., et al. MicroRNA-212 activates hepatic stellate cells and promotes liver fibrosis via targeting SMAD7. Biochem. Biophys. Res. Commun. 2018;496:176–183. doi: 10.1016/j.bbrc.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Zhao Y.R., Tian Z. Roles of hepatic stellate cells in acute liver failure: From the perspective of inflammation and fibrosis. World J. Hepatol. 2019;11:412–420. doi: 10.4254/wjh.v11.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepreux S., Desmouliere A. Human liver myofibroblasts during development and diseases with a focus on portal (myo)fibroblasts. Front. Physiol. 2015;6:173. doi: 10.3389/fphys.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elpek G.O. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman S.L. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 14.Roehlen N., Crouchet E., Baumert T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020;9:875. doi: 10.3390/cells9040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wree A., Eguchi A., McGeough M.D., Pena C.A., Johnson C.D., Canbay A., Hoffman H.M., Feldstein A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHedlidze T., Waldner M., Zopf S., Walker J., Rankin A.L., Schuchmann M., Voehringer D., McKenzie A.N., Neurath M.F., Pflanz S., et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y.S., Kim S.Y., Ko E., Lee J.H., Yi H.S., Yoo Y.J., Je J., Suh S.J., Jung Y.K., Kim J.H., et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci. Rep. 2017;7:3710. doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisseleva T., Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 19.Maretti-Mira A.C., Wang X., Wang L., DeLeve L.D. Incomplete Differentiation of Engrafted Bone Marrow Endothelial Progenitor Cells Initiates Hepatic Fibrosis in the Rat. Hepatology. 2019;69:1259–1272. doi: 10.1002/hep.30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shetty S., Lalor P.F., Adams D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018;15:555–567. doi: 10.1038/s41575-018-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie G., Wang X., Wang L., Wang L., Atkinson R.D., Kanel G.C., Gaarde W.A., Deleve L.D. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918–927.e916. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding B.S., Cao Z., Lis R., Nolan D.J., Guo P., Simons M., Penfold M.E., Shido K., Rabbany S.Y., Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wynn T.A., Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sica A., Invernizzi P., Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 26.Chen P.J., Kuo L.M., Wu Y.H., Chang Y.C., Lai K.H., Hwang T.L. BAY 41-2272 Attenuates CTGF Expression via sGC/cGMP-Independent Pathway in TGFbeta1-Activated Hepatic Stellate Cells. Biomedicines. 2020;8:330. doi: 10.3390/biomedicines8090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Ma L., Wei R., Ye T., Zhou J., Wen M., Men R., Aqeilan R.I., Peng Y., Yang L. Twist1-induced miR-199a-3p promotes liver fibrosis by suppressing caveolin-2 and activating TGF-beta pathway. Signal Transduct. Target. Ther. 2020;5:75. doi: 10.1038/s41392-020-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An S.Y., Petrescu A.D., DeMorrow S. Targeting Certain Interleukins as Novel Treatment Options for Liver Fibrosis. Front. Pharmacol. 2021;12:645703. doi: 10.3389/fphar.2021.645703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Munker S., Mullenbach R., Weng H.L. IL-13 Signaling in Liver Fibrogenesis. Front. Immunol. 2012;3:116. doi: 10.3389/fimmu.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Meyer C., Muller A., Herweck F., Li Q., Mullenbach R., Mertens P.R., Dooley S., Weng H.L. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-beta-independent Smad signaling. J. Immunol. 2011;187:2814–2823. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 31.Higashi T., Friedman S.L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen H., Sheng L., Chen Z., Jiang L., Su H., Yin L., Omary M.B., Rui L. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60:2065–2076. doi: 10.1002/hep.27348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradere J.P., Kluwe J., De Minicis S., Jiao J.J., Gwak G.Y., Dapito D.H., Jang M.K., Guenther N.D., Mederacke I., Friedman R., et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan T., Kisseleva T., Brenner D.A. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS ONE. 2015;10:e0129743. doi: 10.1371/journal.pone.0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurokawa T., Zheng Y.W., Ohkohchi N. Novel functions of platelets in the liver. J. Gastroenterol. Hepatol. 2016;31:745–751. doi: 10.1111/jgh.13244. [DOI] [PubMed] [Google Scholar]
