Abstract
This cohort study reviews enrollment patterns of completed cancer drug trials over the past 20 years to compare sex-specific trial participation with current cancer incidence rates.
Thirty years have passed since the enactment of the National Institutes of Health (NIH) Revitalization Act, which encouraged NIH-funded investigators to include adequate numbers of women in clinical studies.1 Since then, there have been important steps taken to ensure better representation of women and racial and ethnic minority groups in biomedical trials.2 However, lack of representation remains problematic in oncology. Previous research suggests that women represent approximately 30% to 40% of participants in trials leading to drug approvals in the US.3,4
In 2010, the NIH Office of Research on Women’s Health5 set forth a vision to advance the understanding of sex-specific disease differences by 2020. As clinical trials become international in scope, we sought to evaluate the global movement toward this vision in oncology. In this cohort study, we reviewed enrollment patterns of completed cancer drug trials over the past 20 years to compare sex-specific trial participation to current cancer incidence rates. Using data from the International Agency for Research on Cancer (IARC),6 we identified 6 common solid tumor types for women (lung, colon, thyroid, melanoma, kidney, and pancreas).
Methods
We conducted a systematic search of cancer drug trials registered on ClinicalTrials.gov between 2000 and 2020 for 6 common cancers for women (excluding breast and uterus). The investigation of publicly available trial protocols was exempt from institutional review board approval. We included completed drug trials with results for adults (≥18 years). Behavioral, device, procedure, and radiotherapy trials were excluded (eFigure in the Supplement). Pearson χ2 test of independence was used to evaluate the association between female and male enrollment and phase (1, 2, 3), tumor type, and funding source. P values used 2-tailed tests, with P < .05 being significant. We explored changes in sex-specific enrollment patterns over 2 decades by stratifying trials as 2000 to 2010 and 2011 to 2020. Cancer incidence rates were calculated by dividing the number of new male and female cases by the total number of new cases per tumor type (2020).6 Data analysis was performed using R statistical software, version 1.1.463 (R Foundation).
Results
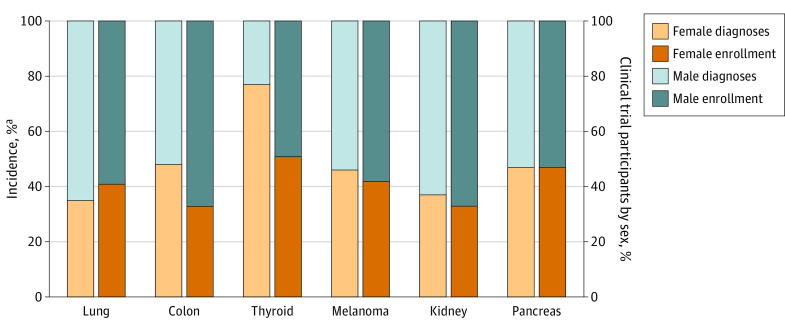
We identified 505 oncology clinical trials that met the eligibility criteria between 2000 and 2020. Of the total 182 416 participants, 73 103 (40%) were women, while 109 313 (60%) were men. We observed significant differences between the enrollment of sexes across all trial phases and tumor types except thyroid (Table). Drug trials for colon and kidney cancer enrolled the least number of women, with 33% participation rates (Figure).
Table. Comparison Between Sex-Specific Enrollment and Clinical Trial Characteristics.
| Characteristic | Sex, No. (%) | P valuea | |
|---|---|---|---|
| Female | Male | ||
| Total enrolled | 73 103 (40) | 109 313 (60) | <.001 |
| Trial phase | |||
| 1 | 3034 (48) | 3322 (52) | .001 |
| 2 | 18 838 (43) | 24 508 (57) | <.001 |
| 3 | 40 139 (38) | 66 611 (62) | <.001 |
| Year | |||
| 2000-2010 | 23 350 (40) | 34 745 (60) | <.001 |
| 2011-2020 | 49 753 (42) | 68 022 (58) | |
| Tumor type | |||
| Lung | 40 829 (41) | 57 979 (59) | <.001 |
| Colon | 7600 (33) | 15 266 (67) | <.001 |
| Thyroid | 904 (51) | 875 (49) | .50 |
| Melanoma | 11 317 (42) | 15 529 (58) | <.001 |
| Kidney | 6586 (33) | 13 127 (67) | <.001 |
| Pancreas | 5867 (47) | 6537 (53) | <.001 |
| Sites | |||
| US | 49 911 (40) | 75 755(60) | <.001 |
| Canada | 29 603 (39) | 45 372 (61) | <.001 |
| China | 23 456 (41) | 33 645 (56) | <.001 |
| United Kingdom | 28 472 (39) | 44 478 (61) | <.001 |
| Australia | 28 505 (39) | 44 332 (61) | <.001 |
| Funding (US) | |||
| Industry | 41 391 (41) | 60 473 (59) | <.001 |
| NIH | 6828 (48) | 7285 (52) | |
Abbreviation: NIH, National Institutes of Health.
P values from Pearson χ2 test of independence.
Figure. Composition of Trial Enrollment and Incidence by Sex per Tumor Type.
aNew cases by sex per International Agency for Research on Cancer 2020 data.6
Comparing 2000 to 2010 with 2011 to 2020, we observed a marginal increase in female participation, from 40% to 42%, respectively (P < .001). We evaluated the association between industry and NIH-sponsored studies in the US (n = 358) and found that NIH-funded trials enrolled a higher proportion of women (48%) compared with industry trials (41%) (P < .001).
Discussion
It is essential that women be enrolled in clinical trials in numbers that, at least, mirror the distribution of the disease in the population so that potential biological differences can be understood. Our analysis, although limited to 6 tumor types, suggests that sex differences persist in clinical trials. In 2020, women represented 77% of newly diagnosed thyroid cancer cases worldwide6 yet comprised only 51% of participants in trials investigating thyroid drugs (Figure). Similarly, women represented 48% of global colon cancer cases6 yet accounted for only 33% of trial participants for colon cancer therapeutics (Figure).
Higher enrollment of women in NIH-funded studies (48%) compared with industry studies (41%) warrants further exploration, but the stagnant rates of women in trials suggest that regulatory initiatives2 over the past 2 decades may be insufficient. Our analysis, although limited to sponsor- and manufacturer-disclosed information, demonstrates that persistent inequities remain in the recruitment of female participants in trials investigating new therapeutics for certain tumor types in oncology.
eFigure. Identification of completed oncology clinical trials for lung, colon, thyroid, melanoma, kidney, and pancreas cancer drugs between 2000-2020
References
- 1.NIH Revitalization Act of 1993 Public Law 103-43. In: Mastroianni AC, Faden R, Federman D, eds. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies: Volume I. National Academies Press; 1994:appendix B. Accessed May 5, 2021. https://www.ncbi.nlm.nih.gov/books/NBK236531/ [PubMed]
- 2.Mazure CM, Jones DP. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health. 2015;15(1):94. doi: 10.1186/s12905-015-0251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 4.Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14(1):e1-e10. doi: 10.1200/JOP.2017.025288 [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Office of Research on Women’s Health . Strategic plan. moving into the future with new dimensions and strategies: a vision for 2020 for women’s health research. Accessed July 22, 2021. https://orwh.od.nih.gov/sites/orwh/files/docs/ORWH_StrategicPlan2020_Vol1.pdf
- 6.Global Cancer Observatory. Accessed June 16, 2021. https://gco.iarc.fr/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Identification of completed oncology clinical trials for lung, colon, thyroid, melanoma, kidney, and pancreas cancer drugs between 2000-2020