- 36.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan W., Liu T., Chen W., Hammad S., Longerich T., Hausser I., Fu Y., Li N., He Y., Liu C., et al. ECM1 Prevents Activation of Transforming Growth Factor beta, Hepatic Stellate Cells, and Fibrogenesis in Mice. Gastroenterology. 2019;157:1352–1367.e1313. doi: 10.1053/j.gastro.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 38.Mazagova M., Wang L., Anfora A.T., Wissmueller M., Lesley S.A., Miyamoto Y., Eckmann L., Dhungana S., Pathmasiri W., Sumner S., et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29:1043–1055. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H., You Y., Hua M., Wu P., Liu Y., Chen Z., Zhang L., Wei H., Li Y., Luo M., et al. Chlorophyllin Modulates Gut Microbiota and Inhibits Intestinal Inflammation to Ameliorate Hepatic Fibrosis in Mice. Front. Physiol. 2018;9:1671. doi: 10.3389/fphys.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Minicis S., Rychlicki C., Agostinelli L., Saccomanno S., Candelaresi C., Trozzi L., Mingarelli E., Facinelli B., Magi G., Palmieri C., et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 41.Stanaway J.D., Flaxman A.D., Naghavi M., Fitzmaurice C., Vos T., Abubakar I., Abu-Raddad L.J., Assadi R., Bhala N., Cowie B., et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zan Y., Zhang Y., Tien P. Hepatitis B virus e antigen induces activation of rat hepatic stellate cells. Biochem. Biophys. Res. Commun. 2013;435:391–396. doi: 10.1016/j.bbrc.2013.04.098. [DOI] [PubMed] [Google Scholar]
- 43.Bai Q., An J., Wu X., You H., Ma H., Liu T., Gao N., Jia J. HBV promotes the proliferation of hepatic stellate cells via the PDGF-B/PDGFR-beta signaling pathway in vitro. Int. J. Mol. Med. 2012;30:1443–1450. doi: 10.3892/ijmm.2012.1148. [DOI] [PubMed] [Google Scholar]
- 44.Florimond A., Chouteau P., Bruscella P., Le Seyec J., Merour E., Ahnou N., Mallat A., Lotersztajn S., Pawlotsky J.M. Human hepatic stellate cells are not permissive for hepatitis C virus entry and replication. Gut. 2015;64:957–965. doi: 10.1136/gutjnl-2013-305634. [DOI] [PubMed] [Google Scholar]
- 45.Preisser L., Miot C., Le Guillou-Guillemette H., Beaumont E., Foucher E.D., Garo E., Blanchard S., Fremaux I., Croue A., Fouchard I., et al. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879–1890. doi: 10.1002/hep.27328. [DOI] [PubMed] [Google Scholar]
- 46.Yi H.S., Lee Y.S., Byun J.S., Seo W., Jeong J.M., Park O., Duester G., Haseba T., Kim S.C., Park K.G., et al. Alcohol dehydrogenase III exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice. Hepatology. 2014;60:1044–1053. doi: 10.1002/hep.27137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khomich O., Ivanov A.V., Bartosch B. Metabolic Hallmarks of Hepatic Stellate Cells in Liver Fibrosis. Cells. 2019;9:24. doi: 10.3390/cells9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ioannou G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2016;27:84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 49.O’Mahony F., Wroblewski K., O’Byrne S.M., Jiang H., Clerkin K., Benhammou J., Blaner W.S., Beaven S.W. Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein. Hepatology. 2015;62:615–626. doi: 10.1002/hep.27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomita K., Teratani T., Suzuki T., Shimizu M., Sato H., Narimatsu K., Usui S., Furuhashi H., Kimura A., Nishiyama K., et al. Acyl-CoA:cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J. Hepatol. 2014;61:98–106. doi: 10.1016/j.jhep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 51.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 52.Ye Q., Zhou Y., Zhao C., Xu L., Ping J. Salidroside Inhibits CCl4-Induced Liver Fibrosis in Mice by Reducing Activation and Migration of HSC Induced by Liver Sinusoidal Endothelial Cell-Derived Exosomal SphK1. Front. Pharmacol. 2021;12:677810. doi: 10.3389/fphar.2021.677810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezhilarasan D. MicroRNA interplay between hepatic stellate cell quiescence and activation. Eur. J. Pharmacol. 2020;885:173507. doi: 10.1016/j.ejphar.2020.173507. [DOI] [PubMed] [Google Scholar]
- 54.Ma L., Yang X., Wei R., Ye T., Zhou J.K., Wen M., Men R., Li P., Dong B., Liu L., et al. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. 2018;9:718. doi: 10.1038/s41419-018-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.You K., Li S.Y., Gong J., Fang J.H., Zhang C., Zhang M., Yuan Y., Yang J., Zhuang S.M. MicroRNA-125b Promotes Hepatic Stellate Cell Activation and Liver Fibrosis by Activating RhoA Signaling. Mol. Ther. Nucleic Acids. 2018;12:57–66. doi: 10.1016/j.omtn.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song L.Y., Ma Y.T., Wu C.F., Wang C.J., Fang W.J., Liu S.K. MicroRNA-195 Activates Hepatic Stellate Cells In Vitro by Targeting Smad7. Biomed. Res. Int. 2017;2017:1945631. doi: 10.1155/2017/1945631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Page A., Paoli P.P., Hill S.J., Howarth R., Wu R., Kweon S.M., French J., White S., Tsukamoto H., Mann D.A., et al. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J. Hepatol. 2015;62:388–397. doi: 10.1016/j.jhep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lackner C., Tiniakos D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019;70:294–304. doi: 10.1016/j.jhep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Ezhilarasan D. Hepatotoxic potentials of methotrexate: Understanding the possible toxicological molecular mechanisms. Toxicology. 2021;458:152840. doi: 10.1016/j.tox.2021.152840. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran A., Jaeschke H. Oxidant Stress and Acetaminophen Hepatotoxicity: Mechanism-Based Drug Development. Antioxid. Redox Signal. 2021 doi: 10.1089/ars.2021.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leite S.B., Roosens T., El Taghdouini A., Mannaerts I., Smout A.J., Najimi M., Sokal E., Noor F., Chesne C., van Grunsven L.A. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016;78:1–10. doi: 10.1016/j.biomaterials.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 62.Mannaerts I., Eysackers N., Anne van Os E., Verhulst S., Roosens T., Smout A., Hierlemann A., Frey O., Leite S.B., van Grunsven L.A. The fibrotic response of primary liver spheroids recapitulates in vivo hepatic stellate cell activation. Biomaterials. 2020;261:120335. doi: 10.1016/j.biomaterials.2020.120335. [DOI] [PubMed] [Google Scholar]
- 63.Chang D., Ramalho L.N., Ramalho F.S., Martinelli A.L., Zucoloto S. Hepatic stellate cells in human schistosomiasis mansoni: A comparative immunohistochemical study with liver cirrhosis. Acta Trop. 2006;97:318–323. doi: 10.1016/j.actatropica.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Carson J.P., Ramm G.A., Robinson M.W., McManus D.P., Gobert G.N. Schistosome-Induced Fibrotic Disease: The Role of Hepatic Stellate Cells. Trends Parasitol. 2018;34:524–540. doi: 10.1016/j.pt.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J., Locksley R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carthy J.M. TGFβ signaling and the control of myofibroblast differentiation: Implications for chronic inflammatory disorders. J. Cell. Physiol. 2018;233:98–106. doi: 10.1002/jcp.25879. [DOI] [PubMed] [Google Scholar]
- 67.Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., Springer T.A. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 69.Kisseleva T., Uchinami H., Feirt N., Quintana-Bustamante O., Segovia J.C., Schwabe R.F., Brenner D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Li C., Kong Y., Wang H., Wang S., Yu H., Liu X., Yang L., Jiang X., Li L., Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 71.Lei X.F., Fu W., Kim-Kaneyama J.R., Omoto T., Miyazaki T., Li B., Miyazaki A. Hic-5 deficiency attenuates the activation of hepatic stellate cells and liver fibrosis through upregulation of Smad7 in mice. J. Hepatol. 2016;64:110–117. doi: 10.1016/j.jhep.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y.M., Noureddin M., Liu C., Ohashi K., Kim S.Y., Ramnath D., Powell E.E., Sweet M.J., Roh Y.S., Hsin I.F., et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci. Transl. Med. 2019;11:eaat9284. doi: 10.1126/scitranslmed.aat9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barondes S.H., Castronovo V., Cooper D.N., Cummings R.D., Drickamer K., Feizi T., Gitt M.A., Hirabayashi J., Hughes C., Kasai K., et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 74.Akahani S., Nangia-Makker P., Inohara H., Kim H.R., Raz A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 75.Henderson N.C., Mackinnon A.C., Farnworth S.L., Poirier F., Russo F.P., Iredale J.P., Haslett C., Simpson K.J., Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu M.H., Chen Y.L., Lee K.H., Chang C.C., Cheng T.M., Wu S.Y., Tu C.C., Tsui W.L. Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-β- and PDGF-like signals in hepatic stellate cells. Sci. Rep. 2017;7:11006. doi: 10.1038/s41598-017-11212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang D.W., Zhao Y.X., Wei D., Li Y.L., Zhang Y., Wu J., Xu J., Chen C., Tang H., Zhang W., et al. HAb18G/CD147 promotes activation of hepatic stellate cells and is a target for antibody therapy of liver fibrosis. J. Hepatol. 2012;57:1283–1291. doi: 10.1016/j.jhep.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 78.Kinoshita K., Iimuro Y., Otogawa K., Saika S., Inagaki Y., Nakajima Y., Kawada N., Fujimoto J., Friedman S.L., Ikeda K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56:706–714. doi: 10.1136/gut.2006.092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hao Z.M., Cai M., Lv Y.F., Huang Y.H., Li H.H. Oral administration of recombinant adeno-associated virus-mediated bone morphogenetic protein-7 suppresses CCl(4)-induced hepatic fibrosis in mice. Mol. Ther. 2012;20:2043–2051. doi: 10.1038/mt.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zou G.L., Zuo S., Lu S., Hu R.H., Lu Y.Y., Yang J., Deng K.S., Wu Y.T., Mu M., Zhu J.J., et al. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J. Gastroenterol. 2019;25:4222–4234. doi: 10.3748/wjg.v25.i30.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arndt S., Wacker E., Dorn C., Koch A., Saugspier M., Thasler W.E., Hartmann A., Bosserhoff A.K., Hellerbrand C. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut. 2015;64:973–981. doi: 10.1136/gutjnl-2014-306968. [DOI] [PubMed] [Google Scholar]
- 82.Meurette O., Mehlen P. Notch Signaling in the Tumor Microenvironment. Cancer Cell. 2018;34:536–548. doi: 10.1016/j.ccell.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Geisler F., Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–392. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sawitza I., Kordes C., Reister S., Haussinger D. The niche of stellate cells within rat liver. Hepatology. 2009;50:1617–1624. doi: 10.1002/hep.23184. [DOI] [PubMed] [Google Scholar]
- 85.Bansal R., van Baarlen J., Storm G., Prakash J. The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci. Rep. 2015;5:18272. doi: 10.1038/srep18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duan J.L., Ruan B., Yan X.C., Liang L., Song P., Yang Z.Y., Liu Y., Dou K.F., Han H., Wang L. Endothelial Notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice. Hepatology. 2018;68:677–690. doi: 10.1002/hep.29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu C., Kim K., Wang X., Bartolome A., Salomao M., Dongiovanni P., Meroni M., Graham M.J., Yates K.P., Diehl A.M., et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci. Transl. Med. 2018;10:eaat0344. doi: 10.1126/scitranslmed.aat0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu C., Ho Y.J., Salomao M.A., Dapito D.H., Bartolome A., Schwabe R.F., Lee J.S., Lowe S.W., Pajvani U.B. Notch activity characterizes a common hepatocellular carcinoma subtype with unique molecular and clinicopathologic features. J. Hepatol. 2021;74:613–626. doi: 10.1016/j.jhep.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richter L.R., Wan Q., Wen D., Zhang Y., Yu J., Kang J.K., Zhu C., McKinnon E.L., Gu Z., Qiang L., et al. Targeted Delivery of Notch Inhibitor Attenuates Obesity-Induced Glucose Intolerance and Liver Fibrosis. ACS Nano. 2020;14:6878–6886. doi: 10.1021/acsnano.0c01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polakis P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barrott J.J., Cash G.M., Smith A.P., Barrow J.R., Murtaugh L.C. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc. Natl. Acad. Sci. USA. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C., Li Y., Semenov M., Han C., Baeg G.H., Tan Y., Zhang Z., Lin X., He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 93.Schunk S.J., Floege J., Fliser D., Speer T. WNT-β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021;17:172–184. doi: 10.1038/s41581-020-00343-w. [DOI] [PubMed] [Google Scholar]
- 94.Shackel N.A., McGuinness P.H., Abbott C.A., Gorrell M.D., McCaughan G.W. Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut. 2001;49:565–576. doi: 10.1136/gut.49.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang F., Parsons C.J., Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J. Hepatol. 2006;45:401–409. doi: 10.1016/j.jhep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 96.Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., MacDougald O.A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 97.Kimura K., Ikoma A., Shibakawa M., Shimoda S., Harada K., Saio M., Imamura J., Osawa Y., Kimura M., Nishikawa K., et al. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/β-Catenin Inhibitor, in Patients with Hepatitis C Virus-related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMedicine. 2017;23:79–87. doi: 10.1016/j.ebiom.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bangs F., Anderson K.V. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb. Perspect. Biol. 2017;9:a028175. doi: 10.1101/cshperspect.a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu C., Tabas I., Schwabe R.F., Pajvani U.B. Maladaptive regeneration—The reawakening of developmental pathways in NASH and fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2021;18:131–142. doi: 10.1038/s41575-020-00365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Omenetti A., Choi S., Michelotti G., Diehl A.M. Hedgehog signaling in the liver. J. Hepatol. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Machado M.V., Diehl A.M. Hedgehog signalling in liver pathophysiology. J. Hepatol. 2018;68:550–562. doi: 10.1016/j.jhep.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sicklick J.K., Li Y.X., Melhem A., Schmelzer E., Zdanowicz M., Huang J., Caballero M., Fair J.H., Ludlow J.W., McClelland R.E., et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G859–G870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 103.Michelotti G.A., Xie G., Swiderska M., Choi S.S., Karaca G., Kruger L., Premont R., Yang L., Syn W.K., Metzger D., et al. Smoothened is a master regulator of adult liver repair. J. Clin. Investig. 2013;123:2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang L., Wang Y., Mao H., Fleig S., Omenetti A., Brown K.D., Sicklick J.K., Li Y.X., Diehl A.M. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J. Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guy C.D., Suzuki A., Zdanowicz M., Abdelmalek M.F., Burchette J., Unalp A., Diehl A.M. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swiderska-Syn M., Syn W.K., Xie G., Krüger L., Machado M.V., Karaca G., Michelotti G.A., Choi S.S., Premont R.T., Diehl A.M. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2014;63:1333–1344. doi: 10.1136/gutjnl-2013-305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guy C.D., Suzuki A., Abdelmalek M.F., Burchette J.L., Diehl A.M. Treatment response in the PIVENS trial is associated with decreased Hedgehog pathway activity. Hepatology. 2015;61:98–107. doi: 10.1002/hep.27235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S.A., Gayyed M.F., Anders R.A., Maitra A., Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mo J.S., Park H.W., Guan K.L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu F., Lagares D., Choi K.M., Stopfer L., Marinković A., Vrbanac V., Probst C.K., Hiemer S.E., Sisson T.H., Horowitz J.C., et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mannaerts I., Leite S.B., Verhulst S., Claerhout S., Eysackers N., Thoen L.F., Hoorens A., Reynaert H., Halder G., van Grunsven L.A. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 112.Mooring M., Fowl B.H., Lum S.Z.C., Liu Y., Yao K., Softic S., Kirchner R., Bernstein A., Singhi A.D., Jay D.G., et al. Hepatocyte Stress Increases Expression of Yes-Associated Protein and Transcriptional Coactivator with PDZ-Binding Motif in Hepatocytes to Promote Parenchymal Inflammation and Fibrosis. Hepatology. 2020;71:1813–1830. doi: 10.1002/hep.30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu H.X., Yao Y., Bu F.T., Chen Y., Wu Y.T., Yang Y., Chen X., Zhu Y., Wang Q., Pan X.Y., et al. Blockade of YAP alleviates hepatic fibrosis through accelerating apoptosis and reversion of activated hepatic stellate cells. Mol. Immunol. 2019;107:29–40. doi: 10.1016/j.molimm.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 114.Anakk S., Bhosale M., Schmidt V.A., Johnson R.L., Finegold M.J., Moore D.D. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bruschi F.V., Tardelli M., Einwallner E., Claudel T., Trauner M. PNPLA3 I148M Up-Regulates Hedgehog and Yap Signaling in Human Hepatic Stellate Cells. Int. J. Mol. Sci. 2020;21:8711. doi: 10.3390/ijms21228711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Konishi T., Schuster R.M., Lentsch A.B. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;314:G471–G482. doi: 10.1152/ajpgi.00153.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Driskill J.H., Pan D. The Hippo Pathway in Liver Homeostasis and Pathophysiology. Annu. Rev. Pathol. 2021;16:299–322. doi: 10.1146/annurev-pathol-030420-105050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koo J.H., Guan K.L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018;28:196–206. doi: 10.1016/j.cmet.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 119.Harvey L.D., Chan S.Y. YAPping About Glutaminolysis in Hepatic Fibrosis. Gastroenterology. 2018;154:1231–1233. doi: 10.1053/j.gastro.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du K., Hyun J., Premont R.T., Choi S.S., Michelotti G.A., Swiderska-Syn M., Dalton G.D., Thelen E., Rizi B.S., Jung Y., et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology. 2018;154:1465–1479.e1413. doi: 10.1053/j.gastro.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pefani D.E., Pankova D., Abraham A.G., Grawenda A.M., Vlahov N., Scrace S., O’Neill E. TGF-β Targets the Hippo Pathway Scaffold RASSF1A to Facilitate YAP/SMAD2 Nuclear Translocation. Mol. Cell. 2016;63:156–166. doi: 10.1016/j.molcel.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 122.Huang Z., Hu J., Pan J., Wang Y., Hu G., Zhou J., Mei L., Xiong W.C. YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development. 2016;143:2398–2409. doi: 10.1242/dev.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y., Shen R.W., Han B., Li Z., Xiong L., Zhang F.Y., Cong B.B., Zhang B. Notch signaling mediated by TGF-β/Smad pathway in concanavalin A-induced liver fibrosis in rats. World J. Gastroenterol. 2017;23:2330–2336. doi: 10.3748/wjg.v23.i13.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aimaiti Y., Yusufukadier M., Li W., Tuerhongjiang T., Shadike A., Meiheriayi A., Abudusalamu A., Wang H., Tuerganaili A., Shao Y., et al. TGF-β1 signaling activates hepatic stellate cells through Notch pathway. Cytotechnology. 2019;71:881–891. doi: 10.1007/s10616-019-00329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tariki M., Dhanyamraju P.K., Fendrich V., Borggrefe T., Feldmann G., Lauth M. The Yes-associated protein controls the cell density regulation of Hedgehog signaling. Oncogenesis. 2014;3:e112. doi: 10.1038/oncsis.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swiderska-Syn M., Xie G., Michelotti G.A., Jewell M.L., Premont R.T., Syn W.K., Diehl A.M. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–244. doi: 10.1002/hep.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Y., Choi S.S., Michelotti G.A., Chan I.S., Swiderska-Syn M., Karaca G.F., Xie G., Moylan C.A., Garibaldi F., Premont R., et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329.e1311. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie G., Karaca G., Swiderska-Syn M., Michelotti G.A., Krüger L., Chen Y., Premont R.T., Choi S.S., Diehl A.M. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58:1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.